1. Introduction
Age-related macular degeneration (AMD) is a leading cause of legal blindness worldwide [
1]. Several factors, such as genetic predisposition, chronic local inflammation, and oxidative stress, contribute to the etiology of AMD; however, the mechanisms leading to AMD development are not fully understood [
2,
3,
4,
5].
Retinal pigment epithelium (RPE) cells are crucial for the maintenance of retinal homeostasis because they participate in several key functions, such as: the transport of nutrients, preservation of the retinal structure, phagocytosis of the outer segments of photoreceptors, and protection of the retina from oxidative damage [
6,
7]. The RPE is a highly oxidized microenvironment generated by a combination of visible light exposure, elevated metabolic activity, and accumulation of oxidized lipoproteins [
8,
9]. Physiological functions and aging processes, in combination with environmental stressors, including smoking or obesity related conditions force RPE cells to support excessive levels of oxidative stress, disturbing cell homeostasis [
10] and causing chronic inflammation [
11].
Genetic predispositions play an essential role in AMD pathogenesis. Different variants of complement system proteins are associated with an increased risk of AMD development and progression [
12]. A single nucleotide polymorphism predicting a Y402H replacement in the gene coding complement factor H (CFH) is an important risk factor for AMD [
13]. CFH is a primary inhibitor of complement alternative pathways. By acting as a cofactor for factor I it prevents the formation of C3 convertase [
14]. CFH is present in human and mouse ocular tissues, such as the RPE and choroid. Moreover, CFH and other complement proteins have been found in the drusen of patients with AMD suggesting that inflammation is an important component of this disease [
15]. However, the mechanism through which the CFH Y402H variant confers an increased risk of AMD remains unclear.
In vitro studies have shown that the at-risk CFH Y402H variant leads to dysregulation of the complement pathway, with increased levels of the C3 protein [
16]. Interestingly, the Y402H polymorphism is located in a short consensus repeat (SCR) 7 of the protein, which is important for mediating CFH binding to polyanions such as heparan sulfate chains, C-reactive proteins, malondialdehyde, and glycosaminoglycans [
17,
18]. This risk variant is associated with high levels of complement activation products and proinflammatory cytokines in the blood of patients with AMD [
19]. Moreover, inefficient binding of the at-risk CFH Y402H variant to malondialdehyde induces macrophages and monocytes to secrete many pro-inflammatory cytokines, suggesting that systemic cytokines may also be associated with genetic factors and thus contribute to AMD [
20].
Similarly, studies in RPE in vitro showed that the poorly binding affinity of CFH Y402H to C-reactive protein resulted in elevated complement activation and altered secretion of the pro-inflammatory cytokines including tumor necrosis factor alpha (TNF-α) [
17,
18] and an upregulation of oxidative stress markers [
21].
Lipid metabolism in AMD is altered and associated with CFH Y402H. It shows impaired binding to malondialdehyde, a product of lipid oxidation. Transgenic aged mice expressing homozygous at-risk CFH variants present an AMD-like phenotype with changes in plasma and eyecup lipoproteins as a consequence of the Y402H polymorphism [
22].
Several animal and in vitro models have been used to investigate the association between CFH and AMD. Mouse models provide a conduit for dissecting the molecular mechanisms linking complement responses to AMD pathobiology [
23]. Given the complexity of the disease, none of the available models fully recapitulate human AMD; however, they have provided important insights into the roles of the complement system and CFH in AMD. The in vitro generation of RPE monolayers from primary donor eye cultures has made it possible to study cell dynamics under experimental conditions, which has increased our knowledge of the cellular and molecular mechanisms of AMD. However, the limited life span and absence of the at-risk CFH variant in normal human RPE cells represents an obstacle for molecular analysis.
Spontaneously generated human RPE cell line (ARPE-19) cells are a valuable source of human RPE cells. As the ARPE-19 cell line has been shown to maintain many of the properties of native cells of the RPE, it has frequently been used as an in vitro model for the study of retinal disorders, such as AMD. Previous assays using ARPE-19 cells showed that an oxidized environment increases the expression of complement receptors and accumulated C3 as well as its regulators complement CFH, properdin and NLRP3, as a mechanism to maintain cell homeostasis following oxidative stress [
24].
With all these backgrounds raised we explored the role of CFH in regulating cell death and inflammation induced by oxidative stress condition in RPE cells culture with the at risk CFH Y402H variant.
2. Materials and Methods
2.1. Cell culture and induction of oxidative stress
The ARPE-19 was purchased from the American Type Culture Collection (ATCC CRL-2302) and cultured in 25 ml flasks containing Dulbecco's modified Eagle's nutrient F12 medium (DMEM / F12, STEMCELL Technologies) supplemented with 10% fetal bovine serum (GIBCO, BRL), 100 U/ml penicillin, 100 mg/ml streptomycin (GIBCO, BRL), 1mM glutamate (Stem cell, Sartorius) and sodium pyruvate (Stem cell, Sartorius). The cell culture was kept under a humid atmosphere of 37 °C and 5% carbon dioxide (CO2). Cells were harvested and adjusted to 2.5 x 105 cells then seeded in 24 wells plates until they reached 80% confluence. To induce an oxidative stress condition, the cells were treated with 400 μM or 800μM hydrogen peroxide (H2O2) for 24 h, harvested, washed, and resuspended in 1X Phosphate-buffered saline (PBS) for treatment and subsequent analysis.
