Submitted:
13 July 2023
Posted:
14 July 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
3. Results
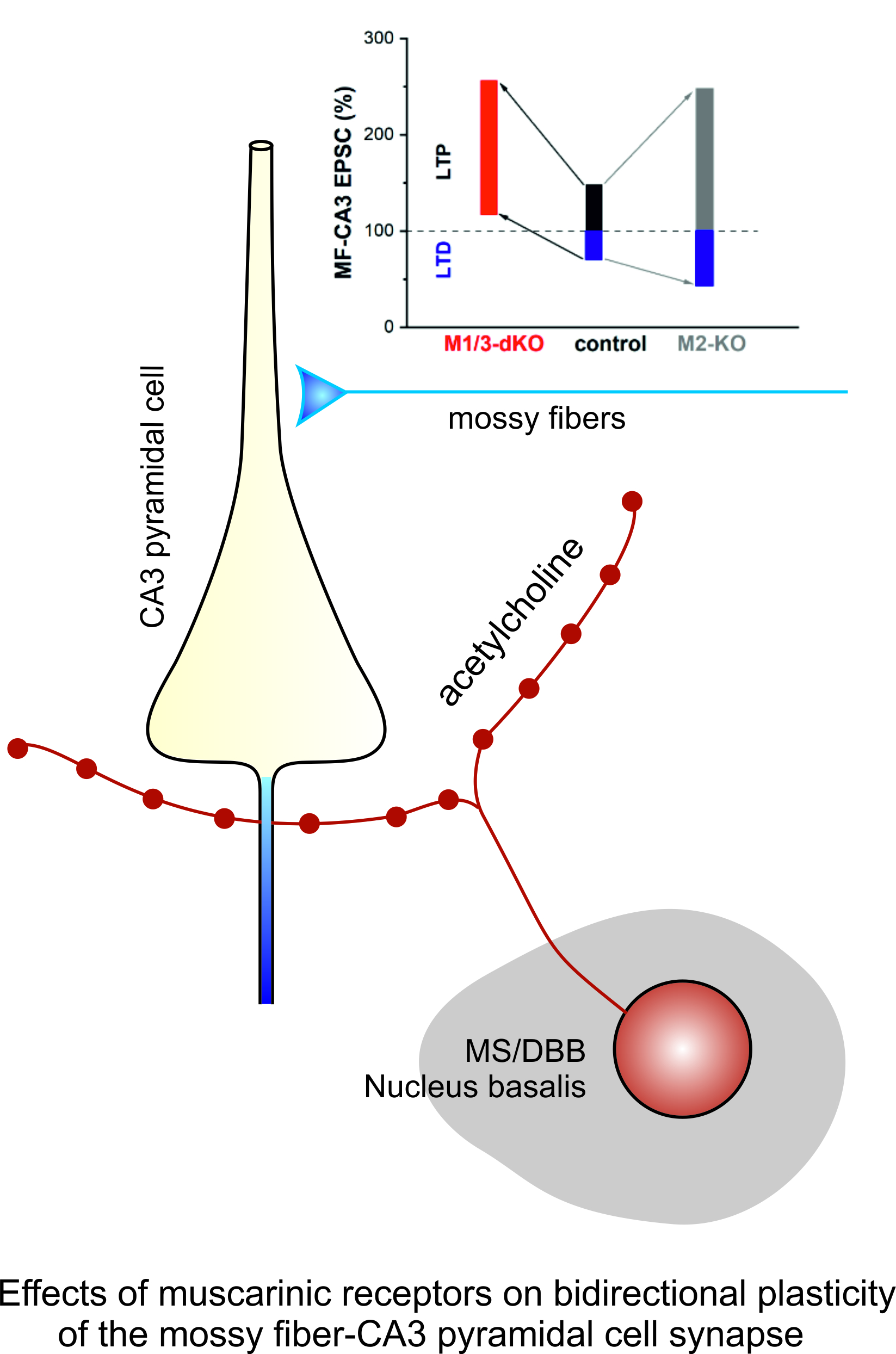
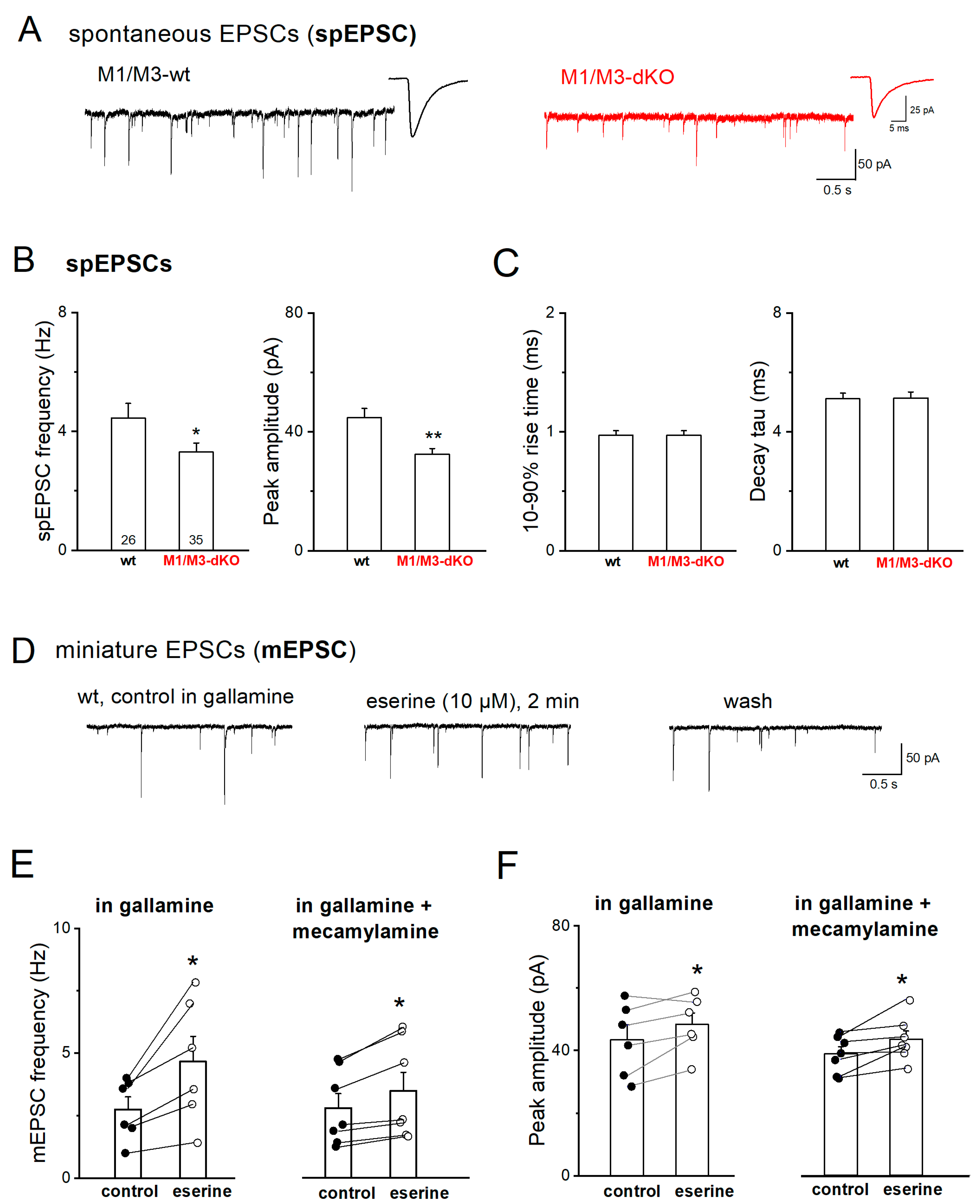
3.1. M1/M3 double KO reduces excitatory synaptic drive onto CA3 pyramidal cells
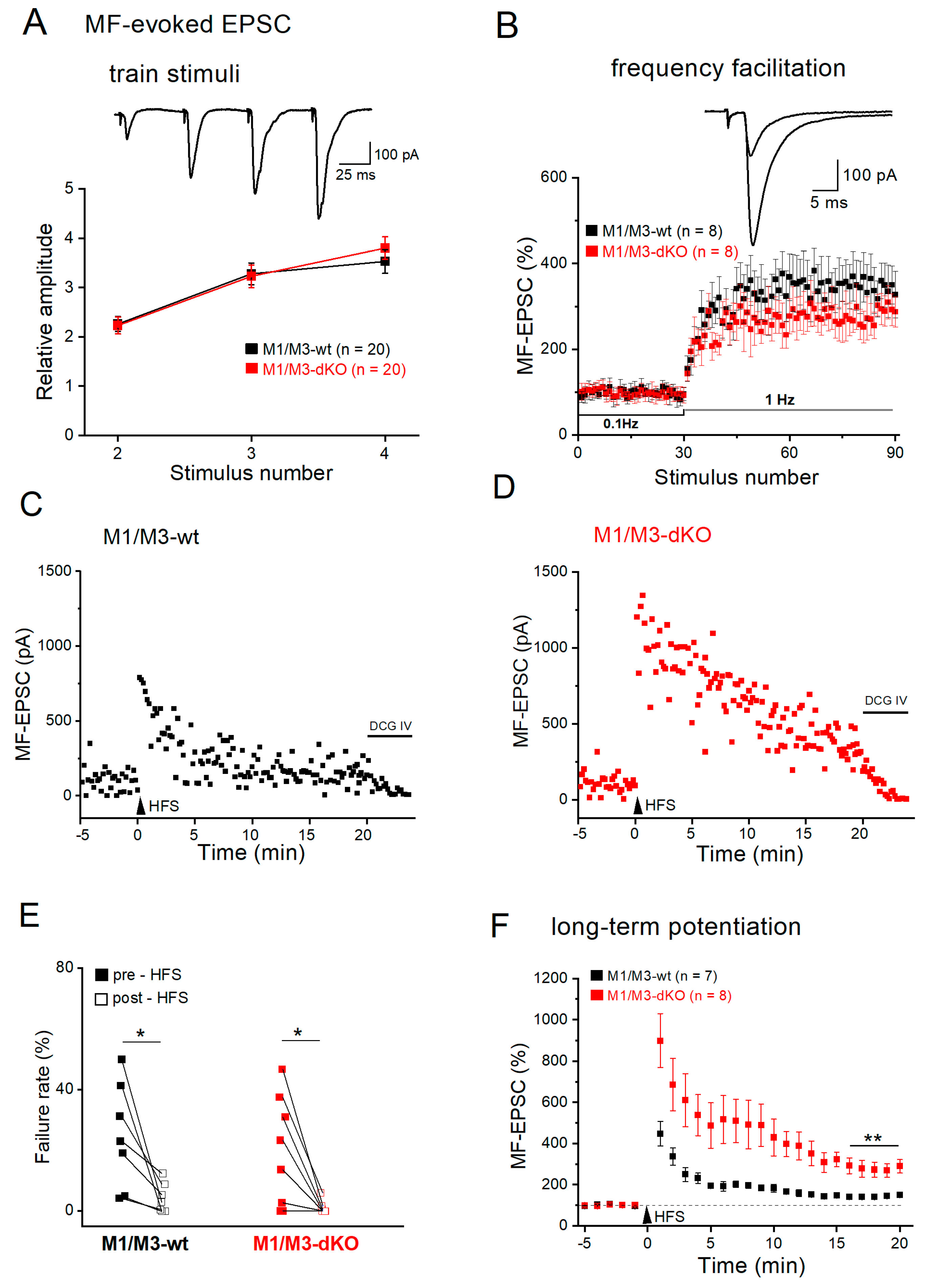
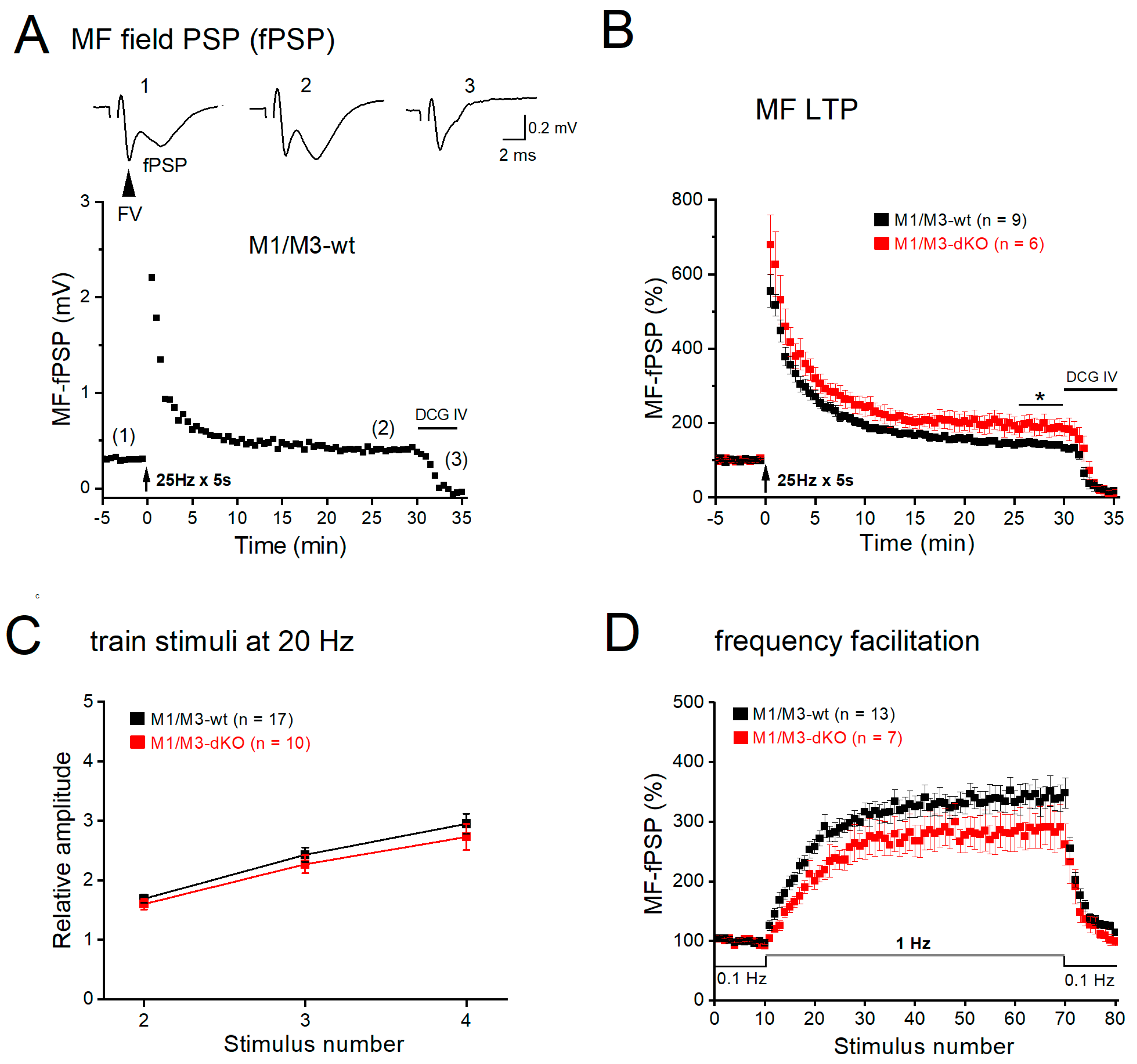
3.2. M1/M3-dKO facilitates LTP of mossy fiber-CA3 synapses
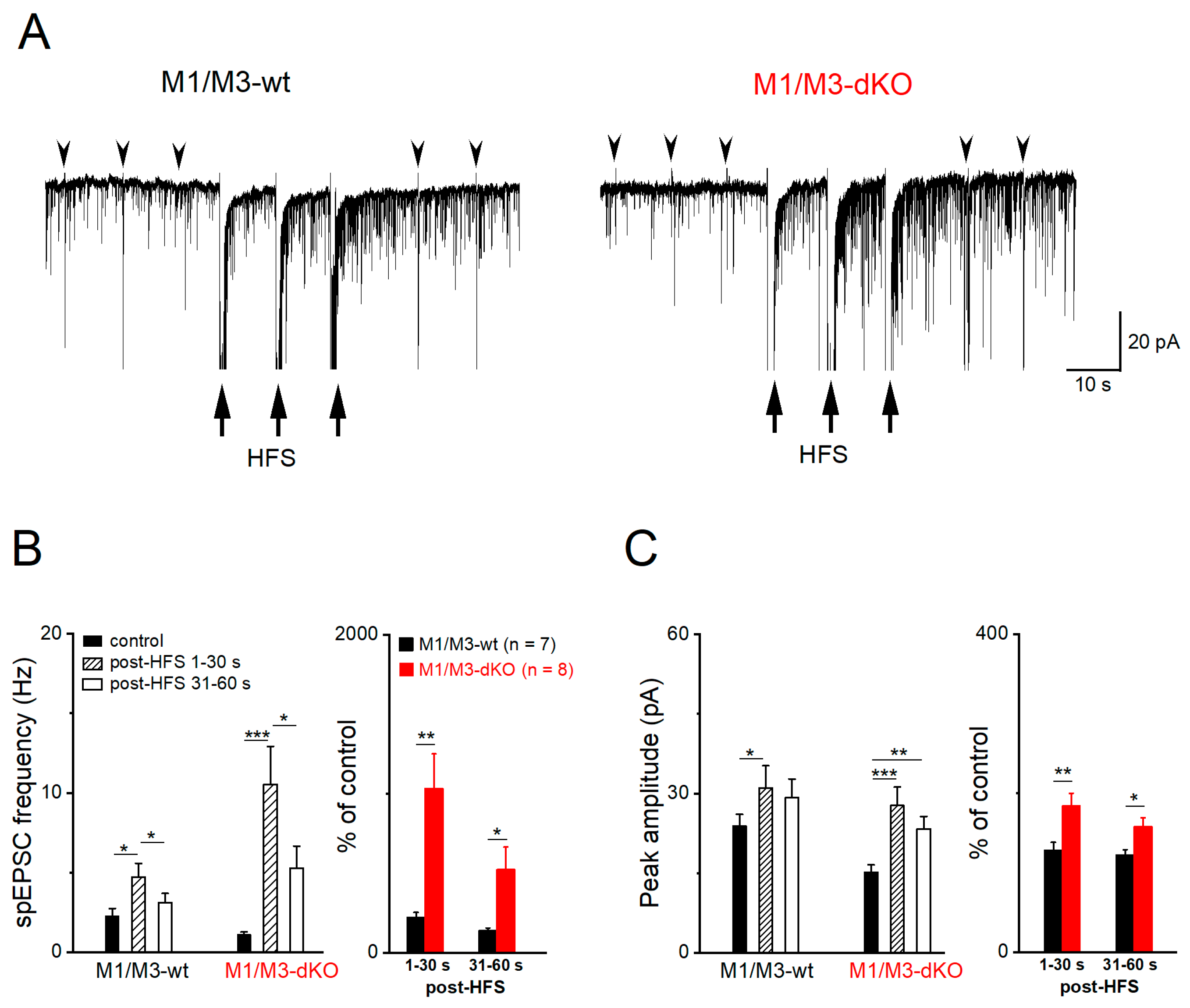
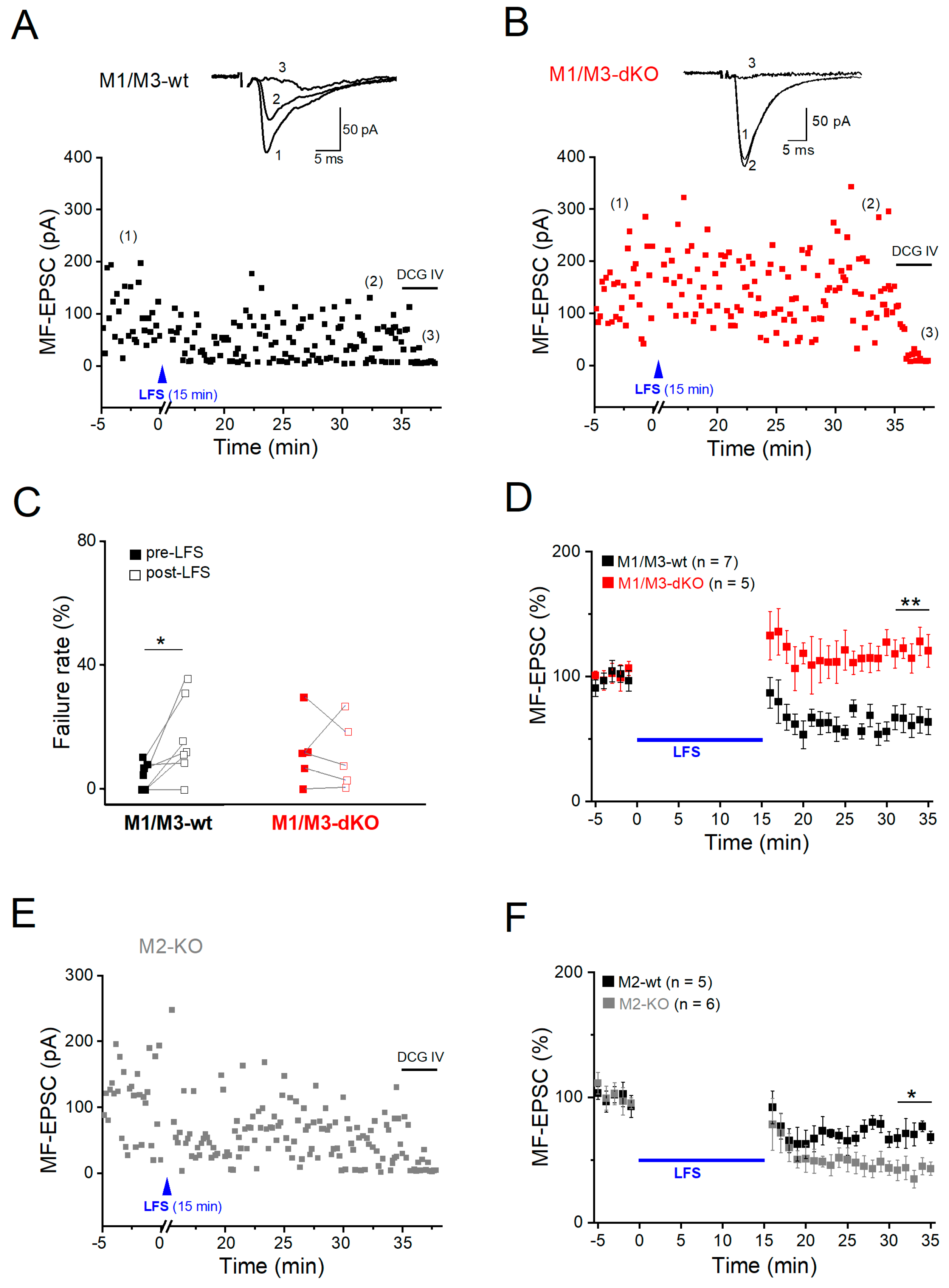
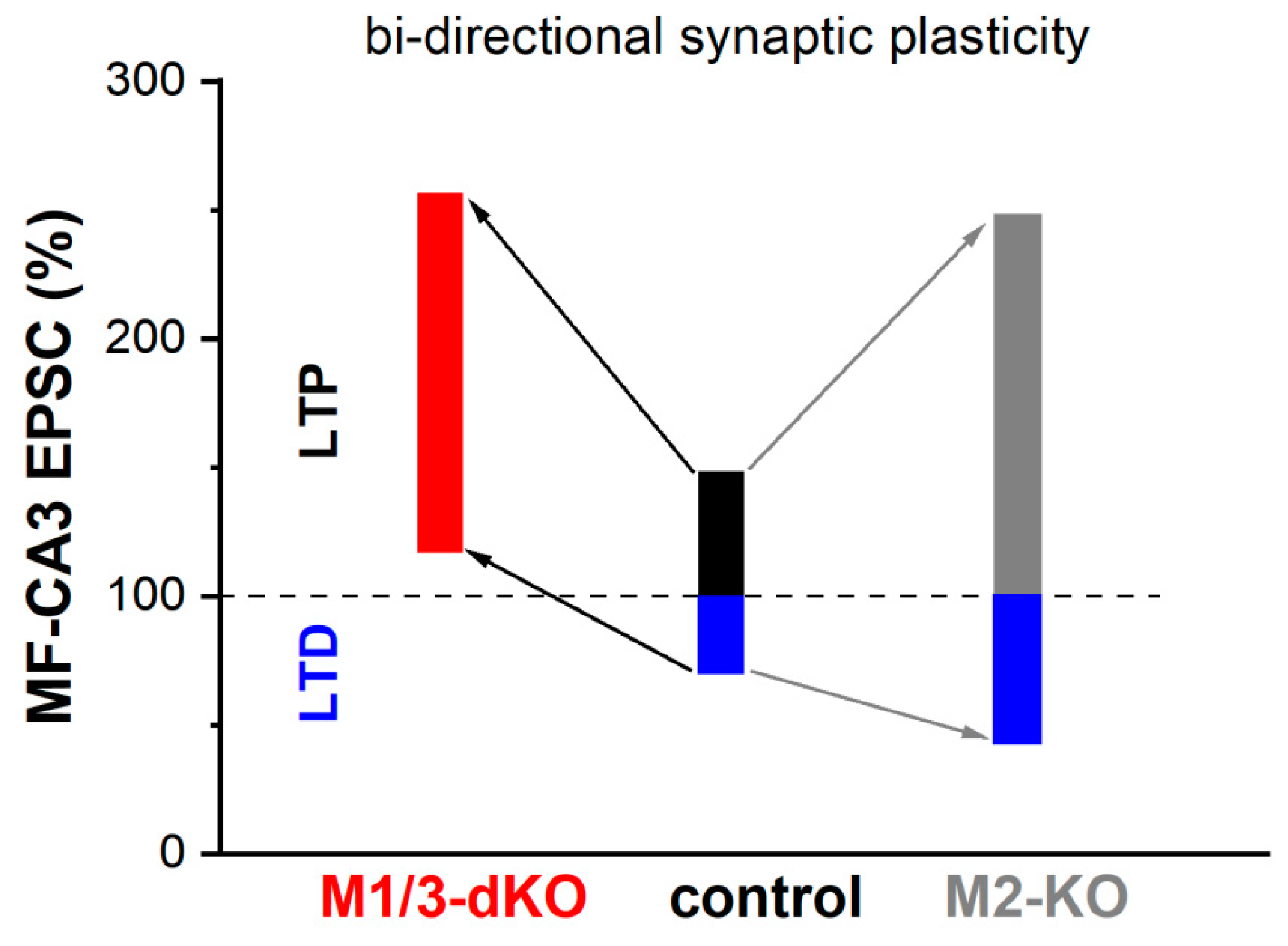
3.3. M1/M3-dKO turns LTD into LTP
4. Discussion
5. Conclusions
Author Contributions
Acknowledgements
Conflicts of Interest
Abbreviations
References
- Zheng, F.; Wess, J.; Alzheimer, C. M2 muscarinic acetylcholine receptors regulate long-term potentiation at hippocampal CA3 pyramidal cell synapses in an input-specific fashion. J. Neurophysiol. 2012, 10, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Levey, A.I. Muscarinic acetylcholine receptor expression in memory circuits: implications for treatment of Alzheimer disease. Proc. Natl. Acad. Sci. USA 1996, 93, 13541–13546. [Google Scholar] [CrossRef] [PubMed]
- Hasselmo, M.E. The role of acetylcholine in learning and memory. Curr. Opin. Neurobiol. 2006, 16, 710–715. [Google Scholar] [CrossRef]
- Thiele, A. Muscarinic signaling in the brain. Annu. Rev. Neurosci. 2013, 36, 271–294. [Google Scholar] [CrossRef] [PubMed]
- Haam, J.; Yakel, J.L. Cholinergic modulation of the hippocampal region and memory function. J. Neurochem. 2017, 142 (Suppl 2), 111–121. [Google Scholar] [CrossRef]
- Ananth, M.R.; Rajebhosale, P.; Kim, R.; Talmage, D.A.; Role, L.W. Basal forebrain cholinergic signalling: development, connectivity and roles in cognition. Nat. Rev. Neurosci. 2023, 24, 233–251. [Google Scholar] [CrossRef]
- Felder, C.C. Muscarinic acetylcholine receptors: signal transduction through multiple effectors. FASEB J. 1995, 9, 619–625. [Google Scholar] [CrossRef]
