1. Introduction
The treatment of cancer has made tremendous strides in the past decade. Despite all of this, there are numerous instances of the curative intention failing in the battle between the tumor and the patient's body; the tumor will prevail and the patient will perish. The statistical evidence that the number of malignant diseases increases annually is also thought-provoking. When treating malignancies, the question arises as to whether it is sufficient to target only the tumor cells with therapy. Evidently not. Like our other organs and tissues, tumor tissue is an ecosystem that employs detrimental solutions to ensure its survival. Therefore, we must consider the formerly "innocent" tumor components that contribute to the survival of cancer cells. These are partially the proteins that compose the extracellular matrix (ECM) and partially the stromal cells, the function of which changes as a result of the tumor cells (fibroblasts, endothelial cells, inflammatory cells), and they promote or do not inhibit the progression of the tumor in a variety of ways. In the present review we aim to provide a concise overlook about the major acellular and cellular components of the extracellular matrix, discuss the role of immune system in creating tumorous microenvironment along with neoangiogenesis. Finally, we provide insight of therapeutic approaches targeting components of the tumorous stroma.
2. Extracellular Matrix Components and Their Functions
2.1. Collagens
The ECM’s principal structural proteins are the collagens. Collagen I and collagen III, two of the more than 28 known types, are the primary structural components of tumors. Long before their oncogenicity was recognized, it was discovered that an increase in their quantity increases the rigidity of the tumor matrix (matrix stiffness). Collagen I and III is one of the main stimulators of DDR1 tyrosine kinase receptors on the surface of tumor cells, which promotes tumor cell proliferation [
1].
2.2. Adhesive Glycoproteins
2.2.1. Fibronectin
Fibronectin is the principal adhesive glycoprotein of the extracellular matrix. Via integrins, it is linked to epithelial cells. It took a long time for its oncogenicity to become evident, and there are still contradictory data available. Fibronectin is also produced by cancer cells. In addition to its myriad physiological functions, a growing body of evidence supports fibronectin’s role in the biological behavior of tumors [
2]. In malignancies, fibroblasts and macrophages are primarily responsible for fibronectin production. It influences tumor cell migration and invasion, as well as tumor angiogenesis [
3].
2.2.2. Laminins
There are 15 different types of laminin known. Their structure consists of three chains (alpha, beta, and gamma). Laminin 5 is one of the primary components of the basement membrane, ensuring the stability of the basement membrane and the epithelial layer. It is linked to epithelial cells through integrin molecules (a6b1, a6b4, respectively). Due to matrix metalloproteases, laminin escapes the adhesion and penetrates the tumor stroma, mainly via its gamma chain, where it promotes the invasion of tumor cells [
4]. Tumor-associated fibroblasts stimulate the progression of cervical cancer by elevating laminin-1 expression in the tumor stroma [
5].
2.3. Proteoglycans
Proteoglycans are composed of a protein chain and glycosaminoglycans that are linked to it via the Ser-Gly amino acid motif. They are found in the ECM, on the surface of epithelial cells, and occasionally in their cytoplasm and nuclei. In the extracellular matrix proteoglycans are responsible for stromal turgor and can bind numerous cytokine and growth factors via their sugar chains. In addition to their numerous physiological functions, they may also contribute to the biological behavior of tumors. Based on their structure, proteoglycans can either stimulate or inhibit tumor growth. The most well-known proteoglycan with antitumor properties is decorin, which inhibits the activity of multiple cell surface tyrosine kinase receptors and TGF-β [
6]. Glypican-3, a cell surface heparan sulfate proteoglycan, is one of the stimulators of liver cancer development [
7]. Agrin, localized in the basement membrane, also promotes the formation of liver cancer, and primarily associated with the YAP-TAZ pathway process of matrix rigidity [
8]. The Spock1/testican-1 is a heparan sulfate proteoglycan that is present in the cytoplasm of numerous epithelial malignancies. It promotes cancer development in part by activating cell surface and intracellular tyrosine kinase receptors and by increasing DNA synthesis [
9].
Hyaluronic acid (HA) is the only molecule that forms a linear non-sulfated glycosaminoglycan chain composed of disaccharide units and does not bind to proteins. Depending on its magnitude, HA’s effects vary significantly. While its large chains serve a crucial role in maintaining connective tissue turgor, inhibiting inflammation, and promoting wound repair, low molecular weight HA variants bind to the CD44 receptor and promote tumor development via the RAS-Raf signal pathway [
10].
2.4. Integrins
Their task is to detect and transmit the signals of certain proteins in the stroma to the cells. They selectively bind particular proteins, such as fibronectin, laminins, collagens, thrombospondin, and numerous other adhesion molecules. The signals they transmit to cells influence several cell functions. Additionally, integrins are expressed on the surface of fibroblasts associated with tumors. The α11β1 integrin promotes the development of matrix stiffness formation, whereas αvβ6, αvβ5 integrins found on tumor cells support tumor cell proliferation and invasion [
11].
