Submitted:
14 June 2023
Posted:
16 June 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
- Anodic inhibitor.
- Cathodic inhibitor.
- Mixed inhibitor: acting on both reactions.
- Absorption inhibitor.
- Passivating inhibitor.
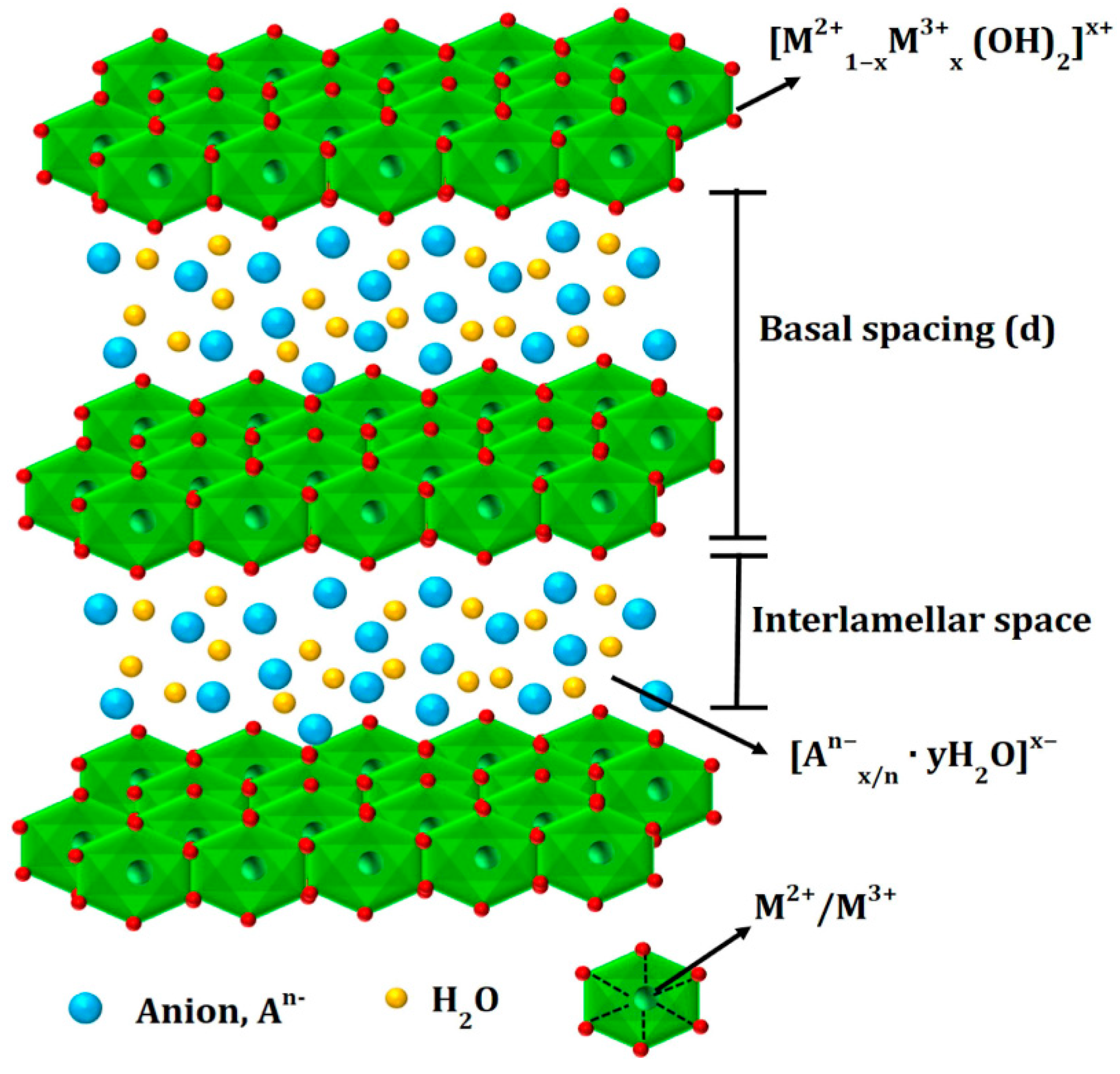
2. Materials and methods
2.1. Preparation of the LDHs
2.2. Steel Samples
2.3. Methods
2.4. Evaluation of inhibitory efficiency
2.4.1. Gravimetric method
2.4.2. Linear voltammetry
3. Results
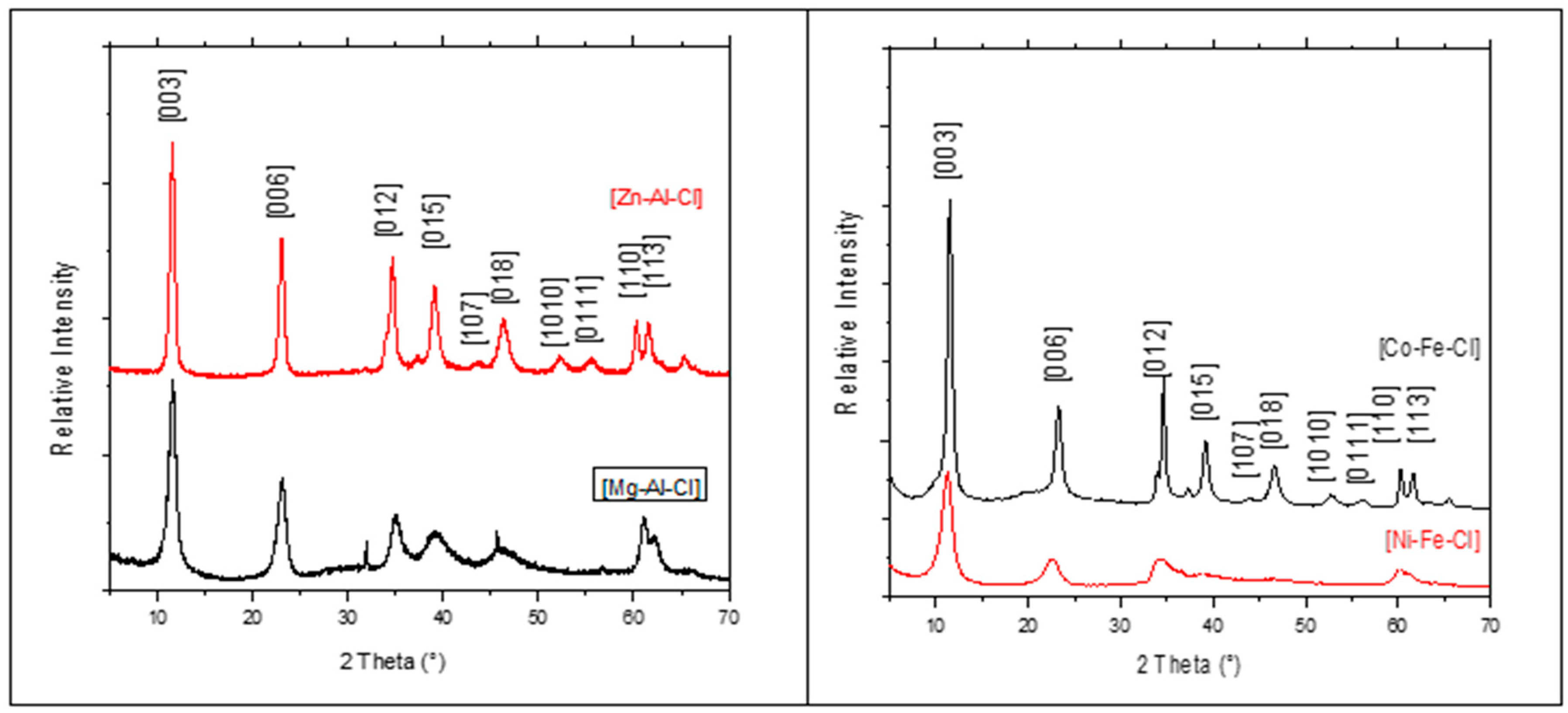
3.1. X-ray diffraction
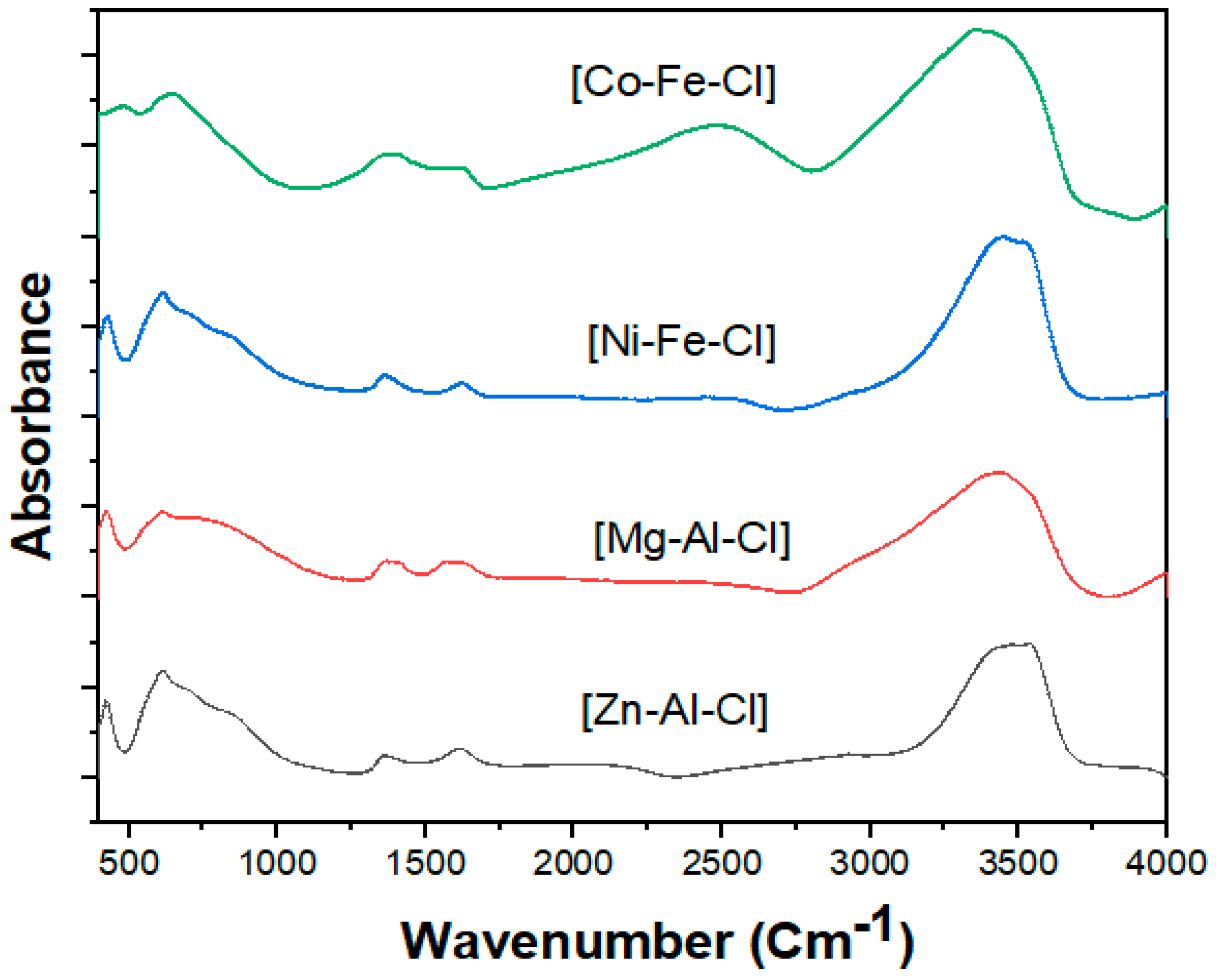
3.2. Infrared spectroscopy
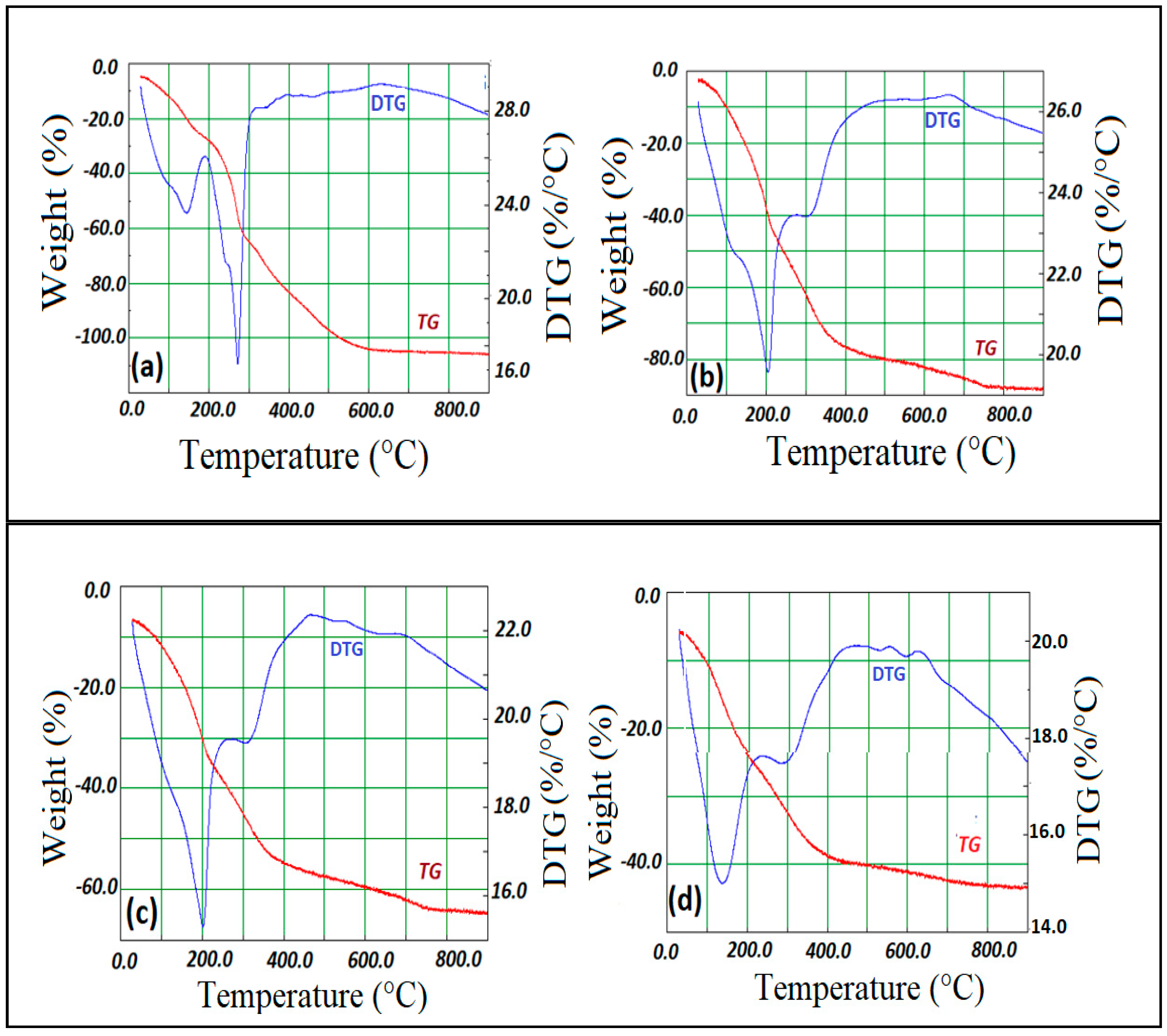
3.3. Thermal analyses
3.4. Evaluation of inhibitory efficiency
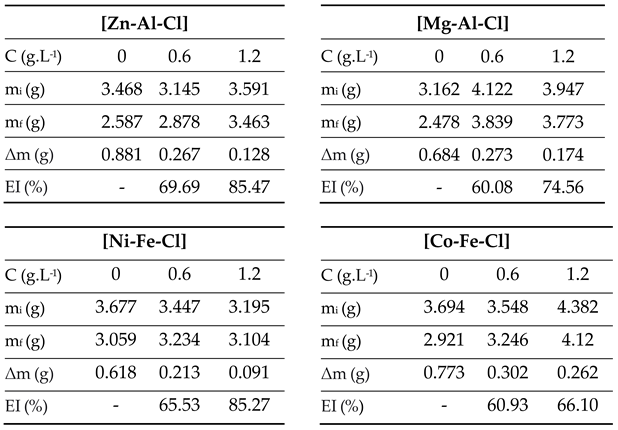
3.4.1. Gravimetry
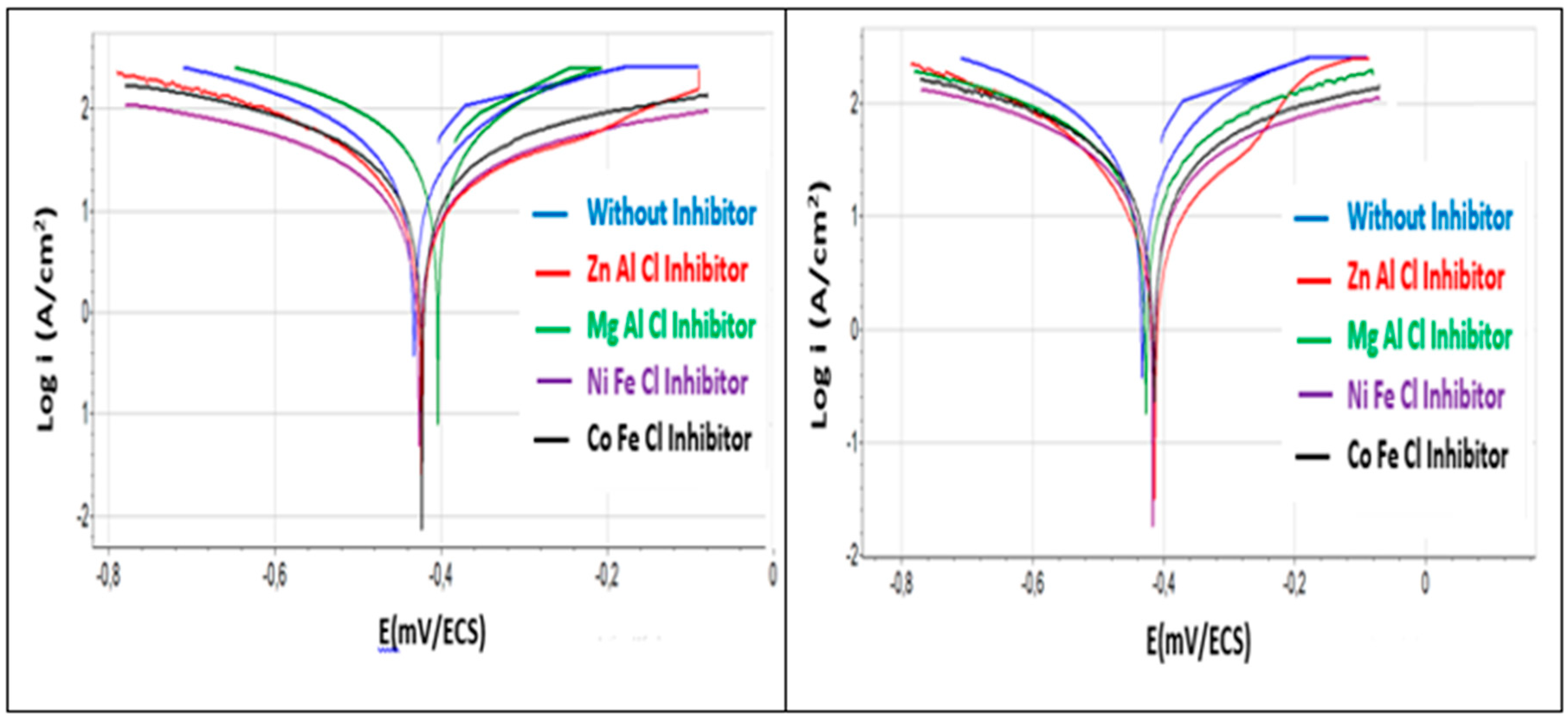
3.4.2. Voltammetry
3.5. Surface analysis by optical microscopy, SEM, and EDS
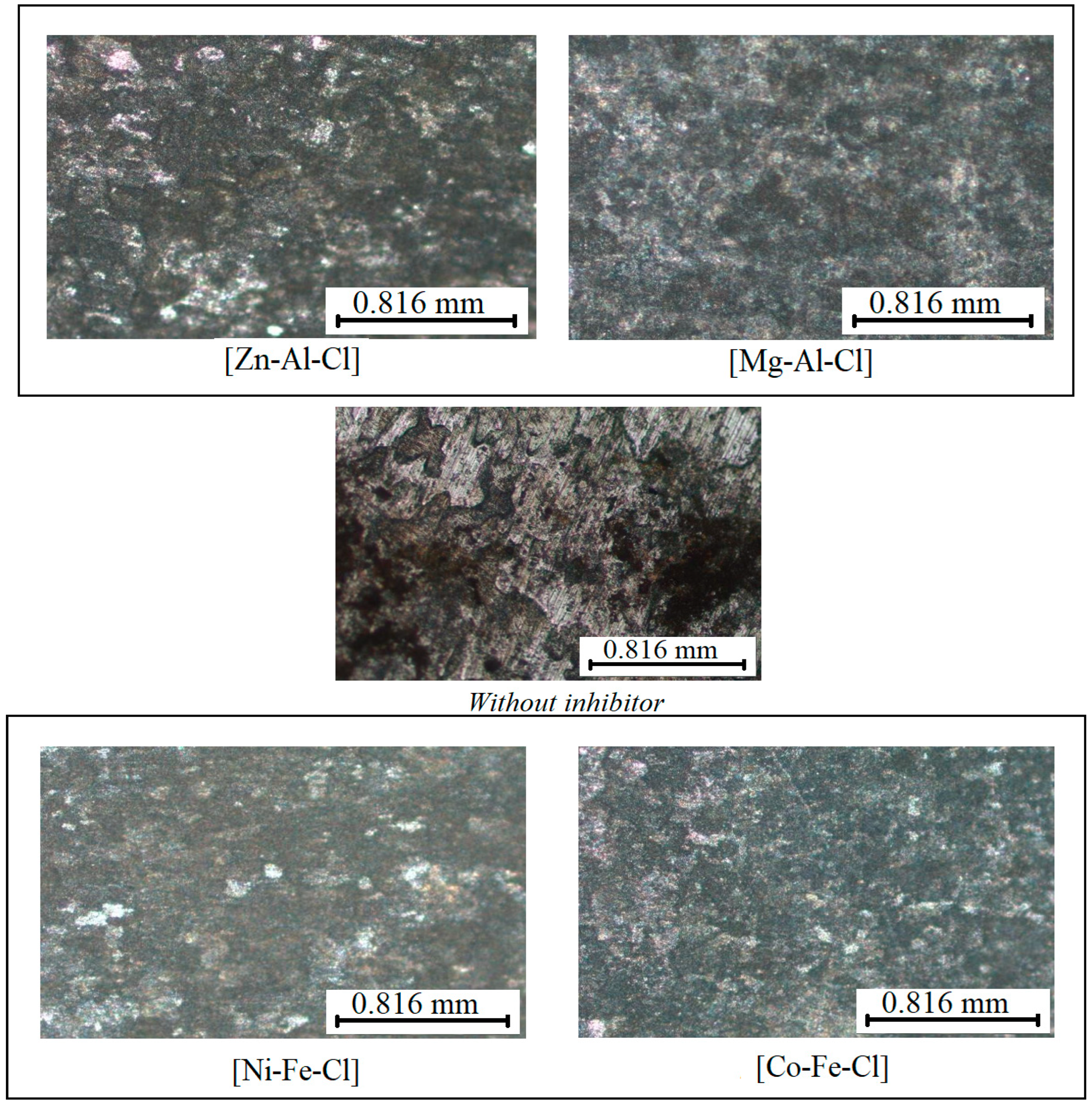
3.5.1. Optical microscopy
3.5.2. Scanning electron microscopy

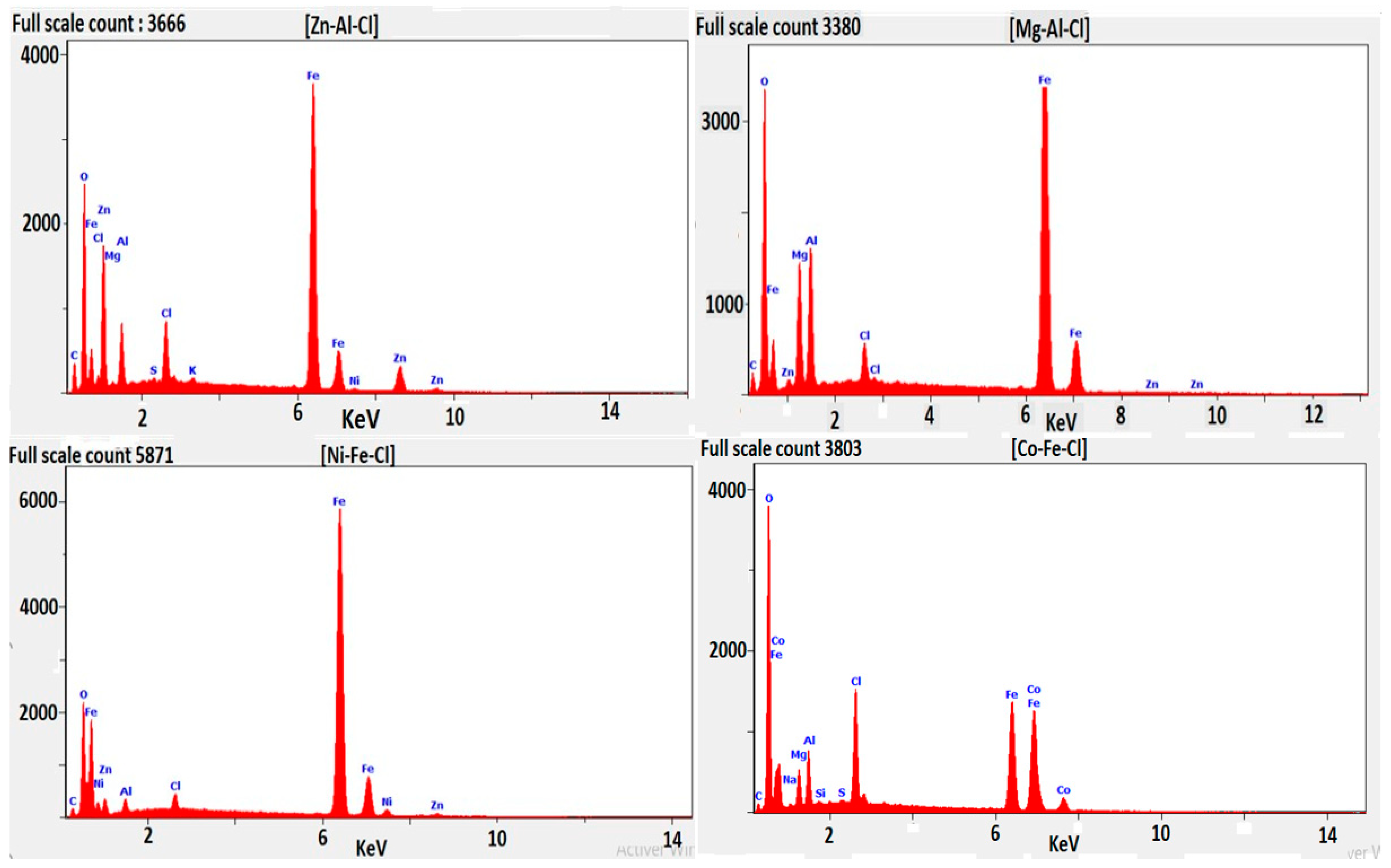
3.5.3. Energy dispersive spectroscopy analysis
4. Conclusions
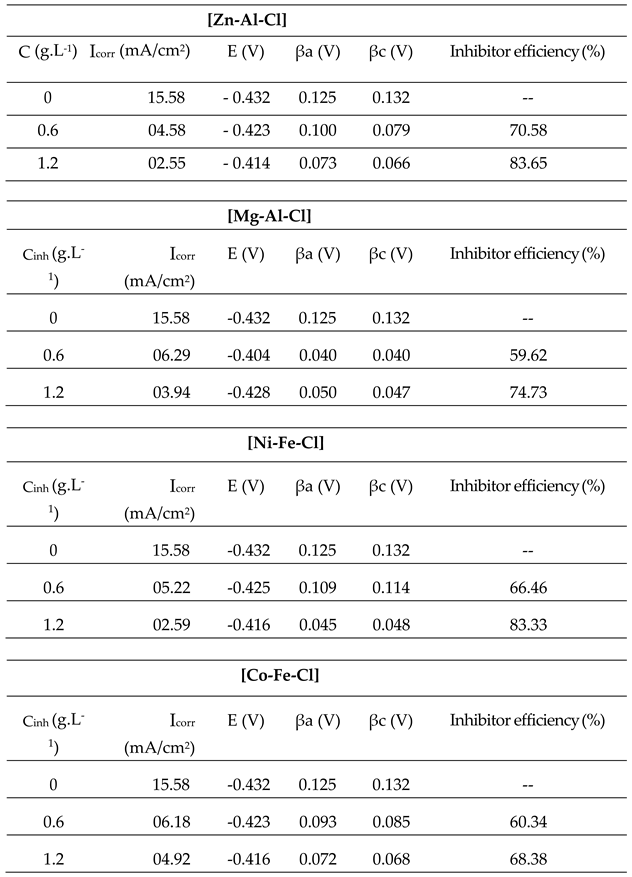
- The best effect is observed with the maximum LDH concentration of 1.2 g.L-1 using [Zn-Al-Cl] and [Ni-Fe-Cl] LDHs which inhibitory efficiency reached 83.65% and 83.33%, respectively.
