Submitted:
18 June 2023
Posted:
19 June 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Material preparation
| Al | Sn | Ta | W | Zr | Mo | Nd | Si | C | Ti |
| 5.9 | 4.0 | 2.0 | 1.0 | 3.5 | 0.3 | 0.3 | 0.35 | 0.05 | Bal. |
2.2. Hot-dip alumininzing
2.3. Solid carburization
2.4. Corrosion experiments
2.5. Analytical characterization
3. Results
3.1. The microstructures of the hot dip aluminum/carburizing composite coating
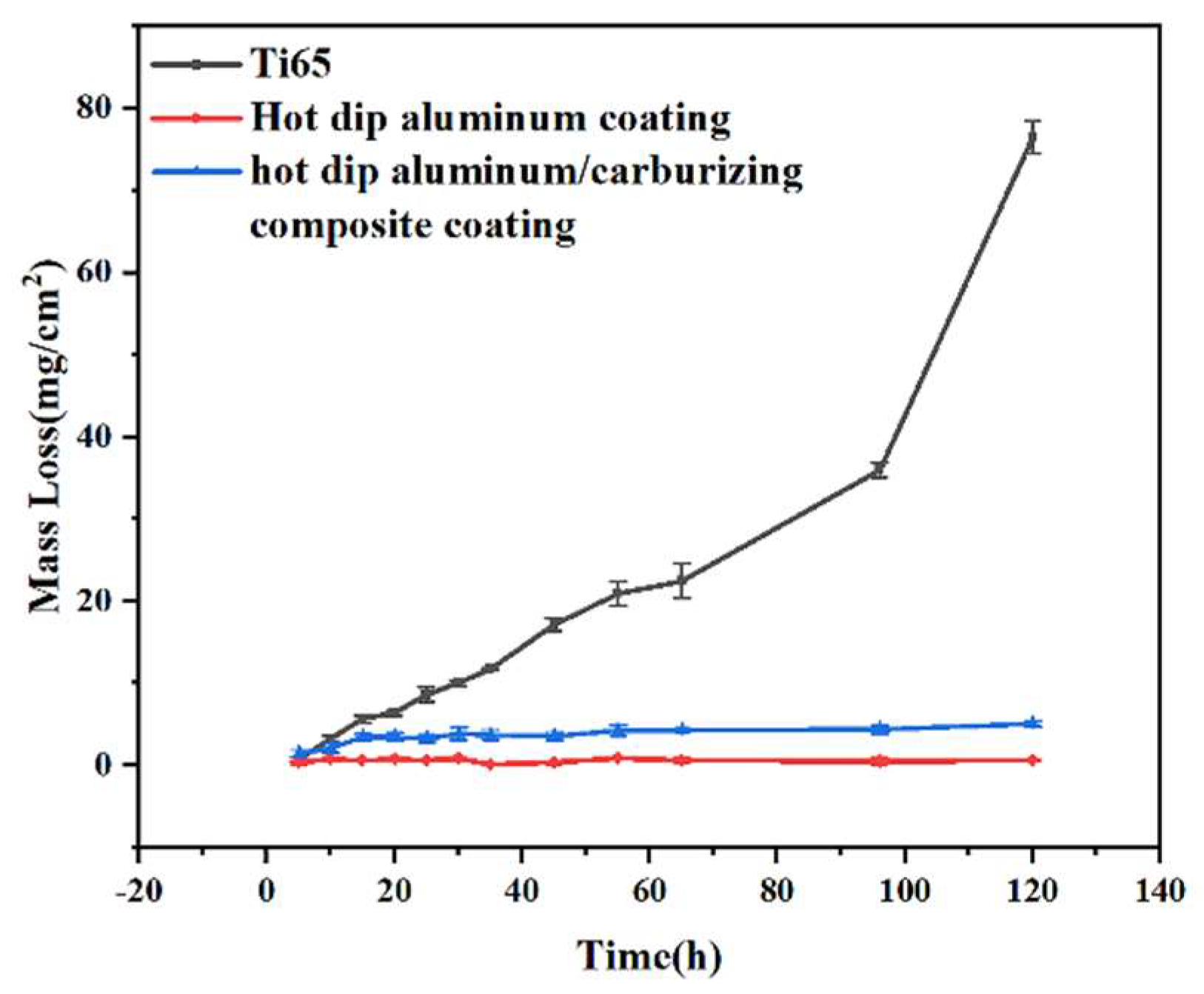
3.2. Molten salt corrosion kinetics
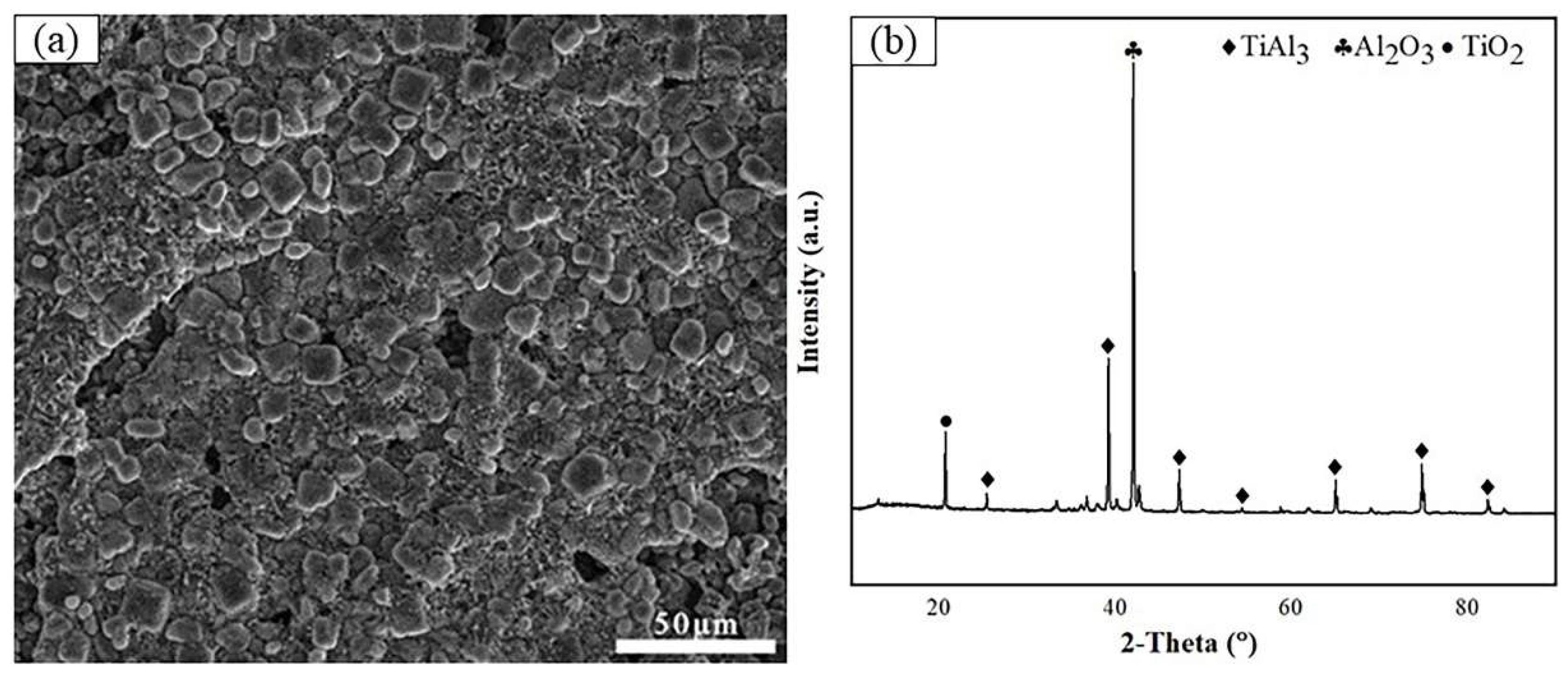
3.3. Cross-sectional morphologies and surface analysis of hot dip aluminum coating after molten salt corrosion
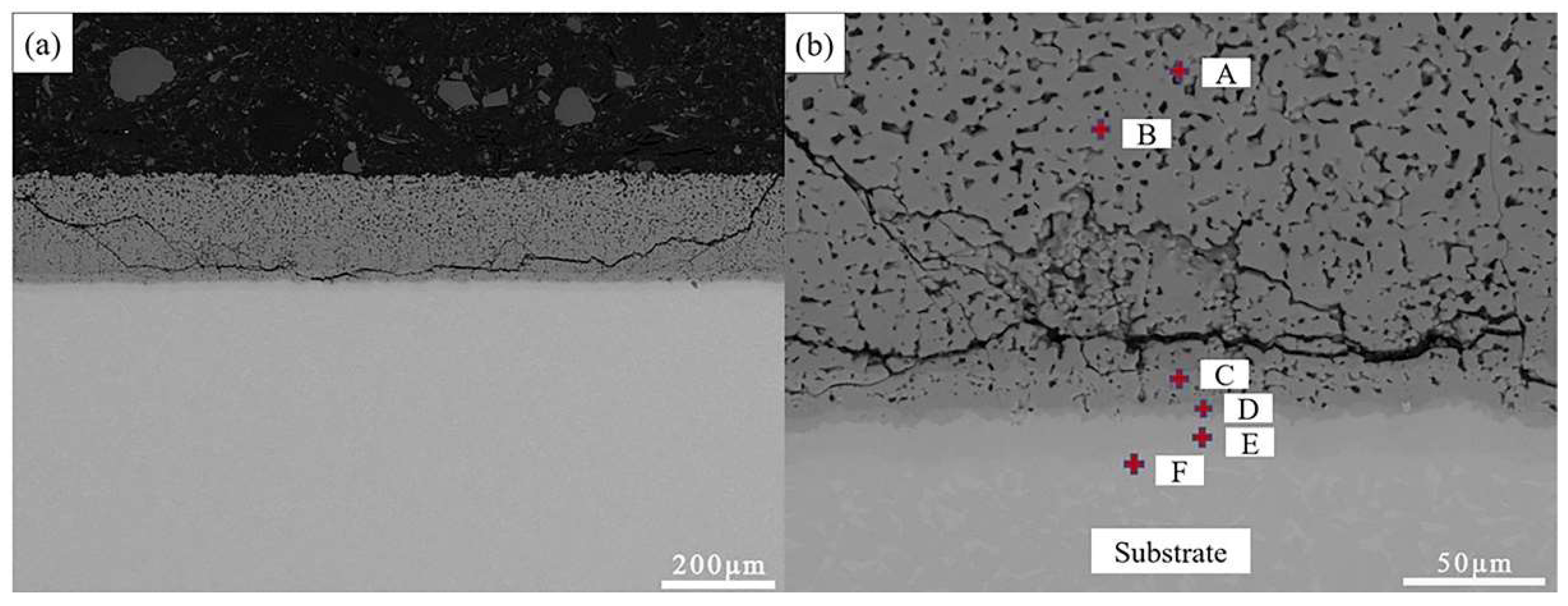
| Point | Ti | Al | O | Possible phase |
| A | 7.58 | 38.41 | 54.01 | Al2O3+Ti-Al |
| B | 24.64 | 55.54 | 31.34 | TiAl3+Al2O3+TiO2 |
| C | 35.67 | 64.33 | / | TiAl2 |
| D | 51.78 | 48.22 | / | TiAl |
| E | 78.81 | 21.19 | / | Ti3Al |
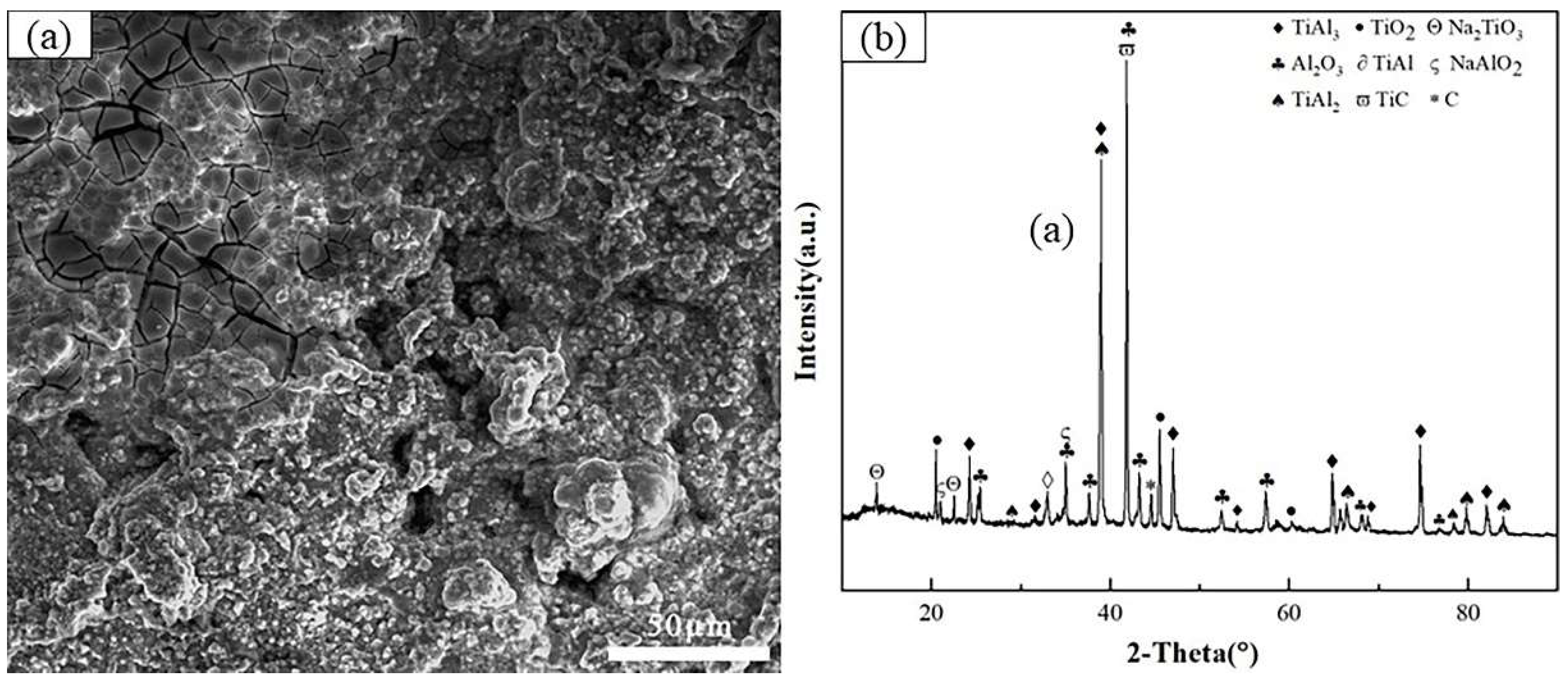
3.4. Cross-sectional morphologies and surface analysis of hot dip aluminum/carburizing composite coatings after molten salt corrosion

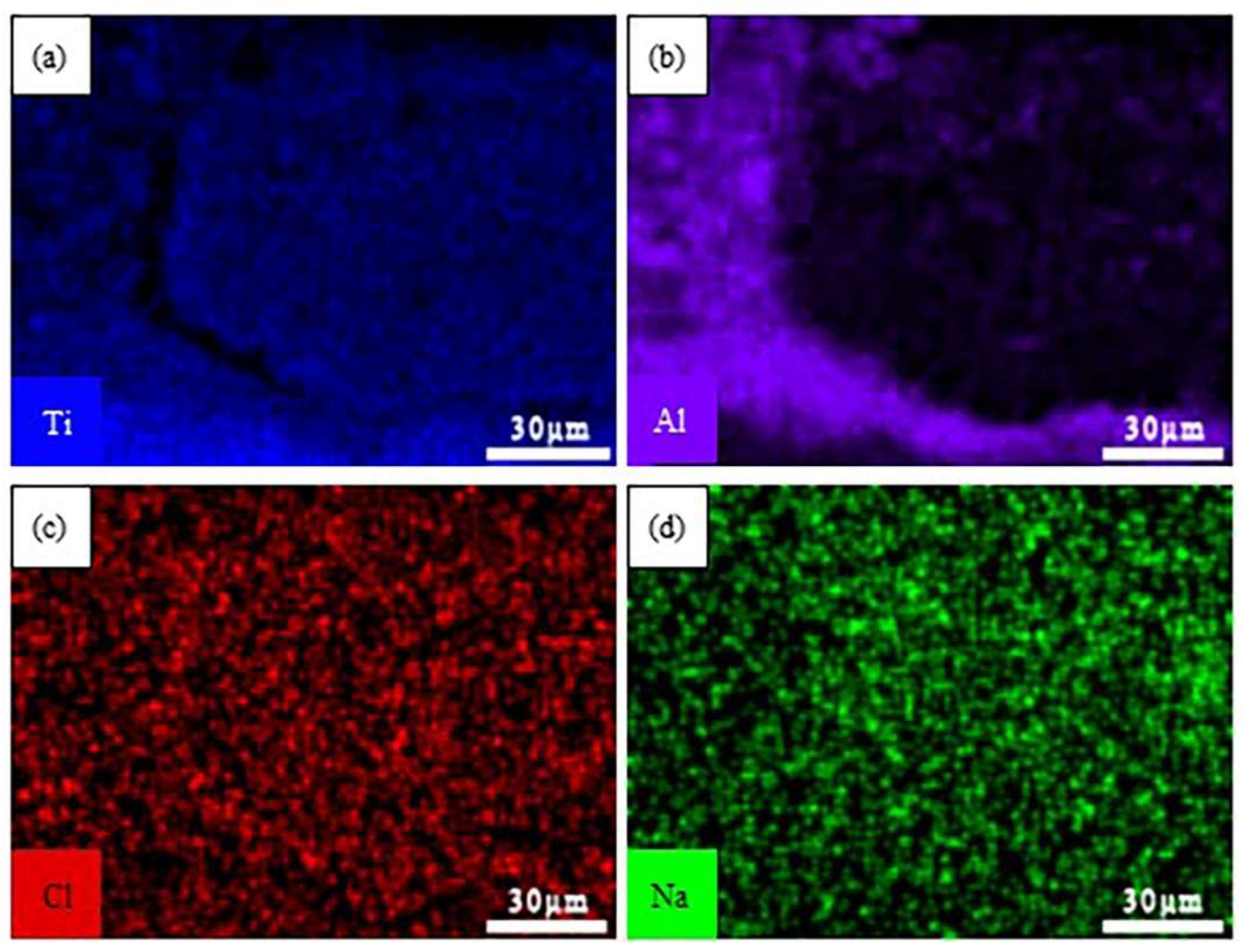
| Point | Ti | Al | O | Cl | Na | Possible phase |
