Submitted:
15 June 2023
Posted:
16 June 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
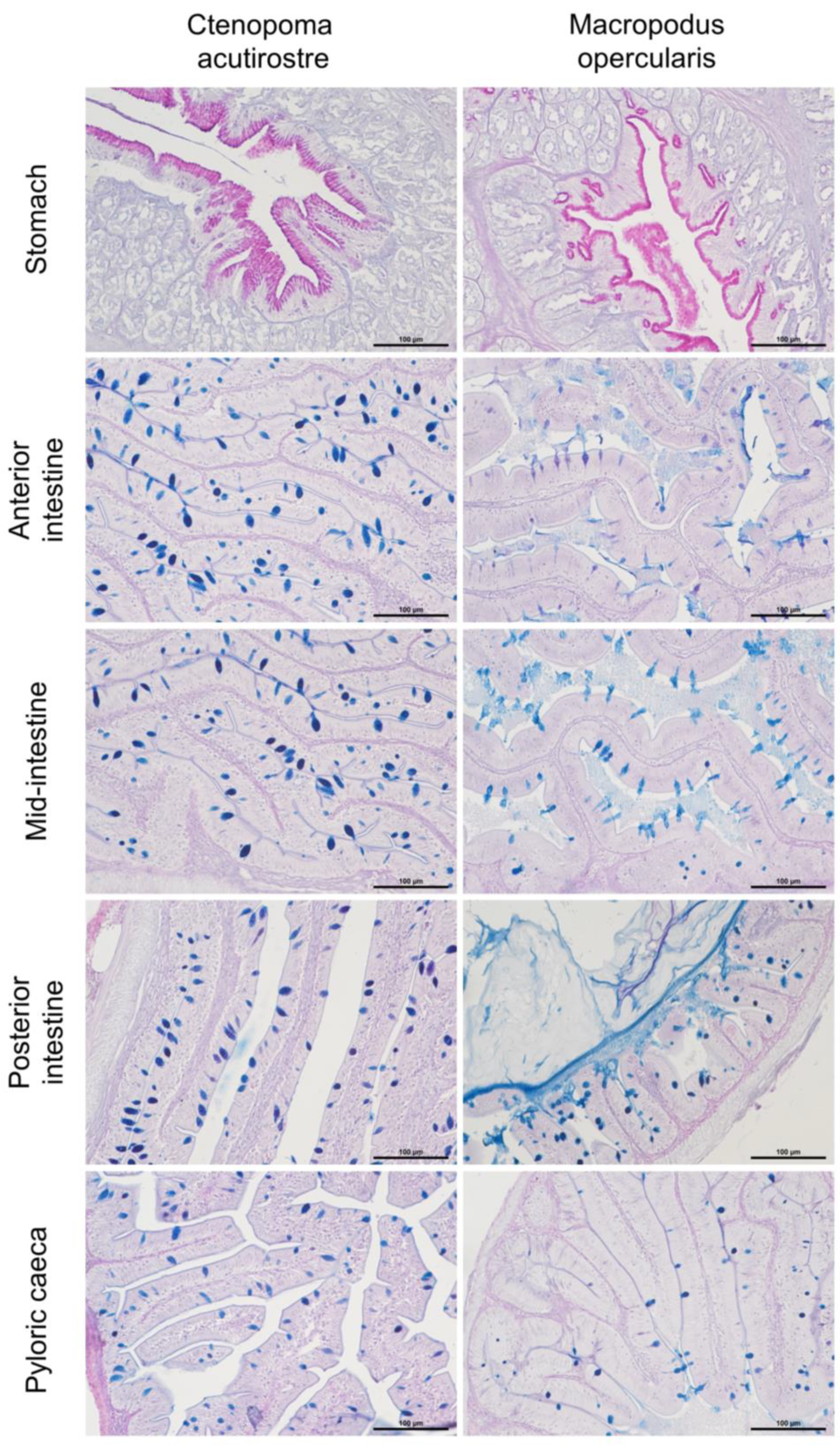
3. Results
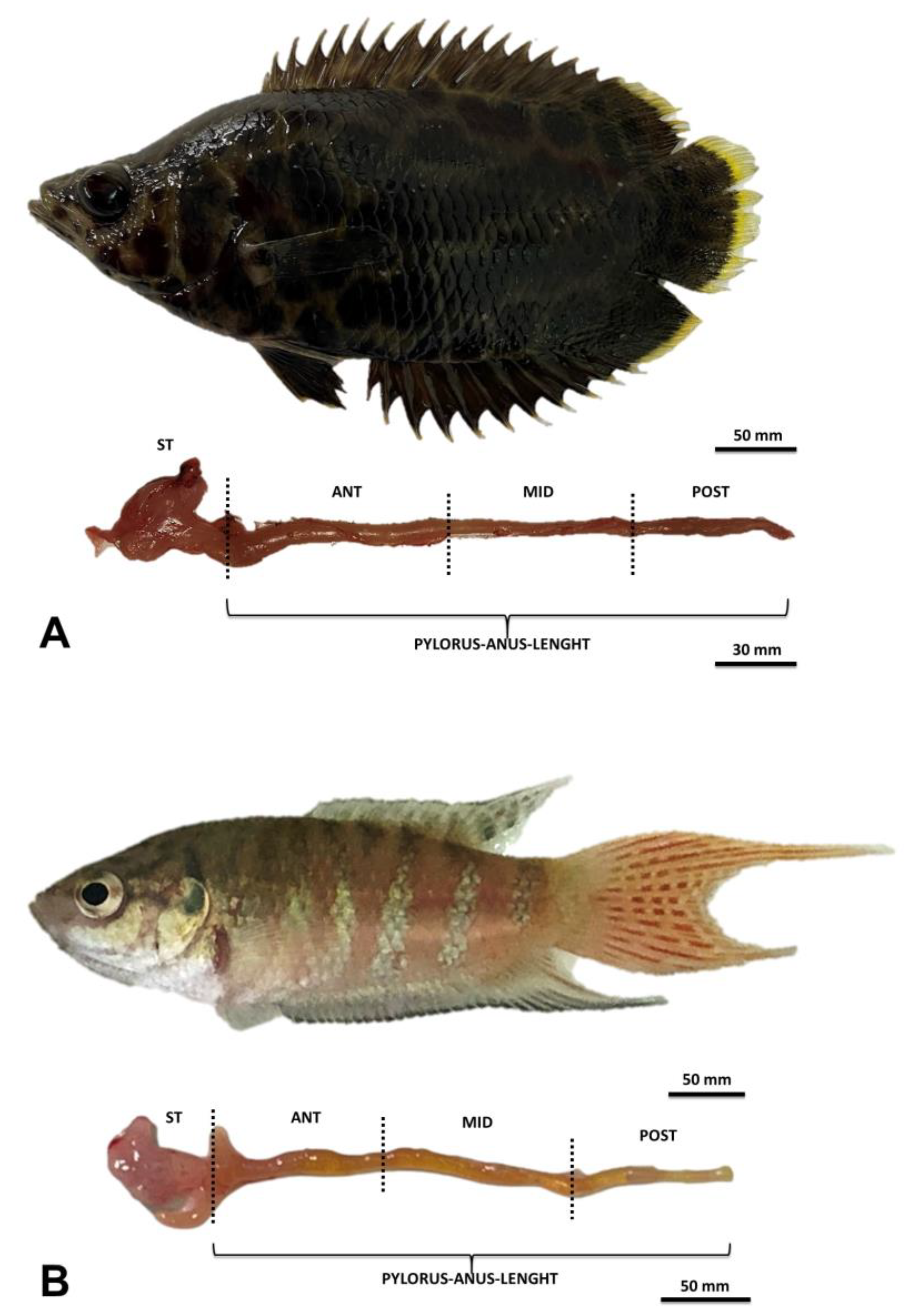
- The general structure of the digestive tract
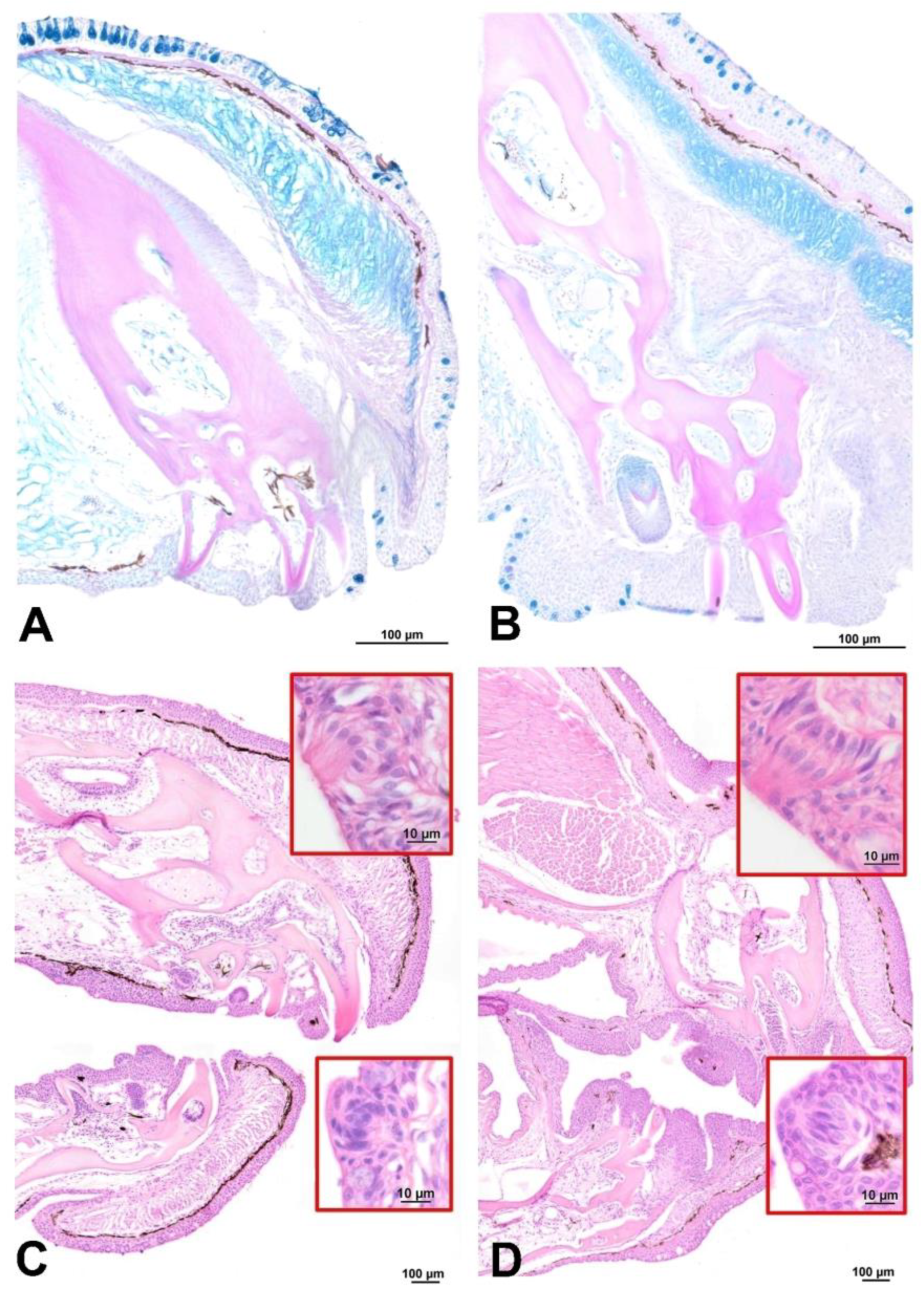
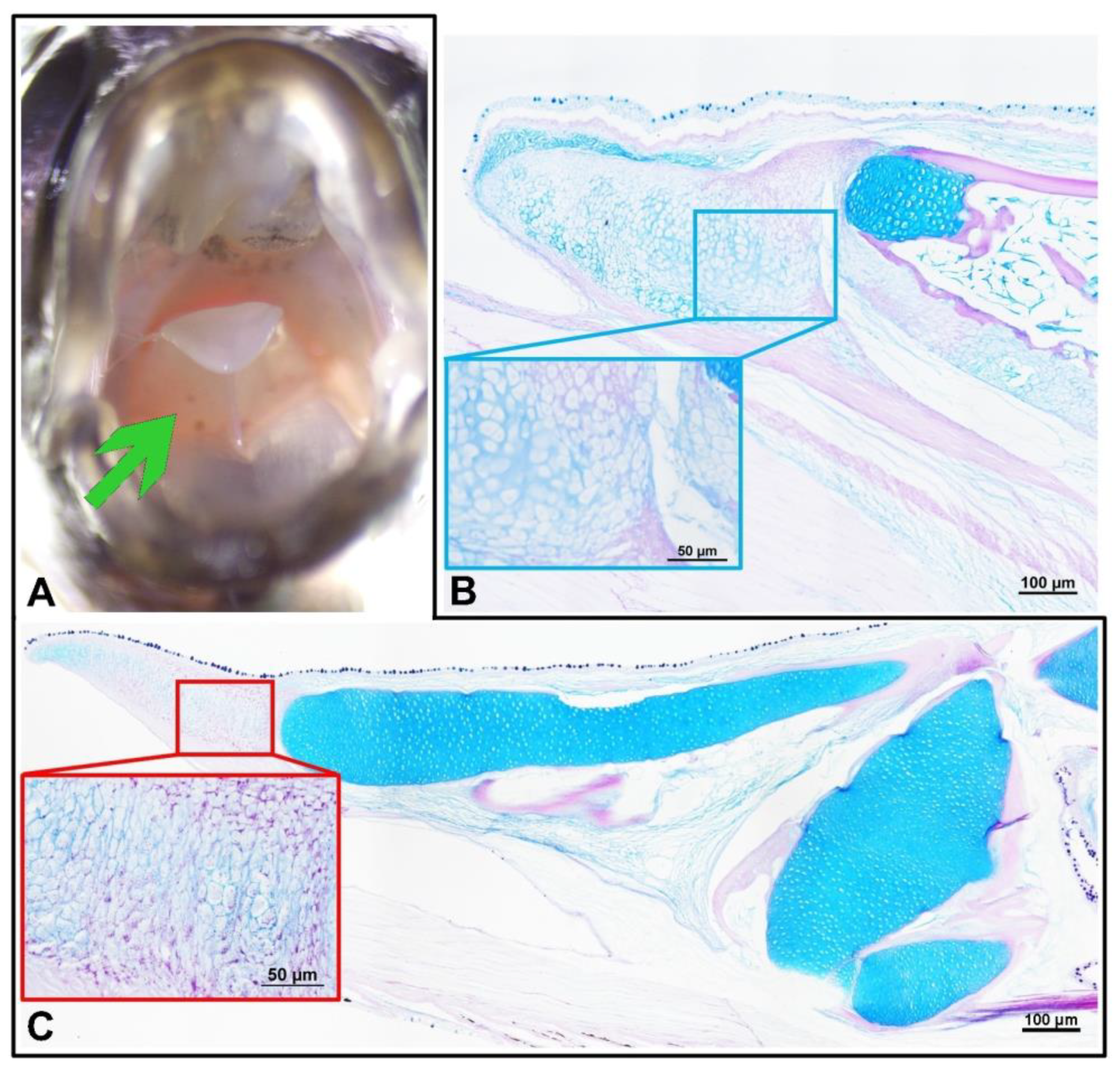
3.1. Headgut
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Yu, T.; Guo, Y. Early Normal Development of the Paradise Fish Macropodus opercularis. Russ. J. Dev. Biol. 2018 494 2018, 49, 240–244. [Google Scholar] [CrossRef]
- Evers, H.G.; Pinnegar, J.K.; Taylor, M.I. Where are they all from? – sources and sustainability in the ornamental freshwater fish trade. J. Fish Biol. 2019, 94, 909–916. [Google Scholar] [CrossRef] [PubMed]
- Jones, M.; Alexander, M.E.; Snellgrove, D.; Smith, P.; Bramhall, S.; Carey, P.; Henriquez, F.L.; McLellan, I.; Sloman, K.A. How should we monitor welfare in the ornamental fish trade? Rev. Aquac. 2022, 14, 770–790. [Google Scholar] [CrossRef]
