Submitted:
14 June 2023
Posted:
15 June 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Animals and Diets
2.2. Samples Collection
2.3. Determination of Biochemical Parameters
2.4. Determination of Malondialdehyde
2.5. Determination of Glycogen
2.6. Statistical Analysis
2.7. Ethical Considerations
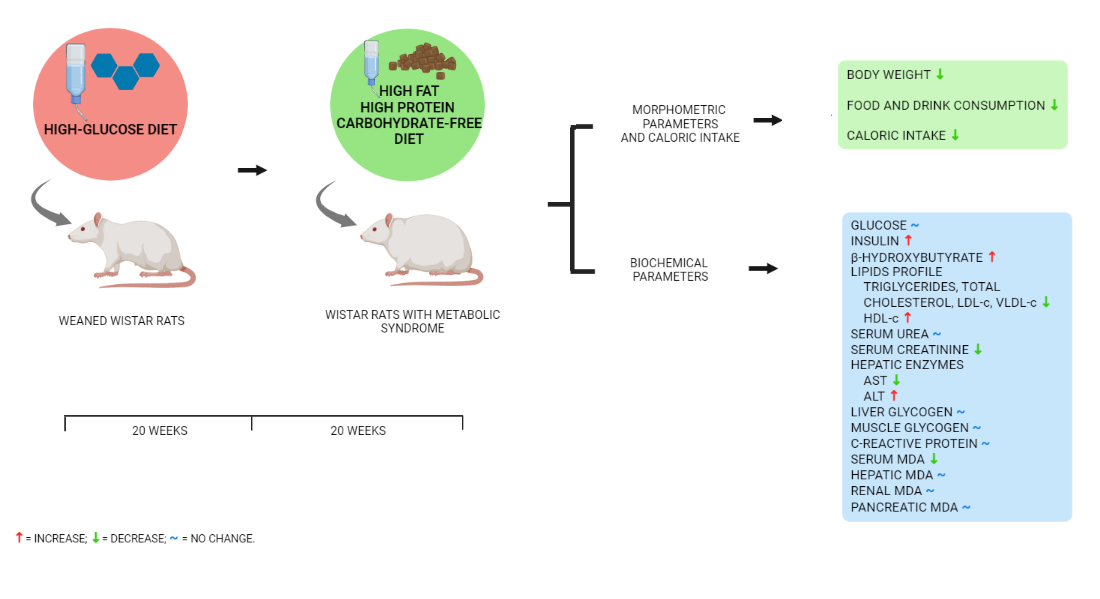
3. Results
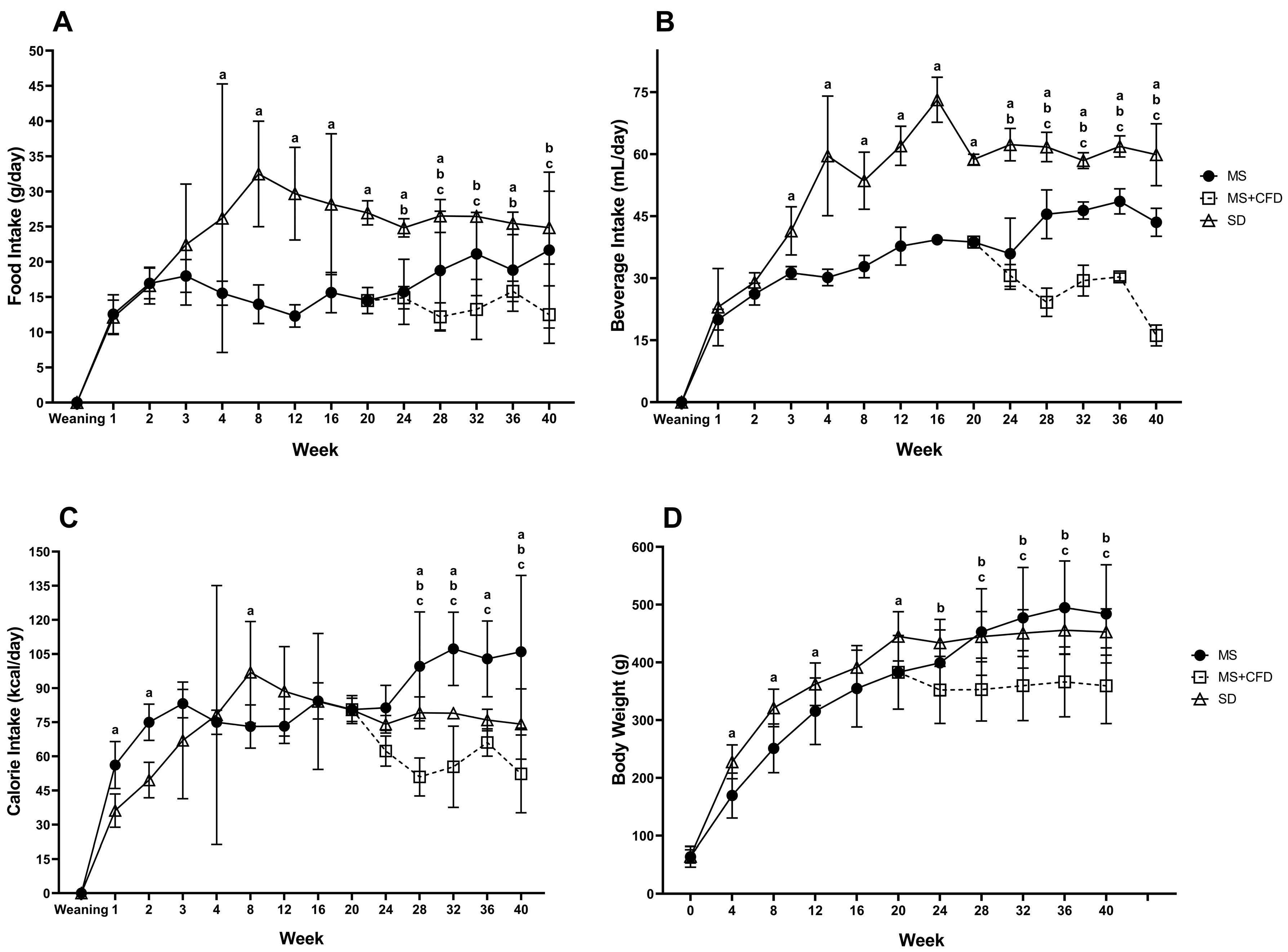
3.1. Morphometric Parameters and Calorie Intake
3.2. Biochemical Parameters
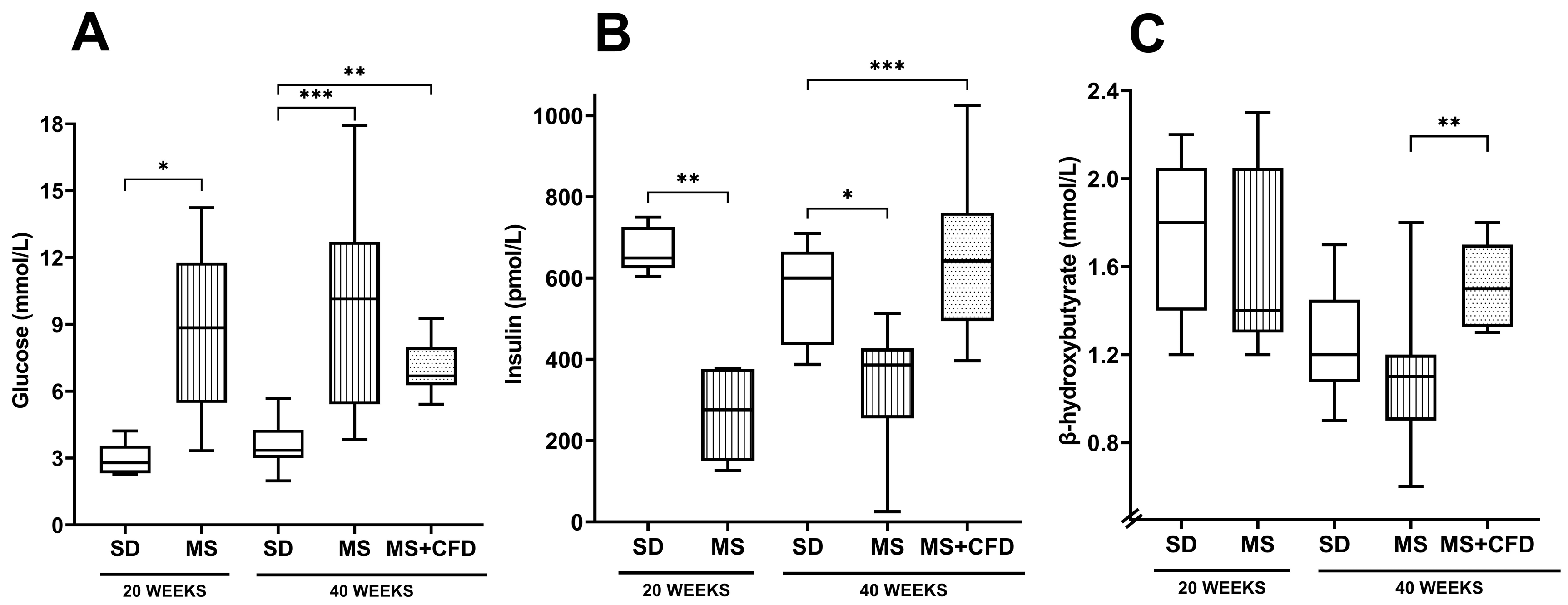
3.2.1. Glucose, Insulin and β-hydroxybutyrate
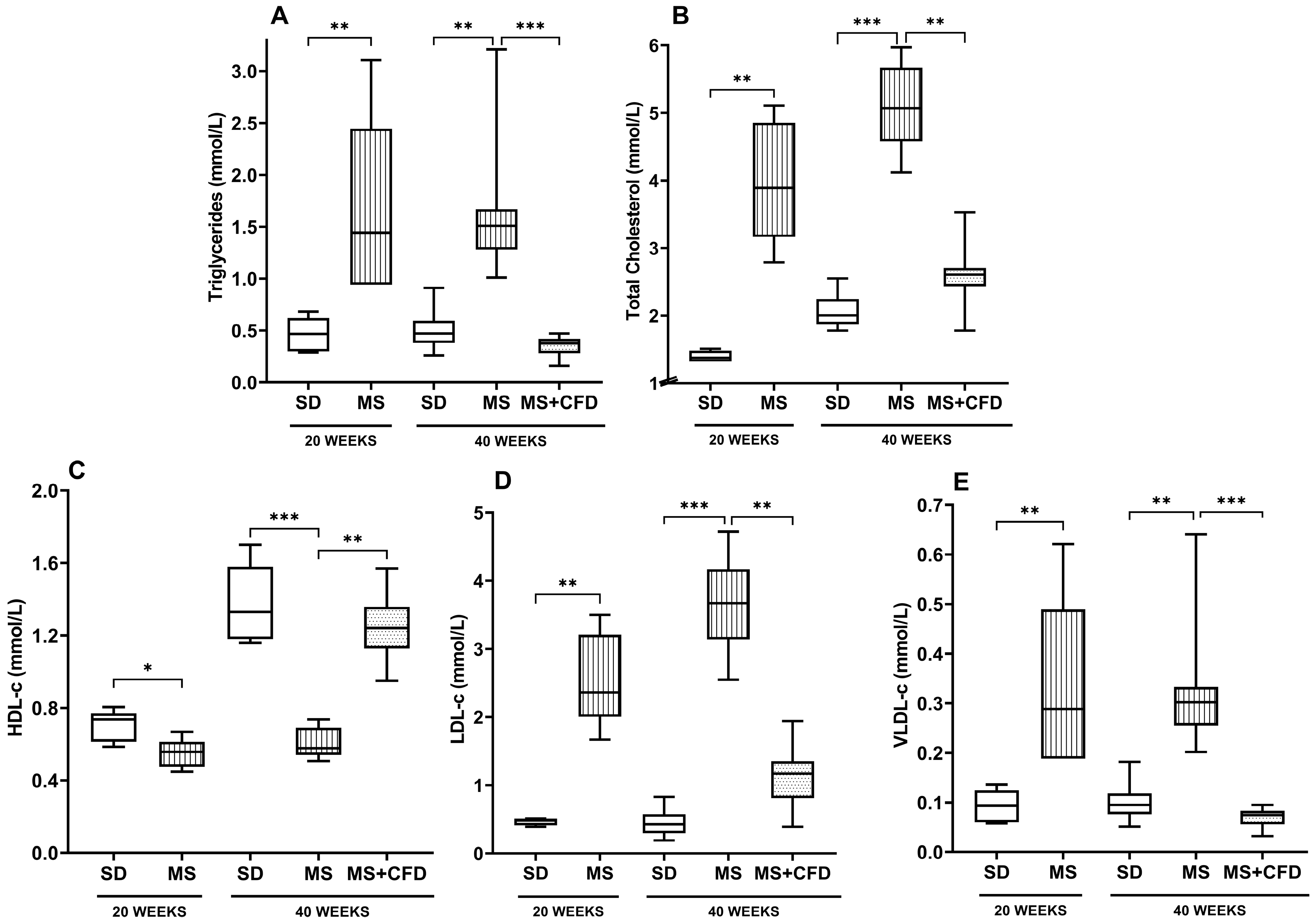
3.2.2. Lipids Profile
3.2.3. C-Reactive Protein
3.2.4. Serum Urea and Creatinine
3.2.5. Hepatic Enzymes and Glycogen
3.2.6. Malondialdehyde
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- McCracken: E.; Monaghan, M.; Sreenivasan, S. Pathophysiology of the Metabolic Syndrome. Clin. Dermatol. 2017, 36, 14–20. [CrossRef]
- Rochlani, Y.; Pothineni, N.V.; Kovelamudi, S. Metabolic Syndrome : Pathophysiology, Management, and Modulation by Natural Compounds. Ther Adv Cardiovasc Dis 2017, 11, 215–225. [CrossRef]
- Sherling, D.H.; Perumareddi, P.; Hennekens, C.H. Metabolic Syndrome: Clinical and Policy Implications of the New Silent Killer. J. Cardiovasc. Pharmacol. Ther. 2017, 22, 365–367. [CrossRef]
- Fahed, G.; Aoun, L.; Bou Zerdan, M.; Allam, S.; Bou Zerdan, M.; Bouferraa, Y.; Assi, H.I. Metabolic Syndrome: Updates on Pathophysiology and Management in 2021. Int. J. Mol. Sci. 2022, 23, 786. [CrossRef]
- Soleimani, M.; Barone, S.; Luo, H.; Zahedi, K. Pathogenesis of Hypertension in Metabolic Syndrome: The Role of Fructose and Salt. Int. J. Mol. Sci. 2023, 24, 4294. [CrossRef]
- Kassi, E.; Pervanidou, P.; Kaltsas, G.; Chrousos, G. Metabolic Syndrome: Definitions and Controversies. BMC Med. 2011, 9, 48. [CrossRef]