| A | 21.96 | 15.58 | 60.71 | / | 1.75 | Al2O3+TiO2 |
| B | 19.47 | 10.39 | 67.10 | / | 3.05 | TiO2+Na2TiO3 |
| C | 18.21 | 30.96 | 50.83 | / | / | Al2O3+TiO2 |
| D | 9.50 | 29.00 | 60.20 | 0.64 | 0.66 | Al2O3+TiAl2 |
| E | 34.33 | 65.57 | / | / | / | TiAl2 |
| F | 46.85 | 53.15 | / | / | / | TiAl |
| G | 80.36 | 19.64 | / | / | / | Ti3Al |
| Mean | 32.95 | 32.06 | 59.71 | 0.64 | 0.92 | / |
4. Discussion
4.1. The microstructures of the hot dip aluminum/carburizing composite coating
4.2. The microstructures of the hot dip aluminum/carburizing composite coating
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huang, L.; An, Q.; Geng, L.; Wang, S.; Jiang, S.; Cui, X.; Zhang, R.; Sun, F.; Jiao, Y.; Chen, X.; Wang, C. Multiscale Architecture and Superior High-Temperature Performance of Discontinuously Reinforced Titanium Matrix Composites. Adv Mater. 2021, 33, e2000688. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Zhou, H.; Liu, J.; Kang, C. Multiaxial fatigue damage and reliability assessment of aero-engine compressor blades made of TC4 titanium alloy. Aerospace Science and Technology. 2021, 119. [Google Scholar] [CrossRef]
