Submitted:
16 June 2023
Posted:
20 June 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
3. Results
3.1. Cisplatin
3.1.1. Cisplatin in lung cancer
3.1.2. Cisplatin in ovarian cancer
3.1.3. Cisplatin in head and neck cancer
3.2. Arsenic Trioxide
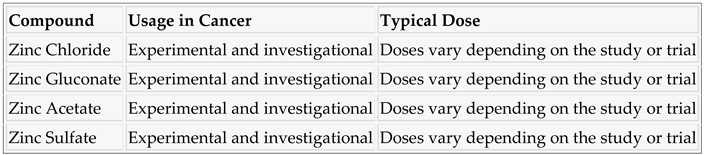
3.3. Zinc and compounds
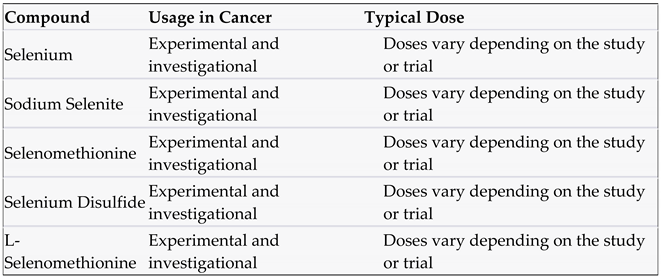
3.4. Selenium and compounds
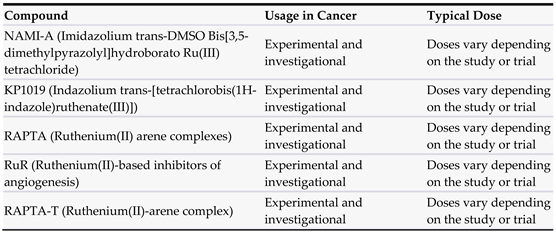
3.5. Ruthenium
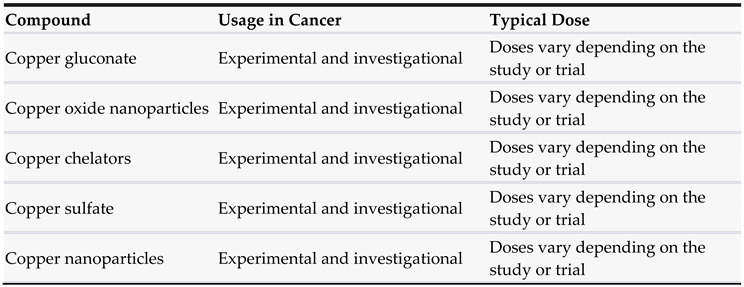
3.6. Copper
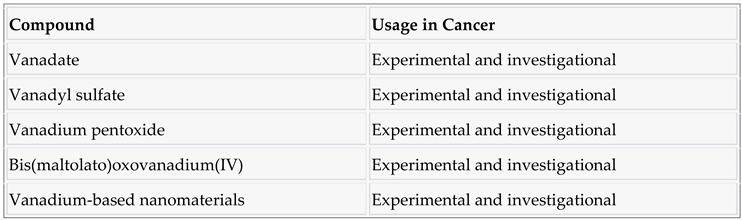
3.7. Vanadium
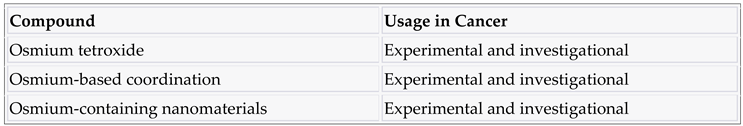
3.8. Osmium
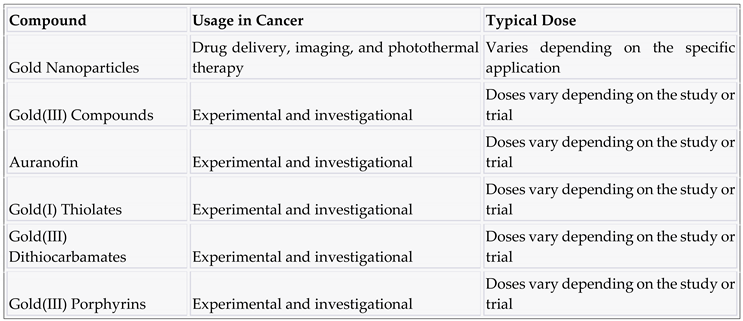
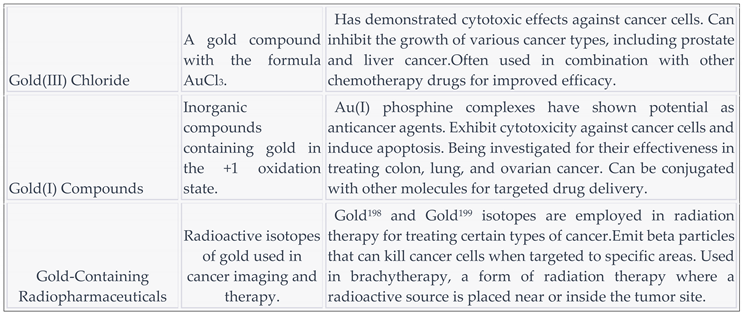
3.9. Gold and compounds
4. Discussion
5. Conclusions
Author Contributions
Conflicts of Interest
References
- IARC. Monographs Evaluate the Carcinogenicity of Occupational Exposure as a Firefighter; International Agency for Research on Cancer (IARC), the Cancer Agency of the World Health Organization; WHO: Lyon, France, 2022; p. 2. [Google Scholar]
- Gavas, S.; Quazi, S.; Karpiński, T.M. Nanoparticles for cancer therapy: Current progress and challenges. Nanoscale Res. Lett. 2021, 16, 173. [Google Scholar] [CrossRef] [PubMed]
- Persano, F.; Leporatti, S. Current Overview of inorganic nanoparticles for the treatment of central nervous system (CNS) diseases. Curr. Nanomater. 2020, 5, 92–110. [Google Scholar] [CrossRef]
- Mousa, M.; Evans, N.D.; Oreffo, R.O.; Dawson, J.I. Clay nanoparticles for regenerative medicine and biomaterial design: A review of clay bioactivity. Biomaterials 2018, 159, 204–214. [Google Scholar] [CrossRef] [PubMed]
- Soteriades, E.S.; Kim, J.; Christophi, C.A.; Kales, S.N. Cancer Incidence and Mortality in Firefighters: A State-of-the-Art Review and Meta-َAnalysis. Asian Pac. J. Cancer Prev. 2019, 20, 3221–3231. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.D.; Li, M.; Yuan, Y.; Mao, N.; Pan, L.Y. Biological comparison of ovarian cancer resistant cell lines to cisplatin and Taxol by two different administrations. Oncol. Rep. 2007, 17, 1163–1169. [Google Scholar] [CrossRef] [PubMed]
- Takakura, M.; Nakamura, M.; Kyo, S.; Hashimoto, M.; Mori, N.; Ikoma, T.; Mizumoto, Y.; Fujiwara, T.; Urata, Y.; Inoue, M. Intraperitoneal administration of telomerase-specific oncolytic adenovirus sensitizes ovarian cancer cells to cisplatin and affects survival in a xenograft model with peritoneal dissemination. Cancer Gene Ther. 2010, 17, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Nounamo, B.; Liem, J.; Cannon, M.; Liu, J. Myxoma Virus Optimizes Cisplatin for the Treatment of Ovarian Cancer In Vitro and in a Syngeneic Murine Dissemination Model. Mol. Ther. Oncolytics 2017, 6, 90–99. [Google Scholar] [CrossRef] [PubMed]
