Submitted:
20 June 2023
Posted:
20 June 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
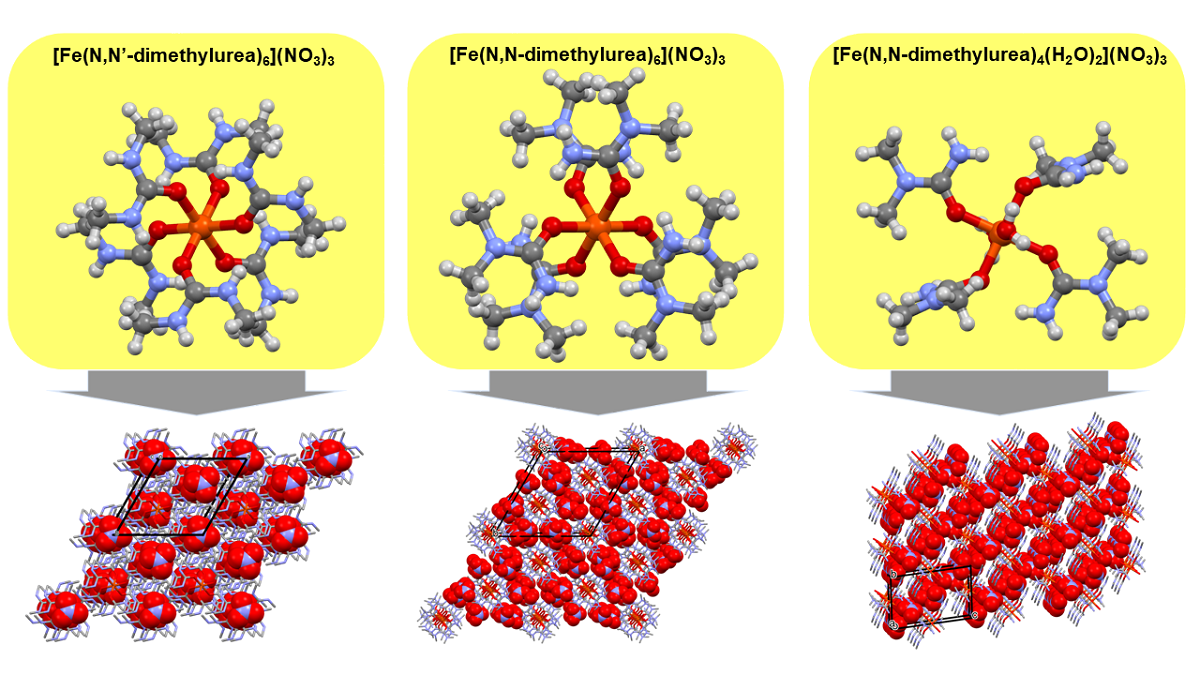
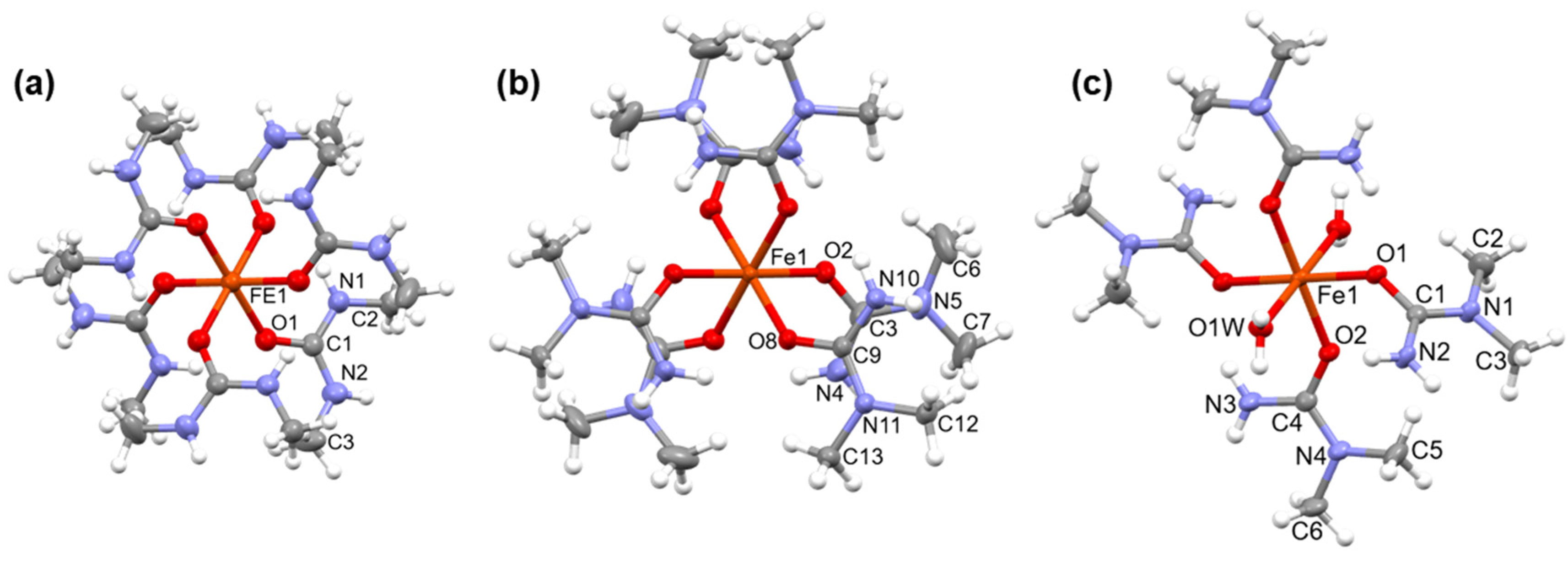