2.2. Genomic DNA extraction (gDNA)
gDNA was obtained from 5 × 106 cells using a Blood and Tissue extraction kit (QIAGEN). The cells cultured in 25 ml culture flasks were dissociated with trypsin/EDTA as previously described, and the cell button was resuspended in 200 μl of 1X PBS to continue with the manufacturer´s protocol. The concentration and purity of the extracted gDNA was determined by spectrophotometry (Nanodrop, Thermofisher) and its integrity was verified by electrophoresis in 1.5% agarose gel, stained with SYBR® Gold Nucleic Acid Gel Stain (Thermofisher Scientific) for visualization under UV light in a photo documenter.
2.3. ARPE-19 genotyping
The region of the CFH gen that contains the single nucleotide polymorphism (SNP) rs1061170 (c.1204T>C; p.Tyr402His) was amplified by Polymerase Chain Reaction (PCR) using the oligonucleotides CFH_Fw:5'-AGT TCG TCT TCA GTT ATA C-3' and CFH_Rv:5'-TGG TCT GCG CTT TTG GAA GAG c-3. ’ The amplification reaction consisted of a mixture of 60 ng of gDNA, 5 l of Buffer A2 KAPA2G 5X, 0.5 l of KAPA dNTP Mix 10mM, 1.25 l of each 10μM oligonucleotide, 0.1 l of KAPA2G Fast DNA Polymerase 5 U/μL and sufficient nuclease-free water to achieve 25 l of reaction. The amplification conditions consisted of an initial denaturation cycle at 95 °C for 3 min, followed by 38 cycles consisting of denaturation at 95 °C for 1 min, hybridization at 59.5 °C for 30 s, extension at 72 °C for 1 min, and a final extension cycle at 72 °C for 3 min. Five microliters of the PCR product were analyzed on a 1.5 % agarose gel and electrophoresed. The remaining samples were purified using a DNA Clean and Concentrator Kit (ZYMO Research). Genotyping of the CFH variant was carried out by automated direct sequencing with the BigDye Terminator Cycle Sequencing kit (Applied Biosystems), following the manufacturer's protocol and using a temperature program consisting of 25 denaturation cycles at 97 °C for 30 s, alignment at 50 °C for 30 s and an extension at 60 °C for 1 min. The samples were analyzed using an ABI Prism 3130 Genetic Analyzer sequencer (Applied Biosystems) and the obtained sequences were compared with a reference published in ENSEMBL (NM_000186.4).
2.4. RNA extraction and cDNA synthesis
Total RNA was extracted from 1 × 105 ARPE-19 cells using Trizol assay [
25]. These samples were treated with RNase-free DNase I (QIAGEN) according to the manufacturer's protocol. cDNA was synthesized from 2 g of total RNA in a final volume of 20 l using the Superscript III First Strand Synthesis System (Thermo Fisher Scientific) with oligo (dT). PCR was carried out with 125 ng of cDNA as a template in a 15 l reaction mix containing Kapa Taq Readymix PCR kit (Sigma-Aldrich) and the specific primers for CRALBP (CRALBP_Fw:5´-TGGTGTCCTCTCTAGTCGGG-3,’ CRALBP_Rv:5'-GTTCAGCTGGCAGGAGATGT-3') and RPE65 (RPE65_Fw:5'-ACAACTTGGCCCTGACTTCC-3,’ RPE65_Rv:5'-TGAAGAGCATGACCACTCGG-3´). The transcripts were analyzed using the GeneAmp PCR System 9700 thermocycler (Applied Biosytem) and PCR conditions of 35 cycles formed by: denaturation at 95 °C for 30 s, hybridization at 60 °C for 15 s, and extension at 72 °C for 1 min, were done. Furthermore, the amplified products were analyzed by electrophoresis.
2.5. Flow cytometry
The cultured ARPE-19 cells were analyzed using flow cytometry. The cells were harvested by treatment with 0.25% trypsin- 0.5% EDTA (Gibco, Carlsbad, CA, USA), washed with 1X PBS and the cell pellet was resuspended in 2% fetal bovine serum in 1X PBS for 1 h at room temperature.
The identification of surface molecules was carried out by incubating the cells with human monoclonal APC-conjugated anti-Toll like receptor 4 (TLR4), FITC- conjugated CD14 and PE- conjugated CD86 antibodies (all from Biolegend, San Diego, CA, USA) for 20 min at 4 °C, according to the manufacturer's recommendations. Cells were washed with 1X PBS and fixed with 4% paraformaldehyde (Sigma-Aldrich, Inc.) for 20 min at room temperature, washed, and acquired with a BD FACSVerse Flow Cytometer (BD Biosciences, San Jose, CA, USA). The results were analyzed using Flow Jo ver. 10 from Beckton Dickinson).
2.6. Cell viability assay
The effect of oxidative stress on ARPE-19 cell viability was evaluated by staining them with 7-Amino-Actinomicin (7-AAD; Molecular Probes, Invitrogen, Carlsbad,CA,USA). ARPE-19 cells were cultured with or without the addition of exogenous CFH (5 µg/ml; Sigma Aldrich) and in the presence or absence of 400μM H2O2 as an inducer of oxidative stress. In some experiments, CFH was added to the cell culture 1 h before or simultaneously with H2O2. Cells with or without stimuli were harvested and washed twice in 1X PBS containing 2% BSA and subsequently resuspended in 100 μL of 1X PBS. The exclusion of viable cells was carried out by staining with 5 μL of 7-AAD for 5 min according to the manufacturer's instructions and subsequently analyzed by flow cytometry.
