Submitted:
16 June 2023
Posted:
16 June 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
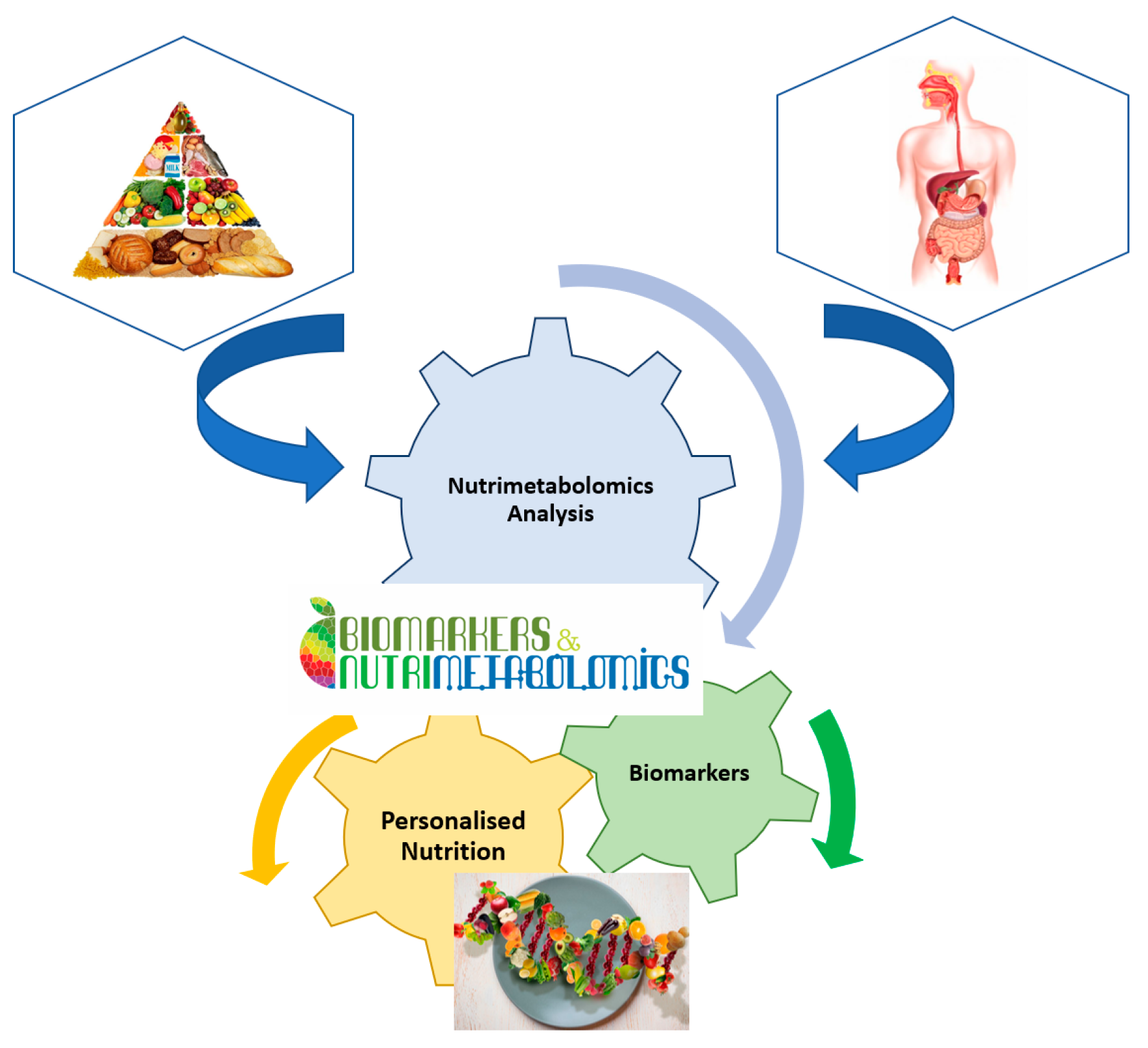
2. Metabolomics and Nutrimetabolomics2
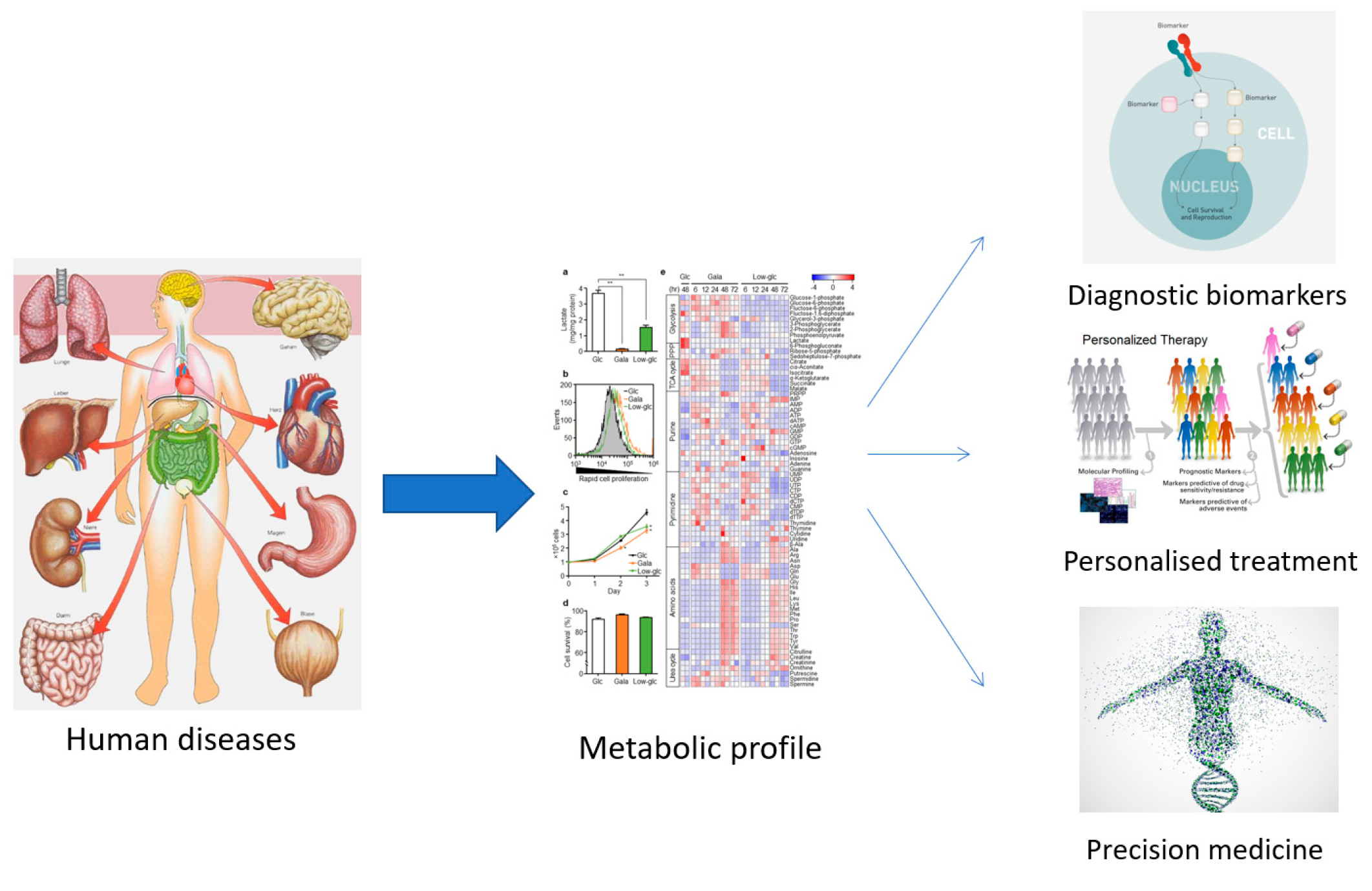
3. (Nutri-)Metabolomics in Human Medicine
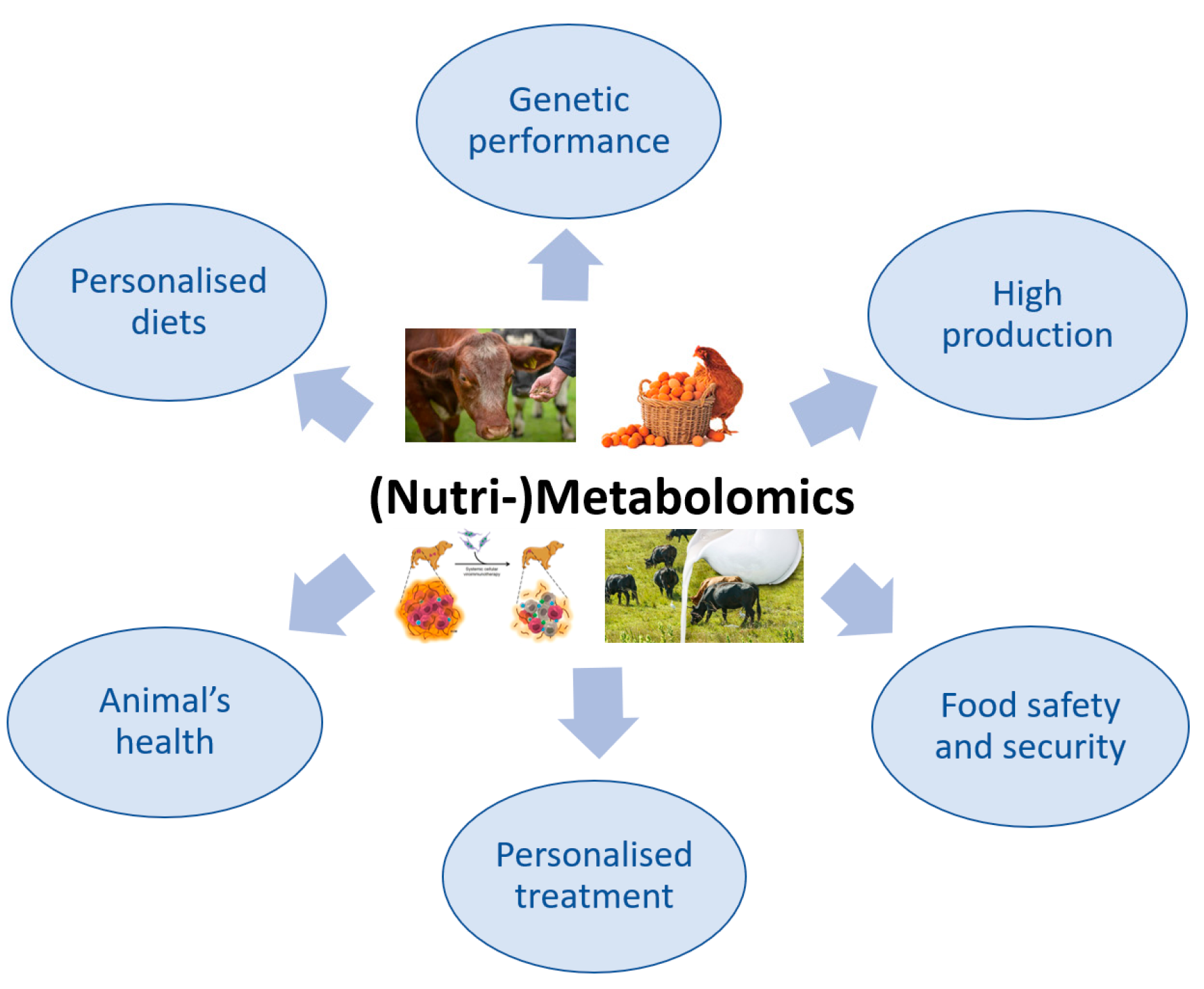
4. (Nutri-)Metabolomics in Veterinary Medicine
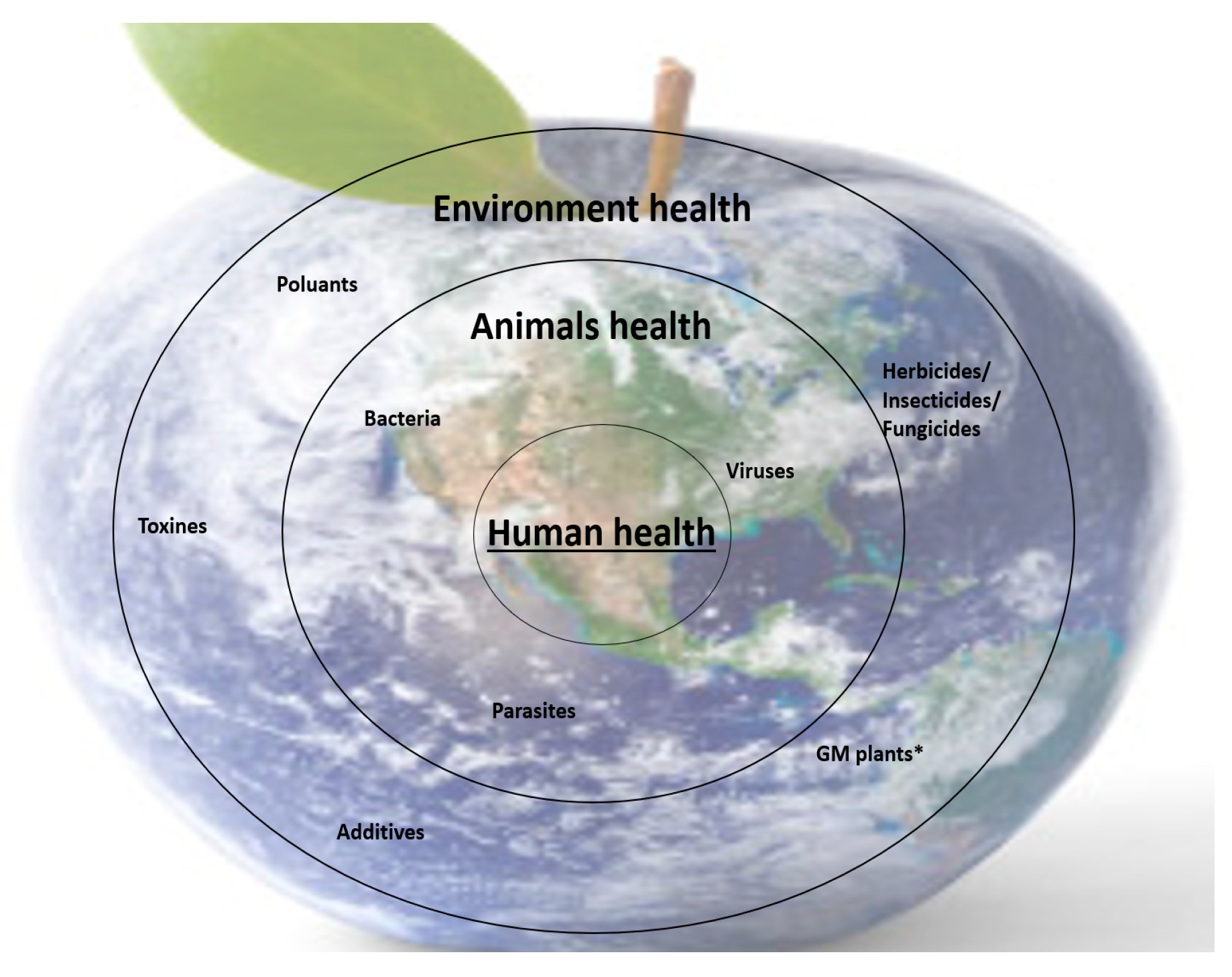
5. (Nutri-)Metabolomics in the Context of One Medicine
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Jones MD, Rainville PD, Isaac G, Wilson ID, Smith NW, Plumb RS. Ultra-high resolution SFC-MS as a high throughput platform for metabolic phenotyping: application to metabolic profiling of rat and dog bile. J Chromatogr B Analyt Technol Biomed Life Sci. 2014 Sep 1; 966:200-7. Epub 2014 Apr 18. [CrossRef] [PubMed]
- Kirwan J. Metabolomics for the practising vet. Diagnostics. 2013, 35(September), 438–445. [CrossRef]
- Carlos G, Dos Santos FP, Fröehlich PE. Canine metabolomics advances. Metabolomics. 2020 Jan 18;16(2):16. [CrossRef] [PubMed]
- Kuehnbaum NL, Britz-McKibbin P. New advances in separation science for metabolomics: resolving chemical diversity in a post-genomic era. Chem Rev. 2013 Apr 10;113(4):2437-68. Epub 2013 Mar 18. [CrossRef] [PubMed]
