1. Introduction
Kingdom Fungi is diverse and includes different types of organisms like molds, yeasts, mushrooms, polypores, plant parasitic rusts, and smuts, totalizing more than 120,000 described species and between 2.2 and 5.1 million estimated species [
1,
2]. This group includes saprotrophic, pathogenic, parasitic, commensal, and symbiotic organisms that can be found in the most variable ecosystems, from marine or freshwater to terrestrial environments [
1,
3,
4]. Their morphological traits allied to an exceptional metabolic diversity enabled them to occupy numerous ecological niches and to create a panoply of interactions with other organisms [
1,
4].
Due to their unique but diverse characteristics, fungi are an essential component for the well-functioning of the ecosystems and have diversified roles in them. In terrestrial environments, fungi are crucial for the soil fertility, decomposing organic matter and facilitating carbon and nitrogen flow, as well for supporting plant species development [
5]. Also, microscopic fungi are an important food-source for soil invertebrates and some of the macroscopic ones are eatable and valuable for humans and animals’ diets [
4]. Furthermore, the observed diverse interaction skills and ability to produce different primary and secondary metabolites turn fungi in an interesting group to pharmaceutical industry [
6]. Most of the plant endophytic fungi produce compounds with both harmful and beneficial effects on plants, which when extracted may have agricultural or even medicinal value [
6,
7]. For example, some secondary metabolites, like alkaloids, may help the plant to resist to pests, to repel herbivorous, and to adapt to the changes on the climatic conditions [
6]. Others, can be used as antibiotics (
e.g., penicillin or cephalosporin), anticancer agents (
e.g., illudin or paclitaxel), immunomodulatory agents (
e.g., cyclosporine or mycophenolic acid), or antiviral agents (
e.g., stachyflin), for example [
7]. So, the fungal metabolites comprise a panoply of promising compounds and applications, but also potential harmful implications, in particular regarding mycotoxins in human and animal health [
6,
7,
8]. The most important mycotoxins are aflatoxins, fumonisins, ochratoxin A (OTA), deoxynivalenol (DON), zearalenone (ZEN), and ergot alkaloids which are mainly produced by
Aspergillus, Penicillium, Fusarium, Stachybotrys, and
Claviceps species [
9,
10].
Mycotoxin contamination of food and feed may result in acute or chronic consequences such as carcinogenic, teratogenic, immunosuppressive, or estrogenic issues in humans and animals [
9], affecting food safety. In this way, the European Commission set the maximum levels in raw materials, feed and processed food [
11], which means that all the products containing high levels of mycotoxins are strongly devalued or even banned from being sold, which also affects the global economy. Furthermore, mycotoxins can also be inhaled and cause adverse human health effects. Exposure by means of inhalation of molds may happen in indoor residential, school, and office environments [
12].
Additionally, in certain conditions, fungi can directly or indirectly, cause allergies [
13] and fungal infections [
14], or infect crops, compromising their yields [
15,
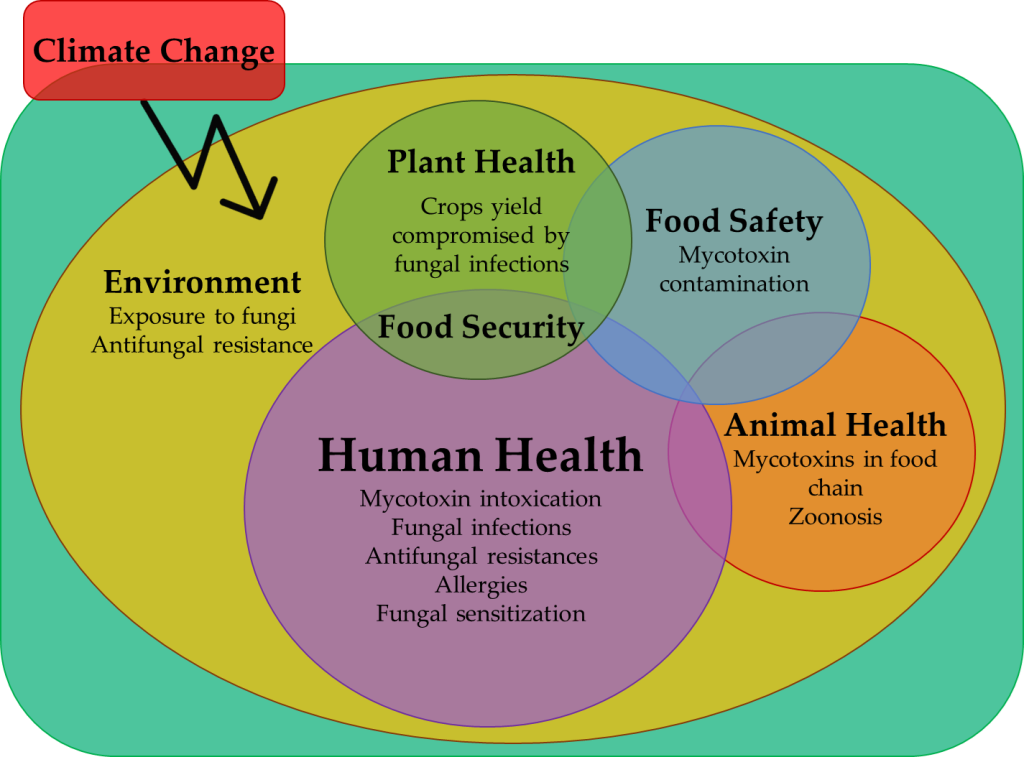
16]. Thus, the contamination of edible plants and of the surrounding environment are other possible routes affecting human and animal health. In this way, this review will explore the negative impact of fungi in human health, under the “One Health” perspective. The “One Health” concept connects the health of plants, humans, animals, and environment in a unifying approach, aiming to balance and optimize them as only one. Its points of action include food security and safety, integrity of ecosystems, fungal infections including zoonosis and antimicrobial resistance control, and promotion of health [
17].
This review covers the most common mycological infestations of plants and animals with effects on human health either directly through dietary exposure or indirectly as environmental contaminants. Additionally, the subject of antifungal resistance induced by non-dietary exposure to plant protection products will be explored and discussed, also in a One Health perspective.
2. Fungal effects on Plant Health
Fungi are an important group of organisms that influence plant health, either by helping them to grow and to resist to some plant pathogens and herbivorous animals, or by causing plant diseases [
6]. The most relevant phytopathogenic fungi are the ones infecting agricultural crops and forestry trees with high impact on human nutrition, life quality, and economy as they may compromise the productions/yields and quality [
18]. Actually, 15 to 20% of the crop losses are caused by plants diseases, of which over than 70% correspond to fungal infections, including on the primary crops, resulting in high levels of direct economic losses [
15,
19].
