Submitted:
16 June 2023
Posted:
19 June 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
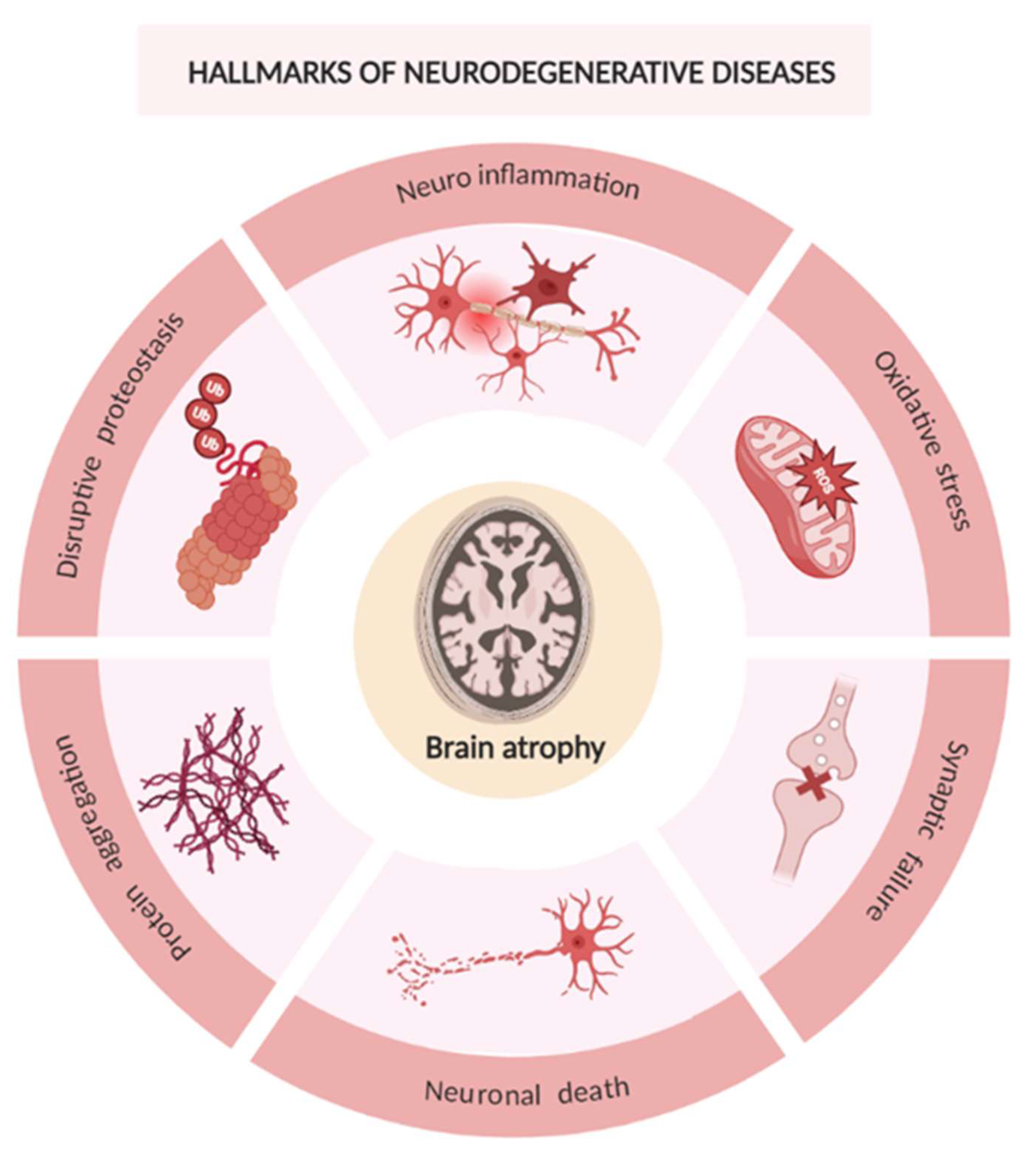
2. Neurodegenerative Diseases
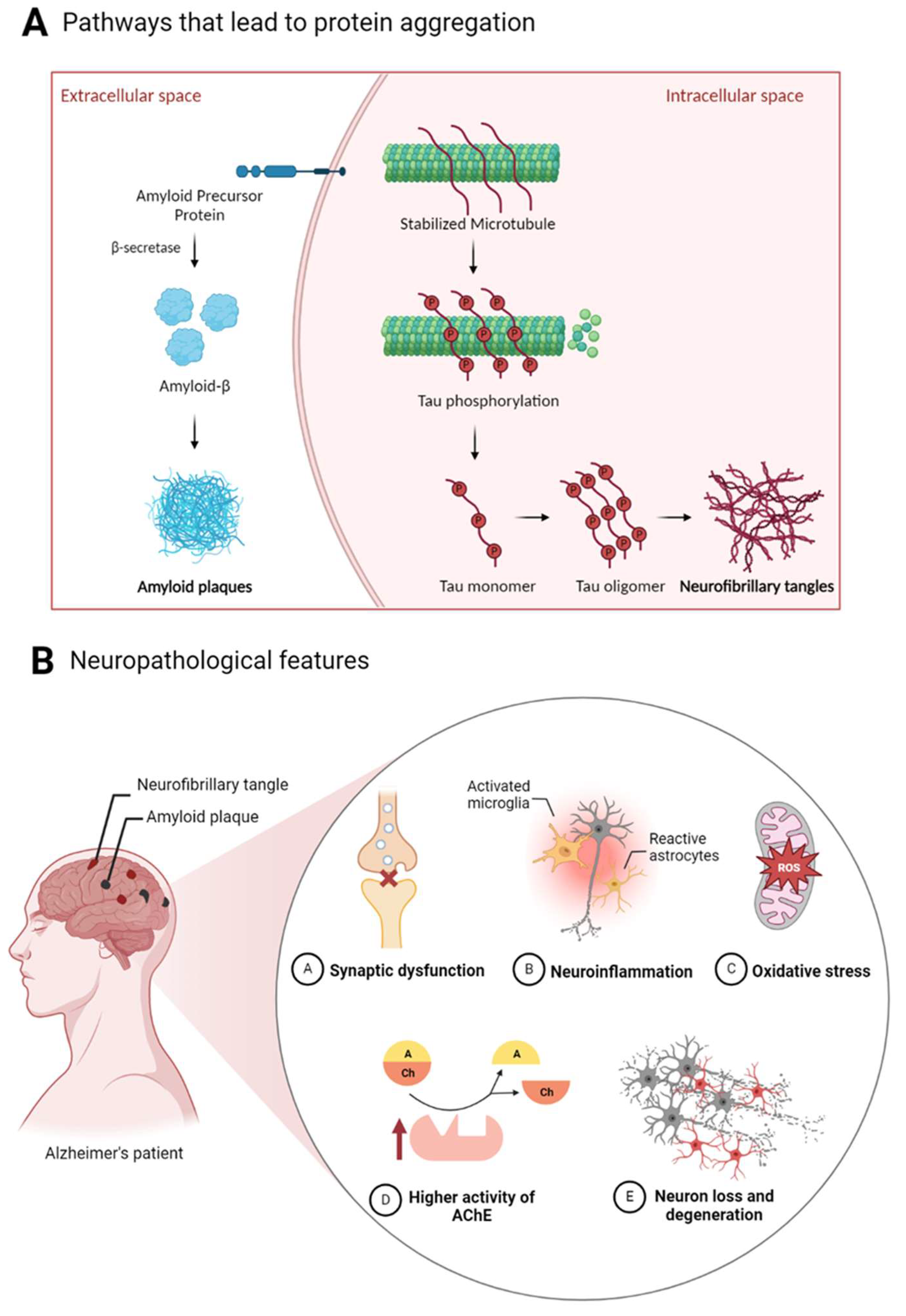
2.1. Alzheimer’s Disease
3. Natural Compounds for AD Treatment
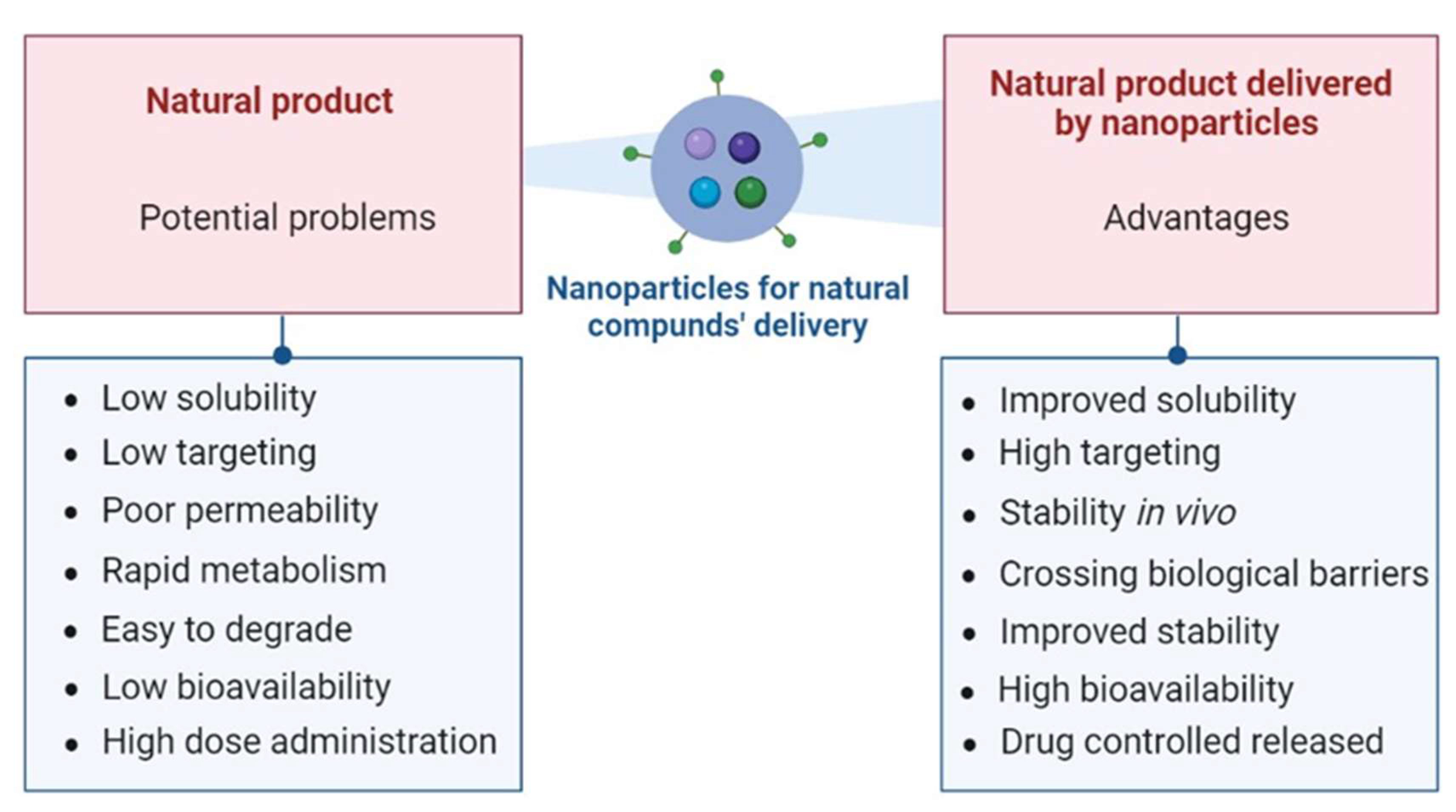
3.1. Overcoming Limitations of Natural Compounds with Delivery Systems
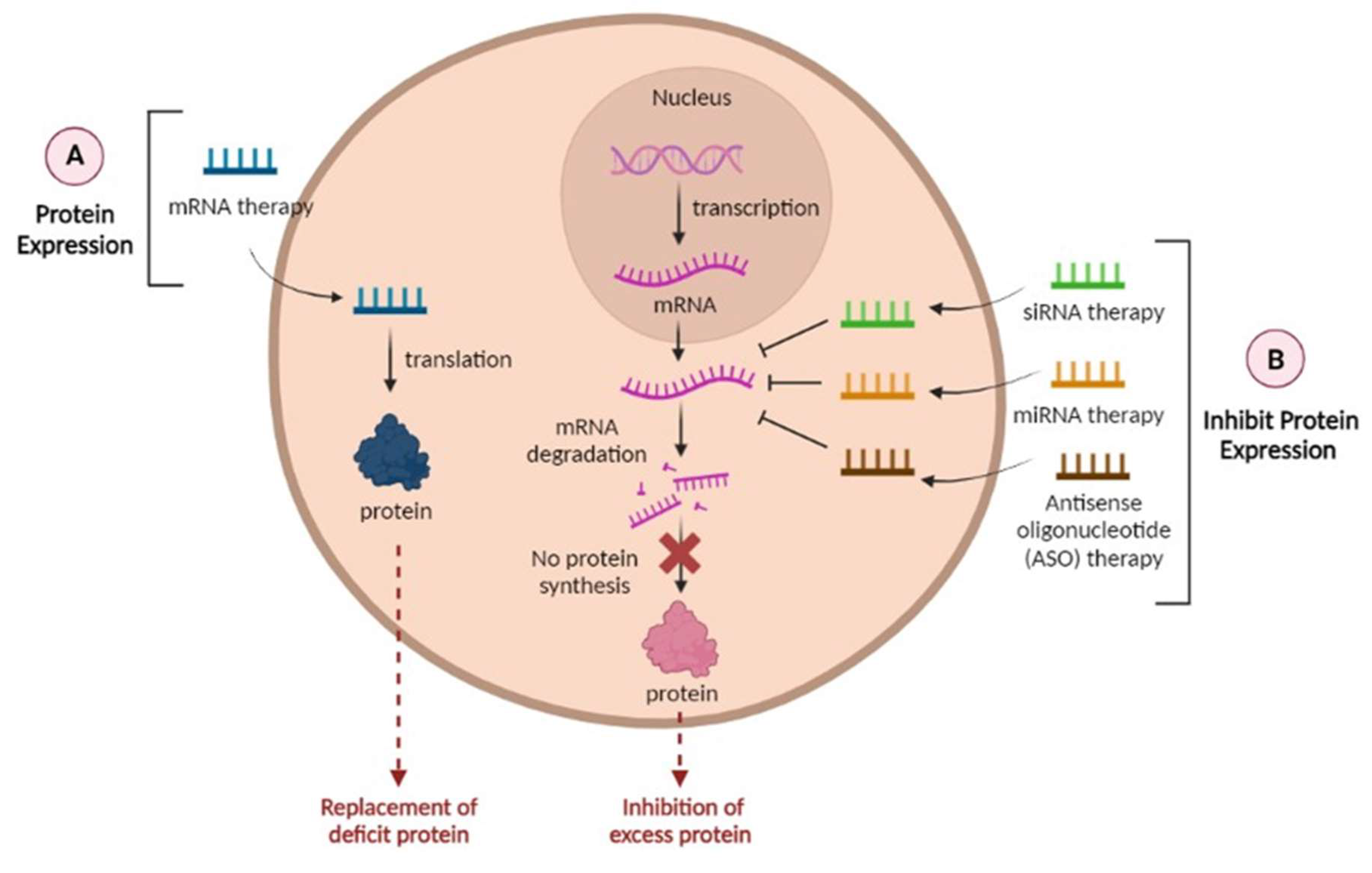
4. RNAs as a Promising Tool in the Treatment of AD
| Role in AD | Reference | |
|---|---|---|
| Types of miRNA | ||
| miR-101 | Significantly reduced the expression of a reporter under control of APP 3’-UTR in HeLa cells. | [95] |
| miR-106b | Overexpression of miR-106b inhibited Aβ1-42-induced tau phosphorylation at Tyr18 in SH-SY5Y cells stably expressing tau. | [96] |
| miR-137 | miR-137 inhibited increased expression levels of p-tau induced by Aβ1-42 in SH-SY5Y and inhibited the hyperphosphorylation of tau protein in a transgenic mouse model of AD. | [97] |
| miR-219 | In a Drosophila model that produces human tau, reduction of miR-219 exacerbated tau toxicity, while overexpression of miR-219 partially annulled toxic effects. | [98] |
| miR-17 | miR-17 inhibits elevated miR-17 in adult AD (5xFAD) mice microglia improves Aβ degradation. | [99] |
| miR-20b-5p | Treatment with miR-20b-5p reduced APP mRNA and protein levels in cultured human neuronal cells. | [100] |
| miR-29c | Over-expression of miR-29c in SH-SY5Y, HEK-293T cell lines and miR-29c in transgenic mice downregulated BACE1 protein levels. | [101] |
| miR-298 | miR-298 is a repressor of APP, BACE1, and the two primary forms of Aβ (Aβ40 and Aβ42) in a primary human cell culture model. Thus, miR-298 significantly reduced levels of ~55 and 50 kDa forms of the tau protein without significant alterations of total tau or other forms. | [102] |
| miR-485-5p | miR-485-5p overexpression facilitated the learning and memory capabilities of APP/PS1 mice and promoted pericyte viability and prohibited pericyte apoptosis in this model. | [103] |
| miR-9-5p | miR-9-5p overexpression inhibited Aβ25-35-induced mitochondrial dysfunction, cell apoptosis, and oxidative stress by regulating GSK-3β expression in HT22 cells. | [104] |
