Submitted:
16 June 2023
Posted:
19 June 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
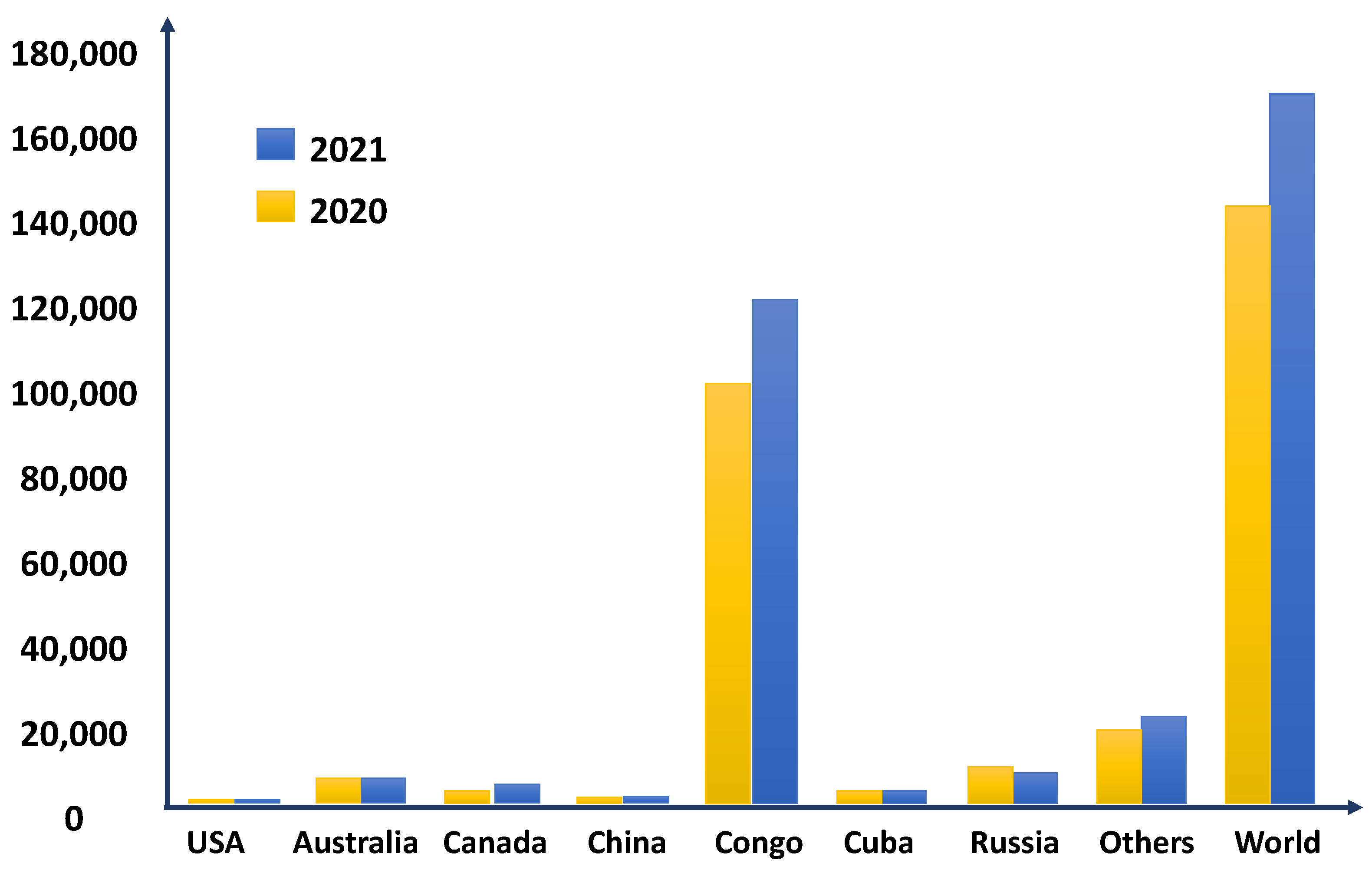
2. Cobalt Demand
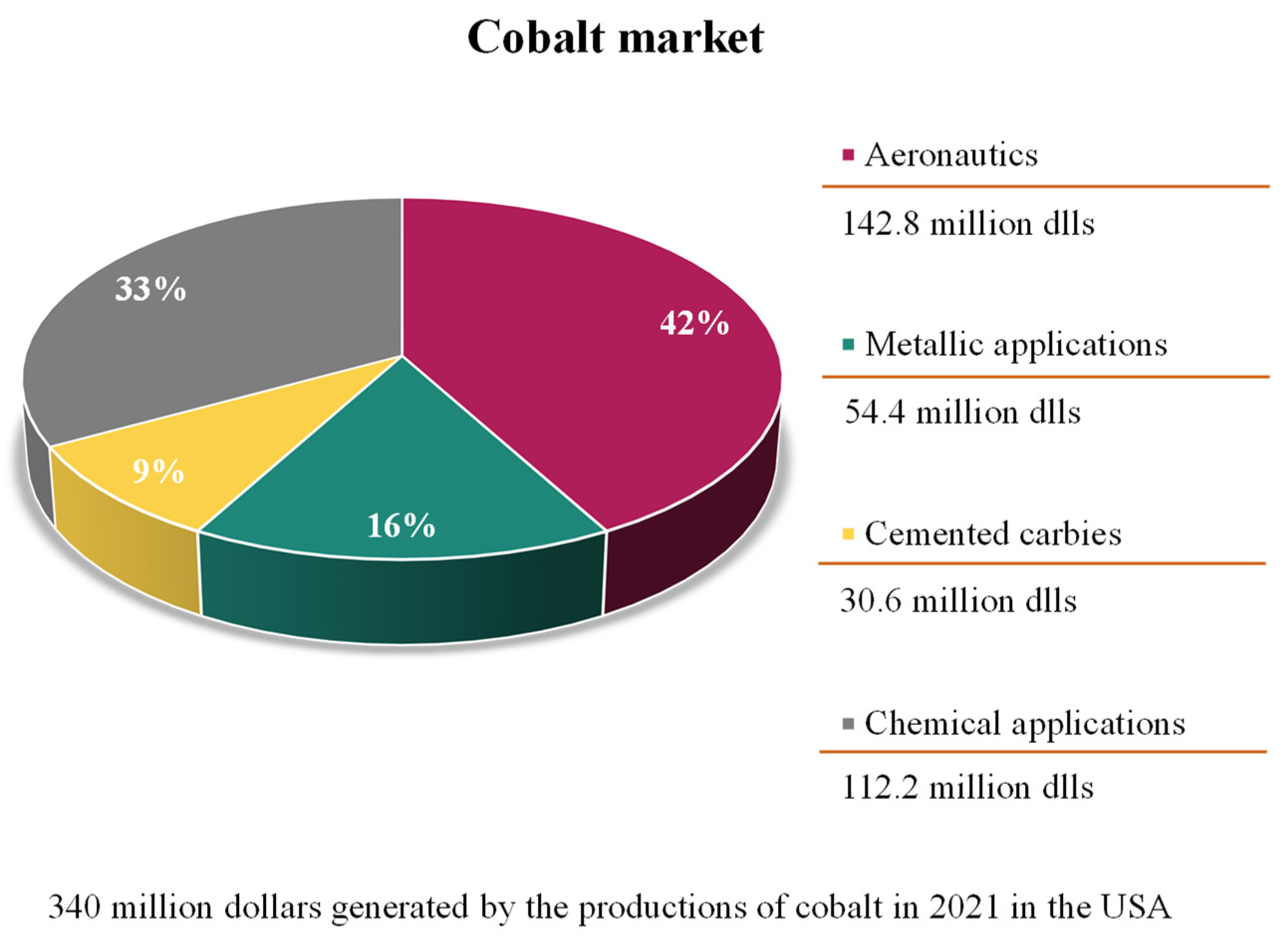
3. Cobalt metal market
4. Cobalt use
5. Cobalt geochemistry
6. Mineralogical properties of cobalt
6.1. Cobalt mineral
7. Geology and cobalt resources
7.1. Cobalt geology and type of mineral deposit
7.2. Cu-Co deposits hosted in stratiform sediments
7.3. Ni-Co laterite deposits
7.4. Ni-Cu-Co sulphide magmatic deposits
7.5. Cobalt mineral resources and ore reserves
- (a)
- Cu-Co deposits hosted in sediments or stratified shale (~58%), found mainly in the DRC [40].
- (b)
- Ni-Co lateritic deposits (~29%) located in Australia, New Caledonia, and Cuba [40].
- (c)
- Magmatic deposits of Ni-Cu-PGE-Co (~9%) found in Australia, Canada, Russia, Finland, and the United States. Based on the current resource estimates based on a geological endowment, if significant seabed resources had been included in this evaluation, they would represent about 80 % of total cobalt reserves [40].
7.6. Secondary Cobalt Resources
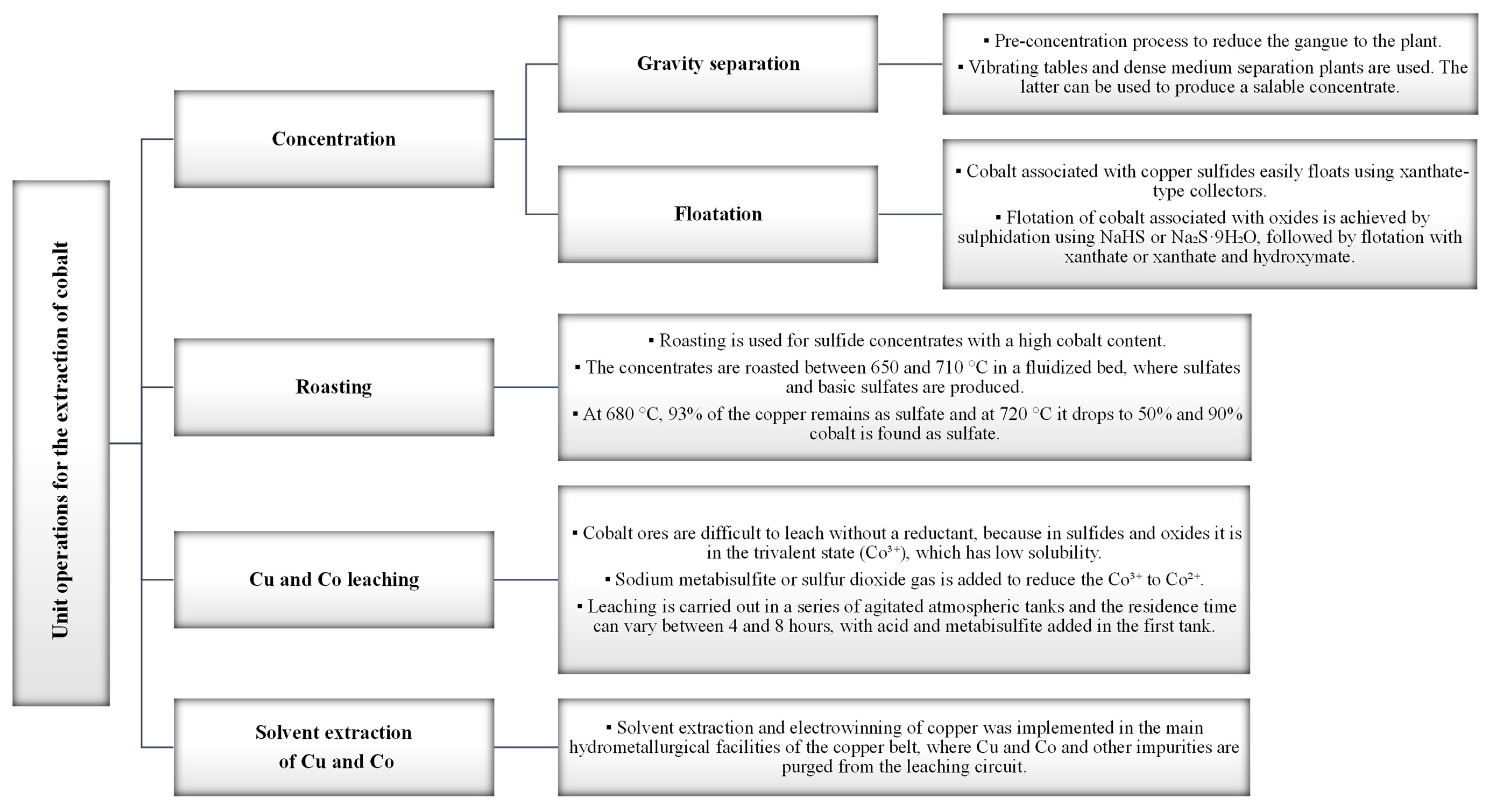
8. Cobalt processing
8.1. Recovery of cobalt from nickel-cobalt ores
8.2. Recovery of cobalt from copper-cobalt ores
8.3. Recovery of cobalt arsenides from Morocco
9. Cobalt recycling
5. Conclusions
References
- USGS. (2020). Mineral Commodity Summaries - Cobalt. [CrossRef]
