Submitted:
15 June 2023
Posted:
15 June 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
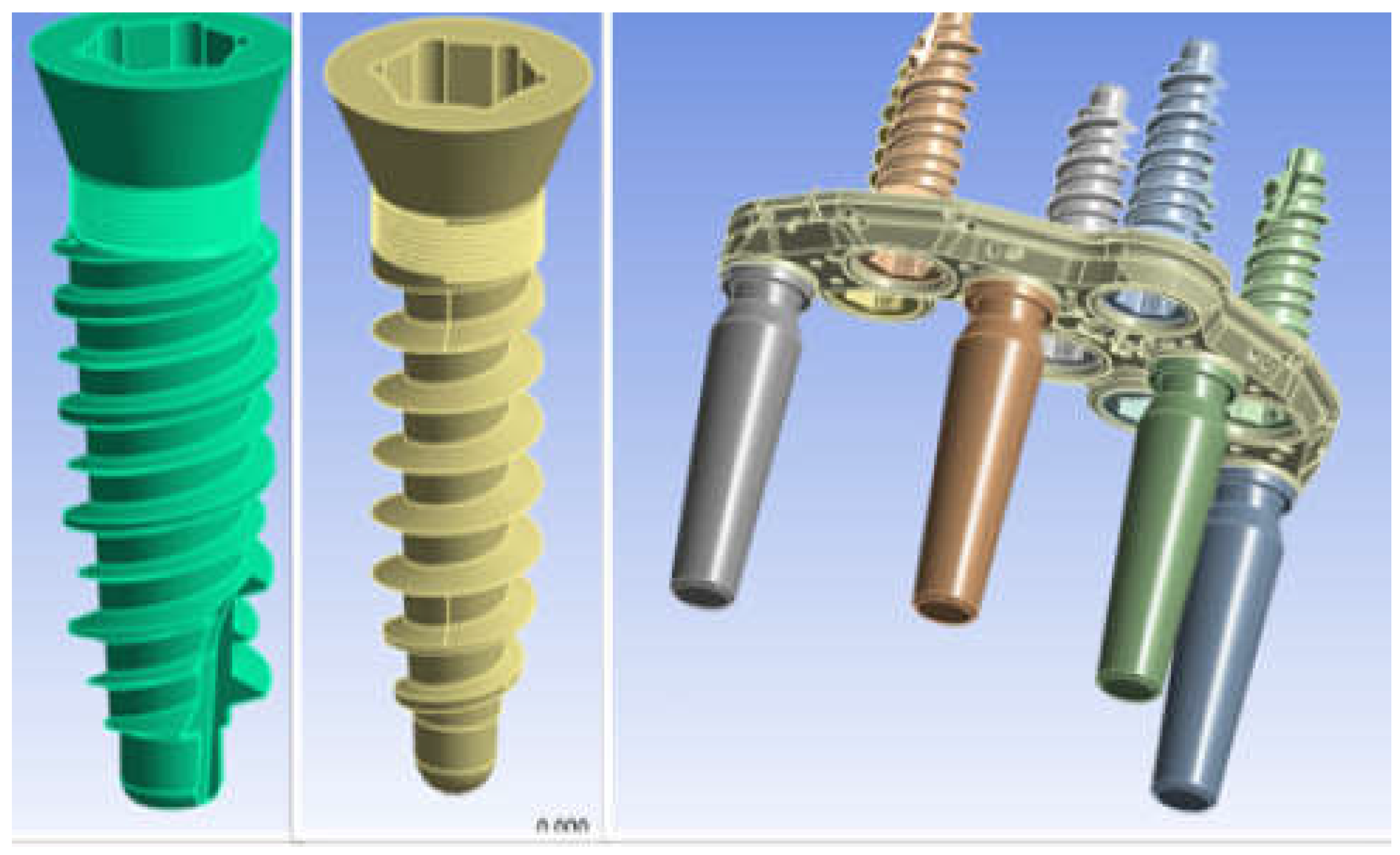
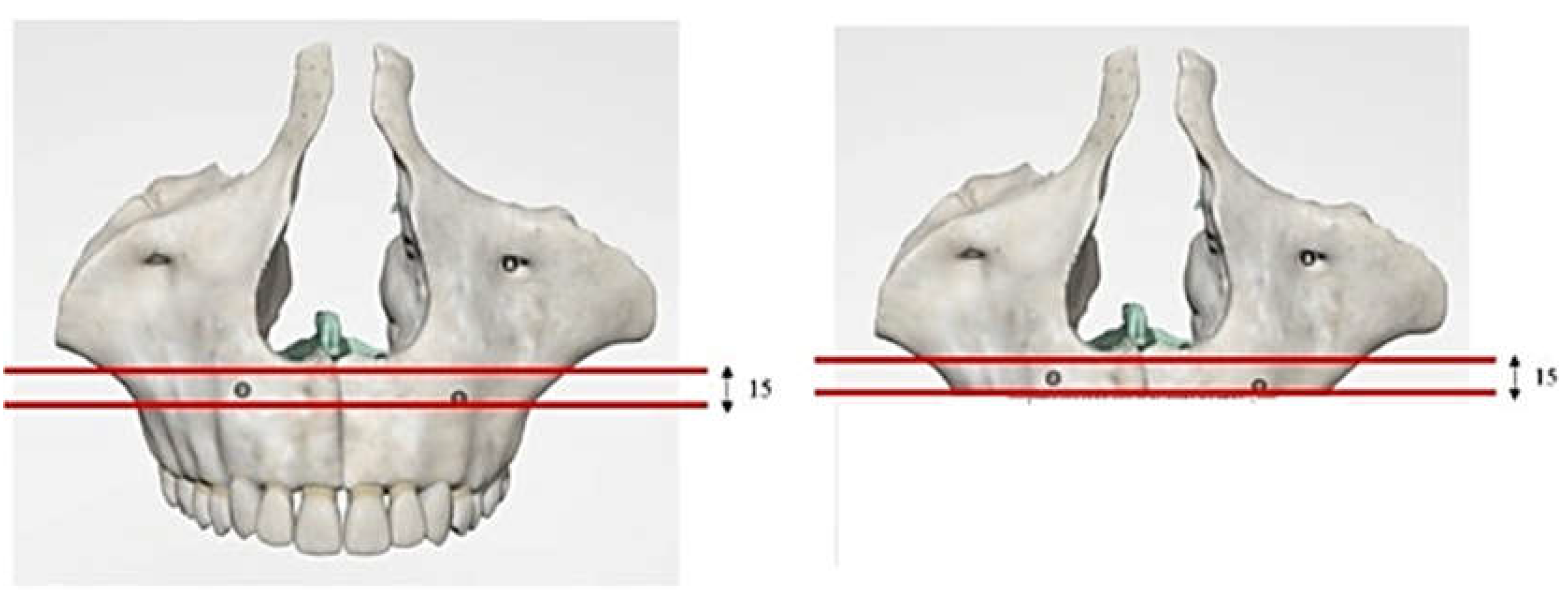
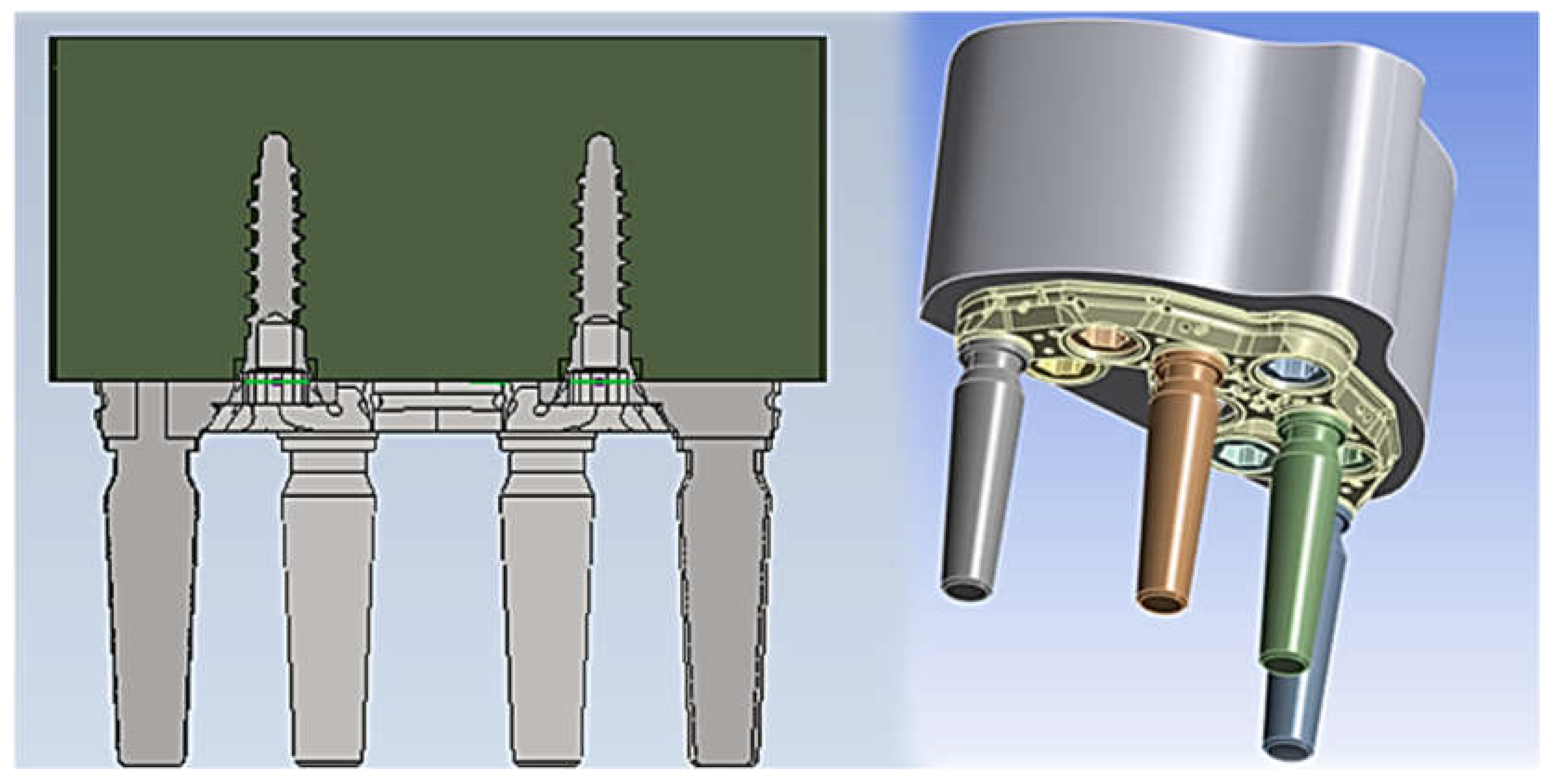
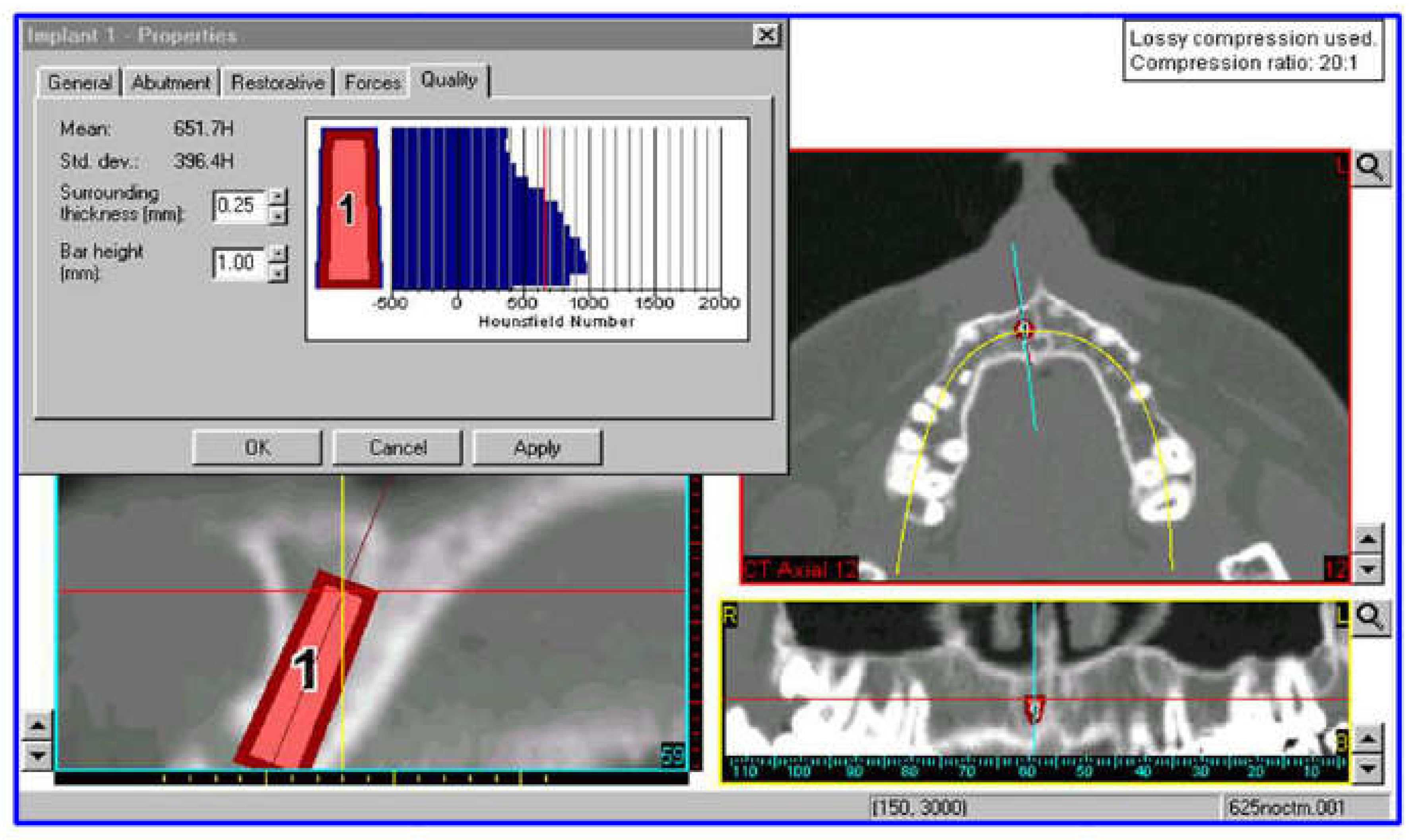
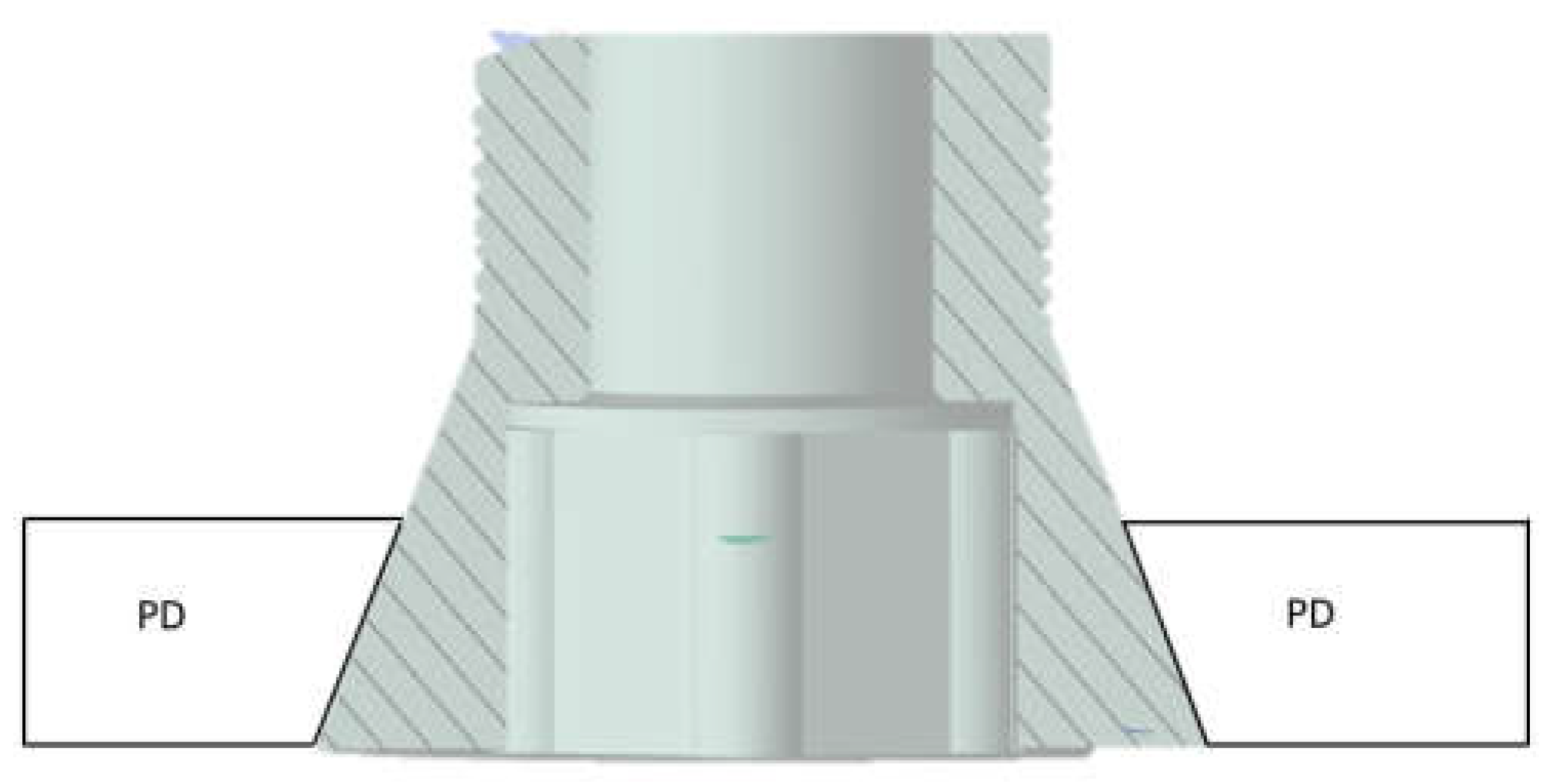
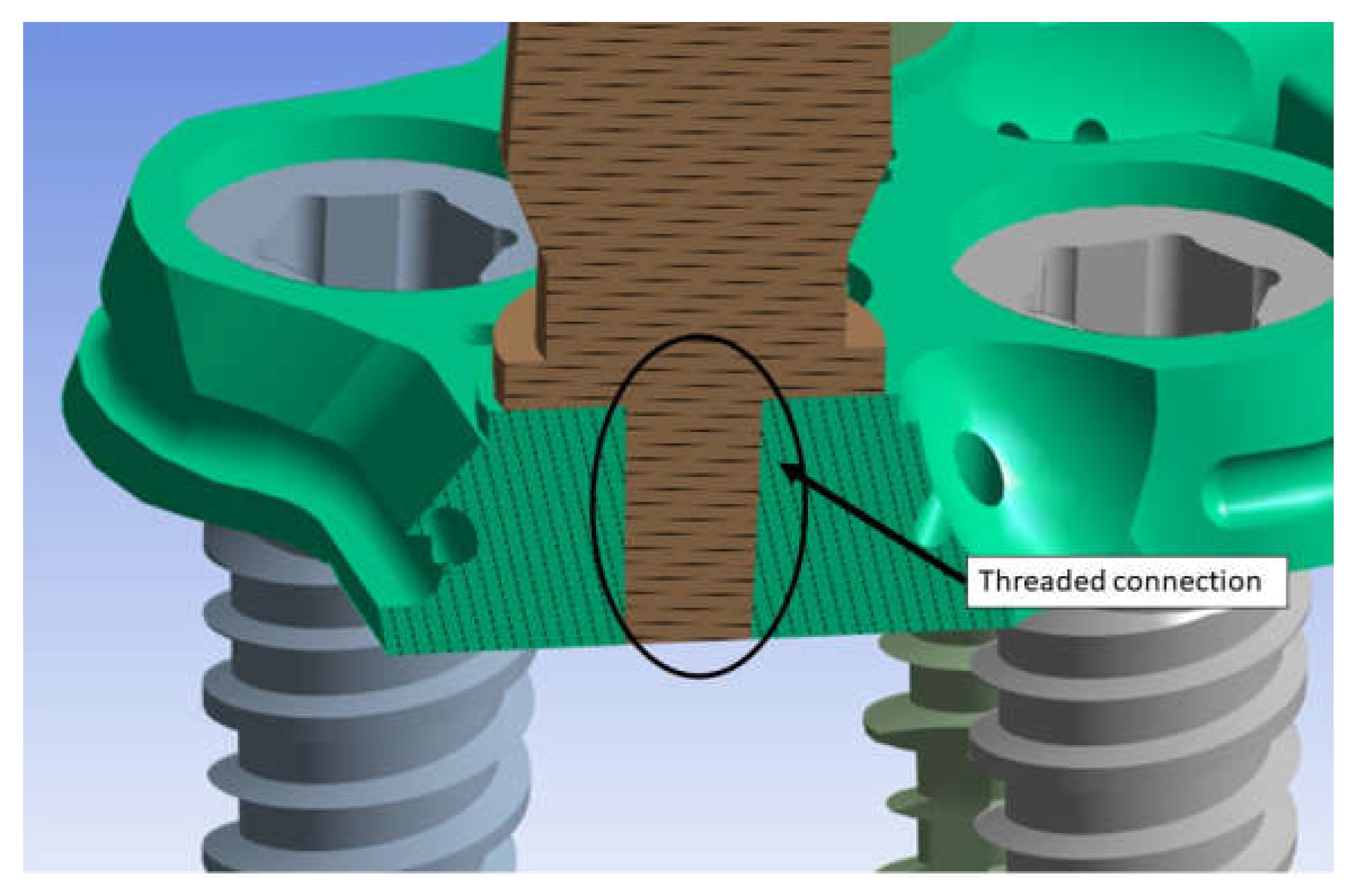
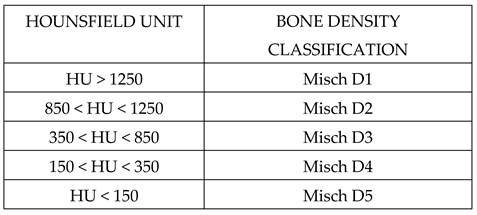
2.1. Modeling
2.2. Materials
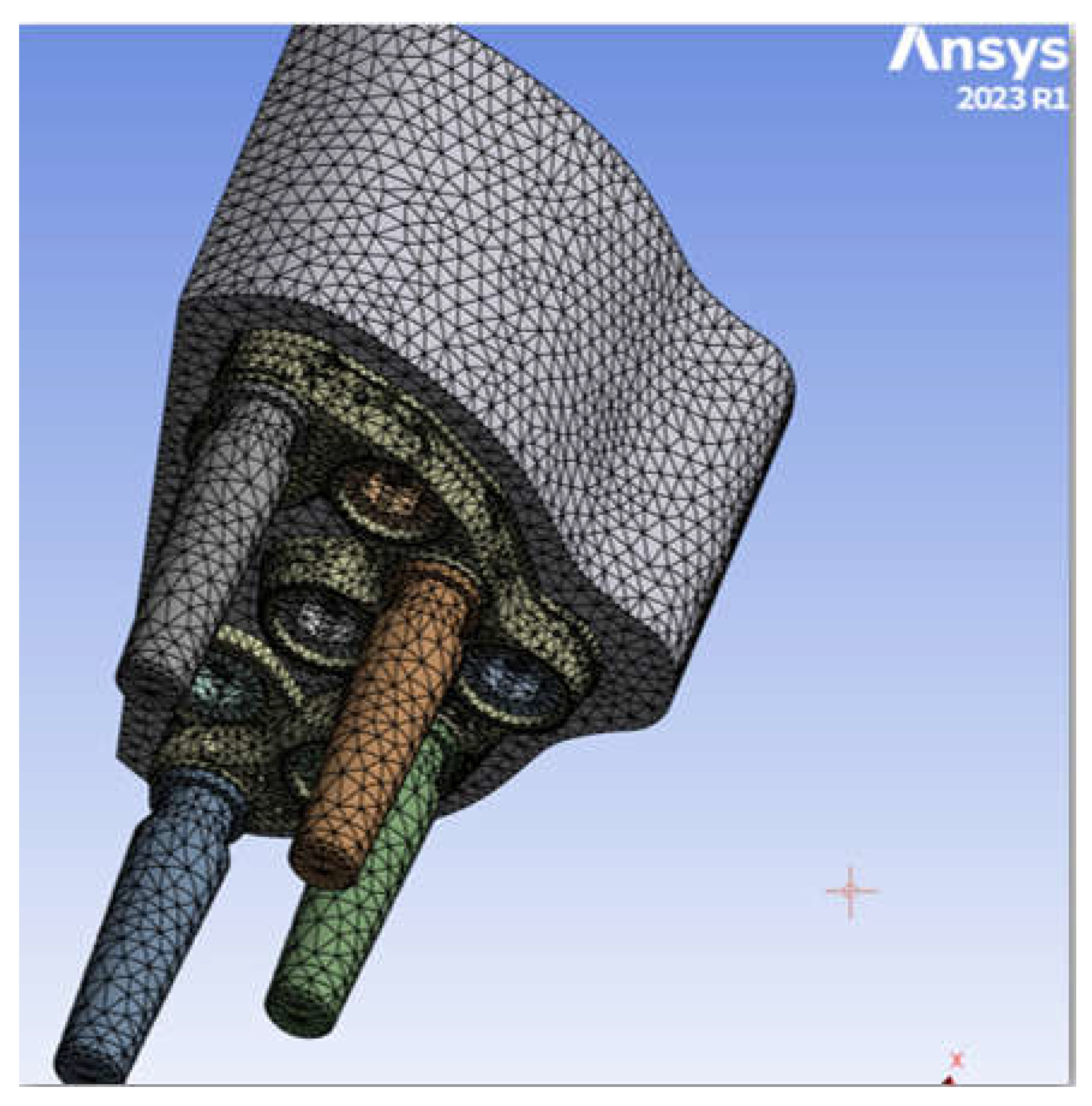
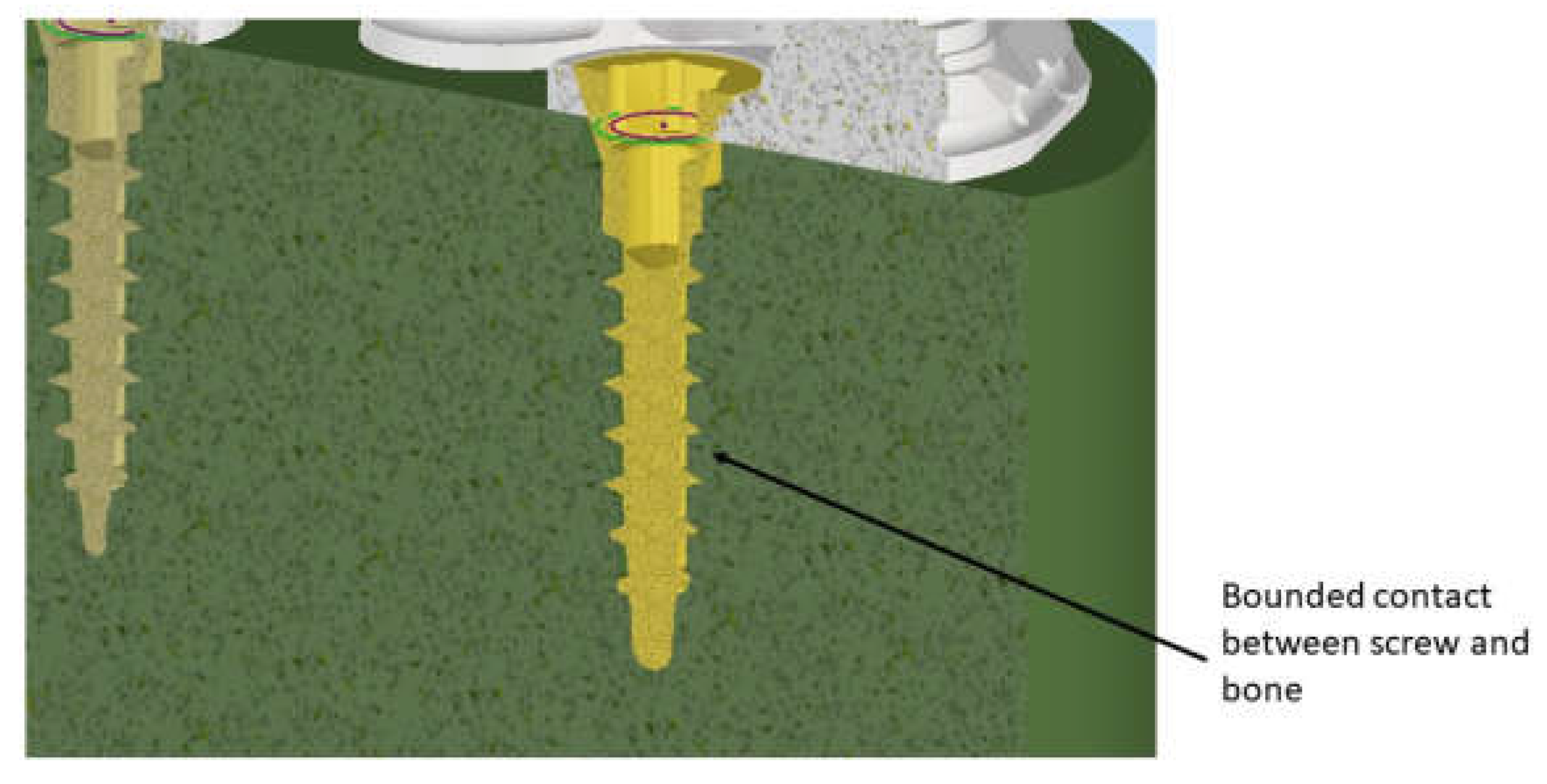
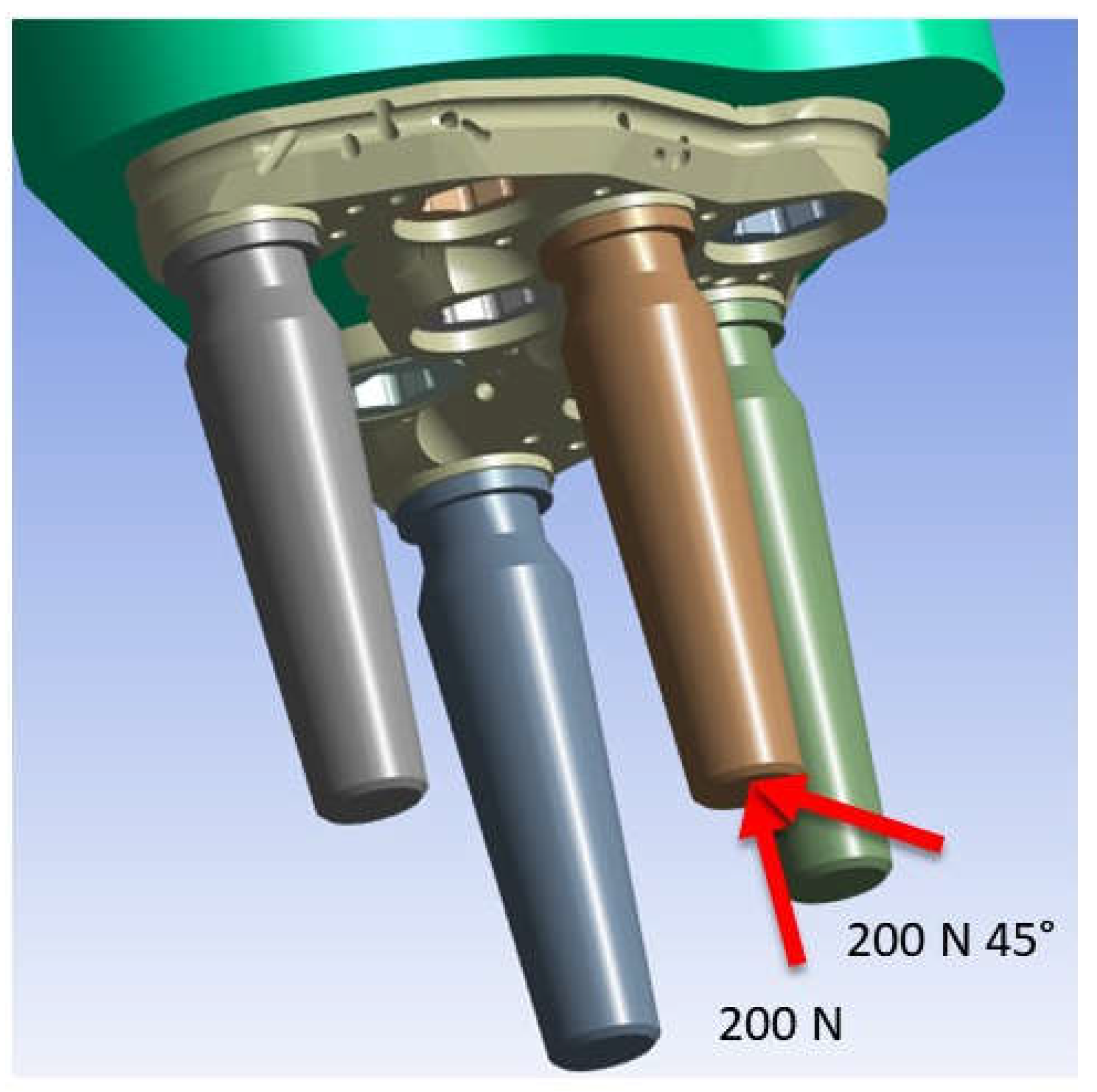
2.3. FEM Modeling
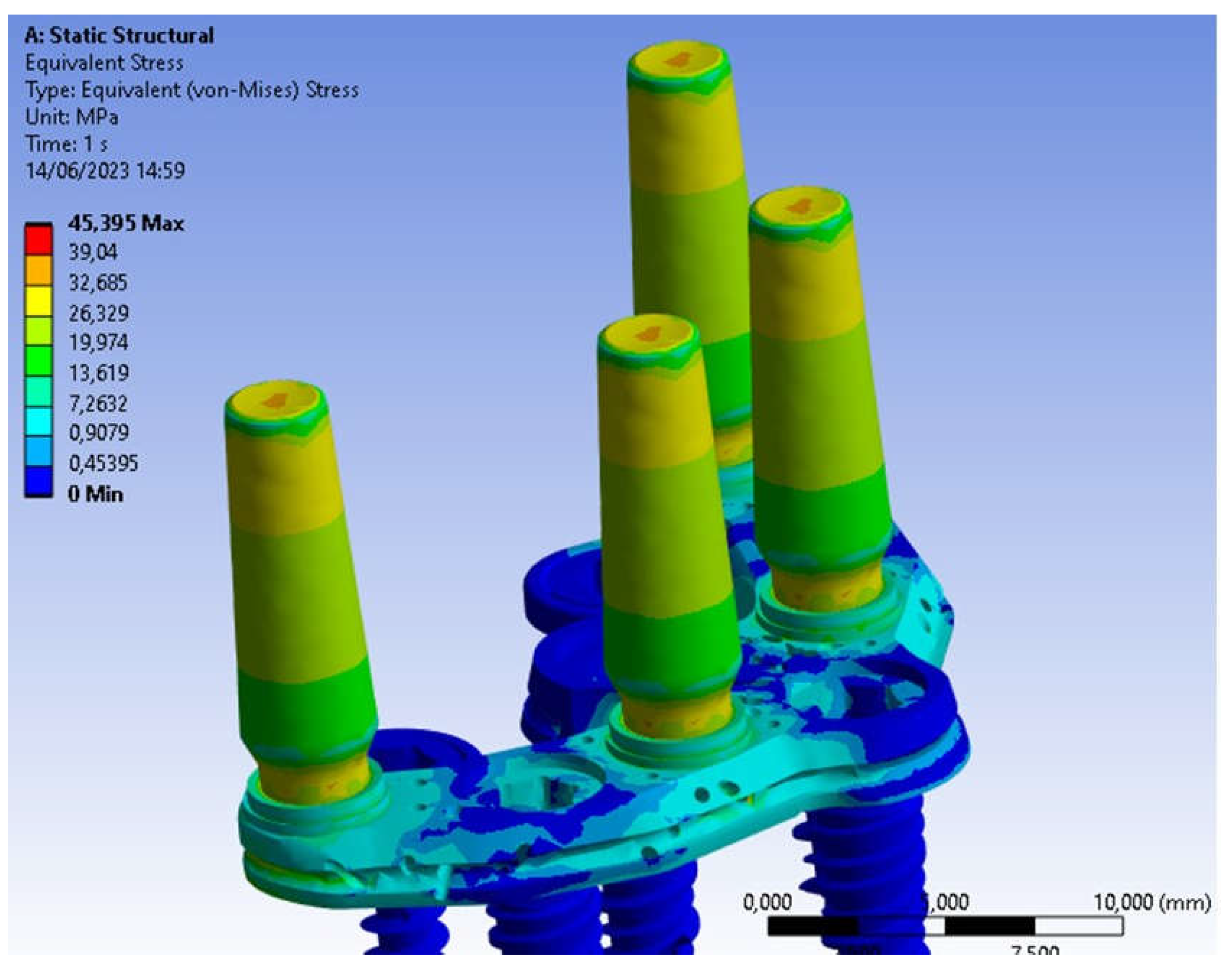
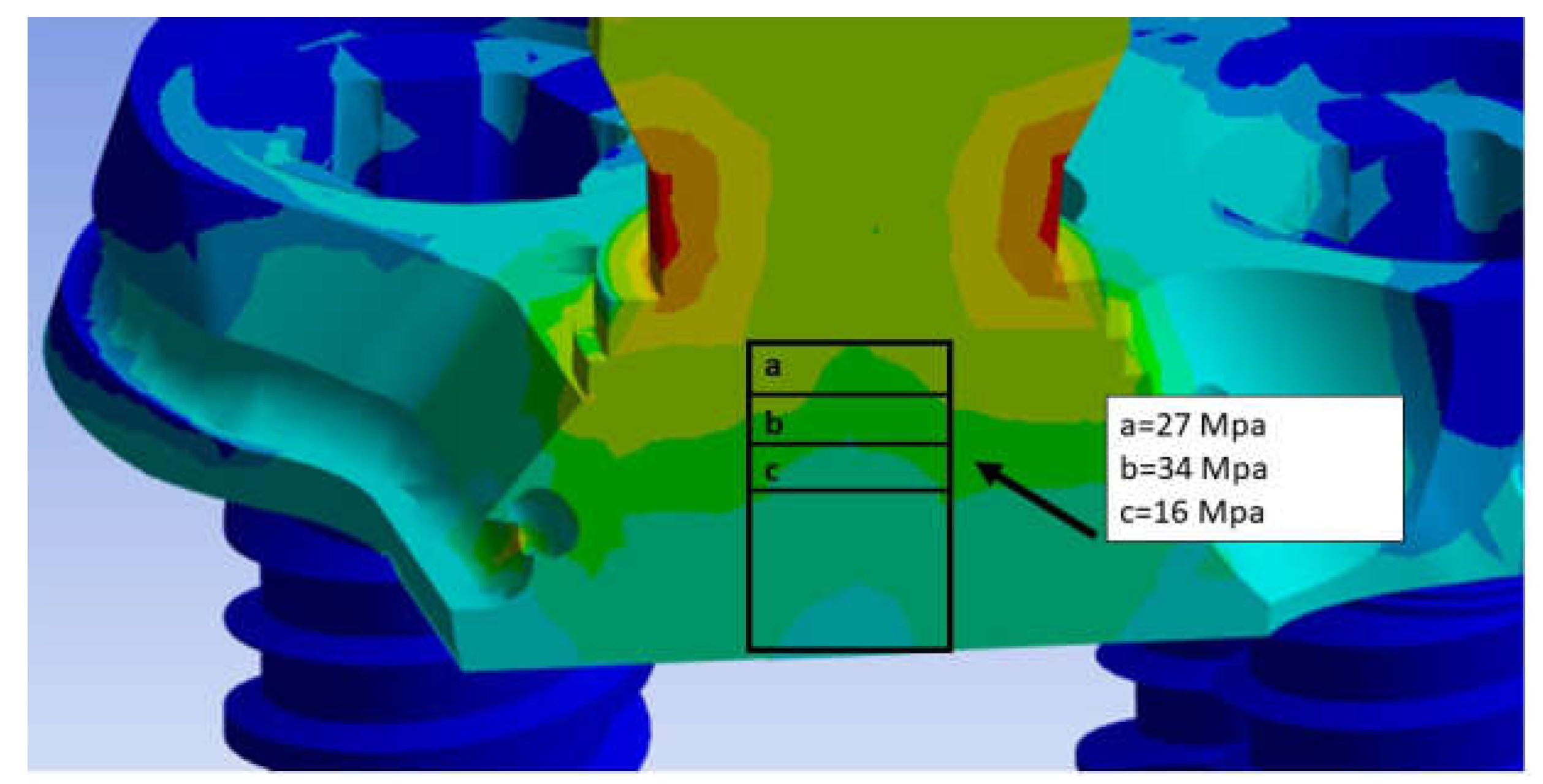
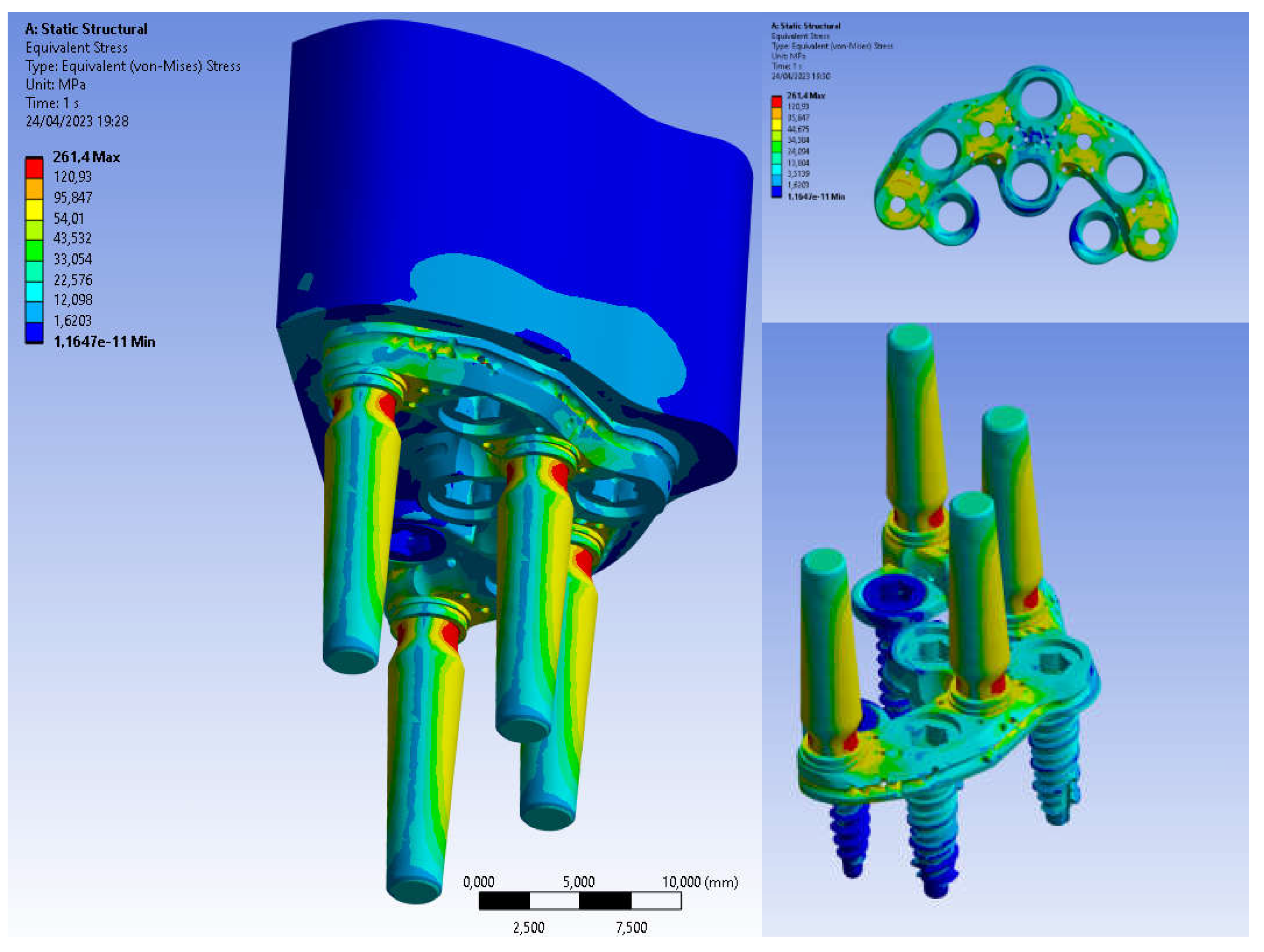
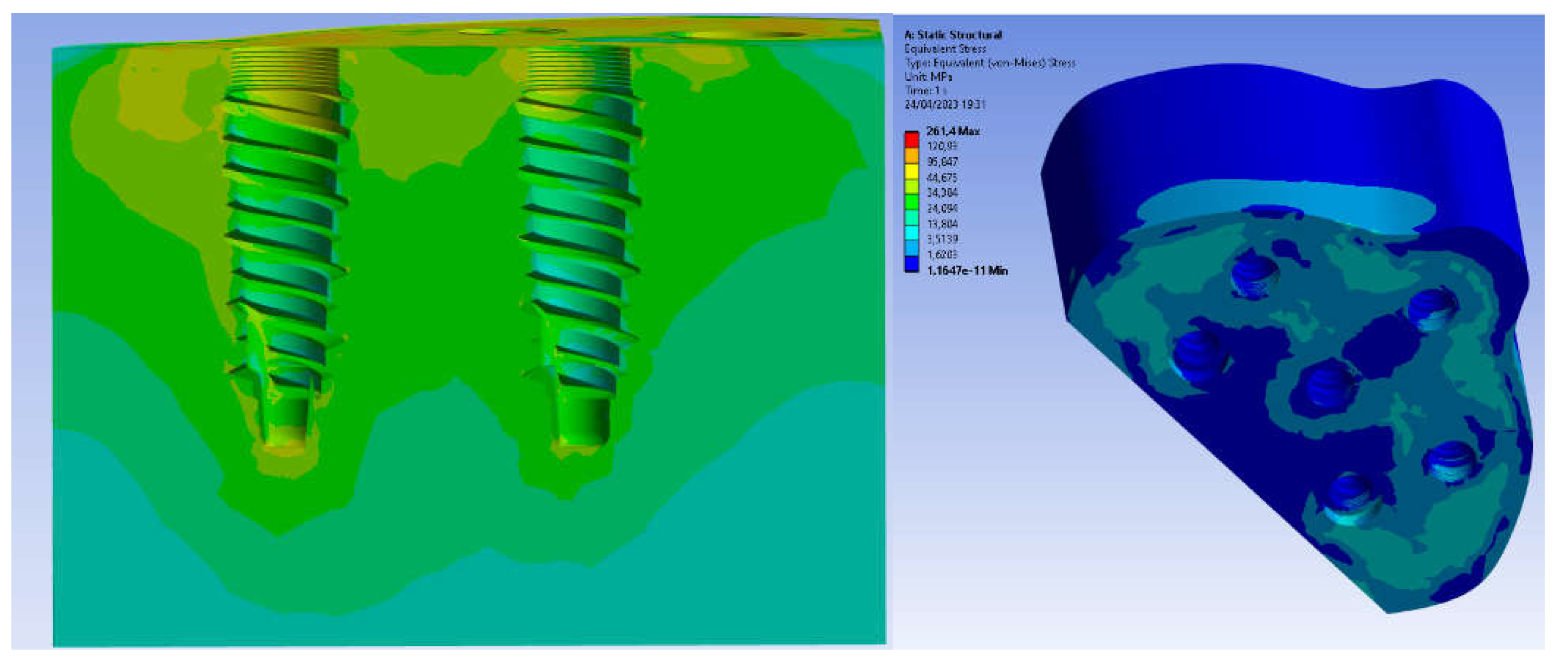
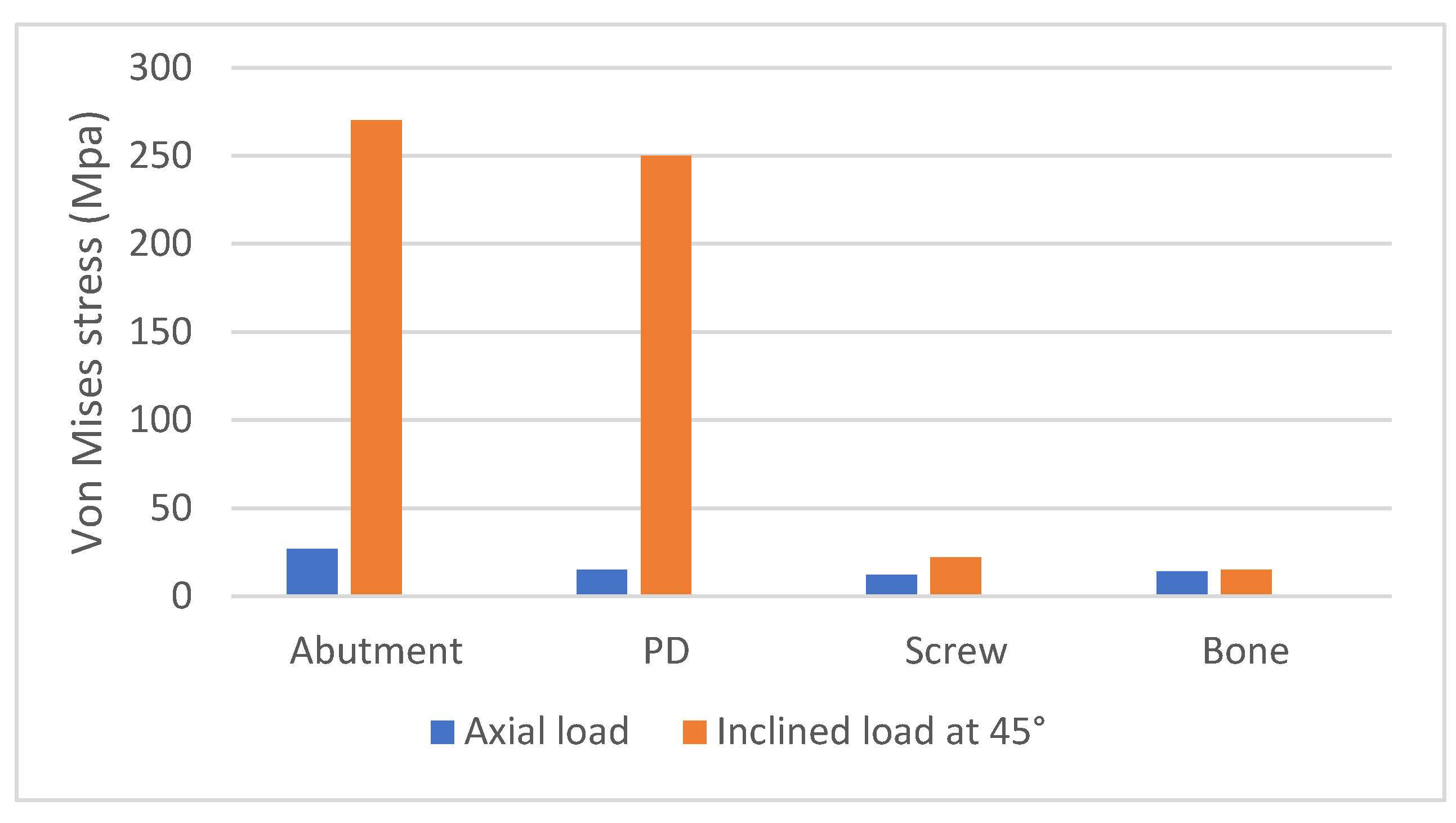
