Introduction
Cardiovascular diseases are the main cause of death worldwide, resulting in around 18 million deaths per year (WHO, 2020) (1), and hypertension is considered as the main contributor to such a global burden (2). Effective blood pressure (BP) control is a mandatory factor in order to improve hypertensive target organ damage and subsequent cardiovascular morbidity and mortality (2).
Functional, mechanical and structural alterations of the microvasculature may be observed in patients with essential hypertension even at very early stages (3-5) and contribute to the development of hypertension complications and cardiovascular prognosis (3,5). In particular, in this setting, microvascular remodeling is a key event in triggering cardiovascular diseases (6,7).
The molecular mechanisms underlying the development of vascular remodeling are only partly understood. However, among the factors that may contribute to microvascular changes, an important role may be played by vascular inflammation with infiltration of inflammatory circulating cells and release of inflammatory cytokines and chemokines, proliferative growth factors, as well as oxidative stress related to both a reduction in nitric oxide bioavailability and increased reaction oxygen species production (5,8,9). These changes are promoted by mechanical, hemodynamic or metabolic vascular insult and result in altered vascular smooth muscle cell phenotype and accumulation of extracellular matrix (9). The present review briefly summarizes current knowledge about microvascular remodeling and its role in hypertension-mediated organ damage and consequent cardiovascular events.
Microvascular remodeling in hypertension
Cardiovascular and metabolic diseases (in particular arterial hypertension), are very commonly associated with alterations in microcirculation (4,6,7); morphological changes may involve small resistance arteries, arterioles, capillaries and post-capillary venules (3,6,10). BP is mainly influenced by vessels resistance and microcirculation is the key element of peripheral resistance regulation. As mentioned, the microcirculation may be subdivided in small arteries, arterioles and capillaries. Small resistance arteries are defined as arteries with a lumen diameter roughly between 350 and 100 µm. Their structure consists in an outer connective tissue adventitia, a smooth muscle cells tunica media and the endothelial layer (5,6). Arterioles are vessels with internal diameter below 100 µm, characterized by a single layer of smooth muscle cells. Small arteries and arterioles account for 45- 50% of peripheral resistance and are defined as resistance arteries. These vessels have the capacity of contracting when transmural pressure increases, a feature called myogenic tone. A 23-30 % of peripheral resistance is to be ascribed to capillaries (internal diameter below 7 µm), whose wall is constituted only by a monolayer of endothelial cells (5,6).
It was proposed several years ago that increases systemic vascular resistance may be a consequence of vascular smooth muscle increase and concomitant narrowing of the arteriolar lumen (3,4,6,7,11). Microcirculation remodeling takes place in primary hypertension (6,10). Remodeling may be classified according to lumen and wall cross sectional area changes (12,13). Hence, remodeling might be inward when internal diameter is reduced and outward when lumen is unchanged (12,13). On the other hand, hypertrophic remodeling occurs when the vessel wall material, i.e. media/wall cross-sectional area, increases along with media/wall thickness whereas if media/wall cross-sectional area does not change or is reduced then remodeling is defined eutrophic or hypotrophic, respectively (12,13). Inward eutrophic remodeling is mainly observed in primary hypertension with media thickness increase and lumen diameter decrease resulting in unchanged media-cross sectional area (11-14). On the contrary, hypertrophic remodeling, which may be outward or inward, it has been shown in secondary form of hypertension (14), and in cardiovascular diseases as diabetes mellitus (15,16) and obesity (17,18) and metabolic syndrome (19), as well as in other endocrine diseases (20,21), independently on the presence of an increase in BP increase. As result of a remodeling process the wall to lumen ratio (WLR) and media to lumen ratio (MLR), defined as the ratio between wall and media thickness respectively and lumen diameter, increase.
Hypertensive patients (4,11) (but also obese (22) or diabetic patients (4) present reduced basal and total capillary density compared to normotensive controls showing a structural anatomical rarefaction of capillaries rather that the presence of non-perfused vessels (4). This reduction in total capillary density may consequently lead to an increase in peripheral resistances thus negatively affecting tissue perfusion and nutrient delivery (23).
The time-course of the development of hypertension in respect with the onset of microvascular alterations is not clear. An increase in the MLR of mesenteric small resistance arteries of spontaneously hypertensive rats may be present in a pre-hypertensive phase (4). Data in humans are obviously difficult to obtain, since very few longitudinal data are presently available (4).