| miR-132 | miR-132 inhibited hippocampal iNOS expression and oxidative stress by inhibiting MAPK1 expression to improve the cognitive function of rats with AD. | [105] |
| miR-153 | Using miR-153 transgenic mouse model, was verified that miR-153 downregulated the expression of APP and APLP2 protein in vivo. | [106], p. 2 |
| Targeted gene silencing by siRNA | ||
| Tau | siRNA against MAPT can effectively suppress tau expression in vitro and in vivo without a specific delivery agent. | [107] |
| BACE1 | Polymeric siRNA nanomedicine targeting BACE1 in APP/PS1 transgenic AD mouse model can efficiently penetrate the BBB via glycemia-controlled glucose transporter-1–mediated transport, ensuring that siRNAs decrease BACE1 expression. | [94] |
| Presenilin1 (PS1) | Down regulation of PS1 and Aβ42 in IMR32 cells transfected with siRNA against PS1 was verified. | [108] |
| APP | Infusion of siRNAs that down-regulated mouse APP protein levels into the ventricular system for 2 weeks down-regulated APP mRNA in mouse brain. | [109] |
| Proteins encoded by mRNA | ||
| mRNA encoding neprilysin | Neprilysin plays a major role in the clearance of Aβ in the brain. New mRNA therapeutic strategy utilizing mRNA encoding the mouse neprilysin protein has shown to decrease Aβ deposition and prevent pathogenic changes in the brain. | [110] |
4.1. Overcoming Limitations of RNA Therapies with Delivery Systems
5. Nanoparticles and the BBB
5.1. Exosomes
5.2. Liposomes
5.3. Exosome-Like Liposomes as a Novel Strategy
- Dendrimers: Dendrimers are highly branched, characterized by defined molecular weights and specific encapsulation properties. This type of delivery system is composed by a symmetrical polymeric macromolecules with large number of reactive surface groups, with three distinctive architectural components, an interior core, an interior layer consisting of repeating units radially attached to the inner core, and functional end groups on the outside layer. Because of these unique features, dendrimers can cross impaired BBB, target astrocytes and microglia after systemic administration in animal models [139].