2.5. Regulatory Molecules
The extracellular matrix contains numerous regulatory molecules, a detailed description of which is beyond the scope of this article. Both cytokines and chemokines play crucial roles in tumor invasion and angiogenesis. The growth factors (EGF, HGF, PDGF, FGF, TGFbeta, etc.) are also important participants as they transmit signals inside the cell by binding to tyrosine kinase and other receptors on the cell surface. As a consequence of their elevated activity, tumors may facilitate pathological signaling. Another group of molecules with distinghised importance are matrikins, cleavage products of matrix proteins. Matrikins can exhibit distinct biological behavior than their parent molecule. Specifically, these molecules are abundant in the regulation of angiogenesis. For example, angiogenesis is stimulated by the perlecan proteoglycan. In contrast, endorepellin, the product of perlecan’s cleavage, inhibits it. Angiostatin derived from plasminogen and endostatin cleaved from collagen XVIII, both inhibit angiogenesis [
12]. Versican and its cleavage product versikin play a role in the inherited and acquired immune response. Myeloid cells produce versican in the tumor microenvironment. Versican aids in the exclusion of T cells from the immune response, whereas versikin promotes the migration of Batf3 dendritic cells and the formation of an immune milieu [
13].
2.6. Proteases
Uncontrolled proteolysis, elevated protease expression, or improper protease activation can all contribute to the development or progression of diseases, including cancer. More than 500 proteases have been identified in the human body, which can be categorized into five groups: metallo-, serine, cysteine, aspartase, and threonine proteases. These enzymes play a role in nearly all aspects of tissue function, with appropriate share of roles [
14]
. They play prominent roles in tumor formation and behavior. For a long time, proteases were attributed a role only in tumor invasion, that is, in penetrating the limiting basement membrane and creating an extracellular matrix that supports the formation of metastases. Currently, it is evident that they participate in carcinogenesis, as well as cell division, apoptosis, autophagy, and inflammation processes. Proteases play a crucial role in promoting epithelial-mesenchymal transformation (EMT), in which the morphology of tumor cells changes losing their epithelial cell characteristics, and acquiring fibroblast-like phenotype favoring their migration and metastasis. Proteases also facilitate their migration by degrading the basement membrane and extracellular matrix [
15]
.
3. Cellular Elements of the Tumor Microenvironment
3.1. Tumor Associated Fibroblasts (CAF)
Tumor-associated fibroblasts are dominant residents of the tumor stroma. Regarding their origin, they are most often transformed from resident fibroblasts induced by various cytokines or growth factors (TGF-β, FGF, PDGF, Il1, IL6) [
16]. CAFs can also originate from the stromal cells of the bone marrow, but there are examples of their transformation from endothelial cells, adipose cells, and pericytes. The term tumor-associated fibroblast does not refer to a well-defined cell type; therefore, it can participate in a variety of functions, the majority of which are supportive of tumor progression. Today, there are at least 20 subtypes of CAF that can be detected in various functions and tumor types. It is evident that they play a role in the overproduction of collagen, which results in the stiffening of matrix that facilitates tumor progression [
17]. This situation can be exacerbated by therapeutic irradiation, which creates an additional stressor. Other factors produced by CAFs can also interfere with the progression of tumors. By reorganizing the extracellular matrix and altering its structure, they facilitate the migration of tumor cells. Furthermore, by causing high connective tissue pressure, they can prevent therapeutic agents from reaching their target. CAFs stimulate tumor angiogenesis and tumor cell proliferation with growth factors (VEGF, HGF), support tumor cell metabolism, and last but not least, inhibit anti-tumor immunity with their cytokines. On the basis of "single cell" sequencing data, CAF subpopulations can be identified in various functions and tumor types [
18,
19]. Therapeutic attempts to inhibit CAFs have so far been unsuccessful.
3.2. The Role of the Immune System in the Defense against Tumors
For many decades, science has been faced with the phenomenon that the immune defense against tumors is ineffective, even when the tumor and its environment are infiltrated by a large number of inflammatory cells. The results of research conducted over the past decade indicate that this inflammatory infiltration contains a variety of cells, including numerous cell types of innate and adaptive immunity with distinct functions. While innate immunity also plays an active role in the development of the pre-tumor state, the cellular elements of the adaptive immunity, namely B and T cells have anti-tumor potential.
3.2.1. Innate Immunity
Our innate immunity is composed of neutrophil cells, monocyte-macrophages, and NK cells, whose primary function is to defend against harmful agents. However, when inflammatory processes become chronic, the body's defense mechanism can go astray, and as a result of the produced cytokines, chemokines, and other mediators, a mutagenic microenvironment is created. As an effect of colony stimulating factor and CCL2 chemokine, immature myeloid cells migrate from the bone marrow to the inflammatory area, where they differentiate into tumor-associated macrophages (TAM) and N2 neutrophil cells, thereby promoting tumor transformation and angiogenesis. Inflammation-induced DNA damage eventually leads to the development of tumors. This is exemplified by tumors that form during chronic viral infections (HPV, HCV, EBV) and colon cancer that develops as a consequence of ulcerative colitis [
20]. Nonetheless, it is important to note that malignant tumors can arise in other ways, such as due to a deficiency in DNA repair enzymes, hereditary genetic mutations, or genotoxic effects with oncogenic potential (e.g. radiation, chemicals, smoking, etc.), in which the inflammatory component in tumors may be much less prominent. Similar to tumor-associated fibroblasts, tumor-associated myeloid cells have multiple (currently 23) subtypes, as revealed by single-cell sequencing. The largest fraction is of monocyte-macrophage origin, but dendritic and neutrophil subtypes are also present. Although anti-tumor effects have also been described, the majority of these cells have a tumor-supporting potential [
21]. Knowing this, it will be possible to utilize them in the future for tumor therapy. TAMs and TANs (tumor-associated macrophages and tumor-associated neutrophils) are responsible for sustaining an inflammatory environment, promoting the proliferation of tumor cells, inhibiting T cells, and transforming the microenvironment to support tumor cell invasion and angiogenesis. Destruction of tumor cells, phagocytosis and removal of debris, activation of NK cells and Th1 immune response are tumor-inhibiting processes [
21,
22].
