1. Introduction
The great advances achieved over the last years in biomass use and transformation have made available a huge amount of new building blocks for the chemical industry, spreading over many different classes of compounds and molecular structures. Among these, carbohydrates represent valuable and abundant starting materials, being the main constituents of cellulose and hemicellulose and therefore potentially deriving from all kinds of lignocellulosic based feedstock. Many alcohols, acids and polyols derived from their stream have been listed by the US National Renewable Laboratory (NREL) among the top value-added chemicals from biomass. [
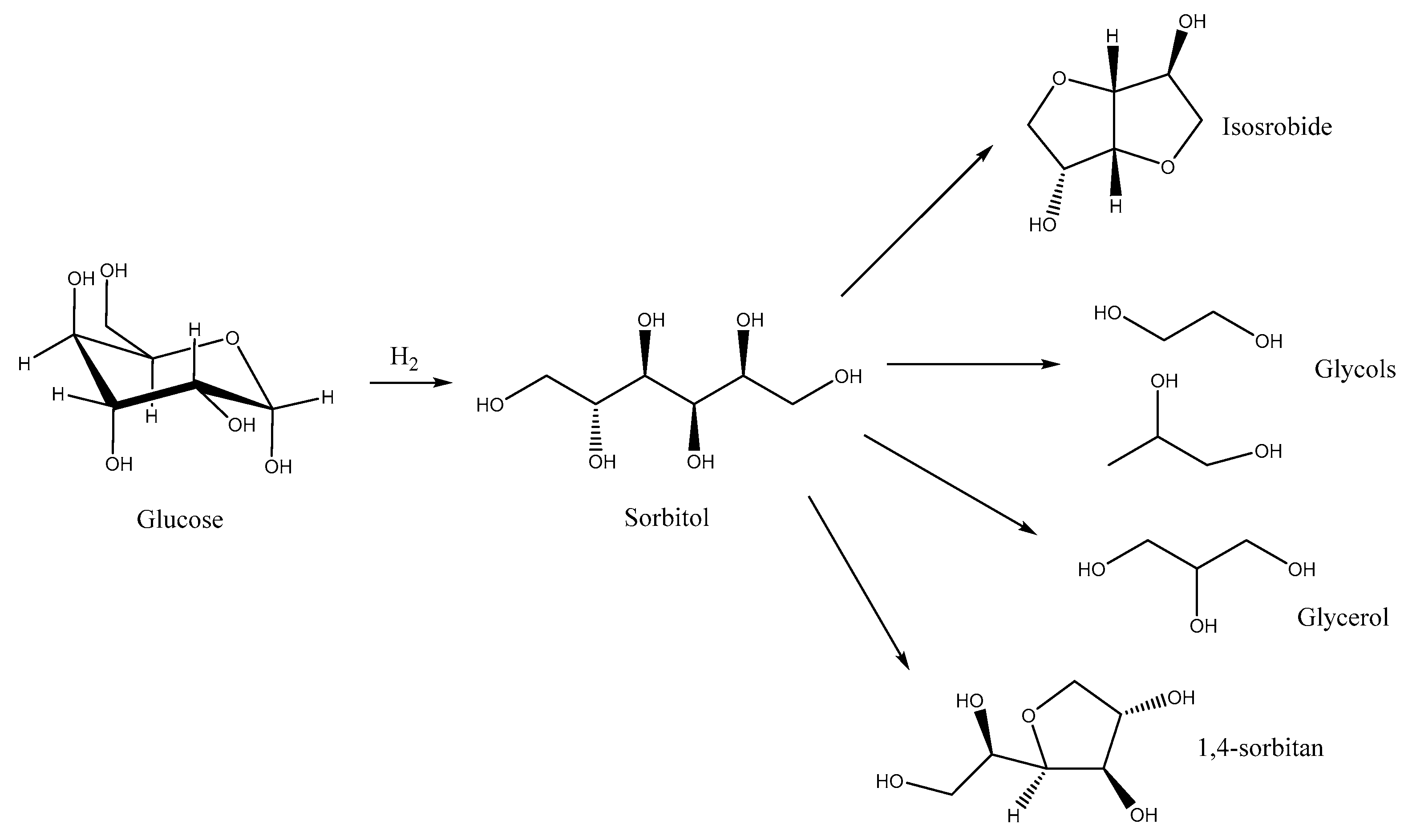
1] Among these, sorbitol can be directly obtained from glucose
via reduction and is considered as a strategic starting material for the preparation of isosorbide, glycols, glycerol, sorbitan and in turn their polymerisation products (
Scheme 1). The technologies to obtain the aforementioned products and intermediates are quite mature and do not present unbreakable critical barriers in the way to their effective application. This overall scenario makes actually sorbitol a very appealing actor within the biorefinery story.