- The displacement of the free potentials towards more anodic values characterizes the formation of a protective layer of the LDHs on the steel surface.
- Increasing the concentration of each of the four inhibitors decreases the corrosion current densities, thus the corrosion rates, especially in the cases of [Zn-Al-Cl] and [Ni-Fe-Cl].
- The surface morphology analyses of the steel after chemical pickling in HCl in the absence of inhibitor allowed to identify the type of corrosion (pitting corrosion) and the damaged state (cracks). These undesirable effects were absent in the presence of the inhibitors, indicating a good protection of the steel against corrosion.
- "The same solutions containing the corrosion inhibitor can be used multiple times for different metal plates."
References
- El Ashry, E.S.; El Nemr, A.; Essawy, S.; Ragab, S. Corrosion Inhibitors - Part II: Quantum Chemical Studies on the Corrosion Inhibitions of Steel in Acidic Medium by Some Triazole, Oxadiazole and Thiadiazole Derivatives. Electrochimica Acta 2006, 51, 3957–3968. https://doi.org/10.1016/j.electacta.2005.11.010. [CrossRef]
- Anionic Oxide-vanadium Schiff Base Amino Acid Complexes as Potent Inhibitors and as Effective Catalysts for Sulfides Oxidation: Experimental Studies Complemented with Quantum Chemical Calculations - ScienceDirect Available online: https://www.sciencedirect.com/science/article/pii/S0167732217345488?casa_token=IZVOSHcKv0gAAAAA:ZHzIeesCXVgE1V1nQuRldrLh7HtIj7QKNqlv1vEee2POeuuHRVlwE1-gl7x2JGMhPxZD8_zOyA (accessed on 8 June 2023). [CrossRef]
