Submitted:
19 June 2023
Posted:
19 June 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Milk
2.1.2. Ultrafiltration membrane
2.1.3. Starters for yoghurt’s production
2.2. Methods
2.2.1. Growth of bacteria
2.2.2. Ultrafiltration
2.2.3. Yoghurts preparation
- Sample A - yoghurt from goat’s milk without membrane concentration (control sample);
- Sample B - yoghurt from double concentrated goat’s milk.
- Sample C - yoghurt from triple concentrated goat’s milk.
2.2.4. Physicochemical analyses
2.2.5. Microbiological analyses
2.2.6. Sensory analyses
2.2.7. Rheological analysis
2.2.8. Statistical Analysis
3. Results
3.1. Physicochemical properties of goat’s milk, retentates and permeate
3.2. Specific microorganisms
3.3. Chemical composition of yoghurts
3.4. Microbiological growth in yoghurts during storage
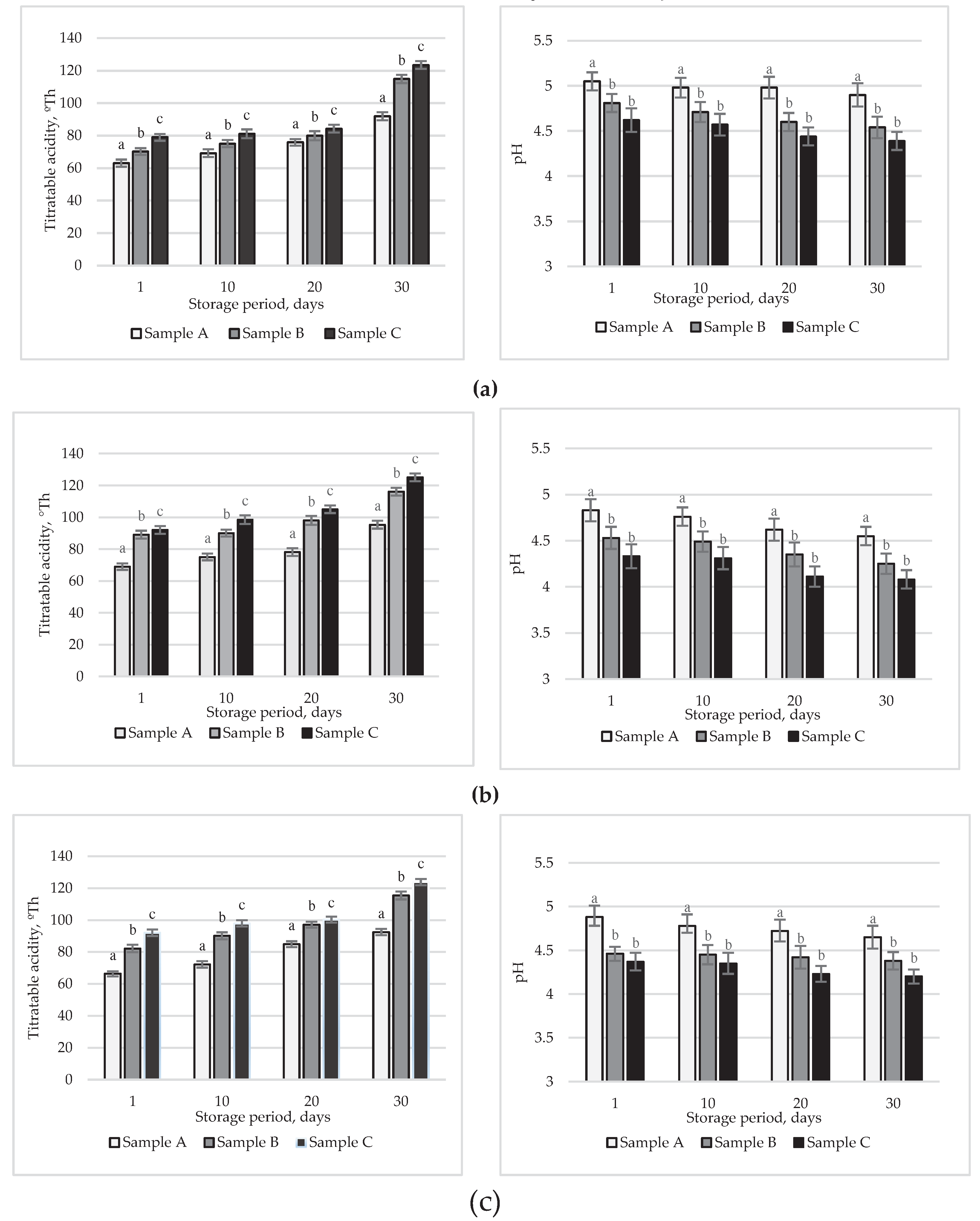
3.6. Titratable and active acidity of yoghurts during storage
3.7. Organoleptic characteristics of yoghurts
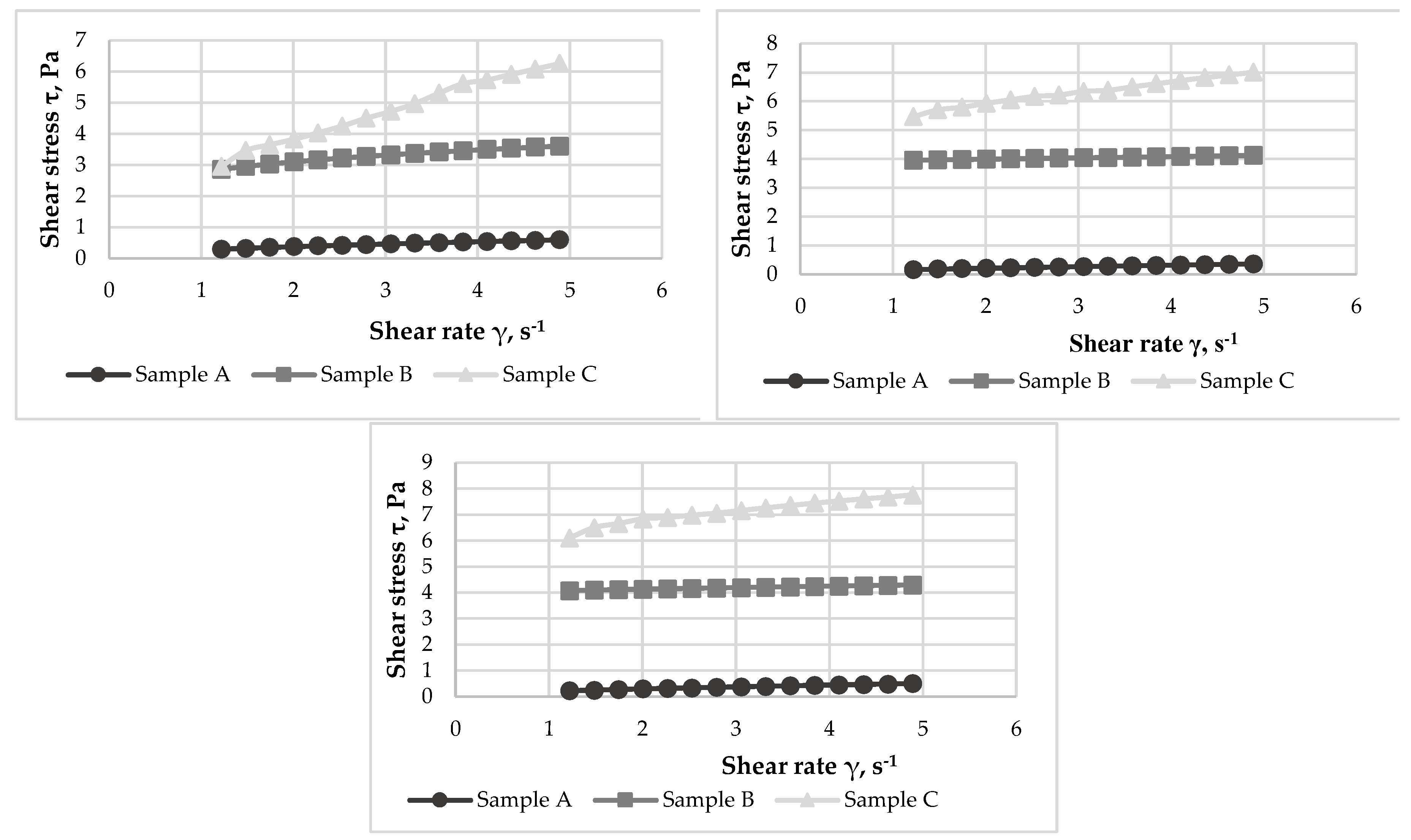
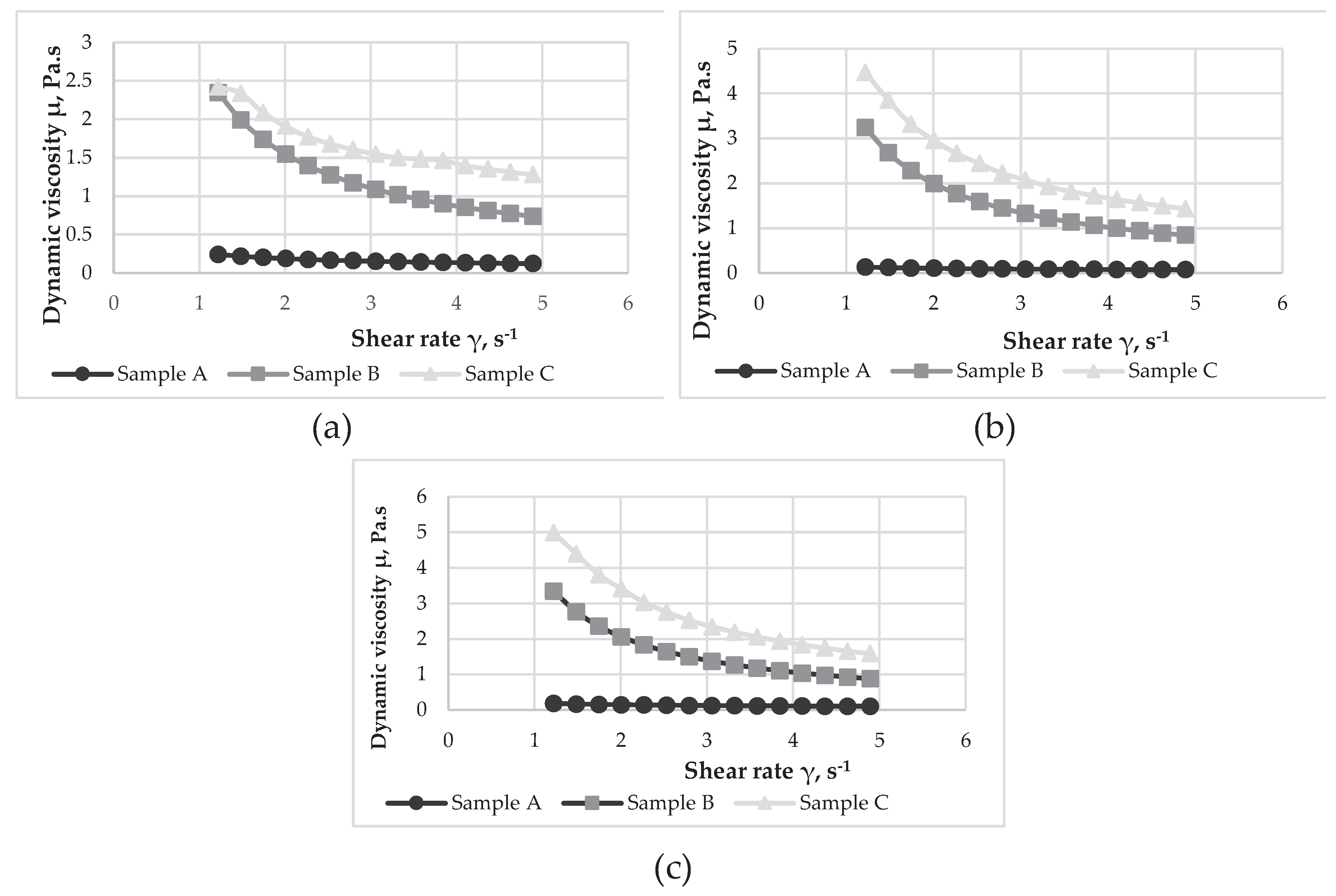
3.8. Rheological properties of yoghurts
4. Discussion
5. Conclusion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gasmalla, M.; Tessema, H.; Salaheldin, A.; Alahmad, K.; Hassanin, H.A.M.; Aboshora, W. Health benefits of milk and functional dairy products. MOJ Food Process. Technol. 2017, 4, 108–111. [Google Scholar] [CrossRef]
- Górska-Warsewicz, H.; Rejman, K.; Laskowski, W.; Czeczotko, M. Milk and dairy products and their nutritional contribution to the average polish diet. Nutrients 2019, 11, 1771. [Google Scholar] [CrossRef] [PubMed]