- Akter, S.; Akhter, H.; Chaudhury, H.S.; Rahman, M.H.; Gorski, A.; Hasan, M.N.; Shin, Y.; Rahman, M.A.; Nguyen, M.N.; Choi, T.G.; et al. Dietary Carbohydrates: Pathogenesis and Potential Therapeutic Targets to Obesity-Associated Metabolic Syndrome. Biofactors 2022, 48, 1036–1059. [CrossRef]
- Xu, H.; Li, X.; Adams, H.; Kubena, K.; Guo, S. Etiology of Metabolic Syndrome and Dietary Intervention. Int. J. Mol. Sci. 2019, 20, 128. [CrossRef]
- Smith, C.J.; Ryckman, K.K. Epigenetic and Developmental Influences on the Risk of Obesity, Diabetes, and Metabolic Syndrome. Diabetes, Metab. Syndr. Obes. Targets Ther. 2015, 8, 295–302. [CrossRef]
- Chan, A.M.L.; Ng, A.M.H.; Mohd Yunus, M.H.; Idrus, R.B.H.; Law, J.X.; Yazid, M.D.; Chin, K.Y.; Shamsuddin, S.A.; Lokanathan, Y. Recent Developments in Rodent Models of High-Fructose Diet-Induced Metabolic Syndrome: A Systematic Review. Nutrients 2021, 13, 1–21. [CrossRef]
- Rodríguez-Monforte, M.; Sánchez, E.; Barrio, F.; Costa, B.; Flores-Mateo, G. Metabolic Syndrome and Dietary Patterns: A Systematic Review and Meta-analysis of Observational Studies. Eur. J. Nutr. 2016, 56, 925–947. [CrossRef]
- Hyde, P.N.; Sapper, T.N.; Crabtree, C.D.; LaFountain, R.A.; Bowling, M.L.; Buga, A.; Fell, B.; McSwiney, F.T.; Dickerson, R.M.; Miller, V.J.; et al. Dietary Carbohydrate Restriction Improves Metabolic Syndrome Independent of Weight Loss. JCI Insight 2019, 4, e128308. [CrossRef]
- Marsset-Baglieri, A.; Fromentin, G.; Tomé, D.; Bensaid, A.; Makkarios, L.; Even, P.C. Increasing the Protein Content in a Carbohydrate-Free Diet Enhances Fat Loss during 35% but Not 75% Energy Restriction in Rats. J. Nutr. 2004, 134, 2646–2652. [CrossRef]
- Volek, J.S.; Feinman, R.D. Carbohydrate Restriction Improves the Features of Metabolic Syndrome. Metabolic Syndrome May Be Defined by the Response to Carbohydrate Restriction. Nutr. Metab. 2005, 2, 1–17. [CrossRef]
