1. Introduction
Life expectancy of people with spinal cord injury (SCI) has improved markedly in recent decades thanks to advances in healthcare and clinical management of the acute post-injury phase [
1]. Thus, many more patients than in the past survive the acute phase and enter the chronic phase of SCI (lasting more than 1 year). In that neurologically stable stage, direct and indirect permanent consequences of neurological damage and disability result in systemic comorbidities that unfavorably impact quality of life and increase cardiovascular mortality. The deep anthropometric and body composition changes following SCI strongly contribute to the increased cardiovascular morbidity and mortality [
2].
The lack of neurotrophic influences of motor nerve projections results in a dramatic and early muscle wasting below the level of the lesion up to sarcopenia [
3]. The loss of muscle trophism and performance capacity underlies a substantial decrease in overall energy expenditure [
3]. This results in a drop in energy expenditure related to basal metabolic rate that worsens the low energy expenditure related to immobility [
2]. On this basis, in people with SCI, body energy balance is oversized, as energy intake easily exceeds energy expenditure, resulting in increased fat mass, largely distributed at the visceral level [
4] . Visceral body fat is highly sensitive to lipolytic stimuli, therefore its accumulation is accompanied by an increased release of non-esterified fatty acids in the blood circulation and their deposition into muscle and liver cells [
5]. This could explain the very high prevalence of non-alcoholic fatty liver disease (NAFLD) [
6,
7], as well as the accumulation of intramuscular fat [
8], peculiar to people with SCI. These pathogenetic processes generate insulin resistance that unfavorably affects glucose and lipid metabolism [
9]. The constellation of visceral obesity and its correlates, including insulin resistance, glycemic dysregulation, dyslipidemia, and hypertension has been referred to as “metabolic syndrome” (MetS), a cluster of clinical and metabolic factors that increase the risk of stroke and cardiovascular disease [
5].
In people with SCI, however, the diagnosis of MetS is challenged by the same alterations in body composition that promote its onset. Because of muscle hypotrophy, BMI underestimates obesity [
10,
11], while due to anterior abdominal wall muscle laxity, waist circumference measurement overestimates visceral fat. In addition, autonomic dysregulation causes blood pressure instability that makes hypertension an unreliable marker of MetS. For these reasons, widely validated diagnostic criteria of MetS in the general population [
12,
13] are not reliable in patients with SCI for whom modified International Diabetes Federation (IDF) criteria have been proposed with the inclusion of a BMI ≥22 kg/m
2 as a surrogate marker of obesity suitable for SCI [
14]. Nevertheless, given the diagnostic challenges, in many patients with SCI, MetS may be identified late or go completely undetected. Failure or delay in taking appropriate lifestyle measures and treatments places these patients at high risk of cardiovascular morbidity and mortality. Identifying clinical variables that could be risk factors for MetS or its early markers would facilitate a timely diagnosis and the implementation of necessary preventive and therapeutic strategies.
On this basis, the present study aims to identify lifestyle and clinical factors independently associated with MetS in people with chronic SCI.
2. Materials and Methods
2.1. Design and Study Population
One hundred sixty-eight consecutive patients (132 men and 36 women; mean age, 54.7±17.2 years) admitted to a rehabilitation program because of traumatic SCI were included in this study. All patients had a documented history of clinically and neurologically stable SCI for more than 1 year.
No patient had acute illness hindering the rehabilitative program. The presence and severity of significant medical comorbidity were scored using a web-based calculator (
http://ww.pmidcalc.org/?sid=7722560&newtest=Y##ath) of the age-adjusted Charlson comorbidity index (CCI): a weighted score was assigned to a number of medical diagnoses, based on the relative risk of 1-year mortality and according to the patient age. Scores were summed to provide a weighted index of medical comorbidity [
15].
The study was approved by the local ethics committee and all enrolled subjects signed an informed consent.