- Polymeric nanoparticles: Polymeric nanoparticles can be produced from synthetic or natural polymers. However, to be applied in brain drug delivery, these nanoparticles need to be biodegradable and biocompatible. PBCA, PLA and PLGA nanoparticles are nanoparticles able to cross the BBB. These nanocarriers possess controlled drug release, targeting efficiency, and they can avoid phagocytosis by the reticuloendothelial system, thus improving the concentration of drugs in the brain [140].
- Gold nanoparticles: Nanoparticles (mostly < 10 nm in size) composed of a gold core and with covalently or non-covalently attached surface ligands. Multiple in vivo studies on rodents have shown that low amounts of this delivery system were able to cross the BBB. However, the greater amount of the administered dose was found in the liver and in the blood.[8] Additionally, Sela et al. proved that gold nanoparticles could penetrate the BBB of rat without the use of external field or surface modification and were found to be distributed uniformly in both hypothalamus and hippocampus indicating there is no selective binding in these regions of brain [141].
- Carbon quantum dots: This delivery system retains a polymeric core structure and various functional groups on the surface, facilitating their conjugation with drug molecules for specific delivery. Carrier with several efficient features for BBB crossing such as excellent biocompatibility and low toxicity due to the lack of metal elements, small size and possess photoluminescence which can be utilized to track the penetration of CDs through the BBB [117].
6. Conclusion
Patents
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bloomingdale P, Karelina T, Ramakrishnan V, et al. Hallmarks of neurodegenerative disease: A systems pharmacology perspective. CPT Pharmacomet Syst Pharmacol. 2022;11(11):1399-1429. [CrossRef]
- 2023 Alzheimer’s disease facts and figures. Alzheimers Dement. Published online March 14, 2023:alz.13016. [CrossRef]
- DeTure MA, Dickson DW. The neuropathological diagnosis of Alzheimer’s disease. Mol Neurodegener. 2019;14(1):32. [CrossRef]
- Zhu Y, Zhu L, Wang X, Jin H. RNA-based therapeutics: an overview and prospectus. Cell Death Dis. 2022;13(7):644. [CrossRef]
- Damase TR, Sukhovershin R, Boada C, Taraballi F, Pettigrew RI, Cooke JP. The Limitless Future of RNA Therapeutics. Front Bioeng Biotechnol. 2021;9:628137. [CrossRef]
- Luo M, Lee LKC, Peng B, Choi CHJ, Tong WY, Voelcker NH. Delivering the Promise of Gene Therapy with Nanomedicines in Treating Central Nervous System Diseases. Adv Sci. 2022;9(26):2201740. [CrossRef]
- Daneman R, Prat A. The Blood–Brain Barrier. Cold Spring Harb Perspect Biol. 2015;7(1):a020412. [CrossRef]
- Lombardo SM, Schneider M, Türeli AE, Günday Türeli N. Key for crossing the BBB with nanoparticles: the rational design. Beilstein J Nanotechnol. 2020;11:866-883. [CrossRef]
- Akbarzadeh A, Rezaei-Sadabady R, Davaran S, et al. Liposome: classification, preparation, and applications. Nanoscale Res Lett. 2013;8(1):102. [CrossRef]
- Zhang Y, Liu Y, Liu H, Tang WH. Exosomes: biogenesis, biologic function and clinical potential. Cell Biosci. 2019;9(1):19. [CrossRef]
- Antimisiaris S, Mourtas S, Marazioti A. Exosomes and Exosome-Inspired Vesicles for Targeted Drug Delivery. Pharmaceutics. 2018;10(4):218. [CrossRef]
- Forrest SL, Kovacs GG. Current Concepts of Mixed Pathologies in Neurodegenerative Diseases. Can J Neurol Sci J Can Sci Neurol. Published online March 31, 2022:1-17. [CrossRef]
- Masoudi Asil S, Ahlawat J, Guillama Barroso G, Narayan M. Nanomaterial based drug delivery systems for the treatment of neurodegenerative diseases. Biomater Sci. 2020;8(15):4109-4128. [CrossRef]
- Wilson DM, Cookson MR, Van Den Bosch L, Zetterberg H, Holtzman DM, Dewachter I. Hallmarks of neurodegenerative diseases. Cell. 2023;186(4):693-714. [CrossRef]
- Roda A, Serra-Mir G, Montoliu-Gaya L, Tiessler L, Villegas S. Amyloid-beta peptide and tau protein crosstalk in Alzheimer’s disease. Neural Regen Res. 2022;17(8):1666. [CrossRef]
- Liang SY, Wang ZT, Tan L, Yu JT. Tau Toxicity in Neurodegeneration. Mol Neurobiol. 2022;59(6):3617-3634. [CrossRef]
- Yu H, Wu J. Amyloid-β: A double agent in Alzheimer’s disease? Biomed Pharmacother. 2021;139:111575. [CrossRef]
- Xiao Y, Wang SK, Zhang Y, et al. Role of extracellular vesicles in neurodegenerative diseases. Prog Neurobiol. 2021;201:102022. [CrossRef]
- Wei Z, Wei M, Yang X, Xu Y, Gao S, Ren K. Synaptic Secretion and Beyond: Targeting Synapse and Neurotransmitters to Treat Neurodegenerative Diseases. Parmar M, ed. Oxid Med Cell Longev. 2022;2022:1-22. [CrossRef]
- Van Den Berge N, Ulusoy A. Animal models of brain-first and body-first Parkinson’s disease. Neurobiol Dis. 2022;163:105599. [CrossRef]
- Sun X, Song J, Huang H, Chen H, Qian K. Modeling hallmark pathology using motor neurons derived from the family and sporadic amyotrophic lateral sclerosis patient-specific iPS cells. Stem Cell Res Ther. 2018;9(1):315. [CrossRef]
- Xiong L, McCoy M, Komuro H, et al. Inflammation-dependent oxidative stress metabolites as a hallmark of amyotrophic lateral sclerosis. Free Radic Biol Med. 2022;178:125-133. [CrossRef]
- Mehler MF, Petronglo JR, Arteaga-Bracho EE, et al. Loss-of-Huntingtin in Medial and Lateral Ganglionic Lineages Differentially Disrupts Regional Interneuron and Projection Neuron Subtypes and Promotes Huntington’s Disease-Associated Behavioral, Cellular, and Pathological Hallmarks. J Neurosci. 2019;39(10):1892-1909. [CrossRef]
- Jimenez-Sanchez M, Licitra F, Underwood BR, Rubinsztein DC. Huntington’s Disease: Mechanisms of Pathogenesis and Therapeutic Strategies. Cold Spring Harb Perspect Med. 2017;7(7):a024240. [CrossRef]
- Machiela E, Southwell AL. Biological Aging and the Cellular Pathogenesis of Huntington’s Disease. J Huntingt Dis. 2020;9(2):115-128. [CrossRef]
- Gallego Villarejo L, Bachmann L, Marks D, Brachthäuser M, Geidies A, Müller T. Role of Intracellular Amyloid β as Pathway Modulator, Biomarker, and Therapy Target. Int J Mol Sci. 2022;23(9):4656. [CrossRef]
- Chen XQ, Mobley WC. Alzheimer Disease Pathogenesis: Insights From Molecular and Cellular Biology Studies of Oligomeric Aβ and Tau Species. Front Neurosci. 2019;13:659. [CrossRef]
