1. Introduction
Artisanal cultures are known as wild consortia of microorganisms which can grow on unconventional fermentation substrates resulting valuable products rich in bioactive compounds (biotics).
Briefly, SCOBY-based membranes are made of a natural consortium of microorganisms which work in mutualistic symbiosis due to a wide variety of species comprising mostly acetic acid bacteria (
Gluconacetobacter ssp., A
cetobacter ssp.,
Gluconobacter ssp.) and yeasts (
Zygosaccharomyces ssp.,
Brettanomyces ssp.,
Saccharomyces ssp.), but also some lactic acid bacteria strains (
Lactobacillus ssp.). The bacterial strains generate a polysaccharide stroma in which the yeasts are attached, causing the development of a membrane with a thickness of several centimetres at the liquid-air border [
1,
2]. This SCOBY-based membrane usually ferments black or green tea supplemented with sucrose. The fermentation took place between 7 to10 days, at 20-25 °C, and as such a beverage with a rich postbiotic composition was achieved. It contained organic acids (acetic, lactic, malic, tartaric, citric, gluconic, glucuronic), and other compounds (substances with antibiotic properties, ethanol, water-soluble vitamins, hydrolytic enzymes, amino acids) [
3,
4].
WKG culture is typically composed of lactic acid bacteria (
Lactobacillus ssp.,
Streptococcus ssp.,
Leuconostoc ssp.), yeasts (
Saccharomyces ssp.,
Dekkera ssp.), and acetic acid bacteria, encapsulated in the polysaccharide dextran (and a limited concentration of levan) matrix, looking like hard granules, small, translucent, and irregular [
5,
6]. These grains are usually cultured in water and sucrose supplemented with dried fruits (often raisins or figs), being afterwards produced a useful beverage [
7]. After 2-3 days of fermentation at 20-25 °C, the FP contains a wide range of synthesized metabolites, including lactic acid, esters, glycerol, carbon dioxide, and acetic acid isoamyl acetate, as well as ethanol, ethyl octanoate, ethyl decanoate, and ethyl hexanoate [
1,
8].
The microbial strains’ symbiotic interaction in the artisanal consortium offers metabolites’ production in the FPs (pre-, pro-, post- and para-probiotics). The diversity of the microbial community ensures the stability and safety of FPs against spoilage and pathogenic microorganisms [
5]. The products fermented with artisanal cultures also exhibit valuable biological activities, including immunomodulatory, anti-inflammatory [
9], antihypertensive, hepatoprotective, cholesterol-lowering, and antioxidant potential [
10]. This study’s goal was the co-cultivation of SCOBY and WKG (liophylised cultures) in a new formulated fermentation medium and optimization of the biotechnological parameters to produce FP with improved bioactive content.
2. Materials and Methods
2.1. Multiplication and freeze-drying of water kefir grains as starter cultures
WKG (Medicer Bios, Romania) were grown on a specific medium based on sterile tap water supplemented with 10% (w/v) sugar and 1% (w/v) raisins, at 25±1°C, for a period of 48 h. Further, the grains firstly were washed using Milli Qwater, and then immersed in a fresh medium. The mixtures were incubated under the same conditions as mentioned before, with successive cultivation steps to allow the granules to multiplicate [
11]. Furthermore, in order to assure the cryoprotection of the viable cells, the granules were washed with ultrapure water and supplemented with 10% (w/v) inulin and lyophilized at −80°C (Christ Alpha 1-4 LD plus, Germany). The cryodesiccated water kefir grains were ground being furtherstored at the temperature of 4°C.
2.2. Multiplication of kombucha’s biofilm as starter culture
The SCOBY-based membranes, purchased from a private household from the Republic of Moldova, were multiplied by successive cultivation in a sugar based medium (7,5%, w/v) with the addition of 3% (w/v) infusion of black tea (Aaro Forstman Oy, Vantaa, Finland) The mixture was incubated for 10 days at room temperature (22±2°C). Then, 20% (w/w) sterile inulin powder was added to 100 g of biofilm that was previously divided in small pieces, the mixture being homogenized and freeze-dried. The resulting biofilm was finally mortared and stored at 4°C.
2.3. Experimental design and optimization of WKG and SCOBY co-cultivation process
2.3.1. The Plackett-Burman Design to select the factors influencing the synergism between WKG and SCOBY
For the screening of the most significant parameters that influence the co-cultivation of WKG and SCOBY, FPs were prepared according to the Plackett-Burman experimental Design (PBD), by varying the following parameters: 1–3% (w/v) black tea leaves (the tea mixture beinginfused for 5 minutes in boiled water and cooled down until 90±1°C), 3–6% (w/v) raisins, 5–10% (w/v) sugar, pH= 6.30 (
Table 1). After the medium’ sterilization at 105°C, for a period of 10 minutes, it was inoculated, after cooling, with the lyophilized starter cultures (0.2-0.5% w/v) and incubated under aerobic and stationary conditions for 5 to 7 days, at 30°C. After fermentation the samples were analysed immediately.
The Minitab software took into consideration 3 central points and 6 factorial and thus generated 15 experimental variants. The following responses were considered: the pH and titratable acidity, the antioxidant capacity and the antimicrobial activities (against above-mentioned microorganisms).
2.3.2. Optimization of WKG and SCOBY co-cultivation by Response Surface Methodology (RSM)
After analysing the responses obtained from PBD experimental runs, the factors influencing the fermentation with WKG and SCOBY were identified, as such: concentration of black tea and raisins, as well as the fermentation time. Subsequently, five variation levels were analysed for the independent variables (
Table 2) included in the RSM analysis. For the statistical validation of the experimental models, a p value of < 0.05 was regarded to be significant.