- Kowalska, A.; Kamaszewski, M.; Czarnowska-Kujawska, M.; Podlasz, P.; Kowalski, R.K. Dietary ARA Improves COX Activity in Broodstock and Offspring Survival Fitness of a Model Organism (Medaka Oryzias latipes). Anim. 2020, Vol. 10, Page 2174 2020, 10, 2174. [Google Scholar] [CrossRef] [PubMed]
- Wiweger, M.; Majewski, L.; Adamek-Urbanska, D.; Wasilewska, I.; Kuznicki, J. npc2-Deficient Zebrafish Reproduce Neurological and Inflammatory Symptoms of Niemann-Pick Type C Disease. Front. Cell. Neurosci. 2021, 15. [Google Scholar] [CrossRef]
- Reeves, G.A.; Singh, P.P.; Brunet, A. Chromatin Accessibility Profiling and Data Analysis using ATAC-seq in Nothobranchius furzeri. 2023. [Google Scholar] [CrossRef]
- Žák, J.; Anil, A.N.; Dyková, I. Dissolved oxygen saturation is crucial for gas bladder inflation in turquoise killifish (Nothobranchius furzeri). Environ. Biol. Fishes 2023, 106, 673–683. [Google Scholar] [CrossRef]
- Rüber, L. Labyrinth fishes (Anabantoidei). Timetree Life 2009, 344–347. [Google Scholar]
- Nelson, J.S.; Grande, T.C.; Wilson, M.V.H. Fishes of the World; John Wiley & Sons, Inc: Hoboken, NJ, USA, 2016; Vol. 27, ISBN 9781119174844. [Google Scholar]
- Adamek-Urbańska, D.; Błażewicz, E.; Sobień, M.; Kasprzak, R.; Kamaszewski, M. Histological study of suprabranchial chamber membranes in anabantoidei and clariidae fishes. Animals 2021, 11, 1158. [Google Scholar] [CrossRef]
- Dadgar Sh., H. M.. Z.D.; A.R., G. Optimum dietary protein requirement of Paradise fish, Macropodus opercularis based on growth and reproduction performances. J. Surv. Fish. Sci. 2020, 7, 91–104. [Google Scholar] [CrossRef]