- Wess, J. Molecular biology of muscarinic acetylcholine receptors. Crit. Rev. Neurobiol. 1996, 10, 69–99. [Google Scholar] [CrossRef]
- Bymaster, F.P.; McKinzie, D.L.; Felder, C.C.; Wess, J. Use of M1-M5 muscarinic receptor knockout mice as novel tools to delineate the physiological roles of the muscarinic cholinergic system. Neurochem. Res. 2003, 28, 437–442. [Google Scholar] [CrossRef]
- Wess, J.; Eglen, R.M.; Gautam, D. Muscarinic acetylcholine receptors: mutant mice provide new insights for drug development. Nat. Rev. Drug Discov. 2007, 6, 721–733. [Google Scholar] [CrossRef]
- Thomsen, M.; Sørensen, G.; Dencker, D. Physiological roles of CNS muscarinic receptors gained from knockout mice. Neuropharmacology 2018, 136(Pt C), 411–420. [Google Scholar] [CrossRef]
- Tzavara, E.T.; Bymaster, F.P.; Felder, C.C.; Wade, M.; Gomeza, J.; Wess, J.; McKinzie, D.L.; Nomikos, G.G. Dysregulated hippocampal acetylcholine neurotransmission and impaired cognition in M2, M4 and M2/M4 muscarinic receptor knockout mice. Mol. Psychiatry 2003, 8, 673–679. [Google Scholar] [CrossRef] [PubMed]
- Seeger, T.; Fedorova, I.; Zheng, F.; Miyakawa, T.; Koustova, E.; Gomeza, J.; Basile, A.S.; Alzheimer, C.; Wess, J. M2 muscarinic acetylcholine receptor knock-out mice show deficits in behavioral flexibility, working memory, and hippocampal plasticity. J. Neurosci. 2004, 24, 10117–10127. [Google Scholar] [CrossRef] [PubMed]
- Bainbridge, N.K.; Koselke, L.R.; Jeon, J.; Bailey, K.R.; Wess, J.; Crawley, J.N.; Wrenn, C.C. Learning and memory impairments in a congenic C57BL/6 strain of mice that lacks the M2 muscarinic acetylcholine receptor subtype. Behav. Brain Res. 2008, 190, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Romberg, C.; Bartko, S.; Wess, J.; Saksida, L.M.; Bussey, T.J. Impaired object-location learning and recognition memory but enhanced sustained attention in M2 muscarinic receptor-deficient mice. Psychopharmacology (Berl) 2018, 235, 3495–3508. [Google Scholar] [CrossRef]