- Schmidt, T., Buchert, M., & Schebek, L. (2016). Investigation of the primary production routes of nickel and cobalt products used for Li-ion batteries. Resour. Conserv. Recycl, 122, 107–122. [CrossRef]
- Darton Commodities Ltd. (2020a). Cobalt Market Review. Guildford.
- Roberts, S., & Gunn, G. (2014a). Cobalt (John Wiley & Sons, Ed.).
- Harper, E. M., Kavlak, G., & Graedel, T. E. (2012). Tracking the metal of the goblins: Cobalt’s cycle of use. Environ. Sci. Technol, 46, 1079–1086. [CrossRef]
- . Azevedo, M., Campagnol, N., Hagenbruch, T., Hoffman, K., Lala, A., & Ramsbottom, O. (2018). Lithium and cobalt - a tale of two commodities. Metals and Mining.
- Smith, C. (2001). Always the bridesmaid, never the bride: cobalt geology and resources. Transactions OfInstitution of Mining and Metallurgy Section B-Applied Earth Science, 110, 75–80. [CrossRef]
- Leblanc, M., & Billard, P. (1982). Cobalt arsenide orebodies related to an upper Proterozoic ophiolite: Bou Azzer (Morocco). Econ. Geol.
- Croxford, N. (1974). Cobalt mineralization at Mount Isa. Miner. Deposita, 9, 105–115. [CrossRef]
- BGS. (2017). World Mineral Production 2016.
- Cobalt Institute. (2022). Cobalt Market Report 2021 (Issue May).
- Chen, Z., Zhang, L., & Xu, Z. (2019). Tracking and Quantifying the Cobalt Flows in Mainland China during 1994-2016: Insights into Use, Trade and Prospective Demand. Sci. Total Environ, 672, 752–762. [CrossRef]