2.7. Intracellular for staining cytochrome c and NF-κB
Nuclear factor kappa b (NF-κB) and release of cytochrome C from the intermembrane space of the mitochondria into the cytosol was measured using a monoclonal fluorescein isothiocyanate (FITC)-conjugated anti NF-κB or FITC-conjugated anti- cytochrome C antibody (Biolegend, San Diego, CA, US). ARPE-19 cells treated with H2O2 for 3, 6, 12, or 24 h were permeabilized and subsequently fixed with 1% PFA in 1X PBS. The mean fluorescence intensity (MFI) for both molecules was measured by flow cytometry and analyzed using FlowJo software, ver.10 (Beckton Dickinson).
2.8. Cytokine measurement
The cytokines produced after the induction of oxidative stress in ARPE-19 cells, with or without the addition of exogenous human plasma Complement Factor H (CFH; Sigma Aldrich, Saint Louis, MO, USA) at different concentrations (1, 2.5, and 5 µg/mL), were determined using the cytometric bead array (CBA) Human Inflammatory Cytokines Kit (BD Biosciences) according to the manufacturer's specifications. The assay allowed the simultaneous and quantitative measurement of cytokines such as interleukin (IL)-8, IL-1β, IL-6, IL-10, TNF-α and IL-12p70 in the cell culture supernatant. The induced culture supernatants were added to a mixture of capture antibodies, bead reagents, and PE-conjugated detection antibodies. The mixture was then incubated at room temperature, in the dark, and washed. The cytokine panel was analyzed using FCAP Array Software (BD Biosciences). Detection limits for this method were as follows: 3.6 pg/mL of IL-8, 7.2 pg/mL of IL-1β, 2.5 pg/mL of IL-6, 3.3 pg/mL of IL-10, 3.7 pg/ml of TNF-α, and 1.9 pg/mL of IL-12p70.
2.9. Statistics
All experiments were performed independently at least three times under each condition. All analyses were performed using GraphPad Prism 5.0 (GraphPad Software Inc., La Jolla, CA, USA). A normality test was performed on all the data obtained from the previous ANOVA and post-hoc statistical analyses. Statistical significance was set at P-value of less than 0.05.
3. Results
3.1. ARPE-19 cell genotyping
Aging, genetic factors, such as the complement factor H (CFH)Y402H polymorphism, and damage to the RPE induced by oxidative stress have been suggested as the main contributors to the development of AMD. As the ARPE-19 cell line has been shown to maintain many of the properties of native cells of the RPE, it has frequently been used as an in vitro model for the study of retinal disorders.
In this study, we used a cell line with an at-risk CFH Y402H variant to investigate the production of pro- and anti-inflammatory cytokines under oxidative stress. Thus, we initially determined the presence of the genetically at-risk CFH Y402H variant in ARPE-19 cells by direct sequencing. The gDNA of ARPE-19 cells was used as a template to amplify exon 9 of CFH by PCR. After sequencing the PCR product, we identified a heterozygous missense variant consisting of a c.1204 T to C transition, which was predicted to change tyrosine (TAT) to histidine (CAT) at residue 402 (
Figure 1).
We characterized the ARPE-19 cell line with respect to the expression of differentiation markers such as retinaldehyde-binding protein (CRALBP) and protein-specific RTE (RPE65) by real time-polymerase chain reaction (RT-PCR). After amplification of the transcripts for differentiation markers, they were separated by electrophoresis into a single transcript of 593 bp for CRALBP and 598 bp for RPE65 (
Supplementary Figure S1). As a negative control (-), the expression of CRALBP was also investigated in peripheral blood monocytes.
The presence of a genetic variant that confers the risk of developing AMD and the expression of differentiation markers validated the idea that the ARPE-19 cell line could be suitable for establishing an in vitro model of oxidative stress and studying the participation of the Y402H genetic variant in the generation of an inflammatory environment.
3.2. Identification of molecules of immunological importance in ARPE-19
It has been shown that the cells of the RTE express Toll-like receptors and co-stimulatory molecules which are considered key factors in ocular inflammation due to their ability to secrete or potentiate various inflammatory mediators [
26,
27].
The expression of molecules of potential immunological importance on the surface of ARPE-19 cells was determined by detection with specific antibodies conjugated to different fluorochromes and analyzed by flow cytometry.
Under stable conditions, and in the absence of external stimuli, ARPE-19 cells constitutively express basal levels of receptors, such as TLR4 and its co-receptor CD14. The co-stimulatory molecule, CD86, was also identified. While CD86 showed low basal expression levels, TLR4 was the most highly expressed molecule, followed by CD14. Representative histograms for the detection of the molecules analyzed in this study are shown in
Supplementary Figure S2.