- Azzout, B.; Chanez, M.; Bois-Joyeux, B.; Peret, J. Gluconeogenesis from Dihydroxyacetone in Rat Hepatocytes during the Shift from a Low Protein, High Carbohydrate to a High Protein, Carbohydrate-Free Diet. J. Nutr. 1984, 114, 2167–2178. [CrossRef]
- Pichon, L.; Huneu, J.; Fromentin, G.; Tomé, D. A High-Protein, High-Fat, Carbohydrate-Free Diet Reduces Energy Intake, Hepatic Lipogenesis, and Adiposity in Rats. Nutr. Physiol. Metab. Nutr. Interact. 2006, 136. [CrossRef]
- Jürgens, H.S.; Neschen, S.; Ortmann, S.; Scherneck, S.; Schmolz, K.; Schüler, G.; Schmidt, S.; Blüher, M.; Klaus, S.; Perez-Tilve, D.; et al. Development of Diabetes in Obese, Insulin-Resistant Mice: Essential Role of Dietary Carbohydrate in Beta Cell Destruction. Diabetologia 2007, 50, 1481–1489. [CrossRef]
- Mirhashemi, F.; Kluth, O.; Scherneck, S.; Vogel, H.; Schürmann, A.; Susanne, H.J. High-Fat , Carbohydrate-Free Diet Markedly Aggravates Obesity but Prevents β -Cell Loss and Diabetes in the Obese , Diabetes-Susceptible Db / Db Strain. 2008, 292–297. [CrossRef]
- Uchiyama, M.; Mihara, M. Determination of Malonaldehyde Precursor in Tissues by Thiobarbituric Acid Test. Anal. Biochem. 1978, 86, 271–278. [CrossRef]
- Fong, J.; Schaffer, F.L.; Kirk, P.L. The Ultramicrodetermination of Glycogen in Liver. Arch. Biochem. Biophys. 1953, 45, 319–326. [CrossRef]
- O’Neill, B.J. Effect of Low-Carbohydrate Diets on Cardiometabolic Risk, Insulin Resistance, and Metabolic Syndrome. Curr. Opin. Endocrinol. Diabetes. Obes. 2020, 27, 301–307. [CrossRef]
- Korkmaz, O.A.; Sadi, G.; Kocabas, A.; Yildirim, O.G.; Sumlu, E.; Koca, H.B.; Nalbantoglu, B.; Pektas, M.B.; Akar, F. Lactobacillus Helveticus and Lactobacillus Plantarum Modulate Renal Antioxidant Status in a Rat Model of Fructose-Induced Metabolic Syndrome. Arch. Biol. Sci. 2019, 71, 265–273. [CrossRef]