- Liu, K., Wang, Y., Long, H., Wu, Y., Cai, Y., & Jiang, J. (2021). Recovery of cobalt and nickel from magnesium-rich sulfate leach liquor with magnesium oxide precipitation method. Minerals Engineering, 169, 1–8. [CrossRef]
- Zeng, X., & Li, J. (2015). On the Sustainability of Cobalt Utilization in China. Resour. Conserv. Recycl, 104, 12–18. [CrossRef]
- Secretaría de Economía. (2022). Data México.
- Survey, U. S. G. (2022). Mineral Commodity Summaries 2022 (Issue 703).
- Kovacheva-Ninova, V., Savov, G., Vassileva, V., Vutova, K., Petrov, E., & Petrov, D. (2018). Trends in the development of cobalt production. Electrotech. Electron, 53, 849–894.
- Dehaine, Q., Flippov, L., Flippova, I., Tijsseling, L., & Glass, H. (2021). Novel approach for processing complex carbonate-rich copper-cobalt mixed ores via reverse flotation. Minerals Engineering, 160, 1–28. [CrossRef]
- USGS. (2022). National Minerals Information Center.
- Darton Commodities Ltd. (2020b). Darton Cobalt Market Research.
- Cobalt Development Institute. (2016). Cobalt Supply & Demand 2015. Cobalt Facts.
- Donaldson, J. D., & Beyersmann, D. (2005). Cobalt and Cobalt Compounds. In Wiley-VCH Verlag GmbH & Co. KGaA (Ed.), Ullmann’s Encyclopedia of Industrial Chemistry.
- Young, R. S. (1957). The geochemistry of cobalt. Geochim. Cosmochim. Acta 13, 28–41. [CrossRef]
- Rudnick, R. L., & Gao, S. (2013). Composition of the Continental Crust. In Treatise on Geochemistry (Second Edition, pp. 1–51). Elsevier.