One of the main issues related with the biomass derived molecules management, primarily with carbohydrates, is their high polarity. Thus, besides their great variability the very high oxygen content present in natural sources, makes them strongly different from the traditional platforms of fossil origin. The reaction conditions and in particular the solvent choice is a not trivial point to look at when designing a chemical process for their transformation and this is even more critical when facing heterogeneously catalyzed processes. Water would actually be the solvent of choice, but it entails several stability drawbacks for metal based catalysts. Some interesting solutions have been found by exploiting a multiphase approach able to grant the contact between substrates and catalysts, while preserving catalyst activity and reagents solubility. This strategy has been applied by pioneering works on biomass derived platform molecules resulting to be particularly efficient in terms of reagent/product separation, as was found to be the case of the production of 5-HMF from sugars[
2] and still represents a viable route to improve the product isolation and catalyst recycling and in preventing metal contamination in the synthesis of final derivatives[
3]. On the other hand, its application needs the use of two solvents, one being an organic one. In this respect and in compliance with the green chemistry approach, the use of the sole water is a definitely desirable route to be pursued, providing the activity and stability of the catalyst.
The hydrogenation of C6 and C5 sugars into the corresponding reduced sugars mainly relies on the use of noble metal-based systems[
4]. The possibility to substitute noble-based metal systems is another important point in setting up more sustainable protocols. A recent review by Redina et al. [
5] sums up the main results obtained with non-noble catalysts. Among these, Nickel-based are definitely the most studied and used ones. Ni Raney, for example, is used in the transformation of glucose into sorbitol in water, even at industrial scale, despite its easy deactivation through metal leaching, that poses purification issues, also considering the toxicity of Nickel [
6]. To improve the performance of Ni Raney, a series of new Ni-based catalysts has been developed in the last years, ranging from unsupported nanoparticles to supported systems, as well as from monometallic Ni catalysts to bi-metallic ones[
5].
Silica is the most common support for Ni for this application, but it has been shown by Silvester et al. that Al
2O
3 is a valid alternative leading to an increase in sorbitol yield from about 20% to 48% starting from glucose[
7]. The stability of the Ni catalysts is one of the major issues to develop an efficient process of hydrogenation of C5 and C6 sugars. In this view, the use of a second metals can help to obtain more stable and recyclable catalysts. This is the case of Fe, that was added to a Ni/SiO
2 or to a Ni/CB (carbon black) with significant improvement both in catalyst activity and stability, in the transformation of xylose to xylitol and glucose to sorbitol, respectively[
8,
9].
Also Cu was studied in combination with Ni to improve the stability of the catalyst[
10] to produce mannitol from fructose. Copper represents also a valid and less toxic alternative to Nickel in a wide range of hydrogenation reactions[
11,
12,
13], but in the case of sugar reduction only few examples are reported so far[
14,
15].
Glucose unit, besides its availability from cellulose deconstruction stream, is one of the main constituents of different natural occurring disaccharides and oligosaccharides. This is the case of saccharose, lactose, raffinose and maltose. The latter is a disaccharide composed of two glucose units linked with a a (1→4) bond. In spite of several reports on the hydrogenation of maltose into its corresponding reduced disaccharide, namely maltitol, widely used as sweetener in the food industry[
16], very few studies rely on the hydrolysis/hydrogenation process for the synthesis of sorbitol directly from maltose indeed considered in most cases as the unwanted side reaction of the maltose to maltitol reduction. A sorbitol yield higher than 90 % was attained with a Ni/Cu/Al/Fe magnetic system in the presence of diluted H
3PO
4[
17] or otherwise few Ru based catalysts are reported[
18,
19,
20].
On the other hand it is worth to underline that maltose can be derived from both agro-industrial residues[
21] and from food waste[
22] and the use of food wastes and/or residues and wastes deriving from agro-industrial chains is a fundamental step towards the transition to the circular economy and to an aware and fruitful exploitation of natural sources[
23].
Here we would like to report on the use of solid copper catalysts for the one-pot conversion of maltose into sorbitol in one pot in water as the unique solvent.
2. Results and Discussion
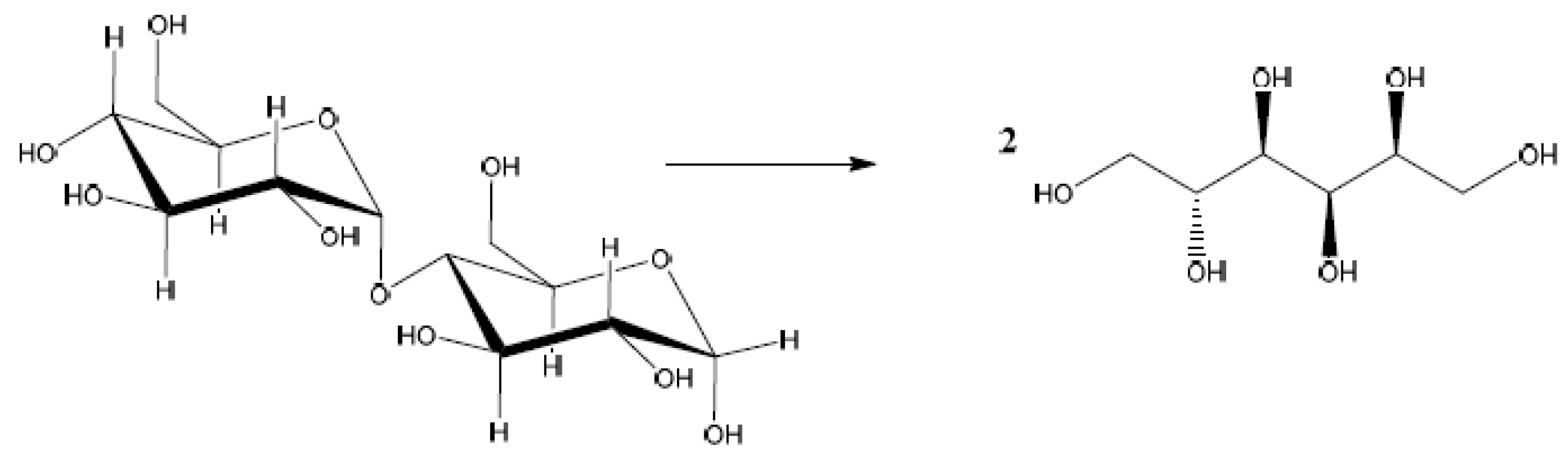
The one-pot transformation of maltose into 2 molecules of sorbitol (
Scheme 2) have been carried out by using a series of heterogeneous copper catalysts prepared with a non-conventional chemisorption-hydrolysis technique[
24]. This method already revealed to be a convenient and reliable one to obtain highly active copper systems for the hydrogenation of a wide variety of substrates, due to the very high dispersion obtained. Actually they can be applied for example to the selective hydrogenation of vegetable oils under mild conditions [
25], in the reduction of platform molecules such as g-valerolactone into 1,4-pentandiol [
26] or in the selective hydrogenation of a,b-unsaturated compounds [
27,
28].