The factors that were not optimized (B, E, F) remained constant, respectively a 5% sugar concentration (w/v), a 0.2 % lyophilized SCOBY concentration (w/v), and a concentration of WKG of 0.2 % (w/v).
2.4. The evaluation of the responses
2.4.1. Acidifying potential
The pH analysis was assessed with a digital pH meter (Mettler Toledo, FiveEasy F20, Greifensee, Switzerland).
The titratable acidity was expressed in Thörner degrees (°Th), using the AOAC method [
12]. In brief, about 4 g of sample was weighted and distilled water was added to reach 50 mL. Aliquots of 10 mL were used for the NaOH 0.1N titration, using phenolphthalein, until the appearance of a weak pink colour.
2.4.2. Evaluation of the antifungal properties of the FPs
The antifungal activity assessment on the indicator strain of
Aspergillus niger MIUG M5, that belongs to the MIUG Collection, from the Faculty of Food Science and Engineering, “Dunărea de Jos” University, Galati, Romania. The incubation of the mold strain was achieved at 25°C, for 96 h, using the Yeast Glucose Chloramphenicol (YGC) Agar. The inoculum was obtained by suspension the spores in sterile saline solution (0.9% NaCl) at a concentration of 1x10
5 spores/mL, by counting with the Thoma chamber. From the fresh FP, a volume of 1 mL was taken and dispersed into a Petri dish and thoroughly mixed with a volume of 20 mL of Potato Dextrose Agar (PDA) medium (Oxoid, England). After solidification, from the spore’s suspension, a volume of 10 μL was inoculated in the centre of the plate and left for incubation at 25°C, for 4 days (96 h). The control was assessed under the above-mentionedconditions but without the FP addition. Subsequent to the incubation time, the diameters of the mould’s growth were determined and the inhibition ratio (RI) was determined using the next Equation (1) [
13]:
where, RI represents the growth inhibition’s ratio, Ac—the mould growth’s diameter of the control sample, At—the mould strain’s diameter on the FP-supplemented medium.
2.4.3. Assessment of the FPs’ antibacterial activity
Antibacterial activity was tested against the indicator strains
Bacillus subtilis MIUG B1,
Escherichia coli ATCC 25922, and
Staphylococcus aureus ATCC 25923, strains that were cultivated on Plate Count Agar (PCA) (Scharlau, Barcelona, Spain), respectively, Mueller II Hinton agar (Biolab, Hungary) for 24 h, at 37°C. Then, the colony was placed into the Nutrient Broth (for
B. subtilis), respectively, Muller Hinton broth (for
E. coli and
S. aureus), with an overnight incubation, at 37°C. Afterwards, the bacterial inoculum was dimensioned spectrophotometrically (OD
600nm) at 0.3, corresponding to a concentration around 2.4×10
8 CFU/mL. Subsequently, a volume of 500 µL of bacterial suspension was added in the Petri dishes with the specific media for each bacterial strain, in the wells made (8 mm diameter), 100 μL of FP were added. Afterwards, the incubation of the plates took placeat 37°C, for 48 h, the inhibition zone being determined and expressed in mm [
14,
15].
2.4.4. Evaluation of the antioxidant properties of the FPs
In order to extract the bioactive from the fermented medium, an ultrasound-assisted method (MRC. Ltd., Holon, Israel) was applied, considering an extraction time of 30 min at 40°C, followed by centrifugation at 7000 rpm and 4°C for 15 min. To obtain the 2,2-diphenyl-1-picrilhydrasyl (DPPH) radical scavenging potential (DPPH) solution, 4 mg of DPPH were transferred in 100 mL of HPLC-grade methanol (≥ 99.9%) and allowed to dissolve. The DPPH solution was prepared daily and stored in dark conditions [
16,
17]. From the supernatant, an aliquot of 0.1 mL sample was homogenized with 3.9 mL DPPH solution and kept in the dark for 90 min, the absorbance was read at 515 nm [
18,
19].
Antioxidant activity was performed by adding using the 2,2-diphenyl-1-picrilhydrasyl (DPPH) radical scavenging potential, allowing to calculate the antioxidant activity in μM TE/mL on a calibration curve based on 6-hydroxy-2,5,7,8-tetramethylcroman-2-carboxylic acid (Trolox). The antioxidant activity was calculated according to the formulas (2) and (3):
where: A
m - absorbance of the control, A
p – the absorbance of the sample analysed.
In brief, 4 mg of DPPH were transferred in 100 mL of HPLC-grade methanol (≥ 99.9%) and allowed to dissolve. The DPPH solution was prepared daily and stored in dark conditions [
16,
17].
2.5. Analysis of organic acids and polyphenolic content in the FP obtained in optimised fermentation conditions
2.5.1. The assessment of the organic acids
The determination was achievedusing an HPLC system, Agilent 1200 (Agilent Technologies, Santa Clara, CA, USA), having a multi-wavelength detector (MWD), with a quaternary pump, autosampler, degasser, and a thermostat. The column was a Hamilton RPR X300 (250 × 4.1 mm, particle size 7 μm, Hamilton, Bonaduz, Switzerland), with a gradient elution composed of mobile phase A – KH
2PO
4, 20 mM, pH 2.4 and phase B – acetonitrile 90% (v/v) (ACN) [
20]. The mobile phases’ mixture had the following steps: min 0 – 80% A, min 10 – 40% A, min 12.5 – 40% A, min 12.6 – 80% A. The organic acids’ separation profile was achieved at 210 nm, injection volume of 20 μL, at 30°C, with a 1.5 mL/min flow-rate [
21]. The data acquisition was assessed with the ChemStation software.