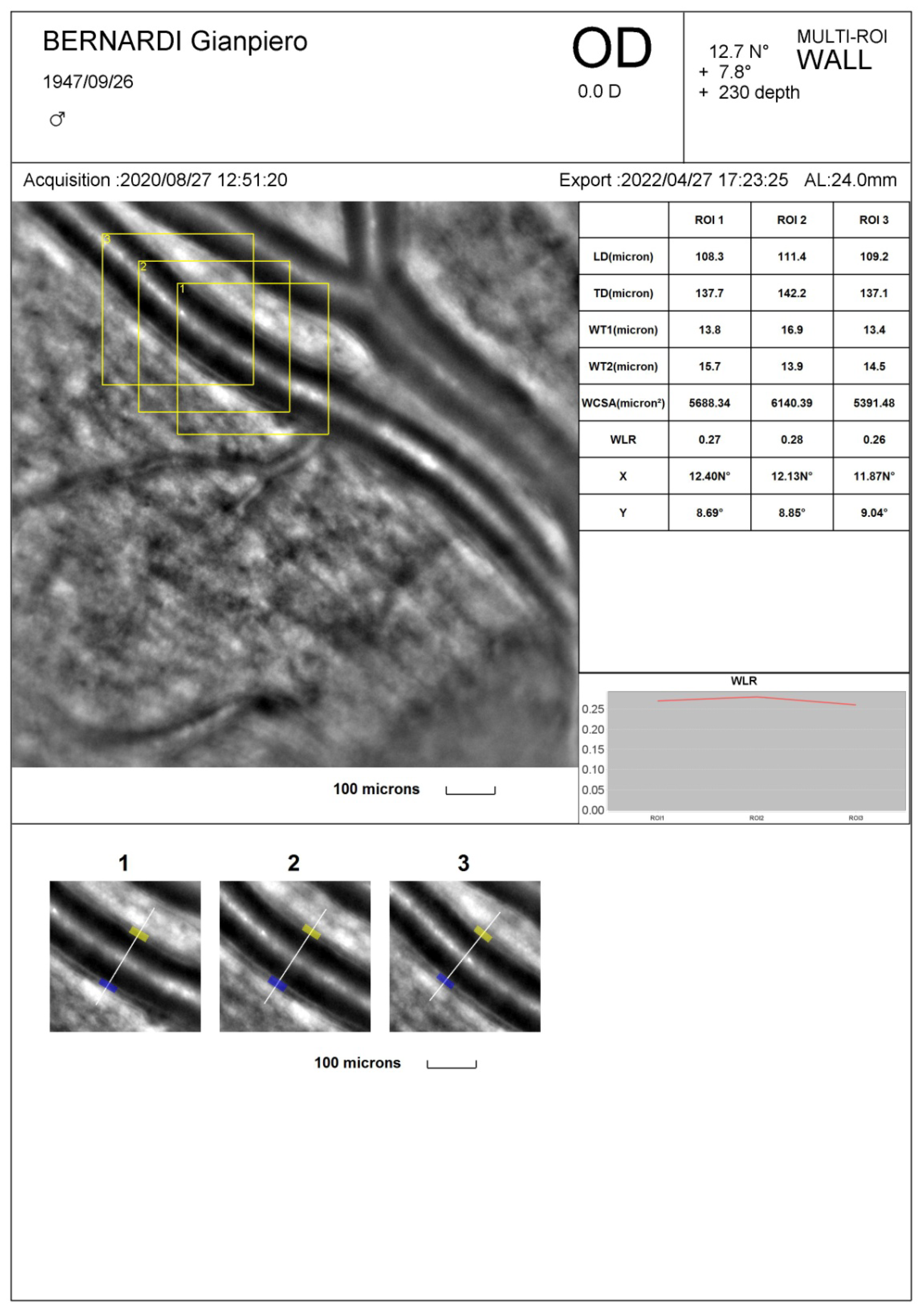
As previously mentioned, the hallmark of a microvascular remodeling process is an increased WLR and/or MLR. These parameters were initially measured in small resistance arteries by using wire or pressure micromyography, an in vitro ex vivo technique in which a small artery is isolated from subcutaneous fat tissue and mounted on wire or glass cannulas and studied in isometric or isobaric condition (24-26). Nowadays, non-invasive techniques have become available (27) such as Scanning Laser Doppler Flowmetry and Adaptive Optics (AO) which allow an accurate visualization and measurement of retinal vessel morphological parameters at high resolution providing similar information compared to the micromyographic system (28). In particular, Scanning Laser Doppler Flowmetry allows to measure the external diameter of retinal arterioles in reflection images and to evaluate the internal diameter in perfusion images according to a laser Doppler technique then providing an automatic estimation of WLR (29,30). With Adaptive Optics internal and external diameter of retinal arterioles are derived though an algorithm detecting the gradient of light’s reflection between the lumen of the vessel and the wall; these data are then used to calculate WLR and wall cross-sectional area (27,31) (
Figure 1). Compared to Scanning Laser Doppler Flowmeter, Adaptive Optics detects retinal arterioles remodeling with a lower intra-observer variability (28) and provides very high quality images (31) (
Figure 1).
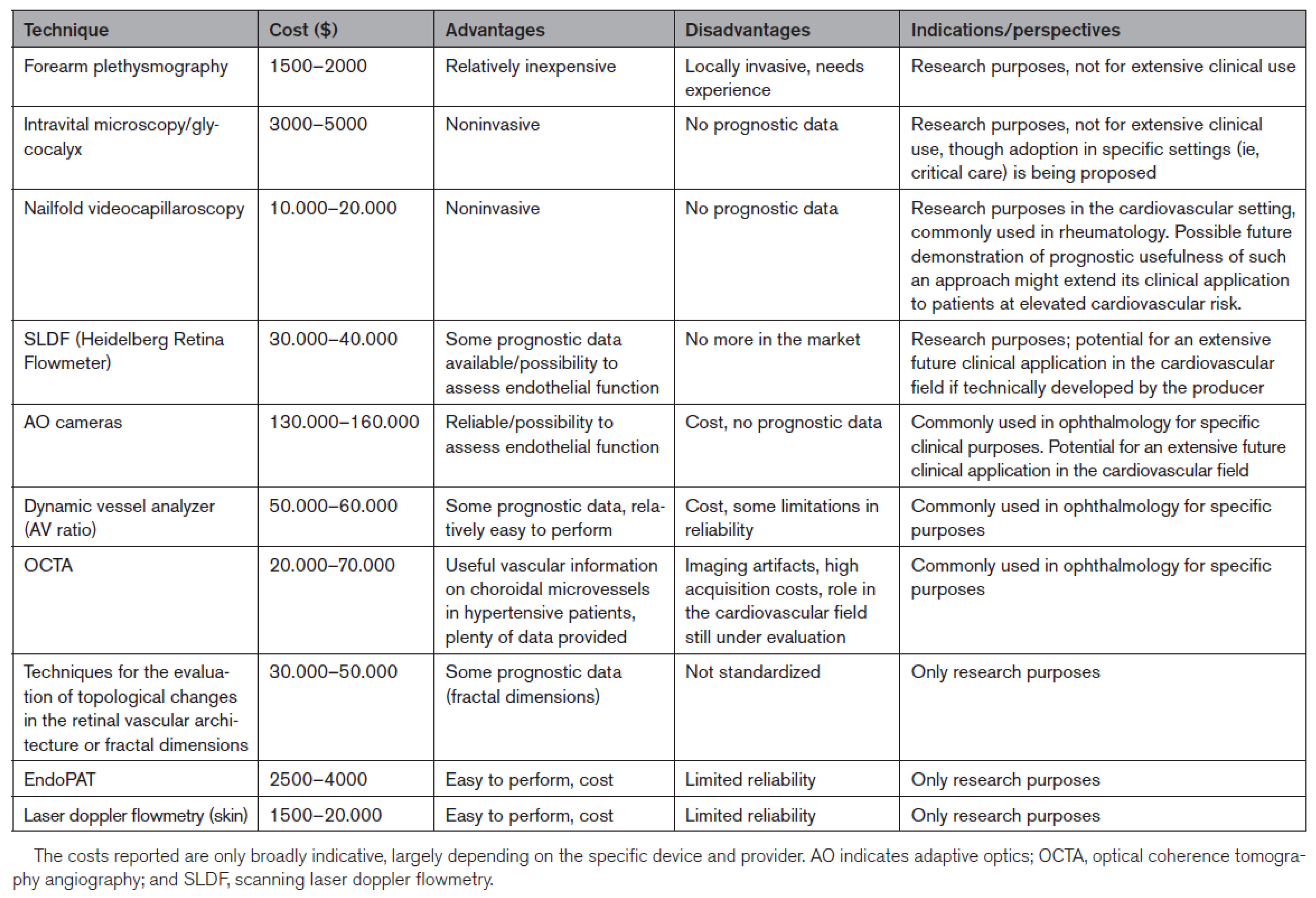
Cost, advantages, disadvantages, and indications/perspectives of the different available techniques for a non-invasive investigation of microvascular structure are summarized in
Figure 2 (27).
Prognostic role of microvascular structural alterations
MLR of subcutaneous small resistance arteries has been suggested to represent the most prevalent and earliest form of arterial damage in essential hypertension (32) and it may be present very early, even in prehypertensive phase at least in experimental model (4).
From a physiological point of view, the reduction of small artery and arteriole lumen is associated to an increase of flow resistance even in condition of maximal dilatation hence impairing the organ flow reserve (33-35). Indeed, a relationship between vasodilating capacity of coronary microcirculation and media to lumen ratio of subcutaneous small arteries was previously demonstrated (36) in patients with mild to moderate hypertension thus suggesting that structural alterations in the subcutaneous vascular district (evaluated by wire micromyography in isolated vessels from fat biopsies taken in the gluteal region) may be representative of similar alterations in coronary microcirculation, leading to a reduced coronary flow reserve (36,37).
Increase MLR of subcutaneous small resistance arteries has been positively related to hypertensive target organ damage such as left ventricular hypertrophy (38,39) or carotid artery structure (39)
