Submitted:
14 June 2023
Posted:
15 June 2023
You are already at the latest version
Abstract
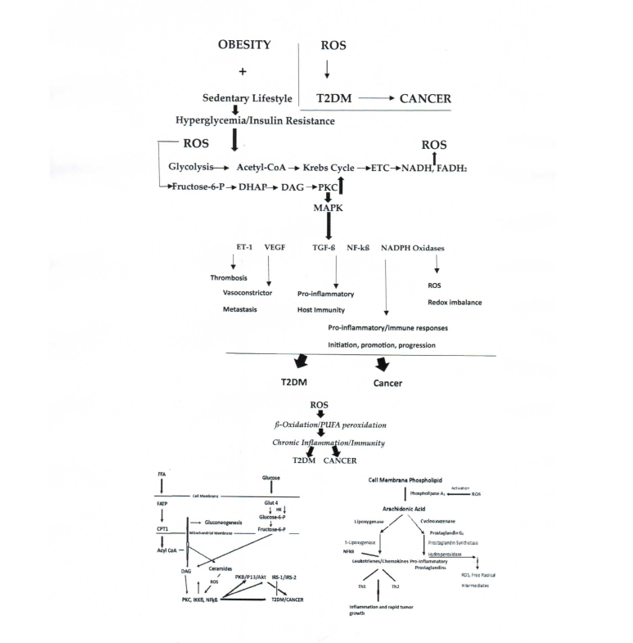
Keywords:
1. Introduction
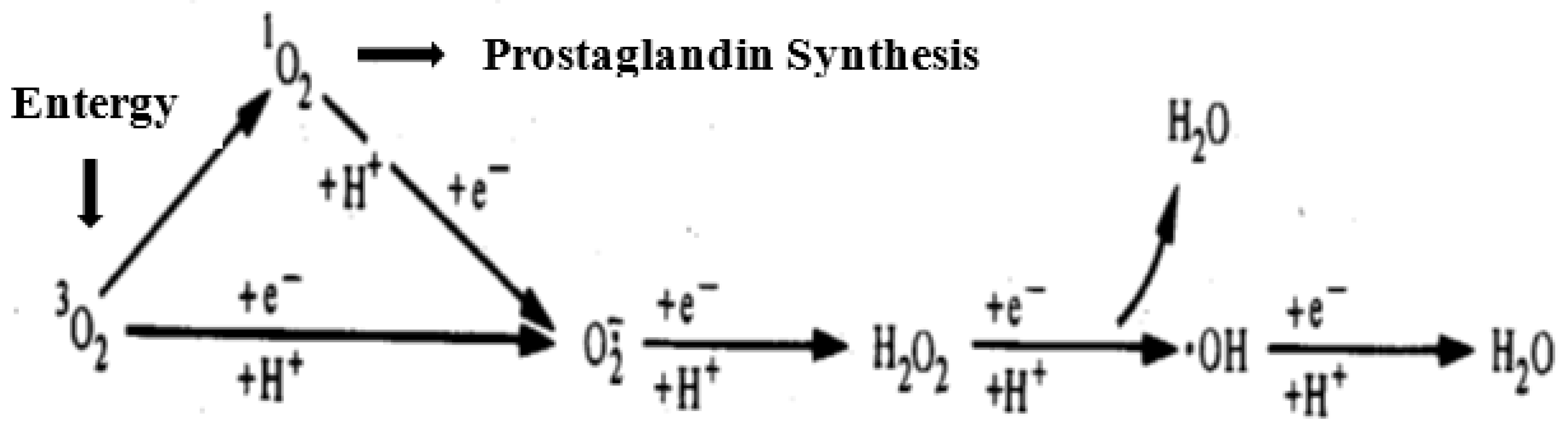
2. Oxidative Stress and Reactive Oxygen Species (ROS)
2.1. Background.
2.2. ROS and endogenous reactive species
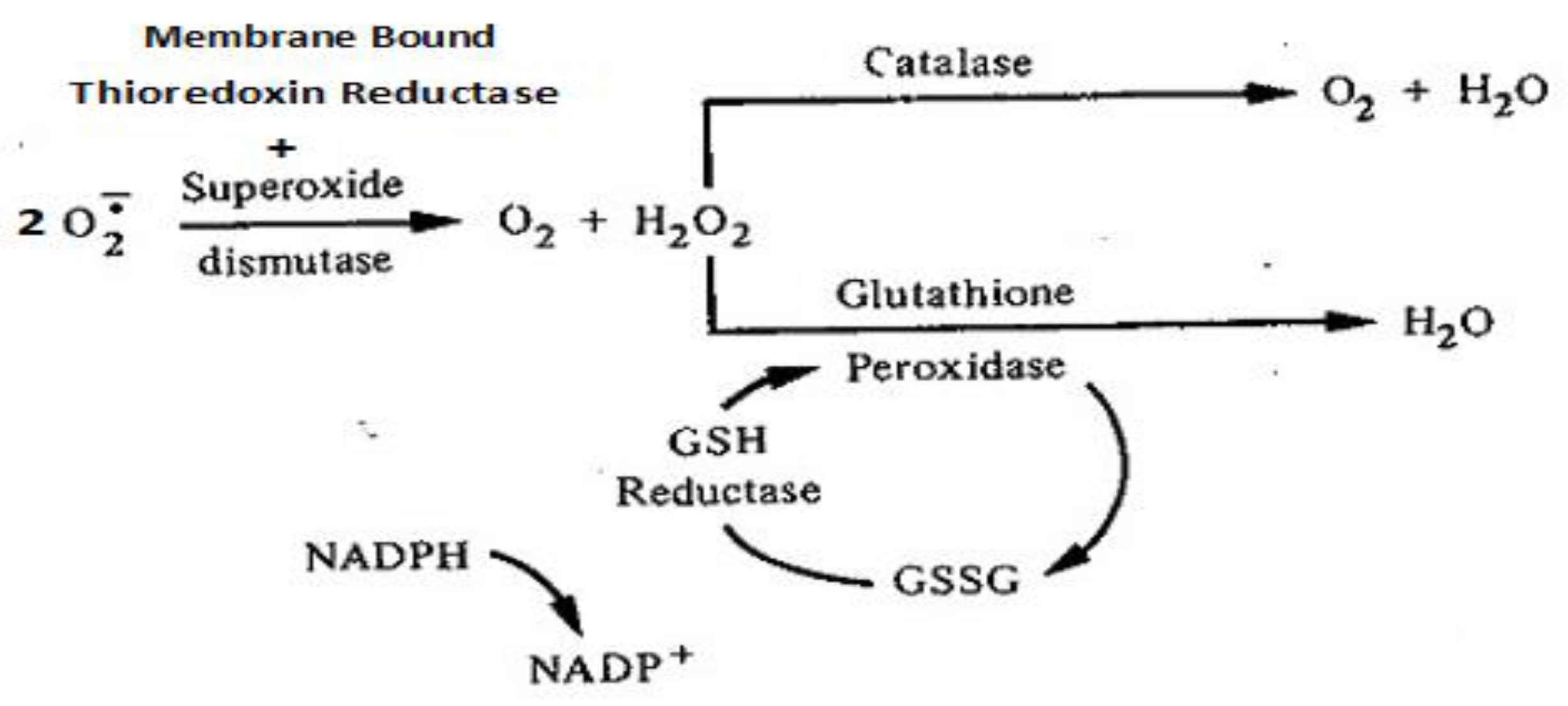
2.3. Antioxidant defense in maintaining Redox balance.
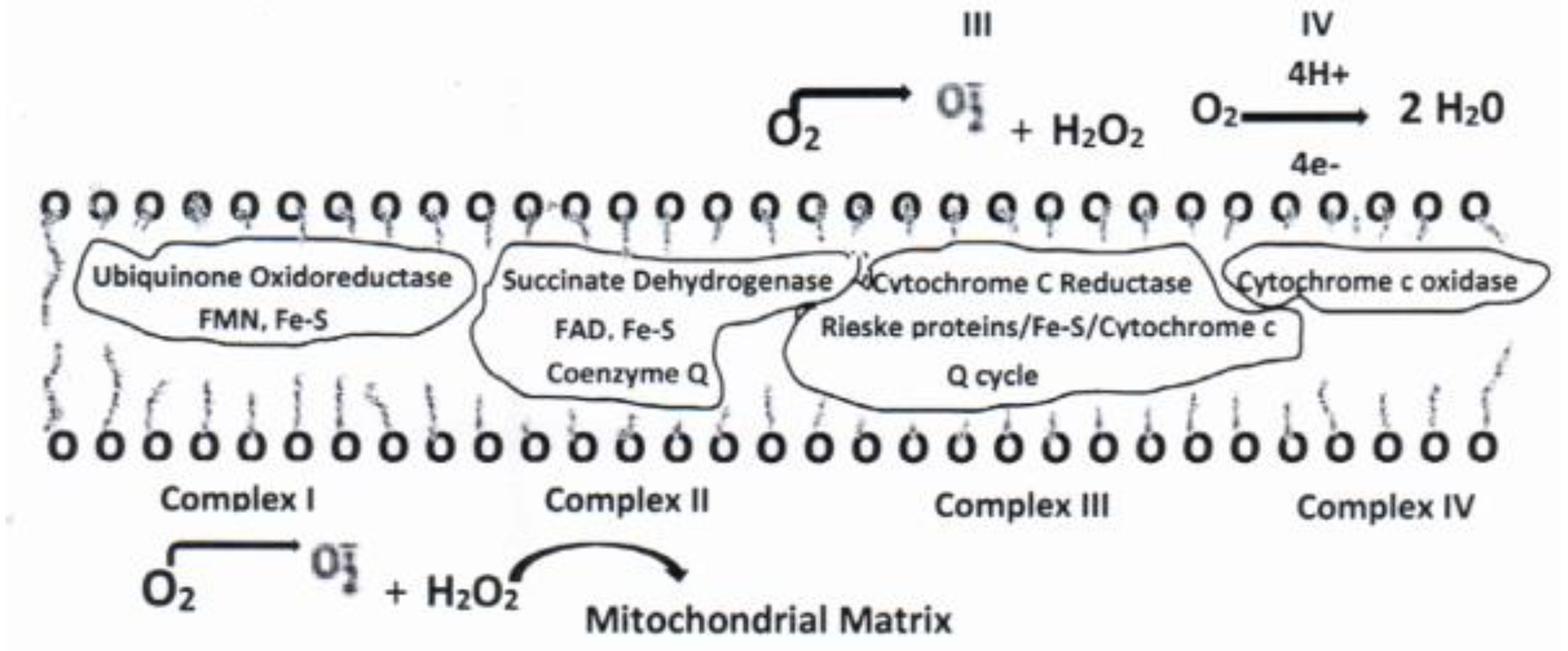
3. Mitochondria, the Electron Transport Chain (ETC) and Respiration.
3.1. Mitochondria and ROS
3.2. Electron Transport Chain.
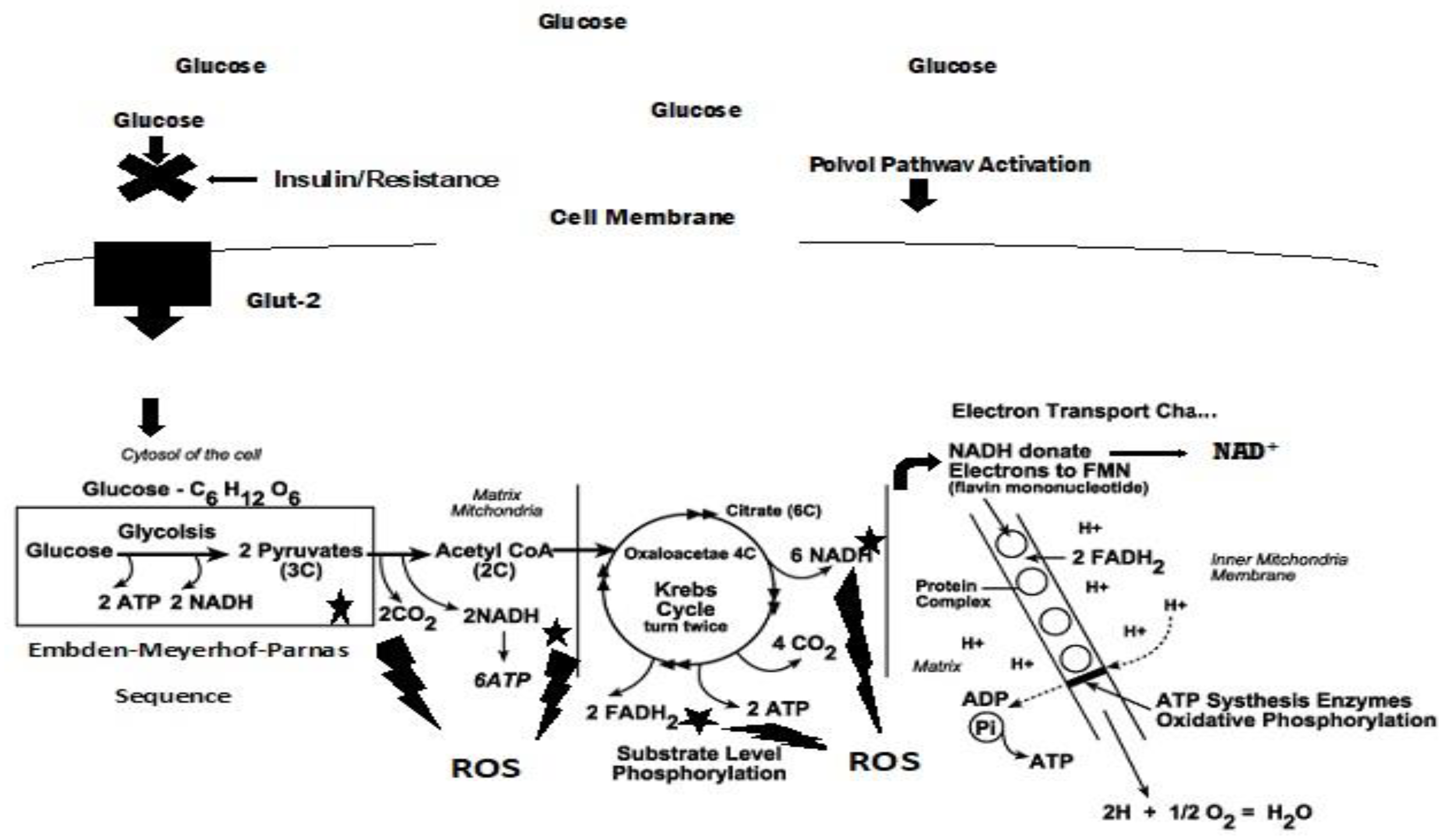
4. Diabetic versus Cancer Metabolism and Antioxidants
4.1. Diabetic metabolism

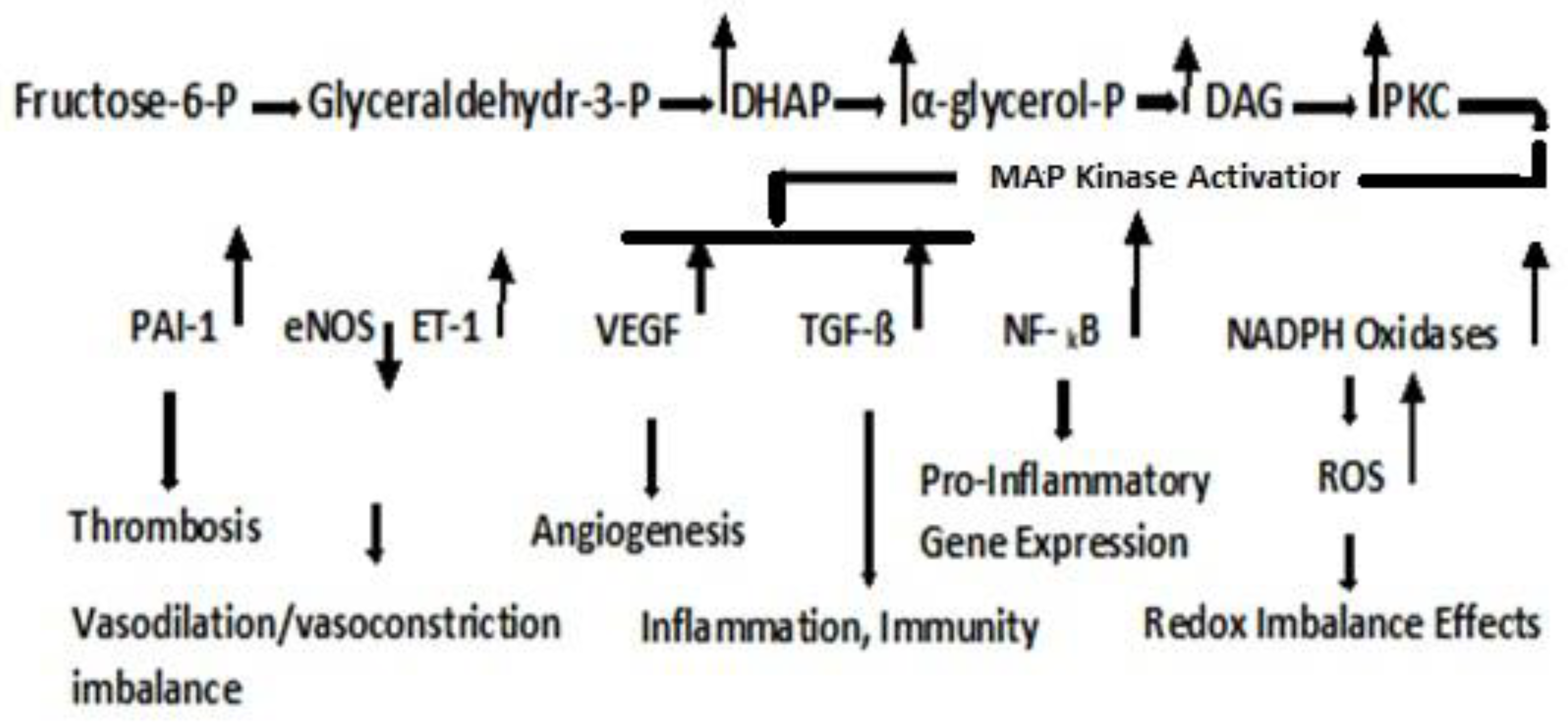
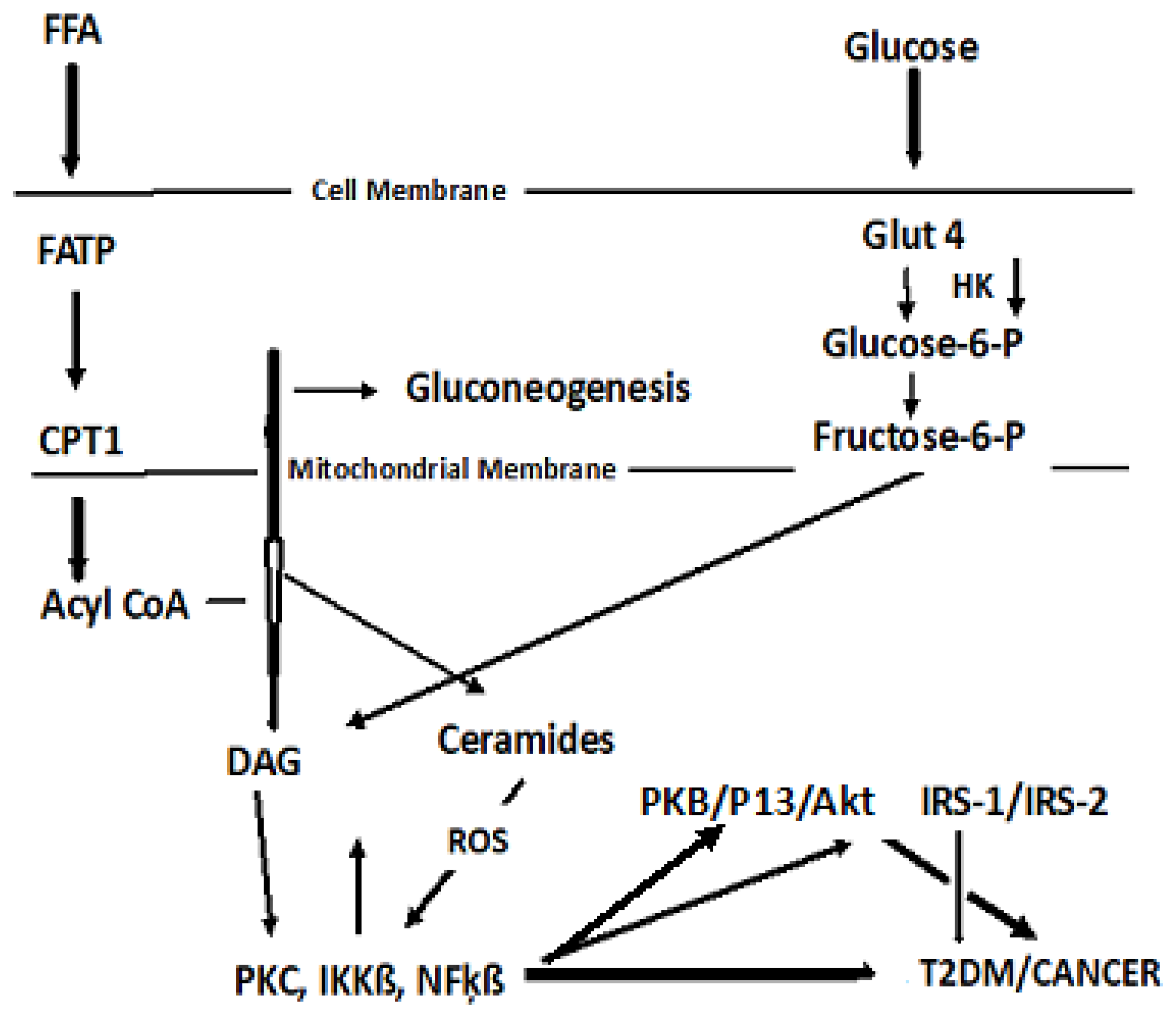
4.2. Dyslipidemia and Cell Signaling
4.3. Cancer Cell Metabolism
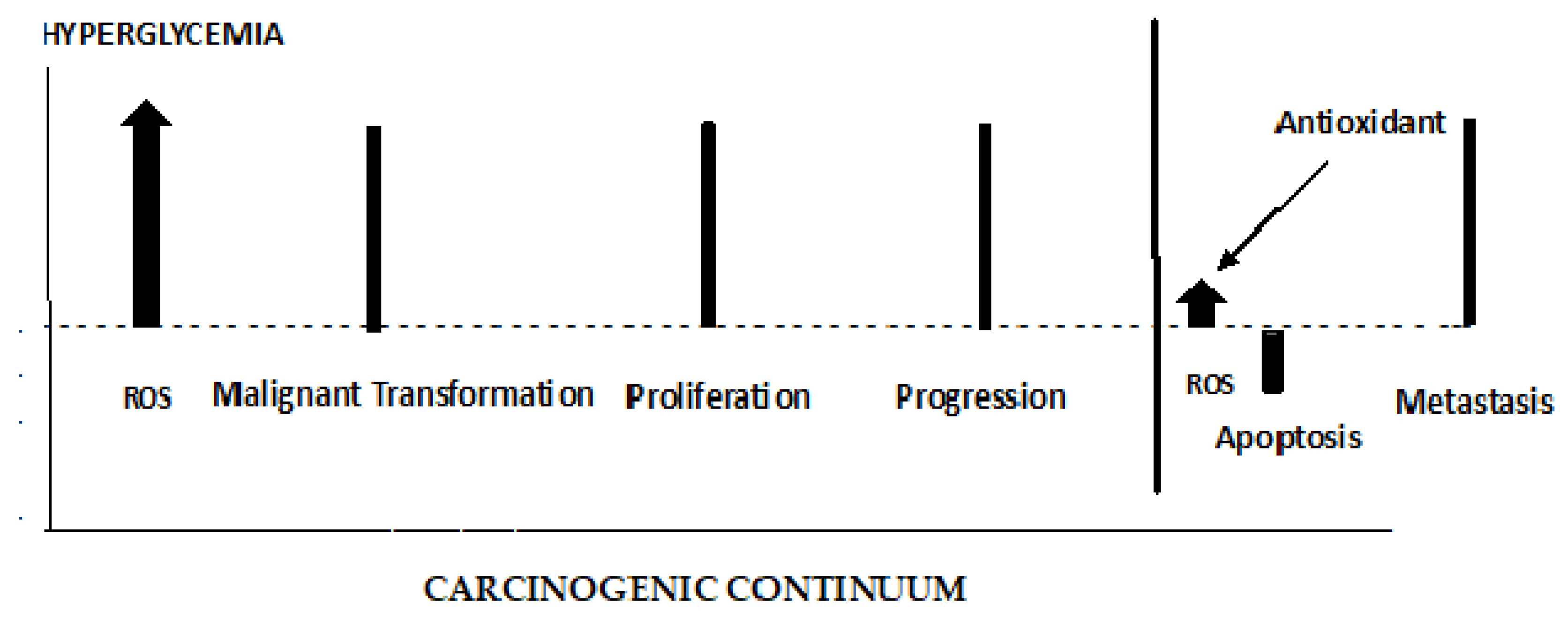
4.4. ROS’ role in early and late stages of cancer
5. Discussion and Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- International Diabetes Federation. IDF Diabetes Atlas, 10th edn. Brussels, Belgium: 2021. https://www.diabetesatlas.org.
- Abudawood, M. ; Diabetes and Cancer: A comprehensive review. J. Res. Med. Sci. 2019, 94. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. National Diabetes Statistics Report, 2020. Atlanta, GA: Centers for Disease Control and Prevention, U.S. Dept of Health and Human Services. https://cdc.gov/diabetes/data/statistics-report/index.html. Accessed 01/25/2022.
- American Diabetes Association. Diabetes Care, J Clin Appl Res Educ 2022, 45: Supplement 1: pp S17-S36.https://www.Diabetes.org/DibetesCare Accessed 02/11/2022.
- Polonsky, K.S. ; The Past 200 Years in Diabetes. N Engl J Med 2012, 367, 1332–1340. [Google Scholar] [CrossRef] [PubMed]
- Siege, l R. L.; Miller, K. D.; Fuchs H.E.; Jemal, A. Cancer Statistics, 2021. CA: A Cancer Journal for Clinicians 2021, 71, 7–33. [Google Scholar] [CrossRef]
- American Cancer Society. Global Cancer Facts & Figures 4th Edition. Atlanta: American Cancer Society; 2018.
- Torre, L.A.; Siegel, R.L.; Ward, E. M.; Jerma, A. Global Cancer Incidence and Mortality Rates and Trends – An Update. Cancer Epidemiol Biomarkers Prev. 2016, 25, 16–27. [Google Scholar] [CrossRef]
- Bray, F. The Evolving Scale and Profile of Cancer Worldwide: Much Ado About Everything. Cancer Surveillance Section, International Agency for Research on Cancer, Lyon, France. [CrossRef]
- World Health Organization. Cancer https://www.who.int/news-room/fact-sheets/detai;/cancer Accessed 11.27/2022.
- Jemal A, Torre L, Soerjomataram I, Bray F (Eds). The Cancer Atlas. Third Ed. Atlanta, GA: American Cancer Society, 2019.: http://www.cancer.org/canceratlas.
- Collins, K.K. ; The Diabetes-Cancer Link. Diabetes Spectrum 2014, 27, 276–280. [Google Scholar] [CrossRef]