3.3. H2O2-induced cell death.
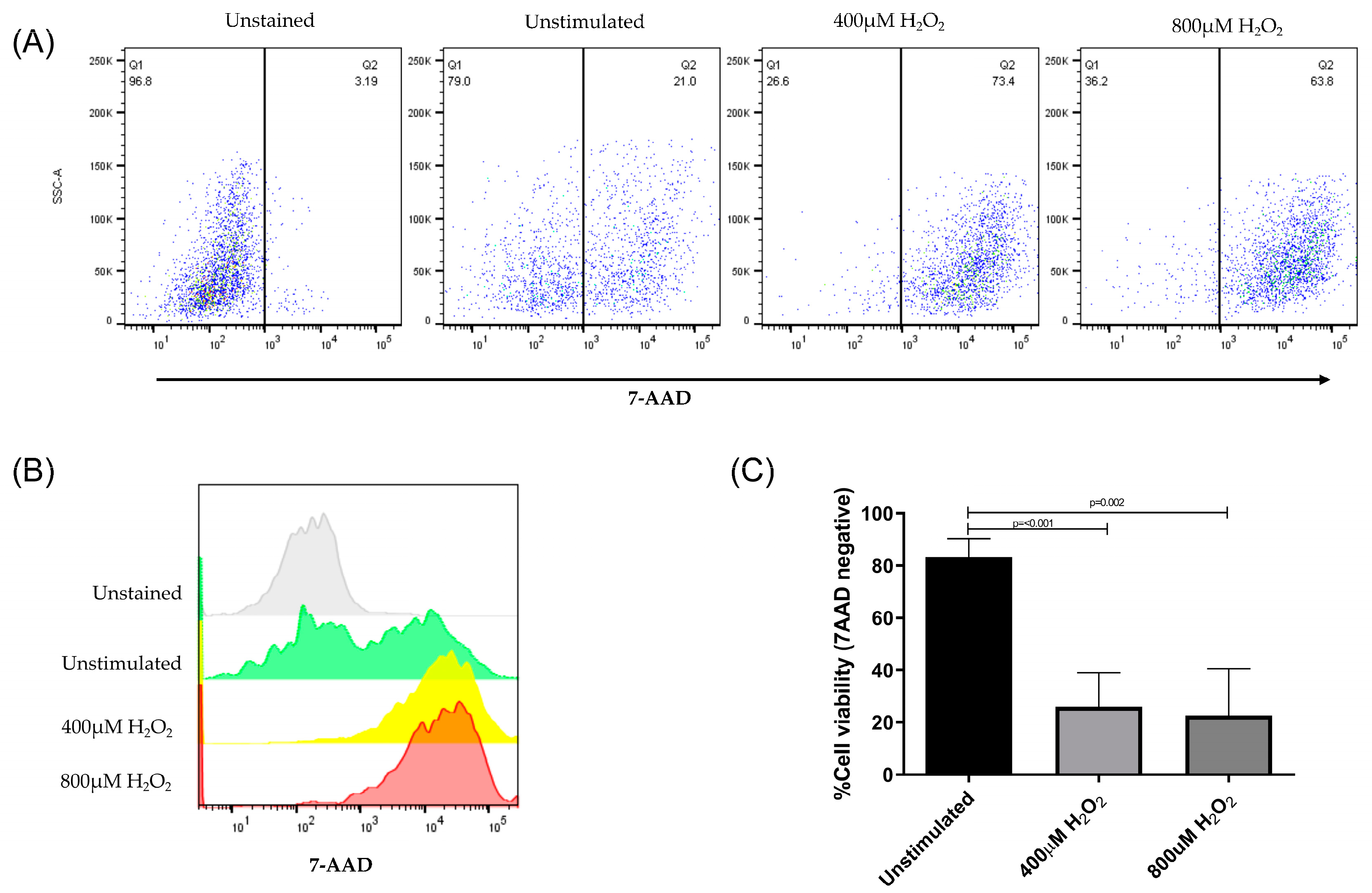
To establish an in vitro model of cell stress induction, ARPE-19 cells were treated with 400μM or 800μM of H2O2 for 24 h. The effect of exposure to oxidative stress was evaluated using a 7-amino-actinomycin-D (7-AAD) dye exclusion test, which allows the evaluation of cell viability by flow cytometry. Living cells keep their membranes intact, which prevents the passage of dyes, such as 7-AAD. In non-viable cells, the membranes are damaged and permeable; therefore, the dye easily penetrates and binds to double-stranded DNA.
ARPE-19 cells without H
2O
2 treatment were used as controls, representing more than 80% viable cells. In contrast, treatment of cells with H
2O
2 significantly decreased the membrane permeability of more than 70% of the cells (
Figure 2).
The representative dot plots in
Figure 2(a) and histograms 2(b) show the distribution of ARPE-19 cells before and after the induction of oxidative damage and subsequent treatment with 7-AAD. All samples were analyzed using the same gates. Within stressed cells, living cells (30%) and apoptotic cells (70%) were identified. Our results indicate that at the H
20
2 concentration used in this assay, induced an oxidative stress state in ARPE-19 cells, which significantly affected membrane permeability and compromised cell viability (
Figure 2c).
3.4. Release of apoptotic and inflammatory markers induced by oxidative stress in ARPE-19 cells
Alterations in the integrity of the plasma and mitochondrial membranes due to oxidative stress leads to the release of apoptogenic factors normally confined to the intermembranous space, such as cytochrome C. This induces an irreversible commitment towards cell death. Therefore, in response to the synthesis of pro-inflammatory cytokines, the canonical NF-κβ pathway also triggers events that lead cells to apoptosis. Hence, we considered it important to evaluate apoptosis markers such as cytochrome C and NF-κβ. Intracellular molecules were identified using specific antibodies and analyzed using flow cytometry. The mean fluorescence intensity (MFI) was determined at different times of oxidative stress induction (
Supplementary Figure S3).