2.2. Clinical Examinations
Patients underwent detailed neurological examination according to the guidelines of the International Standards for Neurological Examination and Functional Classification of Spinal Cord Injury and the American Spinal Injury Association (ASIA) protocol was used to define both level and completeness of the lesion [
16]. According to the ASIA impairment scale, patients with complete lesion and no sensory or motor function preserved in the lowest sacral segment were categorized as A, whereas patients with incomplete lesions were categorized as B–D. Category B indicated sensory incomplete lesion (including segments S4–S5); category C indicated sensory and motor incomplete lesion where more than half of the 10 pairs of key muscles have strength of less than 3 on a scale of 0–5; category D indicated sensory and motor incomplete lesion with at least half of the key muscles having strength greater than or equal to 3.
The Numeral Rating Scale (NRS) was used to assess the presence of pain related to the SCI and to measure its intensity, according to the recommendations of the National Institute on Disability Research [
17]. Patients were asked to verbally rank pain on a scale of 0 to 10, with 0 representing the absence of pain and 10 representing the maximum pain imaginable.
Body weight was taken with patients wearing light clothing, using a professional mechanical chair scale (Wunder SA BI Srl, Monza, Italy). Height was determined by an elastic tape, after placing the patient in a bed, his/her legs were straightened, head was positioned in the Frankfurt plane, and feet were placed in dorsal flexion. Height was determined by an elastic tape, measuring segmentally the heel to knee, the knee to hip, and the hip to head distances. Body mass index (BMI) was calculated in kg/m2.
Diagnosis of MetS complied with the modified IDF criteria validated in people with SCI by Gater and colleagues [
14]: BMI ≥22 kg/m
2 plus any 2 or more of the following: blood pressure ≥130/85 mmHg or on treatment, triglycerides ≥150 mg/dL or on treatment, high-density lipoproteins (HDL) <40 mg/dL for men and <50 mg/dL for women, fasting plasma glucose ≥100 mg/dL or on treatment for type 2 diabetes mellitus.
Functional independence in activity of daily living (ADL) was assessed at admission by the Spinal Cord Independence Measure (SCIM). This is a 19-item instrument to measure the degree of functional independence attained in ADL: the SCIM weighs each function separately, giving a final score that ranges from 0 (totally dependent) to 100 (totally independent) [
18].
Leisure Time Physical Activity (LTPA) includes physical exertion-related activities that people choose to do in their free time, and in case of patients with SCI it includes walking or wheeling, and certain sports played in a gym. These activities were quantified by the LTPA Questionnaire (LTPAQ) for People with SCI [
19,
20]. This is a SCI-specific measure of minutes of LTPA performed at each intensity (mild, moderate, and heavy intensity LTPA) over the previous 7 days. Only total LTPA score was used for analyses, because of its strong correlation with mild, moderate, and heavy intensity sub-scores [
21].
2.3. Laboratory Examinations
A single fasting morning venous blood sample was obtained from each subject between 8:00 and 9:00 a.m. Standard methods and commercial kits (Instrumentation Laboratory Company, Lexington, MA, USA) were used for all the biochemical and hematologic measurements. Insulin resistance was assessed using the homeostatic model assessment of insulin resistance (HOMA-IR), according to the formula: insulin (mU/L) × glucose (m/dL)/405 [
22].
2.4. Statistical Analysis
Statistical analysis was performed using the R statistical software (version 4.0.4, 2021, The R Foundation for Statistical Computing, Vienna, Austria). After ascertaining the non-normal distribution of data (Shapiro–Wilk test), the Wilcoxon rank-sum test (Mann-Whitney U test) was used to compare variables between people with and without MetS. Proportional differences were assessed by the χ2 test. Multiple logistic regression analysis was performed to reveal independent associations with MetS among variables selected by univariate linear regression analyses. Significance of differences in the prevalence of MetS throughout the 1st to the 3rd tertile of increasing NRS score and LTPA levels were assessed by χ2 test for trend (Mantel-Haenszel test). Statistical significance was accepted when p < 0.05.