- Kitagawa, T.; Hosoya, K. Origin of the Ryukyuan paradise fish Macropodus opercularis inferred from literature survey. Biogeography 2016, 18, 11–16. [Google Scholar] [CrossRef]
- Miklosi, A.; Csanyi, V.; Gerlai, R. Antipredator behavior in paradise fish (Macropodus opercularis) larvae: The role of genetic factors and paternal influence. Behav. Genet. 1997, 27, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Dutta, H.M. The suspensorium of Ctenopoma acutirostre: A comparative functional analysis with Anabas testudineus. J. Morphol. 1975, 146, 457–477. [Google Scholar] [CrossRef] [PubMed]
- Prasetya, O.E.S.; Muarif, M.; Mumpuni, F.S. PENGARUH PEMBERIAN PAKAN CACING SUTERA (Tubifex sp.) DAN Daphnia sp. TERHADAP PERTUMBUHAN DAN TINGKAT KELANGSUNGAN HIDUP LARVA IKAN LELE SANGKURIANG (Clarias gariepinus). J. MINA SAINS 2020, 6, 8. [Google Scholar] [CrossRef]
- Santanumurti, M.B.; Nugroho, A.K.; Santoso, L.; Hudaidah, S. Study of Different Protein Content of Feeding of Local Raw Materials on Gourami Fish (Osphronemus goramy Lac.) Aquaculture Performance. IOP Conf. Ser. Earth Environ. Sci. 2022, 1036, 012119. [Google Scholar] [CrossRef]
- Hickley, P.; Bailey, R.G. Food and feeding relationships of fish in the Sudd swamps (River Nile, southern Sudan). J. Fish Biol. 1987, 30, 147–159. [Google Scholar] [CrossRef]
- Louca, V.; Lucas, M.C.; Green, C.; Majambere, S.; Fillinger, U.; Lindsay, S.W. Role of fish as predators of mosquito larvae on the floodplain of the Gambia River. J. Med. Entomol. 2009, 46, 546–556. [Google Scholar] [CrossRef] [PubMed]
- G.B. Kassi; M.G. Zeré; S.Y. Stanislas; V. N’douba Régime alimentaire de Ctenopoma petherici (Perciformes, Anabantidae) dans la rivière Agnéby et dans le lac de barrage hydroélectrique d’Ayamé 2 (Côte d’Ivoire). https://popups.uliege.be/2295-8010 2018. [CrossRef]
- Kilarski, W. Anatomia ryb; 2012; ISBN 9788309010807.