- Qin, ; Qiu, H; Zhang, M.; Zhang, F.; Yang, H.; Yang, L.; Jia, L.; Qin, K.; Jia, L.; Dou, X.; et al. Soluble CD40 ligands sensitize the epithelial ovarian cancer cells to cisplatin treatment. Biomed. Pharmacother. 2016, 79, 166–175. [CrossRef] [PubMed]
- De Brito, R.V.; Mancini, M.W.; Palumbo, M.d.N.; de Moraes, L.H.O.; Rodrigues, G.J.; Cervantes, O.; Sercarz, J.A.; Paiva, M.B. The Rationale for “Laser-Induced Thermal Therapy (LITT) and Intratumoral Cisplatin” Approach for Cancer Treatment. Int. J. Mol. Sci. 2022, 23, 5934. [Google Scholar] [CrossRef] [PubMed]
- Ali, R.; Aouida, M.; Alhaj Sulaiman, A.; Madhusudan, S.; Ramotar, D. Can Cisplatin Therapy Be Improved? Pathways That Can Be Targeted. Int. J. Mol. Sci. 2022, 23, 7241. [Google Scholar] [CrossRef] [PubMed]
- Kitareewan, S.; Roebuck, B.D.; Demidenko, E.; Sloboda, R.D.; Dmitrovsky, E. Lysosomes and Trivalent Arsenic Treatment in Acute Promyelocytic Leukemia. Gynecol. Oncol. 2007, 99, 41–52. [Google Scholar] [CrossRef] [PubMed]
- Jurcic, J.G.; Soignet, S.L.; Maslak, P. Diagnosis and treatment of acute promyelocytic leukemia. Curr. Oncol. Rep. 2007, 9, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Ramaekers, B.L.T.; Riemsma, R.; Grimm, S.; Fayter, D.; Deshpande, S.; Armstrong, N.; Witlox, W.; Pouwels, X.; Duffy, S.; Worthy, G.; et al. Arsenic Trioxide for Treating Acute Promyelocytic Leukaemia: An Evidence Review Group Perspective of a NICE Single Technology Appraisal. Pharmacoeconomics 2018, 37, 887–894. [Google Scholar] [CrossRef] [PubMed]
- Antman, K.H. Introduction: The History of Arsenic Trioxide in Cancer Therapy. Oncologgist 2001, 6, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Paek, H.J.; Lee, Y.J.; Chung, H.E.; Yoo, N.H.; Lee, J.A.; Kim, M.K.; Lee, J.K.; Jeong, J.; Choi, S.J. Modulation of the pharmacokinetics of zinc oxide nanoparticles and their fates in vivo. Nanoscale 2013, 5, 11416–11427. [Google Scholar] [CrossRef] [PubMed]
- Varadharajaperumal, P.; Muthuswamy, S.; Thiruvengadam, S.; Muthuswamy, S.; Mahalingam, S. Biosynthesised Drug-Loaded Silver Nanoparticles: A Vivid Agent for Drug Delivery On Human Breast Carcinoma. Biosci. Biotechnol. Res. Commun. 2021, 14, 1839–1846. [Google Scholar] [CrossRef]
- Sadhukhan, P.; Kundu, M.; Chatterjee, S.; Ghosh, N.; Manna, P.; Das, J.; Sil, P.C. Targeted delivery of quercetin via pH-responsive zinc oxide nanoparticles for breast cancer therapy. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 100, 129–140. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.; Anderson, D.; Dhawan, A. Zinc oxide nanoparticles induce oxidative DNA damage and ROS-triggered mitochondria mediated apoptosis in human liver cells (HepG2). Apoptosis 2012, 17, 852–870. [Google Scholar] [CrossRef] [PubMed]
