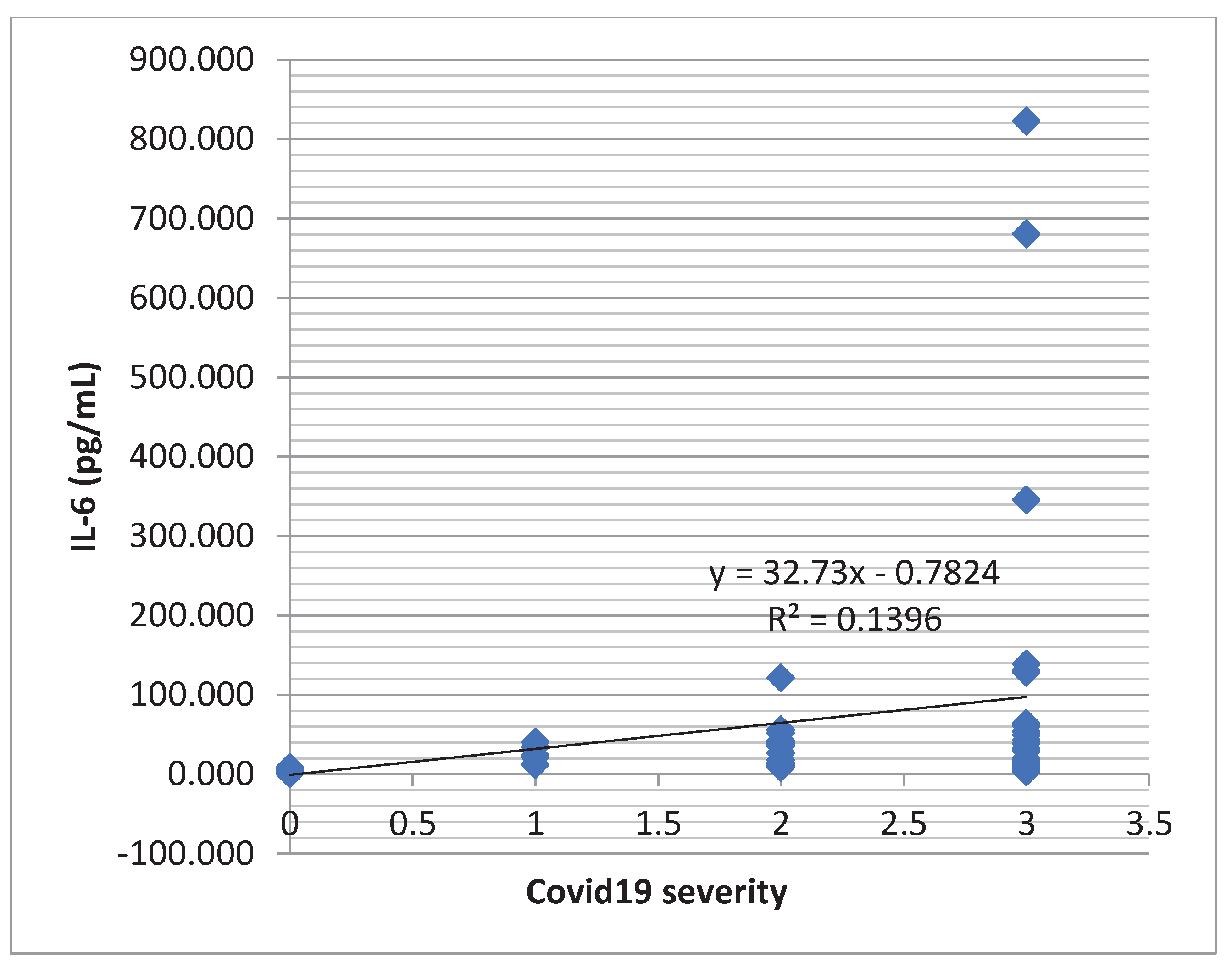1. Introduction
In SARS-CoV-2 virus infection there are certain parameters, presented in the specialized literature, that can be used as markers of severity in infected patients [
1]. Since the beginning of the COVID-19 pandemic, advanced age has been established among the risk factors, as well as the association of certain comorbidities, including type 2 diabetes [
2,
3,
4,
5,
6,
7]. Other severity parameters in the infection with the new coronavirus were: hyperferritinemia, increased d-dimers, increased CRP (C reactive proteine) and decreased number of lymphocytes [
8,
9,
10,
11,
12,
13,
14,
15,
16,
17]. In addition to increased ferritin, regarding iron metabolism in COVID-19, decreased serum iron was associated with the severity of infection [
18,
19,
20]. The link between decreased transferrin, hyperferritinemia and increased CRP was associated with proinflammatory status and progression to severe forms of the disease [
21,
22,
23]. The study of ceruloplasmin in SARS-CoV-2 virus infection is a topic of interest because there are studies that describe the fact that ceruloplasmin can act within the human body, in healthy people, both with an antioxidant role, but also with a pro-oxidant role [
24,
25,
26]. Ceruloplasmin is a glycoprotein synthesized in the liver and has 6 copper atoms in its composition, with a particularly important role in iron metabolism, oxidizing ferrous iron to ferric iron, the ferric iron form being the only one that can be bound to transferrin [
27,
28]. It is known that ceruloplasmin can also act as an acute phase reactant, and ceruloplasmin levels are increased in inflammation, being an independent risk factor for complications associated with diabetes [
29,
30]. Most of the copper in the body,
i.e., 90%, is bound to ceruloplasmin and 10% is bound to albumin [
31]. Type 2 diabetes is a metabolic condition characterized by a chronic inflammatory state in which serum ceruloplasmin is elevated, but ceruloplasmin is not correlated with diabetes severity [
32,
33,
34,
35]. In type 2 diabetes, insulin resistance occurs due to endothelial dysfunction, which is associated with increased ROS (Reactive oxygen species) production [
36,
37,
38]. The imbalance between ROS and antioxidants is represented by oxidative stress, one of the most important factors in the pathogenesis of type 2 diabetes [
39,
40]. Oxidative stress affects pancreatic beta-cell function and apoptosis [
41,
42,
43]. Iron excess is toxic and pro-oxidant, and its accumulation causes inflammation of pancreatic beta cells and is a risk factor for diabetes development and evolution, and a risk of mortality [
44,
45,
46]. Iron homeostasis is regulated by hepcidin and ferroportin, which modulates the flux of iron into the systemic circulation [
47,
48,
49]. Iron deposits are mainly represented by ferritin, which can also play a role as an acute phase reactant, hyperferritinemia being significantly associated with the severity and unfavorable prognosis in the COVID-19 infection. [
50,
51]. Decreased transferrin level was strongly associated with severe forms of SARS-CoV-2 infection, with transferrin representing the transport of circulating iron [
52].
The aim of this study was to identify other possible biomarkers of severity in SARS-CoV-2 virus infection, in patients with type 2 diabetes as an associated comorbidity.
2. Material and Methods
2.1. Study design
The study was based on a total of 90 subjects, divided in a group of 45 patients hospitalized at the Clinical Hospital "Gavril Curteanu" Oradea, between October 21, 2021 and December 31, 2021, randomly selected, having as the main inclusion criteria the positive RT-PCR (Reverse transcription polymerase chain reaction) rapid antigen test for viral infection and the diagnosis of type 2 diabetes. And the control group of 45 healthy persons randomly chosen, with a negative RT-PCR/rapid antigen test as the inclusion criterion and who were not known with diabetes or other associated pathologies. The present study was conducted in accordance with the Declaration of Helsinki. Before taking part in this study, each participant gave a written informed consent for inclusion. The study protocol was approved by the Ethics Committee of the Clinical Hospital "Gavril Curteanu" Oradea (No. 32652/16.11.2020) and by the Ethics Committee of the University of Oradea (No. 5/A, 21.09.2020).
The inclusion criteria in the group of patients were the diagnosis of type 2 diabetes and SARS-CoV-2 virus infection confirmed by positive RT-PCR/rapid antigen test. Exclusion criteria from the group of patients included: absence of type 2 diabetes, absence of infection with the SARS-CoV-2 virus by negative RT-PCR/rapid antigen test.
Inclusion criteria in the control group were healthy individuals, absence of any diseases or medication, including absence of type 2 diabetes and negative RT-PCR/rapid antigen test.
2.2. Data collection
The data were collected in an EXCEL file including the following biomedical parameters: iron biomarkers (ceruloplasmin, serum iron, sideremia, transferrin), anthropometric parameters - age (years), parameters regarding the severity of COVID-19 (absent=0, mild=1, moderate=2, severe=3), Angiotensin-converting enzyme (ACE) and interleukin 6 (IL-6). In the group of patients with type 2 diabetes mellitus and SARS-CoV-2 virus infection, we also evaluated the relationship between certain parameters of iron metabolism (including serum iron, transferrin, ceruloplasmin, hemoglobin, number of erythrocytes, ferritin), coagulation parameters (INR - International normalized ratio, platelets number) and inflammation parameters (CRP, fibrinogen, LDH - Lactate dehydrogenase and lymphocytes number). We analyzed the regression models that reflected the connection and influence of independent parameters such as coagulation and inflammation on serum iron levels at hospital admission. We also evaluated if the gender of the subjects influenced the level of the biomedical parameters considered in our study.
2.3. Methods
Ceruloplasmin determination was achieved by using the nephelometric method (reagent N Antiserum to Human Ceruloplasmin, ref. 10446451, BN ProSpec analyzer, Siemens Healthineers). Spectrophotometric method was employed for sideremia measurement (Iron_2 reagent, ref.10377510, Advia 1800 analyzer, Siemens Healthineers). Transferrin was determined by using the nephelometric method (reagent N Antiserum to Human Transferrin, ref. 10446309, BN ProSpec analyzer, Siemens Healthineers). The ACE biomarker was determined by the spectrophotometric method (Angiotensin Converting Enzyme (ACE) reagent, ref. 12796, analyzer A15, BioSystems SA, Spain). Interleukin 6 was quantified by the electrochemiluminescence method (ECLIA = electrochemiluminescence immunoassay), (IL-6 reagent, ref. 05109442190, Cobas E4111 analyzer, Roche Diagnostics GmbH). All these biomarkers were analyzed from blood samples collected at the time of hospital admission.