3. Results
A diagnosis of MetS was made in 56 of 132 men (42.4%) and 17 of 36 women (47.2%).
Table 1 shows the characteristics of the study population categorized by the presence of MetS. Patients with MetS were significantly older and exhibited higher triglycerides and lower HDL levels, higher HOMA-IR, BMI, CCI, systolic and diastolic blood pressure, NRS score and poorer LTPA. Moreover, in subjects with MetS, the prevalence of complete motor injury was lower, as was the duration of injury (DOI).
As shown in
Table 2, at the univariate regression analyses, putative significant predictors of MetS were an older age, a higher CCI, NRS score and HOMA-IR, as well as a shorter DOI, a poorer LTPA and the presence of incomplete motor injury. At the multiple logistic regression analysis, a significant independent association with MetS only persisted for a poorer LTPA and more severe pain symptoms, as indicated by a higher NRS score.
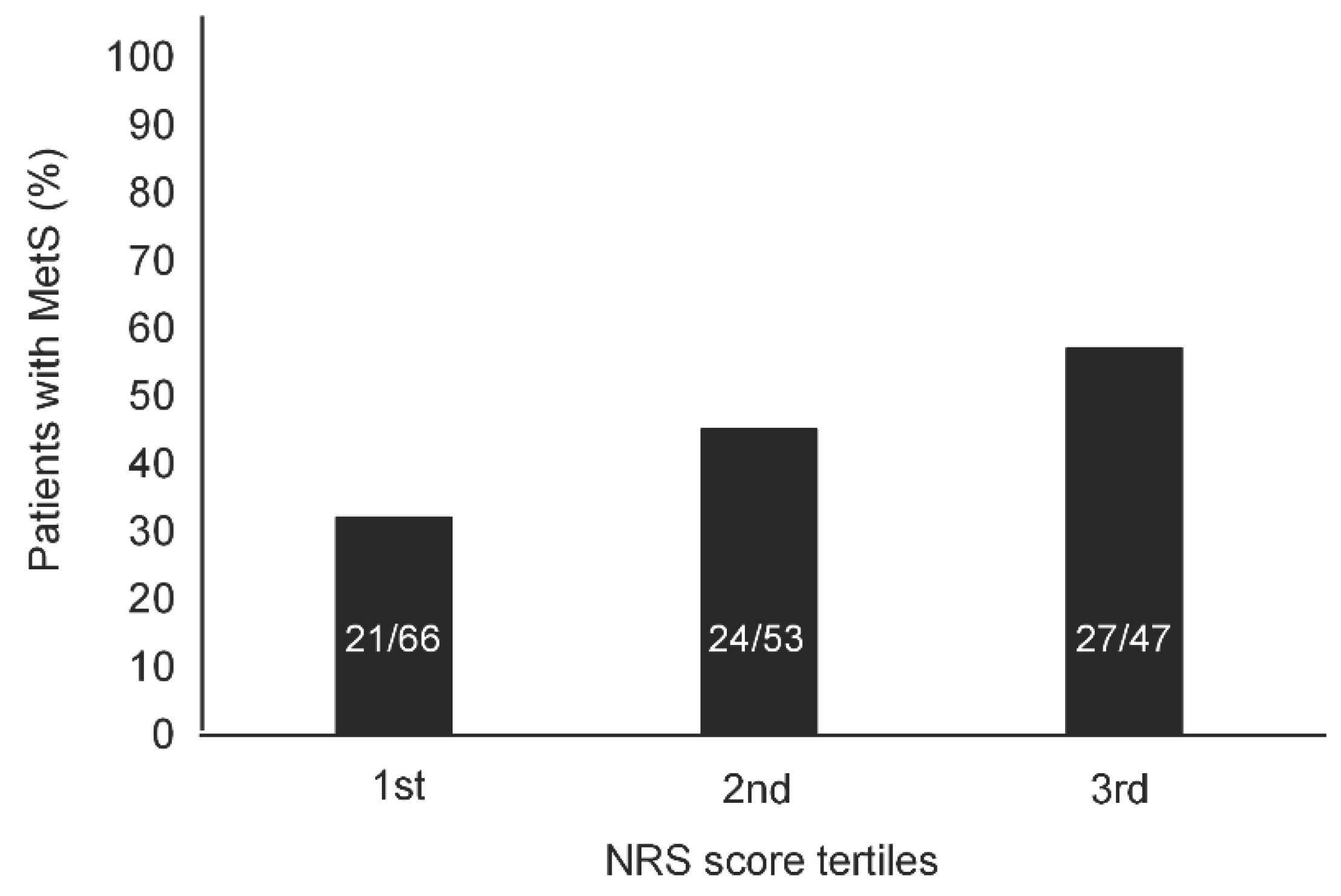
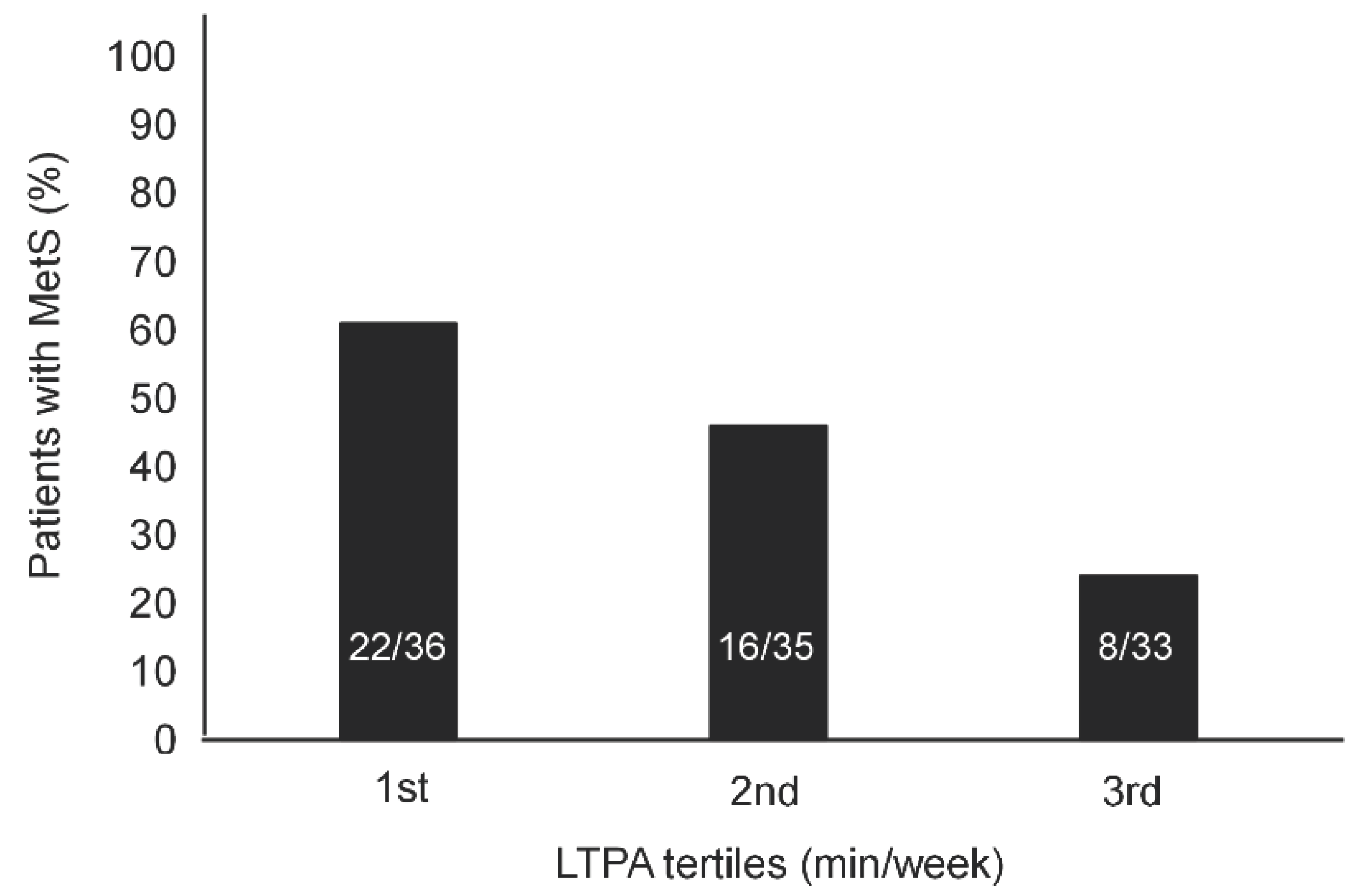
As NRS score and LTPA level increased from the 1
st tertile to the 3
rd tertile, the prevalence of MetS significantly increased (
Figure 1) and decreased (
Figure 2), respectively.