- Baky, N.A.; Faddah, L.M.; Al-Rasheed, N.M.; Al-Rasheed, N.M.; Fatani, A.J. Induction of inflammation, DNA damage and apoptosis in rat heart after oral exposure to zinc oxide nanoparticles and the cardioprotective role of α-lipoic acid and vitamin E. Drug Res. 2013, 63, 228–236. [Google Scholar] [CrossRef] [PubMed]
- Chuang, H.C.; Juan, H.T.; Chang, C.N.; Yan, Y.H.; Yuan, T.H.; Wang, J.S.; Chen, H.C.; Hwang, Y.H.; Lee, C.H.; Cheng, T.J. Cardiopulmonary toxicity of pulmonary exposure to occupationally relevant zinc oxide nanoparticles. Nanotoxicology 2014, 8, 593–604. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.; Sun, K.; Wang, Y.; Dong, S.; Wang, C.; Liu, L.; Wu, Y. Role of Cyt-C/caspases-9,3, Bax/Bcl-2 and the FAS death receptor pathway in apoptosis induced by zinc oxide nanoparticles in human aortic endothelial cells and the protective effect by alpha-lipoic acid. Chem. Biol. Interact. 2016, 258, 40–51. [Google Scholar] [CrossRef] [PubMed]
- Wahab, R.; Siddiqui, M.A.; Saquib, Q.; Dwivedi, S.; Ahmad, J.; Musarrat, J.; Al-Khedhairy, A.A.; Shin, H.S. ZnO nanoparticles induced oxidative stress and apoptosis in HepG2 and MCF-7 cancer cells and their antibacterial activity. Colloids Surf. B 2014, 117, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Bai, D.P.; Zhang, X.F.; Zhang, G.L.; Huang, Y.F.; Gurunathan, S. Zinc oxide nanoparticles induce apoptosis and autophagy in human ovarian cancer cells. Int. J. Nanomed. 2017, 12, 6521–6535. [Google Scholar] [CrossRef] [PubMed]
- Lungu, I.I.; Babarus, I.; Oniciuc, L.; Stefanache, A. A Review of Essential Microelements in the Immune System. International Journal of Immunology 2022, 10(1), 1–4. [Google Scholar]
- Chasteen, T.G.; Bentley, R. Biomethylation of selenium and tellurium: Microorganisms and plants. Chem. Rev. 2003, 103, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Cerwenka, E.A.J.; Cooper, W.C. Toxicology of selenium and tellurium and their compounds. Arch. Environ. Health. 1961, 3, 189–200. [Google Scholar] [CrossRef] [PubMed]
- Garberg, P.; Engman, L.; Tolmachev, V.; Lundqvist, H.; Gerdes, R.G.; Cotgreave, I.A. Binding of tellurium to hepatocellular selenoproteins during incubation with inorganic tellurite: Consequences for the activity of selenium-dependent glutathione peroxidase. Int. J. Biochem. Cell. Biol. 1999, 31, 291–301. [Google Scholar] [CrossRef] [PubMed]
- Kessi, J.; Ramuz, M.; Wehrli, E.; Spycher, M.; Bachofen, R. Reduction of selenite and detoxification of elemental selenium by the phototrophic bacterium Rhodospirillum rubrum. Appl. Environ. Microbiol. 1999, 65, 4734–4740. [Google Scholar] [CrossRef] [PubMed]
- Sors, T.G.; Ellis, D.R.; Salt, D.E. Selenium uptake, translocation, assimilation and metabolic fate in plants. Photosynth. Res. 2005, 86, 373–389. [Google Scholar] [CrossRef] [PubMed]