2.4. Statistical analysis
In the statistical processing of the data, both descriptive statistics procedures were used to calculate averages, standard deviations (for quantitative data), frequencies (for qualitative data), correlation coefficients, graphic representations of the data, as well as statistical analysis procedures. Concerning the data statistical analysis, statistical tests appropriate to the analysis performed were used (Student test, ANOVA test, Student test for paired samples, the chi square test, normality tests (Kolmogorov-Smirnov), non-parametric tests (Kruskal-Wallis), as well as the regression analysis. In order to evaluate the intensity of the correlation of two variables (quantitative and/or order), the following empirical Colton rule was used: for the interpretation of the correlation coefficient value for two quantitative variables, both the parametric Pearson (R - used when both variables are approximately normally distributed), as well as the non-parametric Spearman (Ro - used when at least one of the variables is not normally distributed). The threshold of statistical significance of the tests used in the statistical analyses was the one usually applied in medical research p=0.05 (=5%). The significance threshold p=0.01 was also used for the case when the result of the analysis was strongly significant (p<0.01). To perform the statistical calculations, the EXCEL data file which contained the study data, was converted into an SPSS file, the statistical processing was performed with the statistical software SPSS version 20.
3. Results
In the comparative analysis between infected patients and healthy persons, we denoted with 1 the group of patients with type 2 diabetes mellitus and SARS-CoV-2 virus infection, and with 2 the control group of healthy subjects. In group 1 were 5 mild, 13 moderate and 27 severe forms of SARS-CoV-2 viral infection on CT (Computer tomography) at hospital admission.
We identified a significant statistical difference between the means of the quantitative parameters in the group of infected patients, compared to the group of healthy subjects. The means of IL-6 and ceruloplasmin parameters in patients infected with the SARS-CoV-2 virus were statistically significantly higher than in the control group. Regarding the following parameters such as: serum iron, ACE and transferrin, the means for patients infected with the novel coronavirus were statistically significantly lower than in healthy subjects (
Table 1).
The serum ceruloplasmin was statistically significant inversely correlated with the severity of SARS-CoV-2 infection, but also with IL-6 and serum iron levels. However, ceruloplasmin did not statistically significantly correlate with transferrin, ACE or age (
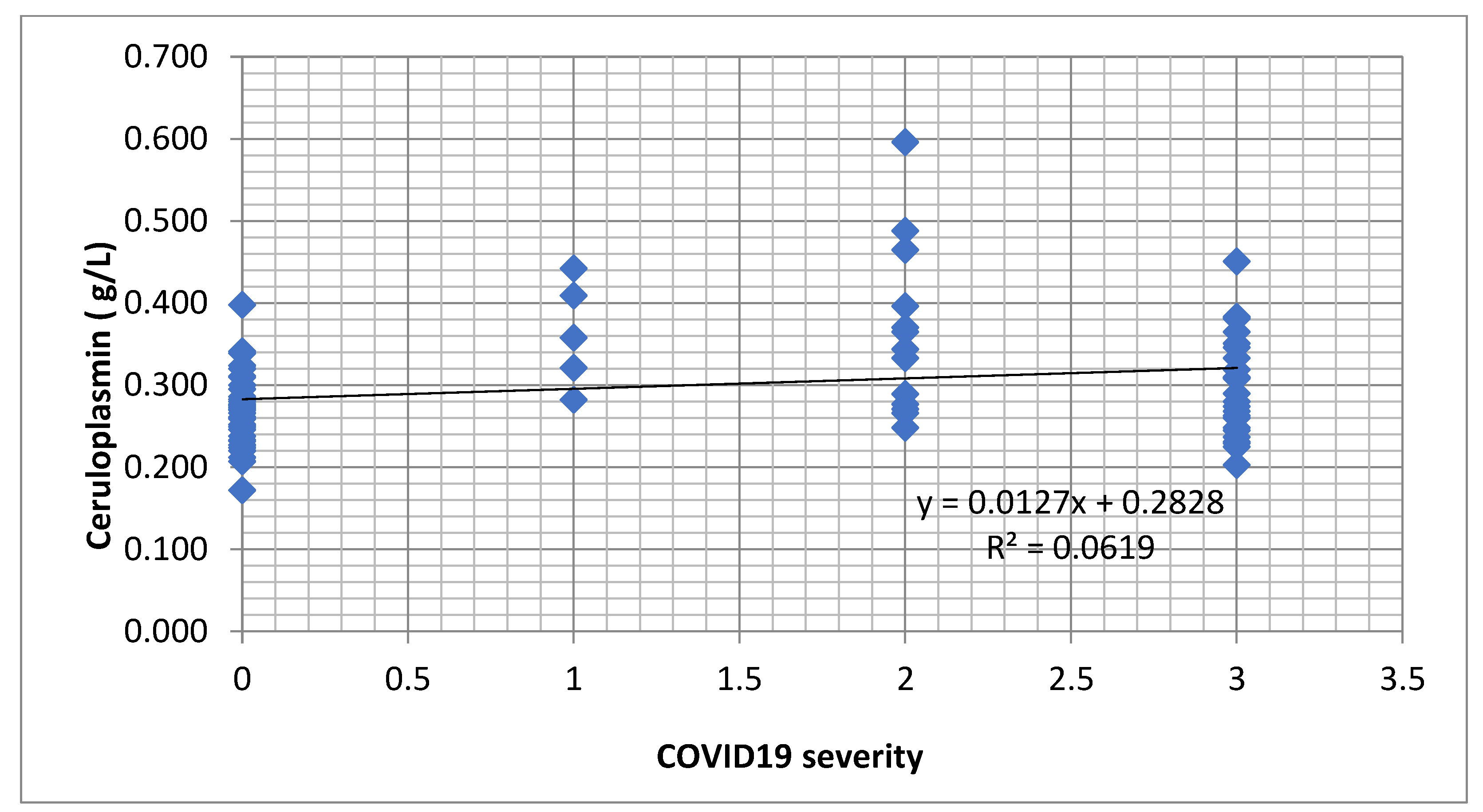
Table 2). We identified an increasing tendency of the ceruloplasmin concentration depending on the level of infection with COVID-19 (
Figure 1).
In the case of serum iron level (sideremia), this parameter correlated strongly significantly (p < 0.001) and inversely with the severity of COVID-19, ceruloplasmin, age and IL-6 (
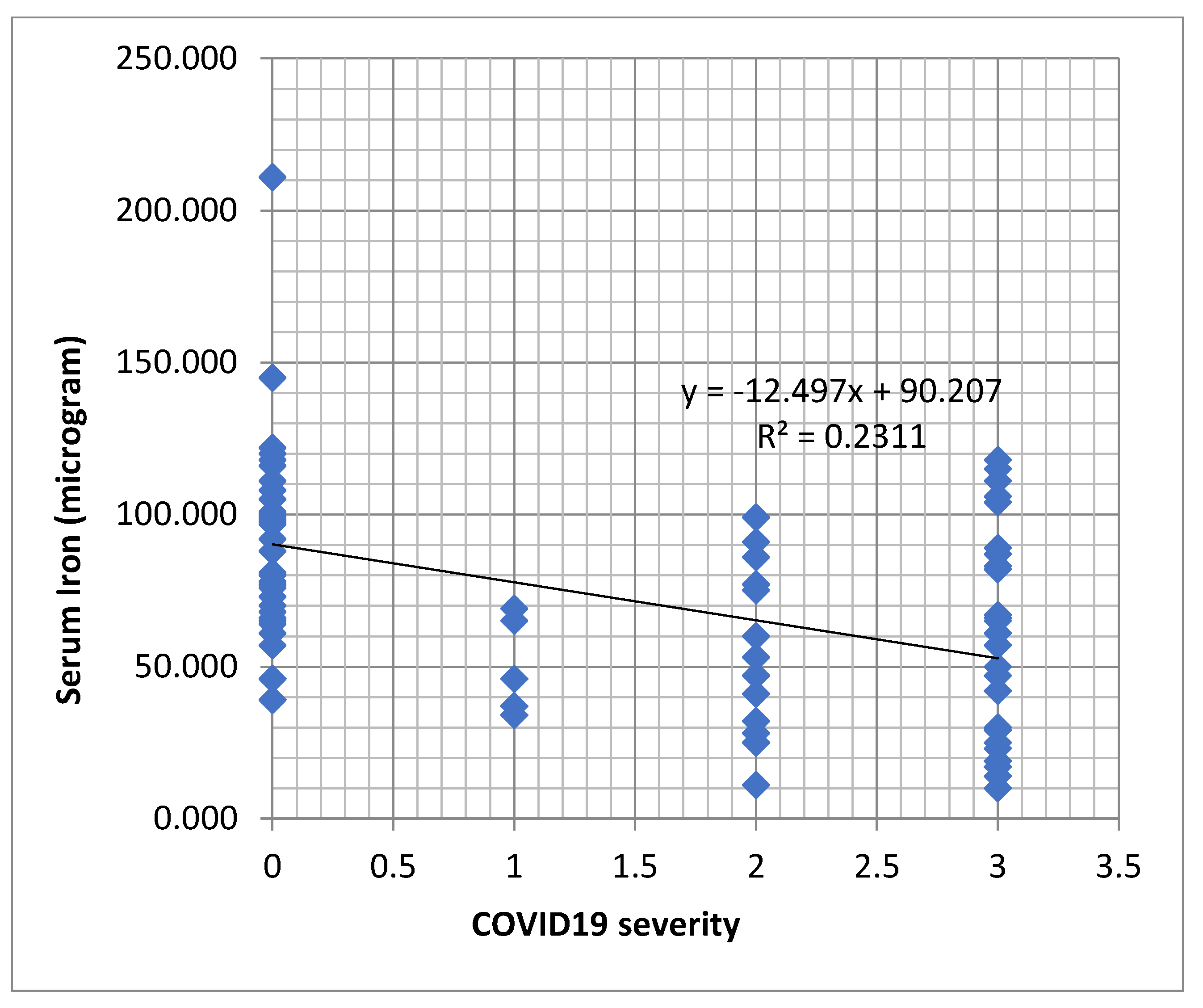
Table 3). A decreasing tendency of serum iron values was observed in dependence with the severity of the COVID-19 infection (
Figure 2).