The discovery of anti-tumor effects has prompted efforts to transform tumor-promoting TAMs into tumor-inhibiting M1 macrophages. Inhibition of the SIRP-1 receptor reactivates the phagocytic ability of TAMs, the CD40 receptor-specific antibody, or Toll-like receptor ligands stimulate the tumor-killing effect of TAMs. Inhibition of tumor intermediate metabolism can reduce the stress level of microenvironment thereby promoting the phenotypic transformation of TAMs.
Several trials are currently active aiming to inhibit tumor associated neutrophils. By inhibiting their surface cytokine receptors, researchers attempted to prevent their migration into the tumor microenvironment and into the tumor itself. Other options, such as reduction of hypoxia in tumors, inhibition of transcription factors (e.g. TGFB1), arose as well. On a subset of neurophils PD-L1 ligand is also detectable, which inhibits the activity of CD8+ lymphocytes [
23].
3.2.2. Adaptive (Acquired) Immunity
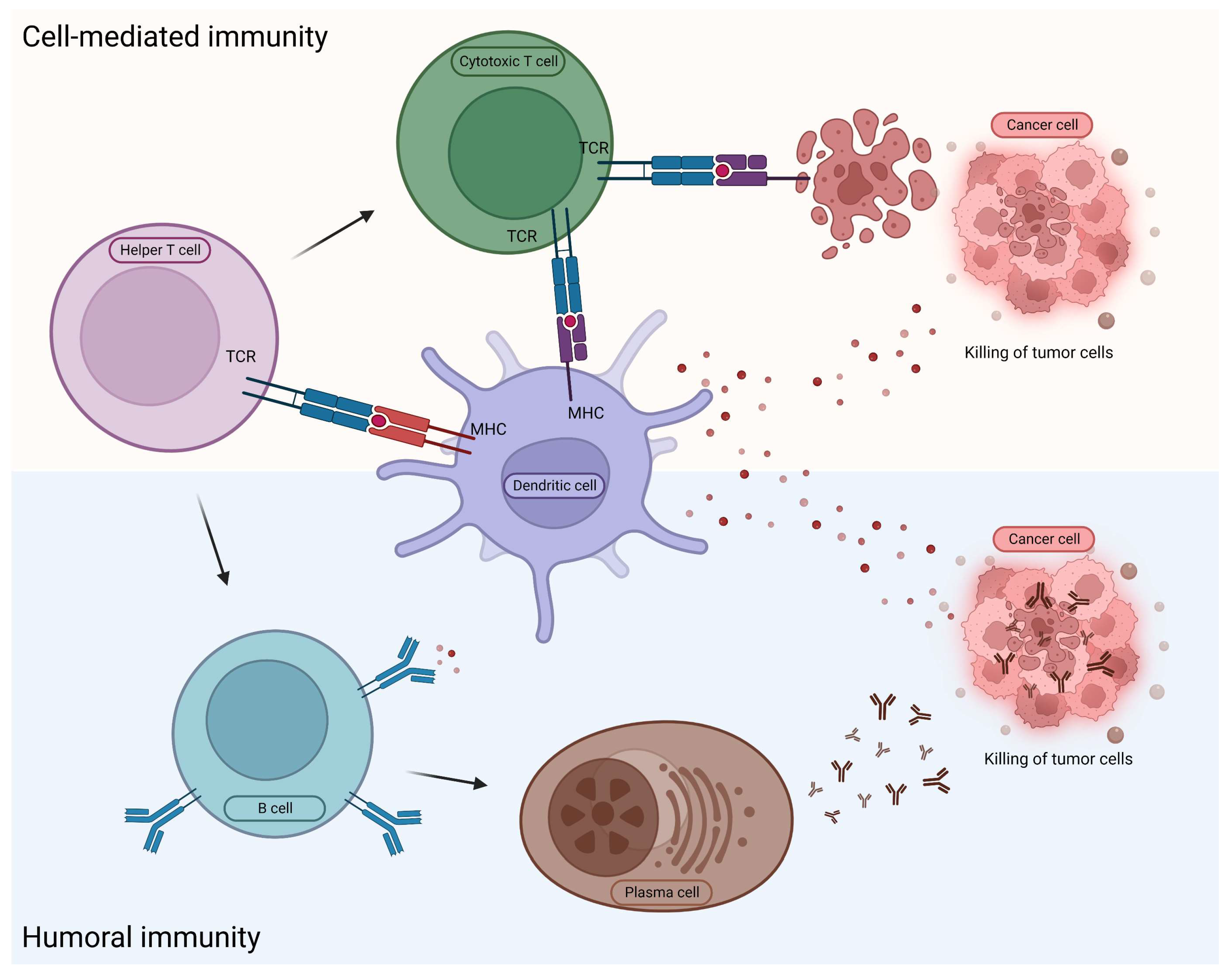
In the case of innate immunity, the reaction against foreign molecules is not specific. In contrast, in adaptive immunity the foreign agent is digested by the antigen-presenting cells, and subsequently their smaller and larger components are presented on their cell surfaces linked to MHC molecules. In humoral immunity, cell components are detected by B lymphocytes and T helper cells through their receptors. Consequently, activated B lymphocytes begin to multiply and, if necessary, differentiate into plasma cells and produce specific antibodies, or transform into memory B cells [
24]. In the case of cellular immunity, the antigen-presenting dendritic cells transmit the information directly to the CD8+ killer cells (
Figure 1).