- Rochfort S. Metabolomics reviewed: a new "omics" platform technology for systems biology and implications for natural products research. J Nat Prod. 2005 Dec;68(12):1813-20. [CrossRef] [PubMed]
- Forster G, Heuberger A, Broeckling C, Bauer J, Ryan E. Consumption of cooked navy bean powders modulate the canine fecal and urine metabolome. Current Metabolomics. 2015. 3(2), 90–101. [CrossRef]
- O'Gorman A, Brennan L. Metabolomic applications in nutritional research: a perspective. J Sci Food Agric. 2015 Oct;95(13):2567-70. Epub 2015 Feb 2. [CrossRef] [PubMed]
- Gibney MJ, Walsh M, Brennan L, Roche HM, German B, van Ommen B. Metabolomics in human nutrition: opportunities and challenges. Am J Clin Nutr. 2005 Sep;82(3):497-503. [CrossRef] [PubMed]
- Nyatanyi T, Wilkes M, McDermott H, Nzietchueng S, Gafarasi I, Mudakikwa A, Kinani JF, Rukelibuga J, Omolo J, Mupfasoni D, Kabeja A, Nyamusore J, Nziza J, Hakizimana JL, Kamugisha J, Nkunda R, Kibuuka R, Rugigana E, Farmer P, Cotton P, Binagwaho A. Implementing One Health as an integrated approach to health in Rwanda. BMJ Glob Health. 2017 Feb 21;2(1): e000121. [CrossRef] [PubMed] [PubMed Central]
- Association AVM. One Health: a new professional imperative, One Health Initiative Task Force: final report. Schaumburg, IL, USA: American Veterinary Medical Association, 2008. http://www avma org/onehealth/default asp.