- Singh, G.; Ramamurty, U. Reprint: Boron modified titanium alloys. Progress in Materials Science. 2021, 120. [Google Scholar] [CrossRef]
- Sun, J.; Qi, M.; Zhang, J.; Li, X.; Wang, H.; Ma, Y.; Xu, D.; Lei, J.; Yang, R. Formation mechanism of α lamellae during β→α transformation in polycrystalline dual-phase Ti alloys. Journal of Materials Science & Technology. 2021, 71, 98–108. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, Y.; Hu, J. Recent advances in the development of aerospace materials. Progress in Aerospace Sciences. 2018, 97, 22–34. [Google Scholar] [CrossRef]
- Ciszak, C.; Popa, I.; Brossard, J.-M.; Monceau, D.; Chevalier, S. NaCl induced corrosion of Ti-6Al-4V alloy at high temperature. Corrosion Science. 2016, 110, 91–104. [Google Scholar] [CrossRef]
- Fan, L.; Liu, L.; Cui, Y.; Cao, M.; Yu, Z.; Oguzie, E.E.; Li, Y.; Wang, F. Effect of streaming water vapor on the corrosion behavior of Ti60 alloy under a solid NaCl deposit in water vapor at 600 °C. Corrosion Science. 2019, 160. [Google Scholar] [CrossRef]
- Li, R.; Liu, L.; Cui, Y.; Liu, R.; Fan, L.; Cao, M.; Yu, Z.; Chen, Z.; Wang, Q.; Wang, F. Corrosion behavior of Ti60 alloy under continuous NaCl solution spraying at 600 °C. Journal of Materials Science & Technology. 2022, 124, 86–101. [Google Scholar] [CrossRef]