- Carr, M. H., & Turekian, K. K. (1961). The geochemistry of cobalt. Geochim. Cosmochim. Acta 23, 9–60. [CrossRef]
- Abaide E. R, Anchieta C. G, Foletto V. S, Reinehr B, Nunes L. F, & Kuhn R. C. (2015). Production of copper and cobalt aluminate spinels and their application as supports for inulinase immobilization. Mat. Res, 18, 1062–1069. [CrossRef]
- Krauskopf, K. B., & Bird, D. K. (1995). Distribution of the elements. Introduction to Geochemistry. McGraw-Hill.
- Gülaçar, O. F., & Delaloye, M. (1976). Geochemistry of nickel, cobalt and copper in alpine-type ultramafic rocks. Chem. Geol, 17, 269–280. [CrossRef]
- Lafuente, B., Downs, R. T., Yang, H., & Stone, N. ,. (2015). The power of databases: The RRUFF project. In T Armbruster and R M Danisi (Ed.), Highlights in Mineralogical Crystallography (pp. 1–29).
- Hazen, R. M., Hystad, G., Golden, J. J., Hummer, D. R., Liu, C., Downs, R. T., Morrison, S. M., Ralph, J., & Grew, E. S. (2017). Cobalt mineral ecology. . Am. Mineral, 102, 108–116. [CrossRef]
- Gauthier, G., & Deliens, M. (1999). Cobalt minerals of the Katanga Crescent, Congo. The Mineralogical Record 30, 255–256.
- Pirard, C., & Hatert, F. (2008). The sulfides and selenides of the Musonoï mine, Kolwezi, Katanga, Democratic Republic of Congo. Can. Mineral, 46, 219–231.
- Decrée, S., Pourret, O., & Baele, J. M. (2015). Rare earth element fractionation in heterogenite (CoOOH): Implication for cobalt oxidized ore in the Katanga Copperbelt (Democratic Republic of Congo). J. Geochem. Explor, 159, 290–301. [CrossRef]
- Vanbrabant, Y., Burlet, C., & Louis, P. (2013). Mineralogical characterization of cobaltic oxides from the democratic republic of congo. In H. Springer (Ed.), Ni-Co 2013 (pp. 243–254). [CrossRef]
- Llorca, S., & Monchoux, P. (1991). Supergene cobalt minerals from New Caledonia. Can. Mineral. , 29, 149–161. (.
- Glasby, G. P., Ren, X., Shi, X., & Pulyaeva, I. A. (2007). Co-rich Mn crusts from the Magellan Seamount cluster. The Long Journey through Time. Geo-Mar. Lett. 27, 315–323.
- Chukhrov, F. V., Gorshkov, A. I., Sivtsov, A. V., & Berezovskaya, V. V. (1985). The Nature and Genesis of Lithiophorite. Int. Geol. Rev, 27, 348–361. [CrossRef]
- Tindall, G. P., & Muir, D. M. (1996). Transformation of iron oxide in nickel laterite processing. . Iron Control and Disposal , 249–262.
- Manceau, A., Llorca, S., & Calas, G. (1987). Crystal chemistry of cobalt and nickel in lithiophorite and asbolane from New Caledonia. Geochim. Cosmochim. Acta 51, 105–113. [CrossRef]
- Slack, J. F., Kimball, B. E., & Shedd, K. B. (2017). Cobalt. In K. J. , D. J. H. , S. R. R. , B. D. C. Schulz (Ed.), Critical Mineral Resources of the United States—Economic and Environmental Geology and Prospects for Future SupplyReston (pp. 1–40).
- Crockett, R. N., Chapman, G. R., & Forrest, M. D. (1987). International Strategic Minerals Inventory Summary Report -Cobalt, US Geological Survey Circular 930. F. US Geological Survey. [CrossRef]
- Mudd, G. M., Weng, Z., Jowitt, S. M., Turnbull, I. D., & Graedel, T. E. (2013). Quantifying the recoverable resources of by-product metals: The case of cobalt. . Ore Geol. Rev., 55, 87–98. [CrossRef]
- Petavratzi, C. E., Gunn, G., & Kresse, C. (2019). Commodity Review: Cobalt.
- Sverdrup, H. U., Ragnarsdottir, K. V., & Koca, D. (2017). Integrated Modelling of the Global Cobalt Extraction, Supply, Price and Depletion of Extractable Resources Using the WORLD6 Model. BioPhysical Economics and Resource Quality, 2, 4. [CrossRef]
- Cailteux, J. L. H., Kampunzu, A. B., Lerouge, C., Kaputo, A. K., & Milesi, J. P. (2005). Genesis of sediment-hosted stratiform copper - cobalt deposits, central African Copperbelt. J. Afr. Earth Sc, 42, 134–158.
- Cailteux, J. L. H., Kampunzu, A. B. H., & Batumike, M. J. (2005). Lithostratigraphic position and petrographic characteristics of R.A.T. (“Roches Argilo-Talqueuses”) Subgroup, Neoproterozoic Katangan Belt (Congo). J. Afr. Earth Sc, 42, 82–94. [CrossRef]
- Kampunzu, A. B., Tembo, F., Matheis, G., Kapenda, D., & Huntsman-Mapila, P. (2000). Geochemistry and Tectonic Setting of Mafic Igneous Units in the Neoproterozoic Katangan Basin, Central Africa: Implications for Rodinia Break-up. Gondwana Res., 3, 125–153. [CrossRef]
- Crundwell, F. K., Moats, M., Ramachandran, V., Robinson, T. G., & Davenport, W. G. (2011). Production of Cobalt from the Copper–Cobalt Ores of the Central African Copperbelt. In Extractive Metallurgy of Nickel, Cobalt and Platinum Group Metals (pp. 377–391).
- Dewaele, S., Muchez, P., Vets, J., Fernandez-Alonzo, M., & Tack, L. (2006). Multiphase origin of the Cu-Co ore deposits in the western part of the Lufilian fold-and-thrust belt, Katanga (Democratic Republic of Congo). J. Afr. Earth Sc, 46, 455–469.