The organic acids were identified and quantified based on external calibration curves using HPLC-grade organic acids’ standard solutions (Sigma Aldrich, Germany).
2.5.2. Evaluation of polyphenols
The Agilent 1200 High-performance liquid chromatography system (Agilent Technologies, Santa Clara, CA, USA) was applied to determine the polyphenolic composition of the analysed samples. Consequently, the compounds of interest were separated by a Synergi Max-RP-80Å column with a guard column (250 × 4.6 mm, particle size of 4 μm, Phenomenex, Torrance, California, USA) using mobile phase A – ultrapure water: acetonitrile: formic acid = 87:3:10, and mobile phase B – ultrapure water: acetonitrile: formic acid = 40:50:10, with the following elution program: min 0 – 94% A, min 20 – 80% A, min 35 – 60% A, min 40 – 40% A, min 45 – 10% A. For the polyphenolic compounds’ separation, at 30°C, a 20 μL volume was deployed into the column, at a of 0.5 mL/min flow rate. The time of the method was 80 min and then the data was processed using ChemStation program version B.04.03 [
13,
22,
23].
The polyphenols were identified and quantified simultaneously at the wavelengths of 280 nm and 320 nm, based on external calibration curves for the available polyphenolic HPLC-grade standards (Sigma Aldrich, Germany).
2.6. Statistical analysis
The design of experiments was assessed with the Minitab 17 software (v. 1.0, LLC, Pennsylvania State University, USA). One-way ANOVA and Tukey test considering a 95% confidence interval (p<0.05) were used to analyze the experimental results, that were considered as averages of triplicate measurements followed by standard deviation.
3. Results and discussions
3.1. The most important parameters selection that influence the fermentation process with artisanal consortia by PBD analysis
This strategy applied for the fermentation process aimed at designing the appropriate culture medium by adjusting the carbon (C) and nitrogen (N) sources, the C/N ratio, minerals, trace elements, growth factors, and the fermentation parameters. In the customised formulas for fermentation, the main source of C was considered, whereas the fresh or dried fruits provided the nitrogen [
24]. The analysed parameters and the interactions between them can be evaluated objectively using statistical methods [
25].
The statistical modelling by PBD generated 15 experimental combinations using the ranges of variation for the chosen factors, as follows: black tea concentration 1-3% (w/v), sugar concentration 5-10% (w/v), raisins concentration 3–6% (w/v), 5-7 days of fermentation, lyophilized SCOBY ranging from 0.2 to 0.5% (w/v) and WKG ranging from 0.2 to 0.5 % (w/v), respectively (
Table 3).
Thus, the following results were obtained: 3.46–3.96 for pH, 20–225°Th for titratable acidity, while for the antioxidant activity a value of 2.388–2.412 μM TE/mL, 0.00–12.67 mm for the antibacterial activity inhibition zone against E. coli, 0.00-14.00 mm against S. aureus, 1.50-14.33 mm against B. subtilis, and 82.06–100% inhibition zone for the antifungal activity.