- Z. Yao, M.M. NaCl-induced hot corrosion of a titanium aluminide alloy Materials Science and Engineering A. 1995, 192/193 6.
- Rubacha, K.; Godlewska, E.; Mars, K. Behaviour of a silicon-rich coating on Ti-46Al-8Ta (at.%) in hot-corrosion environments. Corrosion Science. 2017, 118, 158–167. [Google Scholar] [CrossRef]
- Wu, L.-K.; Wu, J.-J.; Wu, W.-Y.; Yan, H.-J.; Jiang, M.-Y.; Cao, F.-H. Hot corrosion behavior of electrodeposited SiO2 coating on TiAl alloy. Corrosion Science. 2020, 174. [Google Scholar] [CrossRef]
- Hu, Y.-t.; Zheng, L.; Yan, H.-j.; Wu, L.-k.; Lin, X.-j.; Cao, F.-h.; Jiang, M.-y. Improving hot corrosion resistance of aluminized TiAl alloy by anodization and pre-oxidation. Transactions of Nonferrous Metals Society of China. 2021, 31, 193–206. [Google Scholar] [CrossRef]
- Zhang, M.; Liang, Y.; Feng, M.; Jiang, C.; Zhang, Y.; Wang, Y.; Feng, Y.; Ma, L.; Wang, F. Corrosion behaviour of aluminide coatings on Ti-based alloy in the marine environment at high temperature. Corrosion Science. 2022, 209. [Google Scholar] [CrossRef]