Importantly, the changes in small artery structure have a prognostic significance in both primary and secondary hypertension and in type 2 diabetes; indeed, an increased MLR is associated with a reduced event-free survival for cardiovascular events in high risk patients (3,40) as well as in medium risk ones (3,41,42). Moreover, the presence of hypertrophic remodeling seems to be associated to an even worse prognosis compared to eutrophic remodeling (43,44). More details about the prognostic role of structural alterations in subcutaneous small resistance arteries evaluated by micromyography are reported in Reference 3.
On the other hand, WLR evaluated by Scanning Laser Doppler Flowmetry was proved to increase in patients with hypertension and cerebrovascular disease (29) and to directly be associated with urinary albumin excretion (45). Likewise, an increased WLR evaluated with Adaptive Optics correlated with age (46,47), BP (31) and may be improved by reduction of BP values by hypertensive treatment (46). Most recently, a prognostic role of WLR evaluated with AO was demonstrated (48). The event-free survival was significantly worse in 230 normotensive subjects and hypertensive patients with a baseline WLR above the median value of the population (0.28) according to Kaplan-Mayer survival curves and multivariate analysis (Cox’s proportional hazard model) (48). The evidence was confirmed after restricting the analysis to cardiovascular events, excluding deaths and neoplastic diseases (48). Therefore, structural alterations of retinal arterioles evaluated by adaptive optics may predict total and cardiovascular events (48).
Most recently, it was also demonstrated that patients with coronary microvascular dysfunction, defined as the presence of a reduced myocardial flow reserve (≤2), evaluated by Dynamic Single-Photon Emission Computed Tomography (SPECT), had higher rates of adverse outcomes that those without it (49).
However, it is not presently known whether capillary rarefaction may be related to cardiovascular events (3,4).
Possible prevention/regression of microvascular remodeling
The next question to be answered is whether we should aim at correcting the structure of resistance vessels in the treatment of hypertension and whether this could affect the prognosis. Indeed, several drugs have been demonstrated to improve microvascular structure and therefore reduce MLR such as drugs inhibiting the renin-angiotensin-aldosterone system and dihydropyridinic calcium channel blockers whereas diuretic and beta-blocked do not seem to have any relevant effect (5,7,50).
There is clear evidence that angiotensin-converting enzyme (ACE)-inhibitors and angiotensin-receptor blockers (ARB) appear to be more effective than atenolol in terms of microvascular protection also in patients with diabetes mellitus (51-54). Along this line, the direct renin inhibitor aliskiren improved microvascular structural alterations to a similar extent, compared with ramipril, in mild hypertensive patients with type 2 diabetes mellitus (54).