A strongly statistically significant, but inverse correlation with the severity of COVID-19, IL-6 and age was observed in this study (p=0.001). Transferrin did not correlate significantly with serum ceruloplasmin (
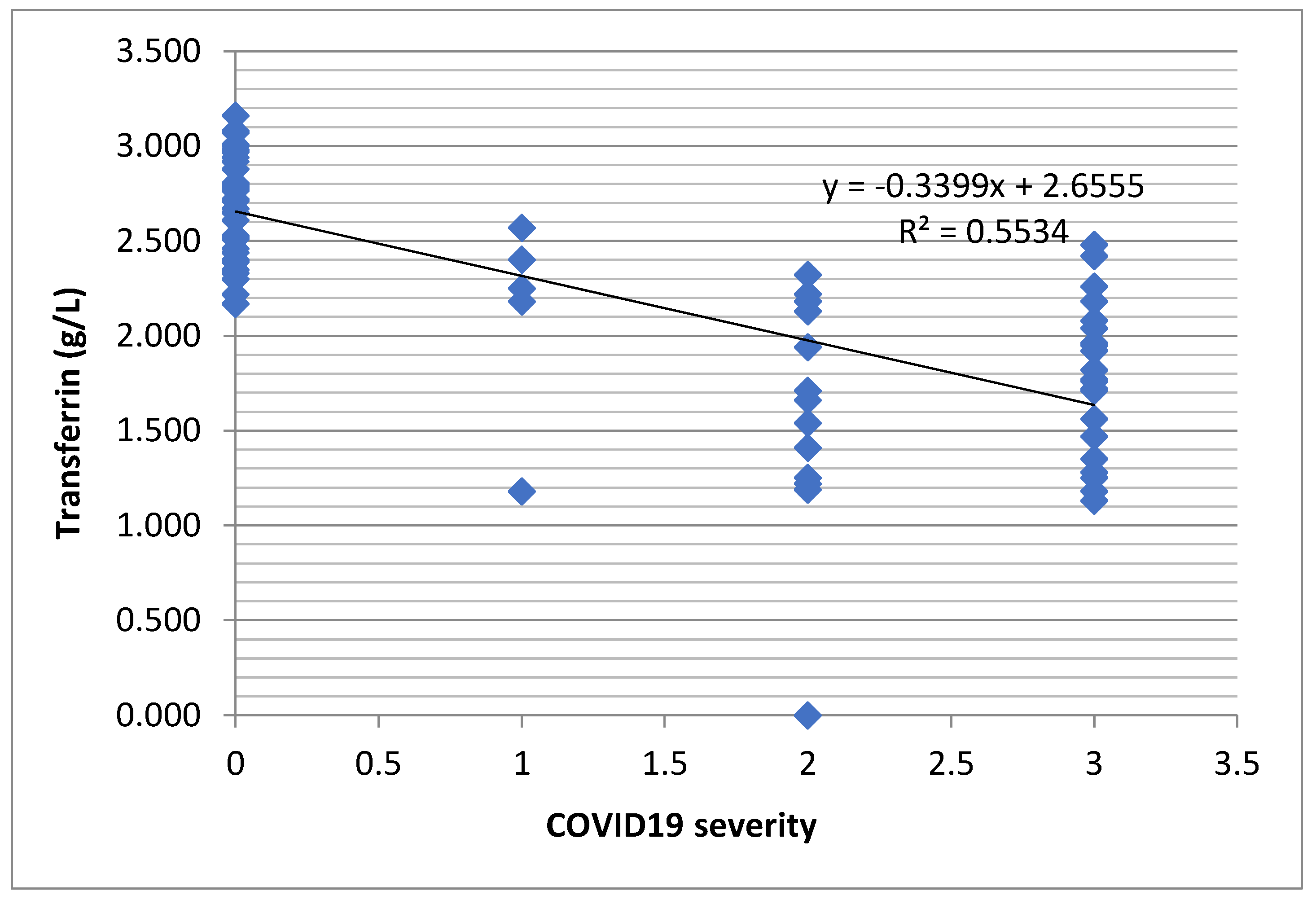
Table 4). However, there was a decreasing tendency of the transferrin level depending on the severity of the COVID-19 infection (
Figure 3).
Age was strongly significant directly correlated with IL-6. However, age correlated strongly significantly, but inversely with ACE, transferrin and serum iron (
Table 5).
The ACE concentration strongly correlated statistically significantly (p=0.001) and inversely with the severity of COVID-19, age, and IL-6 (
Table 6).
IL-6 correlated non-parametrically (Spearman) strongly statistically significant and directly with the severity of the COVID-19 infection, age and ceruloplasmin (p<0.001). An inverse correlation was noticed between IL-6 and transferrin, ACE and serum iron. (
Table 7). An increasing tendency of the IL-6 concentration was observed in dependence with the severity of the COVID-19 infection (
Figure 4).
The severity of COVID-19 correlated statistically significantly with all quantitative parameters of the study. Good (|Ro|>0.5) and very good (|Ro|>0.75) and strongly statistically significant correlations were established between the COVID-19 severity and IL-6, age, and transferrin level. Also, the severity of COVID-19 correlated directly (Ro>0) with IL-6, age, and ceruloplasmin level. Inverse correlation (Ro<0) appeared between the COVID-19 severity and serum iron, ACE and transferrin levels (
Table 8).
Within the group of the diabetic patients with SARS-CoV-2 virus infection, we evaluated possible correlations between the serum level of CRP, LDH, D-dimers, number of lymphocytes, INR, ceruloplasmin and the COVID-19 severity. Ceruloplasmin and lymphocyte count had a statistically significant decreasing tendency in severe forms of SARS-CoV-2 infection (the Spearman correlation coefficient Ro<0), meanwhile for the other parameters, there was a significantly increasing tendency in severe forms of SARS-CoV-2 infection (Ro>0). Moreover, the severity of COVID-19 correlated strongly significantly (p<0.01) with d-Dimers and lymphocytes number (
Table 9).
For the patients with COVID-19, we observed that IL-6 correlated non-parametrically (Spearman) significantly (p<0.05) with the numbers of neutrophils, platelets, and serum iron level. Neutrophils, platelets, and serum iron shown a significantly decreasing tendency when IL-6 increases (Spearman correlation coefficient Ro<0). IL-6 correlated strongly significantly (p<0.01) with CRP (
Table 10).
Serum iron, ACE, ceruloplasmin and transferrin did not differ statistically significantly for the female gender compared to the male gender (p>0.05) (
Table 11). Also, ferritin, hemoglobin, RBC (Red blood cell), glycemia, CRP, LDH and fibrinogen did not differ statistically significantly for the female gender compared to the male gender (p>0.05) (
Table 12).
The multiple linear regression model between serum iron (as dependent parameter) and ceruloplasmin, hemoglobin, RBC, platelets number and fibrinogen (as independent parameters) can be expressed by the following equation:
(Serum iron) = - 164.297 x (Ceruloplasmin) + 15.281 x (Hemoglobin) - 26.98 x RBC + 0.186 x (Platelets number) – 0.186 x (Fibrinogen).
The multiple correlation coefficient (R=0.942, R
square=0.871) in this model was strongly statistically significant (p<0.001) indicating that the serum iron variation was due in a proportion of 87.1% to predictor parameters such as ceruloplasmin, hemoglobin, RBC, platelets number. Therefore, this regression model gave a good enough estimation of serum iron parameter (
Table 13 and
Table 14).
4. Discussions
During the pandemic, certain markers of poor prognosis and increased mortality were identified in patients infected with the SARS-CoV-2 virus [
53,
54,
55,
56]. Among the first common markers of severity recognized in COVID-19 infection were: advancing age, presence of metabolic and cardiovascular comorbidities, increased ferritin, high levels of pro-inflammatory cytokines, especially increased IL-6 [
57,
58]. In the studies of patients with newly diagnosed type 2 diabetes and SARS-CoV-2, hyperferritinemia, increased CRP and decreased lymphocyte counts were more evident than in patients without comorbidity (without type 2 diabetes) [
59,
60,
61]. Associations between significantly elevated levels of serum ferritin, d-dimers and CRP in patients with COVID-19 requiring transfer to intensive care units have been reported in the literature [
62,
63]. In other studies, the link between hyperferritinemia, decreased serum transferrin and increased CRP levels in the pro-inflammatory state from the COVID-19 infection was presented [
64,
65].
Decreased serum transferrin and decreased transferrin saturation, but also increased hepcidin levels were associated with severe forms of COVID-19 infection [
66,
67,
68]. High levels of ferritin, as an acute phase reactant, have been reported in many studies as a negative prognostic marker [
69]. In some studies, the role of transferrin has been suggested as a possible central element of pathogenicity in SARS-CoV-2 infection [
70].
The mechanism of action of serum iron in patients with COVID-19 is not fully elucidated, in the literature it has been assumed that a decrease in serum iron may be associated with an increase in hepcidin level and with hyperferritinemia [
71]. Concerning iron metabolism, there are certain studies regarding the decrease in serum iron as a marker associated with the increased risk of mortality due to SARS-CoV-2 infection [
72,
73]. Decreased serum iron has been shown to be a predictor of severe forms of COVID-19 infection [
74,
75,
76].
In the present study, we evaluated other new possible markers of COVID-19 severity and their associations by comparing serum levels of ceruloplasmin, IL-6, serum iron, transferrin, and ACE in 45 healthy control subjects versus 45 type-2 diabetic patients infected with SARS-CoV-2 virus, randomly chosen. The subjects’ gender did not significantly influence the level of the evaluated biomedical parameters.