- Harada, T.; Takahashi, Y. Origin of the difference in the distribution behavior of tellurium and selenium in a soil-water system. Geochim. Cosmochim. Ac. 2009, 72, 1281–1294. [Google Scholar] [CrossRef]
- Fernández-Llamosas, H.; Castro, L.; Blázquez, M.L.; Díaz, E.; Carmona, M. Speeding up bioproduction of selenium nanoparticles by using Vibrio natriegens as microbial factory. Sci. Rep. 2017, 7, 16046. [Google Scholar] [CrossRef]
- Geering, H.R.; Cary, E.E.; Jones, L.H.P.; Allaway, W.H. Solubility and redox criteria for the possible forms of selenium in soils. Soil Sci. Soc. Am. Proc. 1968, 32, 35–47. [Google Scholar] [CrossRef]
- Kieliszek, M.; Błazejak, S.; Gientka, I.; Bzducha-Wróbel, A. Accumulation and metabolism of selenium by yeast cells. Appl. Microbiol. Biotechnol. 2015, 99, 5373–5382. [Google Scholar] [CrossRef]
- Jiménez-Lamana, J.; Abadálvaro, I.; Bierla, K.; Laborda, F.; Szpunar, J.; Lobinski, R. Detection and characterization of biogenic selenium nanoparticles in selenium-rich yeast by single particle ICPMS. J. Anal. At. Spectrom. 2018, 33, 452–460. [Google Scholar] [CrossRef]
- Grones, J.; Macor, M.; Siekel, P.; Bilska, V. Capability of Escherichia coli and Lactobacillus spp. to accumulate selenium in a biologically utilisable form. Bull. Food Res. 1999, 38, 45–53. [Google Scholar]
- Macor, M.; Grones, J. Genetic basis of selenium incorporation into proteins in bacterial cells. Bull. Food Res. 2001, 40, 101–118. [Google Scholar]
- Bergamo, A.; Gaiddon, C.; Schellens, J.H.M.; Beijnen, J.H.; Sava, G. Approaching tumour therapy beyond platinum drugs: Status of the art and perspectives of ruthenium drug candidates. J. Inorg. Biochem. 2012, 106, 90–99. [Google Scholar] [CrossRef]
- Moreira, T.; Francisco, R.; Comsa, E.; Duban-Deweer, S.; Labas, V.; Teixeira-Gomes, A.-P.; Combes-Soia, L.; Marques, F.; Matos, A.; Favrelle-Huret, A.; et al. Polymer ‘ruthenium-cyclopentadienyl’ conjugates—New emerging anti-cancer drugs. Eur. J. Med. Chem. 2019, 168, 373–384. [Google Scholar] [CrossRef]
- Dougan, S.J.; Sadler, P.J. The design of organometallic ruthenium arene anticancer agents. Chimia 2007, 61, 704–715. [Google Scholar] [CrossRef]
- Murray, B.S.; Babak, M.V.; Hartinger, C.G.; Dyson, P.J. The development of RAPTA compounds for the treatment of tumors. Coord. Chem. Rev. 2016, 306, 86–114. [Google Scholar] [CrossRef]
- Motswainyana, W.M.; Ajibade, P.A. Anticancer Activities of Mononuclear Ruthenium(II) Coordination Complexes. Adv. Chem. 2015, 2015, 859730. [Google Scholar] [CrossRef]
- Valente, A.; Garcia, M.H.; Marques, F.; Miao, Y.; Rousseau, C.; Zinck, P. First polymer ‘ruthenium-cyclopentadienyl’ complex as potential anticancer agent. J. Inorg. Biochem. 2013, 127, 79–81. [Google Scholar] [CrossRef]