- Kubacka, M.; Kotanska, M.; Szafarz, M.; Pociecha, K.; Waszkielewicz, A.M.; Marona, H.; Filipek, B.; Mogilski, S. Beneficial Effects of Non-Quinazoline A1-Adrenolytics on Hypertension and Altered Metabolism in Fructose-Fed Rats. A Comparison with Prazosin. Nutr. Metab. Cardiovasc. Dis. 2019, 29, 751–760. [CrossRef]
- Neeland, I.J.; Turer, A.T.; Ayers, C.R.; Powell-Wiley, T.M.; Vega, G.L.; Farzaneh-Far, R.; Grundy, S.M.; Khera, A.; McGuire, D.K.; de Lemos, J.A. Dysfunctional Adiposity and the Risk of Prediabetes and Type 2 Diabetes in Obese Adults. JAMA 2013, 308, 1150–1159. [CrossRef]
- Bratoeva, K.; Nikolova, S.; Merdzhanova, A.; Stoyanov, G.S.; Dimitrova, E.; Kashlov, J.; Conev, N.; Radanova, M. Association between Serum CK-18 Levels and the Degree of Liver Damage in Fructose-Induced Metabolic Syndrome. Metab. Syndr. Relat. Disord. 2018, 16, 350–357. [CrossRef]
- Ferreira-Santos, P.; Aparicio, R.; Carrón, R.; Montero, M.J.; Sevilla, M.Á. Lycopene-Supplemented Diet Ameliorates Metabolic Syndrome Induced by Fructose in Rats. J. Funct. Foods 2020, 73, 104098. [CrossRef]
- Preguiça, I.; Alves, A.; Nunes, S.; Fernandes, R.; Gomes, P.; Viana, S.D.; Reis, F. Diet-Induced Rodent Models of Obesity-Related Metabolic Disorders—A Guide to a Translational Perspective. Obes. Rev. 2020, 21, 1–29. [CrossRef]
- Moon, J.; Koh, G. Clinical Evidence and Mechanisms of High-Protein Diet-Induced Weight Loss. J. Obes. Metab. Syndr. 2021, 29, 166–173. [CrossRef]
- Pichon, L.; Huneu, J.; Fromentin, G.; Tomé, D. A High-Protein, High-Fat, Carbohydrate-Free Diet Reduces Energy Intake, Hepatic Lipogenesis, and Adiposity in Rats. Nutr. Physiol. Metab. Nutr. Interact. 2006, 136, 1256–1260. [CrossRef]