3. Results
4. Discussion
5. Conclusions
- The PD treatment concept has shown a most favorable biomechanical behavior and can be considered a viable alternative for rehabilitation of severe atrophic maxilla;
- The most rigid materials, such as titanium alloys, showed the most favorable biomechanical behavior and reduced stress levels for bone, implants, screws, and abutments;
- Stress values did not exceed the bone strength limits for basal bone and titanium alloy;
- The application of inclined load increases stress on all areas.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ramezanzade S, Yates J, Tuminelli FJ, Omid Keyhan S , Yousefi P, Lopez-Lopez J Zygomatic implants placed in atrophic maxilla: an overview of current systematic reviews and meta-analysis Maxillofac Plast Reconstr Surg 2021;43:1.
- Sales PH , Gomes MV , Oliveira-Neto OB , de Lima FJ, Leão JC Quality assessment of systematic reviews regarding the effectiveness of zygomatic implants: an overview of systematic reviews Med Oral Patol Oral Cir Bucal 2020;25:e541-e548.
- Bidra AS, Huynh-Ba G Implants in the pterygoid region: a systematic review of the literature Int J Oral Maxillofac Surg 2011;40:773-81.
- Peñarrocha-Oltra D, Candel-Martí E, Ata-Ali J, Peñarrocha-Diago M. Rehabilitation of the atrophic maxilla with tilted implants: review of the literature. J Oral Implantol. 2013 Oct;39(5):625-32. Epub 2011 Nov 28. PMID: 22121829. [CrossRef]