- Zhang, B.; He, C.; Chen, X.; Tian, Z.; Li, F. The Synergistic Effect of Polyamidoamine Dendrimers and Sodium Silicate on the Corrosion of Carbon Steel in Soft Water. Corrosion Science 2015, 90, 585–596. https://doi.org/10.1016/j.corsci.2014.10.054. [CrossRef]
- Synthesis of Polar Unique 3d Metal-Imine Complexes of Salicylidene Anthranilate Sodium Salt. Homogeneous Catalytic and Corrosion Inhibition Performance - ScienceDirect Available online: https://www.sciencedirect.com/science/article/pii/S1876107018302359?casa_token=sq1Q0ndJ3F0AAAAA:fSTEeWIJ4XWSqTGnblXCpQ_OMxQJFjLY2p54Zncv9P1CVFU7h8GwztpC-ifJbwo3JonWJBMuGA (accessed on 8 June 2023). [CrossRef]
- Experimental Investigation of Newly Synthesized Gemini Cationic Surfactants as Corrosion Inhibitors of Mild Steel in 1.0 M HCl | SpringerLink Available online: https://link.springer.com/article/10.1134/S2070205118010173 (accessed on 8 June 2023). [CrossRef]
- Egyptian Petroleum Research Institute (EPRI), Nasr City, Cairo, Egypt; A. Hegazy, M. Corrosion Inhibition Performance of a Novel Cationic Surfactant for Protection of Carbon Steel Pipeline in Acidic Media. Int. J. Electrochem. Sci. 2018, 6824–6842. https://doi.org/10.20964/2018.07.53. [CrossRef]