- Fukazawa, A.; Koike, A.; Karasawa, T.; Tsutsui, M.; Kondo, S.; Terada, S. Effects of a Ketogenic Diet Containing Medium-Chain Triglycerides and Endurance Training on Metabolic Enzyme Adaptations in Rat Skeletal Muscle. Nutrients 2020, 12. [CrossRef]
- Rodríguez-Correa, E.; González-Pérez, I.; Clavel-Pérez, P.I.; Contreras-Vargas, Y.; Carvajal, K. Biochemical and Nutritional Overview of Diet-Induced Metabolic Syndrome Models in Rats: What Is the Best Choice? Nutr. Diabetes 2020, 10, 24. [CrossRef]
- Wong, S.K.; Chin, K.Y.; Suhaimi, F.H.; Fairus, A.; Ima-Nirwana, S. Animal Models of Metabolic Syndrome: A Review. Nutr. Metab. 2016, 13, 1–12. [CrossRef]
- Roberts, C.K.; Hevener, A.L.; Barnard, R.J. Metabolic Syndrome and Insulin Resistance: Underlying Causes and Modification by Exercise Training. Compr. Physiol. 2013, 3, 1–58. [CrossRef]
- Thomas, D.D.; Corkey, B.E.; Istfan, N.W.; Apovian, C.M. Hyperinsulinemia: An Early Indicator of Metabolic Dysfunction. J. Endocr. Soc. 2019, 3, 1727–1747. [CrossRef]
- Brito, S.M.R.C.; Moura, M.A.F.; Kawashita, N.H.; Festuccia, W.T.L.; Garófalo, M.A.R.; Kettelhut, I.C.; Migliorini, R.H. Adaptation to a High Protein, Carbohydrate-Free Diet Induces a Marked Reduction of Fatty Acid Synthesis and Lipogenic Enzymes in Rat Adipose Tissue That Is Rapidly Reverted by a Balanced Diet. Can. J. Physiol. Pharmacol. 2005, 83, 477–482. [CrossRef]
- Veldhorst, M.A.; Westerterp-Plantenga, M.S.; Westerterp, K.R. Gluconeogenesis and Energy Expenditure after a High-Protein, Carbohydrate-Free Diet. Am. J. Clin. Nutr. 2009, 90, 519–526. [CrossRef]
- Sukkar, A.H.; Lett, A.M.; Frost, G.; Chambers, E.S. Regulation of Energy Expenditure and Substrate Oxidation by Short-Chain Fatty Acids. J. Endocrinol. 2019, 242, R1–R8. [CrossRef]
- Locatelli, C.A.A.; Mulvihill, E.E. Islet Health, Hormone Secretion, and Insulin Responsivity with Low-Carbohydrate Feeding in Diabetes. Metabolites 2020, 10, 1–17. [CrossRef]
