Submitted:
16 June 2023
Posted:
16 June 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
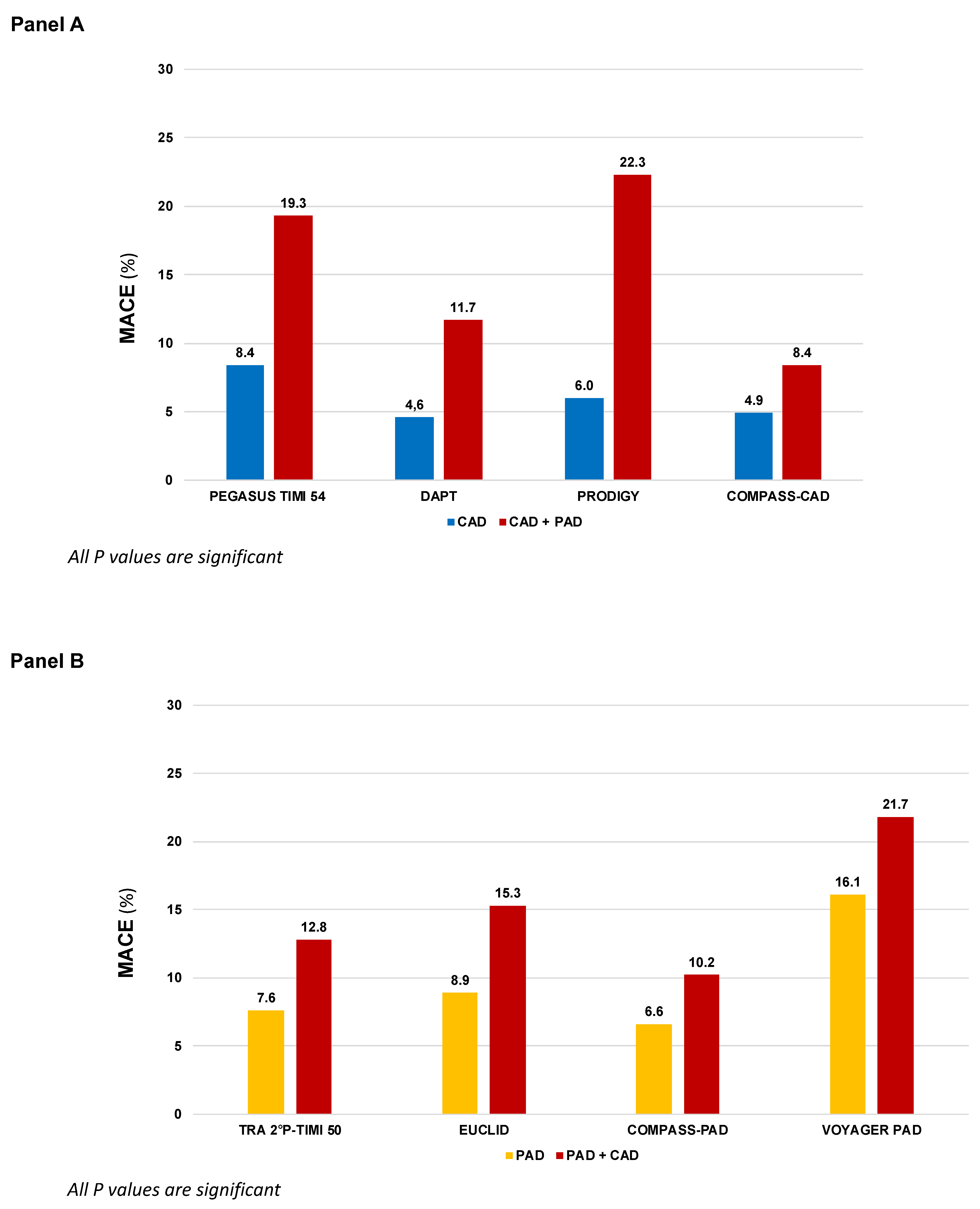
| Trial | First Author, year | Population | Total N | Proportion with both CAD and PAD |
Comparison | Median FUP |
|---|---|---|---|---|---|---|
| Single antiplatelet therapy (more vs. less potent) | ||||||
| CAPRIE | CAPRIE Steering Committee, 1996 | Recent MI (N=6,302) or Symptomatic LE PAD (N=6,452) or Ischemic stroke (N=6,431) |
19,185 | N.A. | Clopidogrel 75 mg vs. Asprin 325 |
22.9 |
| EUCLID | Hiatt WR, 2017 Berger J, 2018 |
Symptomatic LE PAD | 13,885 | 29.0% | Ticagrelor 90 mg b.i.d vs. Clopidogrel 75 mg |
30 |
| DAPT vs. Single antiplatelet therapy | ||||||
| CHARISMA | Bhatt DL, 2006 Cacoub P, 2009 |
CCS (N=5,835) or LE PAD (N=2,838) or Ischemic stroke/TIA (N=4,290) or multiple atherosclerotic risk factors (N=3,284) |
15,603 | N.A. | Clopidogrel 75 mg + Aspirin 75-162 mg vs. Aspirin 75-162 mg |
28 |
| PRODIGY | Valgimigli M, 2012 Franzone A, 2016 |
CCS (N=505) or ACS (N=1,465) |
1,970 | 12.5% | Clopidogrel 75 mg + Aspirin 80-160 mg for 24 months vs. 6 months |
28 |
| DAPT | Secemsky EA, 2017 | Patients free from ischemic and bleeding events 12 months after coronary stenting | 11,648 | 5.57% | Continued thienopyridine (clopidogrel 75 mg or prasugrel 10 mg) + Aspirin therapy for an additional 18 months vs. Aspirin 100 mg | 18 |
| TRA-2°P-TIMI 50 | Morrow D, 2012 Magnani G, 2015 Qamar A, 2020 |
Stable MI (N=17,779) or LE PAD (N=3,787) or Ischemic stroke (N=4,883) |
26,449 | 76.3% of 6,136 patients with PAD regardless of stratum |
Vorapaxar 2.5 mg vs. Placebo | 30 |
| PEGASUS TIMI-54 | Bonaca MP, 2015 & 2016 | Stable MI | 21,162 | CAD + PAD 5.2% |
Ticagrelor 60 or 180 mg b.i.d. + Aspirin 75-150 mg o.d. vs. or Aspirin 75-150 mg o.d. | 33 |
| Therapy with aspirin combined with an anticoagulant drug | ||||||
| COMPASS | Eikelboom JW, 2017 Connolly S, 2018 Anand SS, 2018 |
Stable CAD (N=24,828) or [Symptomatic LE PAD or carotid artery disease or ABI <0.9 with CAD (N=7,470)] |