- Gibbs EP. Emerging zoonotic epidemics in the interconnected global community. Vet Rec. 2005 Nov 26;157(22):673-9. [CrossRef] [PubMed]
- Mwacalimba KK, Green J. 'One health' and development priorities in resource-constrained countries: policy lessons from avian and pandemic influenza preparedness in Zambia. Health Policy Plan. 2015 Mar;30(2):215-22. Epub 2014 Feb 14. [CrossRef] [PubMed]
- Baker M. Metabolomics: from small molecules to big ideas. Nature Methods 2011, 8, 117–121. [CrossRef]
- Gika HG, Theodoridis GA, Plumb RS, Wilson ID. Current practice of liquid chromatography-mass spectrometry in metabolomics and metabonomics. J Pharm Biomed Anal. 2014 Jan; 87:12-25. Epub 2013 Jul 17. [CrossRef] [PubMed]
- Sebedio JL, Brennan L. Metabolomics as a Tool in Nutrition Research. Elsevier Ltd., UK, 2015; 269 pgs.
- Astarita G, Langridge J. An emerging role for metabolomics in nutrition science. J Nutrigenet Nutrigenomics. 2013;6(4-5):181-200. Epub 2013 Aug 31. [CrossRef] [PubMed]
- Patti GJ, Yanes O, Siuzdak G. Innovation: Metabolomics: the apogee of the omics trilogy. Nat Rev Mol Cell Biol. 2012 Mar 22;13(4):263-9. [CrossRef] [PubMed] [PubMed Central]
- Roux A, Lison D, Junot C, Heilier JF. Applications of liquid chromatography coupled to mass spectrometry-based metabolomics in clinical chemistry and toxicology: A review. Clin Biochem. 2011 Jan;44(1):119-35. Epub 2010 Aug 26. [CrossRef] [PubMed]
- Afman L, Müller M. Nutrigenomics: from molecular nutrition to prevention of disease. J Am Diet Assoc. 2006 Apr;106(4):569-76. [CrossRef] [PubMed]
- Trujillo E, Davis C, Milner J. Nutrigenomics, proteomics, metabolomics, and the practice of dietetics. J Am Diet Assoc. 2006 Mar;106(3):403-13. [CrossRef] [PubMed]
- Merched AJ, Chan L. Nutrigenetics and nutrigenomics of atherosclerosis. Curr Atheroscler Rep. 2013 Jun;15(6):328. [CrossRef] [PubMed] [PubMed Central]
- Ganesh V, Hettiarachchy NS. Nutriproteomics: a promising tool to link diet and diseases in nutritional research. Biochim Biophys Acta. 2012 Oct;1824(10):1107-17. Epub 2012 Jun 23. [CrossRef] [PubMed]
- Micha R, Michas G, Lajous M, Mozaffarian D. Processing of meats and cardiovascular risk: time to focus on preservatives. BMC Med. 2013 May 23; 11:136. [CrossRef] [PubMed] [PubMed Central]
- Simopoulos AP. Dietary omega-3 fatty acid deficiency and high fructose intake in the development of metabolic syndrome, brain metabolic abnormalities, and non-alcoholic fatty liver disease. Nutrients. 2013 Jul 26;5(8):2901-23. [CrossRef] [PubMed] [PubMed Central]
- LeMieux M, Al-Jawadi A, Wang S, Moustaid-Moussa N. Metabolic profiling in nutrition and metabolic disorders. Adv Nutr. 2013 Sep 1;4(5):548-50. [CrossRef] [PubMed] [PubMed Central]
- O'Gorman A, Brennan L. The role of metabolomics in determination of new dietary biomarkers. Proc Nutr Soc. 2017 Aug;76(3):295-302. Epub 2017 Jan 16. [CrossRef] [PubMed]
- Cade JE. Measuring diet in the 21st century: use of new technologies. Proc Nutr Soc. 2017 Aug;76(3):276-282. Epub 2016 Dec 15. [CrossRef] [PubMed]
- Stella C, Beckwith-Hall B, Cloarec O, Holmes E, Lindon JC, Powell J, van der Ouderaa F, Bingham S, Cross AJ, Nicholson JK. Susceptibility of human metabolic phenotypes to dietary modulation. J Proteome Res. 2006 Oct;5(10):2780-8. [CrossRef] [PubMed]
- Vasil'ev AV, Sharanova NÉ, Kulakova SN. [Nutrimetabolomics--the new stage of biochemistry of nutrition. The role of nutrilipidomic analysis]. Vopr Pitan. 2014;83(1):4-11. Russian. [PubMed]
- Odriozola L, Corrales FJ. Discovery of nutritional biomarkers: future directions based on omics technologies. Int J Food Sci Nutr. 2015 Jul;66 Suppl 1:S31-40. [CrossRef] [PubMed]
- Regan JA, Shah SH. Obesity Genomics and Metabolomics: a Nexus of Cardiometabolic Risk. Curr Cardiol Rep. 2020 Oct 10;22(12):174. [CrossRef] [PubMed]
- Wu Y, Perng W, Peterson KE. Precision Nutrition and Childhood Obesity: A Scoping Review. Metabolites. 2020 Jun 8;10(6):235. [CrossRef] [PubMed] [PubMed Central]
- Arneth B, Arneth R, Shams M. Metabolomics of Type 1 and Type 2 Diabetes. Int J Mol Sci. 2019 May 18;20(10):2467. [CrossRef] [PubMed] [PubMed Central]
- Wang N, Zhu F, Chen L, Chen K. Proteomics, metabolomics and metagenomics for type 2 diabetes and its complications. Life Sci. 2018 Nov 1; 212:194-202. Epub 2018 Sep 19. [CrossRef] [PubMed]
- Li XS, Wang Z, Cajka T, Buffa JA, Nemet I, Hurd AG, Gu X, Skye SM, Roberts AB, Wu Y, Li L, Shahen CJ, Wagner MA, Hartiala JA, Kerby RL, Romano KA, Han Y, Obeid S, Lüscher TF, Allayee H, Rey FE, DiDonato JA, Fiehn O, Tang WHW, Hazen SL. Untargeted metabolomics identifies trimethyllysine, a TMAO-producing nutrient precursor, as a predictor of incident cardiovascular disease risk. JCI Insight. 2018 Mar 22; 3(6):e99096. [CrossRef] [PubMed] [PubMed Central]
- Onuh JO, Aliani M. Metabolomics profiling in hypertension and blood pressure regulation: a review. Clin Hypertens. 2020 Nov 15;26(1):23. [CrossRef] [PubMed] [PubMed Central]
- Kumar A, Misra BB. Challenges and Opportunities in Cancer Metabolomics. Proteomics. 2019 Nov; 19(21-22):e1900042. Epub 2019 Jun 11. [CrossRef] [PubMed]
- Yang QJ, Zhao JR, Hao J, Li B, Huo Y, Han YL, Wan LL, Li J, Huang J, Lu J, Yang GJ, Guo C. Serum and urine metabolomics study reveals a distinct diagnostic model for cancer cachexia. J Cachexia Sarcopenia Muscle. 2018 Feb;9(1):71-85. Epub 2017 Nov 19. [CrossRef] [PubMed] [PubMed Central]
- Perez-Cornago A, Brennan L, Ibero-Baraibar I, Hermsdorff HH, O'Gorman A, Zulet MA, Martínez JA. Metabolomics identifies changes in fatty acid and amino acid profiles in serum of overweight older adults following a weight loss intervention. J Physiol Biochem. 2014 Jun;70(2):593-602. Epub 2014 Jan 9. [CrossRef] [PubMed]
- Menni C, Fauman E, Erte I, Perry JR, Kastenmüller G, Shin SY, Petersen AK, Hyde C, Psatha M, Ward KJ, Yuan W, Milburn M, Palmer CN, Frayling TM, Trimmer J, Bell JT, Gieger C, Mohney RP, Brosnan MJ, Suhre K, Soranzo N, Spector TD. Biomarkers for type 2 diabetes and impaired fasting glucose using a nontargeted metabolomics approach. Diabetes. 2013 Dec;62(12):4270-6. Epub 2013 Jul 24. [CrossRef] [PubMed] [PubMed Central]