Figure Supplementary S3 (a) and (b) show an increase in the level of cytochrome C released by ARPE-19 cells after exposure to H
2O
2. The release of cytochrome C into the cytosol increases in a time-dependent manner. At the early stages of oxidative stress induction, the cytosolic cytochrome C content was low and increased over time, reaching a maximum at 24 h. The cytosolic content of cytochrome C at 24 h post-oxidative stress was four times higher post-treatment. Furthermore, the induction of NF-κB as shown in
Supplementary Figure S3 (c) and (d), correlated with levels of cytoplasmic cytochrome C. Maximum synthesis of these components was observed 24 h after subjecting ARPE-19 cells to oxidative stress.
Taken together, these results suggest that ARPE-19 cells harboring the at-risk CFH Y402H variant are highly susceptible to oxidative stress-induced damage. In response to oxidative damage, proinflammatory and pro-apoptotic factors, such as cytochrome C and NF-κB, can be detected.
3.5. Exogenous CFH protein protects ARPE -19 cells from damage caused by oxidative stress
As we observed that oxidative stress affected the ARPE-19 cells viability, we considered it important to evaluate whether the presence of exogenous CFH could rescue cells from oxidative damage and prevent apoptosis.
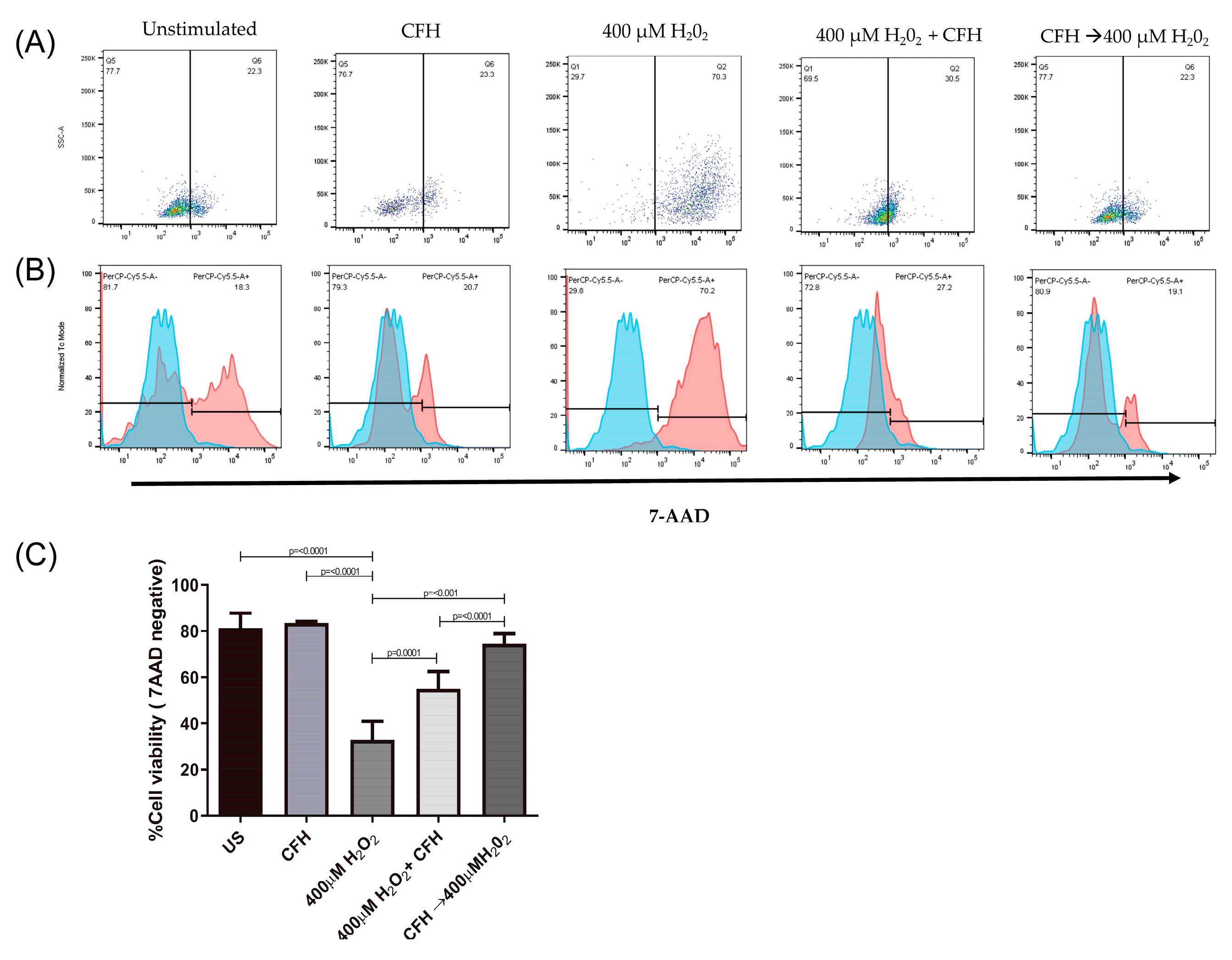
Thus, to evaluate the effect of CFH on cell viability after the induction of oxidative stress, ARPE-19 cells were cultured in the presence of any of the following treatments: a simultaneous combination of exogenous CFH and H2O2 for 24 h, or treatment for 1 h with exogenous CFH followed by H2O2 induced-oxidative stress for 24 h. Cell viability was evaluated by flow cytometry to determine the percentage of cells that were negative for 7-AAD staining. The data was then compared with that obtained from cells cultured in the presence of exogenous CFH or H2O2 as the only stimuli. ARPE-19 cells without any treatment were used as positive controls.