- Guilbert, J. M., & Park, C. F. J. (2007). The Geology of Ore Deposits. . Waveland Press.
- Berger, V. I., Mosier, D. L., Bliss, J. D., & Moring, B. C. (2014). Sediment-hosted gold deposits of the world: database and grade and tonnage models. Open-File Report 51. [CrossRef]
- Gleeson, S. A., Butt, C. R. M., & Elias, M. (2003). Nickel laterites, a review. SEG Newsletter, 54, 11–18.
- Robb, L. (2005). Introduction to ore- forming processes.. Journal of Chemical Information and Modeling.
- Naldrett, A. J. (1999). World-class Ni-Cu-PGE deposits: Key factors in their genesis.. Miner. Deposita, 34, 227–240. [CrossRef]
- Lesher, C. M., & Keays, R. R. (2002). Komatiite-associated Ni-Cu-PGE deposits: Geology, mineralogy, geochemistry and genesis. In L. (Ed. ) abri (Ed.), he Geology, Geochemistry, Mineralogy and Mineral Beneficiation of the Platinum-Group Elements, Canadian Institute Mineral Metallurgy Petroleum (pp. 579–618).
- Crundwell, F. K., Moats, M. S., Ramachandran, V., & Robinson, T. G. (2011). Extractive Metallurgy of Nickel, Cobalt and Platinum-Group Metals . Elsevier. [CrossRef]
- Huang, Y., Zhang, Z., Cao, Y., Han, G., Peng, W., Zhu, X., Zhang, T. an, & Dou, Z. (2020). Overview of cobalt resources and comprehensive analysis of cobalt recovery from zinc plant purification residue- a review. Hydrometallurgy, 193. [CrossRef]
- Alvial-Hein, G., Mahandra, H., & Ghahreman, A. (2021). Separation and recovery of cobalt and nickel from end-of-life products via solvent extraction technique: A review. Journal of Cleaner Production, 297. [CrossRef]
- Nayaka, G. P., Pai, K. V, Santhosh, G., & Manjanna, J. (2016). Recovery of cobalt as cobalt oxalate from spent lithium-ion batteries by using glycine as leaching agent. Journal of Environmental Chemical Engineering, 4(2), 2378–2383. [CrossRef]
- Peeters, N., Binnemans, K., & Riaño, S. (2020). Solvometallurgical recovery of cobalt from lithium-ion battery cathode materials using deep-eutectic solvents. Green Chemistry, 22(13), 4210–4221. [CrossRef]
- Quintero-Almanza, D., Gamiño-Arroyo, Z., Sánchez-Cadena, L. E., Gómez-Castro, F. I., Uribe-Ramírez, A. R., Aguilera-Alvarado, A. F., & Ocampo Carmona, L. M. (2019). Recovery of cobalt from spent lithium-ion mobile phone batteries using liquid–liquid extraction. Batteries, 5(2), 44. [CrossRef]
- Peeters, N., Binnemans, K., & Riaño, S. (2022). Recovery of cobalt from lithium-ion battery cathode material by combining solvoleaching and solvent extraction. Green Chemistry, 24(7), 2839–2852. [CrossRef]
- Liu, F., Peng, C., Porvali, A., Wang, Z., Wilson, B. P., & Lundström, M. (2019). Synergistic recovery of valuable metals from spent nickel–metal hydride batteries and lithium-ion batteries. . ACS Sustainable Chemistry & Engineering, 7(19), 16103–16111. [CrossRef]
- Weshahy, A. R., Sakr, A. K., Gouda, A. A., Atia, B. M., Somaily, H. H., Hanfi, M. Y., Sayyed, M. I., El-Sheikh, R., El-Sheikh, E. M., Radwan, H. A., Cheira, M. F., & Gado, M. A. (2022). Selective Recovery of Cadmium, Cobalt, and Nickel from Spent Ni–Cd Batteries Using Adogen® 464 and Mesoporous Silica Derivatives. . International Journal of Molecular Sciences., 23, 8677.
- Petranikova, M., Ebin, B. , & Tunsu, C. (2019). Selective recovery of cobalt from the secondary streams after NiMH batteries processing using Cyanex 301. . Waste Management, 83, 194–201. [CrossRef]
- Agarwal, V., Khalid, M. K., Porvali, A., Wilson, B. P., & Lundström, M. (2019). Recycling of spent NiMH batteries: Integration of battery leach solution into primary Ni production using solvent extraction. . Sustainable Materials and Technologies, 22. [CrossRef]
- Stopić, S., & Fridrih, B. (2020). Recovery of cobalt from primary and secondary materials-an overiew. . Vojnotehnički Glasnik/Military Technical Courier, 68(2), 321–337.