- Côrte-Real, L.; Karas, B.; Gírio, P.; Moreno, A.; Avecilla, F.; Marques, F.; Buckley, B.T.; Cooper, K.R.; Doherty, C.; Falson, P.; et al. Unprecedented inhibition of P-gp activity by a novel ruthenium-cyclopentadienyl compound bearing a bipyridine-biotin ligand. Eur. J. Med. Chem. 2019, 163, 853–863. [Google Scholar] [CrossRef]
- Pierroz, V.; Joshi, T.; Leonidova, A.; Mari, C.; Schur, J.; Ott, I.; Spiccia, L.; Ferrari, S.; Gasser, G. Molecular and Cellular Characterization of the Biological Effects of Ruthenium(II) Complexes Incorporating 2-Pyridyl-2-pyrimidine-4-carboxylic Acid. J. Am. Chem. Soc. 2012, 134, 20376–20387. [Google Scholar] [CrossRef]
- Tsvetkov, P.; Coy, S.; Petrova, B.; Dreishpoon, M.; Verma, A.; Abdusamad, M.; Rossen, J.; Joesch-Cohen, L.; Humeidi, R.; Spangler, R.D.; et al. Copper induces cell death by targeting lipoylated TCA cycle proteins. Science 2022, 375, 1254–1261. [Google Scholar] [CrossRef]
- Koizumi, M.; Fujii, J.; Suzuki, K.; Inoue, T.; Inoue, T.; Gutteridge, J.M.; Taniguchi, N. A marked increase in free copper levels in the plasma and liver of LEC rats: An animal model for Wilson disease and liver cancer. Free Radic. Res. 1998, 28, 441–450. [Google Scholar] [CrossRef]
- Siddiqui, M.A.; Alhadlaq, H.A.; Ahmad, J.; Al-Khedhairy, A.A.; Musarrat, J.; Ahamed, M. Copper oxide nanoparticles induced mitochondria mediated apoptosis in human hepatocarcinoma cells. PLoS ONE 2013, 8, e69534. [Google Scholar] [CrossRef]
- Polishchuk, E.V.; Merolla, A.; Lichtmannegger, J.; Romano, A.; Indrieri, A.; Ilyechova, E.Y.; Concilli, M.; De Cegli, R.; Crispino, R.; Mariniello, M.; et al. Activation of autophagy, observed in liver tissues from patients with wilson disease and from ATP7B-deficient animals, protects hepatocytes from copper-induced apoptosis. Gastroenterology 2019, 156, 1173–1189.e5. [Google Scholar] [CrossRef]
- Tadini-Buoninsegni, F.; Smeazzetto, S. Mechanisms of charge transfer in human copper ATPases ATP7A and ATP7B. IUBMB Life 2017, 69, 218–225. [Google Scholar] [CrossRef]
- Pessoa, J.C.; Etcheverry, S.; Gambino, D. Vanadium compounds in medicine. Coord. Chem. Rev. 2015, 301–302, 24–48. [Google Scholar] [CrossRef]
- Crans, D.C. Fifteen years of dancing with vanadium. Pure Appl. Chem. 2005, 77, 1497–1527. [Google Scholar] [CrossRef]
- Niu, X.; Xiao, R.; Wang, N.; Wang, Z.; Zhang, Y.; Xia, Q.; Yang, X. The molecular mechanisms and rational design of anti-diabetic vanadium compounds. Curr. Top. Med. Chem. 2016, 16, 811–822. [Google Scholar] [CrossRef] [PubMed]
- Tamrakar, A.K.; Maurya, C.K.; Rai, A.K. PTP1B inhibitors for type 2 diabetes treatment: A patent review (2011–2014). Expert Opin. Ther. Pat. 2014, 24, 1101–1115. [Google Scholar] [CrossRef]
- Heidari, A.; Schmitt, K.; Henderson, M.; Besana, E. Drug delivery systems (DDSs) of osmium nanoparticles on human gum cancer cells, tissues and tumors treatment under synchrotron radiation. Dent. Oral Maxillofac. Res. 2019, 5, 1–18. [Google Scholar] [CrossRef]