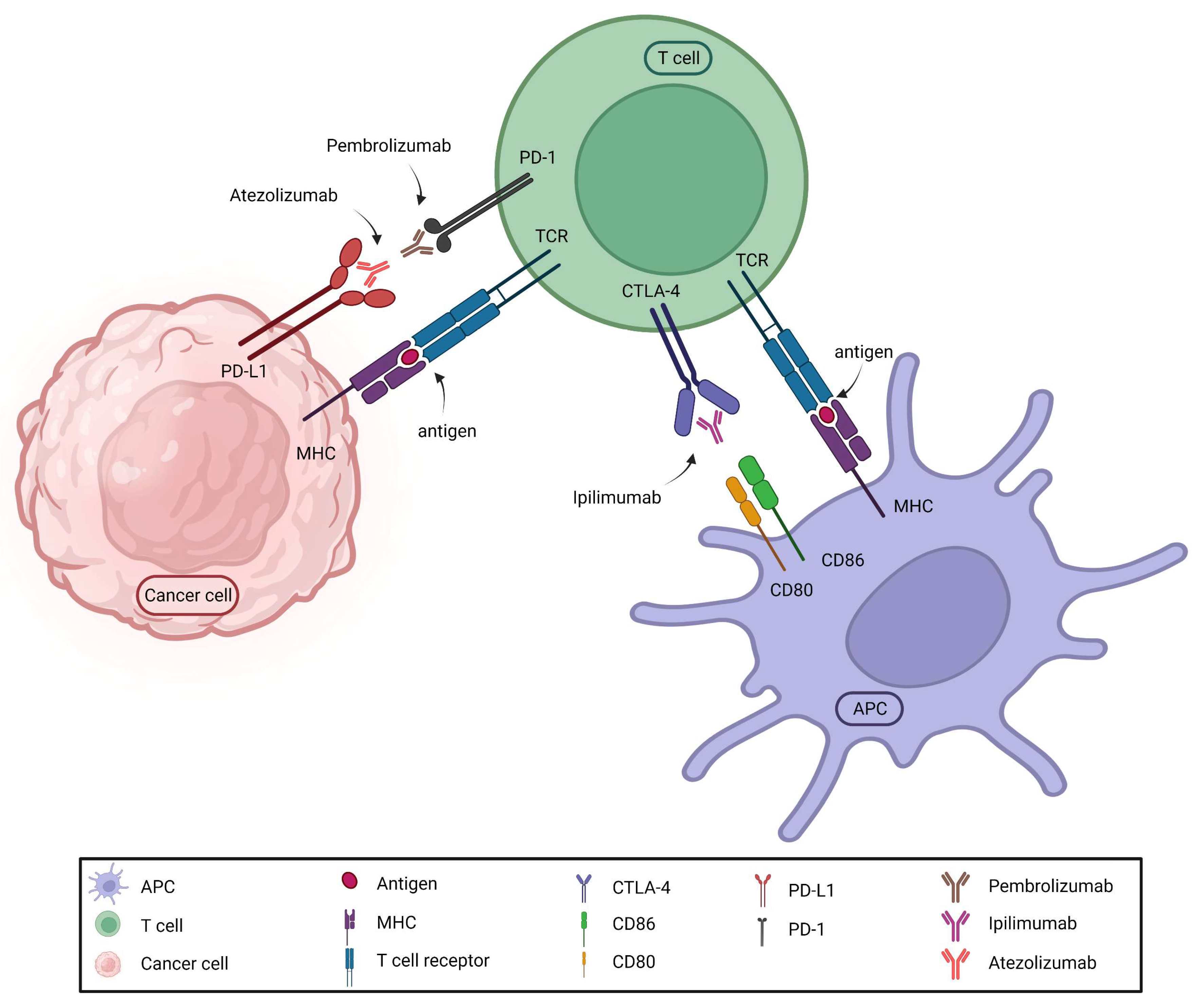
The expanding knowledge of acquired cellular immunity led to the development of adaptive immunotherapy. It has long been understood that tumor cells can neutralize the effects of immune cells acting against them. It required many years to clarify the mechanism and develop effective medications. These so-called immune "checkpoint" inhibitors suspend immune inhibition based on the interaction between immune cells and tumor cells, thereby permitting immune cells to destroy tumor cells. The essence of the discovery was that tumor cells express proteins (ligands) on their cell surface that bind to receptors on CD8+ lymphocytes, deactivating and destroying the immune cell. A number of these medications (immune checkpoint inhibitors) are available on the market today, and their use is associated with various conditions in the case of different tumors (
Figure 2).