Table 1 sums up results obtained in the transformation of maltose into sorbitol by supporting copper with the same protocol onto different matrices, namely oxides and mixed oxides, with different textural and acid-base properties. Copper catalysts prepared over silica alumina, an alumina and two different silicas were compared among them and to the bare supports under the same conditions.
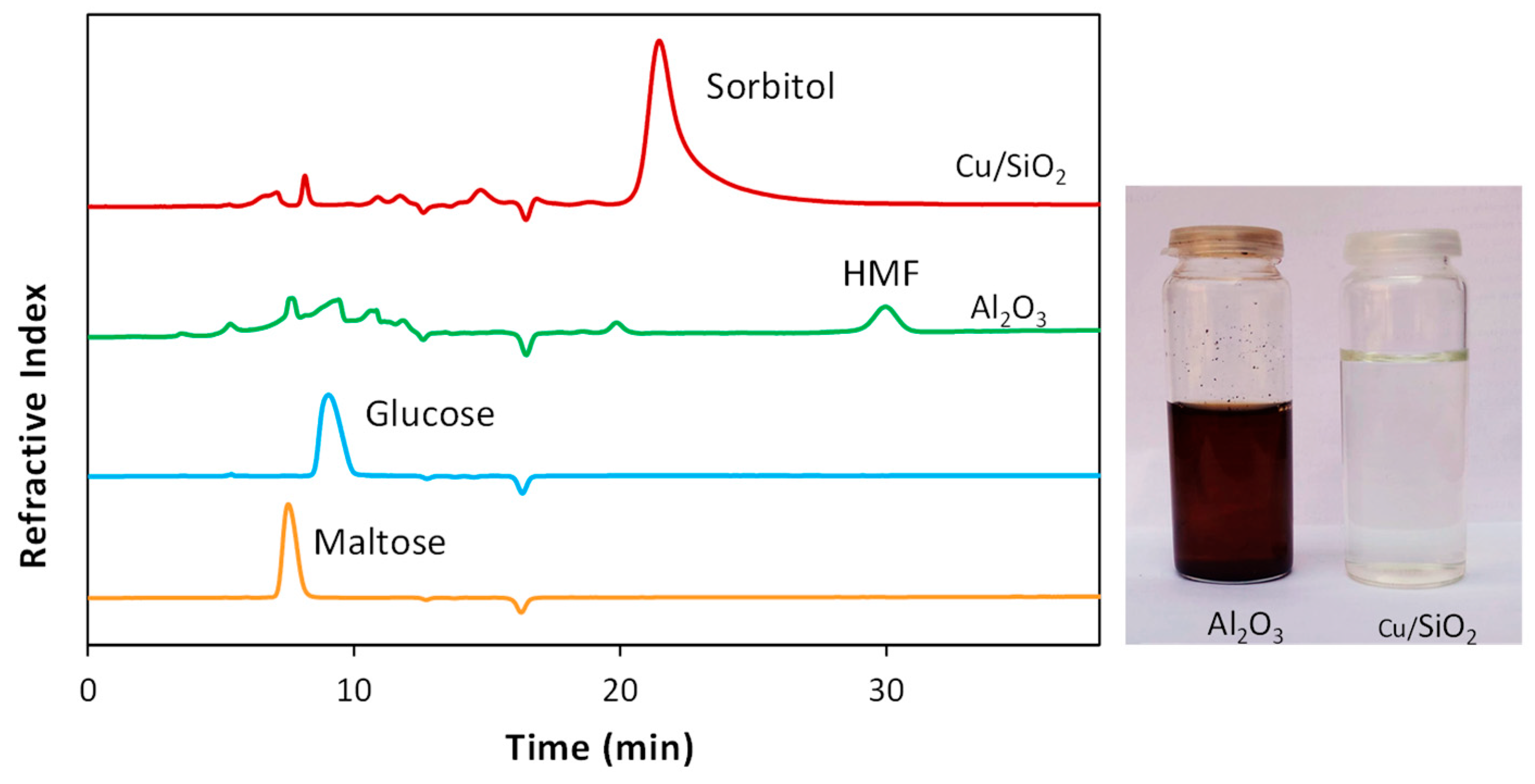
Among the catalysts tested, only the two systems supported over silica allowed us to gain high yields in sorbitol. In all cases the hydrolysis takes place, as well as the glucose conversion, but only Cu/SiO
2 reveals to be active in the desired hydrogenation under the experimental conditions used. With both Cu/Al
2O
3 and Cu/SiO
2-Al
2O
3 a plethora of products was observed in the HPLC chromatogram (see
Figure 1), the main by-product formed being HMF (3-20%). The comparison with the bare supports, as well as the one with the non-catalyzed reaction puts in light the very strong effect of copper supported over the proper matrix in addressing the reaction course. Thus, both the bare supports and the non-catalyzed reaction give rise to a very complicated mixture that with respect to the copper catalyzed ones also present the formation of humins, as clearly shown by the raw-reaction mixtures picture (
Figure 1).