There are several phytopathogenic species and strains in the world, however, their virulence depends on the host species and the susceptibility of the commercial varieties as well on the environmental conditions. One of the most important phytopathogenic fungi for crops is
Fusarium, of which some species can produce mycotoxins or even to cause diseases also in humans. For example,
Fusarium oxysporum causes root and seed rots in rice [
20,
21],
Fusarium wilt in bananas [
22], tomato [
23] and watermelons [
24], and it is also a great producer of moniliformin [
25]. In wheat,
Fusarium graminearum causes “
Fusarium head blight” and is a great producer of DON, nivalenol, and fusarenone [
25]. In Delicious and Fuji apple trees,
F. tricinctum causes a new disease that results in withering and death [
26] and produces moniliformin [
25]. In maize crops, in the cooler temperate regions,
F. graminearum, F. culmorum, F. cerealis, and
F. avenaceum are usually detected causing “
Gibberella ear rot” and contaminating the grains with DON, nivalenol, fusarenone, ZEN, moniliformin, and T-2 toxin derivatives [
25,
27]. Also in maize, but in the warmer temperate regions, the most frequent species belong to
Fusarium fujikuroi complex species, like
F. verticillioides, F. proliferatum, and
F. subglutinans, causing “
Fusarium ear rot” and contaminating the grains with fumonisins, beauvericin, fusaproliferin, and moniliformin [
16,
18,
25,
27,
28].
In 2020, the main crops produced worldwide were sugar cane and cereals, as maize, rice, and wheat. Of fruits, the most important crops were bananas and plantains, watermelons, and apples, while of vegetables was tomato [
29]. As referred, between so many existent fungal genera, one is enough to cause disease and mycotoxins contamination in the most important crops, regardless of their type. In this way, phytopathogenic species have a huge impact in global economy by decreasing crops yield and compromising food security, but also in human health, by contaminating the raw materials and their derivatives products, like flours, breakfast cereals, tortillas, bread, snack-like products, among others, with one or more mycotoxins [
16,
18,
30,
31,
32] and, therefore, compromising the food safety.
Despite the global production of primary crops raised up 9.3 billion tones in 2020 [
29], phytopathogenic fungi diversity also increased and expanded, which constitutes a high risk to food security and global economy, as well to global health and the ecosystems [
19]. There are several reasons for the emergence of these fungal pathogens, including their high adaptive potential, the occurrence of climate and ecological changes, the globalization and international commerce, and the broad use of antifungals [
33]. Some common saprotrophic species, like from
Aspergillus genus, are now emergent as potential pathogenic to plants, as well as to humans.
Post-harvest diseases together with the storage conditions of fruits, vegetables, and commodities are also worrisome, since annually, and depending on the product and country analyzed, 10% to 50% of the production is lost due to microbial spoilage [
34] and may be contaminated with mycotoxins [
18]. So, controlling fungi and their mycotoxins’ production is an emergent concern before and after the harvest, including during the storage.
Fungal diseases are usually treated using chemical fungicides, which have a negative impact in the ecosystems, food safety, and human health [
35]. Azoles are one of the most frequent class of fungicides used in agriculture, which are used in around half of the cereal crops and vineyards of the European Union to control mildew and rust [
36]. The azoles are stable molecules that inhibit the sterol 14α-demethylase enzymes, compromising the ergosterol biosynthesis and the cell structure, which preclude the fungal growth [
37]. Their molecular stability allows them to remain active on water and soil for several months, as well on fruits and vegetables, having adverse ecological effects [
35]. The increasing time of exposure of fungi to them enhances the pressure of positive selection of resistant strains [
35]. The emergence of resistant strains in agriculture is also a major concern to medical and scientific community, since azoles are used as first line therapy to a huge number of fungal infections in humans [
35,
38,
39]. The most common azoles used in agriculture are epoxiconazole, difenoconazole, propiconazole, bromuconazole, and tebuconazole, while in clinic are itraconazole, voriconazole, and posaconazole [
35,
38,
39]. These azoles have similar molecular structure, binding modes, and high levels of cross-resistance, which means a high risk of preclusion of fungal infections treatment in humans [
35,
38,
39].
To prevent and minimize the negative impact of controlling fungi in agriculture using chemical fungicides, research on new and biological alternatives is a rising issue, including the use of bacteria, or other fungal species [
21,
23,
34] for biocontrol of harmful species. For example,
Bacillus spp. may be used to control
Fusarium wilt of tomatoes, caused by
F. oxysporum or
F. solani [
23] or
Fusarium Ear Rot in maize caused by F. verticillioides [
40]. Fungi also can be used as biocontrol in agriculture, due their high reproductive rate, short generation time, and their capability to survive in the environment as parasites or saprotrophs turn them an interesting, potential, and sustainable option to biological control [
34]. Actually, the use of fungi as biocontrol has application in agriculture against multiple organisms, including to control insect pests, like
Metarhizium anisopliae, Beauveria bassiana, Aspergillus flavus, and
Metarhizium flavoviride, which may be used to control locust attacks [
15]. To manage fungal plant diseases, the antagonist species may produce inhibitory metabolites, parasite the pathogenic fungi, induce the plant defense response against the pathogens, promote the plants growth (which reduces the effects of disease), or compete with the pathogenic species [
34].
Trichoderma is one of the greatest fungal genera with potential to control plant fungal diseases, comprising 25 biocontrol agents [
34]. For example,
Trichoderma viride and
Trichoderma harzianum effectively reduce
F. verticillioides growth and fumonisin production in vitro and in planta [
40]. Other genera recognized as with significant potential are
Alternaria,
Aspergillus, Candida, Fusarium, Penicillium, Pichia, Pythium, Talaromyces, and
Verticillium, comprising five or more known antagonistic species [
34]. For example, some species of
Penicillium, namely
P. variabile, P. dendriticum, and
P. duclauxii, were reported as antagonists of
Aspergillus niger, controlling its growth, which may be used as biocontrol to onion black rot [
41]. Moreover, biocontrol antagonists are now also applied to control post-harvest diseases. One example is some
Trichoderma species used for the management of the post-harvest crown rot of banana caused by
Colletotrichum musae,
Fusarium verticillioides, or
Lasiodiplodia theobromae [
34]. Other example is
Clonostachys rosae, that effectively reduces sporulation of
F. verticillioides and
F. proliferatum on maize after and post-harvesting [
40].
Other agricultural practices to manage fungal diseases include the simultaneous use of biocontrol agents and synthetic fungicides [
34], crop rotation, avoid excessive irrigation, and use of resistant or tolerant plant varieties [
23]. In some cases, genetic engineering is also an alternative approach to control fungal diseases,
e.g., introducing genes which encode proteins with antifungal activity into the plant genome. One example is the use of transgenic banana plants which express the rice thaumatin-like protein gene to resist to
Fusarium wilt [
22].