- Kang, S.; Gil, Y.-G.; Yim, G.; Min, D.-H.; Jang, H. Osmium–Tellurium Nanozymes for Pentamodal Combinatorial Cancer Therapy. ACS Appl. Mater. Interfaces 2021, 13, 44124–44135. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.R.K.; Wu, Y.; Ghosh, D.; Do, B.H.; Chen, K.; Dawson, M.R.; Fang, N.; Sulchek, T.A.; El-Sayed, M.A. Nuclear Membrane-Targeted Gold Nanoparticles Inhibit Cancer Cell Migration and Invasion. ACS Nano 2017, 11, 3716. [Google Scholar] [CrossRef]
- Cheheltani, R.; Ezzibdeh, R.M.; Chhour, P.; Pulaparthi, K.; Kim, J.; Jurcova, M.; Hsu, J.C.; Blundell, C.; Litt, H.I.; Ferrari, V.A.; et al. Tunable, biodegradable gold nanoparticles as contrast agents for computed tomography and photoacoustic imaging. Biomaterials 2016, 102, 87–97. [Google Scholar] [CrossRef]
- Her, S.; Jaffray, D.A.; Allen, C. Gold nanoparticles for applications in cancer radiotherapy: Mechanisms and recent advancements. Adv. Drug Deliv. Rev. 2017, 109, 84–101. [Google Scholar] [CrossRef]
- Singh, P.; Pandit, S.; Mokkapati, V.; Garg, A.; Ravikumar, V.; Mijakovic, I. Gold Nanoparticles in Diagnostics and Therapeutics for Human Cancer. Int. J. Mol. Sci. 2018, 19, 1979. [Google Scholar] [CrossRef]
- Luo, D.; Wang, X.; Zeng, S.; Ramamurthy, G.; Burda, C.; Basilion, J.P. Prostate-specific membrane antigen targeted gold nanoparticles for prostate cancer radiotherapy: Does size matter for targeted particles? Chem. Sci. 2019, 10, 8119–8128. [Google Scholar] [CrossRef]
- Tomić, S.; Đokić, J.; Vasilijić, S.; Ogrinc, N.; Rudolf, R.; Pelicon, P.; Vučević, D.; Milosavljević, P.; Janković, S.; Anžel, I.; et al. Size-Dependent Effects of Gold Nanoparticles Uptake on Maturation and Antitumor Functions of Human Dendritic Cells In Vitro. PLoS ONE 2014, 9, e96584. [Google Scholar] [CrossRef] [PubMed]
| Compound | Usage in Cancer | Typical Dose |
|---|---|---|
| Cisplatin | Testicular, ovarian, bladder, lung, head and neck cancers | 20-100 mg/m² every 3-4 weeks |
| Carboplatin | Ovarian, lung, and other types of cancers | 4-6 mg/mL/min every 3-4 weeks |
| Oxaliplatin | Colorectal cancer | 85-130 mg/m² every 2-3 weeks |
| Nedaplatin | Lung, ovarian, and head and neck cancers | 80-120 mg/m² every 3-4 weeks |
| Satraplatin | Prostate, ovarian, and other types of cancers | 80-120 mg/m² every 5-6 weeks |
| Lobaplatin | Lung, ovarian, and other types of cancers | 25-50 mg/m² every 3-4 weeks |
| Heptaplatin | Lung, gastric, and other types of cancers | 100-300 mg/m² every 3-4 weeks |
| Spiroplatin | Ovarian, cervical, and other types of gynecological cancers | 70-100 mg/m² every 3-4 weeks |
| Proplatine | Ovarian, bladder, and other types of cancers | 60-120 mg/m² every 3-4 weeks |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