4. Discussion
Among the many possible determinants and attributes of MetS in patients with SCI, this study has narrowed the field to a limited number of potentially mutually correlating variables. The results of univariate regression analyses showed a significant association of MetS with older age, more comorbidities, lower insulin sensitivity, more intense pain symptoms, a poorer physical activity, a lower degree of neuromotor disability, and a more recent clinical history of SCI.
Advancing age is commonly associated with a progressive decrease in physical activity and worsening overall health status due to the onset of comorbidities, including the MetS components [
23]. Decreased physical activity, quantified in the present study in terms of LTPA, may contribute directly to the increased incidence of comorbidities, insulin resistance and MetS [
2]. An additional factor with a possible pathogenetic role is pain. The prevalence of MetS significantly increased throughout the lowest to the highest tertile of NRS score. Neuropathic and/or nociceptive chronic pain symptoms represent a major complain in people with SCI [
24]. Pain may further limit mobility and thus the patient’s ability to engage in physical activity: the resulting drop in energy expenditure increases fat accumulation and the risk of developing comorbidities and MetS [
2]. Poor physical activity and hypomobility, in turn, could contribute to muscle spasticity that exacerbates pain symptoms [
24], thus triggering a vicious circle. The possible mechanisms by which MetS is associated in our univariate regression analyses with a lower degree of motor disability and a shorter DOI remain speculative but could involve lifestyles. In patients with recent history of SCI, an incomplete psychological and social-relational adjustment to the disabling condition [
25] could result in a propensity to exhibit behavioral correlates of emotional distress [
26,
27] at risk for the development of comorbidity, including MetS components. This might occur, paradoxically, with greater likelihood in patients with incomplete motor SCI: when the greater degree of functional independence requires less caregiver’s intervention, the controlling role he/she exerts on the patient’s lifestyles is also weakened.
Obviously, the cross-sectional design of this study does not allow the causal directionality of the associations under investigation to be established, but some information can be inferred from the multiple logistic regression analysis. When all variables selected by the univariate analyses were included in the same multivariable model, only a poorer LTPA and a higher pain score at the NRS exhibited a significant and independent association with MetS. This finding demonstrated that the relationship between pain and MetS is not necessarily mediated by reduced physical activity. Indeed, the association of MetS with poor LTPA and more intense pain symptoms may be complex and bidirectional: on the one hand, obesity and comorbidities, components of MetS, may constrain a patient’s ability to engage in physical activity; on the other hand, there is preclinical, clinical, and epidemiologic evidence for an association between MetS and sensory peripheral neuropathy independent of prediabetes, diabetes, and glycemic status [
28,
29]. The main mediators of this association would be obesity and dyslipidemia [
28]. Obesity is the hallmark of MetS and is accompanied by the release into circulation of a large pool of long-chain fatty acids (LCFAs) that penetrate the blood-neurogenic barrier, causing oxidative stress-mediated neuroinflammation [
30]. LCFAs alter axonal mitochondrial transport and impair electron chain activity [
31]. The resulting mitochondrial dysfunction results in impaired oxidative phosphorylation with reduced adenosine triphosphate (ATP) production and generation of reactive oxygen species (ROS) [
32,
33]. The nuclear factor kappaB (NF-kB), a transcriptional factor activated by oxidative stress and hyperglycemia, is at the center of neuroinflammation by MetS [
34] as it modulates several downstream pro-inflammatory genes, particularly cyclooxygenase-2 (COX-2) [
35]. These mechanisms as whole underlie neuronal and Schwann cell injury that ultimately contributes to MetS neuropathy resulting in chronic pain [
28].