3. Mycotoxin effects on Animal and Human Health
The consumption of crops-based food and feed contaminated with mycotoxins constitutes a real concern to both animal and human health. Most of the emerging mycotoxins are mainly produced by
Fusarium, Penicillium, Aspergillus, and/or
Alternaria species. For example,
Alternaria alternata is a species considered as vital mycotoxin-producing in fruits and vegetables [
42]. The most important mycotoxins on human health are aflatoxins, OTA, fumonisins, ZEA, nivalenol, DON, and ergot alkaloids [
43].
In the industrialized countries, the majority of the protein consumed
per capita is from animal source, being 41% provided from terrestrial meat animals, 34% from milk, 15% from aquatic meat animals, and 10% from eggs [
44]. On the contrary, in the developing countries, the main protein sources are cereals, followed by milk. Thus, since most of the ingested protein is from farming, the quality of crops and animal feed is an important point for the human health.
In 2022, the DSM World Mycotoxin Survey reported that 57% of the commodities had more than one mycotoxin per sample, being fumonisins and DON the most prevalent, followed by ZEN and aflatoxins [
45]. This tendency has been showed in the last decade, with 52% to 74% of the commodities samples with DON and 53% to 70% with fumonisins [
45]. Furthermore, 75% to 93% of the samples from North America or South Asia countries, and China presented at least one mycotoxin beyond the stablished thresholds (500 ppm of fumonisins, 150 ppm of DON, 50 ppm of ZEN or T-2, 10 ppm of OTA and 2 ppm of aflatoxins) [
45]. This means quality losses and economic costs, estimated by the US Food and Drug Administration (FDA) as an annual mean of
$932 million USD [
46].
Most of the animal feed is from commodities, which may have been exposed to mycotoxin contamination. Actually, the incidences of mycotoxins and multi-mycotoxins in animal feed are high [
46]. Worldwide, most of the feed samples carry mycotoxins, namely fumonisins (B1-82%, B2-73%, and B3-62%), DON (71%), and/or ZEA (67%) [
45]. In 2021 and 2022, DON was the mycotoxin that represented the highest risk to animals in Europe, North America, and Africa, being detected in 46-52%, 69%, and 67-80% of the feed samples, respectively, followed by ZEN (42-45%, 33-41%, and 44-61%, respectively) and fumonisins (34-41%, 43-50%, and 50-59%, respectively) [
45]. In South and Central America, Asia, and Middle East and North Africa, fumonisins were the most frequently associated to feed contamination, detected in 52-64%, 81-87%, and 83-87% of the samples, respectively, followed by DON (43-49%, 70-73%, and 53-81%, respectively) and ZEN (36-43%, 65-73%, and 60-68%, respectively) [
45]. Additionally, some emerging mycotoxins were also detected in most of the feed samples, namely beauvericin (71%) and enniatin (B1-67%, B-58%), which affects the immune system, and moniliformin (63%) that is genotoxic, immunosuppressive, causes heart damage, muscular weakness, and respiratory distress [
45].
As mycotoxins show resistance to the digestive process and to high and low temperatures, they can remain in the meat and milk derivatives and be spread by all food chain [
43]. The exposure to mycotoxins can cause chronic and/or acute health problems, depending on factors as the type and dose of mycotoxin, how long the exposure occur, and the individual health condition, gender, and age [
9,
43]. Mycotoxicosis effects depend on the type of mycotoxin and on the way of exposure, which can occur by inhalation, ingestion, or by skin absorption [
9]. For example, inhaling high concentrations of T-2 toxin may result in human pulmonary edema or lung lesions, while its ingestion may cause direct damage to the intestinal mucosa [
9]. Thus, mycotoxins can directly affect the liver, kidneys, and nervous and immune systems, or, as aflatoxins and fumonisins do, interfere in the protein synthesis, causing cancer [
9,
43].
In animals the consumption of feed contaminated by mycotoxins has been noted in the loss of the body weight and in the decrease in the reproductivity, damage in vital organs, increased illness associated to immunosuppression, and even death [
9,
10]. One example of these situations is the case occurred in 1960 in England where a massive number of turkeys died due to consumption of peanuts contaminated with aflatoxins imported from Brazil [
9]. Mainly produced by
Aspergillus species, as
Aspergillus niger and
A. flavus, aflatoxin B1 is classified also as a human carcinogen, attacking mainly the liver and immune system, and having been reported in the Philippines, Africa, and China as the cause of human gastrointestinal and hepatic neoplasms increment, as well as of the loss of children weight [
9]. Actually, aflatoxins may be detected in several products and inclusive in other forms, like aflatoxin M1, the hydroxylated metabolite of aflatoxin B1, that was reported in pasteurized milk, ultrahigh-treated milk, milk powder, and some milk-based products [
9,
43]. Due its importance, the maximum levels of AFM1 in raw milk is 50 ng/kg in EU and 500 ng/kg in US [
43]. One example of the terrible effect of aflatoxins in the human health occurred in 1988, when 13 Chinese children in the northwestern state of Perak in peninsular Malaysia died due to an outbreak of acute hepatic encephalopathy caused by consuming aflatoxin contaminated Chinese noodles [
47]. Other example occurred in 2004, in Kenya, where 125 individuals died and around 200 needed treatment after consuming maize contaminated with aflatoxins [
43]. Furthermore, in poultry and swine production settings the occurrence of aflatoxins is high, with reported cases of aflatoxin B1 inhalation by workers, as well as the presence of
Aspergillus spp. conidia in the air [
48,
49,
50,
51,
52,
53].
Other
Aspergillus species are also mycotoxigenic, as
A. terreus produces territrems (neurotoxic), and
A. ochraceus produces OTA [
6,
9,
10]. In the same way, various mycotoxins may occur in feed contaminated with
Penicillium species, like
P. verrucosum, including patulin, cyclopiazonic acid, and OTA [
54]. In animals, OTA can cause cancer, morphological defects, lesions on gastrointestinal, renal, and lymphoid tissues, blood clots, and reduction of egg production [
9,
10]. In humans, OTA has teratogenic and genotoxic potential and is toxic to the liver, nephrotoxic, and immunosuppressant, inducing apoptosis lymphocytes and neuronal cells, and may be detected inclusive in human breast milk [
9,
10,
43].