- Poulin, B.; Butcher, A.; McWilliams, P.; Bourgognon, J.M.; Pawlak, R.; Kong, K.C.; Bottrill, A.; Mistry, S.; Wess, J.; Rosethorne, E.M.; Charlton, S.J.; Tobin, A.B. The M3-muscarinic receptor regulates learning and memory in a receptor phosphorylation/arrestin-dependent manner. Proc. Natl. Acad. Sci. USA 2010, 107, 9440–9445. [Google Scholar] [CrossRef]
- Leaderbrand, K.; Chen, H.J.; Corcoran, K.A.; Guedea, A.L.; Jovasevic, V.; Wess, J.; Radulovic, J. Muscarinic acetylcholine receptors act in synergy to facilitate learning and memory. Learn. Mem. 2016, 23, 631–638. [Google Scholar] [CrossRef]
- Miyakawa, T.; Yamada, M.; Duttaroy, A.; Wess, J. Hyperactivity and intact hippocampus-dependent learning in mice lacking the M1 muscarinic acetylcholine receptor. J. Neurosci. 2001, 21, 5239–5250. [Google Scholar] [CrossRef]
- Anagnostaras, S.G.; Murphy, G.G.; Hamilton, S.E.; Mitchell, S.L.; Rahnama, N.P.; Nathanson, N.M.; Silva, A.J. Selective cognitive dysfunction in acetylcholine M1 muscarinic receptor mutant mice. Nat. Neurosci. 2003, 6, 51–58. [Google Scholar] [CrossRef]
- Shinoe, T.; Matsui, M.; Taketo, M.M.; Manabe, T. Modulation of synaptic plasticity by physiological activation of M1 muscarinic acetylcholine receptors in the mouse hippocampus. J. Neurosci. 2005, 25, 11194–11200. [Google Scholar] [CrossRef]
- Kamsler, A.; McHugh, T.J.; Gerber, D.; Huang, S.Y.; Tonegawa, S. Presynaptic m1 muscarinic receptors are necessary for mGluR long-term depression in the hippocampus. Proc. Natl. Acad. Sci. USA 2010, 107, 1618–1623. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, K.; Manabe, T.; Takahashi, T. Presynaptic long-term depression at the hippocampal mossy fiber-CA3 synapse. Science 1996, 273, 648–650. [Google Scholar] [CrossRef] [PubMed]