- Ben, Q,; Xu, M,; Ning, X,; Liu,; Hong, S,; Huang, W,; Zhang, H,; Li, Z: Diabetes mellitus and risk of pancreatic cancer: a meta-analysis of cohort studies. Eur J Cancer 2011, 47, 1928–1937. [CrossRef]
- Jiang, Y,; Ben, Q;, Shen, H;, Lu, W,; Zhang, Y,; Zhu, J: Diabetes mellitus and incidence and mortality of colorectal cancer: a systematic review and meta-analysis of cohort studies. Eur J Epidemiol 2011, 26, 863–876. [CrossRef]
- Wang, C,; Wang, X,; Gong, G,; Ben Q,; Qiu, W,; Chen, Y,; Li, G,; Wang, L: Increased risk of hepatocellular carcinoma in patients with diabetes mellitus: a systematic review and meta-analysis of cohort studies. Int J Cancer 2012, 130, 1639–1648. [CrossRef]
- Larsson SC, Orsini N, Brismar K, Wolk A: Diabetes mellitus and risk of bladder cancer: a meta-analysis. Diabetologia 2006, 49, 2819–2823. [CrossRef]
- Larsson, S.C.; Mantzoros, C.S.; Wolk, A. : Diabetes mellitus and risk of breast cancer: a meta-analysis. Int J Cancer 2007, 121, 856–862. [Google Scholar] [CrossRef] [PubMed]
- Boyle, P,; Bonio, M,; l Koechlin, A,; Robertson,C,; Valentini, F,; Coppens, K,; Boniol, M,; Zheng, T,; Zhang, Y,; M Pasterk, M,; Smans, M,; Curado, M. P,; Mullie,P,; S Gandini, S,; Bota, M,; Bolli, G.B,; Rosenstock, J,; and Autie, P: Diabetes and breast cancer risk: a meta-analysis. Br. J. Cancer 2012, 107, 1608–1617. [Google Scholar] [CrossRef] [PubMed]
- Friberg, E.; Orsini, N.; Mantzoros, C.S. ; Wolk, A: Diabetes mellitus and risk of endometrial cancer: a meta-analysis. Diabetologia 2007, 50, 1365–1374. [Google Scholar] [CrossRef]
- Mitri, J.; Castillo, J.; Pittas, A.G. Diabetes and risk of Non-Hodgkin’s lymphoma: a meta-analysis of observational studies. Diabetes Care 2008, 31, 2391–2397. [Google Scholar] [CrossRef] [PubMed]
- Kasper, J.S.; Giovannucci, E. : A meta-analysis of diabetes mellitus and the risk of prostate cancer. Cancer Epidemiol Biomarkers Prev 2006, 15, 2056–2062. [Google Scholar] [CrossRef] [PubMed]
- Snyder, C. F.; Stein, K.B.; Barone, B. B.; Peairs, K.S.; Yah, H.C.; Derr, R.L.; Wolff, A.C.; Carducci, M.A.; Brancati, F.L. : Does pre-existing diabetes affect prostate cancer prognosis? A systematic review. Prostate Cancer Prostatic Dis 2010, 13, 58–64. [Google Scholar] [CrossRef]
- Bensimon, L.; Yin, H.; Suissa, S.; Pollak, M.N.; Azoulay, L. : Type 2 diabetes and the risk of mortality among patients with prostate cancer. Cancer Causes Control 2014, 25, 329–338. [Google Scholar] [CrossRef]
- Body weight, Physical Activity, Diet & Alcohol. The Cancer Atlas. https://canceratlas.cancer.org/risk-factors/nutrition-and-physical-activity.