On the other hand, fumonisins, DON, and ZEN are mainly produced by
Fusarium species. Fumonisin B1 is genotoxic, inducing chromosomal aberrations, and a cancer promoter, responsible for causing neural tube defects in human babies and linked with the high incidence of human esophageal carcinoma due consumption of fumonisin contaminated maize [
9,
43]. On animals, fumonisins are also toxic to kidneys, the respiratory tract, and liver, being, for example, responsible for porcine pulmonary edema, promote cancer activity, and are neurotoxic, causing, for example, the equine syndrome leukoencephalomalacia, defined by the liquefaction of the cerebral hemisphere of the brain [
9]. ZEN is relevant as an estrogenic mycotoxin with potential to disrupt sex steroid hormone functions, being usually associated with feminizing syndromes or hyper-estrogenic activity in pigs, and having been found in 55.1% and 22.4% of the endometrial tissues from women with endometrial adenocarcinoma and endometrial hyperplasia, respectively [
9,
43]. In turn, DON and T-2 toxin mainly affect bovines and poultry immune and digestive systems, and may be detected in eggs and milk, translating their toxicity in animals as food refusal, weight loss, vomiting, severe dermatitis, abortion, hemorrhage, abnormal feathering, lesions on the edges of the birds' beaks, inhibition of reproductive performance, and death [
9]. In general, monogastric farm animals, like poultry and swine, are more susceptible to mycotoxins than ruminants because their rumen flora have the ability to convert mycotoxins in less carcinogenic metabolites or in compounds without biological action [
9,
10].
As previously mentioned, the same fungal species are able to produce different mycotoxins and considering that more than one mycotoxigenic species may be detected in the same product, the multi-mycotoxin occurrence is an frequent issue, since the co-occurrence of mycotoxins may result in synergistic effects of mycotoxins, which happens when aflatoxins are present together with DON and T2 toxin, or OTA with fumonisins, or fumonisins with DON, for example [
9]. Furthermore, modified mycotoxins have been also reported in commodities. Modified mycotoxins have different structure, solubility, polarity, and molecular mass that confer them less toxicity due to lower absorption by animals and humans [
55]. However, modified mycotoxins may be converted in their free form, which could increase their bioavailability and thus involving higher risks for animal and human health. Therefore, it is important to detect them, evaluate their stability during the feeds’ processing and digestion, as well as their toxicokinetic and toxicodynamic properties [
55].
The mycotoxin management is also a current challenge, having to be systemic and to include all stages of the feed and food supply chain [
55,
56]. Some good farming practices include doing a proper fertilization, crop rotation, an integrated pest management plan, opting to pest and disease resistant/tolerant varieties, harvesting at the optimum maturity stage, ensuring the grain physical integrity, and doing a good post-harvest management, namely controlling humidity, temperature, ventilation, insects and rodents presence, and sanitation [
55,
56,
57]. A combination of new strategies may also be used, such as using bio-competitive fungi as biocontrol agents, varieties resistant to mycotoxins, apply antibody-mediated technology, or even nanomaterials engaged for antifungal or inhibition of mycotoxins [
55]. Also, some physical, thermal, and biological methods may be used, as cleaning and aggressive sorting, non-ionizing and ionizing irradiation, or microorganisms with detoxification activities [
55,
56,
57]. Other methods to control fungal and mycotoxin contamination have been studied, as ozone application that inhibits fungal growth, cold plasma technology which reduces and degrades mycotoxins in food and feed, pulsed light, radio frequency, and microwave for reducing mycotoxin contamination [
55]. However, it is important to keep in mind that even though the several methods existent to prevent and control mycotoxins, it is impossible to eradicate both fungal and mycotoxin contamination [
55], so the aim should be monitoring and reducing them to nonharmful levels to human and animal health. In the livestock production, it is important to choose high-quality feedstuffs and to perform mycotoxins’ monitorization [
55]. Moreover, the silage used for animals’ bedding, the feeding silo, and feeders should be routinely changed, cleaned, and climatized, with insects and rodents presence duly controlled [
43,
55]. Additionally, some binding agents (inorganic or organic) or bio-transforming agents (
e.g., bacteria, yeast, fungi, and enzymes that degrade mycotoxin molecules in non-toxic metabolites) may be used to control fungal proliferation and mycotoxin contamination [
43,
55,
56,
57].
4. Fungal effects in Human Health
Fungal infections is one of the most frequent causes of death worldwide [
58], counting with more than 1,500,000 deaths per year and affecting over than 1,000,000,000 people worldwide [
14]. Fungi cause health issues that can range from allergies to invasive infections, counting for example with over than 10,000,000 of fungal asthma cases per year [
14].
Some of the fungal infections in humans, around 20%-50%, can be transmitted by animals, as cryptococcosis, sporotrichosis, blastomycosis, and dermatophytosis, being this last one the most frequent zoonosis [
59], while sporotrichosis is described as the most prevalent and widespread subcutaneous infection worldwide [
60]. Dermatophytes, they can be transmitted to people from different sources: they can be anthropophilic fungi infecting almost exclusively humans, zoophilic infecting animals and being transmitted to humans, or geophilic, being found in the soil and infecting both hu mans and animals. Dermatophytosis is also known as ringworm or
Tinea, being a superficial and contagious infection of skin, hair, and/or nails, caused by dermatophyte fungi belonging to
Trichophyton or
Microsporum genera, for example [
61].
Tricho phyton spp. is the genera more frequently identified as the etiological agent of dermatophytosis in humans [
61].
Trichophyton rubrum is an anthropophilic dermatophyte fungus, reported in several studies also as the etiological agent of deep and invasive disease in immunosuppressed patients [
60].
Thus, some fungal species are able to cause invasive fungal infections, which are difficult to diagnose, manage, and treat, with an estimated incidence of 6 cases per 100,000 persons per year, also associated to substantial morbidity and mortality [
60,
62]. These species include
Cryptococcus spp.,
Candida spp.,
Aspergillus spp.,
Histoplasma spp., Mucorales,
Fusarium spp.,
Scedosporium spp.,
Lomentospora prolificans,
Coccidioides spp.,
Talaromyces marneffei,
Pneumocystis jirovecii, and
Paracoccidioides spp. [
62].
The main species of
Cryptococcus genus causing cryptococcosis in humans and other animals are C.
gattii, and
C. neoformans, with
C. gattii mainly affecting immunocompetent individuals, causing invasive disease that result in death in 10% to 25% of the cases, and
C. neoformans mainly affecting immunocompromised people, causing cryptococcal meningitis, a disease with very high mortality [
62]. Cryptococcal meningitis accounts for 223,100 cases per year [
14]. Cryptococcosis is usually susceptible to fluconazole, amphotericin B and flucytosine, showing resistance to echinocandins [
62].
The most common nosocomial fungal infections are caused by species generally considered as belonging to the
Candida genus, being
C. auris, C. albicans, Nakaseomyces glabrata (C. glabrata), C. tropicalis, C. parapsilosis, and
Pichia kudriavzeveii (
C. krusei) the most important species [
62,
63].