- Kemp, A.; Manahan-Vaughan, D. Hippocampal long-term depression: master or minion in declarative memory processes? Trends Neurosci. 2007, 30, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Connor, S.A.; Wang, Y.T. A place at the Table: LTD as a Mediator of Memory Genesis. Neuroscientist 2016, 22, 359–371. [Google Scholar] [CrossRef]
- Manabe, T. Two forms of hippocampal long-term depression, the counterpart of long-term potentiation. Rev. Neurosci. 1997, 8, 179–193. [Google Scholar] [CrossRef]
- Nicoll, R.A.; Schmitz, D. Synaptic plasticity at hippocampal mossy fibre synapses. Nat. Rev. Neurosci. 2005, 863–876. [Google Scholar] [CrossRef]
- Vilaró, M.T.; Palacios, J.M.; Mengod, G. Localization of m5 muscarinic receptor mRNA in rat brain examined by in situ hybridization histochemistry. Neurosci. Lett. 1990, 14, 14,154–159. [Google Scholar] [CrossRef]
- Levey, A.I.; Kitt, C.A.; Simonds, W.F.; Price, D.; Brann, M.R. Identification and localization of muscarinic acetylcholine receptor proteins in brain with subtype-specific antibodies. J. Neurosci. 1991, 11, 3218–3226. [Google Scholar] [CrossRef]
- Gautam, D.; Heard, T.S.; Cui, Y.; Miller, G.; Bloodworth, L.; Wess, J. Cholinergic stimulation of salivary secretion studied with M1 and M3 muscarinic receptor single- and double-knockout mice. Mol. Pharmacol. 2004, 66, 260–267. [Google Scholar] [CrossRef]
- Gomeza, J.; Shannon, H.; Kostenis, E.; Felder, C.; Zhang, L.; Brodkin, J.; Grinberg, A.; Sheng, H.; Wess, J. Pronounced pharmacologic deficits in M2 muscarinic acetylcholine receptor knockout mice. Proc. Natl. Acad. Sci. USA 1999, 96, 1692–1697. [Google Scholar] [CrossRef]
- Sydow, A.; Van der Jeugd, A.; Zheng, F.; Ahmed, T.; Balschun, D.; Petrova, O.; Drexler, D.; Zhou, L.; Rune, G.; Mandelkow, E.; D’Hooge, R.; Alzheimer, C.; Mandelkow, E.M. Tau-induced defects in synaptic plasticity, learning and memory are reversible in transgenic mice after switching off the toxic Tau mutant. J. Neurosci. 