- Moats, M. S., & Davenport, W. G. (2014). Nickel and Cobalt Production. In Treatise on Process Metallurgy (Vol. 3, pp. 625–669). Elsevier Ltd. [CrossRef]
- Morcali, M. H., Khajavi, L. T., & Dreisinger, D. B. (2017). Extraction of nickel and cobalt from nickeliferous limonitic laterite ore using borax containing slags. International Journal of Mineral Processing, 167, 27–34. [CrossRef]
- Butt, C. R. M., & Cluzel, D. (2013). Nickel laterite ore deposits: Weathered serpentinites. Elements, 9(2), 123–128. [CrossRef]
- Georgiou, D., & Papangelakis, V. G. (2004). Characterization of limonitic laterite and solids during sulfuric acid pressure leaching using transmission electron microscopy. Minerals Engineering, 17(3), 461–463. 3. [CrossRef]
- Ribeiro, P. P. M., Neumann, R., Santos, I. D. dos, Rezende, M. C., Radino-Rouse, P., & Dutra, A. J. B. (2019). Nickel carriers in laterite ores and their influence on the mechanism of nickel extraction by sulfation-roasting-leaching process. Minerals Engineering, 131, 90–97. [CrossRef]
- Kursunoglu, S., Ichlas, Z. T., & Kaya, M. (2017). Leaching method selection for Caldag lateritic nickel ore by the analytic hierarchy process (AHP). Hydrometallurgy, 171, 179–184. [CrossRef]
- Kursunoglu, S., Ichlas, Z. T., & Kaya, M. (2018). Dissolution of lateritic nickel ore using ascorbic acid as synergistic reagent in sulphuric acid solution. Transactions of Nonferrous Metals Society of China (English Edition), 28(8), 1652–1659. [CrossRef]
- Li, J., Bunney, K., Watling, H. R., & Robinson, D. J. (2013). Thermal pre-treatment of refractory limonite ores to enhance the extraction of nickel and cobalt under heap leaching conditions. Minerals Engineering, 41, 71–78. [CrossRef]
- Liu, K., Wang, Y., Long, H., Cheng, Y., Wu, Y., Cai, Y., & Jiang, J. (2021). Recovery of cobalt and nickel from magnesium-rich sulfate leach liquor with magnesium oxide precipitation method. Minerals Engineering, 169. [CrossRef]
- Feijoo, G. C., Barros, K. S., Scarazzato, T., & Espinosa, D. C. R. (2021). Electrodialysis for concentrating cobalt, chromium, manganese, and magnesium from a synthetic solution based on a nickel laterite processing route. Separation and Purification Technology, 275. [CrossRef]
- Ilyas, S., Srivastava, R. R., Kim, H., Ilyas, N., & Sattar, R. (2020). Extraction of nickel and cobalt from a laterite ore using the carbothermic reduction roasting-ammoniacal leaching process. Separation and Purification Technology, 232. [CrossRef]
- Eksteen, J. J., Oraby, E. A., & Nguyen, V. (2020). Leaching and ion exchange based recovery of nickel and cobalt from a low grade, serpentine-rich sulfide ore using an alkaline glycine lixiviant system. Minerals Engineering, 145. [CrossRef]
- Crundwell, F. K., du Preez, N. B., & Knights, B. D. H. (2020). Production of cobalt from copper-cobalt ores on the African Copperbelt – An overview. Minerals Engineering, 156. [CrossRef]
- Ntakamutshi, P. T., Kime, M. B., Mwema, M. E., Ngenda, B. R., & Kaniki, T. A. (2017). Agitation and column leaching studies of oxidised copper-cobalt ores under reducing conditions. Minerals Engineering, 111, 47–54. [CrossRef]
- Clotilde Apua, M., & Madiba, M. S. (2021). Leaching kinetics and predictive models for elements extraction from copper oxide ore in sulphuric acid. Journal of the Taiwan Institute of Chemical Engineers, 121, 313–320. [CrossRef]
- Zhang, C., Guo, X., Yu, D., Tian, Q., & Cui, F. (2021). Treatment of copper-cobalt alloy with molten magnesium for metal extraction. Journal of Alloys and Compounds, 874. [CrossRef]
- Zhang, M., Zhu, G., Zhao, Y., & Feng, X. (2012). A study of recovery of copper and cobalt from copper-cobalt oxide ores by ammonium salt roasting. Hydrometallurgy, 129–130, 140–144. [CrossRef]
- Giebner, F., Kaden, L., Wiche, O., Tischler, J., Schopf, S., & Schlömann, M. (2019). Bioleaching of cobalt from an arsenidic ore. Minerals Engineering, 131, 73–78. [CrossRef]
- Johnson, D. B., Dybowska, A., Schofield, P. F., Herrington, R. J., Smith, S. L., & Santos, A. L. (2020). Bioleaching of arsenic-rich cobalt mineral resources, and evidence for concurrent biomineralisation of scorodite during oxidative bio-processing of skutterudite. Hydrometallurgy, 195. [CrossRef]
- NS Energy. (2023a). Kamoto Mine.
- NS Energy. (2023b). Tenke Fungurume Copper-Cobalt Mine.
- ERG Africa. (2023). Metalkol RTR.
- Shalina resources. (2023). Geology.
- Shalina resources. (2023a). Etoile mine.
- Shalina resources. (2023c). Process flow.
- Mining Data Solutions. (2023). Ruashi Mine.
- USGS. (2023). Mutoshi.
- Mining Data Solutions. (2023). Ramu Mine.
- Mining Data Solutions. (2023a). Murrin Murrine Mine.
- Mining Techonology. (2023). Murrin Murrin nickel-cobalt project.
- NS Energy. (2023b). Projects.
- Mining Data Solutions. (2023c). Taganito Mine.
- Arnim von Gleich, & Bremen, U. (2006). Outlines of a sustainable metals. In Sustainable Metals Management (pp. 1–37).