- Corrosion Inhibition Behavior of Indazole Derivative as a Green Corrosion Inhibitor for Mild Steel in Hydrochloric Acid: Electrochemical, Weight Loss and DFT Simulations Studies | Chahdi, H. Steli, C. Ad, Y. Ouzidan, E. M. Essassi, A. Chetouani, B. Hammouti | Moroccan Journal of Chemistry Available online: https://revues.imist.ma/index.php/morjchem/article/view/11041 (accessed on 8 June 2023). [CrossRef]
- Al-Taweel, S.; Al-Janabi, K.; Luaibi, H.; Al-Amiery, A.; Gaaz, T. Evaluation and Characterization of the Symbiotic Effect of Benzylidene Derivative with Titanium Dioxide Nanoparticles on the Inhibition of the Chemical Corrosion of Mild Steel. International Journal of Corrosion and Scale Inhibition 2019, 8, 1149–1169. https://doi.org/10.17675/2305-6894-2019-8-4-21. [CrossRef]
- Synergistic of a Coumarin Derivative with Potassium Iodide on the Corrosion Inhibition of Aluminum Alloy in 1.0 M H2SO4 | SpringerLink Available online: https://link.springer.com/article/10.1007/s12540-014-3008-3 (accessed on 8 June 2023). [CrossRef]
- Effect of 1,3,4-Thiadiazole Scaffold on the Corrosion Inhibition of Mild Steel in Acidic Medium: An Experimental and Computational Study | SpringerLink Available online: https://link.springer.com/article/10.1007/s40735-019-0243-7 (accessed on 8 June 2023). [CrossRef]