The statistical models based on some analysed responses, respectively, the antioxidant activity, and the antibacterial activity against S. aureus, were validated in accordance to the regression coefficients higher than 80% and at a p<0.05 value.
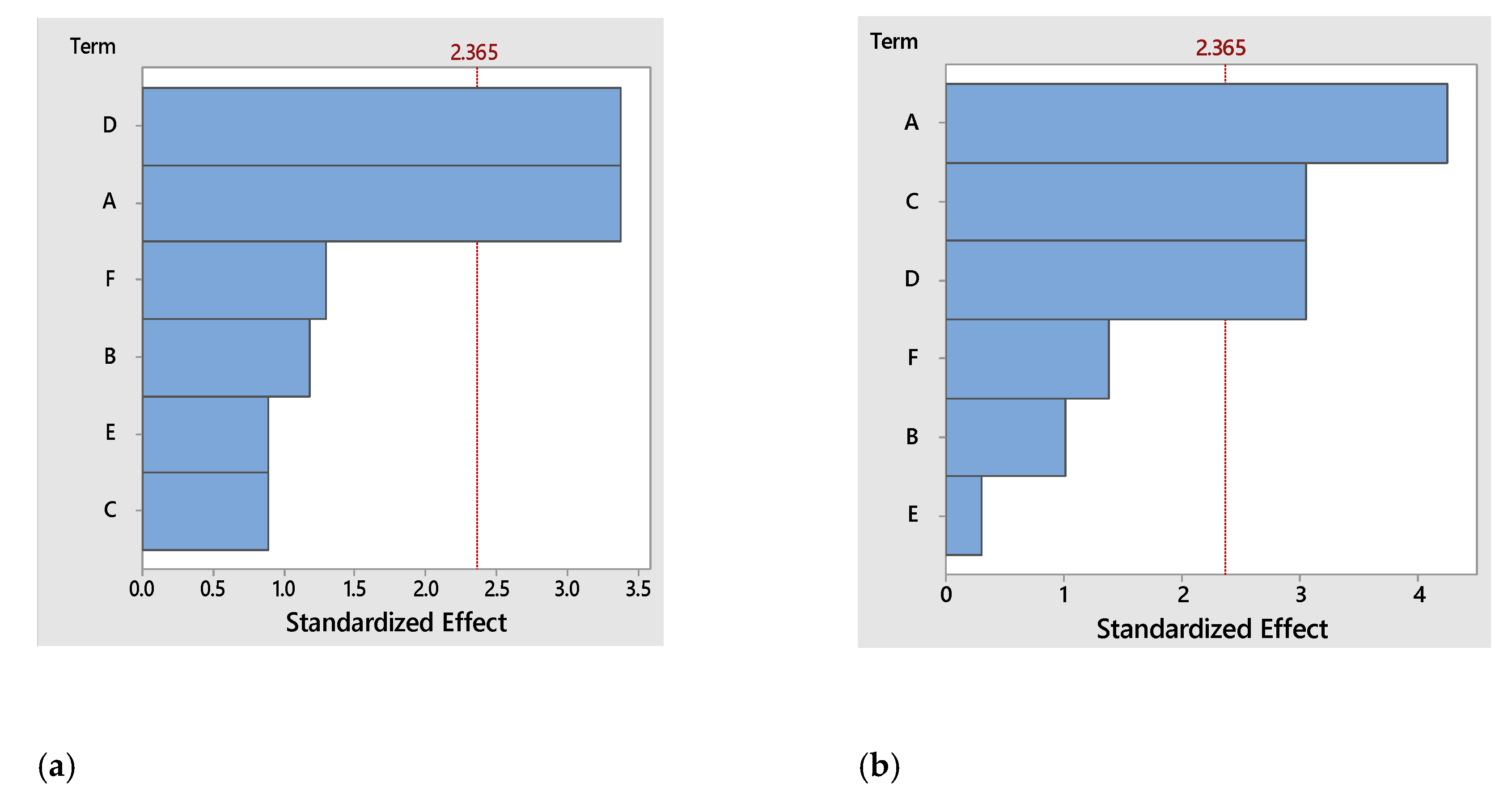
The main parameters that influenced the FPs’ antioxidant activity were the tea’s concentration (A), raisins’ concentration (C) and the time of fermentation (D), their impact on the studied response-variables is shown in the Pareto diagram (
Figure 1a).
Analysing the results comparatively, it can be stated that the FPs, that were involved in the co-culture, were a characterized by a higher bioactive capacity compared to the unfermented medium, due to the polyphenols present in the tea, the metabolites of yeasts and bacteria, including vitamins, organic acids, and extracellular enzymes, that contribute to the structural and compositional changes during the fermentation of kombucha [
26].
The product fermented with WKG had a high antioxidant potential on the account of the presence of lactic and acetic acid bacteria, as well as yeasts, their metabolites and cell lysis’ products that occur during the fermentation [
27].
For the antibacterial activity against
S. aureus, the significant factors were the concentration of tea (A), and the fermentation time (D) as the Pareto diagram in
Figure 1b shows.
It is known that due to the production of post-biotics, FPs (including black tea substrate) showed antibacterial activity of 12-30.2 mm inhibition zone against several pathogens [
28].
For the rest of the analysed responses, no validation of the model was achieved.
The ANOVA results from
Table 4 showed the significant contribution for the concentration of tea (p=0.004), concentration of raisins, and for the fermentation time (p=0.019). Furthermore, this statistical model can be validated based on in the non-significant lack of fit (p=0.923) [
29].
3.2. Optimization of the fermentation process with artisanal co-culture to increase the FPs’ functional potential
The statistical results from the PBD allowed the selection of three important parameters: tea concentration, raisin concentration, and fermentation time. The other factors, namely the concentration of SCOBY lyophilised culture (0.2%), the concentration of WKG lyophilised culture (0.2%), at 30°C, for 5 days of fermentation, remained constant.
The amount of inoculum, the amount of sugar and fruit added, the medium composition, the amount of oxygen, the time and temperature of fermentation are also mentioned as factors that determine the best fermentation of WKG and have an impact on the composition and properties of the FP [
27].
Table 5 presents the experimental matrix obtained by Central Composite Design (CCD) model that generated 20 experimental variants), with the corresponding analysed responses: pH, titratable acidity, antioxidant activity, antibacterial and antifungal activities against the targeted strains.
Following the statistical analysis of the obtained results, two mathematical models, for pH and total acidity, were validated, with a probability value of 0.003, thus highlighting the factors with a significant interaction for each validated response (
Table 6).