2011, 31, 2511–2525. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Z.; Brown, T.H. Complex synaptic current waveforms evoked in hippocampal pyramidal neurons by extracellular stimulation of dentate gyrus. J. Neurophysiol. 1998, 79, 2475–2484. [Google Scholar] [CrossRef] [PubMed]
- Henze, D.A.; Urban, N.N.; Barrionuevo, G. The multifarious hippocampal mossy fiber pathway: a review. Neuroscience 2000, 98, 407–427. [Google Scholar] [CrossRef] [PubMed]
- Langdon, R.B. , Johnson, J.W.; Barrionuevo, G. Asynchrony of mossy fibre inputs and excitatory postsynaptic currents in rat hippocampus. J. Physiol. 1993, 472, 157–176. [Google Scholar] [CrossRef] [PubMed]
- Burke, R.E. Gallamine binding to muscarinic M1 and M2 receptors, studied by inhibition of [3H]pirenzepine and [3H]quinuclidinylbenzilate binding to rat brain membranes. Mol. Pharmacol. 1986, 30, 58–68. [Google Scholar]
- Caulfield, M.P.; Birdsall, N.J. International Union of Pharmacology. XVII. Classification of muscarinic acetylcholine receptors. Pharmacol. Rev. 1998, 50, 279–290. [Google Scholar]
- Vogt, K.E.; Regehr, W.G. Cholinergic modulation of excitatory synaptic transmission in the CA3 area of the hippocampus. J. Neurosci. 2001, 21, 75–83. [Google Scholar] [CrossRef]
- Bacher, I.; Wu, B.; Shytle, D.R.; George, T.P. Mecamylamine—a nicotinic acetylcholine receptor antagonist with potential for the treatment of neuropsychiatric disorders. Expert Opin. Pharmacother. 2009, 20, 2709–2721. [Google Scholar] [CrossRef]
- Pernía-Andrade, A.J.; Jonas, P. Theta-gamma-modulated synaptic currents in hippocampal granule cells in vivo define a mechanism for network oscillations. Neuron 2014, 81, 140–152. [Google Scholar] [CrossRef]
- Williams, S.; Johnston, D. Muscarinic depression of long-term potentiation in CA3 hippocampal neurons. Science 1988, 242, 84–87. [Google Scholar] [CrossRef]