- Cloana, M.; Deng, J.; Nadarajah, A.; Hou, M.; Qiu, Y.; Chen, S.S.J.; Rivas, A.; Banfield, L.; Toor, P,P.; Zhou, F.; et al.: The.
- Prevalence of Obesity Among Children With Type 2 Diabetes A Systematic Review and Meta-analysis. 2022, JAMA Net-work.
- Open. 2002.5(12):e2247186. [CrossRef]
- Stattin, P.; Bjor, O. ; Lukanova, A,; Lenner, P.; Lindahl, B.; Hallmans G.;Kaaks, R.: Prospective study of hyperglycemia and cancer risk. Diabetes Care 2007, 30, 561–567. [Google Scholar] [CrossRef]
- Turrens, J.F.; Freeman, B.A.; Crapo, J.D. : Hyperoxia increases H2O2 release by lung mitochondria and microsomes. Biochem Biophysics 1982, 217, 411–421. [Google Scholar] [CrossRef]
- Black, H.S. : A Synopsis of the Associations of Oxidative Stress, ROS, and Antioxidants with Diabetes Mellitus. Antioxidants 2022, 11, 2003. [Google Scholar] [CrossRef] [PubMed]
- Assi, M. ; The differential role of reactive oxygen species in early and late stages of cancer. Am J Physiol Regul Integr Comp Physiol 2017, 313, R646–R653. [Google Scholar] [CrossRef] [PubMed]
- Anbar, A.D. : Elements and Evolution. Science 2008, 322, 1481–1483. [Google Scholar] [CrossRef] [PubMed]
- Mittler, R. : ROS are good. Trends in Plant Science 2017, 22, 11–19. [Google Scholar] [CrossRef]
- Alper, T.; Howard-Flanders, P. : Role of oxygen in modifying the radiosensitivity of E. coli B. Nature 1956, 178, 978–979. [Google Scholar] [CrossRef] [PubMed]
- Gerschman, R.; Gilbert, D.L.; Mye, S.W.; Dwyer, P.; Fenn, W.O. : Oxygen poisoning and X-irradiation: A mechanism in common. Science 1954, 119, 623–626. [Google Scholar] [CrossRef]
- Wright, E., Jr.; Scism-Bacon, J.L.; Glass, L.C. : Oxidative stress in type 2 diabetes: The role of fasting and postprandial glycaemia. Int. J. Clin. Pract. 2006, 60, 308–314. [Google Scholar] [CrossRef]
- Wang, Y.; Qi, H.; Liu, Y.; Duan, C.; Xia, T.; Chen, D.; Piao, H-l. ; Liu, H-X.: The double-edged roles of ROS in cancer prevention and therapy. Theranostics 2021, 11, 4839–4857. [Google Scholar] [CrossRef]
- Black, H.S. ; Potential involvement of free radical reactions in ultraviolet light-mediated cutaneous damage. Photochem. Photobiol. 1987; 46, 213-221. 1987, 46, 213–221. [Google Scholar]
- Turrens, J.F.; Freeman, B.A.; Crapo, J.D. L: Hyperoxia increases H2O2 release by lung mitochondria and microsomes. Biochem. Biophys. 1982, 217, 411–421. [Google Scholar] [CrossRef]
- Saikolappan, S.; Kumar, B.; Shishodia, G.; Koul, S.; Koul, H.K. : Reactive Oxygen species and cancer: A complex interaction. Cancer Letters. 2019, 452, 132–143. [Google Scholar] [CrossRef] [PubMed]
- Cerutti, P.A. : Prooxidant states and tumor promotion. Science 1954, 119, 623–626. [Google Scholar] [CrossRef]
- Proctor, P.H.; Reynolds, E.S. : Free radicals and disease in man. Physiol. Chem. Phys. Med. NMR 1984, 16, 175–95. [Google Scholar] [PubMed]
- Black, H.S. : New York, USA, 1993; 243-269.carcinogenesis. In Oxidative Stress in Dermatology. Fuchs, J., Packer L., Eds; Marcel Dekker, Inc.: New York, USA, 1993. [Google Scholar]
- Cadenaas, E.; Sies, H. : Singlet oxygen formation detected by low-level chemiluminescence during enzymatic reduction of prostaglandin G2 to H2. Hoppe-Seylers’ Z. Physiol. Chem. 1983, 364, 519–528. [Google Scholar] [CrossRef] [PubMed]
- Bjelakovic, G.; Nikolova, D.; Simonetti, R.G.; Gluud, C. : Antioxidant supplements for prevention of gastrointestinal cancers: a systematic review and meta-analysis. Lancet 2004, 364, 1219–1228. [Google Scholar] [CrossRef]
- The α-Tocopherol, ß-Carotene Cancer Prevention Study Group. The effect of vitamin E and ß-carotene on the incidence of lung cancer and other cancers in male smokers. N Engl J Med. 1996, 330, 1029–1035. [Google Scholar] [CrossRef]
- Black, H.S.; Boehm, F.; Edge, R.; Truscott, T.G. : The benefits and risks of certain dietary carotenoids that exhibit both anti- and pro-oxidative mechanisms – a comprehensive review. Antioxidants 2020, 9, 264. [Google Scholar] [CrossRef]
- Udenfriend, S.; Clark, C.T.; Axelrod, J.; Brodie, B.B. : Ascorbic acid in aromatic hydroxylation.I. A model system for aromatic hydroxylation. J. Biol Chem 1954, 208, 731–739. [Google Scholar] [CrossRef]
- Lee, D-H. ; Folsom, A.R.; Harnack, L.; Halliwell, B.; Jacobs, Jr, D. R.: Does supplemental vitamin C increase cardiovascular disease risk in women with diabetes? Am J Clin Nutr 2004, 80, 1194–2000. [Google Scholar] [CrossRef]
- Le Gal, K.; Ibrahim, M.X.; Wiel, C.; Sayin, V.I.; Akula, M.K.; Karlsson, C.; Dalin, M.G.; Akyürek, L.M.; Lindahl, P.; Nilsson, J.; Bergo, M.O. : Antioxidants can increase melanoma metastasis in mice. Science Translational Medicine 2015, 7, 308re8 www.ScienceTranslationalMedicine.org. [Google Scholar] [CrossRef]
- McArdle, F.; Rhodes, L.E.; Parslew, R.A.G.; Close, G.L.; Jack, C.I.A.; Friedmann, P.S.; Jackson, M.J. : Effects of oral vitamin E and beta-carotene supplementation on ultraviolet radiation-induced oxidative stress in human skin. Am J Clin Nutr. 2004, 80, 1270–1275. [Google Scholar] [CrossRef] [PubMed]
- Berman, A.Y.; Motechin, R.A.; Wiesenfeld, M.Y.; Holz, M.K. : The therapeutic potential of resveratrol: a review of clinical trials. NPJ Precis Oncol. 2017, 1, 35–4. [Google Scholar] [CrossRef] [PubMed]
- AL-Ishag, R.K.; Abotaleb, M.; Kubatka, P.; Kajo, K.; Busselberg, D. : Flavonoids and their anti-diabetic effects: Cellular mechanisms and effects to improve blood sugar levels. Biomolecules 2019, 9, 430. [Google Scholar] [CrossRef]
- Buttuzzi, S.; Brausi, M.; Rizzi, F.; Castagnetti, G.; Peeracchia, G.; Corti, A. : Chemoprevention of human prostate cancer by oral administration of green tea catechins in volunteers with high-grade prostateintraepithelial neoplasia: a preliminary report from a one-year proof-of-principle study. Cancer Res. 2006, 66, 1234–1240. [Google Scholar] [CrossRef] [PubMed]
- Bushman, J.L. : Green tea and cancer in humans: a review of the literature. Nutr Cancer 1998, 31, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.S.; Acharya, A.; Ray, R.S.; Agrawal, R.; Raghuwanshi, R.; Jain, P. : Cellular and molecular mechanisms of curcumin in prevention and treatment of disease. Critical reviews in food science and nutrition. 2020, 60, 887–939. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.H.; Li, Y.Q.; Yang, X.Y. : Protective effects of epigallocatechin gallate on colon preneoplastic lesions induced by 2-amino-3-methylimidazole [4-5 f] quinoline in mice. Molecular Medicine 2018, 31, 88–96. [Google Scholar]
- Sluijs, I.; Cadier, E.; Beulens, J.W.J.; vad der A, D.L.; Spijkerman, A.M.W.; van der Schouw, Y.T. : Dietary intake of carotenoids and risk of type 2 diabetes. Nutr Metabol and Cardiovascular Diseases. 2014, 25, P376–381. [Google Scholar] [CrossRef]
- Kim, Y.; Kim, Y.J.; Lim, Y.; Oh, B.; Kim, J.Y.; Bouwman, J.; Kwon, O. : Combination of Diet Quality Score, Plasma Carotenoids, and Lipid Peroxidation to Monitor Oxidative Stress. Oxid. Med. Cell. Long. 2018, 2018, Art ID 8601028, 11 pgs. [Google Scholar] [CrossRef]
- Marcelino, G.; Machate,D. J.; de Cássia Freitas,K.; Hiane, P.A.; Maldonade, I.R.; Pott, A.; Asato, M.A.; de Cássia Avellaneda Guimarães, R.: β-carotene: preventive role for type 2 diabetes mellitus and obesity: a review. Molecules 2020, 25, 5803. [Google Scholar] [CrossRef]
- Raut, S.K.; Khullar, M. : Oxidative stress in metabolic diseases: current scenario and therapeutic relevance. Mol Cell Biochem. 2023, 478, 185–196. [Google Scholar] [CrossRef]
- Peto, R.; Doll, R.; Buckley, J.D.; Sporn, M.B. : Can dietary β-carotene materially reduce human cancer rates? Nature 1981, 290, 201–208. [Google Scholar] [CrossRef]
- Mathews-Roth, M.M.; Krinsky, N.I. : Carotenoid dose level and protection against UV-B- induced skin tumors. Photochem. Photobiol. 1985, 42, 35–38. [Google Scholar] [CrossRef]
- Black, H.S. : Radical interception by carotenoids and effects on UV carcinogenesis. Nutr. Cancer 1998, 31, 212–217. [Google Scholar] [CrossRef]
- Black, H.S.; Okotie-Eboh, G.; Gerguis, J. : Diet potentiates the UV-carcinogenic response to β-carotene. Nutr. Cancer 2000, 37, 173–178. [Google Scholar] [CrossRef]
- Black, H.S.; Gerguis, J. : Modulation of dietary vitamins E and C fails to ameliorate β-carotene exacerbation of UV Carcinogenesis in mice. Nutr. Cancer 2003, 45, 36–45. [Google Scholar] [CrossRef]
- Burton, G.W.; Ingold, K.U. : β-carotene: An unusual type of lipid antioxidant. Science 1984, 224, 569–573. [Google Scholar] [CrossRef]
- Black, H.S.; Chan, J.T. ; Suppression of ultraviolet light-induced tumor formation by dietary antioxidants. J Invest Dermatol. 1975, 65, 412–414. [Google Scholar] [CrossRef]
- Black, H.S.; Chan, J.T.; Brown, G.E. : Effects of dietary constituents on ultraviolet light-mediated carcinogenesis. Cancer Res. 1978, 38, 1384–1387. [Google Scholar]
- Koone, M.D.; Black, H.S. : A mode of action for butylated hydroxytoluene-mediated photocarcinogenesis. J. Invest Dermatology. 1986, 87, 343–347. [Google Scholar] [CrossRef]
- Black, H.S. : Nutritional lipid and antioxidant supplements: risks versus benefits. Expert Rev of Dermatol. 2012, 7, 483–492. [Google Scholar] [CrossRef]
- Chan, J.T.; Ford, J.O.; Rudolph, A.H.; Black, H.S. : Physiological changes in hairless mice maintained on an antioxidant supplemented diet. Experientia 1977, 33, 41–42. [Google Scholar] [CrossRef]
- Malkinson, A.M. : Review: Putative mutagens and carcinogens in foods. III. Butylated hydroxytoluene (BHT). Environ Mutat. 1983, 5, 353–362. [Google Scholar] [CrossRef]
- Black, H.S. : Gerguis, J.: Use of the Ames test in assessing the relation of dietary lipid and antioxidants to N-2-fluorenylacetamide activation. J Environ Pathol Toxicol. 1980, 4, 131–138. [Google Scholar]
- Witschi, H.; Lock, S. : in Carcinogenesis, Vol. 2. Mechanisms of tumor promotion and carcinogenesis Slaga, T.J., Sivak, A., Boutwell, K. Raven Press, New York, USA, 1978; 465-474.