- Guo Y, Wang Q, Chen S, Xu C. Functions of amyloid precursor protein in metabolic diseases. Metabolism. 2021;115:154454. [CrossRef]
- Liu X, Liu Y, Ji S. Secretases Related to Amyloid Precursor Protein Processing. Membranes. 2021;11(12):983. [CrossRef]
- Zhang T, Chen D, Lee TH. Phosphorylation Signaling in APP Processing in Alzheimer’s Disease. Int J Mol Sci. 2019;21(1):209. [CrossRef]
- Lane CA, Hardy J, Schott JM. Alzheimer’s disease. Eur J Neurol. 2018;25(1):59-70. [CrossRef]
- Beera AM, Seethamraju SM, Nori LP. Alzheimer’s Disease: Perspective on Therapeutic Options and Recent Hallmarks in Clinical Research. Int J Pharm Res Allied Sci. 2021;10(4):110-120. https://doi.org/10.51847/ViC6sAGCyq. [CrossRef]
- Wu M, Zhang M, Yin X, et al. The role of pathological tau in synaptic dysfunction in Alzheimer’s diseases. Transl Neurodegener. 2021;10(1):45. [CrossRef]
- Chu D, Liu F. Pathological Changes of Tau Related to Alzheimer’s Disease. ACS Chem Neurosci. 2019;10(2):931-944. [CrossRef]
- Zhang H, Cao Y, Ma L, Wei Y, Li H. Possible Mechanisms of Tau Spread and Toxicity in Alzheimer’s Disease. Front Cell Dev Biol. 2021;9:707268. https://doi.org/10.3389/fcell.2021.707268. [CrossRef]
- Front Cell Neurosci, Fleeman RM, Proctor EA. Astrocytic Propagation of Tau in the Context of Alzheimer’s Disease. Front Cell Neurosci. 2021;15:645233. [CrossRef]
- Silva MVF, Loures C de MG, Alves LCV, de Souza LC, Borges KBG, Carvalho M das G. Alzheimer’s disease: risk factors and potentially protective measures. J Biomed Sci. 2019;26(1):33. [CrossRef]
- Rawat P, Sehar U, Bisht J, Selman A, Culberson J, Reddy PH. Phosphorylated Tau in Alzheimer’s Disease and Other Tauopathies. Int J Mol Sci. 2022;23(21):12841. [CrossRef]
- DeVos SL, Corjuc BT, Oakley DH, et al. Synaptic Tau Seeding Precedes Tau Pathology in Human Alzheimer’s Disease Brain. Front Neurosci. 2018;12:267. [CrossRef]
- Riscado M, Baptista B, Sousa F. New RNA-Based Breakthroughs in Alzheimer’s Disease Diagnosis and Therapeutics. Published online 2021:27.
- Plascencia-Villa G, Perry G. Neuropathologic Changes Provide Insights into Key Mechanisms of Alzheimer Disease and Related Dementia. Am J Pathol. 2022;192(10):1340-1346. https://doi.org/10.1016/j.ajpath.2022.07.002. [CrossRef]
- Marucci G, Buccioni M, Ben DD, Lambertucci C, Volpini R, Amenta F. Efficacy of acetylcholinesterase inhibitors in Alzheimer’s disease. Neuropharmacology. 2021;190:108352. https://doi.org/10.1016/j.neuropharm.2020.108352. [CrossRef]
- Vecchio I, Sorrentino L, Paoletti A, Marra R, Arbitrio M. The State of The Art on Acetylcholinesterase Inhibitors in the Treatment of Alzheimer’s Disease. J Cent Nerv Syst Dis. 2021;13:117957352110291. https://doi.org/10.1177/11795735211029113. [CrossRef]
- Bennett CF, Kordasiewicz HB, Cleveland DW. Antisense Drugs Make Sense for Neurological Diseases. Annu Rev Pharmacol Toxicol. 2021;61(1):831-852. [CrossRef]
- Angelucci F, Cechova K, Valis M, Kuca K, Zhang B, Hort J. MicroRNAs in Alzheimer’s Disease: Diagnostic Markers or Therapeutic Agents? Front Pharmacol. 2019;10:665. https://doi.org/10.3389/fphar.2019.00665. [CrossRef]
- Noori T, Dehpour AR, Sureda A, Sobarzo-Sanchez E, Shirooie S. Role of natural products for the treatment of Alzheimer’s disease. Eur J Pharmacol. 2021;898:173974. [CrossRef]
- Lee CY, Ryu IS, Ryu JH, Cho HJ. miRNAs as Therapeutic Tools in Alzheimer’s Disease. Int J Mol Sci. 2021;22(23):13012. [CrossRef]
- Ramalho MJ, Andrade S, Loureiro JA, do Carmo Pereira M. Nanotechnology to improve the Alzheimer’s disease therapy with natural compounds. Drug Deliv Transl Res. 2020;10(2):380-402. [CrossRef]
- Alhazmi HA, Albratty M. An update on the novel and approved drugs for Alzheimer disease. Saudi Pharm J. 2022;30(12):1755-1764. [CrossRef]
- Chen X, Drew J, Berney W, Lei W. Neuroprotective Natural Products for Alzheimer’s Disease. Cells. 2021;10(6):1309. [CrossRef]
- Said MM, Rabo MMA. Neuroprotective effects of eugenol against aluminiuminduced toxicity in the rat brain. Arch Ind Hyg Toxicol. 2017;68(1):27-37. [CrossRef]
- Casares N, Alfaro M, Cuadrado-Tejedor M, et al. Improvement of cognitive function in wild-type and Alzheimer´s disease mouse models by the immunomodulatory properties of menthol inhalation or by depletion of T regulatory cells. Front Immunol. 2023;14:1130044. [CrossRef]
- Campos HM, da Costa M, da Silva Moreira LK, et al. Protective effects of chrysin against the neurotoxicity induced by aluminium: In vitro and in vivo studies. Toxicology. 2022;465:153033. [CrossRef]
- Hase T, Shishido S, Yamamoto S, et al. Rosmarinic acid suppresses Alzheimer’s disease development by reducing amyloid β aggregation by increasing monoamine secretion. Sci Rep. 2019;9(1):8711. [CrossRef]
- Guan X, Xu J, Liu J, Wu J, Chen L. Ginkgo biloba preparation prevents and treats senile dementia by inhibiting neuro-inflammatory responses. Trop J Pharm Res. 2019;17(10):1961. [CrossRef]
- Islam F, Nafady MH, Islam MdR, et al. Resveratrol and neuroprotection: an insight into prospective therapeutic approaches against Alzheimer’s disease from bench to bedside. Mol Neurobiol. 2022;59(7):4384-4404. [CrossRef]
- 57. Villegas C, Perez R, Petiz LL, Glaser T, Ulrich H, Paz C. Ginkgolides and Huperzine A for complementary treatment of Alzheimer’s disease. IUBMB Life. 2022;74(8):763-779. [CrossRef]
- Dubey T, Chinnathambi S. Brahmi (Bacopa monnieri): An ayurvedic herb against the Alzheimer’s disease. Arch Biochem Biophys. 2019;676:108153. [CrossRef]
- Snow AD, Castillo GM, Nguyen BP, et al. The Amazon rain forest plant Uncaria tomentosa (cat’s claw) and its specific proanthocyanidin constituents are potent inhibitors and reducers of both brain plaques and tangles. Sci Rep. 2019;9(1):561. [CrossRef]
- Huang M, Jiang X, Liang Y, Liu Q, Chen S, Guo Y. Berberine improves cognitive impairment by promoting autophagic clearance and inhibiting production of β-amyloid in APP/tau/PS1 mouse model of Alzheimer’s disease. Exp Gerontol. 2017;91:25-33. [CrossRef]
- Mani RJ, Mittal K, Katare DP. Protective Effects of Quercetin in Zebrafish Model of Alzheimer’s Disease.