We identified that serum ceruloplasmin increased in severe forms of infection, being a possible new marker of COVID-19 severity. We also found that there was a strong connection between increased serum ceruloplasmin and increased IL-6 levels. Thus, a possible explanation could be the fact that the increased level of ceruloplasmin could also signal a possible negative evolution, with cytokine storm in these patients, through the correlation with the high level of IL-6. Increased IL-6 level in patients with COVID-19 has been associated in other studies with the cytokine storm, increased levels of pro-inflammatory cytokines such as IL-6, IL-7, IL-10, IL-1B being present in patients with severe forms and with a negative impact on the endothelial function [
77,
78,
79,
80]. In our research, increased IL-6 was statistically significant correlated with decreased serum iron, decreased serum ACE, and decreased transferrin. Julian Hackler
et al. presented a possible connection between survival in COVID-19 infection, elevated serum copper levels, and a slight ceruloplasmin increase tendency [
81].
Analysis of serum iron in our study revealed a statistically significant correlation between decreased serum iron and severity of SARS-CoV-2 infection. We also found that the decrease in sideremia was correlated with the increase in serum ceruloplasmin, IL-6 level, and age. IL-6 is involved in the production of hepcidin which is a peptide hormone that ensures iron homeostasis [
82]. Marco Ciotti
et al. presented the association between hepcidin values and the transfer to intensive care in patients infected with SARS-CoV-2 virus, but they did not identify significant differences in serum iron levels between survivors and deceased [
68].
At the patients included in our study, we observed that the decrease in the serum level of ACE was significantly correlated with severe forms of infection and with the increase of IL-6, so the low level of ACE could represent a new possible marker of severity in patients with COVID-19. We have not identified in the literature reliable data related to the role of ACE in patients with COVID-19 or in patients with type-2 diabetes and SARS-CoV-2 virus infection, although the role of ACE 2 (Angiotensin converting enzyme 2) in the infection with the new coronavirus has been intensively studied and presented [
83,
84,
85]. ACE is produced by both kidneys and lungs, being present in vascular structures and being a component of the renin-angiotensin-aldosterone system together with ACE2, important in cardiovascular and renal homeostasis. ACE and ACE2 are metallopeptidases, glycoproteins that metabolize circulating peptides, but ACE and ACE2 are functionally different. ACE mainly acts as a carboxypeptidase, converting angiotensin I into angiotensin II, by removing the terminal dipeptide C; also, through ACE, Angiotensin 1-9 can be converted to Angiotensin 1-7. ACE2 has an enzymatic role by regulating the renin-angiotensin-aldosterone system, but also the kinin-kallikrein system; ACE2 also has a non-enzymatic function representing the entry receptor of the SARS-COV2 virus into the human body. The serum level of ACE did not correlate statistically significantly with the level of serum ceruloplasmin at the patients included in this study [
86,
87].
By analyzing new possible negative prognostic markers, and their association with the others severity markers in the infection with the new coronavirus, we identified a significant inverse statistical correlation between the tendency of decreasing ceruloplasmin (part of iron metabolism), severe forms of COVID-19 and the number of lymphocytes. Lymphopenia, as evidence of immune suppression and a marker of severity in COVID-19 infection, was identified in the patients from our study.
Other studies shown the connection between the decrease in serum iron, hemoglobin and hypoxia in patients with COVID-19, but also the connection between hyperferritinemia and the stimulation of the expression of pro-inflammatory cytokines by macrophages including IL-6 [
88].
One of the finding of our study was the tendency of decreased number of platelets (as coagulation parameters), neutrophils count and association with increased IL-6 cytokine level. On the other hand, the level of IL-6 was directly influenced by the increase in CRP level.
Data from the literature regarding patients with diabetes and COVID-19 infection also mentioned that the predictors of mortality such as lymphopenia, ferritinemia, increase of CRP and D-dimers were more evident than in nondiabetic patients [
89,
90,
91].
Another important finding of our study was that ceruloplasmin and transferrin (as parameters of iron metabolism) and their connection with ACE could show the evolution in the direction of severe forms of COVID-19.
In this research, the regression model for serum iron and ceruloplasmin parameters was also analyzed and it was observed that serum ceruloplasmin, hemoglobin, erythrocytes and platelets count strongly significantly influenced sideremia in patients with SARS-CoV-2 virus at hospital admission.
Among the limitations of this study, it can be mentioned: quite low number of patients, single-center study, parameters evaluated at hospital admission. Future studies on much larger groups are needed.
5. Conclusions
Regarding the severe forms of infection with SARS-CoV-2 virus, we identified a direct relationship between the COVID-19 severity and the level of ceruloplasmin as an antioxidant biomarker, and also a direct relationship between the infection severity and IL-6 levels as a pro-inflammatory cytokine. Moreover, the COVID-19 severity can be influenced by decreased iron deposits, decreased transferrin and decreased ACE levels.
Thus, in patients with type-2 diabetes and viral infection with the new coronavirus, other possible markers independent of the COVID-19 severity such as serum ceruloplasmin, ACE and transferrin levels can be determined at hospital admission. These possible new severity markers may serve to complement all other severity markers already established in COVID-19 infection in patients with type 2 diabetes.
Author Contributions
Conceptualization P.A.R., data collection P.A.R., C.M., I.L.M., methodology P.A.R., O.M., software S.T., P.A.R., study design P.A.R., A.P., writing the manuscript P.A.R., L.F., resources P.A.R., M.E.M., validation L.G.V., T.J., review and supervision E.M., M.E.M. All authors have read and agreed to the published version of the manuscript.
Funding
The research has been funded by the University of Oradea, within the Grants Competition “Scientific Research of Excellence Related to Priority Areas with Capitalization through Technology Transfer INO-TRANSFER-UO”, Project No. 324/2021.
Institutional Review Board Statement
The study was carried out in accordance with the Declaration of Helsinki, and the protocol was approved by the Ethics Committee of the University of Oradea, no. 5/A, September 21, 2020.
Statement of informed consent
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Not applicable.
Conflicts of interest
The authors declare no conflict of interest.
References
- Romero Starke, K.; Petereit-Haack, G.; Schubert, M.; Kämpf, D.; Schliebner, A.; Hegewald, J.; Seidler, A. The Age-Related Risk of Severe Outcomes Due to COVID-19 Infection: A Rapid Review, Meta-Analysis, and Meta-Regression. Int. J. Environ. Res. Public Health 2020, 17, 5974. [Google Scholar] [CrossRef] [PubMed]
- Kaeuffer, C.; Le Hyaric, C.; Fabacher, T.; Mootien, J.; Dervieux, B.; Ruch, Y.; Hugerot, A.; Zhu, Y.-J.; Pointurier, V.; Clere-Jehl, R.; et al. Clinical characteristics and risk factors associated with severe COVID-19: prospective analysis of 1,045 hospitalised cases in North-Eastern France, March 2020. Eurosurveillance 2020, 25. [Google Scholar] [CrossRef]
- Albitar, O.; Ballouze, R.; Ooi, J.P.; Ghadzi, S.M.S. Risk factors for mortality among COVID-19 patients. Diabetes Res. Clin. Pr. 2020, 166, 108293–108293. [Google Scholar] [CrossRef]
- Guo, W.; Li, M.; Dong, Y.; Zhou, H.; Zhang, Z.; Tian, C.; Qin, R.; Wang, H.; Shen, Y.; Du, K.; et al. Diabetes is a risk factor for the progression and prognosis of COVID -19. Diabetes/Metabolism Res. Rev. 2020, 36, e3319. [Google Scholar] [CrossRef]