- Seong WJ, Kim UK, Swift JQ, Heo YC, Hodges JS, Ko CC. Elastic properties and apparent density of human edentulous maxilla and mandible. Int J Oral Maxillofac Surg. 2009 Oct;38(10):1088-93. Epub 2009 Jul 31. PMID: 19647417; PMCID: PMC2743800. [CrossRef]
- Feras Haroun and Oguz Ozan. Evaluation of Stresses on Implant, Bone, and Restorative Materials Caused by Different Opposing Arch Materials in Hybrid Prosthetic Restorations Using the All-on-4 Technique. Materials 2021, 14(15), 4308. [CrossRef]
- Bhering, C.L.B.; Mesquita, M.F.; Kemmoku, D.T.; Noritomi, P.Y.; Consani, R.L.X.; Barão, V.A.R. Comparison between all-on-four and all-on-six treatment concepts and framework material on stress distribution in atrophic maxilla: A prototyping guided 3D-FEA study. Mater. Sci. Eng. C 2016, 69, 715–725. [CrossRef]
- Oh, J.-H.; Kim, Y.-S.; Lim, J.Y.; Choi, B.-H. Stress Distribution on the Prosthetic Screws in the All-on-4 Concept: A Three- Dimensional Finite Element Analysis. J. Oral Implant. 2020, 46, 3–12. [CrossRef]
- Kitamura E, Stegaroiu R, Nomura S, Miyakawa O. Biomechanical aspects of marginal bone resorption around osseointegrated implants: considerations based on a three-dimensional finite element analysis. Clin Oral Implants Res. 2004 Aug;15(4):401-12. PMID: 15248874. [CrossRef]
- Duyck, J.; Vandamme, K. The effect of loading on peri-implant bone: A critical review of the literature. J. Oral Rehabil. 2014, 41, 783–794. [CrossRef]