- Barber MN, Risis S, Yang C, Meikle PJ, Staples M, Febbraio MA, Bruce CR. Plasma lysophosphatidylcholine levels are reduced in obesity and type 2 diabetes. PLoS One. 2012;7(7):e41456. Epub 2012 Jul 25. [CrossRef] [PubMed] [PubMed Central]
- Adams SH. Emerging perspectives on essential amino acid metabolism in obesity and the insulin-resistant state. Adv Nutr. 2011 Nov;2(6):445-56. Epub 2011 Nov 3. [CrossRef] [PubMed] [PubMed Central]
- Basu S, Millett C. Social epidemiology of hypertension in middle-income countries: determinants of prevalence, diagnosis, treatment, and control in the WHO SAGE study. Hypertension. 2013 Jul;62(1):18-26. Epub 2013 May 13. [CrossRef] [PubMed]
- Roberts LD, Gerszten RE. Toward new biomarkers of cardiometabolic diseases. Cell Metab. 2013 Jul 2;18(1):43-50. Epub 2013 Jun 13. [CrossRef] [PubMed] [PubMed Central]
- Magnusson M, Lewis GD, Ericson U, Orho-Melander M, Hedblad B, Engström G, Ostling G, Clish C, Wang TJ, Gerszten RE, Melander O. A diabetes-predictive amino acid score and future cardiovascular disease. Eur Heart J. 2013 Jul;34(26):1982-9. Epub 2012 Dec 13. [CrossRef] [PubMed] [PubMed Central]
- O'Connell TM. The complex role of branched chain amino acids in diabetes and cancer. Metabolites. 2013 Oct 14;3(4):931-45. [CrossRef] [PubMed] [PubMed Central]
- Asemi Z, Samimi M, Tabassi Z, Esmaillzadeh A. The effect of DASH diet on pregnancy outcomes in gestational diabetes: a randomized controlled clinical trial. Eur J Clin Nutr. 2014 Apr;68(4):490-5. Epub 2014 Jan 15. [CrossRef] [PubMed]
- Schwingshackl L, Hoffmann G. Adherence to Mediterranean diet and risk of cancer: a systematic review and meta-analysis of observational studies. Int J Cancer. 2014 Oct 15;135(8):1884-97. Epub 2014 Mar 11. [CrossRef] [PubMed]
- Andersen MB, Rinnan Å, Manach C, Poulsen SK, Pujos-Guillot E, Larsen TM, Astrup A, Dragsted LO. Untargeted metabolomics as a screening tool for estimating compliance to a dietary pattern. J Proteome Res. 2014 Mar 7;13(3):1405-18. Epub 2014 Feb 6. [CrossRef] [PubMed]
- Wang Z, Tang WH, Buffa JA, Fu X, Britt EB, Koeth RA, Levison BS, Fan Y, Wu Y, Hazen SL. Prognostic value of choline and betaine depends on intestinal microbiota-generated metabolite trimethylamine-N-oxide. Eur Heart J. 2014 Apr;35(14):904-10. Epub 2014 Feb 3. [CrossRef] [PubMed] [PubMed Central]
- Dragsted LO. Biomarkers of meat intake and the application of nutrigenomics. Meat Sci. 2010 Feb;84(2):301-7. Epub 2009 Aug 15. [CrossRef] [PubMed]
- Baldrick FR, Woodside JV, Elborn JS, Young IS, McKinley MC. Biomarkers of fruit and vegetable intake in human intervention studies: a systematic review. Crit Rev Food Sci Nutr. 2011 Oct-Nov;51(9):795-815. [CrossRef] [PubMed]
- Marklund M, Landberg R, Andersson A, Åman P, Kamal-Eldin A. Alkylresorcinol metabolites in urine correlate with the intake of whole grains and cereal fibre in free-living Swedish adults. Br J Nutr. 2013 Jan 14;109(1):129-36. Epub 2012 Apr 3. [CrossRef] [PubMed]
- Silva V, Barazzoni R, Singer P. Biomarkers of fish oil omega-3 polyunsaturated fatty acids intake in humans. Nutr Clin Pract. 2014 Feb;29(1):63-72. Epub 2013 Dec 14. [CrossRef] [PubMed]
- Woodside JV, Draper J, Lloyd A, McKinley MC. Use of biomarkers to assess fruit and vegetable intake. Proc Nutr Soc. 2017 Aug;76(3):308-315. Epub 2017 Mar 28. [CrossRef] [PubMed]
- Andersen MB, Kristensen M, Manach C, Pujos-Guillot E, Poulsen SK, Larsen TM, Astrup A, Dragsted L. Discovery and validation of urinary exposure markers for different plant foods by untargeted metabolomics. Anal Bioanal Chem. 2014 Mar;406(7):1829-44. Epub 2014 Jan 4. [CrossRef] [PubMed]
- Heinzmann SS, Brown IJ, Chan Q, Bictash M, Dumas ME, Kochhar S, Stamler J, Holmes E, Elliott P, Nicholson JK. Metabolic profiling strategy for discovery of nutritional biomarkers: proline betaine as a marker of citrus consumption. Am J Clin Nutr. 2010 Aug;92(2):436-43. Epub 2010 Jun 23. [CrossRef] [PubMed] [PubMed Central]
- Lloyd AJ, Beckmann M, Favé G, Mathers JC, Draper J. Proline betaine and its biotransformation products in fasting urine samples are potential biomarkers of habitual citrus fruit consumption. Br J Nutr. 2011 Sep;106(6):812-24. Epub 2011 May 9. [CrossRef] [PubMed]
- May DH, Navarro SL, Ruczinski I, Hogan J, Ogata Y, Schwarz Y, Levy L, Holzman T, McIntosh MW, Lampe JW. Metabolomic profiling of urine: response to a randomised, controlled feeding study of select fruits and vegetables, and application to an observational study. Br J Nutr. 2013 Nov;110(10):1760-70. Epub 2013 May 9. [CrossRef] [PubMed] [PubMed Central]
- Pujos-Guillot E, Hubert J, Martin JF, Lyan B, Quintana M, Claude S, Chabanas B, Rothwell JA, Bennetau-Pelissero C, Scalbert A, Comte B, Hercberg S, Morand C, Galan P, Manach C. Mass spectrometry-based metabolomics for the discovery of biomarkers of fruit and vegetable intake: citrus fruit as a case study. J Proteome Res. 2013 Apr 5;12(4):1645-59. Epub 2013 Mar 5. [CrossRef] [PubMed]
- O'Sullivan A, Gibney MJ, Brennan L. Dietary intake patterns are reflected in metabolomic profiles: potential role in dietary assessment studies. Am J Clin Nutr. 2011 Feb;93(2):314-21. Epub 2010 Dec 22. [CrossRef] [PubMed]
- Nicholson JK, Holmes E, Wilson ID. Gut microorganisms, mammalian metabolism and personalized health care. Nat Rev Microbiol. 2005 May;3(5):431-8. [CrossRef] [PubMed]
- Xie G, Zhang S, Zheng X, Jia W. Metabolomics approaches for characterizing metabolic interactions between host and its commensal microbes. Electrophoresis. 2013 Oct;34(19):2787-98. Epub 2013 Aug 16. [CrossRef] [PubMed]
- Lamichhane S, Sen P, Dickens AM, Orešič M, Bertram HC. Gut metabolome meets microbiome: A methodological perspective to understand the relationship between host and microbe. Methods. 2018 Oct 1; 149:3-12. Epub 2018 Apr 30. [CrossRef] [PubMed]
- Geier B, Sogin EM, Michellod D, Janda M, Kompauer M, Spengler B, Dubilier N, Liebeke M. Spatial metabolomics of in situ host-microbe interactions at the micrometre scale. Nat Microbiol. 2020 Mar;5(3):498-510. Epub 2020 Feb 3. [CrossRef] [PubMed]