- Zhaolin Tang *, F.W., Weitao Wu. <14.pdf>. Materials Science and Engineering A. 2000, 276.
- Huilgol, P.; Udupa, K.R.; Bhat, K.U. Metastable microstructures at the interface between AISI 321 steel and molten aluminum during hot-dip aluminizing. Surface and Coatings Technology. 2018, 348, 22–30. [Google Scholar] [CrossRef]
- Shao, Z.; Yang, H.; Zhang, S.; Liu, W.; Xiao, Z.; Zheng, M. A dense Fe-Al/Al2O3 coating as tritium permeation barrier on CLAM steel by hot-dipping aluminizing. Surface and Coatings Technology. 2022, 440. [Google Scholar] [CrossRef]
- Shibli, S.M.A.; Manu, R.; Dilimon, V.S. Effect of nickel-rich barrier layer on improvement of hot-dip zinc coating. Applied Surface Science. 2005, 245, 179–185. [Google Scholar] [CrossRef]
- Patel, P.; Jamnapara, N.I.; Zala, A.; Kahar, S.D. Investigation of hot-dip aluminized Ti6Al4V alloy processed by different thermal treatments in an oxidizing atmosphere. Surface and Coatings Technology. 2020, 385. [Google Scholar] [CrossRef]
- Wang, Y.; Xiong, J.; Yan, J.; Fan, H.; Wang, J. Oxidation resistance and corrosion behavior of hot-dip aluminized coatings on commercial-purity titanium. Surface and Coatings Technology. 2011, 206, 1277–1282. [Google Scholar] [CrossRef]