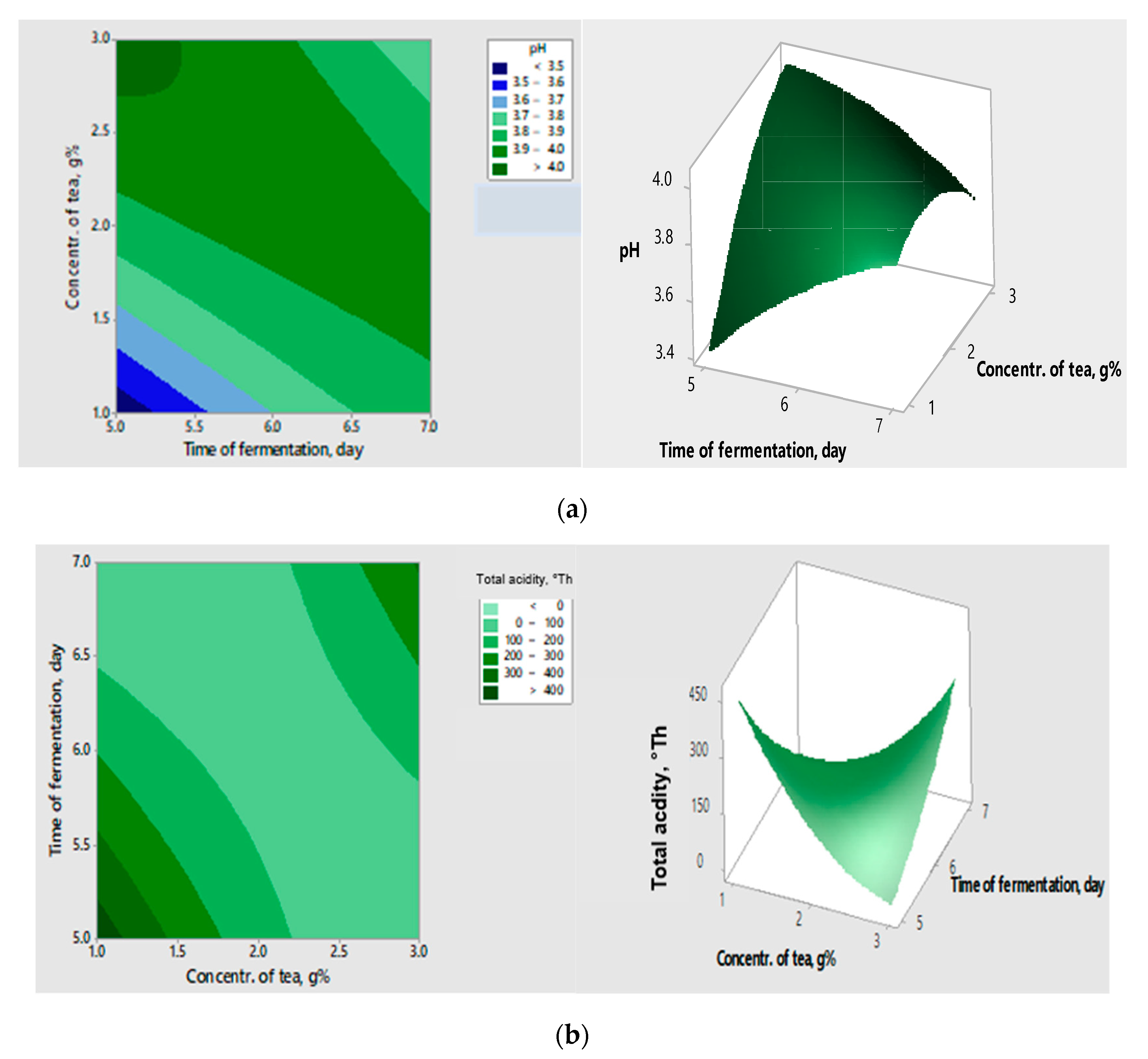
The interactions between the variables, as well as their impact on the response can be visualized in the contour and surface graphs (
Figure 2 a, b), which highlight the correlation between the tea concentration, the fermentation time, the concentration of raisins, and the responses obtained for the validated models.
Analysing the above graphs, the acidification potential increased with increasing the concentration of raisins and decreasing the concentration of tea and the time of fermentation.
According to the CCD experimental data, the following values for the analysed responses were obtained: 3.42 – 3.97 for pH, 51.25 – 637.5°Th, 2.006 – 2.395 μM TE/mL for the antioxidant potential, 0 – 6.67 mm antibacterial activity inhibition zone against E. coli, 0 – 7.67 mm against S. aureus, 5.17 – 18.5 mm against B. subtilis and 70.12–100% inhibition for antifungal activity against A. niger.
Following the analysis of the validated models and the significant factors, an optimized FP with an increased bioactive activity has been designed by the formulated medium based of 3.52 % (w/v) raisins, 1.0 % (w/v) black tea, 5 % (w/v) sugar, inoculated with 0.2% (w/v) WKG lyophilised culture and 0.2% (w/v) SCOBY lyophilised culture co-fermentation at 30°C, for 5 days, under stationary aerobic conditions.
The validation models for pH and total acidity (titratable acidity) were then analysed. The experimental values ranged between the predicted values for a 95% confidence level. Also, the desirability of the model is 0.901, close to 1, which indicates that following the chosen parameters favourable results for the analysed responses can be achieved.
The FP obtained in optimized conditions was characterised by a high acidity of 375.83°Th and a low pH of 3.30 compared to the control (unfermented sample), which had an acidity potential of 9.27°Th and a pH value of 4.69. Also, the control showed no antibacterial activity, for the antifungal inhibition being calculated a ratio of 2.68%. Therefore, the antioxidant activity is higher (2.507 μM TE/mL), due to the polyphenols from the tea. The analysis of the validated models showed that the optimisation of bio-procces improved the FPs’ acidification capacity.
3.3. Organic acids’ and polyphenols’ contents in the optimised FP
3.3.1. Organic acids contents
Using the High-performance liquid chromatography technique, the compounds present in the unfermented medium (control) and the fermented one were quantified (
Table 8).
Due to the symbiosis between yeasts and lactic acid bacteria, the development of the homoexopolysaccharide matrix from WKG, and organic acids’ production were achieved, in this regard yeasts helped bacteria by providing nitrogen as simple assimilable compounds (dipeptides, tripeptides and amino acids) through their proteolytic activity. Also, the carbon source has a key signification in the fermentative capacity of the WKG [
24]. The association and competition between the bacteria and yeasts in kombucha are unique leading to chained reactions from various metabolites, including acetic and lactic acids, up to 6.4 g/L, citric, gluconic, malic and succinic acids, up to 0.5 g/L [
30]. The consortium members’ cooperative association is well established. Less than 30% of the consortium is made of lactic acid bacteria strains, which are recognized for producing both lactic and also gluconic acids, which contribute to the antibacterial and antioxidant characteristics of the FP [
31].