An improvement of microvascular remodeling was also demonstrated in the retinal vasculature. In patients with hypertension aliskiren plus valsartan ameliorated ameliorate the WLR of retinal arterioles measured non-invasively by Scanning Laser Doppler (55). The combination of lercanidipine and enalapril was more effective in reducing the retinal arteriole WLR compared to the combination of lercanidipine and hydrochlorothiazide (56). Using Adaptive Optics a normalization of retinal arteriole structure, was observed after chronic and effective antihypertensive treatment (46). In the contrary, a short-term reduction of blood pressure led only to an increase in the internal diameter of retina arterioles with no change in wall thickness or wall cross-sectional area (46).
A complete regression of the remodeling process is difficult to be obtained in patients with hypertension-mediated organ damage (i.e. left ventricular hypertrophy) or when comorbidities such as diabetes mellitus are present (3,4,7,50). Usually, in such clinical situations, an improvement, but not a full normalization of the MLR of subcutaneous small resistance arteries has been observed, despite effective BP reduction.
Several specific mechanisms may contribute to the beneficial effects of some drugs on small artery structure and on outcome, compared with other pharmacological strategies. Buus NH et al. observed that, in essential hypertension, after one year of treatment with the ACE-inhibitor perindopril coronary flow reserve improved with normalization of small subcutaneous arteries structure (57). In this study, the parallel group treated with the beta-blocker atenolol coronary flow worsened and small subcutaneous artery structure remained unchanged, despite a similar reduction in BP (57). Therefore, it could be speculated that the beneficial effect on hypertensive target organ damage of these drugs may be due also to their ability in improving microvascular structure. Buus NH et al. (58) demonstrated also that MLR represents an independent predictor of cardiovascular events, beyond the extent of blood pressure reduction, in a cohort of moderate-risk essential hypertensive patients including not only untreated patients but also hypertensive patients during long-term effective treatment. This suggests that assessment of microvascular structure may also be important in treated patients, since it may allow to identify those who may benefit from a more aggressive treatment and further risk reduction.
In addition, since vascular damage in hypertension is caused also by the inflammation, oxidative stress and immune system activation, drugs selectively modulating these pathways could represent a potential and interesting strategy of treatment in the near future.
In patients with Conn’s syndrome (primary aldosteronism due to an adenoma of the adrenals), the presence of a marked or persistent vascular remodelling, as indicated by a greater MLR of subcutaneous small resistance arteries was associated with lower chances of blood pressure normalization at long-term follow-up after adrenalectomy. Thus, the severity of structural alterations in subcutaneous small resistance arteries might predict the clinical outcome in these patients with secondary hypertension (59). Therefore, structural alterations of small arteries could possibly be considered an important intermediate endpoint for the evaluation of the efficacy of antihypertensive treatment (4).
Antihypertensive treatment with ACE inhibitors seem to improve capillary rarefaction (56,60), although some methodological caveats were raised (61); in any case it is not yet established whether a reversal of capillary rarefaction ss associated with an improved prognosis (4).
Interrelationships between microvascular and macrovascular remodeling
The large arteries are not only the target of high BP, but a determining factor in the pathogenesis of hypertension, particularly of isolated systolic hypertension and the increase in pulse pressure typical of aging (62,63). The large elastic arteries allow the conduction of blood from the heart to the resistance arteries but also the transformation of the pulsatile flow, generated by cardiac activity, into the continuous flow observed in peripheral circulation. Part of the energy produced by the left ventricular systole is used for the stretching of arteries and it is stored in their walls. During diastole, this energy recoils the aorta and squeeze out blood into microcirculation, ensuring a continuous flow to the tissues (64,65). In essential l hypertension, remodeling occurs not only in small arteries but also in the macrocirculation (66,67). Large conduit arteries (such as aorta and its branches) develop arteriosclerosis of the media. This remodeling is characterized by an increase in intima-media thickness with a lumen enlargement of proximal elastic arteries and it is a compensation mechanism in order to normalize circumferential wall stress (65). Elastic fibers in the media become thinner and frail undergoing fragmentation with a parallel increase in collagen deposition and a consequent reduction in distensibility (62,66). Aging and BP are the two main determinant of arterial stiffness, which is influenced also by diabetes mellitus, obesity and metabolic syndrome. In the Framingham Heart Study, increased large artery stiffness, measured as pulse wave velocity, and aortic root enlargement were seen to be associated with a higher risk of cardiovascular disease (5,68). Pulse wave velocity correlates with hypertensive heart disease and microalbuminuria (69,70).
In fact, arterial stiffness has unfavorable consequences from a hemodynamic point of view. The increase in the speed of the incident and the reflected wave causes them to merge earlier, in the first part of the systole, increasing the systolic BP (with an increased afterload), reducing the diastolic BP (with a reduction in myocardial blood flow), and increasing the pulse pressure (66). In addition, with the elastic arteries becoming more rigid than the muscular ones, a reversion in the normal center-periphery stiffness gradient occurs, which is mainly responsible for the reflection of the sphygmic wave (4,66). Hence, the reflection site moves more distally and the reflected wave is decreased, increasing peripheral transmission of a large incident wave that exposes peripheral arteries and arterioles to harmful levels of pressure plasticity, contributing to alterations of microcirculation and, in the end, of nutrition and oxygenation of peripheral tissues (heart, brain, kidneys, limbs) as well as elimination of waste products (62,66).
It is still difficult to establish a temporal or a linear relationship between small and large artery alterations in essential hypertension. The most likely relationship is actually a cross-talk between micro- and microcirculation that may trigger a vicious circle (62,66). Hypertension causes the degeneration of large arteries and stiffness with consequent higher central systolic and pulse pressure, and microvascular alteration such as eutrophic remodeling, impaired vasodilatation, and microvascular rarefaction. Small arteries remodeling and rarefaction increase total peripheral resistance and amplifies mean BP (62,66). Indeed, in essential hypertension, MLR of small resistance arteries and pulse wave velocity are independent determinants of central systolic BP (71). Moreover, many indices of large artery stiffness (e. g. pulse pressure, pulse wave velocity) are associated with indices of microvascular damage (WLR and MLR respectively) (72,73).