- Grosso, G. Are there any concerns about dairy food consumption and cardiovascular health. Int. J. Food Sci. Nutr. 2021, 72, 429–431. [Google Scholar] [CrossRef] [PubMed]
- Olson, D.W.; Aryana, K.A. Probiotic incorporation into yogurt and various novel yogurt-based products. Appl. Sci. 2022, 12, 12607. [Google Scholar] [CrossRef]
- Rozenberg, S.; Body, J.-J.; Bruyère, O.; Bergmann, P.; Brandi, M.L.; Cooper, C.; Devogelaer, J.-P.; Gielen, E.; Goemaere, S.; Kaufman, J.-M.; et al. Effects of dairy products consumption on health: Benefits and beliefs—A commentary from the Belgian bone club and the European Society for clinical and economic aspects of osteoporosis, osteoarthritis and musculoskeletal diseases. Calcif. Tissue Int. 2016, 98, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Zare, F.; Boye, J.I.; Orsat, V.; Champagne, C.; Simpson, B.K. Microbial, physical and sensory properties of yoghurt supplemented with lentil flour. Food Res. J. 2011, 44, 2482–2488. [Google Scholar] [CrossRef]
- Arioui, F.; Saada, D.A.; Cheriguene, A. Physicochemical and sensory quality of yogurt incorporated with pectin from peel of Citrus sinensis. Food Sci. Nutr. 2017, 5, 358–364. [Google Scholar] [CrossRef]
- Kang, S.S.; Kim, M.K.; Kim, Y.J. Comprehensive evaluation of microbiological and physicochemical properties of commercial drinking yogurts in Korea. Food Sci. Anim. Resour. 2019, 39, 820–830. [Google Scholar] [CrossRef]