- Feng YL, Cao G, Chen DQ, Vaziri ND, Chen L, Zhang J, Wang M, Guo Y, Zhao YY. Microbiome-metabolomics reveals gut microbiota associated with glycine-conjugated metabolites and polyamine metabolism in chronic kidney disease. Cell Mol Life Sci. 2019 Dec;76(24):4961-4978. Epub 2019 May 30. [CrossRef] [PubMed]
- Han J, Antunes LC, Finlay BB, Borchers CH. Metabolomics: towards understanding host-microbe interactions. Future Microbiol. 2010 Feb;5(2):153-61. [CrossRef] [PubMed]
- Rezzi S, Ramadan Z, Martin FP, Fay LB, van Bladeren P, Lindon JC, Nicholson JK, Kochhar S. Human metabolic phenotypes link directly to specific dietary preferences in healthy individuals. J Proteome Res. 2007 Nov;6(11):4469-77. Epub 2007 Oct 12. [CrossRef] [PubMed]
- Herder C, Kowall B, Tabak AG, Rathmann W. The potential of novel biomarkers to improve risk prediction of type 2 diabetes. Diabetologia. 2014 Jan;57(1):16-29. [CrossRef] [PubMed]
- Feng Z, Ding C, Li W, Wang D, Cui D. Applications of metabolomics in the research of soybean plant under abiotic stress. Food Chem. 2020 Apr 25; 310:125914. Epub 2019 Dec 2. [CrossRef] [PubMed]
- Zhu G, Wang S, Huang Z, Zhang S, Liao Q, Zhang C, Lin T, Qin M, Peng M, Yang C, Cao X, Han X, Wang X, van der Knaap E, Zhang Z, Cui X, Klee H, Fernie AR, Luo J, Huang S. Rewiring of the Fruit Metabolome in Tomato Breeding. Cell. 2018 Jan 11;172(1-2):249-261.e12. [CrossRef] [PubMed]
- Tian J, Wang YZ, Yan SX, Sun S, Jia JJ, Hu XX. Metabolomics technology and its applications in agricultural animal and plant research. Yi Chuan. 2020 May 20;42(5):452-465. [CrossRef] [PubMed]
- Goldansaz SA, Guo AC, Sajed T, Steele MA, Plastow GS, Wishart DS. Livestock metabolomics and the livestock metabolome: A systematic review. PLoS One. 2017 May 22;12(5):e0177675. [CrossRef] [PubMed] [PubMed Central]
- Emara S, Amer S, Ali A, Abouleila Y, Oga A, Masujima T. Single-Cell Metabolomics. Adv Exp Med Biol. 2017; 965:323-343. [CrossRef] [PubMed]
- Manach C, Hubert J, Llorach R, Scalbert A. The complex links between dietary phytochemicals and human health deciphered by metabolomics. Mol Nutr Food Res. 2009 Oct;53(10):1303-15. [CrossRef] [PubMed]
- Palmnäs M, Brunius C, Shi L, Rostgaard-Hansen A, Torres NE, González-Domínguez R, Zamora-Ros R, Ye YL, Halkjær J, Tjønneland A, Riccardi G, Giacco R, Costabile G, Vetrani C, Nielsen J, Andres-Lacueva C, Landberg R. Perspective: Metabotyping-A Potential Personalized Nutrition Strategy for Precision Prevention of Cardiometabolic Disease. Adv Nutr. 2020 May 1;11(3):524-532. [CrossRef] [PubMed] [PubMed Central]
- Braconi D, Bernardini G, Millucci L, Santucci A. Foodomics for human health: current status and perspectives. Expert Rev Proteomics. 2018 Feb;15(2):153-164. Epub 2017 Dec 29. [CrossRef] [PubMed]
- Cuadrado-Silva CT, Pozo-Bayón MÁ, Osorio C. Targeted Metabolomic Analysis of Polyphenols with Antioxidant Activity in Sour Guava (Psidium friedrichsthalianum Nied.) Fruit. Molecules. 2016 Dec 23;22(1):11. [CrossRef] [PubMed] [PubMed Central]
- Calumpang CLF, Saigo T, Watanabe M, Tohge T. Cross-Species Comparison of Fruit-Metabolomics to Elucidate Metabolic Regulation of Fruit Polyphenolics Among Solanaceous Crops. Metabolites. 2020 May 19;10(5):209. [CrossRef] [PubMed] [PubMed Central]
- Dixon RA, Gang DR, Charlton AJ, Fiehn O, Kuiper HA, Reynolds TL, Tjeerdema RS, Jeffery EH, German JB, Ridley WP, Seiber JN. Applications of metabolomics in agriculture. J Agric Food Chem. 2006 Nov 29;54(24):8984-94. [CrossRef] [PubMed]
- Simpson MJ, McKelvie JR. Environmental metabolomics: new insights into earthworm ecotoxicity and contaminant bioavailability in soil. Anal Bioanal Chem. 2009 May;394(1):137-49. Epub 2009 Feb 5. [CrossRef] [PubMed]
- Bundy JG, Davey MP, Viant MR. Environmental metabolomics: a critical review and future perspectives. Metabolomics 2009; 5:3–21. [CrossRef]
- Sumner LW, Mendes P, Dixon RA. Plant metabolomics: large-scale phytochemistry in the functional genomics era. Phytochemistry. 2003 Mar;62(6):817-36. [CrossRef] [PubMed]
- Levandi T, Leon C, Kaljurand M, Garcia-Cañas V, Cifuentes A. Capillary electrophoresis time-of-flight mass spectrometry for comparative metabolomics of transgenic versus conventional maize. Anal Chem. 2008 Aug 15;80(16):6329-35. Epub 2008 Jul 9. [CrossRef] [PubMed]
- García-Villalba R, León C, Dinelli G, Segura-Carretero A, Fernández-Gutiérrez A, Garcia-Cañas V, Cifuentes A. Comparative metabolomic study of transgenic versus conventional soybean using capillary electrophoresis-time-of-flight mass spectrometry. J Chromatogr A. 2008 Jun 27;1195(1-2):164-73. Epub 2008 May 14. [CrossRef] [PubMed]
- Jung Y, Lee J, Kwon J, Lee KS, Ryu DH, Hwang GS. Discrimination of the geographical origin of beef by (1)H NMR-based metabolomics. J Agric Food Chem. 2010 Oct 13;58(19):10458-66. [CrossRef] [PubMed]
- Cajka T, Riddellova K, Tomaniova M, Hajslova J. Ambient mass spectrometry employing a DART ion source for metabolomic fingerprinting/profiling: a powerful tool for beer origin recognition. Metabolomics. 2011 7, 500–508. [CrossRef]
- Capanoglu E, Beekwilder J, Boyacioglu D, Hall R, de Vos R. Changes in antioxidant and metabolite profiles during production of tomato paste. J Agric Food Chem. 2008 Feb 13;56(3):964-73. Epub 2008 Jan 19. [CrossRef] [PubMed]
- Boudonck KJ, Mitchell MW, Wulff J. Characterization of the biochemical variability of bovine milk using metabolomics. Metabolomics. 2009 5, 375–386. [CrossRef]
- Castellano P, Pérez Ibarreche M, Blanco Massani M, Fontana C, Vignolo GM. Strategies for Pathogen Biocontrol Using Lactic Acid Bacteria and Their Metabolites: A Focus on Meat Ecosystems and Industrial Environments. Microorganisms. 2017 Jul 11;5(3):38. [CrossRef] [PubMed] [PubMed Central]
- Ercolini D, Russo F, Nasi A, Ferranti P, Villani F. Mesophilic and psychrotrophic bacteria from meat and their spoilage potential in vitro and in beef. Appl Environ Microbiol. 2009 Apr;75(7):1990-2001. Epub 2009 Feb 5. [CrossRef] [PubMed] [PubMed Central]