Drugs that improve microvascular structure are particularly effective also in reducing central blood pressure, thus probably providing an additional benefit (4,62,69,74,75), most likely by slowing down the reflection of BP waves from the distal reflection sites, close to the microcirculation (4,62,66,69,74,75).
Pathophysiological consequences of the regression of small artery remodeling might be: a better blood pressure control, taking advantage of a reduced vascular reactivity (4,35); an improvement of the organ flow reserve, in particular in the heart (4,35,58); and an effective reduction of central blood pressure (4,62,66,69,74,75).
Conclusions
In the hypertensive microcirculation wall thickness is increased in relation to internal lumen, and that this alteration contributes to the increase in peripheral resistance observed at the microvascular level (4). Microvascular structural alterations might also impair organ flow reserve (3,4,10,35,36), and this may play a role in the maintenance/progressive worsening of hypertensive disease. Therefore, an increased MLR in small resistance arteries may predict the development of hypertension-mediated organ damage/cardiovascular events, as well as complications of the disease (43,44).
There is a need for non-invasive techniques, in order to allow for a wider application of the evaluation of microvascular morphology, to better stratify cardiovascular risk and to better evaluate the effects of antihypertensive therapy (4,27). In this regard, techniques that allow an evaluation of retinal artery morphology seem to be a promising approach (27).
In conclusion, the evaluation of microvascular structure is progressively moving from bench to bedside (3,4,27), and could, in the near future, represent an evaluation to be performed in the majority, if not in all hypertensive patients (3,4,27). The most recent demonstration of a possible prognostic relevance of non-invasive measures of microvascular structure by Adaptive Optics represent a relevant contribution, although this evidence has to be confirmed by other studies; in addition, we do need a similar demonstration of a prognostic relevance of changes of indices of microvascular structure evaluated non-invasively observed during antihypertensive treatment.
References
- Cardiovascular diseases (CVDs). World Health Organization (WHO). https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds).
- Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M, Clement DL, Coca A, de Simone G, Dominiczak A, Kahan T, Mahfoud F, Redon J, Ruilope L, Zanchetti A, Kerins M, Kjeldsen SE, Kreutz R, Laurent S, Lip GYH, McManus R, Narkiewicz K, Ruschitzka F, Schmieder RE, Shlyakhto E, Tsioufis C, Aboyans V, Desormais I; Authors/Task Force Members: 2018 ESC/ESH Guidelines for the management of arterial hypertension: The Task Force for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension: The Task Force for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension. J Hypertens 2018; 36):1953-2041.
- Agabiti-Rosei E, Rizzoni D. Microvascular structure as a prognostically relevant endpoint. J Hypertens 2017; 35(5):914-921.
- Rizzoni D, Agabiti-Rosei C, De Ciuceis C. State of the art review: vascular remodeling in hypertension. Am J Hypertens 2023; 36:1-13.
- Chiarini G, Agabiti Rosei C, Lemoli M, Rossini C, Muiesan ML, Rizzoni D, De Ciuceis C. Organ damage in hypertension: role of the microcirculation. Submitted to: Front Cardiovasc Med 2023.
- Mulvany MJ, Aalkjaer C. Structure and function of small arteries. Physiol Rev. 1990; 70: 921-971.
- Schiffrin, EL. Remodeling of resistance arteries in essential hypertension and effects of antihypertensive treatment. Am J Hypertens 2004; 17(12 Pt 1): 1192-1200.
- Rizzoni D, De Ciuceis C, Szczepaniak P, Paradis P, Schiffrin EL, Guzik TJ. Immune system and microvascular remodeling in humans. Hypertension 2022; 79(4):691-705.
- Schiffrin, EL. How structure, mechanics, and function of the vasculature contribute to blood pressure elevation in hypertension. Can J Cardiol 2020; 36(5):648-658.
- Folkow, B. Physiological aspects of primary hypertension. Physiol Rev 1982;62:347-504.
- Rizzoni D, De Ciuceis C, Porteri E, Paiardi S, Boari GE, Mortini P, Cornali C, Cenzato M, Rodella LF, Borsani E, Rizzardi N, Platto C, Rezzani R, Agabiti Rosei E. Altered structure of small cerebral arteries in patients with essential hypertension. J Hypertens 2009; 27: 838-845.
- Heagerty, A.M. , Aalkjaaer C, Bund SJ, Korsgaard N, Mulvany MJ. Small artery structure in hypertension. Dual process of remodeling and growth. Hypertension. 1993; 21: 391-397.
- Mulvany MJ, Baumbach GL, Aalkjaer C, Heagerty AM, Korsgaard N, Schiffrin EL, Heistad DD. Vascular remodeling. Hypertension 1996;28(3):505-6.
- Rizzoni D, Porteri E, Castellano M, Bettoni G, Muiesan ML, Muiesan P, Giulini SM, Agabiti Rosei E. Vascular hypertrophy and remodeling in secondary hypertension. Hypertension 1996; 28: 785-790.
- Rizzoni D, Porteri E, Guelfi D, Muiesan ML, Valentini U, Cimino A, Girelli A, Rodella L, Bianchi R, Sleiman I, Agabiti Rosei E. Structural alterations in subcutaneous small arteries of normotensive and hypertensive patients with non-insulin dependent diabetes mellitus. Circulation. 2001, 103: 1238-1244.