- Møller, N. Ketone Body, 3-Hydroxybutyrate: Minor Metabolite - Major Medical Manifestations. J. Clin. Endocrinol. Metab. 2020, 105, dgaa370. [CrossRef]
- Newman, J.C.; Verdin, E. β -Hydroxybutyrate: Much More than a Metabolite. Diabetes Res. Clin. Pract. 2014, 106, 173–181. [CrossRef]
- Haile, K.; Haile, A.; Timerga, A. Predictors of Lipid Profile Abnormalities Among Patients with Metabolic Syndrome in Southwest Ethiopia: A Cross-Sectional Study. Vasc. Health Risk Manag. 2021, 17, 461–469. [CrossRef]
- Forouhi, N.G.; Krauss, R.M.; Taubes, G.; Willett, W. Dietary Fat and Cardiometabolic Health: Evidence, Controversies, and Consensus for Guidance. BMJ 2018, 361, k2139. [CrossRef]
- Mansoor, N.; Vinknes, K.J.; Veierød, M.B.; Retterstøl, K. Effects of Low-Carbohydrate Diets v. Low-Fat Diets on Body Weight and Cardiovascular Risk Factors: A Meta-Analysis of Randomised Controlled Trials. Br. J. Nutr. 2016, 115, 466–479. [CrossRef]
- Reddy, P.; Lent-Schochet, D.; Ramakrishnan, N.; Mclaughlin, M.; Jialal, I. Metabolic Syndrome Is an Inflammatory Disorder: A Conspiracy between Adipose Tissue and Phagocytes. Clin. Chim. Acta 2019, 496, 35–44. [CrossRef]
- Kwitek, A.E. Rat Models of Metabolic Syndrome. Methods Mol. Biol. 2019, 2018, 269–285. [CrossRef]
- Monteiro, R.; Azevedo, I. Chronic Inflammation in Obesity and the Metabolic Syndrome. Mediators Inflamm. 2010, 2010. [CrossRef]
- Koh, S.; Dupuis, N.; Cit, S.P.; Debr, R. Ketogenic Diet and Neuroinflammation. Epilepsy Res. 2020, 167, 1–8. [CrossRef]
- Valle-Martos, R.; Valle, M.; Martos, R.; Cañete, R.; Jiménez-Reina, L.; Cañete, M.D. Liver Enzimes Correlate with Metabolic Syndrome, Inflammation, and Endothelial Dysfunction in Prepubertal Children with Obesity. Front. Pediatr. 2021, 9, 629346. [CrossRef]