- Leiteritz A, Dilberger B, Wenzel U, Fitzenberger E. Betaine reduces β-amyloid-induced paralysis through activation of cystathionine-β-synthase in an Alzheimer model of Caenorhabditis elegans. Genes Nutr. 2018;13(1):21. [CrossRef]
- Finley JW, Gao S. A Perspective on Crocus sativus L. (Saffron) Constituent Crocin: A Potent Water-Soluble Antioxidant and Potential Therapy for Alzheimer’s Disease. J Agric Food Chem. 2017;65(5):1005-1020. [CrossRef]
- Singh M, Ramassamy C. In vitro screening of neuroprotective activity of Indian medicinal plant Withania somnifera. J Nutr Sci. 2017;6:e54. [CrossRef]
- Kim JK, Bae H, Kim MJ, et al. Inhibitory Effect of Poncirus trifoliate on Acetylcholinesterase and Attenuating Activity against Trimethyltin-Induced Learning and Memory Impairment. Biosci Biotechnol Biochem. 2009;73(5):1105-1112. [CrossRef]
- Bihaqi SW, Sharma M, Singh AP, Tiwari M. Neuroprotective role of Convolvulus pluricaulis on aluminium induced neurotoxicity in rat brain. J Ethnopharmacol. 2009;124(3):409-415. [CrossRef]
- Azimi A, Ghaffari SM, Riazi GH, Arab SS, Tavakol MM, Pooyan S. α-Cyperone of Cyperus rotundus is an effective candidate for reduction of inflammation by destabilization of microtubule fibers in brain. J Ethnopharmacol. 2016;194:219-227. [CrossRef]
- Rivera DS, Lindsay C, Codocedo JF, et al. Andrographolide recovers cognitive impairment in a natural model of Alzheimer’s disease (Octodon degus). Neurobiol Aging. 2016;46:204-220. [CrossRef]
- Balez R, Steiner N, Engel M, et al. Neuroprotective effects of apigenin against inflammation, neuronal excitability and apoptosis in an induced pluripotent stem cell model of Alzheimer’s disease. Sci Rep. 2016;6(1):31450. [CrossRef]
- Shi J, Li Y, Zhang Y, et al. Baicalein Ameliorates Aβ-Induced Memory Deficits and Neuronal Atrophy via Inhibition of PDE2 and PDE4. Front Pharmacol. 2021;12:794458. [CrossRef]
- Celik Topkara K, Kilinc E, Cetinkaya A, Saylan A, Demir S. Therapeutic effects of carvacrol on beta-amyloid-induced impairments in in vitro and in vivo models of Alzheimer’s disease. Eur J Neurosci. 2022;56(9):5714-5726. [CrossRef]
- Li L, Li W, Jung SW, Lee YW, Kim YH. Protective Effects of Decursin and Decursinol Angelate against Amyloid β-Protein-Induced Oxidative Stress in the PC12 Cell Line: The Role of Nrf2 and Antioxidant Enzymes. Biosci Biotechnol Biochem. 2011;75(3):434-442. [CrossRef]
- Duan X, Li Y, Xu F, Ding H. Study on the neuroprotective effects of Genistein on Alzheimer’s disease. Brain Behav. 2021;11(5). https://doi.org/10.1002/brb3.2100. [CrossRef]
- Huang DS, Yu YC, Wu CH, Lin JY. Protective Effects of Wogonin against Alzheimer’s Disease by Inhibition of Amyloidogenic Pathway. Evid Based Complement Alternat Med. 2017;2017:1-13. [CrossRef]
- Xu P xin, Wang S wei, Yu X lin, et al. Rutin improves spatial memory in Alzheimer’s disease transgenic mice by reducing Aβ oligomer level and attenuating oxidative stress and neuroinflammation. Behav Brain Res. 2014;264:173-180. [CrossRef]
- Daily JW, Kang S, Park S. Protection against Alzheimer’s disease by luteolin: Role of brain glucose regulation, anti-inflammatory activity, and the gut microbiota-liver-brain axis. BioFactors. 2021;47(2):218-231. https://doi.org/10.1002/biof.1703. [CrossRef]
- Sabogal-Guáqueta AM, Osorio E, Cardona-Gómez GP. Linalool reverses neuropathological and behavioral impairments in old triple transgenic Alzheimer’s mice. Neuropharmacology. 2016;102:111-120. [CrossRef]
- Asiatic acid nullified aluminium toxicity in in vitro model of Alzheimer’s disease. Accessed May 30, 2023. https://www.imrpress.com/journal/FBE/10/2/10.2741/E823.
- Li Z, Zhao T, Li J, et al. Nanomedicine Based on Natural Products: Improving Clinical Application Potential. Wang R, ed. J Nanomater. 2022;2022:1-11. [CrossRef]
- Woon CK, Hui WK, Abas R, Haron MH, Das S, Lin TS. Natural Product-based Nanomedicine: Recent Advances and Issues for theTreatment of Alzheimer’s Disease. Curr Neuropharmacol. 2022;20(8):1498-1518. [CrossRef]
- Yu AM, Jian C, Yu AH, Tu MJ. RNA therapy: Are we using the right molecules? Pharmacol Ther. 2019;196:91-104. [CrossRef]
- Shin H, Park SJ, Yim Y, et al. Recent Advances in RNA Therapeutics and RNA Delivery Systems Based on Nanoparticles. Adv Ther. 2018;1(7):1800065. [CrossRef]
- Zogg H, Singh R, Ro S. Current Advances in RNA Therapeutics for Human Diseases. Int J Mol Sci. 2022;23(5):2736. [CrossRef]
- Mollocana-Lara EC, Ni M, Agathos SN, Gonzales-Zubiate FA. The infinite possibilities of RNA therapeutics. J Ind Microbiol Biotechnol. 2021;48(9-10):kuab063. [CrossRef]
- DeLong R. Ushering in a new era of RNA-based therapies. Commun Biol. 2021;4(1):577, s42003-021-02150-w. [CrossRef]
- Kim YK. RNA therapy: rich history, various applications and unlimited future prospects. Exp Mol Med. Published online April 19, 2022. [CrossRef]
- Kim YK. RNA Therapy: Current Status and Future Potential. Chonnam Med J. 2020;56(2):87. [CrossRef]
- Lee MJ, Lee I, Wang K. Recent Advances in RNA Therapy and Its Carriers to Treat the Single-Gene Neurological Disorders. Biomedicines. 2022;10(1):158. [CrossRef]
- Anthony K. RNA-based therapeutics for neurological diseases. RNA Biol. 2022;19(1):176-190. [CrossRef]
- Jurcău MC, Andronie-Cioara FL, Jurcău A, et al. The Link between Oxidative Stress, Mitochondrial Dysfunction and Neuroinflammation in the Pathophysiology of Alzheimer’s Disease: Therapeutic Implications and Future Perspectives. Antioxidants. 2022;11(11):2167. [CrossRef]
- Walgrave H, Zhou L, De Strooper B, Salta E. The promise of microRNA-based therapies in Alzheimer’s disease: challenges and perspectives. Mol Neurodegener. 2021;16(1):76. [CrossRef]
- Kreth S, Hübner M, Hinske LC. MicroRNAs as Clinical Biomarkers and Therapeutic Tools in Perioperative Medicine: Anesth Analg. 2018;126(2):670-681. h. [CrossRef]
- Martier R, Konstantinova P. Gene Therapy for Neurodegenerative Diseases: Slowing Down the Ticking Clock. Front Neurosci. 2020;14:580179. [CrossRef]
- Zhou Y, Zhu F, Liu Y, et al. Blood-brain barrier–penetrating siRNA nanomedicine for Alzheimer’s disease therapy. Sci Adv. 2020;6(41):eabc7031. [CrossRef]
- Long JM, Lahiri DK. MicroRNA-101 downregulates Alzheimer’s amyloid-β precursor protein levels in human cell cultures and is differentially expressed. Biochem Biophys Res Commun. 2011;404(4):889-895. [CrossRef]
- Liu W, Zhao J, Lu G. miR-106b inhibits tau phosphorylation at Tyr18 by targeting Fyn in a model of Alzheimer’s disease. Biochem Biophys Res Commun. 2016;478(2):852-857. [CrossRef]
- Jiang Y, Xu B, Chen J, et al. Micro-RNA-137 Inhibits Tau Hyperphosphorylation in Alzheimer’s Disease and Targets the CACNA1C Gene in Transgenic Mice and Human Neuroblastoma SH-SY5Y Cells. Med Sci Monit. 2018;24:5635-5644. [CrossRef]
- Santa-Maria I, Alaniz ME, Renwick N, et al. Dysregulation of microRNA-219 promotes neurodegeneration through post-transcriptional regulation of tau. J Clin Invest. 2015;125(2):681-686. [CrossRef]
- Estfanous S, Daily KP, Eltobgy M, et al. Elevated Expression of MiR-17 in Microglia of Alzheimer’s Disease Patients Abrogates Autophagy-Mediated Amyloid-β Degradation. Front Immunol. 2021;12:705581. [CrossRef]
- Wang R, Chopra N, Nho K, et al. Human microRNA (miR-20b-5p) modulates Alzheimer’s disease pathways and neuronal function, and a specific polymorphism close to the MIR20B gene influences Alzheimer’s biomarkers. Mol Psychiatry. 2022;27(2):1256-1273. [CrossRef]
- Zong Y, Wang H, Dong W, et al. miR-29c regulates BACE1 protein expression. Brain Res. 2011;1395:108-115. [CrossRef]
- Chopra N, Wang R, Maloney B, et al. MicroRNA-298 reduces levels of human amyloid-β precursor protein (APP), β-site APP-converting enzyme 1 (BACE1) and specific tau protein moieties. Mol Psychiatry. 2021;26(10):5636-5657. [CrossRef]
- He C, Su C, Zhang W, Wan Q. miR-485-5p alleviates Alzheimer’s disease progression by targeting PACS1. Transl Neurosci. 2021;12(1):335-345. [CrossRef]
- Liu J, Zuo X, Han J, et al. MiR-9-5p inhibits mitochondrial damage and oxidative stress in AD cell models by targeting GSK-3β. Biosci Biotechnol Biochem. 2020;84(11):2273-2280. [CrossRef]
- Deng Y, Zhang J, Sun X, et al. miR-132 improves the cognitive function of rats with Alzheimer’s disease by inhibiting the MAPK1 signal pathway. Exp Ther Med. 2020;20(6):159. [CrossRef]
- 106. Liang C, Zhu H, Xu Y, et al. MicroRNA-153 negatively regulates the expression of amyloid precursor protein and amyloid precursor-like protein 2. Brain Res. 2012;1455:103-113. [CrossRef]
- Xu H, Rosler T, Carlsson T, et al. Tau Silencing by siRNA in the P301S Mouse Model of Tauopathy. Curr Gene Ther. 2014;14(5):343-351. [CrossRef]
- Kandimalla RJ, Wani WY, Bk B, Gill KD. siRNA against presenilin 1 (PS1) down regulates amyloid b42 production in IMR-32 cells. Published online 2012.