- Norouzi, M.; Norouzi, S.; Ruggiero, A.; Khan, M.S.; Myers, S.; Kavanagh, K.; Vemuri, R. Type-2 Diabetes as a Risk Factor for Severe COVID-19 Infection. Microorganisms 2021, 9, 1211. [Google Scholar] [CrossRef] [PubMed]
- Rubio-Rivas, M.; Corbella, X.; Formiga, F.; Fernández, E.M.; Escalante, M.D.M.; Fernández, I.B.; Fernández, F.A.; Del Corral-Beamonte, E.; Lalueza, A.; Virto, A.P.; et al. Risk Categories in COVID-19 Based on Degrees of Inflammation: Data on More Than 17,000 Patients from the Spanish SEMI-COVID-19 Registry. J. Clin. Med. 2021, 10, 2214. [Google Scholar] [CrossRef] [PubMed]
- Farshbafnadi, M.; Zonouzi, S.K.; Sabahi, M.; Dolatshahi, M.; Aarabi, M.H. Aging & COVID-19 susceptibility, disease severity, and clinical outcomes: The role of entangled risk factors. Exp. Gerontol. 2021, 154, 111507–111507. [Google Scholar] [CrossRef]
- Rueda-Camino, J.A.; Sendín-Martín, V.; Joya-Seijo, M.D.; Angelina-García, M.; Zamarro-García, C.; Gimena-Rodríguez, F.J.; Barba-Martín, R. Plasma D-dimer value corrected by inflammatory markers in patients with SARS-CoV-2 infection: Its prognostic value in the diagnosis of venous thromboembolism. Med Clin 2021, 158, 265–269. [Google Scholar] [CrossRef]
- Trimaille, A.; Thachil, J.; Marchandot, B.; Curtiaud, A.; Leonard-Lorant, I.; Carmona, A.; Matsushita, K.; Sato, C.; Sattler, L.; Grunebaum, L.; et al. D-Dimers Level as a Possible Marker of Extravascular Fibrinolysis in COVID-19 Patients. J. Clin. Med. 2020, 10, 39. [Google Scholar] [CrossRef]
- Thoreau, B.; Galland, J.; Delrue, M.; Neuwirth, M.; Stepanian, A.; Chauvin, A.; Dellal, A.; Nallet, O.; Roriz, M.; Devaux, M.; et al. D-Dimer Level and Neutrophils Count as Predictive and Prognostic Factors of Pulmonary Embolism in Severe Non-ICU COVID-19 Patients. Viruses 2021, 13, 758. [Google Scholar] [CrossRef]
- Yovchevska, I.P.; Trenovski, A.B.; Atanasova, M.H.; Georgiev, M.N.; Tafradjiiska-Hadjiolova, R.K.; Lazarov, S.D.; Yovchevski, P.H. Platelet Distribution Width and Increased D-Dimer at Admission Predicts Subsequent Development of ARDS in COVID-19 Patients. Pathophysiology 2022, 29, 233–242. [Google Scholar] [CrossRef]
- Yamada, H.; Okamoto, M.; Nagasaki, Y.; Yoshio, S.; Nouno, T.; Yano, C.; Tanaka, T.; Watanabe, F.; Shibata, N.; Arimizu, Y.; et al. Analysis of Early Biomarkers Associated with the Development of Critical Respiratory Failure in Coronavirus Disease 2019 (COVID-19). Diagnostics 2022, 12, 339. [Google Scholar] [CrossRef] [PubMed]
- Ceci, F.M.; Fiore, M.; Gavaruzzi, F.; Angeloni, A.; Lucarelli, M.; Scagnolari, C.; Bonci, E.; Gabanella, F.; Di Certo, M.G.; Barbato, C.; et al. Early Routine Biomarkers of SARS-CoV-2 Morbidity and Mortality: Outcomes from an Emergency Section. Diagnostics 2022, 12, 176. [Google Scholar] [CrossRef] [PubMed]
- Ghizlane, E.A.; Manal, M.; Abderrahim, E.K.; Abdelilah, E.; Mohammed, M.; Rajae, A.; Amine, B.M.; Houssam, B.; Naima, A.; Brahim, H. Lymphopenia in Covid-19: A single center retrospective study of 589 cases. Ann. Med. Surg. 2021, 69, 102816. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Park, S.-S.; Kim, T.Y.; Lee, D.-G.; Kim, D.-W. Lymphopenia as a Biological Predictor of Outcomes in COVID-19 Patients: A Nationwide Cohort Study. Cancers 2021, 13, 471. [Google Scholar] [CrossRef]
- Tavakolpour, S.; Rakhshandehroo, T.; Wei, E.X.; Rashidian, M. Lymphopenia during the COVID-19 infection: What it shows and what can be learned. Immunol. Lett. 2020, 225, 31–32. [Google Scholar] [CrossRef]
- Cheng, Y.; Yue, L.; Wang, Z.; Zhang, J.; Xiang, G. Hyperglycemia associated with lymphopenia and disease severity of COVID-19 in type 2 diabetes mellitus. J. Diabetes its Complicat. 2020, 35, 107809–107809. [Google Scholar] [CrossRef]
- Gómez-Pastora, J.; Weigand, M.; Kim, J.; Wu, X.; Strayer, J.; Palmer, A.F.; Zborowski, M.; Yazer, M.; Chalmers, J.J. Hyperferritinemia in critically ill COVID-19 patients – Is ferritin the product of inflammation or a pathogenic mediator? Clin. Chim. Acta 2020, 509, 249–251. [Google Scholar] [CrossRef]
- Bellmann-Weiler, R.; Lanser, L.; Barket, R.; Rangger, L.; Schapfl, A.; Schaber, M.; Fritsche, G.; Wöll, E.; Weiss, G. Prevalence and Predictive Value of Anemia and Dysregulated Iron Homeostasis in Patients with COVID-19 Infection. J. Clin. Med. 2020, 9, 2429. [Google Scholar] [CrossRef]
- Deng, F.; Zhang, L.; Lyu, L.; Lu, Z.; Gao, D.; Ma, X.; Guo, Y.; Wang, R.; Gong, S.; Jiang, W. Increased levels of ferritin on admission predicts intensive care unit mortality in patients with COVID-19. Med Clin 2020, 156, 324–331. [Google Scholar] [CrossRef]
- Zhao, K.; Huang, J.; Dai, D.; Feng, Y.; Liu, L.; Nie, S. Serum Iron Level as a Potential Predictor of Coronavirus Disease 2019 Severity and Mortality: A Retrospective Study. Open Forum Infect. Dis. 2020, 7, ofaa250. [Google Scholar] [CrossRef] [PubMed]
- Edeas, M.; Saleh, J.; Peyssonnaux, C. Iron: Innocent bystander or vicious culprit in COVID-19 pathogenesis? Int. J. Infect. Dis. 2020, 97, 303–305. [Google Scholar] [CrossRef] [PubMed]
- Girelli, D.; Marchi, G.; Busti, F.; Vianello, A. Iron metabolism in infections: Focus on COVID-19. Semin. Hematol. 2021, 58, 182–187. [Google Scholar] [CrossRef]
- Fox, P.L.; Mukhopadhyay, C.; Ehrenwald, E. Structure, oxidant activity, and cardiovascular mechanisms of human ceruloplasmin. Life Sci. 1995, 56, 1749–1758. [Google Scholar] [CrossRef] [PubMed]
- Atanasiu, R.L.; Stea, D.; Mateescu, M.A.; Vergely, C.; Dalloz, F.; Briot, F.; Maupoil, V.; Nadeau, R.; Rochette, L. Direct evidence of caeruloplasmin antioxidant properties. Mol. Cell. Biochem. 1998, 189, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Golizeh, M.; Lee, K.; Ilchenko, S.; Ösme, A.; Bena, J.; Sadygov, R.G.; Kashyap, S.R.; Kasumov, T. Increased serotransferrin and ceruloplasmin turnover in diet-controlled patients with type 2 diabetes. Free. Radic. Biol. Med. 2017, 113, 461–469. [Google Scholar] [CrossRef] [PubMed]
- Eid, C.; Hémadi, M.; Ha-Duong, N.-T.; Chahine, J.-M.E.H. Iron uptake and transfer from ceruloplasmin to transferrin. Biochim. et Biophys. Acta (BBA) - Gen. Subj. 2014, 1840, 1771–1781. [Google Scholar] [CrossRef] [PubMed]
- Kawabata, H. Transferrin and transferrin receptors update. Free. Radic. Biol. Med. 2019, 133, 46–54. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, M.; Zhang, C.; Zhou, S.; Ji, G. Molecular Functions of Ceruloplasmin in Metabolic Disease Pathology. Diabetes, Metab. Syndr. Obesity: Targets Ther. 2022, 15, 695–711. [Google Scholar] [CrossRef]
- Gembillo, G.; Labbozzetta, V.; Giuffrida, A.E.; Peritore, L.; Calabrese, V.; Spinella, C.; Stancanelli, M.R.; Spallino, E.; Visconti, L.; Santoro, D. Potential Role of Copper in Diabetes and Diabetic Kidney Disease. Metabolites 2022, 13, 17. [Google Scholar] [CrossRef]
- Twomey, P.J.; Viljoen, A.; House, I.M.; Reynolds, T.M.; Wierzbicki, A.S. Relationship between Serum Copper, Ceruloplasmin, and Non–Ceruloplasmin-Bound Copper in Routine Clinical Practice. Clin. Chem. 2005, 51, 1558–1559. [Google Scholar] [CrossRef] [PubMed]
- Cunninghamn, J.; Leffell, M.; Mearkle, P.; Harmatz, P. Elevated plasma ceruloplasmin in insulin-dependent diabetes mellitus: Evidence for increased oxidative stress as a variable complication. Metabolism 1995, 44, 996–999. [Google Scholar] [CrossRef] [PubMed]
- Tsalamandris, S.; Antonopoulos, A.S.; Oikonomou, E.; Papamikroulis, G.-A.; Vogiatzi, G.; Papaioannou, S.; Deftereos, S.; Tousoulis, D. The Role of Inflammation in Diabetes: Current Concepts and Future Perspectives. Eur. Cardiol. Rev. 2019, 14, 50–59. [Google Scholar] [CrossRef]
- Mozafari, N.; Azadi, S.; Mehdi-Alamdarlou, S.; Ashrafi, H.; Azadi, A. Inflammation: A bridge between diabetes and COVID-19, and possible management with sitagliptin. Med Hypotheses 2020, 143, 110111–110111. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.J.; Jung, C.H.; Kang, Y.M.; Jang, J.E.; Leem, J.; Park, J.-Y.; Lee, W.J. Serum Ceruloplasmin Level as a Predictor for the Progression of Diabetic Nephropathy in Korean Men with Type 2 Diabetes Mellitus. Diabetes Metab. J. 2015, 39, 230–239. [Google Scholar] [CrossRef]
- Asmat, U.; Abad, K.; Ismail, K. Diabetes mellitus and oxidative stress—A concise review. Saudi Pharm. J. 2015, 24, 547–553. [Google Scholar] [CrossRef]