27,395 | COMPASS-CAD: 17.9% COMPASS-PAD: 44.1% |
Rivaroxaban 2.5 b.i.d. + Aspirin 100 mg or Rivaroxaban 5 mg b.i.d. vs. Aspirin 100 mg |
23 |
| VOYAGER-PAD | Bonaca MP, 2020 | LE PAD with recent peripheral revascularization | 6,564 | PAD + CAD 23.6% | Rivaroxaban 2.5 mg b.i.d + Aspirin 100 mg vs. Aspirin 100 mg | 28 |
2. Single Antiplatelet Strategy
3. Dual Antiplatelet Therapy
4. Therapy with Aspirin Combined with an Anticoagulant Drug
5. A Practical Approach
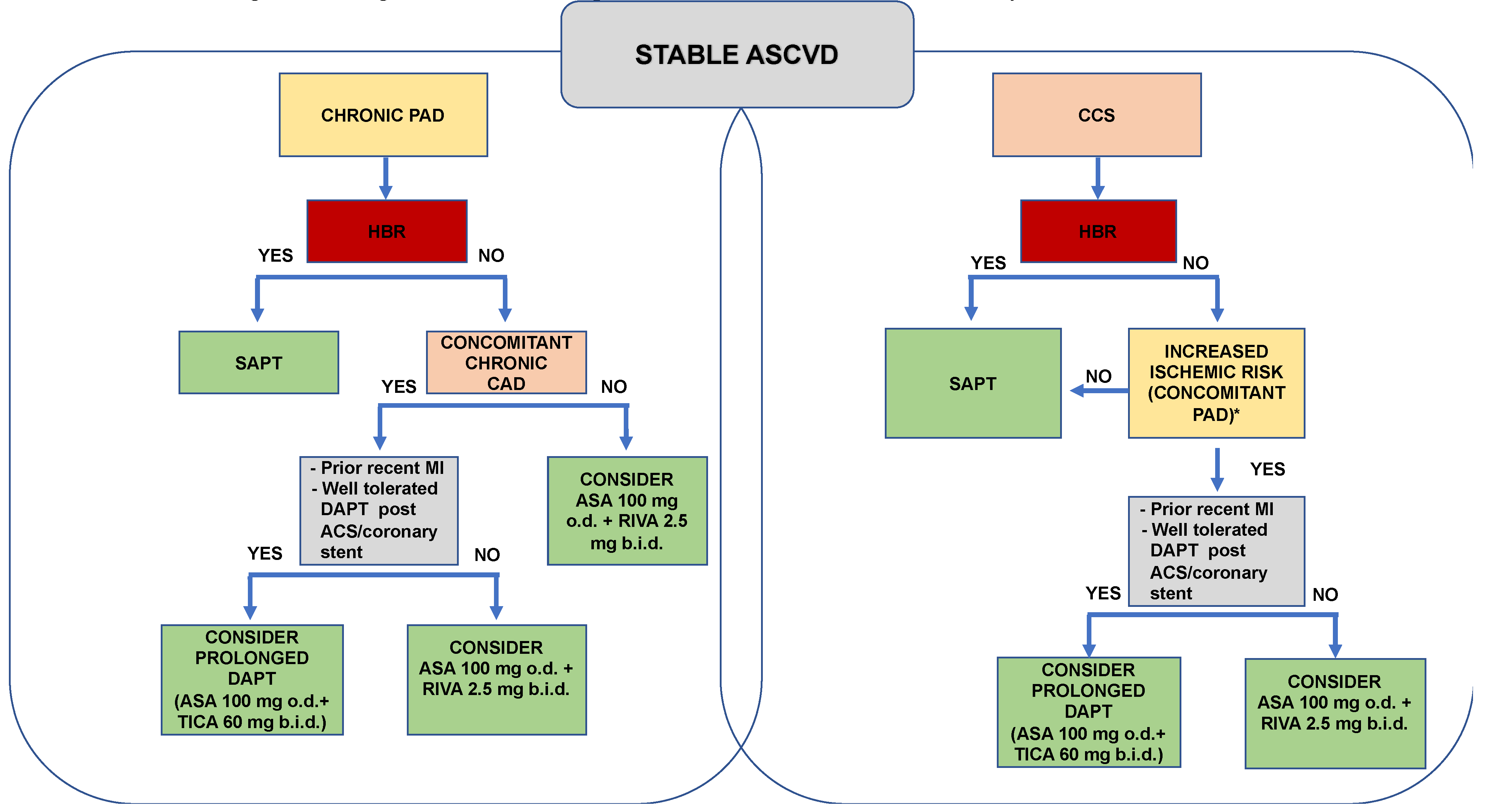
6. Conclusions
References
- Libby, P.; Buring, J.E.; Badimon, L.; Hansson, G.K.; Deanfield, J.; Bittencourt, M.S.; Tokgözoğlu, L.; Lewis, E.F. Atherosclerosis. Nat. Rev. Dis. Primers 2019, 5, 56. [Google Scholar] [CrossRef]
- Suárez, C.; Zeymer, U.; Limbourg, T.; Baumgartner, I.; Cacoub, P.; Poldermans, D.; Röther, J.; Bhatt, D.L.; Steg, P.G.; Investigatorsa, R.R. Influence of polyvascular disease on cardiovascular event rates. Insights from the REACH Registry. Vasc. Med. 2010, 15, 259–265. [Google Scholar] [CrossRef]
- Cotter, G.; Cannon, C.P.; McCabe, C.H.; Michowitz, Y.; Kaluski, E.; Charlesworth, A.; Milo, O.; Bentley, J.; Blatt, A.; Krakover, R.; et al. OPUS-TIMI 16 Investigators. Prior peripheral arterial disease and cerebrovascular disease are independent predictors of adverse outcome in patients with acute coronary syndromes: are we doing enough? Results from the Orbofiban in Patients with Unstable Coronary Syndromes-Thrombolysis In Myocardial Infarction (OPUS-TIMI) 16 study. Am. Hear. J. 2003, 145, 622–627. [Google Scholar] [CrossRef]
- Hess, C.N.; Bonaca, M.P. Contemporary Review of Antithrombotic Therapy in Peripheral Artery Disease. Circ. Cardiovasc. Interv. 2020, 13, e009584. [Google Scholar] [CrossRef] [PubMed]
- Aronow, W.; Ss, A.; F, C.; Jw, E.; J, B.; L, D.; V, A.; Mt, A.; Krh, B.; K, K.; et al. Faculty Opinions recommendation of Major adverse limb events and mortality in patients with peripheral artery disease: the compass trial. J Am Coll Cardiol. 2018, 71, 2306. [Google Scholar] [CrossRef]