- Paoli, A.; Bianco, A.; Grimaldi, K.A.; Lodi, A.; Bosco, G. Long Term Successful Weight Loss with a Combination Biphasic Ketogenic Mediterranean Diet and Mediterranean Diet Maintenance Protocol. Nutrients 2013, 5, 5205–5217. [CrossRef]
- Walton, C.M.; Perry, K.; Hart, R.H.; Berry, S.L.; Bikman, B.T. Improvement in Glycemic and Lipid Profiles in Type 2 Diabetics with a 90-Day Ketogenic Diet. 2019, 2019. [CrossRef]
- Luukkonen, P.K.; Dufour, S.; Lyu, K.; Zhang, X.M.; Hakkarainen, A.; Lehtimäki, T.E.; Cline, G.W.; Petersen, K.F.; Shulman, G.I.; Yki-Järvinen, H. Effect of a Ketogenic Diet on Hepatic Steatosis and Hepatic Mitochondrial Metabolism in Nonalcoholic Fatty Liver Disease. Proc. Natl. Acad. Sci. U. S. A. 2020, 117, 7347–7354. [CrossRef]
- Lum, G. Low Activities of Aspartate and Alanine Aminotransferase: Their Significance in Alcoholic Liver Disease. Lab. Med. 1995, 26, 4–7. [CrossRef]
- Roach, P.J.; Depaoli-Roach, A.A.; Hurley, T.D.; Tagliabracci, V.S. Glycogen and Its Metabolism: Some New Developments and Old Themes. Biochem. J. 2016, 441, 763–787. [CrossRef]
- Al-Daghri, N.M.; Al-Attas, O.S.; Wani, K.; Sabico, S.; Alokail, M.S. Serum Uric Acid to Creatinine Ratio and Risk of Metabolic Syndrome in Saudi Type 2 Diabetic Patients. Sci. Rep. 2017, 1–8. [CrossRef]
- Tang, J.; Yan, H.; Zhuang, S. Inflammation and Oxidative Stress in Obesity-Related Glomerulopathy. Int. J. Nephrol. 2012, 2012. [CrossRef]
- Ko, G.J.; Obi, Y.; Tortoricci, A.R.; Kalantar-Zadeh, K. Dietary Protein Intake and Chronic Kidney Disease. Curr. Opin. Clin. Nutr. Metab. Care 2017, 20, 77–85. [CrossRef]
- Juraschek, S.P.; Chang, A.R.; Appel, L.J.; Anderson, C.A.M.; Crews, D.C.; Thomas, L.; Charleston, J.; Iii, E.R.M. Effect of Glycemic Index and Carbohydrate Intake on Kidney Function in Healthy Adults. BMC Nephrol. 2016, 17, 70. [CrossRef]