- Bauer, A.K.; Dwyer-Nield, L.D.; Hankin, J.A.; Murphy, R.C.; Malkinson, A.M. : The lung tumor promoter, butylated hydroxytoluene (BHT) causes chronic inflammation in promotion-sensitive BALB/cByJ mice but not in promotion-resistant CXB4 mice. Toxicology 2001, 169, 1–15. [Google Scholar] [CrossRef]
- Bajaj, S.; Khan, A. : Antioxidants and diabetes. Indian J. Endocrinol. Metab. 2012, 16 (Suppl 2), S267–S271. [Google Scholar] [CrossRef]
- Szkudlinska, M.A.; von Frankenberg, A.D.; Utzschneider, K.M. : The antioxidant N-Acetylcysteine does not improve glucose tolerance or ß-cell function in type 2 diabetes. J. Diabetes Complications. 2016, 30, 618–622. [Google Scholar] [CrossRef]
- Le Gal, K.; Ibrahim, M.X.; Wiel, C.; Sayin, V.I.; Akula, M.K.; Karlsson, C.; Dalin, M.G.; Akyurek, L.M.; Lindahl, P.; Nilsson, J. ;M. O. Bergo, M.O.: Antioxidants can increase melanoma metastasis in mice. Science Translational Medicine 2015, 7(308), 308re8. [Google Scholar] [CrossRef]
- Sekhar, R.V. : GlyNAC (Glycine and N-Acetylcysteine) supplementation improves impaired mitochondrial fuel oxidation and lowers insulin resistance in patients with type 2 diabetes: Results of a pilot study. Antioxidants 2022, 11, 154. [Google Scholar] [CrossRef]
- Brownlee, M. : The Pathology of Diabetic Complications: A Unifying Mechanism. Diabetes 2005, 54, 1615–1625. [Google Scholar] [CrossRef]
- Bjelakovic, G. ; Nikolova,D.; Gluud, L.L.; Simonetti, R.G.; Christian Gluud,C.;et al: Mortality in randomized trials of antioxidant supplements for primary and secondary prevention: systematic review and meta-analysis. JAMA. 2007, 297(8), 842–857. [Google Scholar] [CrossRef] [PubMed]
- Ristow, M.; Zarse, K.; Oberbach, A.; Kloting, N.; Birringer, M.; Kiehntopf, M.; Stumvoll, M.; Kahn, C.R.; Bluher, M. : Antioxidants prevent health-promoting effects of physical exercise in humans. Proc. Natl. Acad. Sci. USA. 2009, 106(21), 8665–8670. [Google Scholar] [CrossRef] [PubMed]
- Black, H.S. The role of nutritional lipids and antioxidants in UV-induced skin cancer. Frontiers in Bioscience Scholar. 2015, 7, 30–39. [Google Scholar] [CrossRef]
- World Cancer Research Fund/American Institute for Cancer Research. Food, nutrition, physical activity, and the prevention of cancer: A global perspective.; AICR: Washington, DC, USA, 2007. [Google Scholar]
- IARC Working Group on the Evaluation of Cancer-preventive Agents. IARC Handbooks of Cancer Prevention: Carotenoids; International Agency for Research on Cancer: Lyon, France, 1998; Vol. 2. [Google Scholar]
- Black, H.S. : Reassessment of a free radical theory of cancer with emphasis on ultraviolet carcinogenesis. Integr. Cancer Therapies. 2004, 3, 279–293. [Google Scholar] [CrossRef]
- Black, H.S. : Liquid biopsy: A minimally invasive diagnostic tool to identify and characterize cancer cells. J. Integr. Oncol. 2019, 8, 2. [Google Scholar]
- Yan, LJ. : Redox imbalance stress in diabetes mellitus: Role of the polyol pathway. Animal Model Exp Med. 2018, 1, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Schleicher, E.D.; Weigert, C. : Role of hexosamine biosynthetic pathway in diabetic nephropathy. Kidney International. 2000, 58 (suppl 77), S13–S18. [Google Scholar] [CrossRef]
- Ahmad, M.; Wolberg, A.; Kahwaji, C.I. : Biochemistry, Electron Transport Chain. StatPearls [Internet}, Treasure Island (FL): StatPearls Publishing; 2022.https://d.docs.live.net/d89a4d69f41936fc/Documents/CancersMs.docx.