- Knuuti, J.; Wijns, W.; Saraste, A.; Capodanno, D.; Barbato, E.; Funck-Brentano, C.; Prescott, E.; Storey, R.F.; Deaton, C.; Cuisset, T.; et al. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart J. 2020, 41, 407–477. [Google Scholar] [CrossRef] [PubMed]
- Aboyans, V.; Ricco, J.B.; Bartelink, M.E.L.; et al.; ESC Scientific Document Group 2017 ESC Guidelines on the Diagnosis and Treatment of Peripheral Arterial Diseases, in collaboration with the European Society for Vascular Surgery (ESVS): Document covering atherosclerotic disease of extracranial carotid and vertebral, mesenteric, renal, upper and lower extremity arteries Endorsed by: the European Stroke Organization (ESO)The Task Force for the Diagnosis and Treatment of Peripheral Arterial Diseases of the European Society of Cardiology (ESC) and of the European Society for Vascular Surgery (ESVS). Eur Heart J. 2018, 39, 763–816. [Google Scholar]
- Antithrombotic Trialists’ Collaboration. Collaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. BMJ 2002, 324, 71–86. [Google Scholar] [CrossRef] [PubMed]
- Berger, J.S.; Kranty, M.J.; Kittelson, J.M.; et al. Aspirin for the prevention of cardiovascualr events in patients with peripheral artery disease: a meta-analysis of randomized trials. JAMA 2009, 301, 1909–1919. [Google Scholar] [CrossRef]
- CAPRIE Steering Committee. CAPRIE Steering Committee A randomised, blinded, trial of clopidogrel versus aspirin in patients at risk of ischaemic events (CAPRIE). Lancet 1996, 348, 1329–1339. [Google Scholar] [CrossRef]
- Ferreiro, J.L.; Bhatt, D.L.; Ueno, M.; et al. Impact of smoking on long-term outcomes in patients with atherosclerotic vascular disease treated with aspirin or clopidogrel: insights from the CAPRIE trial (Clopidogrel Versus Aspirin in Patients at Risk of Ischemic Events). J Am Coll Cardiol. 2014, 63, 769–777. [Google Scholar] [CrossRef]