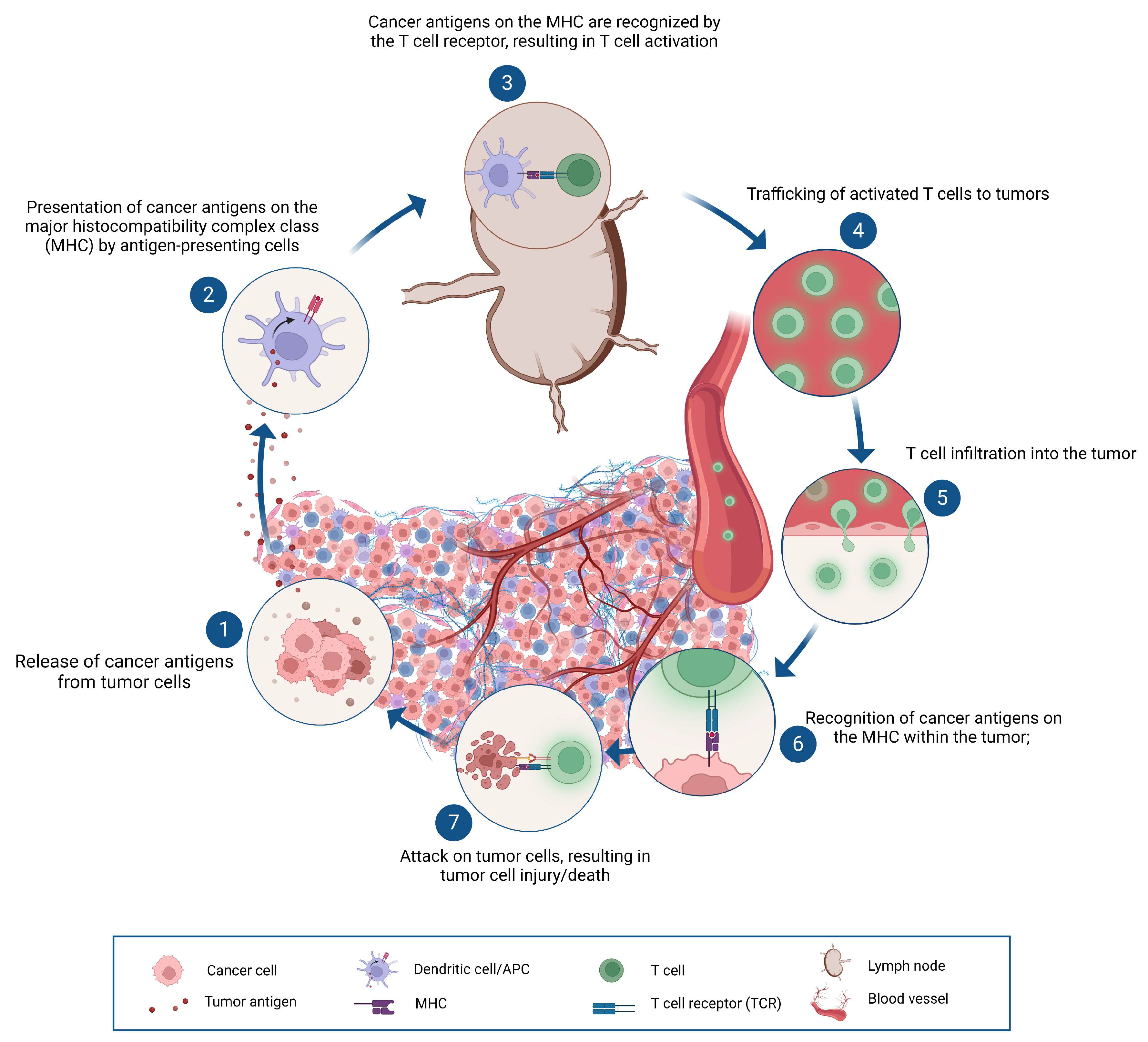
Ipilimumab was the first, but less effective immune checkpoint inhibitor used in malignant melanomas. It inhibited the binding of CTLA4 on the surface of T lymphocytes to the CD80 and CD86 molecules detected on dendritic cells or in the tumor. The breakthrough came with the use of the monoclonal antibodies nivolumab, pembrolizumab and atezolizumab designed to inhibit the connection between the PD1 receptor and the PD-L1 ligand. Nowadays, the current research focuses on the inhibition of novel checkpoint targets. It is fascinating that the discovery of inhibitors occurred only a few years after the original description of the tumor immunity cycle [
25], which elucidated the events of the immune response against tumors (
Figure 3).
Immunotherapy has many triumphs, but not all tumors respond as expected to its application. Immunotherapy can be used in monotherapy, but there are increasing efforts to combine it with other antitumor drugs. In this discipline, there have been both successes and failures, justifying the need for additional studies.
4. Tumor angiogenesis
Tumors, like healthy tissues, require a blood supply. To achieve this, various tumors employ distinct strategies. They are able to create new blood vessels, engulf existing ones and divide them in half, and stimulate the glomerular proliferation of existing veins. The final result is achieved under the influence of factors that stimulate or inhibit angiogenesis. The best and most significant growth factor for cell and anti-tumor therapy is VEGF (vascular endothelial growth factor), which stimulates the proliferation of endothelial cells by binding to its receptor on the cell surface. Several additional cytokines, chemokines, and growth factors are associated with the process, partially stimulating and partially inhibiting tumor vessel formation [
12]. The components of tumor vessels are endothelial cells, pericytes, and the basal membrane that supports the vessel wall. The structure of these blood vessels is imperfect. It is characterized by tortuous, dilated, and frequently blind-ending arteries and capillaries, as well as impaired microcirculation within the vasculature. The endothelial lining is also damaged. Pericytes only partially cover the outer layer of blood vessels. Additionally, the basal membrane is also damaged, its thickness and connection to the endothelial cells is irregular. Consequently, they are partially permeable for the blood circulating in them. These blood vessels are not accompanied by lymphatic vessels. Normal fibroblasts do not produce mediators that stimulate angiogenesis, but tumor-associated fibroblasts generate bFGF, PDGF, and CXCL12 cytokines in addition to VEGF, which further supports tumor neoangiogenesis. The need for nutrients by proliferating tumor cells within the fast growing malignant tumors is substantial, so it is not surprising that the inhibition of angiogenesis is a crucial component of cancer therapy.