Of note, the univariate negative association between MetS and insulin sensitivity was lost in the fully adjusted multiple logistic regression analysis. This finding may reflect the peculiarities of the pathogenesis of MetS in patients with SCI. It is possible to speculate that the SCI-related chronic inflammatory state, in addition to contributing to the onset of neuropathic pain, may promote at the muscle and adipose tissue level mechanisms of glucose and lipid deregulation underlying MetS, regardless of insulin sensitivity degree. Indeed, although HOMA-IR was significantly higher in patients with MetS than in those without MetS, in both groups, its median values were largely within the normal range. Further studies in preclinical models are needed to test this hypothesis.
This study has some limitations. First, the sample size. Despite a relatively sized study population, the high prevalence of the endpoint (e.g., MetS) allowed the inclusion of a fair number of independent variables in the multiple regression model to adjust analysis for possible major confounders. Nevertheless, despite the adjustments, a residual confounding effect from unmeasured variables potentially mediating the revealed associations cannot be ruled out. In this light, factors related to lifestyle and/or psychological functioning could play a major role and deserve to be investigated in targeted studies.
5. Conclusions
In patients with chronic SCI, intense pain symptoms and a poor physical activity may suggest a high likelihood of MetS, regardless of age, DOI, degree of motor disability, insulin sensitivity and comorbidities. Given the challenges in diagnosing MetS in this population [
2], pain and physical inactivity can help health care providers to identify the most at-risk patients early so that all necessary measures for prevention and treatment of cardiovascular implications can be implemented.
Author Contributions
Conceptualization, A.B. and G.F.; methodology, A.B. and C.C.; validation, A.B., F.D.G., D.T. and C.C.; formal analysis, C.C. and F.D.G.; investigation, A.B. and C.C.; data curation, A.B., C.C., F.D.G. and F.A.; writing—original draft preparation, C.C. and F.D.G.; writing—review and editing, A.B. and M.G.B.; supervision, A.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Savic, G.; DeVivo, M.J.; Frankel, H.L.; Jamous, M.A.; Soni, B.M.; Charlifue, S. Causes of death after traumatic spinal cord injury-a 70-year British study. Spinal Cord. 2017, 55, 891–897. [Google Scholar] [CrossRef]
- Barbonetti, A.; Castellini, C.; Francavilla, S.; Francavilla, F.; D’Andrea, S. Metabolic syndrome in spinal cord injury: Impact on health. Academic Press. 2022, 377–388. [Google Scholar] [CrossRef]
- Gorgey, A.S.; Dolbow, D.R.; Dolbow, J.D.; Khalil, R.K.; Castillo, C.; Gater, D.R. ; Effects of spinal cord injury on body composition and metabolic profile - part I. J Spinal Cord Med. 2014, 37, 693–702. [Google Scholar] [CrossRef]
- Cirnigliaro, C.M.; LaFountaine, M.F.; Dengel, D.R.; et al. Visceral adiposity in persons with chronic spinal cord injury determined by dual energy X-ray absorptiometry. Obesity (Silver Spring) 2015, 23, 1811–1817. [Google Scholar] [CrossRef]