It is worth noting that the bare silicas behave as the non-catalyzed reactions (
Table 1, entries 6 and 8
vs entry 9), witnessing the innocent role of this support in comparison with SiO
2-Al
2O
3 and Al
2O
3. With the latter oxides almost complete conversion of glucose was observed differently to the 35-45 % remaining at the end of the reaction carried out with silica or without any catalyst. This means that silica-alumina and alumina favor as themselves the decomposition or isomerization of glucose.
Table 2 summarizes some important features of the catalysts, namely the acidic properties, the mean particle size of the copper particles and the -OH surface density, that could help to have a deeper insight into the parameters affecting the reaction course.
The quantification of specific IR bands ascribed to pyridine used as the probe molecule and adsorbed according to what reported by Emeis [
29] gives account for the density and the character of the acidic sites of the catalytic surfaces. All the systems show the presence of Lewis acid sites only and the internal ranking in terms of sites density is Cu/SiO
2-Al
2O
3 >> Cu/Al
2O
3 ≈ Cu/SiO
2 A > Cu/SiO
2 B. This first aspect puts in evidence the detrimental effect of the different acidity observed in the Cu/SiO
2-Al
2O
3 catalyst. In fact, despite the complete conversion of maltose and glucose no formation of significative amounts of sorbitol was observed (
Table 1, entry 3), thus suggesting that the acidity of the system promotes side reactions but the hydrogenation one. The peculiar effect of the acid properties of this catalysts with respect to silica based ones was already observed in cellulose deconstruction [
30] as well as its poor hydrogenation ability [
31]. It is worth to underline that this catalytic system also has a markedly lower dispersion with respect to the others, showing an inhomogeneous particle size dimension, ranging from 3.2 to 13 nm of mean diameter.