- Hirst, J. : Energy transduction by respiratory complex I—an evaluation of current knowledge. Biochem Soc Trans. 2005, 33, 525–529. [Google Scholar] [CrossRef]
- Cardenas, S. :Mitochondrial uncoupling, ROS generation and cardioprotection. Bioenergetics 2018, 1859, 940–950. [Google Scholar] [CrossRef]
- Hamanaka, R.B.; Chandel, N.S. : Mitochondrial reactive oxygen species regulate cellular signaling and dictate biological outcomes. Trends Biochem Sci. 2010, 35, 505–513. [Google Scholar] [CrossRef]
- Yan, L.J. : Redox imbalance stress in diabetes mellitus: Role of the polyol pathway. Animal Model Exp Med. 2018, 1, 7–13. [Google Scholar] [CrossRef]
- Turkmen, K.; Karagoz, A.; Kucuk, A. : Sirtuins as novel players in the pathogenesis of diabetes mellitus. World J Diabetes. 2014, 5, 894–500. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Jin, Z.; Zheng, H.; Yan, L.J. : Sources and implications of NADH/NAD+ redox imbalance in diabetes and its complications. Diabetes Metab Syndr Obes 2016, 10, 145–153. [Google Scholar] [CrossRef]
- Schleicher, E.D.; Weigert, C. : Role of hexosamine biosynthetic pathway in diabetic nephropathy. Kidney International. 2000, 58 (suppl 77), S13–S18. [Google Scholar] [CrossRef]
- Szabo, C.; Bise,r A. ; Benko, R.; Bottinger, E.; Susztak, K.: Poly (ADP-Ribose) Polymerase Inhibitors Ameliorate Nephropathy of Type 2 Diabetic Leprdb/db Mice. Diabetes 2006, 55, 3004–3012. [Google Scholar] [CrossRef]
- Nishizuka, Y. : Protein kinase C and lipid signaling for sustained cellular responses. Faseb.onlinelibrary.wiley.com/doi/epdf/10.1096/fasebj.9.7.7737456. 7737. [Google Scholar]
- Marasclulo, F.L.; Montagmani, M.; Potenza. M.A.: Endotheliiiiin-1: the yin and yang of vascular function. Curr. Med. Chem. 2006, 13, 1655–1665. [Google Scholar] [CrossRef]
- Aiello, L.P.; Wong, J. :. VEGF –Vascular endothelial growth factor in diabetic vascular complications. Kidney Int. Suppl. 2000, 77, S113–S119. [Google Scholar] [CrossRef] [PubMed]
- Kopfstein L, Veikkola T, Djonov VG, et al. et al. Distinct roles of vascular endothelial growth factor-D in lymphangiogenesis and metastasis. Am J Pathol. 2007, 170, 1348–1361. [Google Scholar] [CrossRef]
- Karnezis, T.; Shayan, R.; Caesar, C.; et al. VEGF-D promotes tumor metastasis by regulating prostaglandins produced by the collecting lymphatic endothelium. Cancer Cell. 2012, 21, 181–195. [Google Scholar] [CrossRef]
- Gomes, K.B.; Rodrigues, K.F.; Fernandes, A.P. : The role of transforming growth factor-beta in Diabetic Nephropathy. Intl. J. Med. Genetics 2014, Article ID 180270. [Google Scholar] [CrossRef]
- Hachana, S.; Larrivée, B. : TGF-β superfamily signaling in the eye: implications for ocular pathologies. Cells. 2022, 29, 11(15):2336. [CrossRef]
- Stacker, S.A.; Achen, M.G. : The VEGF signaling pathway in cancer: The road ahead. Chin J Cancer. 2013; 32: 297-302. [CrossRef]
- Bierie, B.; Moses, H.L. : TGF-ß and cancer. Cytokine & Growth Factor Reviews. 2006; 17: 29-40. [CrossRef]
- Chang, CH.; Pauklin, S. : ROS and TGFβ: from pancreatic tumour growth to metastasis. J Exp Clin Cancer Res 2021; 40: 152. J Exp Clin Cancer Res. [CrossRef]
- Madambath, I.; Appu, A.P. : Role of NF-kapa B (NF-kB) in diabetes. Forum on Immunopathologic Diseases and Therapeutics 2013; 4: 111-132. [CrossRef]
- DiDonato, J.A.; Mercurio, F. Karin, M.: NF-ķß and the link between inflammation and cancer. Immunological Reviews. 2012; 246: 379-400. [CrossRef]
- Karin, M. : NF-ķß and cancer: Mechanisms and targets. Molecular Carcinogenesis 2006; 45: 355-361. [CrossRef]
- Naugler, W.E.; Karin, M. : NF-kappaB and cancer-identifying targets and mechanisms. Curr Opin Genet Dev. 2008; 18(1):19-26. [CrossRef]
- Gao, L.; Mann, G.E. : Vascular NAD(P)H oxidase activation in diabetes: a double-edged sword in redox signaling. Cardiovascular Research 2009; 82: 9-20. [CrossRef]
- Krishnendu, R.; Yongzhong Wu, Y.; Jennifer L Meitzler, J.L.; Agnes Juhasz; Han Liu, H.; et al. : NADPH Oxidases and Cancer. Clin Sci (Lond).2015; 128: 863-875. [CrossRef]
- Weyemi, U.; Redon, C.E.; Parekh, P.R.; Dupuy, C.; Bonner, W.M. : NADPH Oxidases NOXs and DUOXs as putative targets for cancer therapy. Anticancer Agents Med Chem. 2013, 13(3), 502–514. [Google Scholar] [PubMed]
- McGarry, J.D. : Banting Lecture 2001: Dysregulation of fatty acid metabolism in the etiology of Type 2 diabetes. Diabetes 2002, 51, 7–18. [Google Scholar] [CrossRef] [PubMed]
- Reaven, G.M. : Role of Insulin Resistance in Human Disease (Syndrome X): An Expanded Definition. Annual Review of Medicine 1993, 44, 121–131. [Google Scholar] [CrossRef] [PubMed]
- Lau, E.S.; . Paniagua, S.M.; Liu, E.; Jovani, M.; Li, S.X.; Takvorian, K.; et al. : Shared risk factors in cardiovascular disease and cancer. J Am Coll Cardio CardioOncology. 2021, 3, 48–58. [Google Scholar] [CrossRef]
- Koene, R.J.; Prizment, A.E.; Blaes, A.; Konety, S.H. : Shared risk factors in cardiovascular disease and cancer. Circulation 2016, 133, 1104–1114. [Google Scholar] [CrossRef]
- Duarte, C.W. , Lindner V., Francis S.A., Schoormans D. “Visualization of cancer and cardiovascular disease co-occurrence with network methods”. JCO Clin Cancer Inform 2017, 1, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Katzie, V.A.; Sookthai, D.; Johnson, T.; Kühn, T.; Kaaks, R. : Blood lipids and lipoproteins in relation to incidence and mortality risks for CVD and cancer in the prospective epic-Heidelberg cohort. BMC 2017, 19, 218. [Google Scholar] [CrossRef]
- Nomikos,,T.; Panagiotakos, D.; Georgousopoulou, E.; Metaxa, V.; Chrysohoou, C.; Ioannis Skoumas, I. et al: Hierarchical modelling of blood lipids’ profile and 10-year (2002-2012) all-cause mortality and incidence of cardiovascular disease: the Attica study. Lipids Health Dis. 2015, 15, 108. [CrossRef]
- Hasbani, N.R.; Ligthart, S. ; Brown,M.R.; Heath, A.S., Bebo, A., Ashley, K.E., Eds.; et al: American Heart Association’s life’s simple 7: lifestyle recommendations, polygenic risk, and lifetime risk of coronary heart disease. Circulation.2022. [Google Scholar] [CrossRef]
- Mooradian, A.D. : Dyslipidemia in type 2 diabetes mellitus. Nat. Clin. Prac. Endocrinol. Metab. 2009, 5, 150–159. [Google Scholar] [CrossRef]
- Korac, B.; Kalezic, A.; Pekovic-Vaughan, V.; Koras, A. : Redox changes in obesity, metabolic syndrome, and diabetes. Redox Biology 2021, 42, 101887. [Google Scholar] [CrossRef]
- Randle, P.J.; Garland, P.B.; Hales, C.N.; Newsholme, E.A. : The glucose fatty- acid cycle. Its role in insulin sensitivity and the metabolic disturbances of diabetes mellitus. Lancet 1963, 1, 785–789. [Google Scholar] [CrossRef] [PubMed]
- Delarue, J.; Magnan, C. : Free fatty acids and insulin resistance. Curr. Opin. Clin. Nutr. Metab. Care 2007, 10, 142–1448. [Google Scholar] [CrossRef] [PubMed]
- Houten, .SM.; Wanders, R.J.A.: General introduction to the biochemistry of mitochondrial fatty acid ß-oxidation. J. Inherit. Metab. Dis. 2010, 33, 469. [CrossRef] [PubMed]
- Kaur, S.; Auger, C.; Jeschke, M.G. : Adipose tissue metabolic function and dysfunction: Impact of burn injury. Front. Cell Dev. Biol., 2020, 8, - 2020. [Google Scholar] [CrossRef]
- 131. Kang, J-H.: Protein kinase C (PKC) isozymes and cancer. New J. of Science 2014, Article ID 231418. [CrossRef]
- Garg, R.; Benedetti, L.G.; Abera, M.B.; Wang, HB.; Abba, M.; Kazanietz, M.G. : Protein Kinase C and cancer: what we know and what we do not. Oncogene. 2014, 33, 5225–5237. [Google Scholar] [CrossRef]
- Isakov, N. : Protein kinase C (PKC) isoforms in cancer, tumor promotion and tumor suppression. Semin Cancer Biol 2018, 48, 36–52. [Google Scholar] [CrossRef]
- Griner, E.; Kazanietz, M. : Protein kinase C and other diacylglycerol effectors in cancer. Nat Rev Cancer. 2007, 7, 281–294. [Google Scholar] [CrossRef]
- Gupta, A.K.; Galoforo, S.S.; Berns, C.M.; Martinez, A.A.; Guan, K.L.; Lee. : Elevated levels of ERK2 in human breast carcinoma MCF-7 cells transfected with protein kinase Cα,” Cell Proliferation. 1996; 29: 655–663. ; 1996, 29, 655–663. [Google Scholar] [CrossRef]
- Varga, A.; Czifra, A.; Tállai, B.; Németh, T.; Kovács, I.; et al. , “Tumor grade-dependent alterations in the protein kinase C isoform pattern in urinary bladder carcinomas,” European Urology. 2004. 46: 462–465. 2004, 46, 462–465. [Google Scholar] [CrossRef]
- Kho, D.H.; Bae, J.A.; Lee, J.H.; Cho, H.J.; Cho, S.H.; et al. , “KITENIN recruits Dishevelled/PKCd to form a functional complex and controls the migration and invasiveness of colorectal cancer cells,” Gut, 2009; 58: 509–519. Gut 2009, 58, 509–519. [Google Scholar] [CrossRef] [PubMed]
- Lee, M-S.; Kim, T.Y.; Kim, Y-B.; Lee, S-Y.; Ko,S-G.; Jong, H-S.; et al: The Signaling Network of Transforming Growth Factor β1, Protein Kinase Cδ, and Integrin Underlies the Spreading and Invasiveness of Gastric Carcinoma Cells. Molecular and Cellular Biology. 2005, 25, 6921–6936. [CrossRef] [PubMed]
- Mandil, R.; Ashkenazi, E.; Blass, M.; Kronfeld, I.; Kazimirsky, G.; Rosenthal, G. et al.: “Protein kinase Cα and protein kinase Cδ play opposite roles in the proliferation and apoptosis of glioma cells,” Cancer Research, 2001; 61, 4612–4619; cancerres.aacrjournals.