- Wronka, M.; Krzemińska, J.; Młynarska, E.; Rysz, J.; Franczyk, B. The Influence of Lifestyle and Treatment on Oxidative Stress and Inflammation in Diabetes. Int. J. Mol. Sci. 2022, 23, 15743. [Google Scholar] [CrossRef]
- Blesia, V.; Patel, V.B.; Al-Obaidi, H.; Renshaw, D.; Zariwala, M.G. Excessive Iron Induces Oxidative Stress Promoting Cellular Perturbations and Insulin Secretory Dysfunction in MIN6 Beta Cells. Cells 2021, 10, 1141. [Google Scholar] [CrossRef]
- Bhatti, J.S.; Sehrawat, A.; Mishra, J.; Sidhu, I.S.; Navik, U.; Khullar, N.; Kumar, S.; Bhatti, G.K.; Reddy, P.H. Oxidative stress in the pathophysiology of type 2 diabetes and related complications: Current therapeutics strategies and future perspectives. Free. Radic. Biol. Med. 2022, 184, 114–134. [Google Scholar] [CrossRef]
- Kotha, R.R.; Tareq, F.S.; Yildiz, E.; Luthria, D.L. Oxidative Stress and Antioxidants—A Critical Review on In Vitro Antioxidant Assays. Antioxidants 2022, 11, 2388. [Google Scholar] [CrossRef]
- Eguchi, N.; Vaziri, N.D.; Dafoe, D.C.; Ichii, H. The Role of Oxidative Stress in Pancreatic β Cell Dysfunction in Diabetes. Int. J. Mol. Sci. 2021, 22, 1509. [Google Scholar] [CrossRef] [PubMed]
- Dinić, S.; Jovanović, J.A.; Uskoković, A.; Mihailović, M.; Grdović, N.; Tolić, A.; Rajić, J.; Đorđević, M.; Vidaković, M. Oxidative stress-mediated beta cell death and dysfunction as a target for diabetes management. Front. Endocrinol. 2022, 13. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, A.; Muralidharan, C.; May, S.C.; A Tersey, S.; Mirmira, R.G. Inside the β Cell: Molecular Stress Response Pathways in Diabetes Pathogenesis. Endocrinology 2022, 164. [Google Scholar] [CrossRef] [PubMed]
- Marku, A.; Galli, A.; Marciani, P.; Dule, N.; Perego, C.; Castagna, M. Iron Metabolism in Pancreatic Beta-Cell Function and Dysfunction. Cells 2021, 10, 2841. [Google Scholar] [CrossRef]
- Silvestri, L.; Pettinato, M.; Furiosi, V.; Volpe, L.B.; Nai, A.; Pagani, A. Managing the Dual Nature of Iron to Preserve Health. Int. J. Mol. Sci. 2023, 24, 3995. [Google Scholar] [CrossRef]
- Mancardi, D.; Mezzanotte, M.; Arrigo, E.; Barinotti, A.; Roetto, A. Iron Overload, Oxidative Stress, and Ferroptosis in the Failing Heart and Liver. Antioxidants 2021, 10, 1864. [Google Scholar] [CrossRef]
- Jr, R.T.M. Hepcidin, Iron, and COVID-19: Is There an Erythroid Connection? J. Investig. Med. 2022, 70, 861–862. [Google Scholar] [CrossRef]
- Ehsani, S. COVID-19 and iron dysregulation: distant sequence similarity between hepcidin and the novel coronavirus spike glycoprotein. Biol. Direct 2020, 15, 1–13. [Google Scholar] [CrossRef]
- Zeinivand, M.; Jamali-Raeufy, N.; Zavvari, F. The beneficial role of Hepcidin peptide inhibitor in improved the symptoms of COVID-19 in diabetics: anti-inflammatory and potential therapeutic effects. J. Diabetes Metab. Disord. 2022, 21, 1797–1807. [Google Scholar] [CrossRef]
- Kim, J.D.; Lim, D.-M.; Park, K.-Y.; Park, S.E.; Rhee, E.J.; Park, C.-Y.; Lee, W.-Y.; Oh, K.W. Serum Transferrin Predicts New-Onset Type 2 Diabetes in Koreans: A 4-Year Retrospective Longitudinal Study. Endocrinol. Metab. 2020, 35, 610–617. [Google Scholar] [CrossRef]
- Claise, C.; Saleh, J.; Rezek, M.; Vaulont, S.; Peyssonnaux, C.; Edeas, M. Low transferrin levels predict heightened inflammation in patients with COVID-19: New insights. Int. J. Infect. Dis. 2022, 116, 74–79. [Google Scholar] [CrossRef] [PubMed]
- Czempik, P.F.; Wiórek, A. Comparison of Standard and New Iron Status Biomarkers: A Prospective Cohort Study in Sepsis Patients. Healthcare 2023, 11, 995. [Google Scholar] [CrossRef] [PubMed]
- Alsagaby, S.A.; Aljouie, A.; Alshammari, T.H.; Mir, S.A.; Alhumaydhi, F.A.; Al Abdulmonem, W.; Alshaalan, H.; Alomaish, H.; Daghistani, R.; Alsehawi, A.; et al. Haematological and radiological-based prognostic markers of COVID-19. J. Infect. Public Heal. 2021, 14, 1650–1657. [Google Scholar] [CrossRef]
- Pérez, F.M.; del Pino, J.L.; García, N.J.; Ruiz, E.M.; Méndez, C.A.; Jiménez, J.G.; Romero, F.N.; Rodríguez, M.N. Comorbidity and prognostic factors on admission in a COVID-19 cohort of a general hospital. Rev Clin Esp 2020, 221, 529–535. [Google Scholar] [CrossRef]
- Agarwal, S.; Schechter, C.; Southern, W.; Crandall, J.P.; Tomer, Y. Preadmission Diabetes-Specific Risk Factors for Mortality in Hospitalized Patients With Diabetes and Coronavirus Disease 2019. Diabetes Care 2020, 43, 2339–2344. [Google Scholar] [CrossRef] [PubMed]
- Karasneh, R.A.; Khassawneh, B.Y.; Al-Azzam, S.; Al-Mistarehi, A.-H.; Lattyak, W.J.; Aldiab, M.; Kabbaha, S.; Hasan, S.S.; Conway, B.R.; Aldeyab, M.A. Risk Factors Associated with Mortality in COVID-19 Hospitalized Patients: Data from the Middle East. Int. J. Clin. Pr. 2022, 2022, 1–10. [Google Scholar] [CrossRef]
- Costa, F.F.; Rosário, W.R.; Farias, A.C.R.; de Souza, R.G.; Gondim, R.S.D.; Barroso, W.A. Metabolic syndrome and COVID-19: An update on the associated comorbidities and proposed therapies. Diabetes Metab. Syndr. Clin. Res. Rev. 2020, 14, 809–814. [Google Scholar] [CrossRef]
- Rastad, H.; Karim, H.; Ejtahed, H.-S.; Tajbakhsh, R.; Noorisepehr, M.; Babaei, M.; Azimzadeh, M.; Soleimani, A.; Inanloo, S.H.; Hassani, N.S.; et al. Risk and predictors of in-hospital mortality from COVID-19 in patients with diabetes and cardiovascular disease. Diabetol. Metab. Syndr. 2020, 12, 1–11. [Google Scholar] [CrossRef]
- Han, M.; Ma, K.; Wang, X.; Yan, W.; Wang, H.; You, J.; Wang, Q.; Chen, H.; Guo, W.; Chen, T.; et al. Immunological Characteristics in Type 2 Diabetes Mellitus Among COVID-19 Patients. Front. Endocrinol. 2021, 12, 596518. [Google Scholar] [CrossRef]
- Koh, H.; Moh, A.M.C.; Yeoh, E.; Lin, Y.; Low, S.K.M.; Ooi, S.T.; Tan, S.K.; Lin, J.H.X.; Hoong, C.W.S. Diabetes predicts severity of COVID-19 infection in a retrospective cohort: A mediatory role of the inflammatory biomarker C-reactive protein. J. Med Virol. 2021, 93, 3023–3032. [Google Scholar] [CrossRef]
- Alshukry, A.; Abbas, M.B.; Ali, Y.; Alahmad, B.; Al-Shammari, A.A.; Alhamar, G.; Abu-Farha, M.; AbuBaker, J.; Devarajan, S.; Dashti, A.A.; et al. Clinical characteristics and outcomes of COVID-19 patients with diabetes mellitus in Kuwait. Heliyon 2021, 7, e06706–e06706. [Google Scholar] [CrossRef] [PubMed]
- Pérez-García, N.; García-González, J.; Requena-Mullor, M.; Rodríguez-Maresca, M. .; Alarcón-Rodríguez, R. Comparison of Analytical Values D-Dimer, Glucose, Ferritin and C-Reactive Protein of Symptomatic and Asymptomatic COVID-19 Patients. Int. J. Environ. Res. Public Heal. 2022, 19, 5354. [Google Scholar] [CrossRef] [PubMed]
- Shakaroun, D.A.; Lazar, M.H.; Horowitz, J.C.; Jennings, J.H. Serum Ferritin as a Predictor of Outcomes in Hospitalized Patients with Covid-19 Pneumonia. J. Intensiv. Care Med. 2022, 38, 21–26. [Google Scholar] [CrossRef]
- Yu, L.; Yan, J.; Zhang, Q.; Lin, H.; Zhu, L.; Liu, Q.; Zhao, C. Association between Serum Ferritin and Blood Lipids: Influence of Diabetes and hs-CRP Levels. J. Diabetes Res. 2020, 2020, 4138696–12. [Google Scholar] [CrossRef] [PubMed]
- Banchini, F.; Cattaneo, G.M.; Capelli, P. Serum ferritin levels in inflammation: a retrospective comparative analysis between COVID-19 and emergency surgical non-COVID-19 patients. World J. Emerg. Surg. 2021, 16, 1–7. [Google Scholar] [CrossRef] [PubMed]
- McLaughlin, K.-M.; Bechtel, M.; Bojkova, D.; Münch, C.; Ciesek, S.; Wass, M.N.; Michaelis, M.; Cinatl, J. COVID-19-Related Coagulopathy—Is Transferrin a Missing Link? Diagnostics 2020, 10, 539. [Google Scholar] [CrossRef]
- Peng, D.; Gao, Y.; Zhang, L.; Liu, Z.; Wang, H.; Liu, Y. The Relationship Between Hepcidin-Mediated Iron Dysmetabolism and COVID-19 Severity: A Meta-Analysis. Front. Public Heal. 2022, 10, 881412. [Google Scholar] [CrossRef]
- Ciotti, M.; Nuccetelli, M.; Pieri, M.; Petrangeli, C.M.; Giovannelli, A.; Cosio, T.; Rosa, L.; Valenti, P.; Leonardis, F.; Legramante, J.M.; et al. Evaluation of Hepcidin Level in COVID-19 Patients Admitted to the Intensive Care Unit. Diagnostics 2022, 12, 2665. [Google Scholar] [CrossRef]
- Acharya, S.; Hulkoti, V.; Kumar, S.; Talwar, D.; Khanna, S.; Annadatha, A.; Madaan, S.; Verma, V.; Sagar, V.S. Association of serum ferritin with COVID-19 in a cross-sectional study of 200 intensive care unit patients in a rural hospital: Is ferritin the forgotten biomarker of mortality in severe COVID-19? J. Fam. Med. Prim. Care 2022, 11, 2045–2050. [Google Scholar] [CrossRef]