- Maeda, T.; Kaneko, S.; Satoh, M. Bidirectional modulation of long-term potentiation by carbachol via M1 and M2 muscarinic receptors in guinea pig hippocampal mossy fiber-CA3 synapses. Brain Res. 1993, 619, 324–330. [Google Scholar] [CrossRef] [PubMed]
- Gautam, D.; Duttaroy, A.; Cui, Y.; Han, S.J.; Deng, C.; Seeger, T.; Alzheimer, C.; Wess, J. M1-M3 muscarinic acetylcholine receptor-deficient mice: novel phenotypes. J. Mol. Neurosci. 2006, 30, 157–160. [Google Scholar] [CrossRef]
- Araya, R,; Noguchi, T.; Yuhki, M.; Kitamura, N.; Higuchi, M.; Saido, T.C.; Seki, K.; Itohara, S.; Kawano, M.; Tanemura, K.; Takashima, A.; Yamada, K.; Kondoh, Y.; Kanno, I.; Wess, J.; Yamada, M. Loss of M5 muscarinic acetylcholine receptors leads to cerebrovascular and neuronal abnormalities and cognitive deficits in mice. Neurobiol. Dis. 2006, 24, 334–344. [CrossRef]
- Halliwell, J.V.; Adams, P.R. Voltage-clamp analysis of muscarinic excitation in hippocampal neurons. Brain Res. 1982, 250, 71–92. [Google Scholar] [CrossRef] [PubMed]
- Madison, D.V.; Lancaster, B.; Nicoll, R.A. Voltage clamp analysis of cholinergic action in the hippocampus. J. Neurosci. 1987, 7, 733–741. [Google Scholar] [CrossRef]
- Colino, A.; Halliwell, J.V. Carbachol potentiates Q current and activates a calcium-dependent non-specific conductance in rat hippocampus in vitro. Eur. J. Neurosci. 1993, 5, 1198–1209. [Google Scholar] [CrossRef]
- Fisahn, A.; Yamada, M.; Duttaroy, A.; Gan, J.W.; Deng, C.X.; McBain, C.J.; Wess, J. Muscarinic induction of hippocampal gamma oscillations requires coupling of the M1 receptor to two mixed cation currents. Neuron 2002, 33, 615–624. [Google Scholar] [CrossRef]
- Martinello, K.; Huang, Z.; Lujan, R.; Tran, B.; Watanabe, M.; Cooper, E.C.; Brown, D.A.; Shah, M.M. Cholinergic afferent stimulation induces axonal function plasticity in adult hippocampal granule cells. Neuron 2015, 85, 346–363. [Google Scholar] [CrossRef]