- Senadeera, S.S.; Prasanna, P.H.P.; Jayawardana, N.W.I.A.; Gunasekara, D.C.S.; Senadeera, P.; Chandrasekara, A. Antioxidant, physicochemical, microbiological, and sensory properties of probiotic yoghurt incorporated with various Annona species pulp. Heliyon 2018, 4, e00955. [Google Scholar] [CrossRef]
- Temerbayeva, M.; Rebezov, M.; Okuskhanova, E.; Zinina, O.; Gorelik, O.; Vagapova, O.; Beginer, T.; Gritsenko, S.; Serikova, A.; Yessimbekov, Z. Development of yoghurt from combination of goat and cow milk. Annu. Res. Rev. Biol. 2018, 23, 1–7. [Google Scholar] [CrossRef]
- Santis, D.; Giacinti, G.; Chemello, G.; Frangipane, M.T. Improvement of the sensory characteristics of goat milk yogurt. J. Food Sci. 2019, 84, 2289–2296. [Google Scholar] [CrossRef]
- Damunupola, D.A.; Weerathilake, A.D.V.; Sumanasekara, G.S. Evaluation of quality characteristics of goat milk yogurt incorporated with beetroot juice. Int. J. Sci. Res. Publ. 2014, 4, 1–5. [Google Scholar]
- Tafes, A.G. Compositional and technological properties of goat milk and milk products A review. Concepts Dairy Vet. Sci. 2020, 3, 295–300. [Google Scholar]
- Wang, C.; Zhu, Y.; Wang, J. Comparative Study on the Heat Stability of Goat Milk and Cow Milk. Indian J. Anim. Res. 2016, 50, 610–613. https://www.arccjournals.com/journal/indian-journal-of-animal-research/B-320. [CrossRef]
- Stack, H.; Kearney, N.; Stanton, C.; Fitzgerald, G.; Ross, R. Association of beta-glucan endogenous production with increased stresstolerance of intestinal lactobacilli. Appl. Environ. Microbiol. 2010, 76, 500–507. [Google Scholar] [CrossRef]