- Senechal Y, Kelly PH, Cryan JF, Natt F, Dev KK. Amyloid precursor protein knockdown by siRNA impairs spontaneous alternation in adult mice: In vivo knockdown of APP by RNAi. J Neurochem. 2007;102(6):1928-1940. https://doi.org/10.1111/j.1471-4159.2007.04672.x. [CrossRef]
- Lin CY, Perche F, Ikegami M, Uchida S, Kataoka K, Itaka K. Messenger RNA-based therapeutics for brain diseases: An animal study for augmenting clearance of beta-amyloid by intracerebral administration of neprilysin mRNA loaded in polyplex nanomicelles. J Controlled Release. 2016;235:268-275. [CrossRef]
- 111. Lim SA, Cox A, Tung M, Chung EJ. Clinical progress of nanomedicine-based RNA therapies. Bioact Mater. 2022;12:203-213. [CrossRef]
- Gorshkov A, Purvinsh L, Brodskaia A, Vasin A. Exosomes as Natural Nanocarriers for RNA-Based Therapy and Prophylaxis. Nanomaterials. 2022;12(3):524. [CrossRef]
- Tsakiri M, Zivko C, Demetzos C, Mahairaki V. Lipid-based nanoparticles and RNA as innovative neuro-therapeutics. Front Pharmacol. 2022;13:900610. [CrossRef]
- Fernandes F, Dias-Teixeira M, Delerue-Matos C, Grosso C. Critical Review of Lipid-Based Nanoparticles as Carriers of Neuroprotective Drugs and Extracts. Nanomaterials. 2021;11(3):563. [CrossRef]
- Pardridge WM. Treatment of Alzheimer’s Disease and Blood–Brain Barrier Drug Delivery. Pharmaceuticals. 2020;13(11):394. [CrossRef]
- Wohlfart S, Gelperina S, Kreuter J. Transport of drugs across the blood–brain barrier by nanoparticles. J Controlled Release. 2012;161(2):264-273. [CrossRef]
- Zhou Y, Peng Z, Seven ES, Leblanc RM. Crossing the blood-brain barrier with nanoparticles. J Controlled Release. 2018;270:290-303. https://doi.org/10.1016/j.jconrel.2017.12.015. [CrossRef]
- Bors L, Erdő F. Overcoming the Blood–Brain Barrier. Challenges and Tricks for CNS Drug Delivery. Sci Pharm. 2019;87(1):6. [CrossRef]
- Wong K, Riaz M, Xie Y, et al. Review of Current Strategies for Delivering Alzheimer’s Disease Drugs across the Blood-Brain Barrier. Int J Mol Sci. 2019;20(2):381. [CrossRef]
- Zenaro E, Piacentino G, Constantin G. The blood-brain barrier in Alzheimer’s disease. Neurobiol Dis. 2017;107:41-56. [CrossRef]
- Sharma C, Woo H, Kim SR. Addressing Blood–Brain Barrier Impairment in Alzheimer’s Disease. Biomedicines. 2022;10(4):742. [CrossRef]
- Juhairiyah F, de Lange ECM. Understanding Drug Delivery to the Brain Using Liposome-Based Strategies: Studies that Provide Mechanistic Insights Are Essential. AAPS J. 2021;23(6):114. [CrossRef]
- Satapathy MK, Yen TL, Jan JS, et al. Solid Lipid Nanoparticles (SLNs): An Advanced Drug Delivery System Targeting Brain through BBB. Pharmaceutics. 2021;13(8):1183. [CrossRef]
- Musielak E, Feliczak-Guzik A, Nowak I. Synthesis and Potential Applications of Lipid Nanoparticles in Medicine. Materials. 2022;15(2):682. [CrossRef]
- Heidarzadeh M, Gürsoy-Özdemir Y, Kaya M, et al. Exosomal delivery of therapeutic modulators through the blood–brain barrier; promise and pitfalls. Cell Biosci. 2021;11(1):142. [CrossRef]
- Negahdaripour M, Vakili B, Nezafat N. Exosome-based vaccines and their position in next generation vaccines. Int Immunopharmacol. 2022;113:109265. [CrossRef]
- Jafari D, Malih S, Eini M, et al. Improvement, scaling-up, and downstream analysis of exosome production. Crit Rev Biotechnol. 2020;40(8):1098-1112. [CrossRef]
- Hu Q, Su H, Li J, et al. Clinical applications of exosome membrane proteins. Precis Clin Med. 2020;3(1):54-66. https://doi.org/10.1093/pcmedi/pbaa007. [CrossRef]
- Ludwig N, Whiteside TL, Reichert TE. Challenges in Exosome Isolation and Analysis in Health and Disease. Int J Mol Sci. 2019;20(19):4684. [CrossRef]
- H. Rashed M, Bayraktar E, K. Helal G, et al. Exosomes: From Garbage Bins to Promising Therapeutic Targets. Int J Mol Sci. 2017;18(3):538. [CrossRef]
- Zhang N, He F, Li T, et al. Role of Exosomes in Brain Diseases. Front Cell Neurosci. 2021;15:743353. [CrossRef]
- Xu L, Wang X, Liu Y, Yang G, Falconer RJ, Zhao CX. Lipid Nanoparticles for Drug Delivery. Adv NanoBiomed Res. 2022;2(2):2100109. [CrossRef]
- Feng R, Patil S, Zhao X, Miao Z, Qian A. RNA Therapeutics - Research and Clinical Advancements. Front Mol Biosci. 2021;8:710738. [CrossRef]
- Burdușel AC, Andronescu E. Lipid Nanoparticles and Liposomes for Bone Diseases Treatment. Biomedicines. 2022;10(12):3158. [CrossRef]