- Erkmen, E.; Meriç, G.; Kurt, A.; Tunç, Y.; Eser, A. Biomechanical comparison of implant retained fixed partial dentures with fiber reinforced composite versus conventional metal frameworks: A 3D FEA study. J. Mech. Behav. Biomed. Mater. 2011, 4, 107–116. [CrossRef]
- Bijjargi, S.; Chowdhary, R. Stress dissipation in the bone through various crown materials of dental implant restoration: A 2-D finite element analysis. J. Investig. Clin. Dent. 2012, 4, 172–177. [CrossRef]
- Elsayyad, A.A.; Abbas, N.A.; AbdelNabi, N.M.; Osman, R.B. Biomechanics of 3-implant-supported and 4-implant-supported mandibular screw-retained prostheses: A 3D finite element analysis study. J. Prosthet. Dent. 2020, 124, 68.e1–68.e10. [CrossRef]
- Mahmoud Al-Dajani, DDS, MSc, PhD (OMFS), MSc, FRCD(C) (DPH). Recent Trends in Sinus Lift Surgery and Their Clinical Implications. [CrossRef]
- Stephen S Wallace, Dennis P Tarnow, Stuart J Froum, Sang-Choon Cho, Homayoun H Zadeh, Janet Stoupel, Massimo Del Fabbro, Tiziano Testori. Maxillary sinus elevation by lateral window approach: evolution of technology and technique. journal of Evidence Based Dental Practice. [CrossRef]
- Gutiérrez Muñoz D , Obrador Aldover C, Zubizarreta-Macho A, González Menéndez H, Lorrio Castro J, Peñarrocha Oltra D, Montiel-Company JM, Hernández Montero S Survival Rate and Prosthetic and Sinus Complications of Zygomatic Dental Implants for the Rehabilitation of the Atrophic Edentulous Maxilla: A Systematic Review and Meta-Analysis Biology (Basel) 2021;10:601.