- Schofield I, Malik R, Izzard A, Austin C, Heagerty AM. Vascular structural and functional changes in type 2 diabetes mellitus. Evidence for the role of abnormal myogenic responsiveness and dyslipidemia. Circulation 2002; 106: 3037-3043.
- Grassi G, Seravalle G, Scopelliti F, Dell'Oro R, Fattori L, Quarti-Trevano F, Brambilla G, Schiffrin EL, Mancia G. Structural and functional alterations of subcutaneous small resistance arteries in severe human obesity. Obesity 2010; 18: 92-98.
- De Ciuceis C, Porteri E, Rizzoni D, Corbellini C, La Boria E, Boari GE, Pilu A, Mittempergher F, Di Betta E, Casella C, Nascimbeni R, Agabiti Rosei C, Ruggeri G, Caimi L, Agabiti Rosei E. Effects of weight loss on structural and functional alterations of subcutaneous small arteries in obese patients. Hypertension 2011; 58:29-36.
- Grassi G, Seravalle G, Brambilla G, Facchetti R, Bolla G, Mozzi E, Mancia G. Impact of the metabolic syndrome on subcutaneous microcirculation in obese patients. J Hypertens. 2010; 28: 1708-1714.
- Rizzoni D, Porteri E, De Ciuceis C, Rodella LF, Paiardi S, Rizzardi N, Platto C, Boari GE, Pilu A, Tiberio GA, Giulini SM, Favero G, Rezzani R, Agabiti Rosei C, Bulgari G, Avanzi D, Agabiti Rosei E. Hypertrophic remodeling of subcutaneous small resistance arteries in patients with Cushing's syndrome. Clin Endocrinol Metab 2009; 94:5010-5018.
- Rizzoni D, Porteri E, Giustina A, De Ciuceis C, Sleiman I, Boari GE, Castellano M, Muiesan ML, Bonadonna S, Burattin A, Cerudelli B, Agabiti-Rosei E. Acromegalic patients show the presence of hypertrophic remodeling of subcutaneous small resistance arteries. Hypertension 2004; 43:561-565.
- De Ciuceis C, Rossini C, Porteri E, La Boria E, Corbellini C, Mittempergher F, Di Betta E, Petroboni B, Sarkar A, Agabiti-Rosei C, Casella C, Nascimbeni R, Rezzani R, Rodella LF, Bonomini F, Agabiti-Rosei E, Rizzoni D. Circulating endothelial progenitor cells, microvascular density and fibrosis in obesity before and after bariatric surgery. Blood Press 2013; 22:165-172.
- Levy BI, Schiffrin EL, Mourad JJ, Agostini D, Vicaut E, Safar ME, Struijker- Boudier HA. Impaired tissue perfusion: a pathology common to hypertension, obesity, and diabetes mellitus. Circulation 2008; 118:968–976.
- Aalkjaer C, Heagerty AM, Petersen KK, Swales JD, Mulvany MJ. Evidence for increased media thickness, increased neuronal amine uptake, and depressed excitation--contraction coupling in isolated resistance vessels from essential hypertensives. Circ Res 1987; 61: 181–186.
- Schiffrin, E L, Hayoz D. How to assess vascular remodelling in small and medium-sized muscular arteries in humans. J. Hypertens 1997; 15: 571–584.
- Virdis A, Savoia C, Grassi G, Lembo G, Vecchione C, Seravalle G, Taddei S, Volpe M, Aganiti Rosei E, Rizzoni D. Evaluation of microvascular structure in humans: a 'state-of-the-art' document of the Working Group on Macrovascular and Microvascular Alterations of the Italian Society of Arterial Hypertension. J Hypertens 2014; 32:2120-2129.
- Rizzoni D, Mengozzi A, Masi S, Agabiti Rosei C, De Ciuceis C, Virdis A. New noninvasive methods to evaluate microvascular structure and function. Hypertension 2022; 79(5):874-886.
- De Ciuceis C, Agabiti Rosei C, Caletti S, Trapletti V, Coschignano MA, Tiberio GAM, Duse S, Docchio F, Pasinetti S, Zambonardi F, Semeraro F, Porteri E, Solaini L, Sansoni G, Pileri P, Rossini C, Mittempergher F, Portolani N, Ministrini S, Agabiti-Rosei E, Rizzoni D. Comparison between invasive and noninvasive techniques of evaluation of microvascular structural alterations. J Hypertens 2018; 36:1154-1163.
- Harazny JM, Ritt M, Baleanu D, Ott C, Heckmann J, Schlaich MP, Michelson G, Schmieder RE. Increased wall:lumen ratio of retinal arterioles in male patients with a history of a cerebrovascular event. Hypertension 2007; 50:623-829.
- Ritt M, Harazny JM, Ott C, Schlaich MP, Schneider MP, Michelson G, Schmieder RE. Analysis of retinal arteriolar structure in never-treated patients with essential hypertension. J Hypertens 2008; 26:1427-434.
- Koch E, Rosenbaum D, Brolly A, Sahel JA, Chaumet-Riffaud P, Girerd X, Rossant F, Paques M. Morphometric analysis of small arteries in the human retina using adaptive optics imaging: relationship with blood pressure and focal vascular changes. J Hypertens 2014; 32:890-898.
- Park JB1, Schiffrin EL. Small artery remodeling is the most prevalent (earliest?) form of target organ damage in mild essential
hypertension. J Hypertens 2001; 19:921-930.
- Lever, AF. Slow pressor mechanisms in hypertension: a role for hypertrophy of resistance vessels? J Hypertens 1986; 4:515–524.
- Schiffrin EL Reactivity of small blood vessel in hypertension: relation with structural changes. Hypertension 1992; 19(suppl II):II1–II9.