The poor yield in sorbitol observed with Cu/Al
2O
3 (
Table 1, entries 1 vs 2) can be on the other hand ascribed to the amphoteric character of alumina. In this case again complete conversion of maltose and glucose was observed, while minor sorbitol production was obtained. Besides sorbitol, a few amounts of mannitol were detected, corroborating the tendency of the catalyst to facilitate isomerization reaction [
32].
Besides the acid-base properties, as already pointed out in the case of Cu/SiO
2-Al
2O
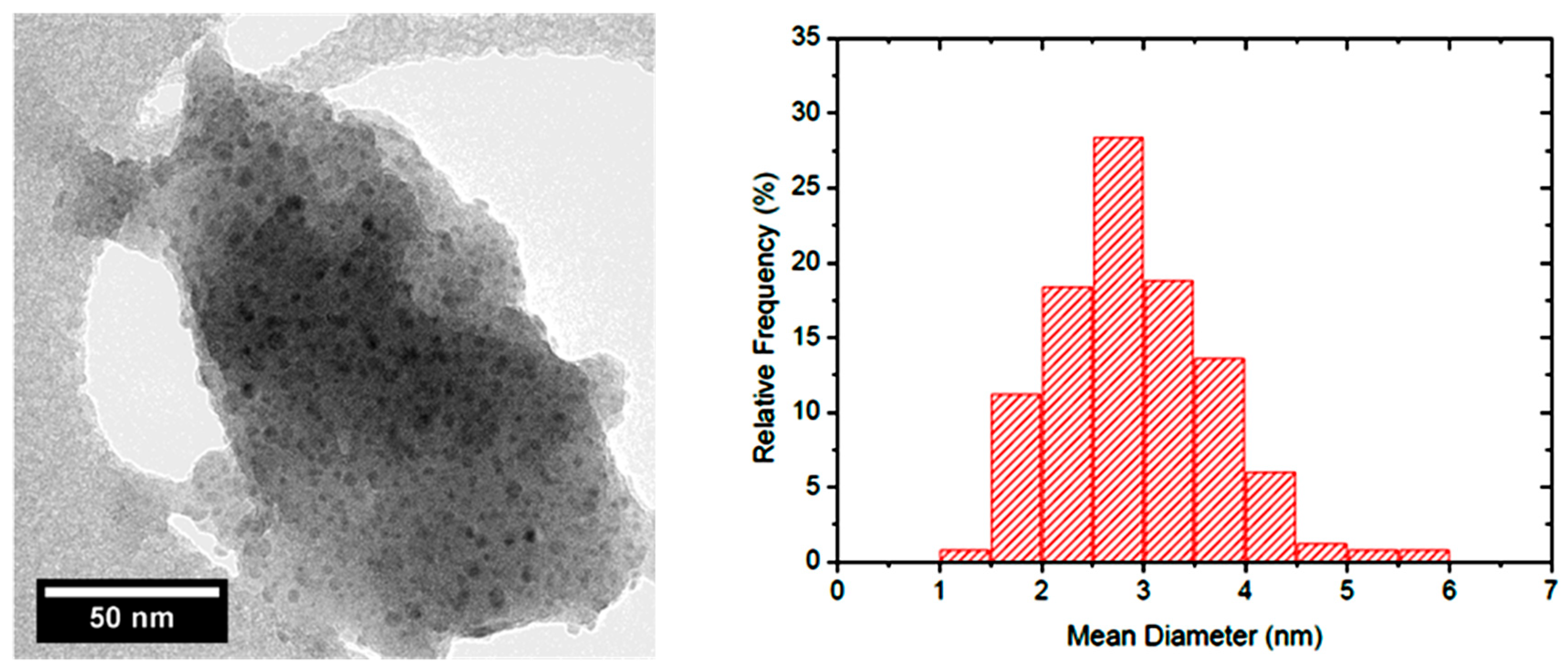
3, the reaction selectivity is strongly influenced by the copper dispersion, being this aspect pivotal in obtaining high hydrogenation activity and therefore in addressing the reaction towards sorbitol formation. This means that when having in hand a material with this hydrogenation capacity, the reduction rules over the isomerization and the cascading reactions. The most selective catalysts were found to be the two supported on silica, both showing particles with a mean diameter lower than 3 nm. In
Figure 2 the TEM micrograph of the system Cu/SiO
2 A is reported.
Another parameter that emerges to be critical for the reaction course is the surface polarity, expressed by the values of -OH group density. This value is related to the hydrophobic/hydrophilic character of the catalyst surface and puts in light another important effect. In particular the two silica materials show a non-negligible difference in this respect. The most performant catalyst results to be the one supported over the silica with the higher -OH density. The importance of the surface polarity in enhancing the reaction selectivity in copper catalyzed hydrogenations has already been highlighted by some of us for different substrates, namely in the reduction of vegetable oils [
25] and of enones [
33] and in the transformation of GVL into 1,4-pentandiol [