Among the identified short-chain fatty acids, acetic acid is characteristic for SCOBY fermentation, being also produced in small amounts as a postbiotic of WKG consortium. As such, the acetic acid concentration increased from 4.34 mg/mL to 8.72 mg/mL. Butyric acid is not frequently found in kombucha-based drinks, but still its presence may occur, as Uţoiu et al. 2018 reported, after 5 days of fermentation, 0.14 g/L butyric acid was determined [
32], compared to the present study, where the amount of butyric acid increased from 37.90 mg/mL to 45.81 mg/mL. Isovaleric acid is a volatile compound that contributes to the flavour of the FP, being the results of the interaction between acetic acid bacteria and yeasts (e.g.,
Acetobacter indonesiensis with
Brettanomyces bruxellensis). The literature highlighted a concentration up to 0.007 mg/mL, instead the present study reports an amount of 0.88 mg/mL in the FP.
Previously, in a FP obtained by co-fermentation with WKG and SCOBY some organic acids such as lactic acid, acetic acid, citric acid, isovaleric acid, and butyric acid that presented the following concentrations, respectively of24.39 mg/mL, 25.21 mg/mL, 5.77 mg/mL, 4.36 mg/mL, 67.33 mg/mL were synthesized by the artisanal cultures [
33].
3.3.2. Content of polyphenols and flavonoids
The major bioactive compounds identified in the product obtained in optimised conditions were caffeic acid 255.64 μg/mL, rutin trihydrate 568.93 μg/mL and epicatechin 1135.69 μg/mL at the wavelength of 280 nm, instead only caffeic acid was found in the control, at a concentration of 16.80 μg/mL at 280 nm, respectively 1.05 μg/mL at 320 nm, being specific to black tea (Table 10). Gallic acid, isorhamnetin, ferulic and chlorogenic acids were present in smaller concentrations. Some compounds have been identified by Vázquez-Cabral et al. 2017, in kombucha beverage, e.g., myricetin - 0.184 mg/L, gallic acid – 54.396 mg/L, caffeic acid -16.213 mg/L, chlorogenic acid - 0.539 mg/L, epicatechin -142.62 mg/L, and rutin - 4.245 mg/L. Our experimental data are confirmed by other results from similar works, that reported in a fermented product with WKG compounds such as chlorogenic acid, caffeic acid, tannins, vitamins C and D, glucosides, various enzymes including lipase, amylase, and protease [
34].
Previously, in our research regarding the co-fermentation with SCOBY and milk kefir grains, in the sample obtained under optimized conditions, several compounds were quantified gallic acid ≅ 71 μg/mL, epicatechin ≅1063 μg/mL, caffeic acid ≅ 315 μg/mL, quercetin ≅ 18 μg/mL, apigenin ≅0.22 μg/mL, isorhamnetin ≅3 μg/mL [
33].
Following the statistical and mathematical modelling analysis, a FP with improved bioactive properties was obtained. In the tested biotechnological conditions, the main independent variables with an influence on the quality of the FP turned out to be the concentration of tea, the fermentation time, the concentration of raisins. Thus, the optimized fermentation conditions are: i) composition of the medium: 3.52% (w/v) raisins, 1.0% (w/v) black tea, 5% (w/v) sugar in sterilized tap water; ii) inoculum: 0.2% (w/v) lyophilised culture of WKG and 0.2% (w/v) lyophilised culture of SCOBY; iii) fermentation process: aerobic conditions, in a stationary system, at a temperature of 30°C, for 5 days.
According to these biotechnological conditions, the obtained FP presented a high acidity potential of 375.83°Th, and a 3.25 pH value. The organic acids were highlighted also in different concentrations: acetic-8.72 mg/mL, butyric-45.81 mg/mL, isovaleric-0.88 mg/mL, respectively polyphenolic compounds such as phenolic acids: caffeic- 255.64 μg/mL, gallic-39.68 μg/mL, ferulic-0.36 μg/mL, chlorogenic-0.25 μg/mL; flavonoids derived from quercetin: rutin trihydrate - 568.93 μg/mL and isorhamnetin-11.94 μg/mL, as well as epicatechin- 1135.69 μg/mL. The presence of these compounds demonstrates the functional potential of the FP.
4. Conclusions
The obtained results confirmed the possibility to use multiple SCOBY and WKG starter cultures to ferment newly formulated black tea, raisins, and sugar-based medium, for improving the postbiotic composition of the FP. The variant of FP with the increased bioactive potential was obtained following statistical techniques for the selection of the parameters (independent variables), and optimisation of the process. The analysed responses demonstrated the ability of the bacteria and yeasts from the SCOBY and WKG microbiome to work in symbiosis. Thus, by variation of the fermentation parameters and exploit PBD and CCD tools it is possible to obtain FPs with different composition and bioactive properties to be used as ingredients for food and feed formulation.