- Hsu YT, Rosen PS, Choksi K, Shih MC, Ninneman S, Lee CT Complications of sinus floor elevation procedure and management strategies: A systematic review Clin Implant Dent Relat Res 2022;24:740-765.
- Agliardi EL, Panigatti S , Romeo D, Sacchi L, Gherlone E Clinical outcomes and biological and mechanical complications of immediate fixed prostheses supported by zygomatic implants: A retrospective analysis from a prospective clinical study with up to 11 years of follow-up Clin Implant Dent Relat Res 2021;23:612-624.
- Marin S, Kirnbauer B, Rugani P, Payer M, Jakse N Potential risk factors for maxillary sinus membrane perforation and treatment outcome analysis ClinImplant Dent Relat Res 2019;21:66-72.
- Solà Pérez A, Pastorino D, Aparicio C, Pegueroles Neyra M, Sannam Khan R, Wright S, Ucer C Success Rates of Zygomatic Implants for the Rehabilitation of Severely Atrophic Maxilla: A Systematic Review Dent J (Basel) 2022;10:151.
- Al-Dajani M Incidence, Risk Factors, and Complications of Schneiderian Membrane Perforation in Sinus Lift Surgery: A Meta-Analysis Implant Dent 2016;25:409-15.
- Yalçın M, Can S, Akbaş M, Dergin G, Garip H, Altuğ Aydil B, Varol A Retrospective Analysis of Zygomatic Implants for Maxillary Prosthetic Rehabilitation Int J Oral Maxillofac Implants 2020;35:750-756.
- Peñarrocha M, Carrillo C, Boronat A, Peñarrocha M Retrospective study of 68 implants placed in the pterygomaxillary region using drills and osteotomes Int J Oral Maxillofac Implants 2009;24:720-6.
- Lan K, Wang F, Huang W, Davó R, Wu Y Quad Zygomatic Implants: A Systematic Review and Meta-analysis on Survival and Complications Int J Oral Maxillofac Implants 2021;36:21-29.
- Barone A, Santini S, Sbordone L, Crespi R, Covani U A clinical study of the outcomes and complications associated with maxillary sinus augmentation Int J Oral Maxillofac Implants 2006;21:81-5.
- Molinero-Mourelle P, Baca-Gonzalez L, Gao B, Saez-Alcaide LM, Helm A, Lopez-Quiles J. Surgical complications in zygomatic implants: A systematic review. Med Oral Patol Oral Cir Bucal. 2016 Nov 1;21(6):e751-e757. PMID: 27694789; PMCID: PMC5116118. [CrossRef]
- Goiato MC, Pellizzer EP, Moreno A, Gennari-Filho H, dos Santos DM, Santiago JF Jr, dos Santos EG. Implants in the zygomatic bone for maxillary prosthetic rehabilitation: a systematic review. Int J Oral Maxillofac Surg. 2014 Jun;43(6):748-57. Epub 2014 Feb 14. PMID: 24530034.. [CrossRef]
- Jokstad A, Sanz M, Ogawa T, Bassi F, Levin L, Wennerberg A, Romanos GE. A Systematic Review of the Role of Implant Design in the Rehabilitation of the Edentulous Maxilla. Int J Oral Maxillofac Implants. 2016;31 Suppl:s43-99. PMID: 27228254. [CrossRef]
- Ramos Chrcanovic B, Nogueira Guimarães Abreu MH Survival and complications of zygomatic implants: a systematic review Oral Maxillofac Surg 2013;17:81-93.
- Araujo MP, Innes NP, Bonifácio CC, Hesse D, Olegário IC, Mendes FM, Raggio DP. Atraumatic restorative treatment compared to the Hall Technique for occluso-proximal carious lesions in primary molars; 36-month follow-up of a randomised control trial in a school setting. BMC Oral Health. 2020 Nov 11;20(1):318. PMID: 33176756; PMCID: PMC7656501. [CrossRef]
- Bai L, Zheng L, Ji P, Wan H, Zhou N, Liu R, Wang C Additively Manufactured Lattice-like Subperiosteal Implants for Rehabilitation of the Severely Atrophic Ridge. ACS Biomater Sci Eng. 2022;8:912-920.
- Wilkirson E, Chandran R, Duan Y Rehabilitation of Atrophic Posterior Maxilla with Pterygoid Implants: A 3D Finite Element Analysis. Int J Oral Maxillofac Implants. 2021;36:e51-e62.
- Leung M, Alghamdi R, Fernandez Guallart I, Bergamini M, Yc Yu P, J Froum SJ, Cho SC Patient-Related Risk Factors for Maxillary Sinus Augmentation Procedures: A Systematic Literature Review Int J Periodontics Restorative Dent 2021;41:e121-e128.
- Candotto V, Gallusi G, Piva A, Baldoni M, Di Girolamo M Complications in sinus lift J Biol Regul Homeost Agents 2020;34(1 Suppl. 1):139-142.
- Stvrtecky RC, Zarate JO, Borgetti ZA. Epithelial adhesion and subperiosteal implants. J Oral Implantol. 1989;15(1):62-5. PMID: 2634781.
- Nemtoi A, Covrig V, Nemtoi A, Stoica G, Vatavu R, Haba D, Zetu I Custom-Made Direct Metal Laser Sintering Titanium Subperiosteal Implants in Oral and Maxillofacial Surgery for Severe Bone-Deficient Patients-A Pilot Study. Diagnostics 2022;12:2531.
- Dimitroulis, Gupta B, Wilson I, Hart C The atrophic edentulous alveolus. A preliminary study on a new generation of subperiosteal implants Oral Maxillofac Surg 2022 Feb 4. [CrossRef]
- Mangano C, Bianchi A, Mangano FG , Dana J , Colombo M , Solop I, Admakin O. Custom-made 3D printed subperiosteal titanium implants for the prosthetic restoration of the atrophic posterior mandible of elderly patients: a case series 3D Print Med 2020;6:1.
- Van den Borre C, Rinaldi M, De Neef B, Loomans NAJ, Nout E, Van Doorne L, Naert I, Politis C, Schouten H, Klomp G, Beckers L, Freilich MM, Mommaerts MY. Patient- and clinician-reported outcomes for the additively manufactured sub-periosteal jaw implant (AMSJI) in the maxilla: a prospective multicentre one-year follow-up study. Int J Oral Maxillofac Surg. 2022;51:243-250.
- James RA, Lozada JL, Truitt PH, Foust BE, Jovanovic SA Subperiosteal implants. CDA J. 1988;16:10-4.
- Shilpa T. Finite element analysis: A boon to dentistry. J Oral Biol Craniofac Res. 2014;4:200–3. [PMC free article].
- El-Anwar MI, El-Zawahry MM. A three dimensional finite element study on dental implant design. J Genet Eng Biotechnol. 2011;9:77–82.
- DeTolla DH, Andreana S, Patra A, Buhite R, Comella B. Role of the finite element model in dental implants. J Oral Implantol. 2000;26:77–81.
- Van Staden RC, Guan H, Loo YC. Application of the finite element method in dental implant research. Comput Methods Biomech Biomed Engin. 2006;9:257–70.
- Rahmitasari F, Ishida Y, Kurahashi K, Matsuda T, Watanabe M, Ichikawa T. PEEK with reinforced materials and modifications for dental implant applications. Dent J (Basel). 2017;5(4):35. [CrossRef]
- Schwitalla A, Abou-Emara M, Spintig T, Lackmann J, Muller W. Finite element analysis of the biomechanical effects of PEEK dental implants on the peri-implant bone. J Biomech. 2015;48(1):1–7. [CrossRef]
- Chen X, Mao B, Zhu Z, et al. A three-dimensional finite element analysis of mechanical function for 4 removable partial denture designs with 3 framework materials: CoCr, Ti-6Al-4V alloy and PEEK. Sci Rep. 2019;9:13975. [CrossRef]
- Tribst JPM, de Morais DC, Alonso AA, Piva AMOD, Borges ALS. Comparative three-dimensional finite element analysis of implant-supported fixed complete arch mandibular prostheses in two materials. J Indian Prosthodont Soc. 2017;17(3):255–60. [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