Candida auris causes invasive candidiasis with high mortality, ranging from 29% to 53%, having rapidly emerged as a major cause of candidemia worldwide and been reported in more than 35 countries, in all continents except Antarctica [
62,
63]. This species is resistant to high temperatures (42°C) and to the most frequently used disinfectants, it appears to survive on surfaces for long periods of time, has high ability for transmission among patients, and is frequently resistant to several antifungals, having a high outbreak potential [
62,
63].
Candida albicans, Nakaseomyces glabrata, C. parapsilosis, and
C. tropicalis are usually present in the healthy human microbiome as commensals, but under certain circumstances they can cause superficial infections or invasive candidiasis, the latter with a mortality of about 20%-50% (
C. albicans and
Nakaseomyces glabrata), 20%-45% (
C. parapsilosis), and 26%-60% (
Candida tropicalis) [
62].
Pichia kudriavzeveii is also an opportunistic pathogenic yeast which can also cause mucosal infections or invasive candidiasis, also with high mortality rate (44%-67%). Invasive candidiasis accounts with around 700,000 cases per year, being usually treated with echinocandins and azoles [
14,
62]. In the last two decades,
C. albicans proportion of invasive candidiasis decreased from 57.4% to 46.4%, being reported a raise of infections caused by other species known as more resistant to antifungals [
58]. The raise of those species is also due to prolonged prophylaxis or therapy with antifungal agents [
64]. One example of resistant species to the available antifungals is
C. auris, that is considered an emerging multidrug-resistant yeast, showing high rates of resistance to fluconazole (87–100%), moderate resistance rates to amphotericin B (8–35%), and low resistance to echinocandins (0–8%) [
62,
63]. Also,
Nakaseomyces glabrata shows high minimum inhibitory concentrations (MIC) to azoles, and rising echinocandin resistance [
62].
C. tropicalis azole-resistance, mainly to fluconazole and itraconazole, usually ranges from 0% to 20%, with some reports of 40–80% [
10,
62]. Antifungal resistance of
C. parapsilosis and
Pichia kudriavzeveii are considered as moderate, being the last one intrinsically resistant to fluconazole [
62]. Recently,
C. parapsilosis isolates resistant to fluconazole are emerging, causing outbreaks in several health institutions [
65].
Candida spp. are also associated to superficial infections, being vaginal candidiasis one of the most frequent infections worldwide, affecting 70-75% of women and resulting in around 134,000,000 infections per year, mainly caused by
C. albicans [
14].
The filamentous fungi more frequently isolated from patients with invasive fungal infections is
Aspergillus, accounting with about 250,000 cases of invasive aspergillosis per year, with a high mortality rate of 45% to >99%, depending on if the patient is treated or not [
14,
39,
60,
66,
67]. Furthermore,
Aspergillus species cause around 3,000,000 cases of chronic pulmonary aspergillosis, and more than 1 million of deaths per year [
14,
39,
60,
66]. Some pulmonary pathologies are related to occupational exposure to
Aspergillus conidia that cause
Aspergillus-induced allergic and asthmatic lung diseases [
66]. An example of occupational disease caused by
Aspergillus was an onion farmer that developed hypersensitivity pneumonitis caused by inhalation of
A. niger cleaning up onion peels with air compressors [
68].
Aspergillus genus is divided in several sections, of which the
Fumigati,
Circumdati,
Terrei,
Nidulantes,
Ornati,
Warcupi,
Candidi,
Restricti,
Usti,
Flavipedes, and
Versicolores sections contain relevant species in clinics [
10]. Section
Fumigati includes the main etiologic agents of aspergillosis:
Aspergillus fumigatus sensu stricto - the major cause of aspergillosis - and its cryptic species
A. lentulus,
A. udagawae,
A. viridinutans,
A. thermomutatus,
A. novofumigatus, and
A. hiratsukae - responsible for 3 to 6% of those infections [
39].
Aspergillus fumigatus sensu stricto, due its small conidia, high thermotolerance, rapid growth, and optimal temperature of 37°C, can affect both the upper and the lower respiratory tract. The damage caused is due to the fungus itself and also due to the host’s inflammatory response, causing a range of health issues that can be an allergic reaction, a colonization or semi-invasive disease, or even an acute invasive aspergillosis, depending on the immune status of the host [
59,
62,
66]. Despite invasive aspergillosis may be rare in immunocompetent individuals, it is a major cause of morbidity and mortality in immunocompromised people [
59,
62,
67]. Recently, two new forms of aspergillosis were reported: Influenza-Associated Pulmonary Aspergillosis (IAPA) and COVID-Associated Pulmonary Aspergillosis (CAPA) [
67]. IAPA was reported in 19% of the patients admitted at the ICU with influenza infection and CAPA was reported in 10% of the mechanically ventilated patients with COVID-19 [
67]. Severe influenza virus infections destruct the respiratory epithelium, while SARS-CoV-2 infections cause diffuse alveolar and endothelial vascular cell damage and are treated with corticosteroids, which both facilitate the
Aspergillus proliferation and invasive aspergillosis development [
67]. Aspergillosis is treated with triazoles, which are responsible by a decrease of more than 50% of invasive aspergillosis mortality [
67]. However, the treatment of aspergillosis has been a concern due to the infections caused by
A. fumigatus resistant to azoles, causing a rise in the mortality rates (47–88%) [
62]. The use of triazoles prophylaxis, treatments with azoles, and the use of azole agricultural fungicides cause the development of acquired resistance in
A. fumigatus isolates, leading to new challenges in therapeutic management of these infections [
35,
36,
38,
66]. As the availability of antifungal drugs to treat fungal infections is limited, the rise in the resistance rates may result in treatment failure and in consequent higher mortality rates [
39]. There are two predominant mutations in
A. fumigatus found in azole-naïve patients - TR34/L98H and TR46/Y121F/T298A (detected in the
cyp51A gene) – which are also present in environmental isolates [
39]. Other species rather than
Fumigati ones are also important pathogens, such as
A. terreus, which causes aspergillosis with high frequency in Austria and that has been reported as resistant to several antifungals, especially to amphotericin B [
66]. Also,
A. flavus,
A. nidulans,
A. sydowii, and
A. alabamensis are less susceptible to amphotericin B, while
A. niger,
A. tubingensis, and
A. calidoustus are less susceptible to azoles [
69]. Other species present an intrinsic resistance to both azoles and amphotericin B, such as
A. fumigatiaffinis,
A. viridinulans, and
A. versicolor, while others are resistant to both caspofungin and amphotericin B, as
A. persii, and
A. tetrazonus [
69]. In turn,
A. lentulus is intrinsically resistant to azoles, amphotericin B, and caspofungin, while
A. udagawae is resistant to voriconazole, and amphotericin B [
69]. Noting that
A. fumigatiaffinis,
A. viridinulans,
A. lentulus and
A. udagawae are all cryptic species within
Fumigati section.