- Jeng, S.-C. Oxidation behavior and microstructural evolution of hot-dipped aluminum coating on Ti-6Al-4V alloy at 800°C. Surface and Coatings Technology. 2013, 235, 867–874. [Google Scholar] [CrossRef]
- Duan, H.-q.; Han, Y.-f.; LÜ, W.-j.; Mao, J.-w.; Wang, L.-q.; Zhang, D. Effect of solid carburization on surface microstructure and hardness of Ti-6Al-4V alloy and (TiB+La2O3)/Ti-6Al-4V composite. Transactions of Nonferrous Metals Society of China. 2016, 26, 1871–1877. [Google Scholar] [CrossRef]
- Liao, C.; Yang, J.; He, Y.; Ming, X. Electrochemical corrosion behavior of the carburized porous TiAl alloy. Journal of Alloys and Compounds. 2015, 619, 221–227. [Google Scholar] [CrossRef]
- Yao, T.; Liu, Y.; Liu, B.; Song, M.; Zhao, K.; Zhang, W.; He, Y. Influence of carburization on oxidation behavior of High Nb contained TiAl alloy. Surface and Coatings Technology. 2015, 277, 210–215. [Google Scholar] [CrossRef]
- Wohlert, S.; Bormann, R. Phase selection governed by different growth velocities in the early stages of the Ti/Al phase reaction. J. Appl. Phys. 1999, 85, 825–832. [Google Scholar] [CrossRef]