- Bauersachs, R.; Wu, O.; Briere, J.-B.; Bowrin, K.; Borkowska, K.; Jakubowska, A.; Taieb, V.; Toumi, M.; Huelsebeck, M. Antithrombotic Treatments in Patients with Chronic Coronary Artery Disease or Peripheral Artery Disease: A Systematic Review of Randomised Controlled Trials. Cardiovasc. Ther. 2020, 2020, 1–11. [Google Scholar] [CrossRef]
- Hess, C.N.; Hiatt, W.R. Antithrombotic Therapy for Peripheral Artery Disease in 2018. JAMA 2018, 319, 2329–2330. [Google Scholar] [CrossRef]
- Clavijo, L.C.; Al-Asady, N.; Dhillon, A.; Matthews, R.V.; Caro, J.; Tun, H.; Rowe, V.; Shavelle, D.M. Prevalence of high on-treatment (aspirin and clopidogrel) platelet reactivity in patients with critical limb ischemia. Cardiovasc. Revascularization Med. 2018, 19, 516–520. [Google Scholar] [CrossRef] [PubMed]
- Hiatt, W.R.; Fowkes, F.G.R.; Heizer, G.; Berger, J.S.; Baumgartner, I.; Held, P.; Katona, B.G.; Mahaffey, K.W.; Norgren, L.; Jones, W.S.; et al. EUCLID Trial Steering Committee and Investigators. Ticagrelor versus Clopidogrel in Symptomatic Peripheral Artery Disease. New Engl. J. Med. 2017, 376, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Capodanno, D.; Bhatt, D.L.; Eikelboom, J.W.; Fox, K.A.A.; Geisler, T.; Gibson, C.M.; Gonzalez-Juanatey, J.R.; James, S.; Lopes, R.D.; Mehran, R.; et al. Dual-pathway inhibition for secondary and tertiary antithrombotic prevention in cardiovascular disease. Nat. Rev. Cardiol. 2020, 17, 242–257. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, D.L.; Fox, K.A.; Hacke, W.; et al.; CHARISMA Investigators Clopidogrel and aspirin versus aspirin alone for the prevention of atherothrombotic events. N Engl J Med. 2006, 354, 1706–1717. [Google Scholar] [CrossRef] [PubMed]
- Cacoub, P.P.; Bhatt, D.L.; Steg, P.; Topol, E.J.; Creager, M.A. ; for the CHARISMA Investigators Patients with peripheral arterial disease in the CHARISMA trial. Eur. Hear. J. 2009, 30, 192–201. [Google Scholar] [CrossRef]
- Bonaca, M.P.; Bhatt, D.L.; Storey, R.F.; et al. Faculty Opinions recommendation of Ticagrelor for prevention of ischemic events after myocardial infarction in patients with peripheral artery disease. J Am Coll Cardiol. 2016, 67, 2719–2728. [Google Scholar] [CrossRef]