- Rizzoni D, Agabiti-Rosei C, Agabiti-Rosei E. Hemodynamic consequences of changes in microvascular structure. Am J Hypertens 2017; 30(10):939-946.
- Rizzoni D, Palombo C, Porteri E, Muiesan ML, Kozàkovà M, La Canna G, Nardi M, Guelfi D, Salvetti M, Morizzo C, Vittone F, Agabiti Rosei E. Relationships between coronary vasodilator capacity and small artery remodeling in hypertensive patients. J Hypertens 2003; 21: 625-632.
- Agabiti Rosei E, Rizzoni D, Castellano M, Porteri E, Zulli R, Muiesan ML, Bettoni G, Salvetti M, Muiesan P, Giulini SM. Media: lumen ratio in human small resistance arteries is related to forearm minimal vascular resistance. J Hypertens 1995; 13:341-347.
- Muiesan ML, Rizzoni D, Salvetti M, Porteri E, Monteduro C, Guelfi D, Castellano M, Garavelli G, Agabiti-Rosei E. Structural changes in small resistance arteries and left ventricular geometry in patients with primary and secondary hypertension. J Hypertens 2002; 20:1439-1444.
- Rizzoni D1, Muiesan ML, Porteri E, Salvetti M, Castellano M, Bettoni G, Tiberio G, Giulini SM, Monteduro C, Garavelli G, Agabiti-Rosei E. Relations between cardiac and vascular structure in patients with primary and secondary hypertension. J Am Coll Cardiol 1998; 32:985-992.
- Rizzoni D, Porteri E, Boari GEM, De Ciuceis C, Sleiman I, Muiesan ML, Castellano M, Miclini M, Agabiti-Rosei E. Prognostic significance of small artery structure in hypertension. Circulation 2003; 108: 2230-2235.
- De Ciuceis C, Porteri E, Rizzoni D, Rizzardi N, Paiardi S, Boari GEM, Miclini M, Zani F, Muiesan ML, Donato F, Salvetti M, Castellano M, Tiberio GA, Giulini SM, Agabiti Rosei E. Structural alterations of subcutaneous small arteries may predict major cardiovascular events in hypertensive patients. Am J Hypertens 2007, 20:846-852.
- Mathiassen ON, Buus NH, Sihm I, Thybo NK, Mørn B, Schroeder AP, Thygesen K, Aalkjaer C, Lederballe O, Mulvany MJ, Christensen KL. Small artery structure is an independent predictor of cardiovascular events in essential hypertension. J Hypertens 2007; 25:1021-1026.
- Izzard AS, Rizzoni D, Agabiti-Rosei E, Heagerty AM. Small artery structure and hypertension: adaptive changes and target
organ damage. J Hypertens 2005, 23:247-250.
- Heagerty, AM. Predicting hypertension complications from small artery structure. J Hypertens 2007; 25:939-940.
- Ritt M, Harazny JM, Ott C, Schneider MP, Schlaich MP, Michelson G, Schmieder RE. Wall-to-lumen ratio of retinal arterioles is related with urinary albumin excretion and altered vascular reactivity to infusion of the nitric oxide synthase inhibitor N-monomethyl-L-arginine. J Hypertens 2009; 27: 2201-2218.
- Rosenbaum D, Mattina A, Kock E, Rossant F, Gallo A, Kachenoura N, Paques M, Redheuil A, Gired X Effects of age, blood pressure and antihypertensive treatment on retinal arterioles remodeling assessed by adaptive optics. J Hpertens 2016; 34: 1115-1122.
- Meixner E, Michelson G. Measurement of retinal wall-to-lumen ratio by adaptive optics retinal camera: a clinical research. Graefe’s Arch Clin Exp Ophthalmol 2015; 253: 1985–1995.
- De Ciuceis C, Agabiti-Rosei C, Malerba P, Rossini C, Chiarini G, Brami V, Famà F, Gaggero A, Nardin M, Lemoli M, Baresi M, Petelca A, Bortoluzzi C, Porteri E, Salvetti M, Muiesan ML, Agabiti-Rosei E, Rizzoni D. Prognostic significance of the wall to lumen ratio of retinal arterioles evaluated by adaptive optics in human hypertension. Submitted to Hypertension, 2023.
- Kopeva K, Grakova E, Maltseva A, Mochula A, Gusakova A, Smorgon A, Zavadovsky K. Coronary microvascular dysfunction: features and prognostic value. J Clin Med 2023; 12:2964.
- Agabiti-Rosei E, Heagerty AM, Rizzoni D. Effects of antihypertensive treatment on small artery remodelling. J Hypertens 2009; 27:1107-1114.
- Savoia C, Touyz RM, Endemann DH, Pu Q, Ko EA, De Ciuceis C, Schiffrin EL. Angiotensin receptor blocker added to previous antihypertensive agents on arteries of diabetic hypertensive patients. Hypertension 2006; 48:271-277.
- Rizzoni D, Porteri E, De Ciuceis C, Sleiman I, Rodella L, Rezzani R, Paiardi S, Bianchi R, Ruggeri G, Boari GEM, Muiesan ML, Salvetti M, Zani F, Miclini M, Agabiti Rosei E. Effects of treatment with candesartan or enalapril on subcutaneous small resistance artery structure in hypertensive patients with NIDDM. Hypertension 2005, 45: 659-665.
- Rizzoni D, Agabiti Rosei E. Small artery remodeling in diabetes mellitus. Nutr Metab Cardiovasc Dis 2009; 19:587-592.
- De Ciuceis C, Savoia C, Arrabito E, Porteri E, Mazza M, Rossini C, Duse S, Semeraro F, Agabiti Rosei C, Alonzo A, Sada L, La Boria E, Sarkar A, Petroboni B, Mercantini P, Volpe M, Rizzoni D, Agabiti Rosei E. Effects of a long-term treatment with aliskiren or ramipril on structural alterations of subcutaneous small-resistance arteries of diabetic hypertensive patients. Hypertension 2014; 64:717–724.