- Tratnik, L.; Bozanic, R.; Herceg, Z.; Drgalic, I. The quality of plain andsupplemented kefir from goat’s and cow’s milk. Int. J. Dairy Technol. 2006, 59, 40–46. [Google Scholar] [CrossRef]
- Dec, B.; Kiełczewska, K.; Smoczynski, M.; Baranowska, M.; Kowalik, J. Properties and fractal analysis of high-protein milk powders. Appl. Sci. 2023, 13, 3573. [Google Scholar] [CrossRef]
- Zhang, Y.; Munir, M.; Udugama, I.; Yu, W.; Young, B. Modelling of a milk powder falling film evaporator for predicting process trends and comparison of energy consumption. J. Food Eng. 2018, 225, 26–33. [Google Scholar] [CrossRef]
- Park, C.W.; Drake, M.A. Condensed milk storage and evaporation affect the flavor of nonfat dry milk. J. Dairy Sci. 2016, 99, 9586–9597. [Google Scholar] [CrossRef]
- Bergillos-Meca, T.; Cabrera-Viquea, C.; Artachoa, R.; Moreno-Montoro, M.; Navarro-Alarcóna, M.; Olalla, M.; Giméneza, R.; Ruiz-López, D. Influence of milk ultrafiltration on Ca, Mg, Zn and P levels in fermented goat’s milk. Small Rumin. Res. 2015, 124, 95–100. [Google Scholar] [CrossRef]
- Chen, G.Q.; Leong, T.S.H.; Kentish, S.E.; Ashokkumar, M.; Martin, G.J.O. Separation of Functional Molecules in Food by Membrane Technology; Academic Press Ltd.; Elsevier Science Ltd.: New York, NY, USA, 2019. [Google Scholar]
- Corredig, M.; Nair, P.K.; Li, Y.; Eshpari, H.; Zhao, Z. Invited review: Understanding the behavior of caseins in milk concentrates. J. Dairy Sci. 2019, 102, 4772–4782. [Google Scholar] [CrossRef] [PubMed]
- Dhineshkumar, V.; Ramasamy, D. Review on membrane technology applications in food and dairy processing. J. Appl. Biotechnol. Bioeng. 2017, 3, 399–407. [Google Scholar] [CrossRef]