- Secemsky, E.A.; Yeh, R.W.; Kereiakes, D.J.; et al.; Dual Antiplatelet Therapy Study Investigators Extended Duration Dual Antiplatelet Therapy After Coronary Stenting Among Patients With Peripheral Arterial Disease: A Subanalysis of the Dual Antiplatelet Therapy Study. JACC Cardiovasc Interv. 2017, 10, 942–954. [Google Scholar] [CrossRef]
- Franzone, A.; Piccolo, R.; Gargiulo, G.; et al. Prolonged vs Short Duration of Dual Antiplatelet Therapy After Percutaneous Coronary Intervention in Patients With or Without Peripheral Arterial Disease: A Subgroup Analysis of the PRODIGY Randomiyed Clinical Trial. JAMA Cardiol 2016, 1, 795–803. [Google Scholar] [CrossRef] [PubMed]
- Bonaca, M.P.; Creager, M.A.; Olin, J.; et al. Peripheral Revascularization in Patients With Peripheral Artery Disease With Vorapaxar: Insights From the TRA 2°P-TIMI 50 Trial. JACC Cardiovasc Interv. 2016, 9, 2157–2164. [Google Scholar] [CrossRef]
- Qamar, A.; A Morrow, D.; A Creager, M.; Scirica, B.M.; Olin, J.W.; A Beckman, J.; A Murphy, S.; Bonaca, M.P. Effect of vorapaxar on cardiovascular and limb outcomes in patients with peripheral artery disease with and without coronary artery disease: Analysis from the TRA 2°P-TIMI 50 trial. Vasc. Med. 2020, 25, 124–132. [Google Scholar] [CrossRef] [PubMed]
- Criqui, M.H.; Matsushita, K.; Aboyans, V.; et al.; American Heart Association Council on Epidemiology and Prevention; Council on Arteriosclerosis, Thrombosis and Vascular Biology; Council on Cardiovascular Radiology and Intervention; Council on Lifestyle and Cardiometabolic Health; Council on Peripheral Vascular Disease; and Stroke Council Lower Extremity Peripheral Artery Disease: Contemporary Epidemiology, Management Gaps, and Future Directions: A Scientific Statement From the American Heart Association. Circulation 2021, 144, e171–e191. [Google Scholar] [CrossRef] [PubMed]