- Gaiatto, A.C.M.; Bibo, T.A.; Moreira, N.d.G.; Raimundo, J.R.S.; Alves, B.d.C.A.; Gascón, T.; Carvalho, S.S.; Pereira, E.C.; Fonseca, F.L.A.; da Veiga, G.L. COVID-19 compromises iron homeostasis: Transferrin as a target of investigation. J. Trace Elements Med. Biol. 2023, 76, 127109–127109. [Google Scholar] [CrossRef]
- Lanser, L.; Burkert, F.R.; Bellmann-Weiler, R.; Schroll, A.; Wildner, S.; Fritsche, G.; Weiss, G. Dynamics in Anemia Development and Dysregulation of Iron Homeostasis in Hospitalized Patients with COVID-19. Metabolites 2021, 11, 653. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Bai, M.; You, Q. Associations between Serum Interleukins (IL-1β, IL-2, IL-4, IL-6, IL-8, and IL-10) and Disease Severity of COVID-19: A Systematic Review and Meta-Analysis. BioMed Res. Int. 2022, 2022, 1–15. [Google Scholar] [CrossRef] [PubMed]
- McGonagle, D.; Sharif, K.; O'Regan, A.; Bridgewood, C. The Role of Cytokines including Interleukin-6 in COVID-19 induced Pneumonia and Macrophage Activation Syndrome-Like Disease. Autoimmun. Rev. 2020, 19, 102537–102537. [Google Scholar] [CrossRef]
- Oh, S.M.; Skendelas, J.P.; Macdonald, E.; Bergamini, M.; Goel, S.; Choi, J.; Segal, K.R.; Vivek, K.; Nair, S.; Leff, J. On-admission anemia predicts mortality in COVID-19 patients: A single center, retrospective cohort study. Am. J. Emerg. Med. 2021, 48, 140–147. [Google Scholar] [CrossRef]
- Sonnweber, T.; Boehm, A.; Sahanic, S.; Pizzini, A.; Aichner, M.; Sonnweber, B.; Kurz, K.; Koppelstätter, S.; Haschka, D.; Petzer, V.; et al. Persisting alterations of iron homeostasis in COVID-19 are associated with non-resolving lung pathologies and poor patients’ performance: a prospective observational cohort study. Respir. Res. 2020, 21, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.; Chen, L.; Liang, X.; Liu, X.; Gao, M.; Wang, Q.; Wei, Q.; Liu, L. Association between iron status and the risk of adverse outcomes in COVID-19. Clin. Nutr. 2020, 40, 3462–3469. [Google Scholar] [CrossRef]
- Mehta, P.; McAuley, D.F.; Brown, M.; Sanchez, E.; Tattersall, R.S.; Manson, J.J.; on behalf of theHLH Across Speciality Collaboration, UK. COVID-19: Consider cytokine storm syndromes and immunosuppression. Lancet 2020, 395, 1033–1034. [Google Scholar] [CrossRef]
- Basheer, M.; Saad, E.; Assy, N. The Cytokine Storm in COVID-19: The Strongest Link to Morbidity and Mortality in the Current Epidemic. COVID 2022, 2, 540–552. [Google Scholar] [CrossRef]
- Jain, V.; Kumar, P.; Panda, P.K.; Suresh, M.; Kaushal, K.; Mirza, A.A.; Raina, R.; Saha, S.; Omar, B.J.; Subbiah, V. Utility of IL-6 in the Diagnosis, Treatment and Prognosis of COVID-19 Patients: A Longitudinal Study. Vaccines 2022, 10, 1786. [Google Scholar] [CrossRef]
- Chen, L.Y.; Biggs, C.M.; Jamal, S.; Stukas, S.; Wellington, C.L.; Sekhon, M.S. Soluble interleukin-6 receptor in the COVID-19 cytokine storm syndrome. Cell Rep. Med. 2021, 2, 100269. [Google Scholar] [CrossRef]
- Hackler, J.; Heller, R.A.; Sun, Q.; Schwarzer, M.; Diegmann, J.; Bachmann, M.; Moghaddam, A.; Schomburg, L. Relation of Serum Copper Status to Survival in COVID-19. Nutrients 2021, 13, 1898. [Google Scholar] [CrossRef]
- Nemeth, E.; Rivera, S.; Gabayan, V.; Keller, C.; Taudorf, S.; Pedersen, B.K.; Ganz, T. IL-6 mediates hypoferremia of in-flammation by inducing the synthesis of the iron regulatory hormone hepcidin. J. Clin. Invest. 2004, 113, 1271–1276. [Google Scholar] [CrossRef] [PubMed]
- Rouaud, F.; Méan, I.; Citi, S. The ACE2 Receptor for Coronavirus Entry Is Localized at Apical Cell—Cell Junctions of Epithelial Cells. Cells 2022, 11, 627. [Google Scholar] [CrossRef] [PubMed]
- Henry, B.M.; Benoit, J.L.; Rose, J.; de Oliveira, M.H.S.; Lippi, G.; Benoit, S.W. Serum ACE activity and plasma ACE concentration in patients with SARS-CoV-2 infection. Scand. J. Clin. Lab. Investig. 2021, 81, 272–275. [Google Scholar] [CrossRef]
- Goren, T.; Yilmaz, A.; Uluturk, M.; Sabirli, R.; Kemanci, A.; Seyit, M.; Ozen, M.; Oskay, A.; Koseler, A.; Turkcuer, I. Investigation of Serum Angiotensin-Converting Enzyme (ACE) Concentration and ACE Gene Polymorphism in Patients With SARS-CoV-2 Pneumonia Admitted to the Emergency Department. Cureus 2022, 14. [Google Scholar] [CrossRef] [PubMed]
- Oz, M.; Lorke, D.E. Multifunctional angiotensin converting enzyme 2, the SARS-CoV-2 entry receptor, and critical appraisal of its role in acute lung injury. Biomed. Pharmacother. 2021, 136, 111193–111193. [Google Scholar] [CrossRef] [PubMed]
- Zemlin, A.E.; Wiese, O.J. Coronavirus disease 2019 (COVID-19) and the renin-angiotensin system: A closer look at angiotensin-converting enzyme 2 (ACE2). Ann. Clin. Biochem. Int. J. Biochem. Lab. Med. 2020, 57, 339–350. [Google Scholar] [CrossRef]
- Maira, D.; Duca, L.; Busti, F.; Consonni, D.; Salvatici, M.; Vianello, A.; Milani, A.; Guzzardella, A.; Di Pierro, E.; Aliberti, S.; et al. The role of hypoxia and inflammation in the regulation of iron metabolism and erythropoiesis in COVID-19: The IRONCOVID study. Am. J. Hematol. 2022, 97, 1404–1412. [Google Scholar] [CrossRef]
- Wu, D.; Gao, S. Analysis of the lymphocyte count in type 2 diabetic patients with coronavirus disease (COVID-19): A retrospective study in a centralized treatment center. Diabetes Res. Clin. Pr. 2020, 166, 108340–108340. [Google Scholar] [CrossRef]
- Zhang, Q.; Wei, Y.; Chen, M.; Wan, Q.; Chen, X. Clinical analysis of risk factors for severe COVID-19 patients with type 2 diabetes. J. Diabetes its Complicat. 2020, 34, 107666–107666. [Google Scholar] [CrossRef]
- Fritea, L.; Sipponen, M.; Antonescu, A.; Miere, F.G.; Chirla, R.; Vesa, C.; Cavalu, S.; Ganea, M.; Horvath, T.; Petchesi, C.; et al. Relationship between Pre-Existing Conditions in Covid-19 Patients and Inflammation. Pharmacophore 2022, 13, 41–48. [Google Scholar] [CrossRef]
Figure 1.
Regression function associated with the scatter plot for ceruloplasmin and severity of COVID-19 (an increasing tendency of the ceruloplasmin concentration depending on the level of infection).
Figure 1.
Regression function associated with the scatter plot for ceruloplasmin and severity of COVID-19 (an increasing tendency of the ceruloplasmin concentration depending on the level of infection).
Figure 2.
Regression function associated with the scatter plot for serum iron and the severity of COVID-19 (a decreasing tendency of serum iron values in dependence with the severity of the infection).
Figure 2.
Regression function associated with the scatter plot for serum iron and the severity of COVID-19 (a decreasing tendency of serum iron values in dependence with the severity of the infection).
Figure 3.
Regression function associated with the scatter plot for transferrin and severity of COVID-19 (the decreasing tendency of transferrin level depending on the severity of infection).
Figure 3.
Regression function associated with the scatter plot for transferrin and severity of COVID-19 (the decreasing tendency of transferrin level depending on the severity of infection).
Figure 4.
Regression function associated with the scatter plot for IL-6 and severity of COVID-19 (the increasing tendency of the IL-6 level depending on the severity of the infection).
Figure 4.
Regression function associated with the scatter plot for IL-6 and severity of COVID-19 (the increasing tendency of the IL-6 level depending on the severity of the infection).
Table 1.
Comparison between patients with COVID-19 and healthy subjects for the following parameters (age, IL-6, serum iron, ACE, ceruloplasmin, transferrin).
Table 1.
Comparison between patients with COVID-19 and healthy subjects for the following parameters (age, IL-6, serum iron, ACE, ceruloplasmin, transferrin).
| SAMPLE |
N |
Mean |
Std. Deviation |
p |
Test |
| Age (years) |
1 |
45 |
69.600 |
11.727 |
0.000 |
T |
| 2 |
45 |
55.000 |
3.126 |
|
|
| IL-6 (pg/mL) |
1 |
45 |
77.843 |
158.320 |
0.000 |
M-W |
| 2 |
45 |
2.052 |
1.448 |
|
|
| Serum iron (µg/dL) |
1 |
45 |
56.156 |
31.321 |
0.000 |
T |
| 2 |
45 |
93.155 |
28.035 |
|
|
| ACE (U/L) |
1 |
44 |
20.891 |
17.312 |
0.018 |
T |
| 2 |
45 |
28.099 |
9.616 |
|
|
| Ceruloplasmin (g/L) |
1 |
44 |
0.325 |
0.081 |
0.000 |
T |
| 2 |
45 |
0.273 |
0.040 |
|
|
| Transferrin (g/L) |
1 |
44 |
1.761 |
0.486 |
0.000 |
T |
| 2 |
45 |
2.707 |
0.254 |
|
|
Table 2.