Figure 3 shows the percentage of viable cells after 24 h of treatment. ARPE-19 cells treated with exogenous CFH did not show deleterious changes and maintained a viability similar to that of unstimulated cells (80%). In contrast, the induction of oxidative stress by H
2O
2 significantly affected cell survival (20% of viable cells), as shown in previous studies.
The addition of exogenous CFH to the cell culture increased the percentage of viable cells when added simultaneously or before the induction of oxidative stress. The simultaneous addition of exogenous CFH and H2O2 to the cell culture increased the percentage viability (55%) almost two-fold with respect to the condition where only oxidative stress was induced. Similarly, when the cultures were treated with exogenous CFH and subsequently subjected to oxidative stress, the percentage of viable cells (74%) increased 2.3 times with respect to cells treated only with H2O2 or 1.3 times with respect to cells treated simultaneously with exogenous CFH and H2O2.
Therefore, CFH synthesized by ARPE-19 cells possessing the at-risk CFH Y402H variant may not be completely efficient in modulating the oxidative effect caused by the addition of H2O2. However, when exogenous CFH was incorporated into the culture medium, ARPE-19 cells could modulate the effect of oxidative stress. This cellular protection was more effective when CFH was present in the cell culture before stimulation with oxidative stress. Furthermore, although exogenous CFH contributed to the protection of cells against oxidative damage, the percentage of viable cells did not reach the level observed in untreated cells.
3.6 Effect of exogenous CFH on the synthesis of pro-inflammatory cytokines induced by oxidative stress
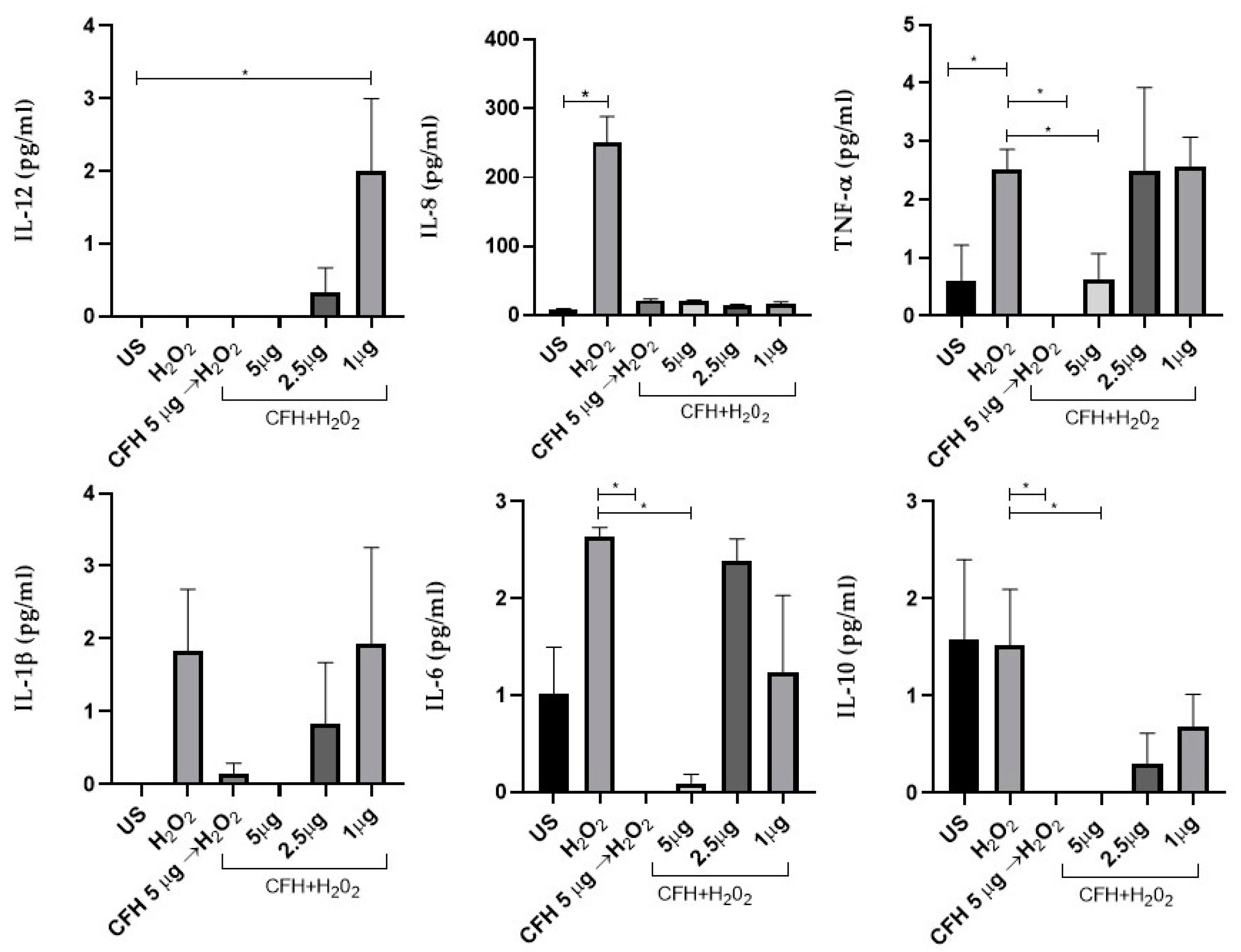
Oxidative stress and the inflammatory process are known to be associated with AMD. When a cell damage signal is generated, caspase activation mechanisms are triggered, which subsequently activate the synthesis of pro-inflammatory cytokines. Since our previous results showed that the addition of exogenous CFH to the cell culture prevented apoptosis induced by exposure to oxidative stress, we evaluated whether this protection was driven by the modulation of pro-inflammatory cytokines.