- Jahadi, M.; Ehsani, M.; Paidari, S. Characterization of milk proteins in ultrafiltration permeate and their rejection coefficients. J. Food Biosci. Technol. 2018, 8, 49–54. [Google Scholar]
- Valencia, A.P.; Doyen, A.; Benoit, S.; Margni, M.; Pouliot, Y. Effect of ultrafiltration of milk prior to fermentation on mass balance and process efficiency in greek-style yogurt manufacture. Foods 2018, 7, 144. [Google Scholar] [CrossRef]
- Ali, A.; Drioli, E.; Macedonio, F. Membrane engineering for sustainable development: a perspective. Appl. Sci. 2017, 7(10), 1026. [Google Scholar] [CrossRef]
- Macedonio, F.; Drioli, E. Membrane engineering for green process engineering. Engineering. [CrossRef]
- KoŁakowski, P.; Malinowska, M.; Gerlich, J. The use of transoligosaccharide (TOS) propionate agar medium with mupirocin for selective enumeration of bifidobacteria in dairy cultures and in fermented dairy products. Milchwissenschaft 2010, 65, 380–384. [Google Scholar]
- Kodinova, S.; Dushkova, M.; Miteva-Petrova, M.; Yanakieva, V.; Petrov, S.; Denkova, Z. Production of probiotic Bulgarian yoghurts obtained from an ultrafiltered cow’s milk. Irish J. Agric. Food Res. 2021, 59, 1–11. [Google Scholar] [CrossRef]
- Batawy, O.; Khalil, O. Manufacture and properties of low-fat bio yoghurt containing probiotic strains and maltodextrin as prebiotic. J. Probiotics Health 2018, 6, 1–9. [Google Scholar] [CrossRef]
- Bulgarian Standard. (2010). Bulgarian yoghurt; BS 12:2010.