- Hsu, I.C.; Wu, S.K.; Lin, R.Y. A study of aluminum cladding on Ti50Al50 intermetallics by liquid aluminizing. Materials Chemistry and Physics. 1997, 49, 184–190. [Google Scholar] [CrossRef]
- Jiang, S.-y.; Li, S.-c.; Zhang, L. Microstructure evolution of Al–Ti liquid–solid interface. Transactions of Nonferrous Metals Society of China. 2013, 23, 3545–3552. [Google Scholar] [CrossRef]
- Alam, M.Z.; Das, D.K. Effect of cracking in diffusion aluminide coatings on their cyclic oxidation performance on Ti-based IMI-834 alloy. Corrosion Science. 2009, 51, 1405–1412. [Google Scholar] [CrossRef]
- Ciszak, C.; Abdallah, I.; Popa, I.; Brossard, J.-M.; Vande Put, A.; Monceau, D.; Chevalier, S. Degradation mechanism of Ti-6Al-2Sn-4Zr-2Mo-Si alloy exposed to solid NaCl deposit at high temperature. Corrosion Science. 2020, 172. [Google Scholar] [CrossRef]
- Wang, Z.; Ma, G.; Li, Z.; Ruan, H.; Yuan, J.; Wang, L.; Ke, P.; Wang, A. Corrosion mechanism of Ti2AlC MAX phase coatings under the synergistic effects of water vapor and solid NaCl at 600 °C. Corrosion Science. 2021, 192. [Google Scholar] [CrossRef]
- Li, R.; Wang, S.; Zhou, D.; Pu, J.; Yu, M.; Guo, W. A new insight into the NaCl-induced hot corrosion mechanism of TiN coatings at 500 °C. Corrosion Science. 2020, 174. [Google Scholar] [CrossRef]