- Xu Y, Cheung W, Winder CL, Goodacre R. VOC-based metabolic profiling for food spoilage detection with the application to detecting Salmonella typhimurium-contaminated pork. Anal Bioanal Chem. 2010 Jul;397(6):2439-49. Epub 2010 May 16. [CrossRef] [PubMed]
- Herrero M, Simó C, García-Cañas V, Ibáñez E, Cifuentes A. Foodomics: MS-based strategies in modern food science and nutrition. Mass Spectrom Rev. 2012 Jan-Feb;31(1):49-69. Epub 2011 Mar 3. [CrossRef] [PubMed]
- van Ruth SM, Alewijn M, Rogers K, Newton-Smith E, Tena N, Bollen M, Koot A. Authentication of organic and conventional eggs by carotenoid profiling. Food Chem. 2011, 126, 1299–1305. [CrossRef]
- Ceciliani F, Roccabianca P, Giudice C, Lecchi C. Application of post-genomic techniques in dog cancer research. Mol Biosyst. 2016 Aug 16;12(9):2665-79. [CrossRef] [PubMed]
- de Godoy MR, Hervera M, Swanson KS, Fahey GC Jr. Innovations in Canine and Feline Nutrition: Technologies for Food and Nutrition Assessment. Annu Rev Anim Biosci. 2016; 4:311-33. [CrossRef] [PubMed]
- Honneffer JB, Steiner JM, Lidbury JA, Suchodolski JS. Variation of the microbiota and metabolome along the canine gastrointestinal tract. Metabolomics 2017, 13, 26-31. [CrossRef]
- Zhang S, Nagana Gowda GA, Asiago V, Shanaiah N, Barbas C, Raftery D. Correlative and quantitative 1H NMR-based metabolomics reveals specific metabolic pathway disturbances in diabetic rats. Anal Biochem. 2008 Dec 1;383(1):76-84. Epub 2008 Aug 20. Erratum in: Anal Biochem. 2009 Feb 15;385(2):392. [CrossRef] [PubMed] [PubMed Central]
- O'Kell AL, Garrett TJ, Wasserfall C, Atkinson MA. Untargeted metabolomic analysis in naturally occurring canine diabetes mellitus identifies similarities to human Type 1 Diabetes. Sci Rep. 2017 Aug 25;7(1):9467. [CrossRef] [PubMed] [PubMed Central]
- O'Kell AL, Garrett TJ, Wasserfall C, Atkinson MA. Untargeted metabolomic analysis in non-fasted diabetic dogs by UHPLC-HRMS. Metabolomics. 2019 Jan 22;15(2):15. [CrossRef] [PubMed] [PubMed Central]
- Zhang A, Sun H, Wang P, Han Y, Wang X. Recent and potential developments of biofluid analyses in metabolomics. J Proteomics. 2012 Feb 2;75(4):1079-88. Epub 2011 Nov 4. [CrossRef] [PubMed]
- Tamai R, Furuya M, Hatoya S, Akiyoshi H, Yamamoto R, Komori Y, Yokoi S, Tani K, Hirano Y, Komori M, Takenaka S. Profiling of serum metabolites in canine lymphoma using gas chromatography mass spectrometry. J Vet Med Sci. 2014 Nov;76(11):1513-8. Epub 2014 Aug 13. [CrossRef] [PubMed] [PubMed Central]
- Minamoto Y, Otoni CC, Steelman SM, Büyükleblebici O, Steiner JM, Jergens AE, Suchodolski JS. Alteration of the fecal microbiota and serum metabolite profiles in dogs with idiopathic inflammatory bowel disease. Gut Microbes. 2015; 6(1):33-47. Epub 2015 Jan 7. [CrossRef] [PubMed] [PubMed Central]
- Abdullah M, Kornegay JN, Honcoop A, Parry TL, Balog-Alvarez CJ, O'Neal SK, Bain JR, Muehlbauer MJ, Newgard CB, Patterson C, Willis MS. Non-Targeted Metabolomics Analysis of Golden Retriever Muscular Dystrophy-Affected Muscles Reveals Alterations in Arginine and Proline Metabolism, and Elevations in Glutamic and Oleic Acid In Vivo. Metabolites. 2017 Jul 29;7(3):38. [CrossRef] [PubMed] [PubMed Central]
- Martini FM, Brandstetter de Bellesini A, Miolo A, Del Coco L, Fanizzi FP, Crovace A. Combining a joint health supplement with tibial plateau leveling osteotomy in dogs with cranial cruciate ligament rupture. An exploratory controlled trial. Int J Vet Sci Med. 2017 Oct 6;5(2):105-112. [CrossRef] [PubMed] [PubMed Central]
- Wang Y, Lawler D, Larson B, Ramadan Z, Kochhar S, Holmes E, Nicholson JK. Metabonomic investigations of aging and caloric restriction in a life-long dog study. J Proteome Res. 2007 May;6(5):1846-54. Epub 2007 Apr 6. [CrossRef] [PubMed]
- Hall JA, Brockman JA, Jewell DE. Dietary fish oil alters the lysophospholipid metabolomic profile and decreases urinary 11-dehydro thromboxane B₂ concentration in healthy Beagles. Vet Immunol Immunopathol. 2011 Dec 15;144(3-4):355-65. Epub 2011 Aug 17. [CrossRef] [PubMed]
- Allaway D, Kamlage B, Gilham MS, Hewson-Hughes AK, Wiemer JC, Colyer A. Efects of dietary glucose supplementation on the fasted plasma metabolome in cats and dogs. Metabolomics, 2013, 9(5), 1096–1108. [CrossRef]
- Schmidt M, Unterer S, Suchodolski JS, Honneffer JB, Guard BC, Lidbury JA, Steiner JM, Fritz J, Kölle P. The fecal microbiome and metabolome differs between dogs fed Bones and Raw Food (BARF) diets and dogs fed commercial diets. PLoS One. 2018 Aug 15; 13(8):e0201279. [CrossRef] [PubMed] [PubMed Central]
- Yan G, Zhao Y, Deng P, Lv L, Wang Y, Bu Q, Liu B, Hu C, Zhuo Y, Yang X, Wang L, Cen X. Investigation of toxicological effects of Shuanghuanglian injection in Beagle dogs by metabonomic and traditional approaches. Exp Biol Med (Maywood). 2010 Nov;235(11):1356-64. Epub 2010 Sep 23. [CrossRef] [PubMed]
- Zhang H, Patrone L, Kozlosky J, Tomlinson L, Cosma G, Horvath J. Pooled sample strategy in conjunction with high-resolution liquid chromatography-mass spectrometry-based background subtraction to identify toxicological markers in dogs treated with ibipinabant. Anal Chem. 2010 May 1;82(9):3834-9. [CrossRef] [PubMed]
- Osaki T, Azuma K, Kurozumi S, Takamori Y, Tsuka T, Imagawa T, Okamoto Y, Minami S. Metabolomic analyses of blood plasma after oral administration of D-glucosamine hydrochloride to dogs. Mar Drugs. 2012 Aug;10(8):1873-82. Epub 2012 Aug 22. [CrossRef] [PubMed] [PubMed Central]
- Kararli TT. Comparison of the gastrointestinal anatomy, physiology, and biochemistry of humans and commonly used laboratory animals. Biopharm Drug Dispos. 1995 Jul;16(5):351-80. [CrossRef] [PubMed]
- Kang CI, Kim SH, Park WB, Lee KD, Kim HB, Kim EC, Oh MD, Choe KW. Bloodstream infections caused by antibiotic-resistant gram-negative bacilli: risk factors for mortality and impact of inappropriate initial antimicrobial therapy on outcome. Antimicrob Agents Chemother. 2005 Feb;49(2):760-6. [CrossRef] [PubMed] [PubMed Central]
- German JB, Roberts MA, Watkins SM. Personal metabolomics as a next generation nutritional assessment. J Nutr. 2003 Dec;133(12):4260-6. [CrossRef] [PubMed]
- Suhre K. Genetics Meets Metabolomics - from Experiment to Systems Biology. Springer, 2012, 318 pgs.