- Zafar, U.; Khaliq, S.; Ahmad, H.U.; Manzoor, S.; Lone, K.P. ; Metabolic syndrome: an update on diagnostic criteria, pathogenesis, and genetic links. Hormones (Athens). 2018, 17, 299–313. [Google Scholar] [CrossRef]
- Shin, J.C.; Park, C.I.; Kim, S.H.; Yang, E.J.; Kim, E.J.; Rha, D.W. Abdominal ultrasonography findings in patients with spinal cord injury in Korea. J Korean Med Sci. 2006, 21, 21,927–931. [Google Scholar] [CrossRef]
- Barbonetti, A.; Caterina Vassallo, M.R.; Cotugno, M.; Felzani, G.; Francavilla., S.; Francavilla, F. Low testosterone and non-alcoholic fatty liver disease: Evidence for their independent association in men with chronic spinal cord injury. J Spinal Cord Med 2016, 39, 443–449. [Google Scholar] [CrossRef]
- Elder, C.P.; Apple, D.F.; Bickel, C.S.; Meyer, R.A.; Dudley, G.A. ; Intramuscular fat and glucose tolerance after spinal cord injury--a cross-sectional study. Spinal Cord. 2004, 42, 711–716. [Google Scholar] [CrossRef]
- Gallagher, E.J.; Leroith, D.; Karnieli, E. ; The metabolic syndrome--from insulin resistance to obesity and diabetes. Med Clin North Am. 2011, 95, 855–873. [Google Scholar] [CrossRef]
- Jones, L.M.; Legge, M.; Goulding, A. ; Healthy body mass index values often underestimate body fat in men with spinal cord injury. Arch Phys Med Rehabil. 2003, 84, 1068–1071. [Google Scholar] [CrossRef]
- Nash, M.S.; Groah, S.L.; Gater, D.R.; et al. Identification and Management of Cardiometabolic Risk after Spinal Cord Injury. J Spinal Cord Med. 2019, 42, 643–677. [Google Scholar] [CrossRef]
- Alberti, K.G.; Zimmet, P.Z. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med 1998, 15, 539–553. [Google Scholar] [CrossRef]
- Ford, E.S. Prevalence of the metabolic syndrome defined by the International Diabetes Federation among adults in the U.S. Diabetes Care 2005, 28, 2745–2749. [Google Scholar] [CrossRef]
- Gater, D.R. Jr; Farkas, G.J.; Berg, A.S.; Castillo, C. Prevalence of metabolic syndrome in veterans with spinal cord injury. J Spinal Cord Med. 2019, 42, 86–93. [Google Scholar] [CrossRef]
- Charlson, M; Szatrowski, TP; Peterson, J; Gold, J.; Validation of a combined comorbidity index. J Clin Epidemiol 1994, 47, 1245–1251. [CrossRef]
- Maynard, F.M. Jr; Bracken, M.B.; Creasey, G.; et al. International Standards for Neurological and Functional Classification of Spinal Cord Injury. American Spinal Injury Association. Spinal Cord. 1997, 35, 266–274. [Google Scholar] [CrossRef]
- Bryce, T.N.; Budh, C.N.; Cardenas, D.D.; et al. Pain after spinal cord injury: an evidence-based review for clinical practice and research. Report of the National Institute on Disability and Rehabilitation Research Spinal Cord Injury Measures meeting. J Spinal Cord Med. 2007, 30, 421–440. [Google Scholar] [CrossRef]
- Anderson, K.; Aito, S.; Atkins, M.; et al. Functional recovery measures for spinal cord injury: an evidence-based review for clinical practice and research. J Spinal Cord Med. 2008, 31, 133–144. [Google Scholar] [CrossRef]
- Martin Ginis, K.A.; Phang, S.H.; Latimer, A.E.; Arbour-Nicitopoulos, K.P. Reliability and validity tests of the leisure time physical activity questionnaire for people with spinal cord injury. Arch Phys Med Rehabil. 2012, 93, 677–682. [Google Scholar] [CrossRef]