- Chang, L.M.; Liu, J.H.; Zhang, R.J. Corrosion behaviour of electrodeposited Ni/Al2O3 composite coating covered with a NaCl salt film at 800 degrees C. Materials and Corrosion-Werkstoffe Und Korrosion. 2011, 62, 920–925. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, X.; Wu, Y.; Chen, C.; Li, Y.; Zhou, W.; Ren, X. Effect of surface morphology and microstructure on the hot corrosion behavior of TiC/IN625 coatings prepared by extreme high-speed laser cladding. Corrosion Science. 2022, 201. [Google Scholar] [CrossRef]
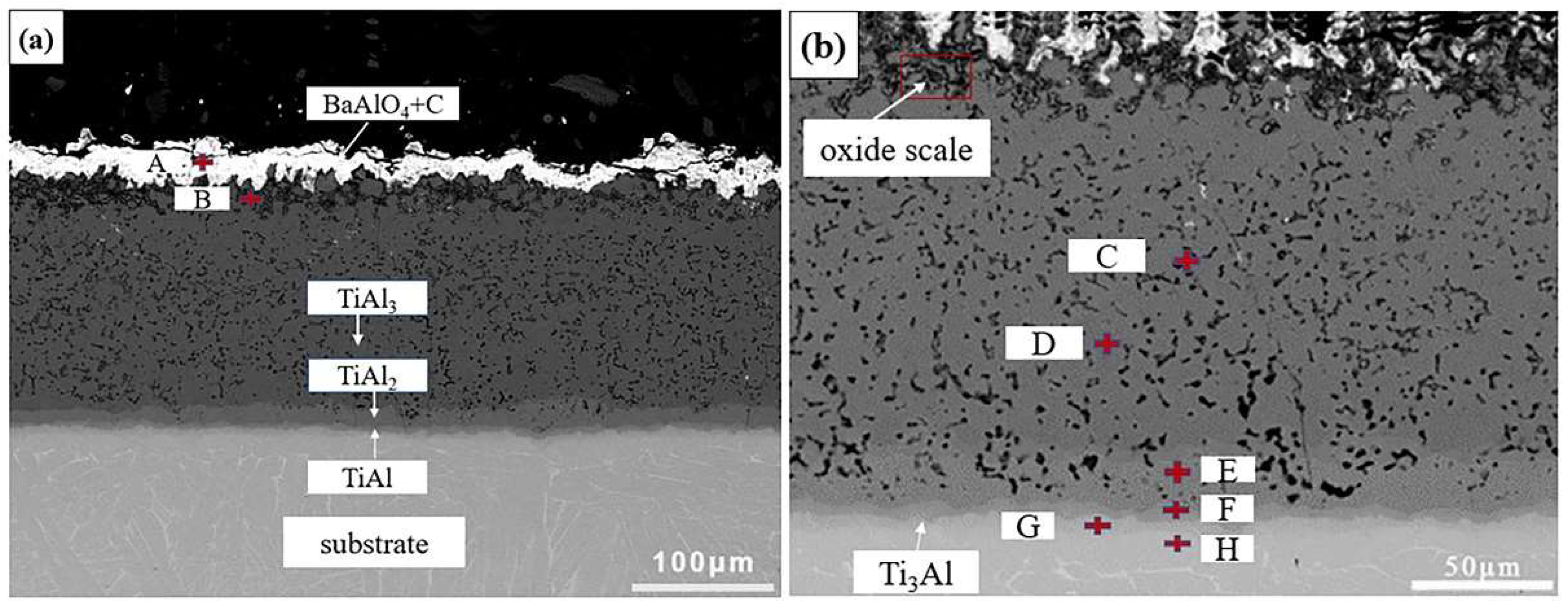
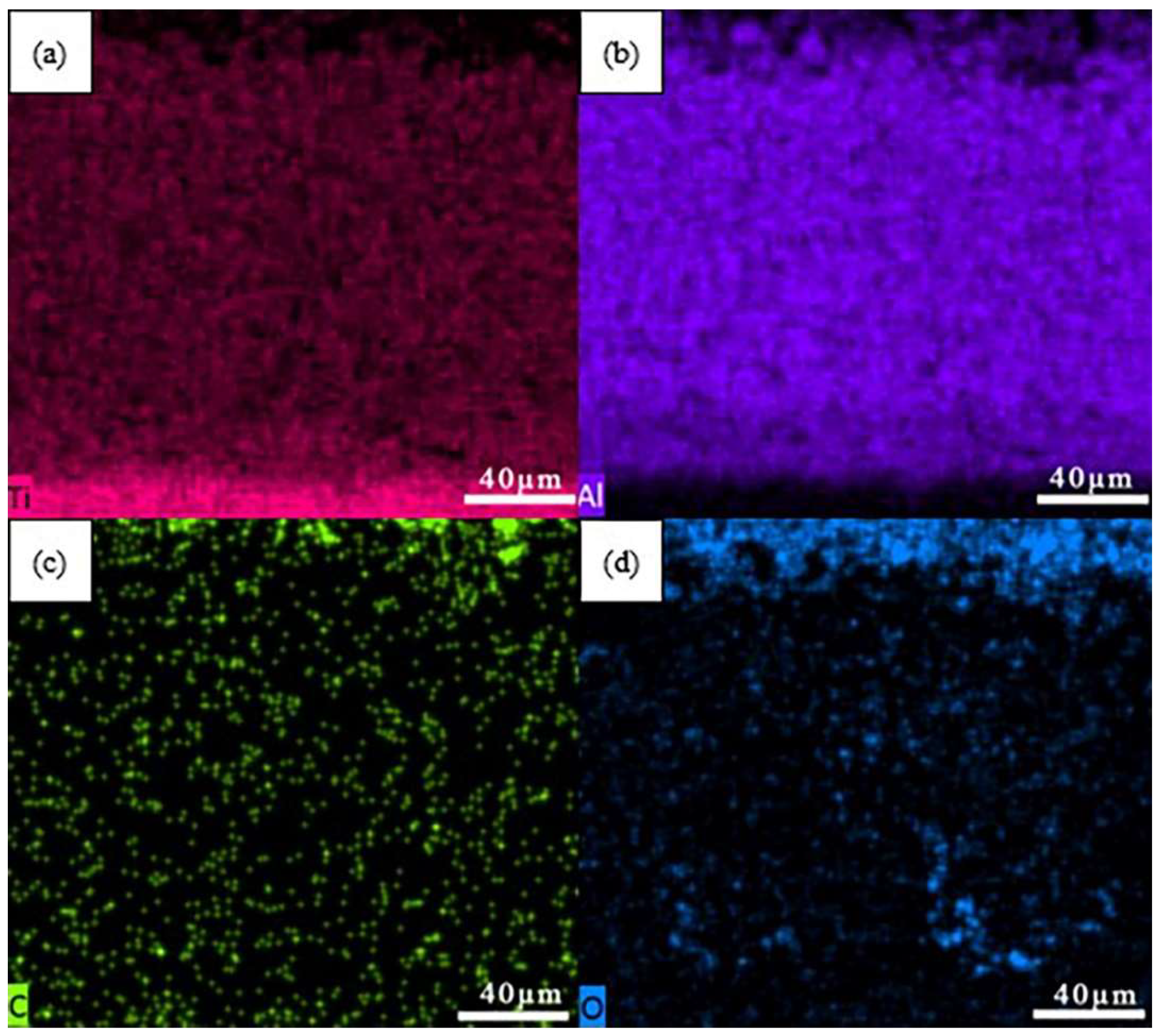
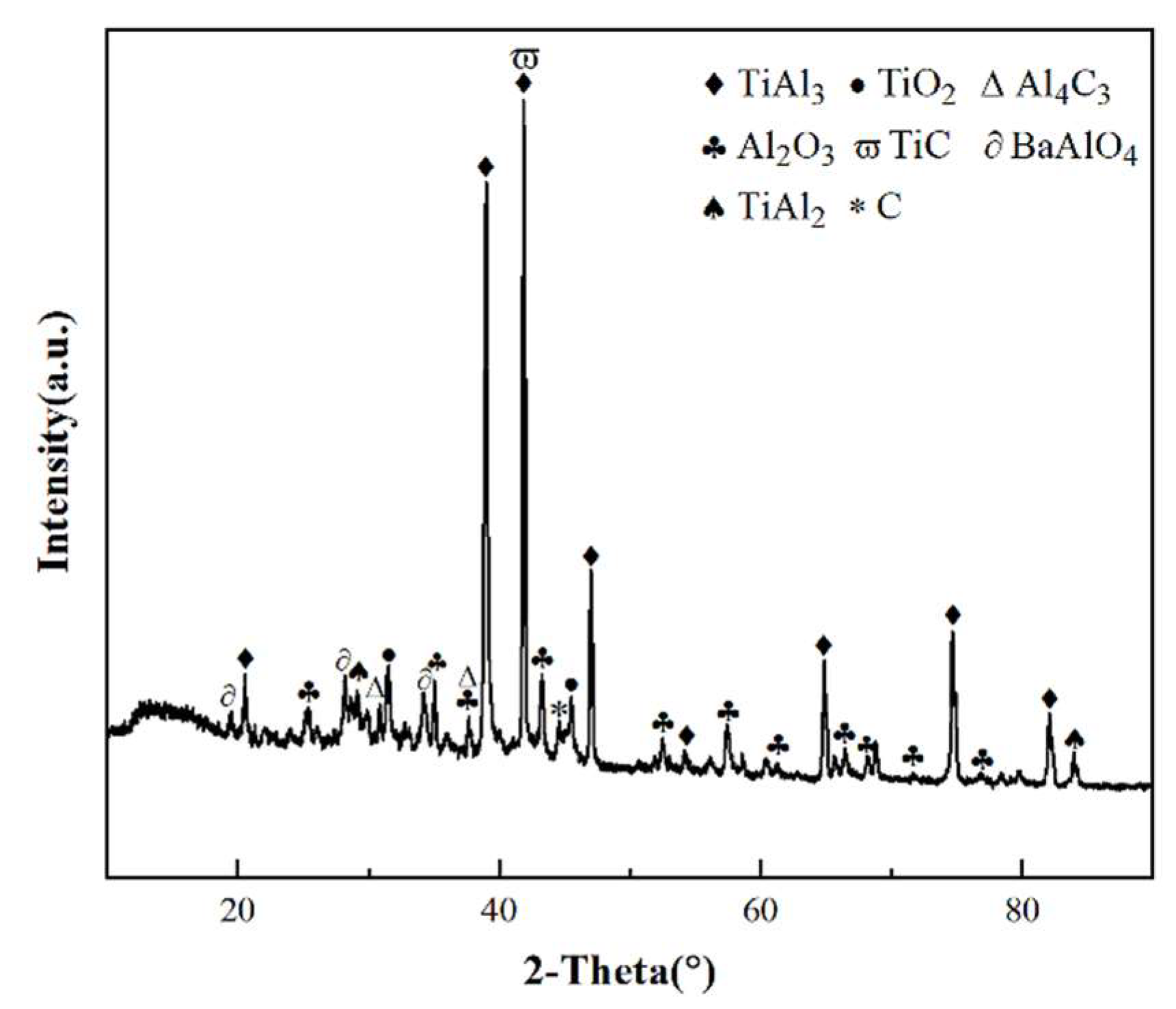

| Point | Ti | Al | C | O | Ba | Possible Phase |
| A | 2.38 | 12.62 | 22.20 | 51.20 | 11.51 | C+BaAlO4 |
| B | 6.85 | 24.72 | 15.31 | 53.12 | / | Al2O3+TiO2+TiC+Al4C3 |
| C | 15.94 | 60.62 | / | 25.45 | / | Al2O3+Ti-Al |
| D | 29.87 | 70.13 | / | / | / | TiAl3 |
| E | 33.19 | 66.81 | / | / | / | TiAl2 |
| F | 49.39 | 50.61 | / | / | / | TiAl |
| G | 70.52 | 29.48 | / | / | / | Ti3Al |
| H | 83.71 | 16.29 | / | / | / | Ti3Al+Ti |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