- Duan Y, Dhar A, Patel C, et al. A brief review on solid lipid nanoparticles: part and parcel of contemporary drug delivery systems. RSC Adv. 2020;10(45):26777-26791. [CrossRef]
- Tenchov R, Bird R, Curtze AE, Zhou Q. Lipid Nanoparticles─From Liposomes to mRNA Vaccine Delivery, a Landscape of Research Diversity and Advancement. ACS Nano. 2021;15(11):16982-17015. [CrossRef]
- Abdellatif AAH, Younis MA, Alsowinea AF, et al. Lipid nanoparticles technology in vaccines: Shaping the future of prophylactic medicine. Colloids Surf B Biointerfaces. 2023;222:113111. [CrossRef]
- Vieira D, Gamarra L. Getting into the brain: liposome-based strategies for effective drug delivery across the blood–brain barrier. Int J Nanomedicine. 2016;Volume 11:5381-5414. [CrossRef]
- Gauro R, Nandave M, Jain VK, Jain K. Advances in dendrimer-mediated targeted drug delivery to the brain. J Nanoparticle Res. 2021;23(3):76. [CrossRef]
- Teleanu D, Chircov C, Grumezescu A, Volceanov A, Teleanu R. Blood-Brain Delivery Methods Using Nanotechnology. Pharmaceutics. 2018;10(4):269. [CrossRef]
- Sela H, Cohen H, Elia P, Zach R, Karpas Z, Zeiri Y. Spontaneous penetration of gold nanoparticles through the blood brain barrier (BBB). J Nanobiotechnology. 2015;13(1):71. [CrossRef]
- Li X, Corbett AL, Taatizadeh E, et al. Challenges and opportunities in exosome research—Perspectives from biology, engineering, and cancer therapy. APL Bioeng. 2019;3(1):011503. [CrossRef]
- Schiffelers R, Kooijmans S, Vader, van Dommelen, Van Solinge. Exosome mimetics: a novel class of drug delivery systems. Int J Nanomedicine. Published online March 2012:1525. [CrossRef]
- Fernandes M, Lopes I, Magalhães L, et al. Novel concept of exosome-like liposomes for the treatment of Alzheimer’s disease. J Controlled Release. 2021;336:130-143. [CrossRef]
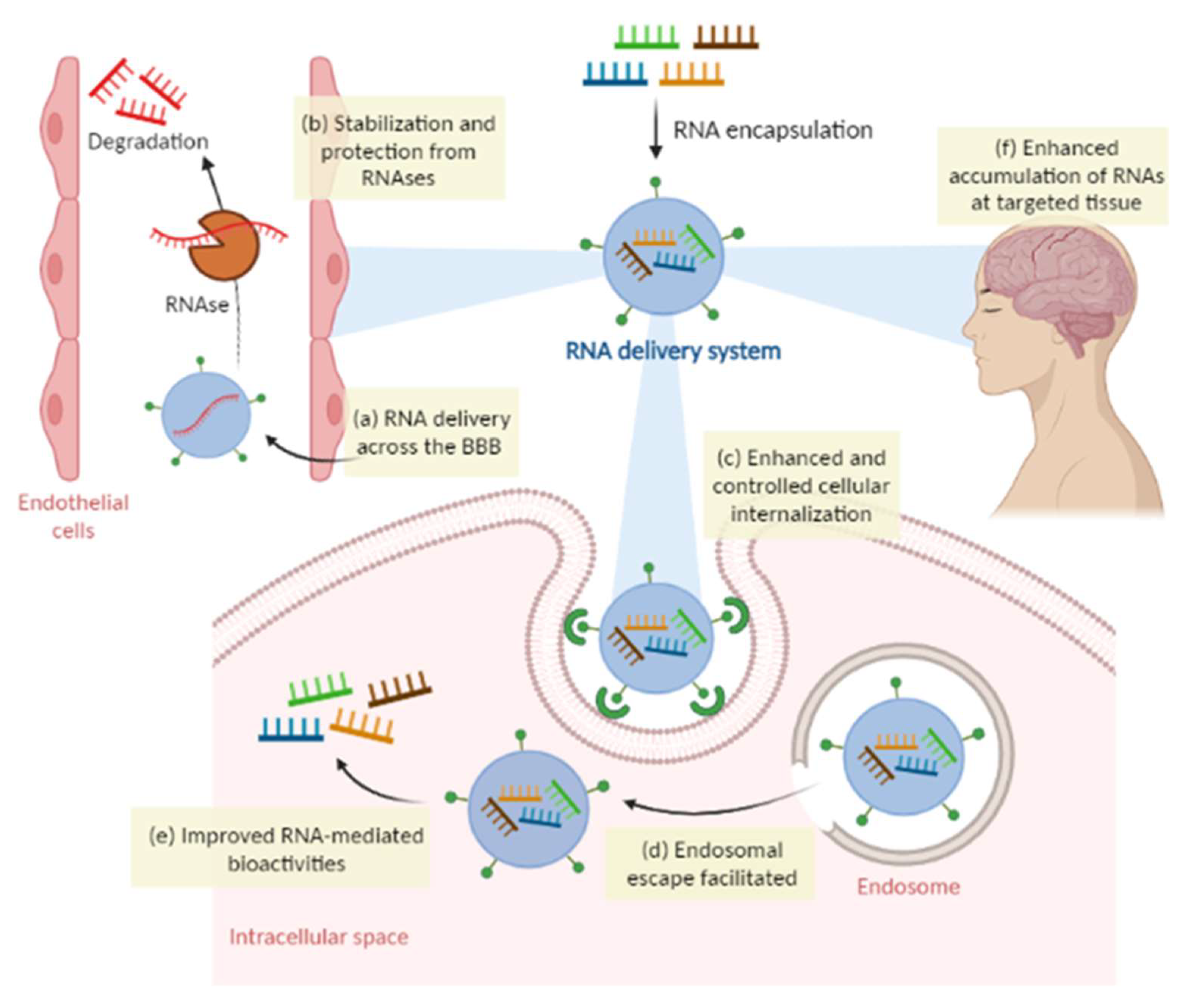
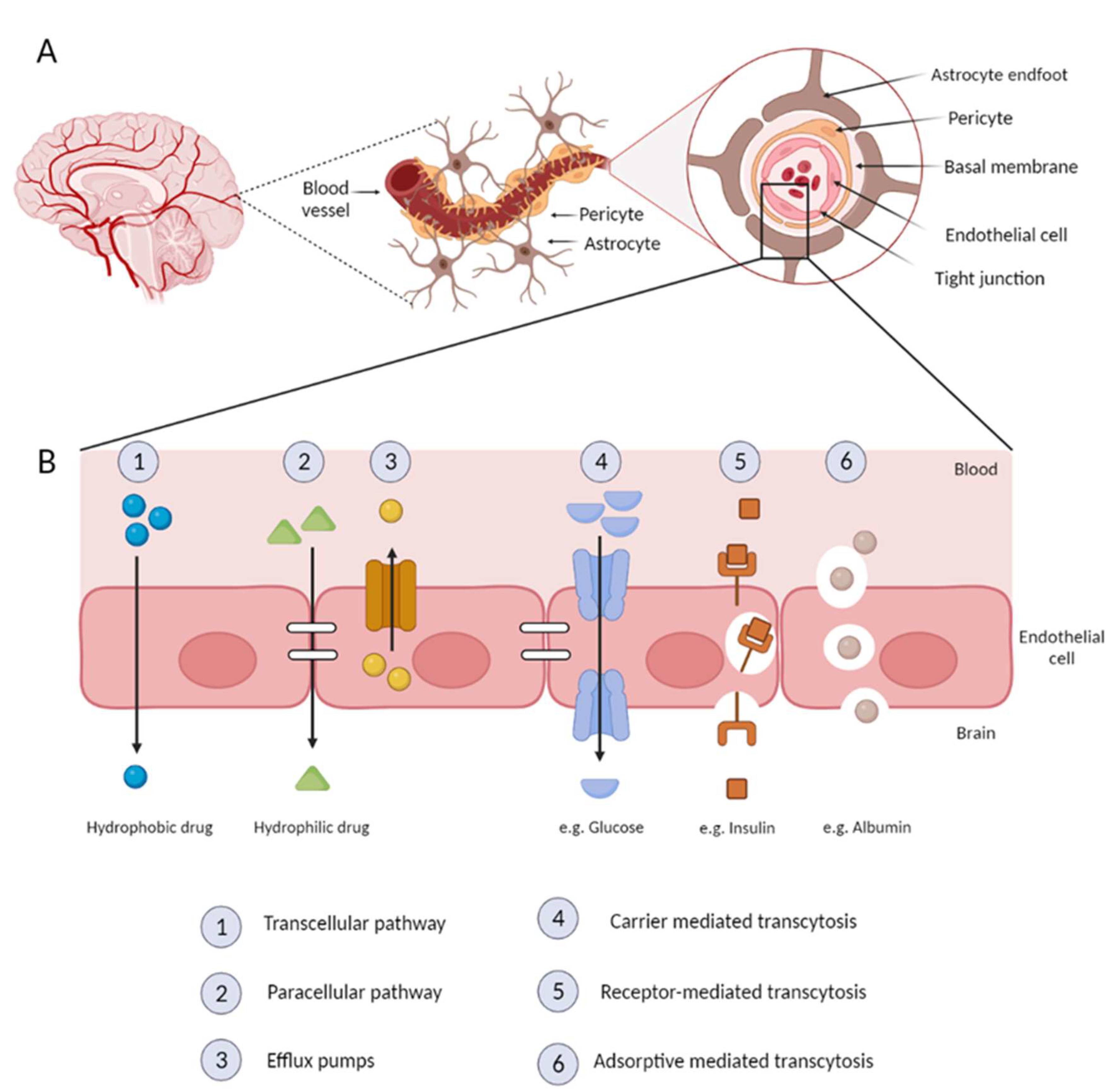
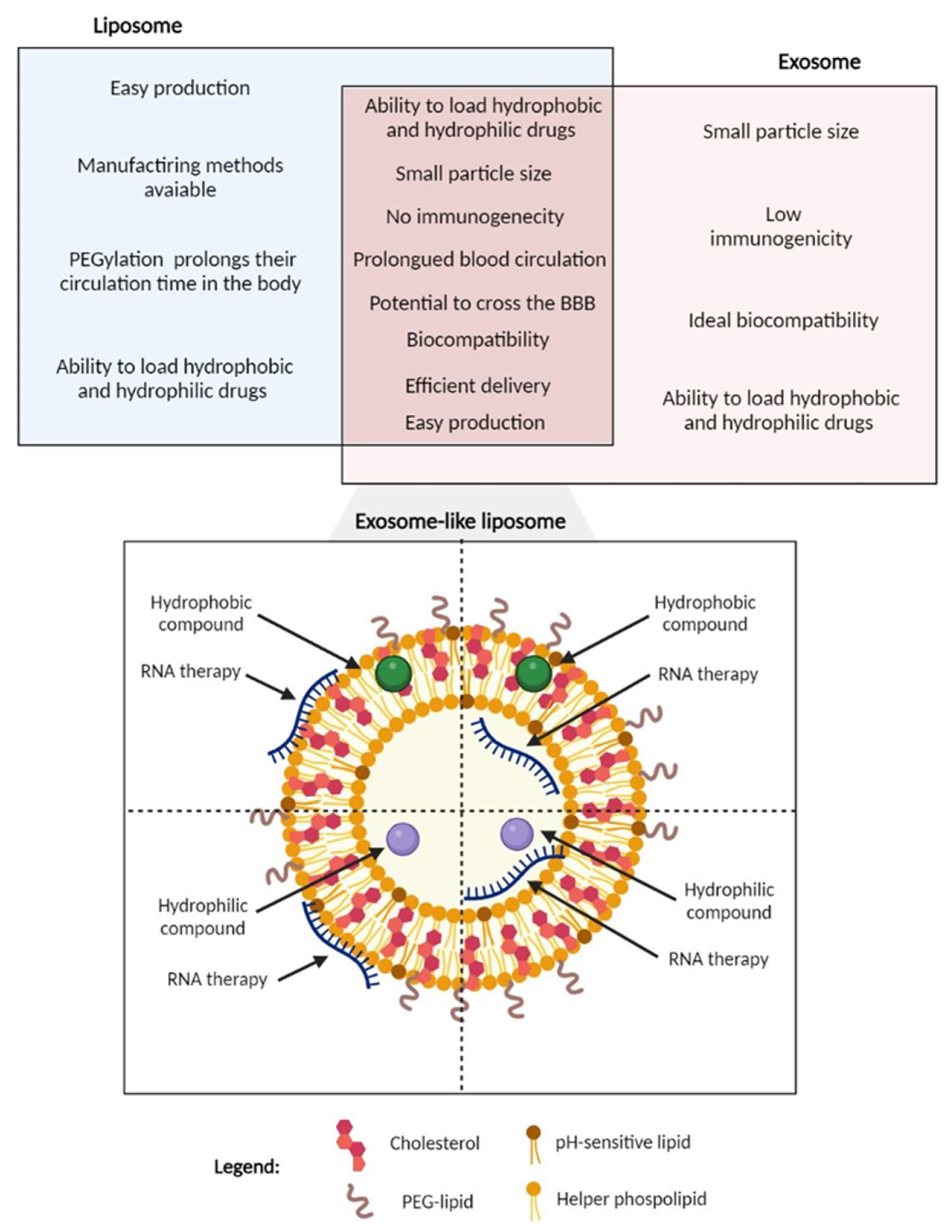
| Natural Compound | Role in AD | Reference |
|---|---|---|
| Eugenol | Rats were fed aluminium, a neurotoxic metal that leads to oxidative brain injury and enhanced lipid peroxidation, disruption of neurotrophic, cholinergic, and serotonergic functions, and induce apoptosis with ultimate neuronal and astrocyte damages. A neuroprotective role of eugenol against the aluminium effects, was verified through its antioxidant, antiapoptotic potential and its neurotrophic properties. | [51] |
| Menthol | Menthol inhalation by mice (1 week per month, for 6 months) prevented cognitive impairment in the APP/PS1 mouse model of Alzheimer’s. | [52] |
| Chrysin | Chrysin showed the ability to act as a membrane shield against early oxidative events mediated by O2˙- and other ROS that contribute to neuronal death triggered by AlCl3 exposure, showing chrysin neuroprotective action. | [53] |
| Rosmarinic acid | Suppresses amyloid β accumulation in mice. | [54] |
| Ginkgo biloba | Ginkgo biloba improves microcirculation, inhibits the expression of inflammatory factors, and reduces inflammatory damage to neurons, thereby improving the spatial exploration memory of dementia model rats. | [55] |
| Resveratrol | Multiple studies demonstrated that resveratrol has a neuroprotective, anti-inflammatory, and antioxidant characteristics and the ability to minimize Aβ peptide aggregation and toxicity in the hippocampus of Alzheimer’s patients, stimulating neurogenesis and inhibiting hippocampal degeneration. Furthermore, resveratrol’s antioxidant effect promotes neuronal development by activating the silent information regulator-1 (SIRT1), which can protect against the detrimental effects of oxidative stress. | [56] |
| Huperzine A | Huperzine A is natural, potent, highly specific reversible inhibitor of acetylcholinesterase, with the ability to cross the BBB. | [57] |
| Brahmi | The neuroprotective properties of Brahmi include the reduction of ROS and neuroinflammation, the inhibition of the aggregation of Aβ and the improvement of cognitive and learning behaviour. | [58] |
| Uncaria tomentosa | Inhibits plaques and tangles formation. | [59] |
| Berberine | Berberine has antioxidant activity and promotes AChE and monoamine oxidase inhibition. Berberine has been shown to improve memory, lower Aβ and APP concentration, and diminish Aβ plaque accumulation. | [60] |