Dimorphic fungi, considered as true pathogenic fungi, are a concern to humans’ health, being disseminated in the environment in the mold form and changing to the yeast form inside the host, such as
Histoplasma spp.,
Paracoccidioides spp.,
Coccidioides spp.,
Blastomyces spp.,
Emergomyces spp., and
Talaromyces spp.. Histoplasmosis is an example of a relevant disease in humans, caused by dimorphic fungi, mainly by
Histoplasma capsulatum var. capsulatum, affecting the lungs and with ability to disseminate in the body, through to the central nervous system, or blood, resulting in approximately 100,000 cases of disseminated histoplasmosis annually [
14,
59,
62].
Paracoccidioides species are also pathogenic dimorphic fungi, that can be inhaled or be introduced into the skin by injury, affecting mainly the lungs, mucous membranes, and skin. Paracoccidioidomycosis can disseminate to the lymph nodes and other organs of the reticuloendothelial system, having a mortality of 3%-23% and being usually treated with itraconazole, amphotericin B, or cotrimoxazole [
62]. In endemic regions of
Paracoccidioides spp. and
Histoplasma spp., as the Americas, Africa, and Southeast Asia, histoplasmosis and paracoccidioidomycosis tend to be the most frequently detected endemic mycoses, with about 10 million people infected with
Paracoccidioides spp. in Latin America [
60].
Coccidioides spp., mainly
C. immitis and
C. posadasii are some of the most virulent fungal pathogens, causing invasive coccidioidomycosis with high mortality in immunocompromised patients and being their antifungal resistance is of concern, with high MIC for fluconazole [
62].
Blastomyces spp., mainly
B. dermatitidis and
B. gilchristii, can result in acute or chronic pulmonary infection, and more rarely in hematogenous dissemination disease, being treated with itraconazole [
70,
71].
Emergomyces spp. infect essentially immunocompromised patients, causing pulmonary diseases and skin lesions, being treated using amphotericin B followed by itraconazole or only using itraconazole, with some strains presenting high MIC to fluconazole [
70,
71]. The only
Talaromyces species that is considered pathogenic is
T. marneffei, which affects mainly HIV patients, and can cause lymphadenopathy, hepatomegaly, splenomegaly, respiratory and gastrointestinal abnormalities, and skin lesions [
71]. Disseminated talaromycosis is fatal if untreated and the mortality rate is around 30% even with itraconazole administration [
70].
The large group of different fungal genera belonging to Mucorales order, includes
Rhizopus, Mucor, Rhizomucor, Absidia, Lichtheimia, Apophysomyces, Cunninghamella, and
Saksenaea, and can infect immunocompromised and patients with metabolic disorders, like diabetes mellitus [
58]. Infection may occur by spore inhalation or by skin and soft-tissue injuries. Invasive mucormycosis is a very serious disease, with mortality rates ranging between 23% and 80% [
62]. The available treatments include surgery and antifungal administration. In general, Mucorales are susceptible to amphotericin B and isavuconazole, but show resistance to fluconazole, voriconazole, and echinocandins [
62].
Other pathogenic fungi known by their ability to cause invasive infections and to show resistance to antifungals are
Lomentospora prolificans, and
Scedosporium spp..
Lomentospora prolificans causes invasive lomentosporiosis in immunocompromised patients and its mortality rates range from 50% to 71%, with high antifungal resistance described [
62].
Scedosporium spp. cause invasive scedosporiosis, with mortality rates of 42–46%, usually showing reduced susceptibility to amphotericin B, itraconazole, isavuconazole, and echinocandins, being usually treated with voriconazole [
62].
Other fungi found in soil and water, as
Madurella spp.,
Falciformispora senegalensis,
Curvularia lunata,
Scedosporium spp.,
Zopfia rosatii,
Sarocladium spp. and
Fusarium spp., can also cause infections in humans, namely eumycetoma, a serious deep tissue infection that results in amputation in up to 39% of the cases, with special risk to farmers [
62]. These species are opportunistic human pathogens infecting mainly immunocompromised people. For example,
Fusarium spp. causes fusariosis mainly in people who was subject to invasive surgery, organ transplantation, chronic steroid treatment, or aggressive cytotoxic therapy [
72].
Fusarium species are responsible for causing respiratory infections, keratitis, and invasive fusariosis that can reach, by hematogenous dissemination, the central nervous system, and other organs, as well, with a mortality rate of 43%-67%, especially when caused by species belonging to the
F. solani complex [
62]. Fusariosis treatment is difficult due to
Fusarium spp. innate resistance to most of the antifungals [
62]. Keratitis has an incidence of around 1,000,000 cases per year, mainly caused by
F. oxysporum [
14].
Fusarium species are intrinsically resistant to azole antifungals and resistant to almost all currently used antifungals as echinocandins and polyenes [
72]. Infections caused by the emergent fungi
Fusarium spp.,
Scedosporium spp. and Mucorales show an increasing frequency and are more frequently isolated from invasive infections, especially in immunocompromised patients [
60].
Pneumocystis jirovecii is a different infectious agent, only with a intracellular life cycle, with ability to be transmitted from person to person, and causing around 500,000 cases of pneumonia per year [
14,
62]. It is an opportunistic fungal pathogen, transmitted by the air and that can also affect healthy individuals but mostly affecting immunocompromised patients, with highly variable mortality rates [
59,
62].
Despite all these potential pathogenic fungal species, it is important to keep in mind that some fungal species belong to the human mycobiome. Although representing less than 0.1% of the microbiome, it maintains the microbial community structure, metabolic function, and host homeostasis, as well as being an essential source of antigens to the immune system training and responses [
73,
74]. The composition of the human mycobiome changes substantially during the lifespan, having high interindividual and intraindividual variability, being shaped since birth, with the mother and the environment as sources, and then being influenced by factors like diet, body weight, age, sex, and antibiotic and antifungal therapy [
73,
74].