- Korhonen, J., Nuur, C., Feldmann, A., & Birkie, S. E. (2018). Circular economy as an essentially contested concept. Journal of Cleaner Production, 175, 544–552. [CrossRef]
- Ulusoy, U. (2019). Review of the Recovery of Cobalt from Secondary Resources.. Critical and Rare Earth Elements (Issue December). [CrossRef]
- Chandra, M., Yu, D., Tian, Q., & Guo, X. (2022). Recovery of Cobalt from Secondary Resources: A Comprehensive Review. . Mineral Processing and Extractive Metallurgy Review. [CrossRef]
- Hamza, M. F., Roux, J. C., & Guibal, E. (2019). Metal valorization from the waste produced in the manufacturing of Co/Mo catalysts: leaching and selective precipitation. Journal of Material Cycles and Waste Management, 21(3), 525–538. [CrossRef]
- Chae, S., Yoo, K., Tabelin, C. B., & Tabelin, C. B. (2020). Hydrochloric acid leaching behaviors of copper and antimony in speiss obtained from top submerged lance furnace. Metals, 10(10), 1–8. [CrossRef]
- Xue, M., Kendall, A., Xu, Z., & Schoenung, J. M. (2015). Waste management of printed wiring boards: A life cycle assessment of the metals recycling chain from liberation through refining. Environmental Science and Technology, 49(2), 940–947. [CrossRef]
- Cheng, J., Lu, T., Wu, X., Zhang, H., Zhang, C., Peng, C. A., & Huang, S. (2019). Extraction of cobalt(ii) by methyltrioctylammonium chloride in nickel(ii)-containing chloride solution from spent lithium ion batteries. RSC Advances, 9(39), 22729–22739. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions, and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
| Rock type | Co content (ppm) | Ni/Co ratio | Cu/Co ratio | Reference |
|---|---|---|---|---|
| Igneous rocks Ultramafic | 200 | 10 | 0.1 | [27] |
| Dunita | 108.6 | 21.5 | 0.2 | [28] |
| Pyroxenite | 55.2 | 8.1 | 5.1 | [28] |
| Serpentinite | 115.1 | 18.2 | 0.7 | [28] |
| Mafic | 45 | 3.6 | 2.2 | [27] |
| Gabbro | 51 | 2.6 | - | [22] |
| Basalt | 41 | 2.5 | - | [22] |
| Diabase | 47 | 1.6 | 2.3 | [27] |
| Intermediate igneous rocks | 10 | 5.5 | 3.5 | [27] |
| Felsic | 5 | 1.6 | 4 | [27] |
| Granite | 47 | 0.4 | 5.4 | [27] |
| Shales | 19 | 3.6 | 2.4 | [27] |
| Sandstone | 0.3 | 6.7 | 1 | [27] |
| Carbonates | 0.1 | 200 | 1 | [27] |
| Metamorphic rocks | 40 | - | - | [25] |
| Quartzite | 0.3 | - | - | [25] |
| Operation name | Type of deposit | Country | Tonnage (Mt) | Co grade (%) | Co (kt) |
|---|---|---|---|---|---|
| BOSS Mining | SSH | DRC | 75 | 0.2 | 150 |
| Comide | SSH | DRC | 53 | 0.2 | 106 |
| Etoile | SSH | DRC | 21 | 0.4 | 88 |
| KCCd | SSH | DRC | 574 | 0.51 | 2908 |
| Musonoi | SSH | DRC | 32 | 0.9 | 289 |
| Mutanda | SSH | DRC | 918 | 0.39 | 3584 |
| Mutoshi | SSH | DRC | 300 | ||
| Ruashi | SSH | DRC | 33 | 0.3 | 100 |
| Tenke Fungurume | SSH | DRC | 1015 | 0.29 | 2919 |
| Sotkamo | SSH | Finland | 1525 | 0.02 | 290 |
| Boleo | SSH | Mexico | 424 | 0.05 | 221 |
| Bornite | SSH | USA | 182 | 0.02 | 35 |
| Iron Creek | SSH | USA | 4 | 0.24 | 12 |
| Konkola (KCM) | SSH | Zambia | 12 | 0.34 | 42 |
| Mopani | SSH | Zambia | 361 | 0.08 | 289 |
| Nova-Bollinger | Magmatic | Australia | 24 | 0.07 | 16 |
| Fortaleza de Minas | Magmatic | Brazil | 10 | 0.2 | 21 |
| Santa Rita | Magmatic | Brazil | 159 | 0.02 | 24 |
| Raglan | Magmatic | Canada | 46 | 0.07 | 31 |
| Sudbury (Glencore) | Magmatic | Canada | 69 | 0.04 | 28 |
| Sudbury (Vale) | Magmatic | Canada | 61 | 0.03 | 19 |
| Thompson | Magmatic | Canada | 39 | 0.09 | 35 |
| Voisey’s Bay | Magmatic | Canada | 55 | 0.09 | 49 |
| Jinchuan | Magmatic | China | 515 | 0.02 | 98 |
| Kevitsa | Magmatic | Finland | 286 | 0.01 | 30 |
| Sakatti | Magmatic | Finland | 44 | 0.05 | 20 |
| Noril’sk area | Magmatic | Rusia | 1309 | 0.06 | 785 |
| Nkomati | Magmatic | South Africa | 302 | 0.02 | 60 |
| Kabanga | Magmatic | Tanzania | 58 | 0.2 | 116 |
| Eagle | Magmatic | USA | 4 | 0.067 | 4 |
| Murrin-Murrin | Laterite | Australia | 332 | 0.08 | 261 |
| Musgrave | Laterite | Australia | 25 | 0.07 | 151 |
| Owendale | Laterite | Australia | 16 | 0.12 | 20 |
| Ravensthorpe | Laterite | Australia | 535 | 0.03 | 149 |
| Sunrise | Laterite | Australia | 101 | 0.12 | 129 |
| Barro Alto | Laterite | Brazil | 86 | - | - |
| Jacaré | Laterite | Brazil | 185 | 0.19 | 352 |
| Niquelandia | Laterite | Brazil | 56 | 0.06 | 34 |