- Puurunen J, Tiira K, Lehtonen M, Hanhineva K, Lohi H. Non-targeted metabolite profiling reveals changes in oxidative stress, tryptophan and lipid metabolisms in fearful dogs. Behav Brain Funct. 2016 Feb 12;12(1):7. [CrossRef] [PubMed] [PubMed Central]
- González-Ramírez MT. Compatibility between Humans and Their Dogs: Benefits for Both. Animals (Basel). 2019 Sep 12;9(9):674. [CrossRef] [PubMed] [PubMed Central]
- De Souza AI, Cardin S, Wait R, Chung YL, Vijayakumar M, Maguy A, Camm AJ, Nattel S. Proteomic and metabolomic analysis of atrial profibrillatory remodelling in congestive heart failure. J Mol Cell Cardiol. 2010 Nov;49(5):851-63. Epub 2010 Jul 23. [CrossRef] [PubMed]
- Shibayama J, Taylor TG, Venable PW, Rhodes NL, Gil RB, Warren M, Wende AR, Abel ED, Cox J, Spitzer KW, Zaitsev AV. Metabolic determinants of electrical failure in ex-vivo canine model of cardiac arrest: evidence for the protective role of inorganic pyrophosphate. PLoS One. 2013; 8(3):e57821. Epub 2013 Mar 8. [CrossRef] [PubMed] [PubMed Central]
- Shibayama J, Yuzyuk TN, Cox J, Makaju A, Miller M, Lichter J, Li H, Leavy JD, Franklin S, Zaitsev AV. Metabolic remodeling in moderate synchronous versus dyssynchronous pacing-induced heart failure: integrated metabolomics and proteomics study. PLoS One. 2015 Mar 19;10(3):e0118974. [CrossRef] [PubMed] [PubMed Central]
- Li Q, Freeman LM, Rush JE, Huggins GS, Kennedy AD, Labuda JA, Laflamme DP, Hannah SS. Veterinary Medicine and Multi-Omics Research for Future Nutrition Targets: Metabolomics and Transcriptomics of the Common Degenerative Mitral Valve Disease in Dogs. OMICS. 2015 Aug;19(8):461-70. Epub 2015 Jul 8. [CrossRef] [PubMed]
- Whitfeld PD, Noble PJM, Major H, Beynon RJ, Burrow R, Freeman AI. Metabolomics as a diagnostic tool for hepatology: Validation in a naturally occurring canine model. Metabolomics, 2005, 1(3), 215–225. [CrossRef]
- Kafsack BF, Llinás M. Eating at the table of another: metabolomics of host-parasite interactions. Cell Host Microbe. 2010 Feb 18;7(2):90-9. [CrossRef] [PubMed] [PubMed Central]
- Kloehn J, Blume M, Cobbold SA, Saunders EC, Dagley MJ, McConville MJ. Using metabolomics to dissect host-parasite interactions. Curr Opin Microbiol. 2016 Aug; 32:59-65. Epub 2016 May 17. [CrossRef] [PubMed]
- Puurunen J, Sulkama S, Tiira K, Araujo C, Lehtonen M, Hanhineva K, Lohi H. A non-targeted metabolite profiling pilot study suggests that tryptophan and lipid metabolisms are linked with ADHD-like behaviours in dogs. Behav Brain Funct. 2016 Sep 29;12(1):27. [CrossRef] [PubMed] [PubMed Central]
- Colyer A, Gilham MS, Kamlage B, Rein D, Allaway D. Identification of intra- and inter-individual metabolite variation in plasma metabolite profiles of cats and dogs. Br J Nutr. 2011 Oct;106 Suppl 1:S146-9. [CrossRef] [PubMed]
- Viant MR, Ludwig C, Rhodes S, Günther UL, Allaway D. Validation of a urine metabolome fngerprint in dog for phenotypic classifcation. Metabolomics, 2007, 3(4), 453–463. [CrossRef]
- Lloyd AJ, Beckmann M, Tailliart K, Brown WY, Draper J, Allaway D. Characterisation of the main drivers of intra- and inter- breed variability in the plasma metabolome of dogs. Metabolomics. 2016; 12:72. Epub 2016 Mar 8. [CrossRef] [PubMed] [PubMed Central]
- Lloyd AJ, Beckmann M, Wilson T, Tailliart K, Allaway D, Draper J. Ultra-high performance liquid chromatography-high resolution mass spectrometry plasma lipidomics can distinguish between canine breeds despite uncontrolled environmental variability and non-standardized diets. Metabolomics. 2017; 13(2):15. Epub 2017 Jan 5. [CrossRef] [PubMed] [PubMed Central]
- Osaki T, Kurozumi S, Sato K, Terashi T, Azuma K, Murahata Y, Tsuka T, Ito N, Imagawa T, Minami S, Okamoto Y. Metabolomic Analysis of Blood Plasma after Oral Administration of N-acetyl-d-Glucosamine in Dogs. Mar Drugs. 2015 Aug 7;13(8):5007-15. [CrossRef] [PubMed] [PubMed Central]
- Wagoner MP, Yang Y, McDuffie JE, Klapczynski M, Buck W, Cheatham L, Eisinger D, Sace F, Lynch KM, Sonee M, Ma JY, Chen Y, Marshall K, Damour M, Stephen L, Dragan YP, Fikes J, Snook S, Kinter LB. Evaluation of Temporal Changes in Urine-based Metabolomic and Kidney Injury Markers to Detect Compound Induced Acute Kidney Tubular Toxicity in Beagle Dogs. Curr Top Med Chem. 2017; 17(24):2767-2780. [CrossRef] [PubMed]
- Söbbeler FJ, Carrera I, Pasloske K, Ranasinghe MG, Kircher P, Kästner SBR. Effects of isoflurane, sevoflurane, propofol and alfaxalone on brain metabolism in dogs assessed by proton magnetic resonance spectroscopy (1H MRS). BMC Vet Res. 2018 Mar 5;14(1):69. [CrossRef] [PubMed] [PubMed Central]
- Musteata M, Nicolescu A, Solcan G, Deleanu C. The 1H NMR profile of healthy dog cerebrospinal fluid. PLoS One. 2013 Dec 23;8(12):e81192. [CrossRef] [PubMed] [PubMed Central]
- Hasegawa T, Sumita M, Horitani Y, Tamai R, Tanaka K, Komori M, Takenaka S. Gas chromatography-mass spectrometry-based metabolic profiling of cerebrospinal fluid from epileptic dogs. J Vet Med Sci. 2014 Apr;76(4):517-22. Epub 2013 Dec 13. [CrossRef] [PubMed] [PubMed Central]
- Yanibada B, Hohenester U, Pétéra M, Canlet C, Durand S, Jourdan F, Boccard J, Martin C, Eugène M, Morgavi DP, Boudra H. Inhibition of enteric methanogenesis in dairy cows induces changes in plasma metabolome highlighting metabolic shifts and potential markers of emission. Sci Rep. 2020 Sep 24;10(1):15591. [CrossRef] [PubMed] [PubMed Central]
- Zhou M, Jing JH, Mao RH, Guo J, Wang ZP. [Applications of metabonomics in animal genetics and breeding]. Yi Chuan. 2019 Feb 20;41(2):111-124. Chinese. [CrossRef] [PubMed]
- Long JA. The 'omics' revolution: Use of genomic, transcriptomic, proteomic and metabolomic tools to predict male reproductive traits that impact fertility in livestock and poultry. Anim Reprod Sci. 2020 Sep; 220:106354. Epub 2020 May 19. [CrossRef] [PubMed]
- Bertram HC, Jakobsen LMA. Nutrimetabolomics: integrating metabolomics in nutrition to disentangle intake of animal-based foods. Metabolomics. 2018 Feb 14;14(3):34. [CrossRef] [PubMed]
- Pugliese A, Gruppillo A, Di Pietro S. Clinical nutrition in gerontology: chronic renal disorders of the dog and cat. Vet Res Commun. 2005 Aug;29 Suppl 2:57-63. [CrossRef] [PubMed]
- Moore RE, Kirwan J, Doherty MK, Whitfield PD. Biomarker discovery in animal health and disease: the application of post-genomic technologies. Biomark Insights. 2007 Jul 10; 2:185-96. [PubMed] [PubMed Central]
- Whitfield PD, German AJ, Noble PJ. Metabolomics: an emerging post-genomic tool for nutrition. Br J Nutr. 2004 Oct;92(4):549-55. [CrossRef] [PubMed]
- Zeisel SH. Nutrigenomics and metabolomics will change clinical nutrition and public health practice: insights from studies on dietary requirements for choline. Am J Clin Nutr. 2007 Sep;86(3):542-8. [CrossRef] [PubMed] [PubMed Central]
- One Health Initiative - http://www.onehealthinitiative.com/ (accessed on 08.12.2020).