- Experimental and Computational Investigation on the Corrosion Inhibition Characteristics of Mild Steel by Some Novel Synthesized Imines in Hydrochloric Acid Solutions - ScienceDirect Available online: https://www.sciencedirect.com/science/article/pii/S0010938X14005617?casa_token=lOzkxqmREXwAAAAA:Bz75m6r6vSOszxkK5ye5C7t76gSTa8ojor-dMAccEHlMil2aW-N643Holk9lAwmwHf0mCZbzcA (accessed on 8 June 2023). [CrossRef]
- Muthukumar, N. Chapter 21 - Petroleum Products Transporting Pipeline Corrosion—A Review. In The Role of Colloidal Systems in Environmental Protection; Fanun, M., Ed.; Elsevier: Amsterdam, 2014; pp. 527–571 ISBN 978-0-444-63283-8.
- Bai, Y.; Bai, Q. Subsea Corrosion and Scale. Subsea Engineering Handbook, 2nd ed.; Gulf Professional Publishing: Oxford, UK 2019, 455–487.
- Arya, S.B.; Joseph, F.J. Chapter 3 - Electrochemical Methods in Tribocorrosion. In Tribocorrosion; Siddaiah, A., Ramachandran, R., Menezes, P.L., Eds.; Academic Press, 2021; pp. 43–77 ISBN 978-0-12-818916-0.
- Murariu, M.; Oancea, A.-V.; Ursu, C.; Rusu, B.G.; Cotofana, C.; Simionescu, B.; Olaru, M. Chapter 20 - Surface Properties of POSS Nanocomposites. In Polyhedral Oligomeric Silsesquioxane (POSS) Polymer Nanocomposites; Thomas, S., Somasekharan, L., Eds.; Elsevier, 2021; pp. 421–448 ISBN 978-0-12-821347-6.
- Norman, E.H. NACE Glossary of Corrosion Terms. Materials Protection 1965, 4, 79.