- Barbonetti, A.; Sperandio, A.; Micillo, A.; et al. Independent Association of Vitamin D With Physical Function in People With Chronic Spinal Cord Injury. Arch Phys Med Rehabil. 2016, 97, 726–732. [Google Scholar] [CrossRef]
- Barbonetti, A.; D’Andrea, S.; Castellini, C.; et al. Erectile Dysfunction Is the Main Correlate of Depression in Men with Chronic Spinal Cord Injury. J Clin Med. 2021, 10, 2090. [Google Scholar] [CrossRef]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985, 28, 412–419. [Google Scholar] [CrossRef]
- Kuk, J.L.; Ardern, C.I. ; Age and sex differences in the clustering of metabolic syndrome factors: association with mortality risk. Diabetes Care. 2010, 33, 2457–2461. [Google Scholar] [CrossRef]
- Stampacchia, G.; Gerini, A.; Morganti, R.; et al. Pain characteristics in Italian people with spinal cord injury: a multicentre study. Spinal Cord. 2022, 60, 604–611. [Google Scholar] [CrossRef]
- Craig, A.R.; Hancock, K.M.; Dickson, H.G. A longitudinal investigation into anxiety and depression in the first 2 years following a spinal cord injury. Paraplegia. 1994, 32, 675–679. [Google Scholar] [CrossRef]
- Macleod, A.D. Self-neglect of spinal injured patients. Paraplegia. 1988, 26, 340–349. [Google Scholar] [CrossRef]
- DeVivo, M.J.; Black, K.J.; Richards, J.S.; Stover, S.L. Suicide following spinal cord injury. Paraplegia. 1991, 29, 620–627. [Google Scholar] [CrossRef]
- Kazamel, M.; Stino, A.M.; Smith, A.G. Metabolic syndrome and peripheral neuropathy. Muscle Nerve. 2021, 63, 285–293. [Google Scholar] [CrossRef]
- Bonomo, R.; Kramer, S.; Aubert, V.M. Obesity-Associated Neuropathy: Recent Preclinical Studies and Proposed Mechanisms. Antioxid Redox Signal. 2022, 37, 597–612. [Google Scholar] [CrossRef]
- Stavniichuk, R.; Shevalye, H.; Lupachyk, S.; et al. Peroxynitrite and protein nitration in the pathogenesis of diabetic peripheral neuropathy. Diabetes Metab Res Rev. 2014, 30, 669–678. [Google Scholar] [CrossRef]
- Rumora, A.E.; LoGrasso, G.; Haidar, J.A.; Dolkowski, J.J.; Lentz, S.I.; Feldman, E.L. Chain length of saturated fatty acids regulates mitochondrial trafficking and function in sensory neurons. J Lipid Res. 2019, 60, 58–70. [Google Scholar] [CrossRef]
- Chowdhury, S.K.; Smith, D.R.; Fernyhough, P. The role of aberrant mitochondrial bioenergetics in diabetic neuropathy. Neurobiol Dis. 2013, 51, 56–65. [Google Scholar] [CrossRef]
- Fernyhough, P. Mitochondrial dysfunction in diabetic neuropathy: a series of unfortunate metabolic events. Curr Diab Rep. 2015, 15, 89. [Google Scholar] [CrossRef]
- Shoelson, S.E.; Lee, J.; Goldfine, A.B. Inflammation and insulin resistance. J Clin Invest. 2006, 116, 1793–1801. [Google Scholar] [CrossRef]
- Kellogg, A.P.; Wiggin, T.D.; Larkin, D.D.; Hayes, J.M.; Stevens, M.J.; Pop-Busui, R. Protective effects of cyclooxygenase-2 gene inactivation against peripheral nerve dysfunction and intraepidermal nerve fiber loss in experimental diabetes. Diabetes. 2007, 56, 2997–3005. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).