5. Tumor stroma as a therapeutic target
Among the components of the tumorous ECM, collagen, fibronectin, certain integrins, and hyaluronic acid are expected to be of interest in terms of therapeutic intervention. Collagen forms the structural basis of the extracellular matrix of the tumor. Accordingly, most therapeutic attempts were aimed at reducing the increased amount of collagen present in the tumor matrix, partially inhibiting the factors that stimulate their production, increasing their degradation or inhibition of their cross-binding. The trials were conducted primarily on animals, but there were also human trials. The fungal derivative called halofungin reduced the overproduction of collagen by inhibiting TGF-β in a human breast cancer orthotopic mouse model [
26], while others used collagenase to reduce the stiffness of the matrix for better drug penetration [
27]. Looking back on the various enthusiastic attempts, it appears that a significant proportion of them have failed so far. During drug trials, novel TGF-β-inhibiting strategies demonstrated some efficacy against various solid tumors, providing an incentive for further testing [
28]. Recently, inhibition of lysyl oxidase has been attempted in colorectal and pancreatic cancers by binding FOLFIRI or gemcitabine to the enzyme-recognizing humanized monoclonal antibody [
29,
30]. In another trial, losartan (ACE inhibitor) and paclitaxel packed in liposomes were administered to the lungs, inhibiting breast cancer metastases in 80% of cases. The procedure achieved a reduction in collagen and lysyl oxidase levels, and also inhibited TGF-β1 [
31]. It is a known fact that the CD44 variant binds hyaluronic acid, which has a tumor-supporting effect. Moreover, apoptosis is inhibited in malignancies that generate lactic acid via aerobic glycolysis. Dichloroacetate inhibits lactic acid production by reactivating mitochondrial function, and this, in conjunction with the inhibition of hyaluronic acid synthesis by 4-methylumbelliferone had a dual antitumor effect in the case of aerobic glycolysis of the tumor [
32].
A communication from two years ago recommending a therapeutic strategy for colon cancer presents an entirely novel approach. In this publication, the tumors were divided into four groups based on their transcriptome/protein profile, and the stroma of each group was examined. [
33]. The first group (CMS1) mainly contained hypermutated MSI positive tumors with BRAF mutation or CpG island methylation. APC mutant hereditary tumors with WNT signaling and consequent Myc activation were included in the second group (CMS2). Group 3 (CMS3) tumors were distinguished by metabolic dysregulation with frequent KRAS mutations and a very poor prognosis. Group 4 (CMS4) was characterized by epithelial-mesenchymal transition, activation of TGF-β signaling, increased angiogenesis, and remodeling of the matrix. Studying the extracellular matrix of the groups revealed that it is also different. Type 1 stroma contained many proteins that are involved in the regulation of T cells, with the presence of highly differentiated Th1 and T8 cytotoxic cells, and the expression of CXCL13 was significant. These tumors expressed large amounts of immune checkpoint molecules (PD1 and CTLA, etc.), so immune checkpoint inhibitors were recommended for their treatment. In the stroma of group 2, few lymphocytes, macrophages, endothelial cells and fibroblasts were detected. In accordance with their known gene mutation, β-catenin inhibition was recommended. The stroma of the third group was characterized by sparse immune cell infiltration, and along with chemotherapy, an EGFR inhibitor was suggested, and in the case of KRAS mutation, angiogenesis inhibition, possibly adoptive T cell therapy. Tumors in group 4 contained immune checkpoint molecules, their stroma was infiltrated by macrophages and myeloid-derived suppressor cells. The large amount of chemokines in their stroma induced the migration of myeloid cells. The presence of tertiary lymphoid structures and inflammatory genes indicated a disorganized antitumor reaction. In these cases, in addition to immune checkpoint therapy, TGF-β inhibition, angiogenesis inhibition, and macrophage inhibition were recommended. Of course, it is again questionable how these proposals will work in the future. In any case, they reflect the attempts based on which the status of the stromal resident cells, the role of cytokines, chemokines and matrikines can participate in the selection of therapy.
6. Conclusions
Although tumors originate from healthy tissue, during their malignant transformation they create their own environment involving all necessary participants. Therefore, a tumor is not equal to cancer cells. The tumor cell is only one component of the tumor. Next to it, virtually all types of tissue cells line up with the initial intention of defending themselves, and then resigning to the change in the circumstances. This process results in the formation of tumor-associated fibroblasts, blood vessels, and immune cells with altered tumor-supporting functions. The cells that can still be activated in the defense (e.g. CD8 lymphocytes) are neutralized by immune check point inhibitory molecules. A precise understanding of the tumor stroma is in progress. If we follow the events of the last 10 years, the expansion of immunotherapy, we should observe that in addition to tumor angiogenesis, the factors involved in immunotherapy are also the components of the tumor stroma. Even if we have not faced this fact so far, it gives us reason to hope that an improved understanding of the tumor microenvironment will yield more novel outcomes.
Author Contributions
Writing—original draft preparation, K.B; writing—review and editing, I.K.; visualization, A.R. All authors have read and agreed to the published version of the manuscript.”