- Kahn LH, Kaplan B, Steele JH. Confronting zoonoses through closer collaboration between medicine and veterinary medicine (as 'one medicine'). Vet Ital. 2007 Jan-Mar;43(1):5-19. [PubMed]
- Cecaro M. Food Borne Illness and One Medicine Approach. J Mass Communicat Journalism 2013, S1. [CrossRef]
- Mersha C, Tewodros F. One Health One Medicine One World: Co-joint of Animal and Human Medicine with Perspectives, A review. Vet. World. 2011 5(4):238-243. [CrossRef]
- Zinsstag J., Schelling E., Schelling E., Waltner-Toews D., Tanner M. One Health: The Theory and Practice of Integrated Health Approaches, CABI, 2015, 478 pgs.
- Frank D. One world, one health, one medicine. Can Vet J. 2008 Nov;49(11):1063-5. [PubMed] [PubMed Central]
- Karesh WB, Dobson A, Lloyd-Smith JO, Lubroth J, Dixon MA, Bennett M, Aldrich S, Harrington T, Formenty P, Loh EH, Machalaba CC, Thomas MJ, Heymann DL. Ecology of zoonoses: natural and unnatural histories. Lancet. 2012 Dec 1;380(9857):1936-45. [CrossRef] [PubMed] [PubMed Central]
- Dubey JP, Hotea I, Olariu TR, Jones JL, Dărăbuş G. Epidemiological review of toxoplasmosis in humans and animals in Romania. Parasitology. 2014 Mar;141(3):311-25. [CrossRef] [PubMed]
- World Health Organization. Noncommunicable diseases. 2014. Fact sheet 355. Available at: http://www. who.int/mediacentre/factsheets/fs355/en (accessed 16.12.2020).
- Chandler M, Cunningham S, Lund EM, Khanna C, Naramore R, Patel A, Day MJ. Obesity and Associated Comorbidities in People and Companion Animals: A One Health Perspective. J Comp Pathol. 2017 May;156(4):296-309. Epub 2017 Apr 28. [CrossRef] [PubMed]
- Muñoz-Prieto A, Nielsen LR, Dąbrowski R, Bjørnvad CR, Söder J, Lamy E, Monkeviciene I, Ljubić BB, Vasiu I, Savic S, Busato F, Yilmaz Z, Bravo-Cantero AF, Öhlund M, Lucena S, Zelvyte R, Aladrović J, Lopez-Jornet P, Caldin M, Lavrador C, Karveliene B, Mrljak V, Mazeikiene J, Tvarijonaviciute A. European dog owner perceptions of obesity and factors associated with human and canine obesity. Sci Rep. 2018 Sep 6;8(1):13353. [CrossRef] [PubMed] [PubMed Central]
- Delicano RA, Hammar U, Egenvall A, Westgarth C, Mubanga M, Byberg L, Fall T, Kennedy B. The shared risk of diabetes between dog and cat owners and their pets: register based cohort study. BMJ. 2020 Dec 10;371:m4337. [CrossRef] [PubMed] [PubMed Central]
- Thibault M, Houlbrèque F, Lorrain A, Vidal E. Seabirds: Sentinels beyond the oceans. Science. 2019 Nov 15;366(6467):813. [CrossRef] [PubMed]
- Schmidt PL. Companion animals as sentinels for public health. Vet Clin North Am Small Anim Pract. 2009 Mar;39(2):241-50. [CrossRef] [PubMed]
- Reif JS. Animal sentinels for environmental and public health. Public Health Rep. 2011 May-Jun;126 Suppl 1(Suppl 1):50-7. [CrossRef] [PubMed] [PubMed Central]
- National Research Council (US) Committee on Animals as Monitors of Environmental Hazards. Animals as Sentinels of Environmental Health Hazards. Washington (DC): National Academies Press (US); 1991. [PubMed]
- Scorpio DG. Do cats serve as good sentinels for Bartonella species infection risk in people and animals? Vet Rec. 2017 Apr 1;180(13):322-324. [CrossRef] [PubMed]
- Ellis K.H. One Health Initiative will unite veterinary, human medicine. Infectious Disease News, Feb.2008.
- Mobasheri A. COVID-19, Companion Animals, Comparative Medicine, and One Health. Front Vet Sci. 2020 Aug 14; 7:522. [CrossRef] [PubMed] [PubMed Central]
- Thamm D, Dow S. How companion animals contribute to the fight against cancer in humans. Vet Ital. 2009 Jan-Mar;45(1):111-20. [PubMed]
- Jacob M, Lopata AL, Dasouki M, Abdel Rahman AM. Metabolomics toward personalized medicine. Mass Spectrom Rev. 2019 May;38(3):221-238. Epub 2017 Oct 26. [CrossRef] [PubMed]
- Sandøe P, Palmer C, Corr S, Astrup A, Bjørnvad CR. Canine and feline obesity: a One Health perspective. Vet Rec. 2014 Dec 20-27;175(24):610-6. [CrossRef] [PubMed]
- Chijiiwa H, Takagi S, Arahori M, Hori Y, Saito A, Kuroshima H, Fujita K. Dogs and cats prioritize human action: choosing a now-empty instead of a still-baited container. Anim Cogn. 2021 Jan;24(1):65-73. Epub 2020 Jul 23. [CrossRef] [PubMed]
- Scotch M, Odofin L, Rabinowitz P. Linkages between animal and human health sentinel data. BMC Vet Res. 2009 Apr 23; 5:15. [CrossRef] [PubMed] [PubMed Central]
- Harding JC. Genomics, animal models, and emerging diseases: relevance to One Health and food security. Genome. 2015 Dec;58(12):499-502. Epub 2015 Nov 18. [CrossRef] [PubMed]
- European Commission – Food Safety Overwiew, https://ec.europa.eu/food/overview_en (accessed on 15.12.2021).
- Brennan L. Metabolomics: A Powerful Tool to Enrich our Understanding of the Impact of Food on Health. Mol Nutr Food Res. 2019 Jan;63(1):e1870087. [CrossRef] [PubMed]
- FAO. Rome Declaration on World Food Security and World Food Summit Plan of Action. 1996. Available at: http://www.fao.org/docrep/003/W3613E/W3613E00.htm (accessed 13.12.2020).
- EFSA GMO Panel Working Group on Animal Feeding Trials. Safety and nutritional assessment of GM plants and derived food and feed: the role of animal feeding trials. Food Chem Toxicol. 2008 Mar;46 Suppl 1:S2-70. Epub 2008 Feb 13. [CrossRef] [PubMed]
- Sun W, Chen Z, Hong J, Shi J. Promoting Human Nutrition and Health through Plant Metabolomics: Current Status and Challenges. Biology (Basel). 2020 Dec 31;10(1):20. [CrossRef] [PubMed] [PubMed Central]
- Hemler EC, Hu FB. Plant-Based Diets for Personal, Population, and Planetary Health. Adv Nutr. 2019 Nov 1;10(Suppl_4):S275-S283. [CrossRef] [PubMed] [PubMed Central]
- Gupta SM, Arora S, Mirza N, Pande A, Lata C, Puranik S, Kumar J, Kumar A. Finger Millet: A "Certain" Crop for an "Uncertain" Future and a Solution to Food Insecurity and Hidden Hunger under Stressful Environments. Front Plant Sci. 2017 Apr 25; 8:643. [CrossRef] [PubMed] [PubMed Central]
- Alberti G. Noncommunicable diseases: tomorrow's pandemics. Bull World Health Organ. 2001;79(10):907. Epub 2001 Nov 1. [PubMed] [PubMed Central]
- Beran D, Pedersen HB, Robertson J. Noncommunicable diseases, access to essential medicines and universal health coverage. Glob Health Action. 2019; 12(1):1670014. [CrossRef] [PubMed] [PubMed Central]
- Gibbons H, O'Gorman A, Brennan L. Metabolomics as a tool in nutritional research. Curr Opin Lipidol. 2015 Feb;26(1):30-4. [CrossRef] [PubMed]
| 1 | The term ‘(Nutri-)Metabolomics’ is used to refer to both, metabolomics and nutrimetabolomics. |
| 2 | Given that nutrimetabolomics is a branch of metabolomics, in this study, one term or the other will be used, depending on the context, although the study refers to both technologies - metabolomics and nutrimetabolomics |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).