ARPE-19 cells were treated for 1 h with exogenous CFH (5 µg / ml) prior to the addition of H
2O
2, or simultaneous to the addition of H2O2, maintaining the stress condition for 12 h. Additionally, we evaluated the cytokine profile depending on the concentration of exogenous CFH added simultaneously with H2O2 to the cell culture. Cytokines in the supernatants of the ARPE-19 cell cultures were quantified using the CBA method (
Figure 4). It is important to note that under some conditions, the evaluated cytokines showed concentrations below the estimated detection limit.
Oxidative stress did not induce significative changes in the synthesis of IL-1β. IL-1β was not detected in the supernatant of ARPE-19 cells treated simultaneously with 5 µg/ml of exogenous CFH and H2O2. Even though the synthesis of IL-1β showed an increased expression trend with respect to unstimulated cells, it was not statistically significant.
The induction of oxidative stress triggered the significant overexpression of IL-8, which increased 30 times with respect to non-stimulated cells or those stimulated with exogenous CFH. This shows that the synthesis of IL-8 is inhibited by CFH, regardless of the concentration. Similarly, exogenous CFH regulates IL-8 synthesis when present in the cell culture before or simultaneously with H2O2. Furthermore, IL-6 also significantly increased after the induction of oxidative stress compared with when 5μg exogenous CFH was added previously or simultaneously with H2O2.
The behavior of TNF-α, was similar to that observed for IL-6. The synthesis of TNF-α was increased 2.3 times in ARPE-19 cells under oxidative stress. Treatment of cells with exogenous CFH at the highest concentration tested, added before or simultaneously with H2O2, significantly decreased (approximately 3 times) the synthesis of TNF-α with respect to treatment of cells with only H2O2.
Finally, IL-10, an anti-inflammatory cytokine, was synthesized in response to oxidative stress similar to levels in untreated cells. Lower IL-10 concentrations were observed when exogenous CFH was added in combination with H2O2. The addition of exogenous CFH to cell cultures inhibited IL-10 synthesis induced by oxidative damage.
These results indicate that oxidative stress induced in ARPE-19 cells results in the expression of pro-inflammatory cytokines, which is regulated by exogenous CFH, suggesting that CFH is important for maintaining cell homeostatic function (
Figure 5).
4. Discussion
The RPE performs different complex functions that are necessary to maintain photoreceptor homeostasis. Alterations in the RPE cell layer lead to deleterious effects and photoreceptor death, triggering diseases, such as AMD.
The risk of developing AMD is mainly due to advanced age, environmental stress, and genetic factors [
5,
13,
16,
28]. Among genetic factors, the at-risk CFH Y402H variant is strongly associated with AMD [
29,
30,
31]. However, little is known about its impact on disease pathophysiology.
The in vitro generation of RPE monolayers has enabled the study of cell dynamics under experimental conditions, which has increased our knowledge of the cellular and molecular mechanisms of AMD. The ARPE-19 cell line used in this study maintained many of the differentiation characteristics of RPE, such as the expression of RPE65 and CRALBP, which are involved in the vitamin A cycle and regeneration of visual pigment, respectively. Molecules of immunological importance, such as TLR4, its CD14 co-receptor, and co-stimulatory molecules such as CD86 on the surface of ARPE-19 cells, were also identified as part of their cellular characterization. It is well known that the Toll-like receptors and co-stimulatory molecules expressed in RPE cells are important for inducing ocular inflammation mechanisms because of their ability to secrete or potentiate various inflammatory mediators [
26,
32,
33]. Additionally, direct sequencing of CFH confirmed the heterozygous presence of the Y402H variant in ARPE-19 cells, one of the main genetic variants associated with AMD development. These characteristics of ARPE-19 cells allowed us to consider them an ideal cell model for studying the effect of the at-risk CFH variant on the inflammatory response to oxidative stress.
It is known that one of the main generators of oxidative stress in the RPE cells is H
2O
2. In this study, we used H
2O
2 to mimic the conditions of physiological oxidative stress in vitro and evaluate the role of the at-risk CFH Y402H variant in ARPE-19 cells in generating the inflammatory cytokine profile under these conditions. The RPE is susceptible to oxidative stress but is protected by the production of antioxidant agents and its ability to regulate apoptosis under certain conditions [
8]. However, the inadequate neutralization of oxidative stress affects mitochondrial function and can lead to cellular damage. The main event after damage to the mitochondria is increased permeability and loss of membrane potential, which allows the release of pro-apoptotic molecules such as cytochrome C into the cytoplasm [
34,
35,
36]. Furthermore, the activation of caspases 9 and 3 has been demonstrated in RPE cells treated with H
2O
2 [
35].
In our oxidative stress model using H2O2, ARPE-19 cells showed different degrees of alteration in the permeability of their plasma membrane, which correlated with the amount of the internalized fluorescent marker (7AAD), and allowed the identification of living cells with intact plasma membranes and apoptotic cells. The increased release of cytochrome C in stressed cells confirmed apoptosis. This release increased in a time-dependent manner, which correlated with the permeability of the plasma membrane.