- Study by Acoustic Emission and Electrochemical Methods of the..|INIS Available online: https://inis.iaea.org/search/search.aspx?orig_q=RN:36034413 (accessed on 8 June 2023).
- Corrosion and Anticorrosion. Industrial Practice|INIS Available online: https://inis.iaea.org/search/search.aspx?orig_q=RN:33045680 (accessed on 8 June 2023).
- Development of a Predictive Model for Corrosion Inhibition of Carbon Steel by Imidazole and Benzimidazole Derivatives - ScienceDirect Available online: https://www.sciencedirect.com/science/article/pii/S0010938X16300798?casa_token=zEaB5l6WGxoAAAAA:QZodXnBZNaWr5OFrbcDdiLHWJVg2hrtDr1zs1TkppjfXzdc9MWcR-EOjw92iEK501_W6gw0vBw (accessed on 8 June 2023). [CrossRef]
- School of Chemistry and Chemical Engineering, Chongqing University, Chongqing 400044, P.R. China; Luo, L. Study on 1-Allyl-3-Butylimidazalium Bromine as Corrosion Inhibitor for X65 Steel in 0.5 M H2SO4 Solution. Int. J. Electrochem. Sci. 2016, 8177–8192. https://doi.org/10.20964/2016.10.52. [CrossRef]
- Hamner, N.E. Scope and Importance of Inhibitor Technology. Corrosion Inhibitors, Houston, NACE 1994.
- COROZIUNEA MATERIALELOR METALICE - S.ZAMFIR, R. VIDU SI V. BRINZOI Available online: https://www.galeriile-cismigiu.ro/coroziunea-materialelor-metalice-szamfir-r-vidu-si-v-brinzoi-p26618 (accessed on 8 June 2023).