- ISO 6731:2010; Milk, cream and evaporated milk – Determination of total solids content (Reference method). 2010.
- ISO 8968-1:2014; Milk and milk products – Determination of nitrogen content – Part 1: Kjeldahl principle and crude protein calculation. 2014.
- ISO 2446:2008; Milk Determination of fat content. 2008.
- Bulgarian Standard BS 6154:1974; Methods for determination of ash content. 1974.
- ISO/TS 222113:2012; Milk and milk products – Determination of titratable acidity of milk fat. 2012.
- ISO 4833-1:2013; Microbiology of the food chain - Horizontal method for the enumeration of microorganisms - Part 1: Colony count at 30 ºC by the pour plate technique. 2013.
- ISO 16649-2:2014; Microbiology of food and animal feeding stuffs – Horizontal method for the enumeration of beta-glucoronidase-positive Escherichia coli – Part 2: Colony count technique at 44 ºС using 5-bromo – 4 – chloro – 3 -indolul beta-D – glucuronide (BS EN). 2014.
- ISO 6888-1:2005+A1; Microbiology of food and animal feeding stuffs - Horizontal method for the enumeration of coagulase-positive staphylococci (Staphylococcus aureus and other species) - Part 1: Technique using Baird-Parker agar medium - Amendment 1: Inclusion of precision data. 2005.
- ISO 6579:2003; Microbiology of food and animal feeding stuffs - Horizontal method for the detection of Salmonella spp. 2003.
- ISO 6611:2004; Milk and milk products — Enumeration of colony-forming units of yeasts and/or moulds — Colony-count technique at 25 degrees C. 2004.
- Dushkova, M.; Kodinova, S.; Denkova, Z.; Yanakieva, V.; Menkov, N.D. Physicochemical, microbiological, and sensory characteristics of probiotic Bulgarian yoghurts obtained by ultrafiltration of goat’s milk. Z. Naturforsch., C, J. Biosci. 2021; 76, pp. 481–489. [Google Scholar] [CrossRef]
- Cancino-Padilla, N.; Fellenberg, M.A.; Franco, W.; Ibáñez, R.A.; Vargas-Bello-Pérez, E. Foodborne bacteria in dairy products: Detection by molecular techniques. Cienc. Investig. Agrar. 2017, 44, 215–229. [Google Scholar] [CrossRef]
- Meena, P.K.; Gupta, V.K.; Meena, G.S.; Raju, P.N.; Parmar, P.T. Application of ultrafiltration technique for the quality improvement of dahi. J. Food Sci. Technol. 2015, 52, 7974–7983. [Google Scholar] [CrossRef]
- Tamime, A.Y.; Kalab, M.; Davies, G.; Mahdi, H.A. Microstructure and firmness of labneh (high solids yogurt) made from cow’s, goat’s and sheep’s milks by a traditional method or by ultrafiltration. Food Struct. 1991, 10, 37–44. [Google Scholar]
- Shekin, J.J. Applications of ultrafiltration, reverse osmosis, nanofiltration, and microfiltration in dairy and food industry. Extensive Rev. 2021, 1, 39–48. [Google Scholar] [CrossRef]
- Gavazzi-April, C.; Benoit, S.; Doyen, A.; Britten, M.; Pouliot, Y. Preparation of milk protein concentrates by ultrafiltration andcontinuous diafiltration: Effect of process design on overall efficiency. J. Dairy Sci. 2018, 101, 9670–9679. [Google Scholar] [CrossRef]