Our assays showed that release of cytochrome C was accompanied by the activation of the NF-kB transcription factor. These results suggest that oxidative stress triggers a signal transduction cascade that induces the expression of genes encoding NF-kB-dependent proinflammatory cytokines. However, further studies are needed to investigate the possible signaling pathways that lead to apoptosis.
Intervention with caspases and the proteolytic pathway of the Bcl-2 family could be involved in cytochrome C release. This could be feasible considering that in the mitochondria, Bcl-2 proteins modulate the apoptotic signal that releases apoptotic factors such as cytochrome C and activates effectors such as caspases 9 and 3 that promote cell death [
37,
38]. Additionally, signal transduction through Fas or TNF-α receptors with caspase 8 activation also stimulates cytochrome C release through the cleavage pathway of Bcl-2 family products [
39].
The synthesis of pro-inflammatory cytokines is dependent on the activation of transcription factors, or the action of other cytokines leading to the establishment of a state of inflammation that contributes to the elimination of agents that cause cell damage; however, under disease conditions, exacerbation of the inflammatory response leads to cell and tissue damage. In RPE cells, the expression of IL-6 and IL-8 for example, is induced in vitro by TNF-α, a potent initiator of the immune response. The synthesis of these cytokines by RPE cells plays an important role in the immune processes of the eye to achieve homeostasis in stressful situations; however, its exacerbated or poorly controlled action induces severe tissue damage, leading to the development of diseases such as AMD [
40].
Our results showed that ARPE-19 cells were highly susceptible to damage caused by oxidative stress, with increased levels of inflammatory mediators and pro-apoptotic factors that lead to cell death. However, it is unknown whether the presence of Y402H polymorphism is related to the inability of cells to achieve homeostasis under oxidative stress. Therefore, we evaluated whether the presence of exogenous wild type CFH could restore the ability of cells to modulate inflammatory responses and survive under prolonged conditions of oxidative stress.
Exogenous CFH incorporated into the cell culture prior to the induction of oxidative stress prevented damage and cell death. This protective effect was related to the negative regulation of pro-inflammatory cytokines induced by exogenous CFH. In the absence of exogenous CFH, the synthesis of pro- and anti-inflammatory cytokines significantly increased, causing deleterious effects on cell viability. Similar alterations in cytokine profiles were observed when ARPE-19 cells were grown simultaneously in the presence of both stimuli. However, it is important to note that, in some cases, the levels of cytokines were dependent on the concentration of exogenous CFH such as, IL-1β, IL-12, or TNF-α; high concentrations of exogenous CFH inhibited the synthesis of these cytokines.
In basal conditions, RPE cells express very low levels of IL-1β which can be slightly induced by treatment with TNF-α or antibodies cross-linking of CD48 on the surface of cells, suggesting a minor role in the inflammatory process of the posterior segment of the eye [
41]. However, in our study, no significant differences were found in the levels of IL-1β synthesis in the ARPE-19 cell supernatant after any treatment. These results may indicate that the availability of CFH in the environment before an oxidative insult occurs is required to modulate the pro-inflammatory cytokine response and protect cells from oxidative damage.
Therefore, it is possible that the elevation of pro-inflammatory cytokines under oxidative stress observed in this study was related to the inability of the at-risk Y402H variant of CFH to regulate complement activation. Although our assays did not elucidate the mechanisms by which the at-risk variant in CFH promotes the dysregulated expression of pro-inflammatory cytokines, a plethora of evidence in the literature has been reported. Oxidative stress triggers the inflammatory response and activates death signals. The activation of complements and their bioactive fragments is also a potent stimulator of cytokine secretion that leads to the amplification of inflammatory responses [
42].
Earlier studies have shown that the CFH Y402H polymorphism may be associated with increased activation of the complement cascade, where activation of complement proteins such as C3a and C5a may regulate the expression of proinflammatory cytokines [
43,
44]. Studies on ARPE-19 cells have shown increased intracellular concentrations of complement regulators CFH, properdin, and central complement protein C3 following oxidative stress induction. Properdin serves as a positive complement regulator; therefore, a higher concentration of this protein in ARPE-19 cells results in enhanced cellular C3 cleavage [
24]. It is potentially feasible that properdin activity prevails over that of the negative complement regulator CFH, which can be functionally decreased by the presence of the risk variant Y402H.
Other studies have reported that the complement activation product C5a is associated with the at-risk variant in the CFH gen and promotes the activation of the NF-kB pathway in RPE in vitro [
45]. Concurrently, the NF-kB pathway mediates the expression of classic inflammatory cytokines (IL-6, IL-8, TNF-α) and those dependent of the activation of the NLRP3 inflammasomes such as IL-1β and IL-18 [
46]. The addition of H
2O
2 to ARPE-19 cells increases the inflammasome activation NLRP3 and subsequently enhances the secretion of proinflammatory cytokines [
24]. Thus, it has been suggested that the CFH Y402H variant contributes to AMD through increased activation of complement and NF-kβ pathways that result in exacerbated secretion of inflammatory cytokines.
Finally, our results show that CFH is important for modulating the synthesis of proinflammatory cytokines and preventing cell damage in response to oxidative stress.
Further studies are needed to verify the signaling pathway(s) induced by exogenous CFH to regulate the secretion of proinflammatory cytokines, and to investigate the endogenous expression of CFH at the mRNA and protein level.