- Jumar A, Ott C, Kistner I, Friedrich S, Schmidt S, Harazny JM, Schmieder RE. Effect of aliskiren on vascular remodelling in
small retinal circulation. J Hypertens 2015; 33:2491-9.
- De Ciuceis C, Salvetti M, Rossini C, Muiesan ML, Paini A, Duse S, La Boria E, Semeraro F, Cancarini A, Agabiti Rosei C, Sarkar A, Ruggeri G, Caimi L, Ricotta D, Rizzoni D, Agabiti Rosei E. Effect of antihypertensive treatment on microvascular structure, central blood pressure and oxidative stress in patients with mild essential hypertension. J Hypertens 2014; 32:565-574.
- Buus NH, Mathiassen ON, Fenger-Grøn M, Præstholm MN, Sihm I, Thybo NK, Schroeder AP, Thygesen K, Aalkjær C, Pedersen OL, Mulvany MJ, Christensen KL. Small artery structure during antihypertensive therapy is an independent predictor of cardiovascular events in essential hypertension. J Hypertens 2013; 31:791-797.
- Buus NH, Bøttcher M, Jørgensen CG, Christensen KL, Thygesen K, Nielsen TT, Mulvany MJ. Myocardial perfusion during long-term angiotensin-converting enzyme inhibition or beta-blockade in patients with essential hypertension. Hypertension 2004; 44:465-470.
- Rossi GP, Bolognesi M, Rizzoni D, Seccia TM, Piva A, Porteri E, Tiberio GA, Giulini SM, Agabiti-Rosei E, Pessina AC. Vascular remodeling and duration of hypertension predict outcome of adrenalectomy in primary aldosteronism patients. Hypertension 2008; 51:1366-1371.
- Debbabi H, Uzan L, Mourad JJ, Safar M, Levy BI, Tibirica` E. Increased skin capillary density in treated essential hypertensive patients. Am J Hypertens 2006; 19:477–483.
- Antonios, TF. Microvascular rarefaction in hypertension--reversal or over-correction by treatment? Am J Hypertens 2006, 19:484-485.
- Laurent S, Agabiti-Rosei C, Bruno RM, Rizzoni D. Microcirculation and macrocirculation in hypertension: a dangerous cross-link? Hypertension 2022; 79(3):479-490.
- Laurent S, Boutouyrie P. Arterial stiffness and hypertension in the elderly. Front Cardiovasc. Med 2020; Oct 29;7:544302.
- Rizzoni D, Rizzoni M, Nardin M, Chiarini G, Agabiti-Rosei C, Aggiusti C, Paini A, Salvetti M, Muiesan ML. Vascular aging and disease of the small vessels. High Blood Press Cardiovasc Prev 2019; 26(3):183-189.
- Laurent S, Boutouyrie P. The structural factor of hypertension. Circ Res 2015; 116, 1007–1021.
- Laurent S, Agabiti-Rosei E. The cross-talk between the macro- and the microcirculation. In: Early Vascular Aging (EVA): new
directions in cardiovascular protection. Nilsson P, Olsen MH & Laurent S Eds. Pag. 105-118, 2015.
- Sasaki R, Yamano S, Yamamoto Y, Minami S, Yamamoto J, Nakashima T, Takaoka M, Hashimoto T. Vascular remodeling of the carotid artery in patients with untreated essential hypertension increases with age. Hypertens Res 2002; 25(3):373-9.
- Vasan RS, Pan S, Xanthakis V, Beiser A, Larson MG, Seshadri S, Mitchell GF. Arterial Stiffness and long-term risk of health outcomes: The Framingham Heart Study. Hypertension 2022; 79:1045-1056.
- Laurent S, Briet M, Boutouyrie P. Large and small artery cross-talk and recent morbidity-mortality trials in hypertension. Hypertension 2009; 54:388-392.
- Mulè G, Cottone S, Vadalà A, Volpe V, Mezzatesta G, Mongiovì R, Piazza G, Nardi E, Andronico G, Cerasola G. Relationship between albumin excretion rate and aortic stiffness in untreated essential hypertensive patients. J Intern Med 2004; 256:22-9.
- Muiesan ML, Salvetti M, Rizzoni D, Paini A, Agabiti-Rosei C, Aggiusti C, Bertacchini F, Stassaldi D, Gavazzi A, Porteri E, De Ciuceis C, Agabiti-Rosei E. Pulsatile hemodynamics and microcirculation: evidence for a close relationship in hypertensive patients. Hypertension 2013; 61:130-136.
- Ott C, Raff U, Harazny JM, Michelson G, Schmieder RE. Central pulse pressure is an independent determinant of vascular remodeling in the retinal circulation. Hypertension 2013; 61, 1340–1345.
- Salvetti M, Agabiti Rosei C, Paini A, Aggiusti C, Cancarini A, Duse S, Semeraro F, Rizzoni D, Agabiti Rosei E, Muiesan ML. Relationship of wall-to-lumen ratio of retinal arterioles with clinic and 24-hour blood pressure. Hypertension 2014; 63:1110-1115.
- Rizzoni D, Muiesan ML, Porteri E, De Ciuceis C, Boari GE, Salvetti M, Paini A, Agabiti Rosei E. Vascular remodeling, macro- and microvessels: therapeutic implications. Blood Press 2009; 18:242-246.
- Laurent, S. & Rizzoni D. Targeting central blood pressure through the micro-and macrocirculation cross-talk. In: Early Vascular Aging (EVA): new directions in cardiovascular protection. Nilsson P, Olsen MH & Laurent S Eds. Pag. 297-306, 2015.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).