Author Contributions
Conceptualization, G.E.B, M.P., and M.K.; methodology, M.C, M.P., B.P.-B, and N.S.; software, D.B., M.P. and B.P.-B.; validation, D.B., M.C., B.P.-B.; formal analysis, M.P., L.G.G and B.P.-B.; investigation, M.P., M.C., B.P.-B. and N.S.; resources, G.E.B.; data curation, M.P., M.C., L.G.G., and D.B.; writing—original draft preparation, M.P.; writing—review and editing, M.P., M.C., M.K. and G.E.B.; visualization, M.C., D.B., N.S. and G.E.B.; supervision, M.C. and G.E.B.; project administration, G.E.B.; funding acquisition, G.E.B. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by a grant of the Ministry of Research, Innovation and Digitiza- tion, CNCS/CCCDI—UEFISCDI, project number PCE 159/2021, within PNCDI III.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Data is contained within the article.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Pihurov, M.; Păcularu-Burada, B.; Cotârleţ, M.; Vasile, M.A.; Bahrim, G.E. Novel Insights for Metabiotics Production by Using Artisanal Probiotic Cultures. Microorganisms 2021, 9. [Google Scholar] [CrossRef] [PubMed]
- Nikolaev, Y.A.; Plakunov, V.K. Biofilm-”city of Microbes” or an Analogue of Multicellular Organisms? Microbiology 2007, 76, 125–138. [Google Scholar] [CrossRef]
- Jayabalan, R.; Waisundara, V.Y. Kombucha as a Functional Beverage. In Functional and Medicinal Beverages: Volume 11: The Science of Beverages; Elsevier Inc., 2019; pp. 413–446. ISBN 9780128163979.
- Oliveira, Í.A.C.L. de; Rolim, V.A. de O.; Gaspar, R.P.L.; Rossini, D.Q.; de Souza, R.; Bogsan, C.S.B. The Technological Perspectives of Kombucha and Its Implications for Production. Fermentation 2022, 8, 185. [Google Scholar] [CrossRef]
- Moretti, A.F.; Moure, M.C.; Quiñoy, F.; Esposito, F.; Simonelli, N.; Medrano, M.; León-Peláez, Á. Water Kefir, a Fermented Beverage Containing Probiotic Microorganisms: From Ancient and Artisanal Manufacture to Industrialized and Regulated Commercialization. Futur. Foods 2022, 5, 100123. [Google Scholar] [CrossRef]
- Wang, X.; Wang, P. Red Beetroot Juice Fermented by Water Kefir Grains: Physicochemical, Antioxidant Profile and Anticancer Activity. Eur. Food Res. Technol. 2022, 249, 939–950. [Google Scholar] [CrossRef]
- Havva¸safak, H.H.; Gün, I.; Kalit, M.T.; Kalit, S. Physico-Chemical, Microbiological and Sensory Properties of Water Kefir Drinks Produced from Demineralized Whey and Dimrit and Shiraz Grape Varieties. Foods 2023, Vol. 12, Page 1851 2023, 12, 1851. [Google Scholar] [CrossRef]
- Spizzirri, U.G.; Loizzo, M.R.; Aiello, F.; Prencipe, S.A.; Restuccia, D. Non-Dairy Kefir Beverages: Formulation, Composition, and Main Features. J. Food Compos. Anal. 2023, 117, 105130. [Google Scholar] [CrossRef]
- Moreira, M.E.C.; Santos, M.H.D.; Zolini, G.P.P.; Wouters, A.T.B.; Carvalho, J.C.T.; Schneedorf, J.M. Anti-Inflammatory and Cicatrizing Activities of a Carbohydrate Fraction Isolated from Sugary Kefir. J. Med. Food 2008, 11, 356–361. [Google Scholar] [CrossRef]
- M. Alsayadi, Y. Jawfi, M.B. et al. Antioxidant Potency of Water Kefir. J. Microbiol. Biotechnol. Food Sci. 2013, 2, 2444–2447. [Google Scholar]
- Laureys, D.; Aerts, M.; Vandamme, P.; De Vuyst, L. The Buffer Capacity and Calcium Concentration of Water Influence the Microbial Species Diversity, Grain Growth, and Metabolite Production During Water Kefir Fermentation. Front. Microbiol. 2019, 0, 2876. [Google Scholar] [CrossRef]
- Mirani, A.; Goli, M. Optimization of Cupcake Formulation by Replacement of Wheat Flour with Different Levels of Eggplant Fiber Using Response Surface Methodology. Food Sci. Technol. 2022, 42, 1–9. [Google Scholar] [CrossRef]
- Păcularu-Burada, B.; Turturică, M.; Rocha, J.M.; Bahrim, G.E. Statistical Approach to Potentially Enhance the Postbiotication of Gluten-Free Sourdough. Appl. Sci. 2021, 11, 1–25. [Google Scholar] [CrossRef]
- Balouiri, M.; Sadiki, M.; Ibnsouda, S.K. Methods for in Vitro Evaluating Antimicrobial Activity: A Review. J. Pharm. Anal. 2016, 6, 71. [Google Scholar] [CrossRef] [PubMed]
- Cotârleț, M.; Stănciuc, N.; Bahrim, G.E. Yarrowia Lipolytica and Lactobacillus Paracasei Solid State Fermentation as a Valuable Biotechnological Tool for the Pork Lard and Okara’s Biotransformation. Microorganisms 2020, 8, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Moraes Filho, M.L.; Busanello, M.; Garcia, S. Optimization of the Fermentation Parameters for the Growth of Lactobacillus in Soymilk with Okara Flour. LWT - Food Sci. Technol. 2016, 74, 456–464. [Google Scholar] [CrossRef]
- Vohra, B.M.; Fazry, S.; Sairi, F.; Babul-Airianah, O. Effects of Medium Variation and Fermentation Time on the Antioxidant and Antimicrobial Properties of Kombucha. Malaysian J. Fundam. Appl. Sci. 2019, 15, 298–302. [Google Scholar] [CrossRef]
- Diguță, C.F.; Nițoi, G.D.; Matei, F.; Luță, G.; Cornea, C.P. The Biotechnological Potential of Pediococcus Spp. Isolated from Kombucha Microbial Consortium. Foods 2020, 9, 1780. [Google Scholar] [CrossRef] [PubMed]
- Bogdan, M. STUDII ASUPRA POTENȚIALULUI DE VALORIFICARE A CONSORȚIULUI DE MICROORGANISME DE TIP KOMBUCHA, 2021.