| Vermelho | Laterite | Brazil | 148 | 0.05 | 74 |
| Musongati | Laterite | Burundi | 150 | 0.09 | 135 |
| Nkamouna | Laterite | Cameroon | 391 | 0.22 | 860 |
| Cerro Matoso | Laterite | Colombia | 95 | 0.1 | 95 |
| Moa Bay | Laterite | Cuba | 89 | 0.12 | 110 |
| Cyclops Ni-Co | Laterite | Indonesia | 37 | 0.11 | 41 |
| Sorowako | Laterite | Indonesia | 116 | - | - |
| Ambatovy-Analamay | Laterite | Madagascar | 251 | 0.08 | 201 |
| Goro | Laterite | New Caledonia | 323 | 0.11 | 355 |
| Ramu | Laterite | Papua New Guinea | 175 | 0.1 | 175 |
| Agata | Laterite | Philippines | 45 | 0.05 | 23 |
| Berong | Laterite | Philippines | 18 | 0.08 | 15 |
| Mindoro | Laterite | Philippines | 315 | 0.06 | 189 |
| Rio Tuba (Coral Bay) | Laterite | Philippines | 57 | 0.012 | 69 |
| Taganito/Adlay | Laterite | Philippines | 123 | 0.012 | 146 |
| Çaldag | Laterite | Turkey | 37 | 0.05 | 119 |
| Olympic Dam | Hydro & volc | Australia | 605 | 0.02 | 121 |
| NICO | Hydro & volc | Canada | 64 | 0.012 | 74 |
| Kylylahti | Hydro & volc | Finland | 8 | 0.012 | 10 |
| Rompas-Rajapalot | Hydro & volc | Finland | 4 | 0.04 | 2 |
| Bou Azzer (district) | Hydro & volc | Morocco | 45 | 1.5 | 686 |
| Blackbird (district) | Hydro & volc | USA | 16 | 0.74 | 124 |
| Idaho Cobalt Operation | Hydro & volc | USA | 5 | 0.55 | 31 |
| DRC, Democratic Republic of the Congo | |||||
| USA, United State of America | |||||
| Type of waste | Leaching reagents | Extractant | Conditions | Co species extracted | Extraction | Reference |
|---|---|---|---|---|---|---|
| Lithium ion battery(LIB) | Glycine (0.5 M), Absorbic Acid (0.02) | - | 80˚ C, 360 min | Co (II) | 95% | [59] |
| Cathode materials for lithium ion batteries | Choline Chloride Citric Acid DES | LIX 984 / Aliquat 336 | 40˚ C, 60 min | Co (II) | 81% | [60] |
| Used Lithium Ion Batteries For Mobile Phones | Sulfuric acid and hydrogen peroxide | Cyanex 272 dissolved in kerosene | 75˚ C, 90 min | Co | 97-99% | [61] |
| Lithium ion battery(LIB) | - | Di-(2-ethylhexyl) phosphoric acid (D2EHPA) | 80˚ C, 360 min | Co | 90% | [62] |
| NaOH y Na₂SO₄ | Cynaex 272 | 70˚ C, 60 min | Co | 98% | [63] | |
| Nickel-metal hydride (NiMH) batteries | ||||||
| Used Ni-Cd Batteries | H₂SO₄ | Adogen® 464 | 80˚ C, 360 min | Co(OH)₂ | 100% | [64] |
| Used NiMH Batteries | - | Cyanex 301 | 23˚C, 10 min | Co | 79.60% | [65] |
| Solvent 70 (Statoil) | ||||||
| Used NiMH Batteries | Di-2-ethylheixl phosphoric acid (D2EHPA) | - | 50˚ C, 15 min | Co (II) | 3,7 g/L | [66] |
| Company | Location |
|---|---|
| Umicore | Belgium |
| Xstrata Nickel | Canada |
| Accurec | Germany |
| Inmetco | USA |
| S.N.A.M | France |
| Sony-Sumitomo | Japan |
| USA, United State of America | |
| Company | Total production, t | Location | Project type | Reserves, Mt | Grade, Co | Products | Reference |
|---|---|---|---|---|---|---|---|
| Kamoto | 23,900 | Lualaba, DRC | Subterranean mine | 26,6 | 0,54 % | Copper and cobalt | [87,88] |
| Tenke Fungurume | 15,440 | Lualaba, DRC | Open sky | 176,8 | 0,30 % | Cu cathode and Co hydroxide | [87,89] |
| Metalkol RTR | 10,500 | Haut-Katanga, DRC | Tailings | - | - | Co hydroxide and metallic Co | [87,90] |
| Etoile | 7,000 | Katanga, DRC | Open sky | 648,046 | 0,35% | Copper and cobalt ore | [87,91,92,93] |
| Luiswishi | 5,390 | Katanga, DRC | Open sky | 12,4 | 0,95% | - | [87] |
| Ruashi | 5,090 | DRC | Open sky | 5,5 | 0,2% | Co hydroxide | [94] |
| Lubumbashi Slag Hill | 4,000 | DRC | - | - | - | Co alloy and Zn oxide powder | [87] |
| Mutoshi | 4,000 | DRC | Surface mining | - | - | - | [95] |
| Cuba’s Moa Bay | 3,370 | Cuba | Open sky | - | - | - | [87] |
| Papua New Guinea’s Ramu | 2,940 | New Guinea | Open sky | - | 0.09% | Co and Ni metal concentrate | [96] |
| Murrin Murrin Mine | 2,900 | Australia | Open sky | 145 | 0,085% | Cobalt and nickel | [97,98] |
| Ambatovy | 2,860 | Madagascar | Open sky | 152.1 | - | Co concentrates | [99] |
| Polar Division | 2,700 | Russia | - | - | - | - | [87] |
| The Phillipines Taganito | 2,550 | Philippines | Open sky | - | - | Mixed nickel-cobalt sulfide | [99] |
| Morocco’s Bou-Azzer | 2,420 | Morocco | - | - | - | - | [87] |
| t, ton | |||||||
| DRC, Democratic Republic of the Congo | |||||||
| Mt, Millions of tons | |||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