- Merlini, P.A.; Bauer, K.A.; Oltrona, L.; Ardissino, D.; Cattaneo, M.; Belli, C.; Mannucci, P.M.; Rosenberg, R.D. Persistent activation of coagulation mechanism in unstable angina and myocardial infarction. Circulation 1994, 90, 61–68. [Google Scholar] [CrossRef]
- Ardissino, D.; Merlini, P.A.; Bauer, K.A.; Galvani, M.; Ottani, F.; Franchi, F.; Bertocchi, F.; Rosenberg, R.D.; Mannucci, P.M. Coagulation activation and long-term outcome in acute coronary syndromes. Blood 2003, 102, 2731–2735. [Google Scholar] [CrossRef]
- Weitz, J.I. Insights into the role of thrombin in the pathogenesis of recurrent ischaemia after acute coronary syndrome. Thromb. Haemost. 2014, 112, 924–931. [Google Scholar] [CrossRef]
- Mega, J.L.; Braunwald, E.; Wiviott, S.D.; Bassand, J.-P.; Bhatt, D.L.; Bode, C.; Burton, P.; Cohen, M.; Cook-Bruns, N.; Fox, K.A.A.; et al. ATLAS ACS 2–TIMI 51 Investigators. Rivaroxaban in Patients with a Recent Acute Coronary Syndrome. N. Engl. J. Med. 2012, 366, 9–19. [Google Scholar] [CrossRef]
- Eikelboom, J.W.; Connolly, S.J.; Bosch, J.; et al. ; COMPASS Investigators. Rivaroxaban with or without Aspirin in Stable Cardiovascular Disease. N Engl J Med. 2017, 377, 1319–1330. [Google Scholar]
- Bonaca, M.P.; Bauersachs, R.M.; Anand, S.S.; et al. Rivaroxaban in Peripheral Artery Disease after Revascularization. N Engl J Med. 2020, 382, 1994–2004. [Google Scholar] [CrossRef]
- Magnani, G.; Ardissino, D.; Im, K.; Budaj, A.; Storey, R.F.; Steg, P.G.; Bhatt, D.L.; Cohen, M.; Ophius, T.O.; Goudev, A.; et al. Predictors, Type, and Impact of Bleeding on the Net Clinical Benefit of Long-Term Ticagrelor in Stable Patients With Prior Myocardial Infarction. J. Am. Hear. Assoc. 2021, 10, e017008. [Google Scholar] [CrossRef] [PubMed]
- Bonaca, M.P.; Im, K.; Magnani, G.; Bansilal, S.; Dellborg, M.; Storey, R.F.; Bhatt, D.L.; Steg, P.G.; Cohen, M.; Johanson, P.; et al. Patient selection for long-term secondary prevention with ticagrelor: insights from PEGASUS-TIMI 54. Eur. Hear. J. 2022, 43, 5037–5044. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