- Kasprzak, R.; Grzeszkiewicz, A.B.; Górecka, A. Performance of Co-Housed Neon Tetras ( Paracheirodon innesi) and Glowlight Rasboras ( Trigonostigma hengeli) Fed Commercial Flakes and Lyophilized Natural Food. Anim. an open access J. from MDPI 2021, 11. [Google Scholar] [CrossRef]
- Ray, A.K.; Ringø, E. The Gastrointestinal Tract of Fish. Aquac. Nutr. Gut Heal. Probiotics Prebiotics 2014, 1–13. [Google Scholar] [CrossRef]
- Karachle, P.K.; Stergiou, K.I. Intestine morphometrics: a compilation and analysis of bibliographic data. Acta Ichthyol. Piscat. 2010, 40, 45–54. [Google Scholar] [CrossRef]
- German, D.P.; Horn, M.H. Gut length and mass in herbivorous and carnivorous prickleback fishes (Teleostei: Stichaeidae): ontogenetic, dietary, and phylogenetic effects. Mar. Biol. 2006, 148, 1123–1134. [Google Scholar] [CrossRef]
- Cho, J.H.; Park, J.W.; Ryu, Y.W.; Kim, K.W.; Hur, S.W. Morphology, Histology, and Histochemistry of the Digestive Tract of the Marbled Flounder Pseudopleuronectes yokohamae. Anim. 2023, Vol. 13, Page 936 2023, 13, 936. [Google Scholar] [CrossRef] [PubMed]
- Karachle, P.K.; Stergiou, K.I. Gut length for several marine fish: relationships with body length and trophic implications. Mar. Biodivers. Rec. 2010, 3, e106. [Google Scholar] [CrossRef]
- Omoniyi, I.T.; Agbon, A.O.; Akegbejo-Samsons, Y. The Food Habits of Ctenopoma pethereci, Gunther (Pisces: Anabantidae) in River Oluwa, Ondo State, Nigeria. West African J. Appl. Ecol. 2012, 19. [Google Scholar] [CrossRef]
- Skelton, P.H. The taxonomic identity of dwarf or blackspot Ctenospoma (Pisces, Anabantidae) in Southern Africa. Cybium (Paris) 1988, 12, 73–89. [Google Scholar]
- Buddington, R.K.; Kuz’mina, V. Digestive System. Lab. Fish 2000, 379–384. [Google Scholar] [CrossRef]
- Meunier, F.J.; Brito, P.M.; Leal, M.-E.C. Morphological and histological data on the structure of the lingual toothplate of Arapaima gigas (Osteoglossidae; Teleostei). Cybium 2013, 37, 263–271. [Google Scholar]
- El Bakary, N.E.S.R. Morphological study of the asymmetrical buccal cavity of the flatfish common solea (Solea solea) and its relation to the type of feeding. Asian Pac. J. Trop. Biomed. 2014, 4, 13. [Google Scholar] [CrossRef]
- MEYER-ROCHOW, V.B. Fish tongues—surface fine structures and ecological considerations. Zool. J. Linn. Soc. 1981, 71, 413–426. [Google Scholar] [CrossRef]
- Levanti, M.; Germanà, A.; Montalbano, G.; Guerrera, M.C.; Cavallaro, M.; Abbate, F. The Tongue Dorsal Surface in Fish: A Comparison Among Three Farmed Species. Anat. Histol. Embryol. 2017, 46, 103–109. [Google Scholar] [CrossRef]
- Abbate, F.; Guerrera, M.C.; Montalbano, G.; Ciriaco, E.; Germanà, A. Morphology of the tongue dorsal surface of gilthead seabream (Sparus aurata). Microsc. Res. Tech. 2012, 75, 1666–1671. [Google Scholar] [CrossRef] [PubMed]
- Abbate, F.; Guerrera, M.C.; Montalbano, G.; De Carlos, F.; Suárez, A.Á.; Ciriaco, E.; Germanà, A. Morphology of the european sea bass (dicentrarchus labrax) tongue. Microsc. Res. Tech. 2012, 75, 643–649. [Google Scholar] [CrossRef] [PubMed]
- Camp, A.L.; Brainerd, E.L. Role of axial muscles in powering mouth expansion during suction feeding in largemouth bass (Micropterus salmoides). J. Exp. Biol. 2014, 217, 1333–1345. [Google Scholar] [CrossRef] [PubMed]
- Sadeghinezhad, J.; Rahmati-Holasoo, H.; Fayyaz, S.; Zargar, A. Morphological study of the northern pike (Esox lucius) tongue. Anat. Sci. Int. 2015, 90, 235–239. [Google Scholar] [CrossRef] [PubMed]
- Palasai, A.; Lampang, P.N.; Gerald Plumley, F.; Kettratad, J.; Senarat, S.; Kangwanrangsan, N.; Jiraungkoorskul, W. Feeding apparatus, digestive system structure, and gut contents of Priapium fish, Neostethus lankesteri Regan 1916. Songklanakarin J. Sci. Technol 44, 602–608.