- Ohno-Shosaku, T.; Matsui, M. , Fukudome, Y; Shosaku, J.; Tsubokawa, H.; Taketo, M.M.; Manabe, T.; Kano, M. Postsynaptic M1 and M3 receptors are responsible for the muscarinic enhancement of retrograde endocannabinoid signalling in the hippocampus. Eur. J. Neurosci. 2003, 18, 109–116. [Google Scholar] [CrossRef]
- Straiker, A.; Mackie, K. Depolarization-induced suppression of excitation in murine autaptic hippocampal neurons. J. Physiol. 2005, 569, 501–517. [Google Scholar] [CrossRef]
- Ruiz, A.; Campanac, E.; Scott, R.S.; Rusakov, D.A.; Kullmann, D.M. Presynaptic GABAA receptors enhance transmission and LTP induction at hippocampal mossy fiber synapses. Nat. Neurosci. 2010, 13, 431–438. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.B.; Castillo, P.E. Long-term potentiation selectively expressed by NMDA receptors at hippocampal mossy fiber synapses. Neuron 2008, 57, 108–120. [Google Scholar] [CrossRef]
- Rebola, N.; Lujan, R.; Cunha, R.A.; Mulle, C. Adenosine A2A receptors are essential for long-term potentiation of NMDA-EPSCs at hippocampal mossy fiber synapses. Neuron 2008, 57, 121–134. [Google Scholar] [CrossRef] [PubMed]
- Bischofberger, J.; Engel, D.; Frotscher, M.; Jonas, P. Timing and efficacy of transmitter release at mossy fiber synapses in the hippocampal network. Pflug. Arch. 2006, 453, 361–372. [Google Scholar] [CrossRef] [PubMed]
- Origlia, N.; Kuczewski, N.; Aztiria, E.; Gautam, D.; Wess, J.; Domenici, L. Muscarinic acetylcholine receptor knockout mice show distinct synaptic plasticity impairments in the visual cortex. J. Physiol. 2006, 577(Pt 3) Pt 3, 829–840. [Google Scholar] [CrossRef]
- Rosenzweig, E.S.; Barnes, C.A.; McNaughton, B.L. Making room for new memories. Nat. Neurosci. 2002, 5, 6–8. [Google Scholar] [CrossRef]
- Nicholls, R.E.; Alarcon, J.M.; Malleret, G.; Carroll, R.C.; Grody, M.; Vronskaya, S.; Kandel, E.R. Transgenic mice lacking NMDAR-dependent LTD exhibit deficits in behavioral flexibility. Neuron 2008, 58, 104–117. [Google Scholar] [CrossRef]