- Kostogrys, R.B.; Franczyk-Żarów, M.; Maślak, E.; Topolska, K. Effect of Low Carbohydrate High Protein (LCHP) Diet on Lipid Metabolism, Liver and Kidney Function in Rats. Environ. Toxicol. Pharmacol. 2015, 39, 713–719. [CrossRef]
- Martin, W.F.; Armstrong, L.E.; Rodriguez, N.R. Dietary Protein Intake and Renal Function. Nutr. Metab. 2005, 2, 25. [CrossRef]
- Onyango, A.N. Lipid Peroxidation as a Link between Unhealthy Diets and the Metabolic Syndrome. In Accenting Lipid Peroxidation; Atukeren, P., Ed.; 2021.
- Panchal, S.K.; Poudyal, H.; Iyer, A.; Nazer, R.; Alam, A.; Diwan, V.; Kauter, K.; Sernia, C.; Campbell, F.; Ward, L.; et al. High-Carbohydrate High-Fat Diet-Induced Metabolic Syndrome and Cardiovascular Remodeling in Rats. J. Cardiovasc. Pharmacol. 2011, 57, 51–64. [CrossRef]
- Avelar, T.M.T.; Storch, A.S.; Castro, L.A.; Azevedo, G.V.M.M.; Ferraz, L.; Lopes, P.F. Oxidative Stress in the Pathophysiology of Metabolic Syndrome : Which Mechanisms Are Involved ? 2015, 231–239. [CrossRef]
- Chimienti, G.; Orlando, A.; Lezza, A.M.S.; D’Attoma, B.; Notarnicola, M.; Gigante, I.; Pesce, V.; Russo, F. The Ketogenic Diet Reduces the Harmful Effects of Stress on Gut Mitochondrial Biogenesis in a Rat Model of Irritable Bowel Syndrome. Int. J. Mol. Sci. 2021, 22, 3498. [CrossRef]
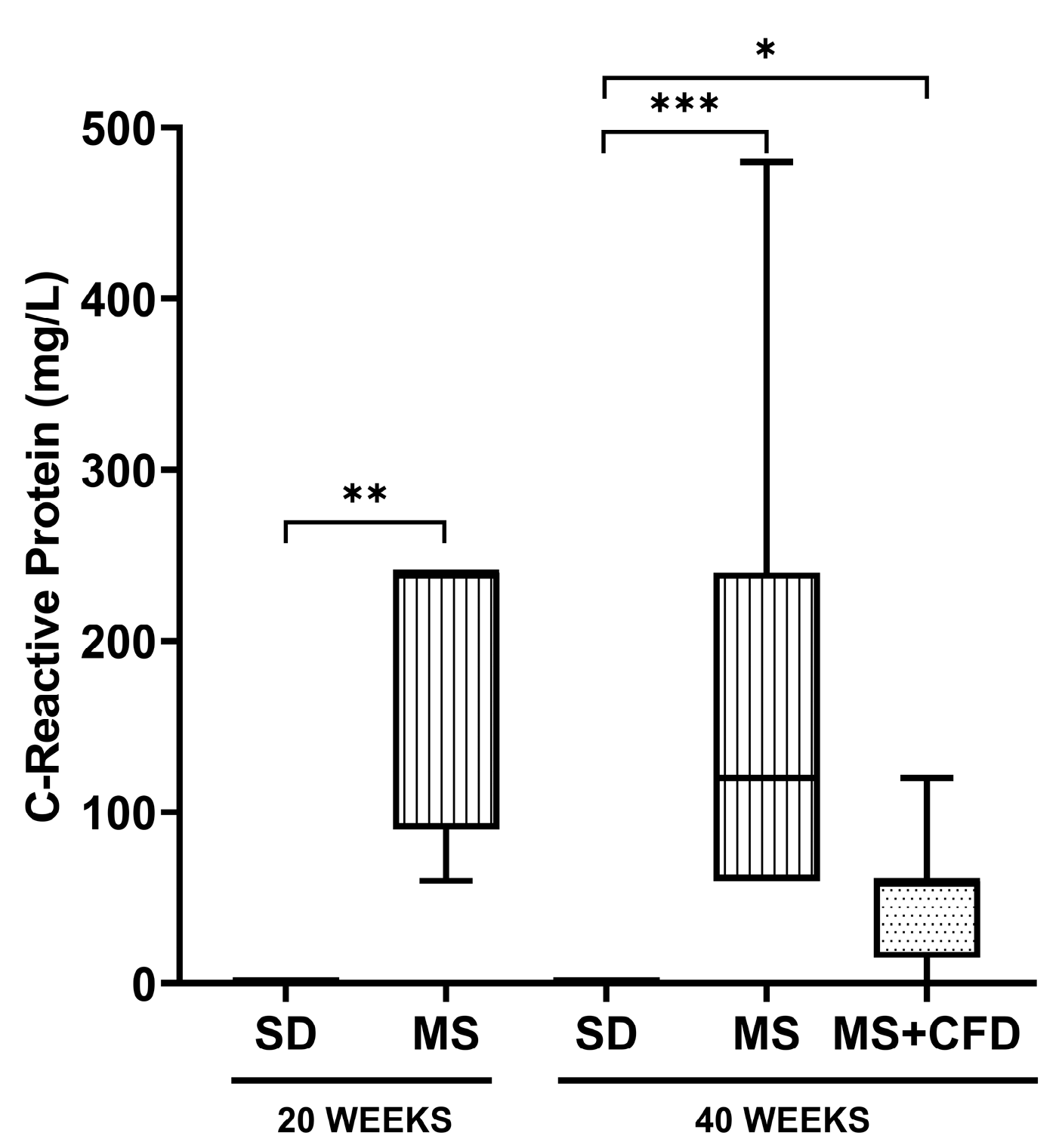
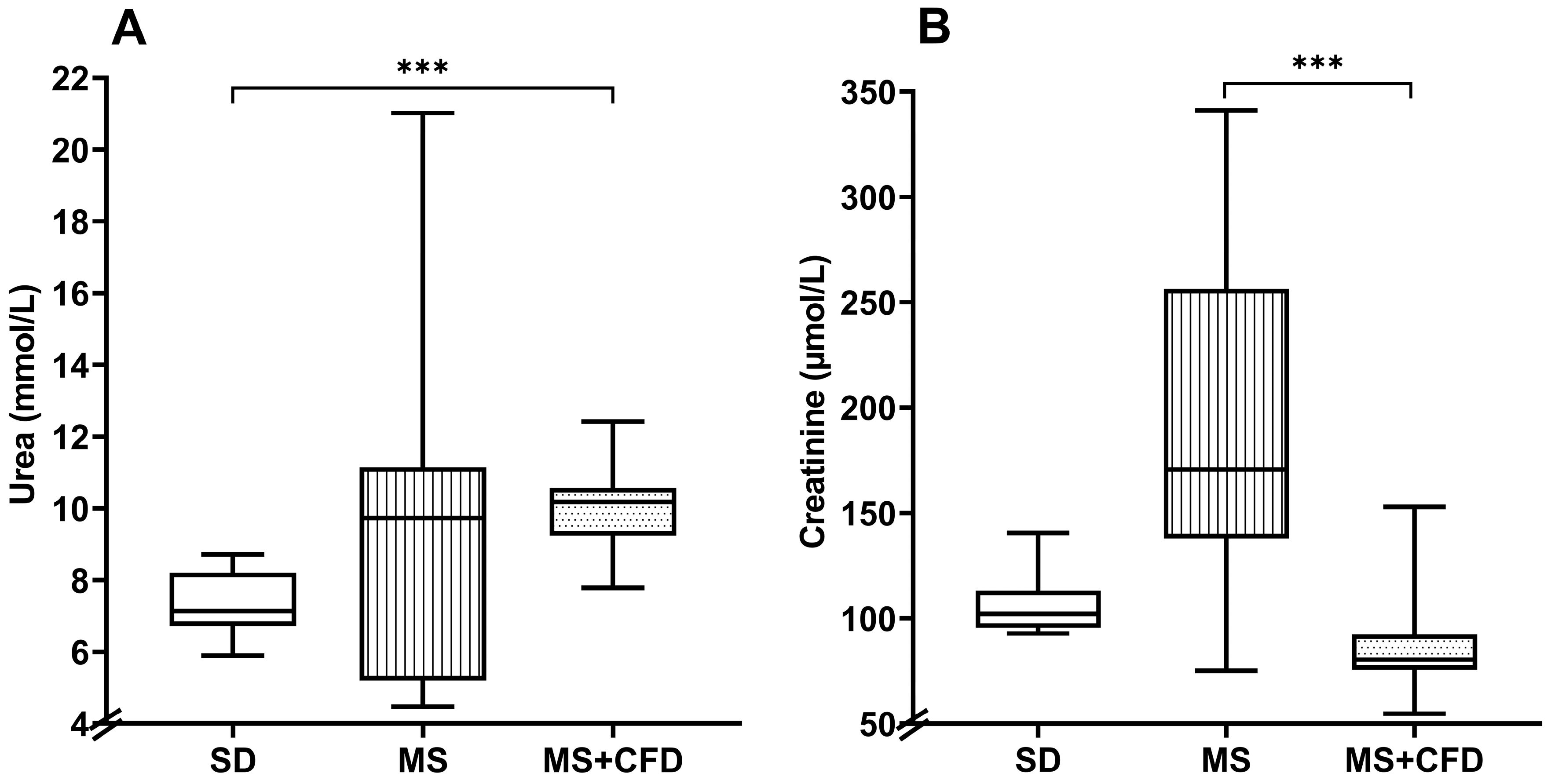
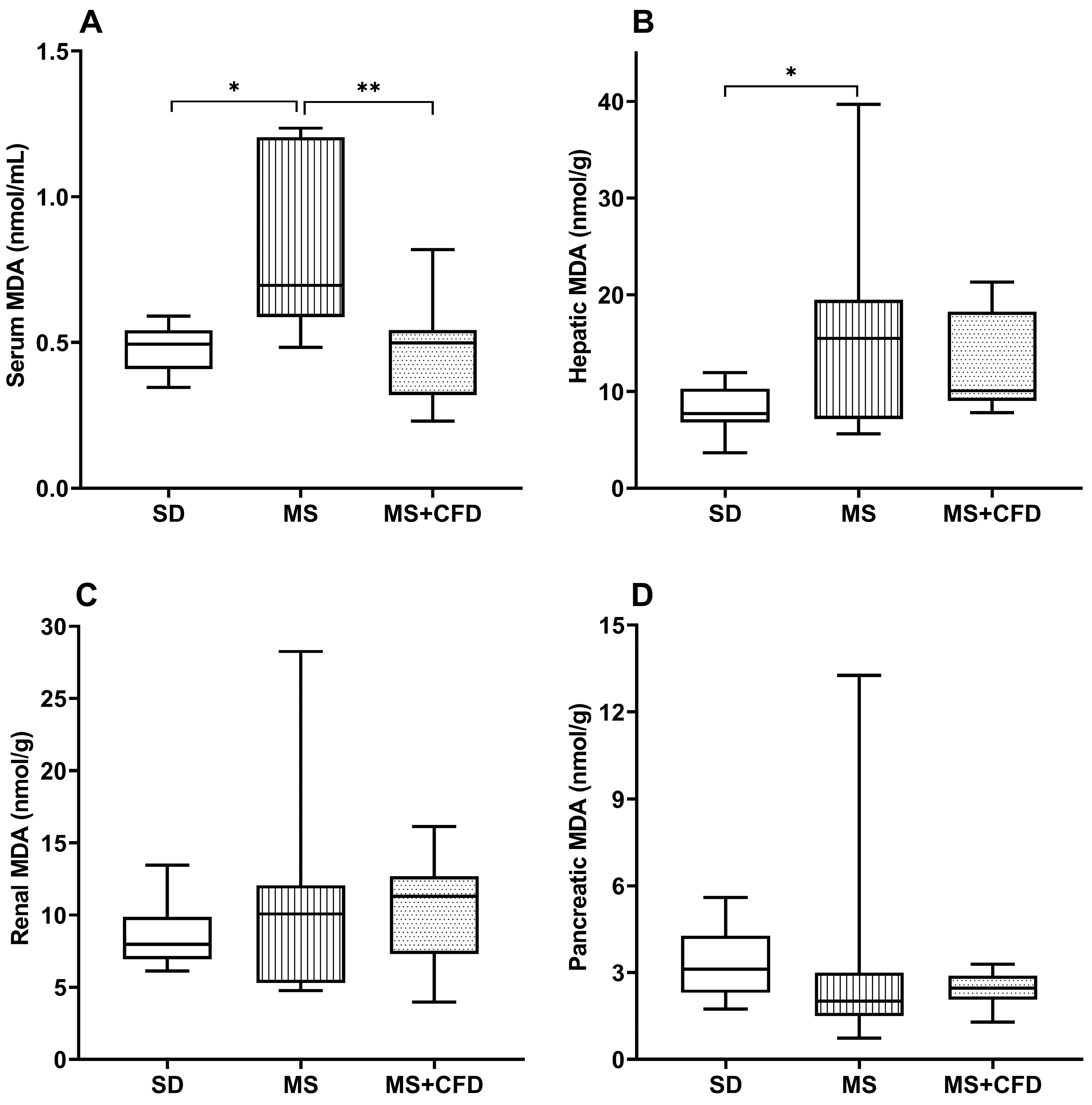
| Biochemical Parameter | SD Group | MS Group | MS+CFD Group | p Value |
|---|---|---|---|---|
| AST (U/L) | 84.29 (72.92-214.08) | 75.25 (54.83-155.75) | 44.63 (11.66-88.08) | 0.00082 0.0133 |
| ALT (U/L) | 49.88 (39.08-99.17) | 16.33 (13.42-24.50) | 31.79 (7.00-66.50) | 0.0001 0.0213 |
| Liver Glycogen (µmol/L) | 1.82 (1.41-2.85) | 6.47 (2.69-22.33) | 3.13 (0.14-5.23) | 0.0001 |
| Muscle Glycogen (µmol/L) | 2.64 (1.51-6.0) | 4.23 (1.84-5.75) | 2.85 (0.22-5.49) | ns |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