- Molybdate and Tungstate as Corrosion Inhibitors for Cold Rolling Steel in Hydrochloric Acid Solution - ScienceDirect Available online: https://www.sciencedirect.com/science/article/pii/S0010938X05000521?casa_token=ScW1ATJ62SIAAAAA:-A-4H9dTGAEaeWkT_HkF49IZlgOiG_EpPlqkGvm1_Z7RtAN3cJRpvioF6gmB8sDxXXaaFkNlxw (accessed on 8 June 2023). [CrossRef]
- Effects of Chromate and Chromate Conversion Coatings on Corrosion of Aluminum Alloy 2024-T3 - ScienceDirect Available online: https://www.sciencedirect.com/science/article/abs/pii/S0257897201010039 (accessed on 8 June 2023). [CrossRef]
- Bastos, A.C.; Zheludkevich, M.L.; Ferreira, M.G.S. A SVET Investigation on the Modification of Zinc Dust Reactivity. Progress in Organic Coatings 2008, 63, 282–290. https://doi.org/10.1016/j.porgcoat.2008.01.013. [CrossRef]
- Effect of Various Cations on the Formation of Calcium Carbonate and Barium Sulfate Scale with and without Scale Inhibitors | Industrial & Engineering Chemistry Research Available online: https://pubs.acs.org/doi/abs/10.1021/ie2003494 (accessed on 8 June 2023). [CrossRef]
- Corrosion Inhibition of AA2024-T3 By Sodium Silicate | Request PDF Available online: https://www.researchgate.net/publication/261139143_Corrosion_Inhibition_of_AA2024-T3_By_Sodium_Silicate (accessed on 8 June 2023). [CrossRef]
- Yohai, L.; Vázquez, M.; Valcarce, M.B. Phosphate Ions as Corrosion Inhibitors for Reinforcement Steel in Chloride-Rich Environments. Electrochimica Acta 2013, 102, 88–96. https://doi.org/10.1016/j.electacta.2013.03.180. [CrossRef]
- Lafont, M.C.; Pebere, N.; Moran, F.; Bleriot, P. Inhibition de La Corrosion d’un Acier Au Carbone Par Des Produits Dérivés de Phosphonates En Association Avec Des Sels de Zinc. rseau 2005, 6, 97–112. https://doi.org/10.7202/705168ar. [CrossRef]
- Bensaada, S.; Bouziane, M.T.; Mohammedi, F.; Zergui, B.; Bouras, A. EFFET DES INHIBITEURS DE CORROSION ZnCl2, Na2MoO4 ET ZnCl2 +Na2MoO4 SUR LE COMPORTEMENT DE L’ACIER POUR ARMATURE A BETON EN MILIEU OXYDANT NaCl. LARHYSS Journal P-ISSN 1112-3680 / E-ISSN 2521-9782 2013.
- Quantum Chemical Studies of Some Rhodanine Azosulpha Drugs as Corrosion Inhibitors for Mild Steel in Acidic Medium - Ebenso - 2010 - International Journal of Quantum Chemistry - Wiley Online Library Available online: https://onlinelibrary.wiley.com/doi/abs/10.1002/qua.22249 (accessed on 8 June 2023). [CrossRef]
- Pillaring of Layered Double Hydroxides (LDH’s) by Polyoxometalate Anions | Journal of the American Chemical Society Available online: https://pubs.acs.org/doi/pdf/10.1021/ja00219a048 (accessed on 8 June 2023). [CrossRef]
- Cavani, F.; Trifirò, F.; Vaccari, A. Hydrotalcite-Type Anionic Clays: Preparation, Properties and Applications. Catalysis Today 1991, 11, 173–301. https://doi.org/10.1016/0920-5861(91)80068-K. [CrossRef]
- Playle, A.C.; Gunning, S.R.; Llewellyn, A.F. The in Vitro Antacid and Anti-Pepsin Activity of Hydrotalcite. Pharm Acta Helv 1974, 49, 298–302.
- Mishra, G.; Dash, B.; Pandey, S. Layered Double Hydroxides: A Brief Review from Fundamentals to Application as Evolving Biomaterials. Applied Clay Science 2018, 153, 172–186. https://doi.org/10.1016/j.clay.2017.12.021. [CrossRef]
- Mousty, C.; Therias, S.; Forano, C.; Besse, J.-P. Anion-Exchanging Clay-Modified Electrodes: Synthetic Layered Double Hydroxides Intercalated with Electroactive Organic Anions. Journal of Electroanalytical Chemistry 1994, 374, 63–69. https://doi.org/10.1016/0022-0728(94)03346-3. [CrossRef]
- Badreddine, M.; Khaldi, M.; Legrouri, A.; Barroug, A.; Chaouch, M.; De Roy, A.; Besse, J.P. Chloride-Hydrogenophosphate Ion Exchange into the Zinc–Aluminium–Chloride Layered Double Hydroxide. Materials Chemistry and Physics 1998, 52, 235–239. https://doi.org/10.1016/S0254-0584(97)02050-6. [CrossRef]
- Ookubo, A.; Ooi, K.; Hayashi, H. Hydrotalcites as Potential Adsorbents of Intestinal Phosphate. J Pharm Sci 1992, 81, 1139–1140. https://doi.org/10.1002/jps.2600811120. [CrossRef]
- Buchheit, R.G.; Guan, H.; Mahajanam, S.; Wong, F. Active Corrosion Protection and Corrosion Sensing in Chromate-Free Organic Coatings. Progress in Organic Coatings 2003, 47, 174–182. https://doi.org/10.1016/j.porgcoat.2003.08.003. [CrossRef]
- Characterization of Inhibitor Release from Zn-Al-[V10O28]6− Hydrotalcite Pigments and Corrosion Protection from Hydrotalcite-Pigmented Epoxy Coatings | CORROSION Available online: https://meridian.allenpress.com/corrosion/article-abstract/64/3/230/162739/Characterization-of-Inhibitor-Release-from-Zn-Al (accessed on 8 June 2023). [CrossRef]
- Formation of Mg,Al-Hydrotalcite Conversion Coating on Mg Alloy in Aqueous HCO3−/CO32− and Corresponding Protection against Corrosion by the Coating - ScienceDirect Available online: https://www.sciencedirect.com/science/article/pii/S0010938X09000535?casa_token=nrOuPpnd3N0AAAAA:OpkLPTiwcx7FLFfMci1AhGfkjEp7dlIEM_ey2WTox-nBpSm0KsmMRMYeNsxEl0aSzjnBZUPH79M (accessed on 9 June 2023). [CrossRef]
- Jing, C.; Dong, B.; Raza, A.; Zhang, T.; Zhang, Y. Corrosion Inhibition of Layered Double Hydroxides for Metal-Based Systems. Nano Materials Science 2021, 3, 47–67. https://doi.org/10.1016/j.nanoms.2020.12.001. [CrossRef]
- Gastuche, M.C.; Brown, G.; Mortland, M.M. Mixed Magnesium-Aluminiun Hydroxides. I. Preparation and Characterization of Compounds Formed in Dialysed Systems. Clay Minerals 1967, 7, 177–192. https://doi.org/10.1180/claymin.1967.007.2.05. [CrossRef]
- Miyata, S. The Syntheses of Hydrotalcite-Like Compounds and Their Structures and Physico-Chemical Properties—I: The Systems Mg2+-Al3+-NO3−, Mg2+-Al3+-Cl−, Mg2+-Al3+-ClO4−, Ni2+-Al3+-Cl− and Zn2+-Al3+-Cl−. Clays Clay Miner. 1975, 23, 369–375. https://doi.org/10.1346/CCMN.1975.0230508. [CrossRef]
- Legrouri, A.; Badreddine, M.; Barroug, A. Influence of PH on the Synthesis of the Zn–Al–Nitrate Layered Double Hydroxide and the Exchange of Nitrate by Phosphate Ions.