- Domagala, J. Instrumental texture, syneresis, and microstructure of yoghurts prepared from ultrafiltered goat milk: Effect of degree of concentration. Int. J. Food Prop. 2012, 5, 558–568. [Google Scholar] [CrossRef]
- Li, Y.; Corredig, M. Calcium release from milk concentrated by ultrafiltration and diafiltration. J. Dairy Sci. 2014, 97, 5294–5302. [Google Scholar] [CrossRef]
- Jørgensen, C.E.; Abrahamsen, R.K.; Rukke, E.O.; Hoffmann, T.K.; Johansen, A.G.; Skeie, S.B. Processing of high-protein yoghurt—A review. Int. Dairy J. 2019, 88, 42–59. [Google Scholar] [CrossRef]
- Ordonez, A.; Jeon, I.J.; Roberts, H.A. Manufacture of frozen yogurt with ultrafiltered milk. J. Food Process. Preserv. 2007, 24, 163–176. [Google Scholar] [CrossRef]
- Moineau-Jean, A.; Champagne, C.P.; Roy, D.; Raymond, Y.; La Pointe, G. Effect of Greek-style yoghurt manufacturing processes on starter and probiotic bacteria populations during storage. Int. Dairy J. 2019, 93, 35–44. [Google Scholar] [CrossRef]
- Özer, B.H.; Robinson, R.K. The behaviour of starter cultures in concentrated yoghurt (Labneh) produced by different techniques. LWT 1999, 32, 391–395. [Google Scholar] [CrossRef]
- Alirezalu, K.; Rita, S.I.; Hesari, J.; Remize, F.; Nemati, Z.; Saraiva, J.A.; Barba, F.J.; Sant’Ana, A.; Lorenzo, J.M. Nutritional, chemical, syneresis, sensory properties, and shelf life of Iranian traditional yoghurts during storage. LWT 2018, 114, 108417. [Google Scholar] [CrossRef]
- Qingli, Z.; Bao, Y.; Brashears, M.M.; Zhimin, Y.; Mouming, Z.; Ning, L.; Li, Y. Influence of casein hydrolysates on exopolysaccharide synthesis by Streptococcus thermophilus and Lactobacillus delbrueckii ssp. bulgaricus. J. Sci.Food Agric. 2014, 94, 1366–1372. [Google Scholar] [CrossRef]
- Özcelik, S.; Kuley, E.; Özogul, F. Formation of lactic, acetic, succinic, propionic, formic and butyric acid by lactic acid bacteria. LWT 2016, 73, 536–542. [Google Scholar] [CrossRef]
- Qingli, Z.; Brashears, M.M.; Zhimin, Y.; Jiaoyan, R.; Yinjuan, L.; Mouming, Z. Effect of ultrafiltered fractions from casein on lactic acid biosynthesis and enzyme activity in yoghurt starter cultures. Int. J. Food Sci. Technol. 2013, 48, 1474–1482. [Google Scholar] [CrossRef]
- Özer, B.H. Production of concentrated products. In Fermented Milks; Tamime, A., Ed.; W Blackwell Publishing Ltd.: Oxford, UK, 2006; pp. 128–155. [Google Scholar] [CrossRef]
- Skriver, A.; Roemer, H.; Qvist, K.B. Rheological characterization of stirred yoghurt: Viscometry. J. Texture Stud. 1993, 24, 185–198. [Google Scholar] [CrossRef]
- Prasanna, P.H.P.; Grandison, A.S.; Charalampopoulos, D. Microbiological, chemical and rheological properties of low fat set yoghurt produced with exopolysaccharide (EPS) producing Bifidobacterium strains. Food Res. Int. 2013, 51, 15–22. [Google Scholar] [CrossRef]
- Girard, M.; Schaffer-Lequart, C. Gelation of skim milk containing anionic exopolysaccharides and recovery of texture after shearing. Food Hydrocoll. 2007, 21, 1031–1040. [Google Scholar] [CrossRef]