26]. In all these cases the effect of the different surface polarity of the supports plays a role in addressing selectivity due to its effect on the reagents and/or products adsorption and desorption phenomena.
Therefore, the combination of the very high copper dispersion imparting moderate acidity to the catalyst while expressing a very high hydrogenation activity, with a proper surface polarity reveals to be the right way to obtain complete conversion and very high selectivity to the reduced carbohydrates. The water tolerance of the catalyst is worth underlining. The metal-based systems stability in aqueous medium it is generally not trivial and this is even more true in the case of non-noble metals such as copper [
34,
35,
36]. The proposed Cu/SiO
2 not only allows one to obtain very high yields in sorbitol, but it is also stable and recyclable. Recycle experiments have been carried out by only spilling the solution after decanting the reaction mixture and charging a new maltose solution without any treatment or new reduction of the catalyst between two following runs.
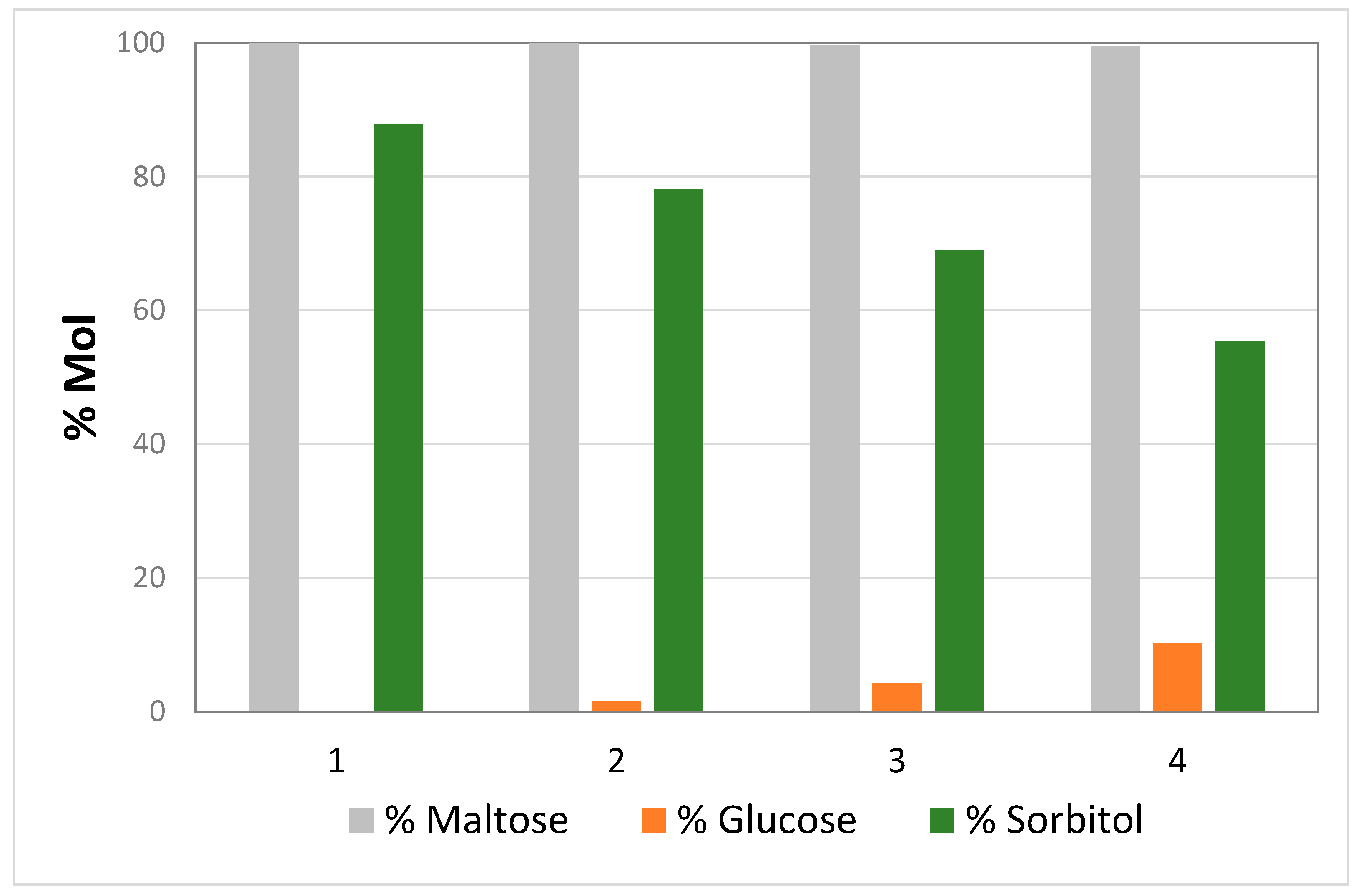
Figure 3 shows the good stability of the catalyst for at least three runs carried out under the above-described conditions.
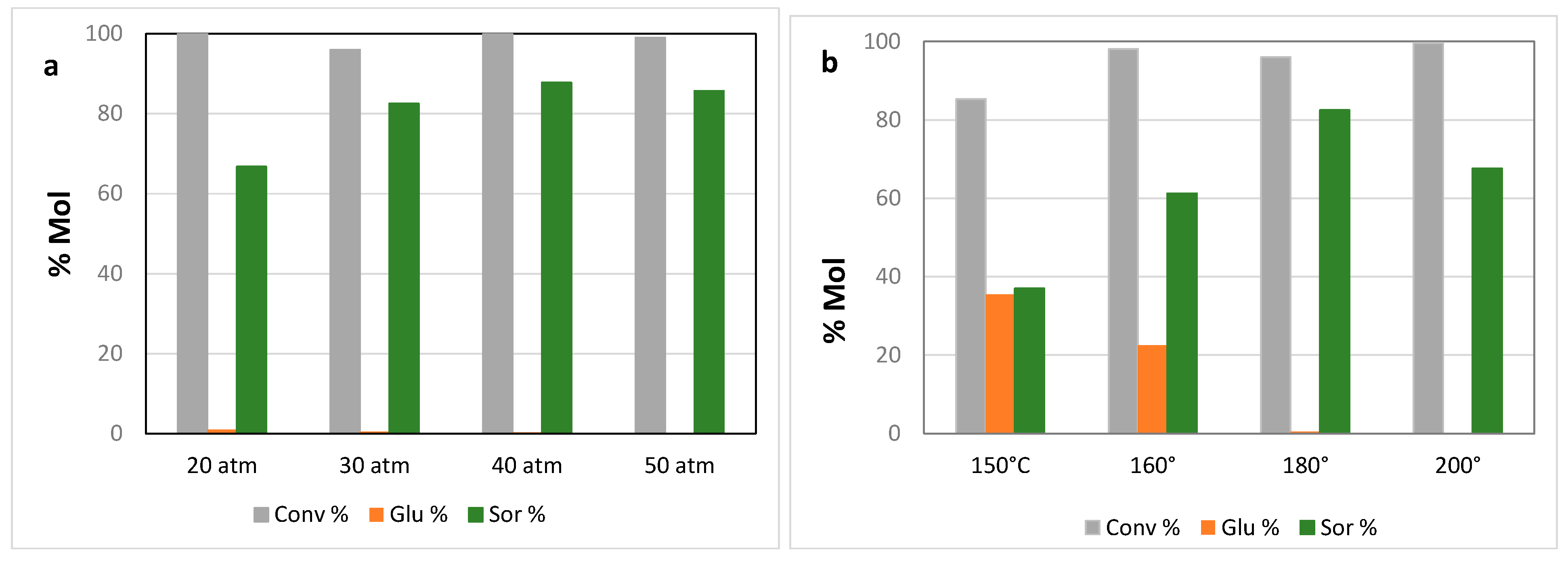
Figure 4 shows the results obtained by using the 16% copper loaded catalyst prepared over SiO
2 A under different conditions in terms of hydrogen pressure and temperature. Data reported clearly show that hydrogen pressure has a significant effect on sorbitol yield. Thus, by increasing the pressure from 20 to 40 atm the sorbitol content increases from 67 to 87%. A further increase in hydrogen pressure does not translate in a higher yield in sorbitol. It is interesting to note that conversion of both maltose and glucose are almost complete in all the pressure range explored, revealing that the hydrogen pressure strongly helps to address the reaction towards the desired hydrogenation limiting side products.