Spearman correlations (Ro) of ceruloplasmin.
Table 2.
Spearman correlations (Ro) of ceruloplasmin.
| Evaluated parameters |
Ceruloplasmin |
| Ro |
p |
N |
| COVID-19 severity |
.222* |
.037 |
89 |
| Age (years) |
.162 |
.130 |
89 |
| IL-6 (pg/mL) |
.425** |
.000 |
89 |
| Serum iron (µg/dL) |
-.530** |
.000 |
89 |
| ACE (U/L) |
-.105 |
.329 |
89 |
| Transferrin (g/L) |
-.055 |
.610 |
89 |
Table 3.
Spearman correlations (Ro) of the serum iron level.
Table 3.
Spearman correlations (Ro) of the serum iron level.
| Evaluated parameters |
Serum iron |
| Ro |
p |
N |
| COVID-19 severity |
-.476** |
.000 |
90 |
| Age (years) |
-.358** |
.001 |
90 |
| IL-6 (pg/mL) |
-.617** |
.000 |
90 |
| Ceruloplasmin (g/L) |
-.530** |
.000 |
89 |
Table 4.
Spearman correlations (Ro) of the transferrin level.
Table 4.
Spearman correlations (Ro) of the transferrin level.
| Evaluated parameters |
Transferrin |
| Ro |
p |
N |
| COVID-19 severity |
-.791** |
.000 |
89 |
| Age (years) |
-.504** |
.000 |
89 |
| IL-6 (pg/mL) |
-.668** |
.000 |
89 |
| Ceruloplasmin (g/L) |
-.055 |
.610 |
89 |
| |
|
|
|
Table 5.
Spearman correlations (Ro) of age.
Table 5.
Spearman correlations (Ro) of age.
| Evaluated parameters |
Age |
| Ro |
p |
N |
| IL-6 (pg/mL) |
.497** |
.000 |
90 |
| Serum iron (µg/dL) |
-.358** |
.001 |
90 |
| ACE (U/L) |
-.346** |
.001 |
89 |
| Ceruloplasmin (g/L) |
.162 |
.130 |
89 |
| Transferrin (g/L) |
-.504** |
.000 |
89 |
Table 6.
Spearman correlations (Ro) of ACE level.
Table 6.
Spearman correlations (Ro) of ACE level.
| Evaluated parameters |
Angiotensin-converting enzyme |
| Ro |
p |
N |
| COVID-19 severity |
-.348** |
.001 |
89 |
| Age (years) |
-.346** |
.001 |
89 |
| IL-6 (pg/mL) |
-.349** |
.001 |
89 |
| Ceruloplasmin (g/L) |
-.105 |
.329 |
89 |
Table 7.
Spearman correlations (Ro) of the IL-6 level.
Table 7.
Spearman correlations (Ro) of the IL-6 level.
| Evaluated parameters |
IL-6 |
| Ro |
p |
N |
| COVID-19 severity |
.819** |
.000 |
90 |
| Age (years) |
.497** |
.000 |
90 |
| Serum iron (µg/dL) |
-.617** |
.000 |
.000 |
90 |
| ACE (U/L) |
-.349** |
.001 |
89 |
| Ceruloplasmin (g/L) |
.425** |
.000 |
89 |
| Transferrin (g/L) |
-.668** |
.000 |
89 |
Table 8.
Spearman correlations (Ro) of the clinical form of SARS-CoV-2 disease and the quantitative parameters.
Table 8.
Spearman correlations (Ro) of the clinical form of SARS-CoV-2 disease and the quantitative parameters.
| Evaluated parameters |
COVID-19 severity |
| Ro |
p |
N |
| Age (years) |
.605** |
.000 |
90 |
| IL-6 (pg/mL) |
.819** |
.000 |
90 |
| Serum iron (µg/dL) |
-.476** |
.000 |
90 |
| ACE (U/L) |
-.348** |
.001 |
89 |
| Ceruloplasmin (g/L) |
.222*
|
.037 |
89 |
| Transferrin (g/L) |
-.791** |
.000 |
89 |
Table 9.
Correlation between COVID-19 severity and ceruloplasmin, CRP, LDH, D-Dimer, Lymphocytes and INR.
Table 9.
Correlation between COVID-19 severity and ceruloplasmin, CRP, LDH, D-Dimer, Lymphocytes and INR.
| Evaluated parameters |
COVID-19 severity |
| |
Spearman Ro |
p |
N |
| Ceruloplasmin (g/L) |
-.380*
|
.011 |
44 |
| CRP (mg/L) |
.375*
|
.013 |
43 |
| LDH (U/L) |
.370*
|
.015 |
43 |
| D-Dimer (ng/mL) |
.393**
|
.009 |
43 |
| Lymphocytes number (*1000/µL) |
-.471**
|
.001 |
43 |
| INR |
.313*
|
.043 |
42 |
Table 10.
Correlation between IL-6 and CRP, neutrophils and platelets numbers.
Table 10.
Correlation between IL-6 and CRP, neutrophils and platelets numbers.
| Evaluated parameters |
IL-6 |
| |
Spearman Ro |
p |
N |
| Serum iron (µg/dL) |
-.512**
|
.000 |
45 |
| CRP (mg/L) |
.395**
|
.009 |
43 |
| Neutrophils number (*1000/µL) |
-.364*
|
.016 |
43 |
| Platelets number (*1000/µL) |
-.343*
|
.026 |
42 |
Table 11.
Serum iron, IL-6, ACE, ceruloplasmin and transferrin variation according to the patients gender.
Table 11.
Serum iron, IL-6, ACE, ceruloplasmin and transferrin variation according to the patients gender.
| Parameters |
Gender |
N |
Mean |
Std. deviation |
Test t, p |
| Serum iron (µg/dL) |
W |
29 |
56.310 |
33.495 |
0.965 |
| M |
16 |
55.875 |
27.985 |
|
| IL-6 (pg/mL) |
W |
29 |
94.028 |
187.189 |
0.245 |
| M |
16 |
48.508 |
81.807 |
|
| ACE (U/L) |
W |
29 |
19.462 |
17.039 |
0.453 |
| M |
15 |
23.653 |
18.097 |
|
| Ceruloplasmin (g/L) |
W |
29 |
0.321 |
0.070 |
0.670 |
| M |
15 |
0.332 |
0.100 |
|
| Transferrin (g/L) |
W |
29 |
1.789 |
0.536 |
0.603 |
| M |
15 |
1.70733 |
.383321 |
|
Table 12.
Other parameters variation according to the patients gender.
Table 12.
Other parameters variation according to the patients gender.
| Other Parameters |
Gender |
N |
Mean |
Deviatia std. |
Testul t, p |
| Ferritin (ng/ml) |
W |
26 |
538.081 |
379.858 |
.079 |
| M |
16 |
836.506 |
696.056 |
|
| Hemoglobin (g/dL) |
W |
27 |
12.733 |
1.684 |
.126 |
| M |
16 |
13.606 |
1.914 |
|
| RBC(*1003/µL) |
W |
27 |
4.444 |
0.568 |
.572 |
| M |
16 |
4.551 |
0.642 |
|
| Glycemia (mg/dL) |
W |
26 |
224.192 |
87.761 |
.678 |
| M |
16 |
211.875 |
100.572 |
|
| CRP (mg/L) |
W |
27 |
101.613 |
76.581 |
.837 |
| M |
16 |
96.806 |
67.662 |
|
| LDH (U/L) |
W |
27 |
382.259 |
146.793 |
.569 |
| M |
16 |
416.625 |
247.522 |
|
| Fibrinogen (mg/dL) |
W |
27 |
470.300 |
119.607 |
.435 |
| M |
16 |
504.463 |
163.552 |
|
| d-Dimer (ng/mL) |
W |
27 |
1135.111 |
2241.639 |
.546 |
| M |
16 |
1959.875 |
3820.193 |
|
| Lymfocytes number ( *1000/µL) |
W |
27 |
0.933 |
0.604 |
.440 |
| M |
16 |
1.169 |
1.111 |
|
Table 13.
The multiple linear regression model between serum iron (as dependent parameter) and ceruloplasmin, hemoglobin, RBC, platelets number and fibrinogen (as independent parameters).
Table 13.
The multiple linear regression model between serum iron (as dependent parameter) and ceruloplasmin, hemoglobin, RBC, platelets number and fibrinogen (as independent parameters).
| Model |
Unstandardized Coefficients |
Standardized Coefficients |
p |
95.0% Confidence Interval for B |
| B |
Beta |
Lower Bound |
Upper Bound |
| 1 |
Ceruloplasmin (g/L) |
-164.297 |
-.850 |
.001 |
-252.774 |
-75.819 |
| Hemoglobin(g/dL) |
15.281 |
3.066 |
.001 |
6.911 |
23.652 |
| RBC (*1003/µL) |
-26.980 |
-1.864 |
.031 |
-51.324 |
-2.636 |
| Fibrinogen(mg/dL) |
-.016 |
-.127 |
.545 |
-.070 |
.038 |
| Platets number (*1000/µL) |
.186 |
.680 |
.000 |
.097 |
.275 |
Table 14.
The coefficient of multiple correlation in the linear regression model.
Table 14.
The coefficient of multiple correlation in the linear regression model.
| Model |
R |
R Squareb |
Adjusted R Square |
Change Statistics |
| R Square Change |
Sig. F Change |
| 1 |
.942a |
.887 |
.871 |
.887 |
.000 |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).