- Krzeminski, A.; Großhable, K.; Hinrichs, J. Structural properties of stirred yoghurt as influenced by whey proteins. LWT 2011, 44, 2134–2140. [Google Scholar] [CrossRef]
- Miocinovic, J.; Miloradovic, Z.; Josipovic, M.; Nedeljkovic, A.; Radovanovic, M.; Pudja, P. Rheological and textural properties of goat and cow milk set type yoghurts. Int. Dairy J. 2016, 58, 43–45. [Google Scholar] [CrossRef]
- Damin, M.; Alcantara, M.; Nunes, A.; Oliveira, M. Effect of milk supplementation with skim milk powder, whey protein concentrate and sodium caseinate on acidification kinetics, rheological properties and structure of nonfat stirred yoghurt. LWT 2008, 42, 1744–1750. [Google Scholar] [CrossRef]
- Shaker, R.R.; Jumah, R.Y.; Abu-Jdayil, B. Rheological properties of plain yogurt during coagulation process: Impact of fat content and preheat treatment of milk. J. Food Eng. 2000, 44, 175–180. [Google Scholar] [CrossRef]
- Delgado, K.F.; Frasao, B.S.; Costa, M.P.; Junior, C.A.C. Different alternatives to improve rheological and textural characteristics of fermented goat products—A review. Rheol. Open Access 2017, 1, 106. [Google Scholar]



| Indice | Goat’s milk | VRR 2 | VRR 3 | Permeate |
|---|---|---|---|---|
| Dry matter, % | 12.30 ± 0.26a | 16.48 ± 0.90b | 23.00 ± 0.15c | 5.19 ± 0.08d |
| Proteins, % | 3.53 ± 0.10a | 6.01 ± 0.12b | 9.60± 0.05c | 0.25 ± 0.09d |
| Fat, % | 3.50 ± 0.10a | 6.00 ± 0.10b | 9.50 ± 0.20c | - |
| Ash, % | 0.87 ± 0.02a | 0.98 ± 0.01b | 1.32 ± 0.03c | 0.61 ± 0.02d |
| Titratable acidity, °Тh | 17.40 ± 0.90a | 25.20 ± 1.50b | 33.00 ± 1.20c | 8.30 ± 1.00d |
| pH | 6.76 ± 0.07 a | 6.51 ± 0.07 b | 6.20 ± 0.04 c | 6.36 ± 0.11d |
| Sample | Dry matter, % | Total protein, % | Fat, % |
|---|---|---|---|
| A1* | 12,27 ± 0,16a | 3,59 ± 0,10 a | 3,50 ± 0,10 a |
| A2* | 11,92 ± 0,24a | 3,64 ± 0,12 a | 3,40 ± 0,12 a |
| A3* | 12,08 ± 0,27a | 3,50 ± 0,08 a | 3,50 ± 0,14 a |
| B1 | 16,68 ± 1,49b | 5,95 ± 0,12 b | 5,20 ± 0,14 b |
| B2 | 16,53 ± 0,49b | 6,20 ± 0,10 b | 5,40 ± 0,12 b |
| B3 | 16,39 ± 0,32b | 5,90 ± 0,20 b | 5,50 ± 0,20 b |
| C1 | 23,12 ± 0,13c | 10,00 ± 0,14 c | 9,70 ± 0,16 c |
| C2 | 23,02 ± 0,14c | 10,00 ± 0,12 c | 9,80 ± 0,18 c |
| Sample | Bingham | Casson | Ostwald-de-Waele | Herschel-Bulkley | |||||
|---|---|---|---|---|---|---|---|---|---|
| τ0, Pa | ηB, Pa.s | τ0, Pa | ηca, Pa.s | K, Pa.sn | n | τ0, Pa | K, Pa.sn | n | |
| А1 | 0.67 | 0.031 | 0.28 | 0.019 | 0.229 | 0.58 | 0.07 | 0.199 | 0.61 |
| В1 | 4.02 | 0.032 | 3.23 | 0.065 | 2.623 | 0.19 | 0.98 | 1.793 | 0.24 |
| С1 | 2.04 | 0.886 | 0.95 | 0.483 | 2.672 | 0.53 | 1.49 | 1.33 | 0.81 |
| А2 | 0.28 | 0.035 | 0.06 | 0.028 | 0.096 | 0.78 | 0.07 | 0.077 | 0.83 |
| В2 | 3.98 | 0.036 | 3.16 | 0.078 | 2.562 | 0.21 | 3.89 | 0.526 | 0.92 |
| С2 | 5.10 | 0.393 | 4.22 | 0.07 | 5.263 | 0.17 | 4.5 | 0.903 | 0.64 |
| А3 | 0.54 | 0.033 | 0.19 | 0.022 | 0.177 | 0.64 | 0.04 | 0.162 | 0.66 |
| В3 | 4.27 | 0.033 | 3.49 | 0.006 | 2.873 | 0.18 | 3.96 | 0.103 | 0.75 |
| С3 | 5.98 | 0.377 | 5.10 | 0.058 | 6.111 | 0.15 | 3.29 | 2.861 | 0.28 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