The human mycobiome inhabits the oral cavity, gastrointestinal tract, respiratory tract, urogenital tract, and skin. The oral adult mycobiome includes approximately 81 genera and 101 species of fungi, belonging mostly to
Candida,
Penicillium,
Cladosporium,
Aspergillus,
Alternaria,
Rhodotorula,
Malassezia, and
Cryptococcus genera [
74]. The gut mycobiome comprises approximately 400 fungal species, with Ascomycota phylum covering 48% to 99% of all present species, while Basidiomycota phylum covers 0.5% to 14%, followed by the phylum Mucoromycota [
74]. The most common species belong to
Candida,
Saccharomyces,
Paecilomyces,
Cladosporium,
Aspergillus,
Penicillium genera,
Malassezia,
Cryptococcus,
Rhodotorula,
Mucor, and
Rhizopus [
73,
74]. The human respiratory tract mycobiome is mostly composed by
Saccharomyces cerevisiae,
Candida,
Pichia jadinii,
Debaryomyces,
Cladosporium,
Aspergillus,
Alternaria, and
Penicillium species [
74]. Genitourinary tract mycobiome comprises mainly Ascomycota and Basidiomycota species from 22 genera, being
C. albicans,
C. glabrata, and
S. cerevisiae the most common species, while the human skin mycobiome includes at least 168 fungal genera, with
Malassezia genus representing more than 57% of the microscopic colonizers on average [
74].
5. Fungal effects in Animal Health
Fungi may affect animal health in several ways, being the mycotoxin contamination, associated with agricultural components of their feed, and the fungal infections the most relevant examples.
Some fungal species can infect both animals and humans, causing diseases, but not being transmitted between them. However, animals can transmit certain fungal diseases to humans (fungal zoonosis), as happen with dermatophytosis, cryptococcosis, sporotrichosis, or blastomycosis, and diseases caused by Malassezia spp., or Encephalitozoon cuniculi.
Aspergillosis can truly affect several animal species, but certain avian species are even more susceptible, being aspergillosis one of the major causes of morbidity and mortality in birds occurring in the wild (
e.g., albatrosses and penguins) and in poultry production settings, which causes ecological damage and economic loses (e.g., in turkey production it can cost US
$ 11 million per year) [
10,
39,
75]. Only
Aspergillus fumigatus sensu stricto is responsible for up to 90% of deaths in birds with aspergillosis [
39]. Due to the constant travels between agricultural fields, and between natural environments and urban settings, birds substantially contribute to the dispersion of
Aspergillus spp. conidia, including of azole-resistant strains [
39].
Aspergillus spp. also affect invertebrates, like sea corals, honeybees (causing stonebrood disease over all larval stages), and reptiles (causing cutaneous and disseminated infections) [
10]. In horses,
A. fumigatus also causes disease, namely equine guttural pouch mycosis, while in cows, it causes mycotic pneumonia, gastroenteritis, mastitis, placentitis, and abortions [
10,
75]. Cats and dogs are also susceptible to aspergillosis, but mostly the immunocompromised ones, causing sinonasal, bronchopulmonary, and disseminated infections [
10,
59,
75].
Other opportunistic pathogens, as Mucorales
, also infect cattle, horses, birds, cats, and dogs, causing mucormycotic ruminitis, lymphadenitis and abortions in cows, mainly affecting the respiratory system and gastrointestinal tract in horses and birds, and causing enteritis or systemic mucormycosis in cats and dogs [
10,
58]. Mucorales also infect wild animals such as dolphins, bison, and seals [
58].
Dogs and cats are also susceptible to other fungal diseases that can also affect humans but that are not transmitted to them, which are caused by dimorphic soil-borne fungi. Some examples are coccidioidomycosis, histoplasmosis, and bastomycosis. Coccidioidomycosis is caused by
Coccidioides immitis or
Coccidioides posadasii, and may infect a panoply of susceptible animals species, but being mainly reported in pet dogs, resulting in subclinical to severe or disseminated infections [
10,
59,
71]. Histoplasmosis is caused by species belonging to
Histoplasma capsulatum complex, resulting in infections in cats, dogs, primates, and equines that include skin and subcutaneous lymphatic system injury, or disseminated diseases [
10,
59,
71,
76]. Other example of fungal diseases in animals caused by dimorphic yeast is blastomycosis, caused by B
lastomyces dermatitidis, that highly affects dogs and, usually, is not transmitted to humans by direct contact with an infected animal, unless if there is injury, like a bite [
59,
71]. Occasionally, blastomycosis can occur in other mammals such as cats and horses [
10,
71]. It may disseminate and affect various organs and systems [
77].
Some commensal fungi also can cause infections in animals if the host immune system is disturbed.
Candida spp., mainly
C. albicans, can cause mucosal oral and gastrointestinal candidiasis in chickens, horses, cattle, dogs, cats, and pigs, usually associated with antibiotic use or immunosuppression [
10,
58]. Also,
Malassezia spp., mainly
M. pachydermatis, which colonizes dogs’ external ear canal, lip, interdigital skin, anus, nose, and vagina, can cause dermatitis and otitis externa [
59,
78].
Malassezia pachydermatis. can be transmitted to humans, but with low incidence [
59].
Dermatophytosis is considered the most common zoonotic disease in the world, with cats as the major source to humans, especially colonized or infected with
Microsporum canis [
59]. Actually,
M. canis colonization is estimated in up to 36% of healthy dogs (affecting mostly puppies) and up to 54% of healthy cats [
59]. Other dermatophytes and animal hosts had also been reported, as
Trichophyton benhamiae in guinea pig, rabbits, dogs, or cats [
61],
T. mentagrophytes and
Nannizia gypsea in cats and dogs,
T. verrucosum in cattle, and
T. equinum in horses, all zoophilic fungi that can be transmitted from animals to humans [
59].
Tinea cor poris, tinea manuum, capitis, unguium, and
barbae may be treated by applying topical antifungals or, in case of extensive lesions, using also systemic treatment [
61].
Other concerning infection of animals to human transmission is the one caused by
Encephalitozoon cuniculi, a microsporidium, an important cause of neurological disease in rabbits, starting in their kidneys and then spreading to the brain, and that may also infect dogs [
59].
In the same way, cryptococcosis affects domestic animals, as cats and birds, with possible subsequent hematogenous dissemination and tropism for the central nervous system [
59]. Cryptococcosis is caused by inhalation of the encapsulated yeasts
Cryptococcus neoformans or
Cryptococcus gattii, causing severe infections in both immunocompromised and immunocompetent individuals [
59,
79]. A large diversity of animals is affected by
C. neoformans, from lower invertebrates to higher mammals, infecting most frequently cats and affecting their respiratory tracts or causing subcutaneous granulomata or disseminated infections [
10,
58]. The same can occur in dogs, but usually the central nervous system is the most affected, while in dairy animals causes mastitis and in horses respiratory infections [
10,
58].
C. gattii infects different animal species, such as marine mammals, ferrets, and llamas, but also cats and dogs, causing upper respiratory tract infections and subcutaneous masses. However, the central nervous system, lymphatic tissue, lungs, oral cavity, and eyes can also be affected [
10].