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors are thankful for all their hard work and support of their colleagues during the last twenty years.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Chen, L.; Kong, X.; Fang, Y.; Paunikar, S.; Wang, X.; Brown, J.A.L.; Bourke, E.; Li, X.; Wang, J. Recent Advances in the Role of Discoidin Domain Receptor Tyrosine Kinase 1 and Discoidin Domain Receptor Tyrosine Kinase 2 in Breast and Ovarian Cancer. Frontiers in cell and developmental biology 2021, 9, 747314. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.-C.; Yang, C.-H.; Cheng, L.-H.; Chang, W.-T.; Lin, Y.-R.; Cheng, H.-C. Fibronectin in Cancer: Friend or Foe. Cells 2020, 9, 27. [Google Scholar] [CrossRef] [PubMed]
- Erdogan, B.; Ao, M.; White, L.M.; Means, A.L.; Brewer, B.M.; Yang, L.; Washington, M.K.; Shi, C.; Franco, O.E.; Weaver, A.M.; et al. Cancer-associated fibroblasts promote directional cancer cell migration by aligning fibronectin. The Journal of cell biology 2017, 216, 3799–3816. [Google Scholar] [CrossRef] [PubMed]
- Giannelli, G.; Antonaci, S. Biological and clinical relevance of Laminin-5 in cancer. Clinical & Experimental Metastasis 2000, 18, 439–443. [Google Scholar] [CrossRef]
- Fullár, A.; Dudás, J.; Oláh, L.; Hollósi, P.; Papp, Z.; Sobel, G.; Karászi, K.; Paku, S.; Baghy, K.; Kovalszky, I. Remodeling of extracellular matrix by normal and tumor-associated fibroblasts promotes cervical cancer progression. BMC cancer 2015, 15, 256. [Google Scholar] [CrossRef]
- Reszegi, A.; Horváth, Z.; Karászi, K.; Regős, E.; Postniková, V.; Tátrai, P.; Kiss, A.; Schaff, Z.; Kovalszky, I.; Baghy, K. The Protective Role of Decorin in Hepatic Metastasis of Colorectal Carcinoma. Biomolecules 2020, 10. [Google Scholar] [CrossRef]
- Pang, N.; Shi, J.; Qin, L.; Chen, A.; Tang, Y.; Yang, H.; Huang, Y.; Wu, Q.; Li, X.; He, B.; et al. IL-7 and CCL19-secreting CAR-T cell therapy for tumors with positive glypican-3 or mesothelin. Journal of hematology & oncology 2021, 14, 118. [Google Scholar] [CrossRef]
- Chakraborty, S.; Hong, W. Linking Extracellular Matrix Agrin to the Hippo Pathway in Liver Cancer and Beyond. Cancers 2018, 10. [Google Scholar] [CrossRef]
- Váncza, L.; Karászi, K.; Péterfia, B.; Turiák, L.; Dezső, K.; Sebestyén, A.; Reszegi, A.; Petővári, G.; Kiss, A.; Schaff, Z.; et al. SPOCK1 Promotes the Development of Hepatocellular Carcinoma. Frontiers in oncology 2022, 12, 819883. [Google Scholar] [CrossRef]
- Tavianatou, A.G.; Caon, I.; Franchi, M.; Piperigkou, Z.; Galesso, D.; Karamanos, N.K. Hyaluronan: molecular size-dependent signaling and biological functions in inflammation and cancer. The FEBS journal 2019, 286, 2883–2908. [Google Scholar] [CrossRef]
- Zeltz, C.; Lu, N.; Heljasvaara, R.; Gullberg, D. Integrins in Cancer: Refocusing on the Tumor Microenvironment. In The Extracellular Matrix and the Tumor Microenvironment, Kovalszky, I., Franchi, M., Alaniz, L.D., Eds.; Springer International Publishing: Cham, 2022; pp. 279–314. [Google Scholar]
- Kovalszky, I.; Váncza, L.; Reszegi, A.; Tátrai, P.; Baghy, K. Cancer Angiogenesis and Its Master Regulator Perlecan. In The Extracellular Matrix and the Tumor Microenvironment, Kovalszky, I., Franchi, M., Alaniz, L.D., Eds.; Springer International Publishing: Cham, 2022; pp. 399–419. [Google Scholar]
- Papadas, A.; Arauz, G.; Cicala, A.; Wiesner, J.; Asimakopoulos, F. Versican and Versican-matrikines in Cancer Progression, Inflammation, and Immunity. The journal of histochemistry and cytochemistry : official journal of the Histochemistry Society 2020, 68, 871–885. [Google Scholar] [CrossRef] [PubMed]
- Vizovisek, M.; Ristanovic, D.; Menghini, S.; Christiansen, M.G.; Schuerle, S. The Tumor Proteolytic Landscape: A Challenging Frontier in Cancer Diagnosis and Therapy. International journal of molecular sciences 2021, 22. [Google Scholar] [CrossRef]
- Mitschke, J.; Burk, U.C.; Reinheckel, T. The role of proteases in epithelial-to-mesenchymal cell transitions in cancer. Cancer and Metastasis Reviews 2019, 38, 431–444. [Google Scholar] [CrossRef]
- Park, D.; Sahai, E.; Rullan, A. SnapShot: Cancer-Associated Fibroblasts. Cell 2020, 181, 486–486. [Google Scholar] [CrossRef]