- Hagena, H.; Manahan-Vaughan, D. Learning-facilitated synaptic plasticity at CA3 mossy fiber and commissural-associational synapses reveals different roles in information processing. Cereb. Cortex 2011, 21, 2442–2449. [Google Scholar] [CrossRef]
- Cooper, L.N.; Bear, M.F. The BCM theory of synapse modification at 30: interaction of theory with experiment. Nat. Rev. Neurosci. 2012, 13, 798–810. [Google Scholar] [CrossRef]
- Bienenstock, E.L.; Cooper, L.N.; Munro, P.W. Theory for the development of neuron selectivity: orientation specificity and binocular interaction in visual cortex. J. Neurosci. 1982, 2, 32–48. [Google Scholar] [CrossRef]
- Philpot, B.D.; Sekhar, A.K.; Shouval, H.Z.; Bear, M.F. Visual experience and deprivation bidirectionally modify the composition and function of NMDA receptors in visual cortex. Neuron 2001, 29, 157–169. [Google Scholar] [CrossRef] [PubMed]
- Mayford, M.; Wang, J.; Kandel, E.R.; O’Dell, T.J. CaMKII regulates the frequency-response func-tion of hippocampal synapses for the production of both LTD and LTP. Cell 1995, 81, 891–904. [Google Scholar] [CrossRef] [PubMed]
- Hulme, S.R.; Jones, O.D.; Ireland, D.R.; Abraham, W.C. Calcium-dependent but action potential-independent BCM-like metaplasticity in the hippocampus. J. Neurosci 2012, 32, 6785–6794. [Google Scholar] [CrossRef]
- Narayanan R, Johnston D. The h current is a candidate mechanism for regulating the sliding modification threshold in a BCM-like synaptic learning rule. J. Neurophysiol. 2010, 104, 1020–1033. [CrossRef] [PubMed]
- Fukaya, R.; Hirai, H.; Sakamoto, H.; Hashimotodani, Y.; Hirose, K.; Sakaba, T. Increased vesicle fusion competence underlies long-term potentiation at hippocampal mossy fiber synapses. Sci. Adv. 2023, 9, eadd3616. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