Other current fungal infection in animals is sporotrichosis, which in cats may vary from a subclinical to a systemic infection with hematogenous dissemination of
Sporothrix schenckii (species complex) and can be easily transmitted to humans by cats’ scratches or bites [
80]. For example, in 2000, in Brazil more than 750 people were diagnosed with sporotrichosis, 83.4% of them having reported contact with infected cats, 56% had suffered a cat scratch or bite [
80]. Other species have clinical relevance, such as
S. brasiliensis,
S. globosa, and
S. luriei, showing different response to the antifungals which means that the antifungal susceptibility differs between species, as well as the recommended treatment [
81].
In wild animal species, other panoply of fungi can cause infections. Some examples include
Batrachochytrium dendrobatidis and
B. salamandrivorans infecting amphibians,
Pseudogymnoascus destructans causing white-nose syndrome in bats, as well as melanized fungal species causing severe phaeohyphomycoses, chromoblastomycosis, and mycetoma in crustaceans, fish, amphibians, and other cold-blooded vertebrates [
10].
The options of treatment applied to the different animal diseases are mainly based on azole administration, which also promotes the emergence of azole-resistant strains of the different species and can restrict the treatment options. The emergence of azole-resistant strains has been a problem since the last century due azoles have been the largest and most widely used class of antifungals [
82], existing increasingly species and strains with azole-resistance reported. For example, strains of several
Aspergillus species are resistant to different azoles [
35,
36,
37,
83], as well as strains of
M. pachydermatis [
78]. There are also strains of
Histoplasma capsulatum reported as fluconazole-resistant [
82], and strains of
S. brasiliensis showing a growing resistance to amphotericin B and itraconazole [
81]. Moreover, several fungal species have intrinsic resistance to specific antifungals.
6. Climate Change influence on Fungi
Climate change have a significant role in fungal development, proliferation and distribution, susceptibility pattern, and pathogenicity. Climate change on fungi leads to new environmental pressures that result in the emergence of novel pathogens to plants, animals, and humans, and consequently of new fungal diseases, which may compromise production yield, public health, and wildlife biodiversity [
33,
84,
85,
86]. Species considered as emergent pathogens are the ones which are causal agents of new diseases, have changes in pathogenicity, exhibit geographic expansion, increase their incidence, and/or infect novel hosts [
84]. Climate change also promote the increase of natural disasters such as floods, hurricanes, and tsunamis, which contributes to the distribution, aerosolization, and proliferation of fungi and to the increment of fungal infections via traumatic wounds, caused by previously rare or unknown fungal species [
33]. For example, coccidioidomycosis incidence has increased after environmental disasters such as earthquakes, volcano eruption, wildfires, and dust storms [
71]. These situations could be observed in 1977, when a severe dust-storm dispersed
Coccidioides immitis from their endemic area in Bakersfield to Sacramento Country, where it was rare, causing more than 100 infections [
33] or more recently, in the cluster of cases of cutaneous mucormycosis among Joplin tornado survivors [
87]. Furthermore, climate change, and in particular the global warming, can favour the heat-tolerant fungal species and increase the susceptibility of hosts to fungal pathogens [
33,
88]. Examples include the emergence of
Candida auris in humans,
Pseudogymnoascus destructans in bats, and
Batrachochytrium dendrobatidis and
Batrachochytrium salamandrivorans in amphibians [
33,
85,
86].
Candida auris is hypothesized as being a completely new pathogenic fungal species that had an environmental reservoir [
89] and emerged due to climate changes, in particular due to the global warming [
58].
Batrachochytrium dendrobatidis and
Batrachochytrium salamandrivorans are emergent species causing significant outbreaks in amphibians, since global warming is compromising their immune system and raising their susceptibility to these pathogens [
33,
86]. The projected mean global temperature increase of around 2 to 5°C in the next decades can cause a strong selective pressure to rise heat-tolerant species and will decrease the temperature gradient between environment and mammals, increasing the prevalence of fungal diseases [
88]. In the same way, higher day and night temperatures and milder winters can increase proliferation and pathogenicity of foliar fungi [
84] and others. For example, the rise in temperature leads to an increased evaporation in certain forest environments, which promotes cloud formation and the decreasing of the daytime temperature by blocking the sun, while at night the cloud cover serves as insulation to raise the night time temperature from its normal range, which may lead the chytridiomycosis proliferation in amphibians [
90]. Moreover, climate change can promote the emergence of new virulent fungal lineages and long-distance spore dispersal, as happen with the geographical expansion of
Fusarium wilt of bananas [
91]. Also,
Puccinia striiformis causing yellow stripe rust in wheat originally in cooler regions is now reported also in warmer regions, but with more aggressivity and thermotolerance [
33,
84]. Other example is
Fusarium head blight in cereals crops in cooler regions that is more and more caused by
F. graminearum instead of
F. culmorum, resulting in an increment of yield losses of up to 75% and of mycotoxins contamination, affecting food safety and security [
33]. Thus, the substitution of species by others more thermotolerant and mycotoxigenic is predicted with the climate change [
92]. For example, is predicted that the climate change promotes the substitution of
Penicillium species by aflatoxin-producing
Aspergillus species [
93], as
A. flavus and
A. parasiticus that can persist in the most extreme climate warming conditions, having high optimum temperatures to produce aflatoxins [
92]. Also, extreme climatic conditions can promote the mycotoxin production as happened between 2003 and 2016, when high levels of aflatoxins were reported in maize from Italy and Serbia, both associated to the extreme climatic conditions [
92]. The same happened in France in 2015, when 6% of maize fields were contaminated by aflatoxins, due to exceptionally hot and dry climatic conditions and high prevalence of
A. flavus [
93]. In this way, the climate change may be a real concern to agriculture in the future, turning the crops more susceptible to fungal and mycotoxin contamination due plants will be subjected to suboptimal climatic conditions and phytopathogenic and mycotoxigenic fungi will be subjected to optimal ones [
92]. Coffee cultivation is an example, with a predicted reduction of 50% of production area and increase of mycotoxin contamination due the climate change [
93].
Prediction of where and when emergent pathogens will appear is ever more difficult with climate change acceleration [
84]. Moreover, the risk of the emergence of new potential pathogenic species is a concern due to the few antifungals and fungicides available, which in association with the emergence of antifungal resistant strains is a huge problem in short and long-term [
88]. In this way, reduce carbon emissions and control the climate change advances are important steps to control fungal species adaptation and pathogens emergence. Control the excessive use of antifungals and fungicides and the increasing efforts on developing vaccines are also main actions to promote the reduction of fungal infections [
33,
88]. More epidemiological studies and mathematical models are also needed to predict the incidence of emergent pathogens in long-term [
84].