- Calvo, F.; Ege, N.; Grande-Garcia, A.; Hooper, S.; Jenkins, R.P.; Chaudhry, S.I.; Harrington, K.; Williamson, P.; Moeendarbary, E.; Charras, G.; et al. Mechanotransduction and YAP-dependent matrix remodelling is required for the generation and maintenance of cancer-associated fibroblasts. Nature cell biology 2013, 15, 637–646. [Google Scholar] [CrossRef] [PubMed]
- Costa, A.; Kieffer, Y.; Scholer-Dahirel, A.; Pelon, F.; Bourachot, B.; Cardon, M.; Sirven, P.; Magagna, I.; Fuhrmann, L.; Bernard, C.; et al. Fibroblast Heterogeneity and Immunosuppressive Environment in Human Breast Cancer. Cancer cell 2018, 33, 463–479. [Google Scholar] [CrossRef]
- Chen, Y.; McAndrews, K.M.; Kalluri, R. Clinical and therapeutic relevance of cancer-associated fibroblasts. Nature reviews. Clinical oncology 2021, 18, 792–804. [Google Scholar] [CrossRef] [PubMed]
- Hagerling, C.; Casbon, A.J.; Werb, Z. Balancing the innate immune system in tumor development. Trends in cell biology 2015, 25, 214–220. [Google Scholar] [CrossRef] [PubMed]
- Weissleder, R.; Pittet, M.J. The expanding landscape of inflammatory cells affecting cancer therapy. Nature Biomedical Engineering 2020, 4, 489–498. [Google Scholar] [CrossRef]
- Mantovani, A.; Marchesi, F.; Jaillon, S.; Garlanda, C.; Allavena, P. Tumor-associated myeloid cells: diversity and therapeutic targeting. Cellular & molecular immunology 2021, 18, 566–578. [Google Scholar] [CrossRef]
- He, G.; Zhang, H.; Zhou, J.; Wang, B.; Chen, Y.; Kong, Y.; Xie, X.; Wang, X.; Fei, R.; Wei, L.; et al. Peritumoural neutrophils negatively regulate adaptive immunity via the PD-L1/PD-1 signalling pathway in hepatocellular carcinoma. Journal of Experimental & Clinical Cancer Research 2015, 34, 141. [Google Scholar] [CrossRef]
- Akkaya, M.; Kwak, K.; Pierce, S.K. B cell memory: building two walls of protection against pathogens. Nature Reviews Immunology 2020, 20, 229–238. [Google Scholar] [CrossRef]
- Chen, Daniel S. ; Mellman, I. Oncology Meets Immunology: The Cancer-Immunity Cycle. Immunity 2013, 39, 1–10. [Google Scholar] [CrossRef]
- Liu, J.; Liao, S.; Diop-Frimpong, B.; Chen, W.; Goel, S.; Naxerova, K.; Ancukiewicz, M.; Boucher, Y.; Jain, R.K.; Xu, L. TGF-β blockade improves the distribution and efficacy of therapeutics in breast carcinoma by normalizing the tumor stroma. Proceedings of the National Academy of Sciences of the United States of America 2012, 109, 16618–16623. [Google Scholar] [CrossRef] [PubMed]
- Dolor, A.; Szoka, F.C., Jr. Digesting a Path Forward: The Utility of Collagenase Tumor Treatment for Improved Drug Delivery. Molecular pharmaceutics 2018, 15, 2069–2083. [Google Scholar] [CrossRef]
- Haque, S.; Morris, J.C. Transforming growth factor-β: A therapeutic target for cancer. Human vaccines & immunotherapeutics 2017, 13, 1741–1750. [Google Scholar] [CrossRef]
- Benson, A.B., 3rd; Wainberg, Z.A.; Hecht, J.R.; Vyushkov, D.; Dong, H.; Bendell, J.; Kudrik, F. A Phase II Randomized, Double-Blind, Placebo-Controlled Study of Simtuzumab or Placebo in Combination with Gemcitabine for the First-Line Treatment of Pancreatic Adenocarcinoma. The oncologist 2017, 22, 241–e215. [Google Scholar] [CrossRef]
- Hecht, J.R.; Benson, A.B., 3rd; Vyushkov, D.; Yang, Y.; Bendell, J.; Verma, U. A Phase II, Randomized, Double-Blind, Placebo-Controlled Study of Simtuzumab in Combination with FOLFIRI for the Second-Line Treatment of Metastatic KRAS Mutant Colorectal Adenocarcinoma. The oncologist 2017, 22, 243–e223. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, Y.; Xia, T.; Yu, Q.; Zhang, Q.; Yang, Y.; Cun, X.; Lu, L.; Gao, H.; Zhang, Z.; et al. Suppression for lung metastasis by depletion of collagen I and lysyl oxidase via losartan assisted with paclitaxel-loaded pH-sensitive liposomes in breast cancer. Drug delivery 2016, 23, 2970–2979. [Google Scholar] [CrossRef] [PubMed]
- Twarock, S.; Reichert, C.; Bach, K.; Reiners, O.; Kretschmer, I.; Gorski, D.J.; Gorges, K.; Grandoch, M.; Fischer, J.W. Inhibition of the hyaluronan matrix enhances metabolic anticancer therapy by dichloroacetate in vitro and in vivo. British journal of pharmacology 2019, 176, 4474–4490. [Google Scholar] [CrossRef] [PubMed]
- Fridman, W.H.; Miller, I.; Sautès-Fridman, C.; Byrne, A.T. Therapeutic Targeting of the Colorectal Tumor Stroma. Gastroenterology 2020, 158, 303–321. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).