- Le, H.T.M.D.; Shao, X.; Krogdahl, Å.; Kortner, T.M.; Lein, I.; Kousoulaki, K.; Lie, K.K.; Sæle, Ø. Intestinal function of the stomachless fish, ballan wrasse (Labrus bergylta). Front. Mar. Sci. 2019, 6, 438019. [Google Scholar] [CrossRef]
- Y, H. INFLUENCES OF GUARDING TERRITORY ON REPRODUCTIVE ACTIVITY IN THE MALE DWARF GOURAMI, COLISA LALIA. Indian J. Sci. Technol. 2011, 4, 113–113. [Google Scholar] [CrossRef]
- Nugraha, A.A.; Yustiati, A.; Bangkit, I.; Andriani, Y. Growth performance and survival rate of giant gourami fingerlings (Osphronemus goramy Lacepede, 1801) with potassium diformate addition. World Sci. News 2020, 143. [Google Scholar]
- Boonanuntanasarn, S.; Jangprai, A.; Yoshizaki, G. Characterization of proopiomelanocortin in the snakeskin gourami (Trichopodus pectoralis) and its expression in relation to food intake. Domest. Anim. Endocrinol. 2015, 50, 1–13. [Google Scholar] [CrossRef]
- Tan, X.; Hoang, L.; Du, S.J. Characterization of muscle-regulatory genes, Myf5 and Myogenin, from striped bass and promoter analysis of muscle-specific expression. Mar. Biotechnol. 2002, 4, 537–545. [Google Scholar] [CrossRef]
- Moreira, E.; Novillo, · Manuel; Eastman, J.T.; Barrera-Oro, E. Degree of herbivory and intestinal morphology in nine notothenioid fishes from the western Antarctic Peninsula. 2020, 43, 535–544. [CrossRef]
- Rust, M.B. Nutritional Physiology. Fish Nutr. 2003, 367–452. [Google Scholar] [CrossRef]
| Total length [cm] | Standard length [cm] | Fork length [cm] | Body weight [g] | |
|---|---|---|---|---|
| Ctenopoma acutirostre | 5,39 ± 0.23 | 4,58 ± 0.24 | 0,82 ± 0.09 | 2,88 ± 0.56 |
| Macropodus opercularis | 5.47 ± 0.49 | 3,68 ± 0.18 | 1,79 ± 0.37 | 1,30 ± 0.49 |
| Length [cm] | Ctenopoma acutirostre | Macropodus opercularis |
|---|---|---|
| SL [cm] | 4.58 ± 0.24 | 3.68 ± 0.18 |
| IL [cm] | 3.38 ± 0.47 | 4.14 ± 0.70 |
| Pyloric caeca length [cm] | 0.25 ± 0.13 | 0.41 ± 0.09 |
| IL/SL | 0.74 ± 0.9 | 1.13 ± 0.14 |
| Range of IL/SL | 0.59-0.83 | 0.93-1.31 |
| % of body length | 59-83 | 93-131 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