On the other hand, the reaction temperature has a different effect. By carrying out the reaction at 160°C and 40 atm of H2 results in a complete transformation of maltose, but in a lower yield in sorbitol due to a lower hydrogenation activity. By further lowering the temperature, that means by working at 150°C even the conversion does not result do be complete. On the other hand, a temperature higher such as 200°C leads to less sorbitol due to the partial formation of degradation products.
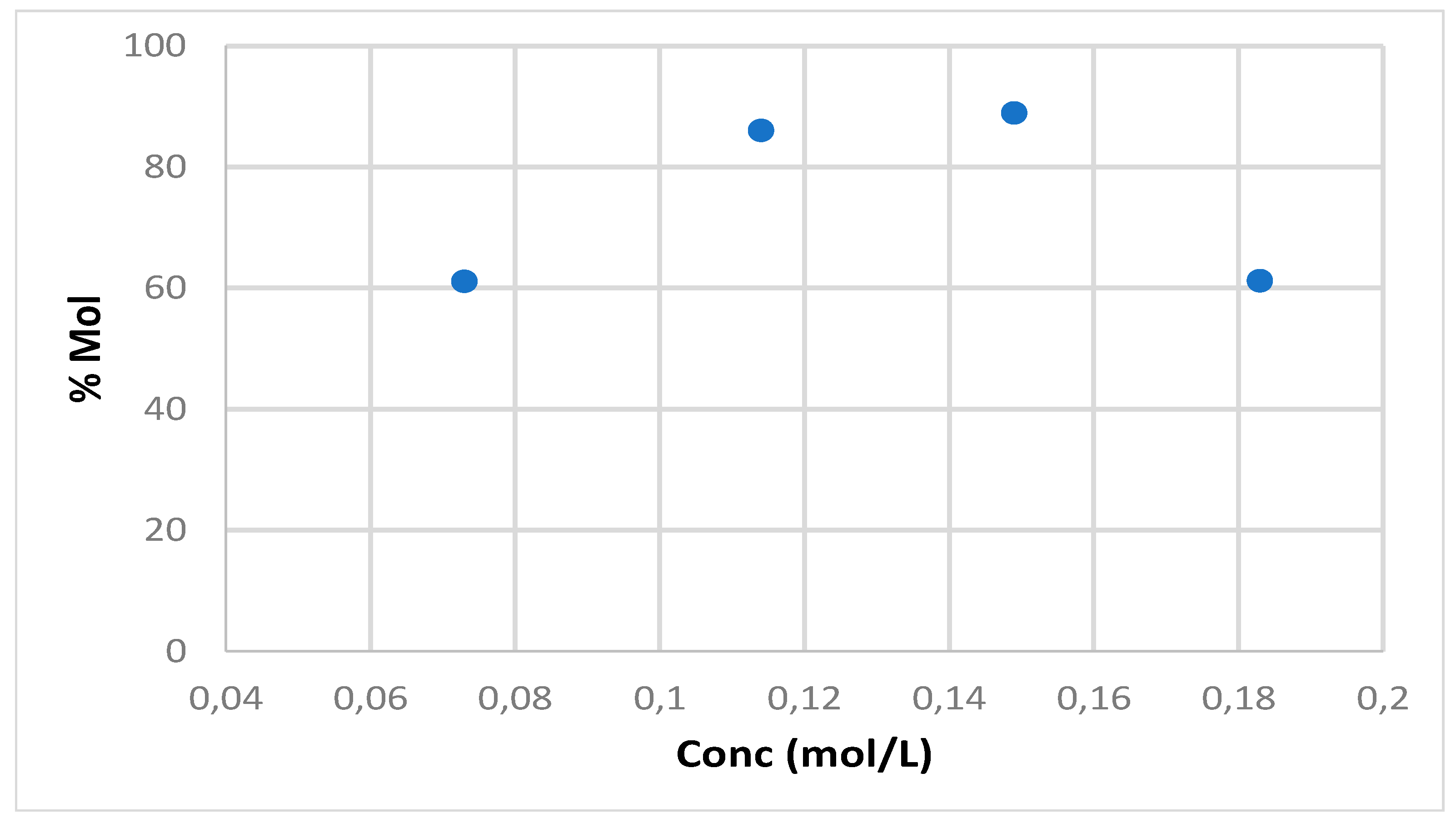
An interesting behavior was observed by varying the concentration of the starting maltose solution. In
Figure 5 a maximum sorbitol yield was obtained by increasing the maltose concentration up to 0.15 mol/L. On the other hand, after this value a significant drop in the product formation was detected.