- M. Kim, M.; R. Kim, R.;C. Yoon, C.; An, S.; Hwang, S-G.; Suh, Y.; et al.: Importance of PKCδ signaling in fractionatedradiation-induced expansion of glioma-initiating cells and resistance to cancer treatment. Journal of Cell Science 2011, 124, 3084–3094. [CrossRef] [PubMed]
- Yang,Y-L.; Chu, J-Y.; Luo, M-L.; Wu, Y-P.; Zhang, Y.; Feng,Y-B.; et al: Amplification of PRKCI, located in 3q26, is associated with lymph node metastasis in esophageal squamous cell carcinoma. Genes Chromosomes and Cancer 2008, 47, 127–136. [CrossRef]
- S. Liu, B.; Wang, B.; Y. Jiang, Y.; Zhang, T-T.; Shi, Z-Z.; Yang, Y.; et al.: Atypical protein kinase Cι (PKCι) promotes metastasis of esophageal squamous cell carcinoma by enhancing resistance to anoikis via PKCι-SKP2-AKT Pathway. Molecular Cancer Research 2011, 9, 390–402. [CrossRef]
- Lahn ,M.; Su, C.; Li, S.; Chedid, M.; KR Hanna, K.R.; JR Graff, J.R.; et al.: Expression levels of protein kinase C-α in non-small-cell lung cancer. Clinical Lung Cancer 2004, 6, 184–189. [CrossRef]
- Bae, K.; Wang, H.; Jiang, G.; Chen, M.G.; Lu, L.; Xiao, L. : Protein kinase Cε is overexpressed in primary human non-small cell lung cancers and functionally required for proliferation of non-small cell lung cancer cells in a p21/Cip1-dependent manner,” Cancer Research, 2007; 67: 6053–6063. [CrossRef]
- Oka, M.; Kikkawa, U. : Protein kinase C in melanoma, Cancer and Metastasis Reviews, 2005; 24: 287–300. [CrossRef]
- la Porta, C.A.M.; di Dio, A.; Porro, D.; Comolli, R. : Overexpression of novel protein kinase C δ in BL6 murine melanoma cells inhibits the proliferative capacity in vitro but enhances the metastatic potential in vivo. Melanoma Research 2000, 10, 93–102. [Google Scholar] [CrossRef]
- Abrams, S.T.; Lakum, T.; Lin, K. ; Abrams,S.T.; Lakum, T.; K. Lin, K.; et al. B-cell receptor signaling in chronic lymphocytic leukemia cells is regulated by overexpressed active protein kinase CβII,” Blood, 2007; 109:1193–1201. [CrossRef]
- Holler, C..; Piñón, J.D.; Denk, U.; Holler, C.; Piñón, J.D.; et al.: PKCβ is essential for the development of chronic lymphocytic leukemia in the TCL1 transgenic mouse model: validation of PKCβ as a therapeutic target in chronic lymphocytic leukemia. Blood 2009, 113, 2791–2794. [CrossRef]
- Kabir,N.N.; Rönnstrand, L.;. Kazi, J.U.: Protein kinase C expression is deregulated in chronic lymphocytic leukemia. Leukemia & Lymphoma 2013, 54, 2288–2290. [CrossRef]
- Espinosa, I.; Briones, J.; Bordes, R.; Brunet, S.; Martino, R. Espinosa, I.; Briones, J.; Bordes, R.; Brunet, S.; Martino, R.; Sureda, A,; et al: Membrane PKC-beta 2 protein expression predicts for poor response to chemotherapy and survival in patients with diffuse large B-cell lymphoma. Ann Hematol, 2006, 85, 597–603. [Google Scholar] [CrossRef]
- Nicolle, A.; Zhang, Y.; Belguise, K. : The emerging function of PKCtheta in cancer. Biomolecules, 2021; 11: 221. [CrossRef]
- Page, A.; Navarro, M.; Suarez-Cabrera, C.; Bravo, A.; Ramirez, A. : Context-dependent role of IKKß in cancer. Genes, 2017; 8: 376. [CrossRef]
- Israël, A. : The IKK complex, a central regulator of NF-kappaB activation. Cold Spring Harb Perspect Biol. 2010; 2(3): a000158. [CrossRef]
- Viatour, P. ; Merville, M-P.; Bours, V.; Chariot, A.: Phosphorylation of NF-ķß and Ikß proteins: imp;ications in cancer and inflammation. Trends in Biochemical Sciences, 2005; 30. [CrossRef]
- Taniguchi, K.; Karin, M. : NF-κB, inflammation, immunity and cancer: coming of age. Nat Rev Immunol 2018; 18: 309–324. [CrossRef]
- Morrison, D.K. : MAP kinase pathways. Cold Spring Harb Perspect Biol. 2012;1;4(11):a011254. [CrossRef]
- Lawlor, M.; Alessi, D.R. : PKB/AKT: a key mediator of cell proliferation, survival and insulin responses? J. Cell Sci.2001; 114: 2903-2910. [CrossRef]
- Li, W.; Liang, X., Zeng, Z.; Yu, K.; Zhan, S.; Su, Q.; et al.: Simvastatin inhibits glucose uptake activity and GLUT4 translocation through suppression of the IR/IRS-1/Akt signaling in C2C12 myotubes. Biomed Pharmacother, 2016, 83, 194–200. [CrossRef] [PubMed]
- Cantley, L.C. : The phosphoinositide 3-kinase pathway. Science. 2002;296:1655-7. [CrossRef]
- Zhang, C.; Yang, N.; Yang, C.H.; Ding, H.S.; Luo, C.; Zhang, Y.; et al. : A novel anticancer agent, exerts its anti-proliferative activity by interfering with both PI3K-Akt-mTOR signaling and microtubule cytoskeleton. PloS One. 2009; 4: e4881. 4881. [Google Scholar] [CrossRef]
- Revathidevi, S.; Munirajan, A. : Akt in cancer: Mediator and more. Semin Cancer Biol. 2019; 59: 80-91. [CrossRef]
- Ponnusamy, S.; Meyers-Needham; Senkal, C.E.; Saddoughi, S.A.; Sentelle, D.; Selvam, S.P.; et al. : Sphingolipids and cancer: ceramide and sphingosine-1-phosphate in the regulation of cell death and drug resistance. Future Oncol. 2010; 10:1603-24. [CrossRef]