| Quercetin | Behavioural and biochemical tests confirm that quercetin promotes the reduction in oxidative stress and increased cognition in zebrafish AD models induced with aluminium chloride. | [61] |
| Betaine | Betaine has shown to decrease homocysteine levels and Aβ toxicity in Caenorhabditis elegans AD model. | [62] |
| Curcumin | Curcumin is known to be a potent antioxidant, anti-inflammatory and anti-amyloidogenic compound, that plays a beneficial role in treating AD through several mechanisms. Curcumin can promote a significant reduction Aβ oligomers and fibril formation. | [46] |
| Crocin | Crocin, the main constituent of Crocus sativus L., has a multifunctional role in protecting brain cells, modulating aggregation of Aβ and tau proteins, attenuating cognitive and memory impairments, and improving oxidative stress. | [63] |
| Withania somnifera | Withania somnifera (WS) extract can protect against Aβ peptide- and acrolein-induced toxicity. Treatment with WS extract significantly protected against Aβ peptide and acrolein, in various cell survival assays with the human neuroblastoma cell line SK-N-SH , significantly reduced the generation of reactive oxygen species and demonstrated to be a potent inhibitor of acetylcholinesterase activity. | [64] |
| Poncirus trifoliate | The extract of Poncirus trifoliate is a naturally occurring AChE inhibitor. It showed a 47.31% inhibitory effect on the activity of acetylcholine. | [65] |
| Convolvulus pluricaulis | Convolvulus pluricaulis prevented aluminium induced neurotoxicity in rat cerebral cortex. | [66] |
| α-Cyperone | α-Cyperone binds and interacts with tubulin, being capable of destabilizing microtubule polymerization. The effect of this interaction could result in reduction of inflammation. | [67] |
| Andrographolide | Andrographolide has beneficial effects in the recovery of spatial memory and learning performance, recovery of synaptic basal transmission, partial or complete protection of certain synaptic proteins and shows a specific neuroprotective effect, that includes the reduction of phosphorylated tau and amyloid beta aggregate maturation, in aged degus. | [68] |
| Apigenin | Apigenin has been shown to have anti-inflammatory and neuroprotective properties in a number of cell and animal models. This compound is also able to protect human induced pluripotent stem cell-derived AD neurons via multiple pathways, by reducing the frequency of spontaneous Ca2+ signals and significantly reducing caspase-3/7 mediated apoptosis. | [69] |
| Baicalein | Baicalein has antioxidant and anti-inflammatory effects. | [70] |
| Carvacrol | Carvacrol possesses anti-acetylcholinesterase, antioxidant, and neuroprotective properties. This compound alleviated Aβ-induced deficits by reducing cellular neurotoxicity and oxidative stress in the SH-SY5Y cell line, and by reducing oxidative stress and memory impairment in a rat model of AD. | [71] |
| Decursin/ Decursinol angelate | Decursin and decursinol angelate increase cellular resistance to Aβ-induced oxidative injury in PC12 cells. | [72] |
| Genistein | In vivo studies have shown that genistein improves brain function, antagonizes the toxicity of Aβ and has neuroprotective effects. | [73] |
| Wogonin | Wogonin has various neuroprotective and neurotrophic activities, such as inducing neurite outgrowth. | [74] |
| Rutin | Rutin is an antioxidant, anti-inflammatory, and has the capacity of reducing Aβ oligomer activities. | [75] |
| Luteolin | Luteolin has the capacity to cross the BBB and can inhibit β- and γ-secretase to decrease Aβ. It can also reduce neuroinflammation, and attenuate the phosphorylation of tau and the formation of tangles. | [76] |
| Linalool | A linalool-treated mice model of AD showed improved learning and spatial memory. This compound reverses the histopathological hallmarks of AD and restores cognitive and emotional functions via an anti-inflammatory effect. | [77] |
| Asiatic acid | Pre-treatment with Asiatic Acid enhanced cell viability, attenuated rotenone-induced ROS, mitochondrial membrane dysfunction and apoptosis regulating AKT/GSK-3β signalling pathway, after aluminium maltolate neurotoxicity induction in SH-SY5Y neuroblastoma cells. | [78] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