The use of Cu/SiO
2 is therefore a viable route to selectively obtain sorbitol directly from maltose and the system proposed to the best of our knowledge represents the first example of copper-based catalyst for this purpose among the very few reported for this application so far. It is worth noting that under the optimized conditions in terms of H
2 pressure, temperature and maltose concentration Cu/SiO
2 A allows one to obtain up to 89% of sorbitol that is even higher than the copper systems reported for the only glucose to sorbitol hydrogenation. In fact a Cu/SiO
2 catalyst is reported to promote glucose hydrogenation with 89% of selectivity at 45% of glucose conversion [
14].
3. Materials and Methods
3.1. Materials
γ-Al2O3 (SSA = 210 m2/g) was purchased by Sasol; SiO2-Al2O3 (SSA = 496 m2/g) SiO2 A (SSA = 460 m2/g) were purchased from Sigma Aldrich; SiO2 B (SSA = 267 m2/g) was kindly supplied from Evonik.
Maltose was purchased from Sigma-Aldrich (Merck Life Science S.r.l.) and used without further purification.
3.2. Methods
Copper catalysts were prepared through the Chemisoprtion-Hydrolysis method [
24]. A [Cu(NH
3)
4]
2+ solution was prepared by dropping NH
4OH to a Cu(NO
3)
2 solution until reaching pH = 9. Then, the support was added and let under magnetic stirring for 20 min; the slurry was therefore diluted with 3 L of water in an ice bath at 0 °C, under mechanical stirring. After that, the solid was filtrated with a Büchner funnel, washed with water, dried overnight at 120 °C and finally calcined under air atmosphere at 350 °C for 4 h. The metal loading was confirmed by Inductively Coupled Plasma (ICP) analysis.
The catalysts were pre-reduced before the catalytic tests at 270 °C for 20 min under air and for 20 min under vacuum and then reduced through three hydrogen (1 bar) and vacuum cycles (5 mins each) at the same temperature.
Maltose reduction reactions were carried out in a stainless-steel Parr Instrument autoclave with an internal volume of 0.1 L. The pressure reactor was loaded with a slurry composed of the pre-reduced copper catalyst (0.2 g) and a solution of 1 g of maltose in 40 mL of distilled water. The autoclave was sealed, evacuated, and filled with hydrogen three times, then and charged with the desired pressure of hydrogen. The system was heated at the desired temperature and mechanically stirred (700 rpm). At the end of the catalytic test the autoclave was vented, and the catalyst separated by filtration. The solution was therefore analyzed by HPLC.
The HPLC analyses were performed by using an Agilent Technologies 1260 Infinity LC system (Santa Clara, CA, USA) equipped with a Quaternary gradient pump unit, an UV variable wavelength detector, a refractive index (RID) detector, and a Repromer Ca 9µm, 300 x 8.0 mm column hyphenated with a Repromer Ca 10 × 4.6 mm Guard Column (Dr. Maisch, High Performance LC GmbH, Germany). MilliQ water was used as the mobile phase and the separation was carried for 1 hours under the following conditions: flow rate 0.75 mL/min, column temperature 70 °C, wavelength detection λ 210 nm, RID temperature 35 °C. Before analysis, each sample was filtered through a 0.22 μm microporous membrane, diluted with milliQ water (0.1 ml in 1.500 ml) and injected (20 μl) with a 50 μL glass syringe (Agilent Technologies, Inc.). Conversions, yields, and selectivity were obtained by using the linear calibration curves (r2 > 0.99) of commercial maltose, sorbitol, and glucose as the standards in concentration ranging from 1*10-3 to 1*10-5 mmol/ml.
3.3. Catalyst characterisation
Transmission Electron Microscopy (TEM) analysis was performed using a ZEISS LIBRA200FE. The samples were gently smashed in an agate mortar, suspended in isopropyl alcohol for 20 min in an ultrasonic bath and dropped onto a lacey carbon-coated copper TEM grid. The samples were analysed after complete solvent evaporation.
Py-adsorption FT-IR- Studies of pyridine adsorption and desorption were carried out with an FTS-60 spectrophotometer equipped with mid-IR MCT detector purchased from BioRad. The experiments were performed on a sample disk (15–20 mg) after catalyst reduction (270°C, 20 min air + 20 min under vacuum + 3 H
2/vacuum cycles). One spectrum was collected before probe molecule adsorption as a blank experiment. Therefore, pyridine adsorption was carried out at room temperature, and the following desorption steps were performed from room temperature to 250°C. The spectrum of each desorption step was acquired every 50°C after cooling the sample. For quantitative analysis the amount of adsorbed pyridine (mmol
Py/g
cat) was calculated on the basis of the relationship reported by Emeis [
29] evaluated in the spectra registered at 150°C.
Thermogravimetric analysis (TGA) was performed on Perkin Elmer 7 HT thermobalance. Analyses were performed by heating the sample from 50–1000 °C with a temperature ramp of 5 °C/min. The water loss was evaluated in the range of 250–900 °C and mmolOH/gcat was calculated by the following formula: [(2 × Δwt %) × 10]/18 (g/mol).