- Khaldi, M.; Badreddine, M.; Legrouri, A.; Chaouch, M.; Barroug, A.; De Roy, A.; Besse, J.P. Preparation of a Well-Ordered Layered Nanocomposite from Zinc–Aluminum–Chloride Layered Double Hydroxide and Hydrogenophosphate by Ion Exchange. Materials Research Bulletin 1998, 33, 1835–1843. https://doi.org/10.1016/S0025-5408(98)00185-8. [CrossRef]
- Synthesis and Characterization of Intercalated Layered Double Metal Hydroxides With Interlayer Aromatic Molecular Anions: Intercalation of 9,10-Anthraquinonedisulfonates Between Layers of Mg and Al Layered Double Hydroxides(MgAl-LDH): Molecular Crystals and Liquid Crystals Science and Technology. Section A. Molecular Crystals and Liquid Crystals: Vol 286, No 1 Available online: https://www.tandfonline.com/doi/abs/10.1080/10587259608042280 (accessed on 8 June 2023). [CrossRef]
- Badreddine, M.; Legrouri, A.; Barroug, A.; De Roy, A.; Besse, J.-P. Influence of PH on Phosphate Intercalation in Zinc-Aluminum Layered Double Hydroxide. Collect. Czech. Chem. Commun. 1998, 63, 741–748. https://doi.org/10.1135/cccc19980741. [CrossRef]
- Newman, S.P.; Jones, W. Synthesis, Characterization and Applications of Layered Double Hydroxides Containing Organic Guests. New J. Chem. 1998, 22, 105–115. https://doi.org/10.1039/A708319J. [CrossRef]
- Bonnet, S. Synthese et Caracterisation de Materiaux Hybrides de Type Hdl-Porphyrine. These de doctorat, Clermont-Ferrand 2, 1997.
- Microwave-Assisted Reconstruction of Ni,Al Hydrotalcite-like Compounds - ScienceDirect Available online: https://www.sciencedirect.com/science/article/pii/S0022459608000856?casa_token=flo2t0p-gtgAAAAA:sNmk4tqI-YywIbFUK-ozWRHod497kkZHsxJk27YYvjSf6Oydh9nY5PCDUj2Ve1qCDNscxsSqrWI (accessed on 8 June 2023). [CrossRef]
- Gimbert, F.; Morin-Crini, N.; Renault, F.; Badot, P.-M.; Crini, G. Adsorption Isotherm Models for Dye Removal by Cationized Starch-Based Material in a Single Component System: Error Analysis. Journal of Hazardous Materials 2008, 157, 34–46. https://doi.org/10.1016/j.jhazmat.2007.12.072. [CrossRef]
- del Arco, M.; Malet, P.; Trujillano, R.; Rives, V. Synthesis and Characterization of Hydrotalcites Containing Ni(II) and Fe(III) and Their Calcination Products. Chem. Mater. 1999, 11, 624–633. https://doi.org/10.1021/cm9804923. [CrossRef]
- Stählin, W.; Oswald, H.R. The Infrared Spectrum and Thermal Analysis of Zinc Hydroxide Nitrate. Journal of Solid State Chemistry 1971, 3, 252–255. https://doi.org/10.1016/0022-4596(71)90037-5. [CrossRef]
- Morpurgo, S.; Lo Jacono, M.; Porta, P. Copper—Zinc—Cobalt—Chromium Hydroxycarbonates and Oxides. Journal of Solid State Chemistry 1995, 119, 246–253. https://doi.org/10.1016/0022-4596(95)80038-Q. [CrossRef]
- Khan, A.I.; Williams, G.R.; Hu, G.; Rees, N.H.; O’Hare, D. The Intercalation of Bicyclic and Tricyclic Carboxylates into Layered Double Hydroxides. Journal of Solid State Chemistry 2010, 183, 2877–2885. https://doi.org/10.1016/j.jssc.2010.09.036. [CrossRef]
- PAHO-Water Treatment: Principles and Design; Water Treatment: Principles and Design Available online: https://bases.bireme.br/cgi-bin/wxislind.exe/iah/online/?IsisScript=iah/iah.xis&src=google&base=PAHO&lang=p&nextAction=lnk&exprSearch=577&indexSearch=ID (accessed on 8 June 2023).
- Suzuki, M. Adsorption Engineering; Chemical engineering monographs; Kodansha ; Elsevier: Tokyo : Amsterdam ; New York, 1990; ISBN 978-0-444-98802-7.
| Element | Fe | Cu | C | Mn | Si | P | S | Ni | Cr | V |
| Weight percent (%) | 99.41 | 0.2 | 0.04 | 0.18 | 0.025 | 0.02 | 0.015 | 0.03 | 0.03 | 0.05 |
| Matrix | a (nm) | c (nm) |
|---|---|---|
| [Zn-Al-Cl] | 0.307 | 2.321 |
| [Mg-Al-Cl] | 0.305 | 2.313 |
| [Ni-Fe-Cl] | 0.318 | 2.349 |
| [Co-Fe-Cl] | 0.309 | 2.301 |
| Temperature (°C) |
Mass loss (%) |
DTG signal (°C) |
Allocation | |
|---|---|---|---|---|
| 25-200 | 21.0 | 140 | Loss of physisorbed and interlayer water | |
| 200-280 | 33.1 | 270 | Dehydroxylation of LDH layers | |
| [Zn-Al-Cl] | 280-570 | 40.2 | 390 | Loss of chloride ions in form of HCl gas |
| > 570 | 630 | Formation of ZnO and ZnAl2O4 | ||
| 25-210 | 40.1 | 130 | Loss of physisorbed and interlayer water | |
| 210-380 | 25.1 | 205 | Dehydroxylation of LDH layers | |
| [Mg-Al-Cl] | 380-670 | 10.2 | 310 | Loss of chloride ions in form of HCl gas |
| > 670 | 670 | Formation of MgO and MgAl2O4 | ||
| 25-210 | 30.1 | 130 | Loss of physisorbed and interlayer water | |
| [Ni-Fe-Cl] | 210-350 | 20.1 | 200 | Dehydroxylation of LDH layers |
| 350-710 | 10.3 | 310 | Loss of chloride ions in form of HCl gas | |
| > 710 | 470-690 | Formation of NiO and NiFe2O4 | ||
| 25-180 | 19.2 | 140 | Loss of physisorbed and interlayer water | |
| [Co-Fe-Cl] | 180-410 | 36.1 | 285 | Dehydroxylation of LDH layers |
| 410-720 | 9.1 | 460 | Loss of chloride ions in form of HCl gas | |
| > 570 | 550-630 | Formation of CoO and CoFe2O4 |
 |
 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