- Sheridan, M; Ogretmen, B.: The Role of Ceramide Metabolism and Signaling in the Regulation of Mitophagy and Cancer Therapy. Cancers, 2021; 13: 2475. [CrossRef]
- Li, Z.; Zhang, L.; Liu, D.; Wang, C. : Ceramide glycosylation and related enzymes in cancer signaling and therapy. Biomed.Pharmacotherapy. 2021; 139: 111565. [CrossRef]
- Coussens, L.M.; Werb, Z. : Inflammation and cancer. Nature. 2002; 420: 860-867. [CrossRef]
- Berger, A. : Th1 and Th2 responses: what are they? Brit. Med. J. 2000; 321: 424. [CrossRef]
- Hughes, C.E.; Nibbs, R.J.B. : A guide to chemokines and their receptors. FEBS J. 2018; ;285: 2944-2971. [CrossRef]
- Prescott, J,;A, Cook, S.J.: Targeting IKKβ in Cancer: Challenges and Opportunities for the Therapeutic Utilisation of IKKβ Inhibitors. Cells. 2018; 23:115. [CrossRef]
- Jezek, J.; Jaburek, M.; Zelenka, J.; Jezek, P. Mitochondrial phospholipase A2 activated by reactive oxygen species in heart mitochondria induces mild uncoupling. Physiol. Res. 2010, 59, 737–747. [Google Scholar] [CrossRef]
- Ferreira, L.M. : Cancer metabolism: the Warburg effect today. Exp Mol Pathol. 2010; 89: 372-80. [CrossRef]
- Warburg, O. : On the origin of cancer cells. Science 1956; 123: 309-314. [CrossRef]
- Garcia-Heredia, J.M.; Carnero, A. : Decoding Warburg’s hypothesis: tumor-related mutations in the mitochondrial respiratory chain. Oncotarget 2015; 6: 41582-41599. [CrossRef]
- Ward, P.S.; Thompson, C.B. : Metabolic reprogramming: A cancer hallmark even Warburg did not anticipate. Cancer Cell 2012; 20: 297-308. [CrossRef]
- Ishikawa, K.; Takenaga, K.; Akimoto, M.; Koshikawa, N.; Yamaguchi, A.; Imanishi, H.; et al. : ROS-generating mitochondrial DNA mutations can regulate tumor cell metastasis. Science. 2008; 320: 661-664. [CrossRef]
- Zielonka, J.; Kalyanaraman, B. : ROS-generating mitochondrial DNA mutations can regulate tumor cell metastasis – a critical commentary. Free Rad. Biol. Med. 2008; 45: 1217-1719. [CrossRef]
- Wang, G.L.; Jiang, B.H.; Rue, E.A. ; Semenza,.GL: “Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. PNAS, USA.1995; 92: 5510–5514. [CrossRef]
- Muz, B.; de la Puente, P.; Azab, F.; Azab, A.K. : The role of hypoxia in cancer progression, angiogenesis, metastasis, and resistance to therapy. Hypoxia (Auckl). 2015;3:83-92. [CrossRef]
- Noman, M.Z.; Hasmim, M.; Messai, Y.; Terry, S.; Kieda, C.; Janji, B.; Chouaib, S. : Hypoxia: a key player in antitumor immune response. A Review in the Theme: Cellular Responses to Hypoxia. Am J Physiol Cell Physiol. 2015; 309:C569-79. [CrossRef]
- Hagen, T.; Vidal-Puig, A. : Mitochondrial uncoupling proteins in human physiology and disease. Minerva Med. 2002, 93, 41–57. [Google Scholar]
- Echtay, K.S.; Roussel, D.; St-Pierre, J.; Jekabsons, M.B.; Cardenas, S.; Stuart, J.A.; Harper, J.A.; Roebuck, S.J.; Morrison, A.; Pickering, S.; et al. Superoxide activates mitochondrial uncoupling proteins. Nature 2002;, 415: 96–99. [CrossRef]
- Zhao, R.-Z.; Jiang, S.; Zhang, L.; Yu, Z.-B. :. Mitochondrial electron transport chain, ROS generation and uncoupling (Review). Int. J. Mol. Med. 2019; 44: 3–15. [CrossRef]
- Huang, J.; Wang, G.; Liao, K.; Xie, N.; Deng, K. : UCP1 modulates immune infiltration level and survival outcome in ovarian cancer patients. J Ovarian Res 2022; 15: 16. [CrossRef]
- Vozza, A.; Parisi, G.; De Leonardis, F.; Lasorsa, F.M.; Castegna, A.; Amorese, D.; et al. : UCP2 transports C4 metabolites out of mitochondria, regulating glucose and glutamine oxidation. Proc Natl Acad Sci U S A. 2014; 21: 960-065. [CrossRef]
- Li, W.; Zhang, C.; Jackson, K.; Shen, X.; Jin, R.; Li, G.; et al. : UCP2 knockout suppresses mouse skin carcinogenesis. Cancer Prev Res 2015; 8:487-491. [CrossRef]
- Yang, Y.; Karakhanova, S.; Hartwig, W.; D'Haese, J.G.; Philippov, P.P. ; Werne,r J.; Bazhin, A.V.: Mitochondria and Mitochondrial ROS in Cancer: Novel Targets for Anticancer Therapy. J Cell Physiol. 2016; 231: 2570-2581. [CrossRef]
- Aggarwal, V.; Tuli, H.S.; Varol, A.; Thakral, F.; Yerer, M.B.; Sak, K.; et al. : Role of Reactive Oxygen Species in Cancer Progression: Molecular Mechanisms and Recent Advancements. Biomolecules. 2019; 13; 735. [CrossRef]
- Hibino, M.; Maeki, M.; Tokeshi, M.; Ishitsuka, Y.; Harashima, H.; Yamada, Y. : A system that delivers an antioxidant to mitochondria for the treatment of drug-induced liver injury. Nature Scientific Reports 2023; 13: 6961. 6961. [Google Scholar] [CrossRef]

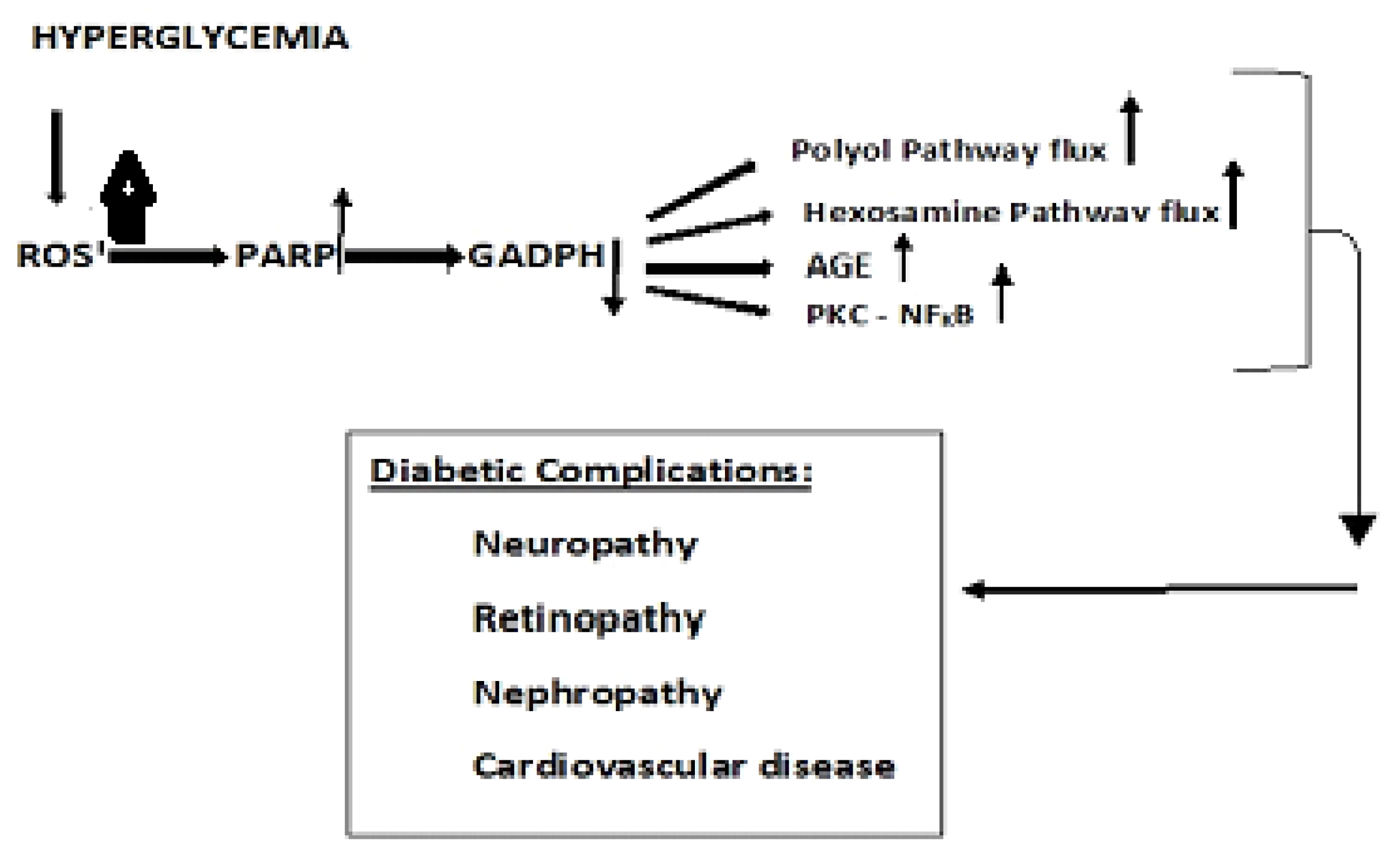
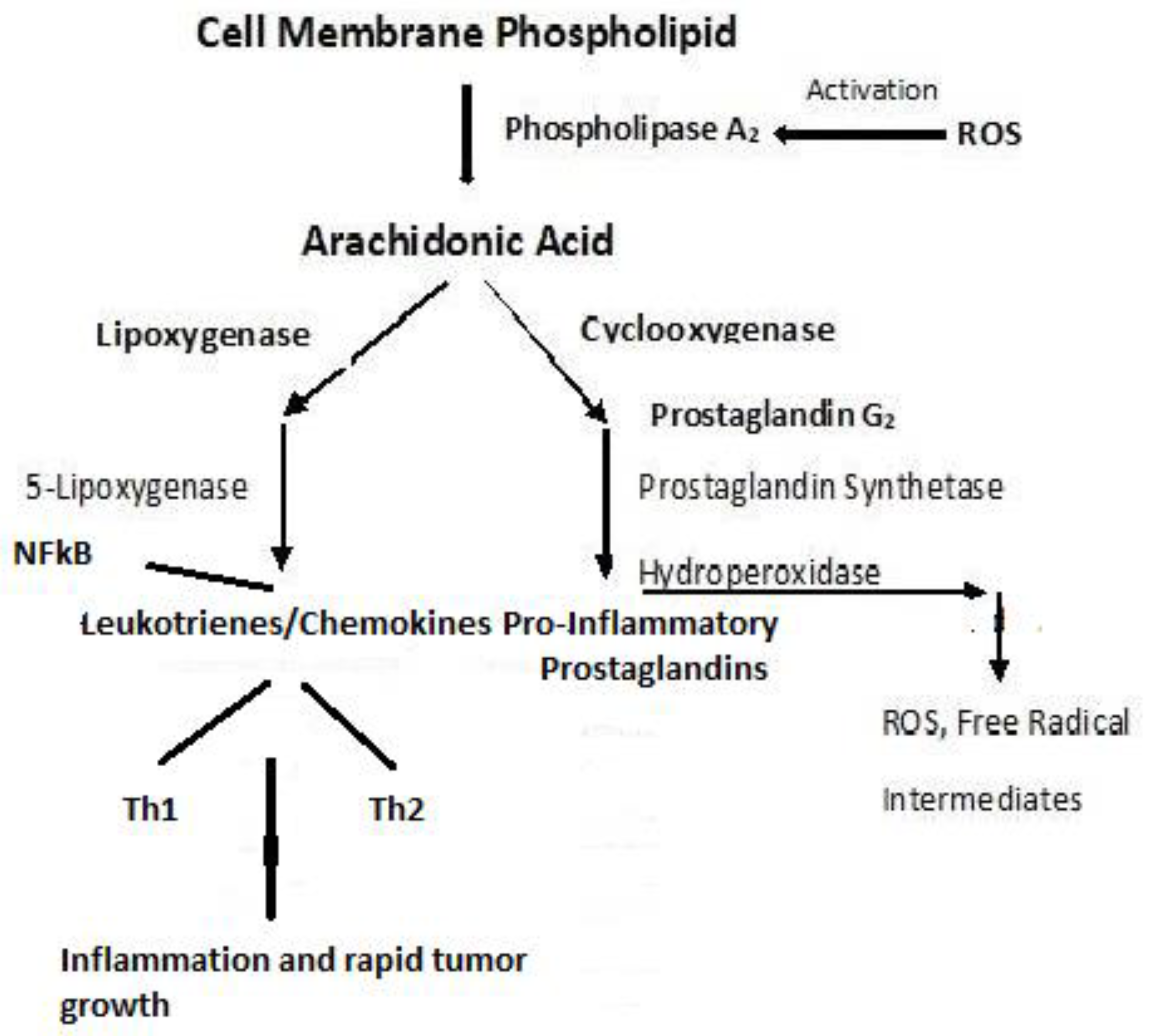
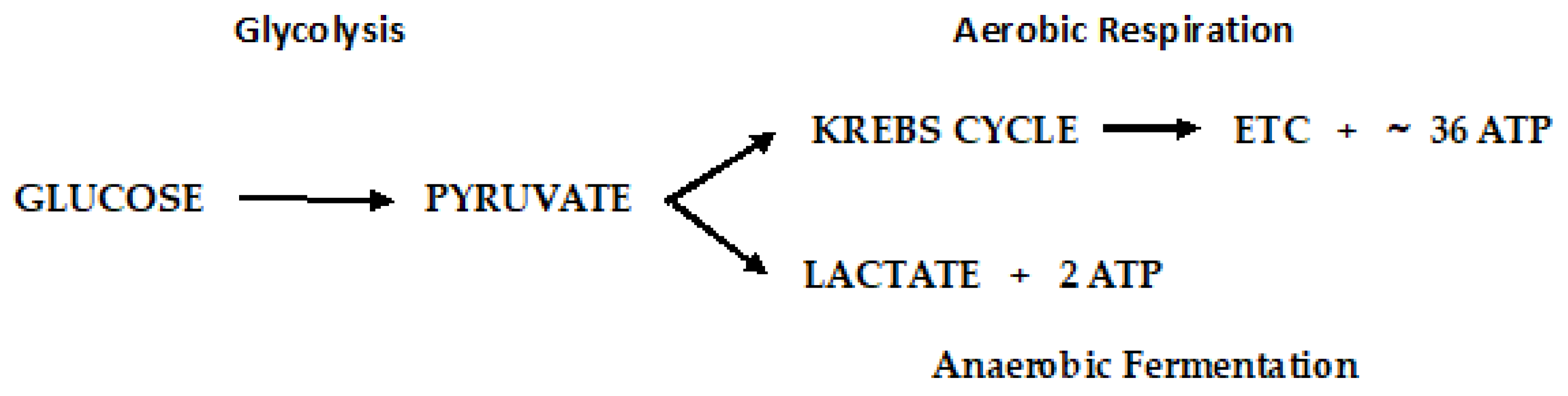
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).