- Cawthray, G.R. An Improved Reversed-Phase Liquid Chromatographic Method for the Analysis of Low-Molecular Mass Organic Acids in Plant Root Exudates. J. Chromatogr. A 2003, 1011, 233–240. [Google Scholar] [CrossRef]
- Neffe-Skocińska, K.; Sionek, B.; Ścibisz, I.; Kołożyn-Krajewska, D. Acid Contents and the Effect of Fermentation Condition of Kombucha Tea Beverages on Physicochemical, Microbiological and Sensory Properties. CYTA - J. Food 2017, 15, 601–607. [Google Scholar] [CrossRef]
- Khaleil, M.M. A BIOPROCESS DEVELOPMENT STUDY OF POLYPHENOL PROFILE, ANTIOXIDANT AND ANTIMICROBIAL ACTIVITIES OF KOMBUCHA ENRICHED WITH Psidium Guajava L. J. Microbiol. Biotechnol. Food Sci. 2020, 9, 1204–1210. [Google Scholar] [CrossRef]
- Mizzi, L.; Chatzitzika, C.; Gatt, R.; Valdramidis, V. HPLC Analysis of Phenolic Compounds and Flavonoids with Overlapping Peaks. Food Technol. Biotechnol. 2020, 58, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Zannini, E.; Lynch, K.M.; Nyhan, L.; Sahin, A.W.; O’ Riordan, P.; Luk, D.; Arendt, E.K. Influence of Substrate on the Fermentation Characteristics and Culture-Dependent Microbial Composition of Water Kefir. Fermentation 2022, 9, 28. [Google Scholar] [CrossRef]
- Sitanggang, A.B.; Wu, H.S.; Wang, S.S.; Lan, J.C.W. Fermentation Strategies: Nutritional Requirements. Ind. Ferment. Food Process. Nutr. Sources Prod. Strateg. 2010, 217–247. [Google Scholar]
- Ivanišová, E.; Meňhartová, K.; Terentjeva, M.; Godočíková, L.; Árvay, J.; Kačániová, M. Kombucha Tea Beverage: Microbiological Characteristic, Antioxidant Activity, and Phytochemical Composition. Acta Aliment. 2019, 48, 324–331. [Google Scholar] [CrossRef]
- Dolores, M.; Ana, P.; Bengoa, A.; Iraporda, C.; Medrano, M.; Garrote, G.L.; Abraham, A.G. Water Kefir : Factors Affecting Grain Growth and Promoting Properties of the Fermented Beverage. 2022, 162–180. [CrossRef]
- Subardjo, M.V.K. Black Tea Water Kefir Beverage, Massey University, Albany, New Zealand, 2017.
- Atalar, I.; Dervisoglu, M. Optimization of Spray Drying Process Parameters for Kefir Powder Using Response Surface Methodology. Lwt 2015, 60, 751–757. [Google Scholar] [CrossRef]
- Andreson, M.; Kazantseva, J.; Kuldjärv, R.; Malv, E.; Vaikma, H.; Kaleda, A.; Kütt, M.L.; Vilu, R. Characterisation of Chemical, Microbial and Sensory Profiles of Commercial Kombuchas. Int. J. Food Microbiol. 2022, 373, 109715. [Google Scholar] [CrossRef]
- Ferremi Leali, N.; Binati, R.L.; Martelli, F.; Gatto, V.; Luzzini, G.; Salini, A.; Slaghenaufi, D.; Fusco, S.; Ugliano, M.; Torriani, S.; et al. Reconstruction of Simplified Microbial Consortia to Modulate Sensory Quality of Kombucha Tea. Foods 2022, 11, 3045. [Google Scholar] [CrossRef]
- Uțoiu, E.; Matei, F.; Toma, A.; Diguță, C.F.; Ștefan, L.M.; Mănoiu, S.; Vrăjmașu, V.V.; Moraru, I.; Oancea, A.; Israel-Roming, F.; et al. Bee Collected Pollen with Enhanced Health Benefits, Produced by Fermentation with a Kombucha Consortium. Nutrients 2018, 10, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Pihurov, M.; Păcularu-Burada, B.; Cotârleț, M.; Bahrim, G.E. Tailoring the Optimized Fermentation Conditions of SCOBY-Based Membranes and Milk Kefir Grains to Promote Various Functional Properties. Foods 2022, 11. [Google Scholar] [CrossRef] [PubMed]
- Vázquez-Cabral, B.D.; Larrosa-Pérez, M.; Gallegos-Infante, J.A.; Moreno-Jiménez, M.R.; González-Laredo, R.F.; Rutiaga-Quiñones, J.G.; Gamboa-Gómez, C.I.; Rocha-Guzmán, N.E. Oak Kombucha Protects against Oxidative Stress and Inflammatory Processes. Chem. Biol. Interact. 2017, 272, 1–9. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).