2.1. [Hexakis(N,N’-dimethyurea-O)iron(III)] nitrate (compound 1)
2.2. [Diaquatetrakis(N,N-dimethyurea-O)iron(III)] nitrate (compound 2) and [hexakis(N,N-dimethyurea-O)iron(III)] nitrate trihidrate (compound 3)
2.3. Powder X-ray Diffractometry
2.4. Single crystal X-ray diffraction
2.5. Infrared Spectroscopy
2.6. Raman Spectroscopy
2.7. UV−Vis Spectroscopy
3. Results and Discussion
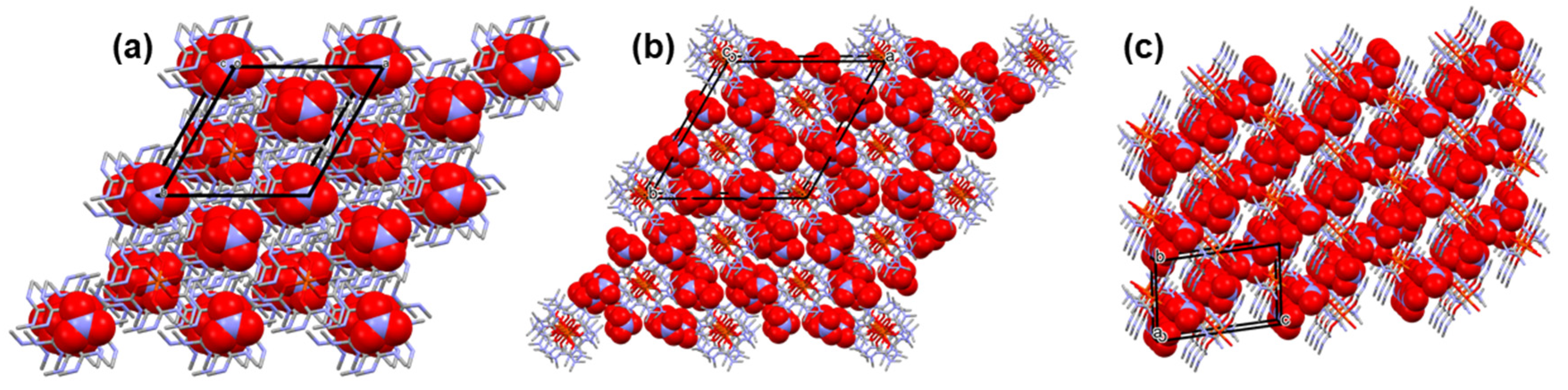
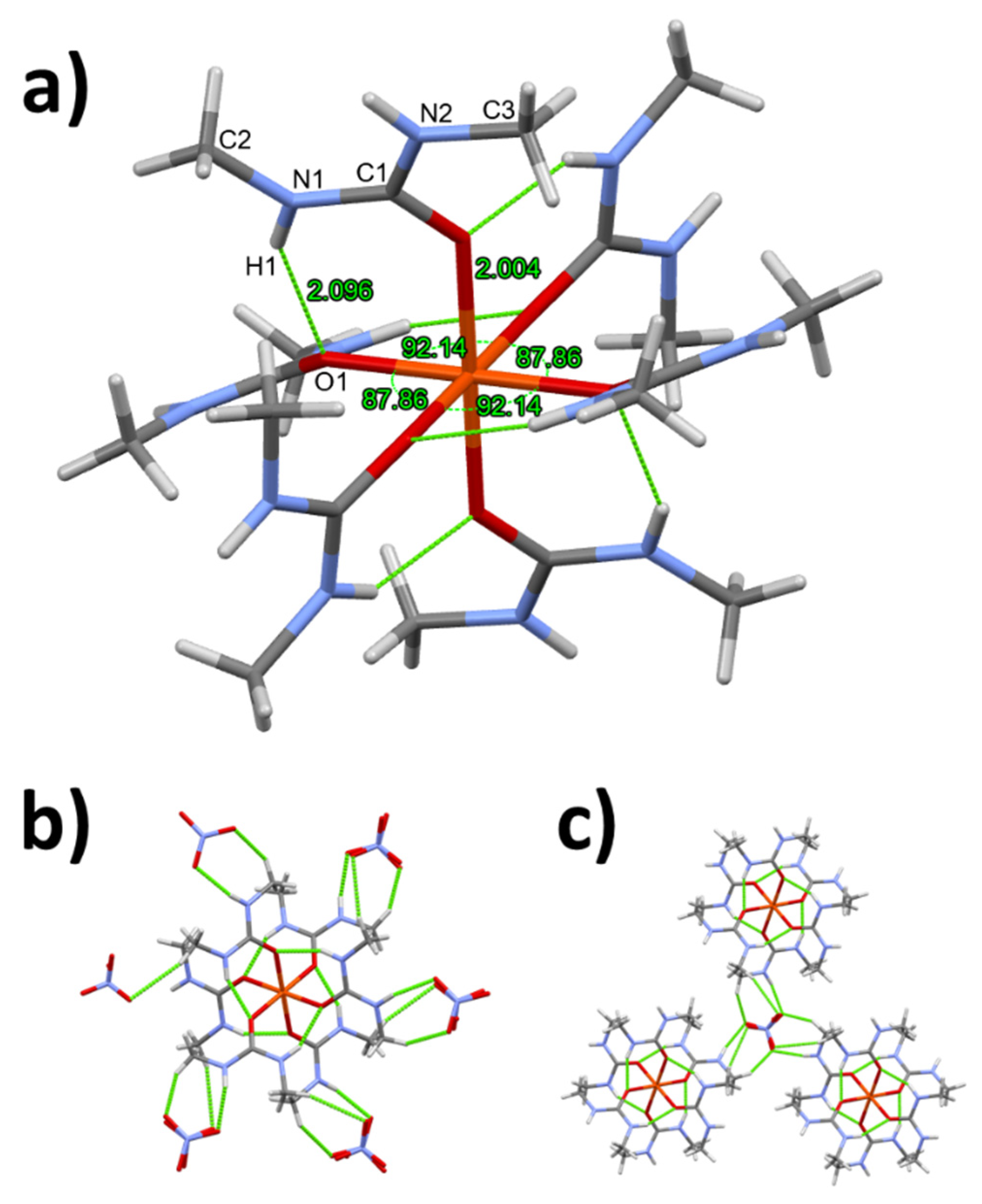
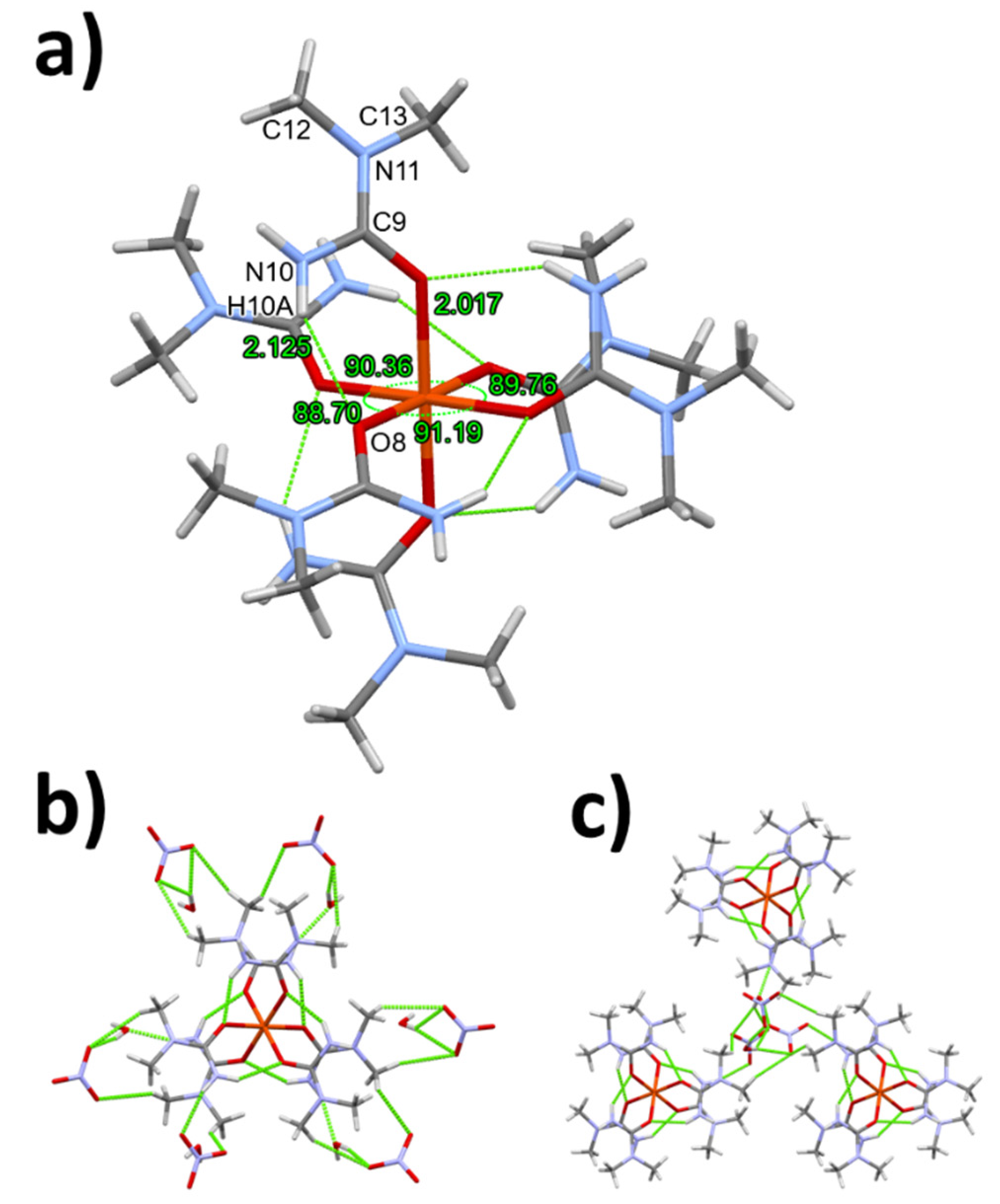
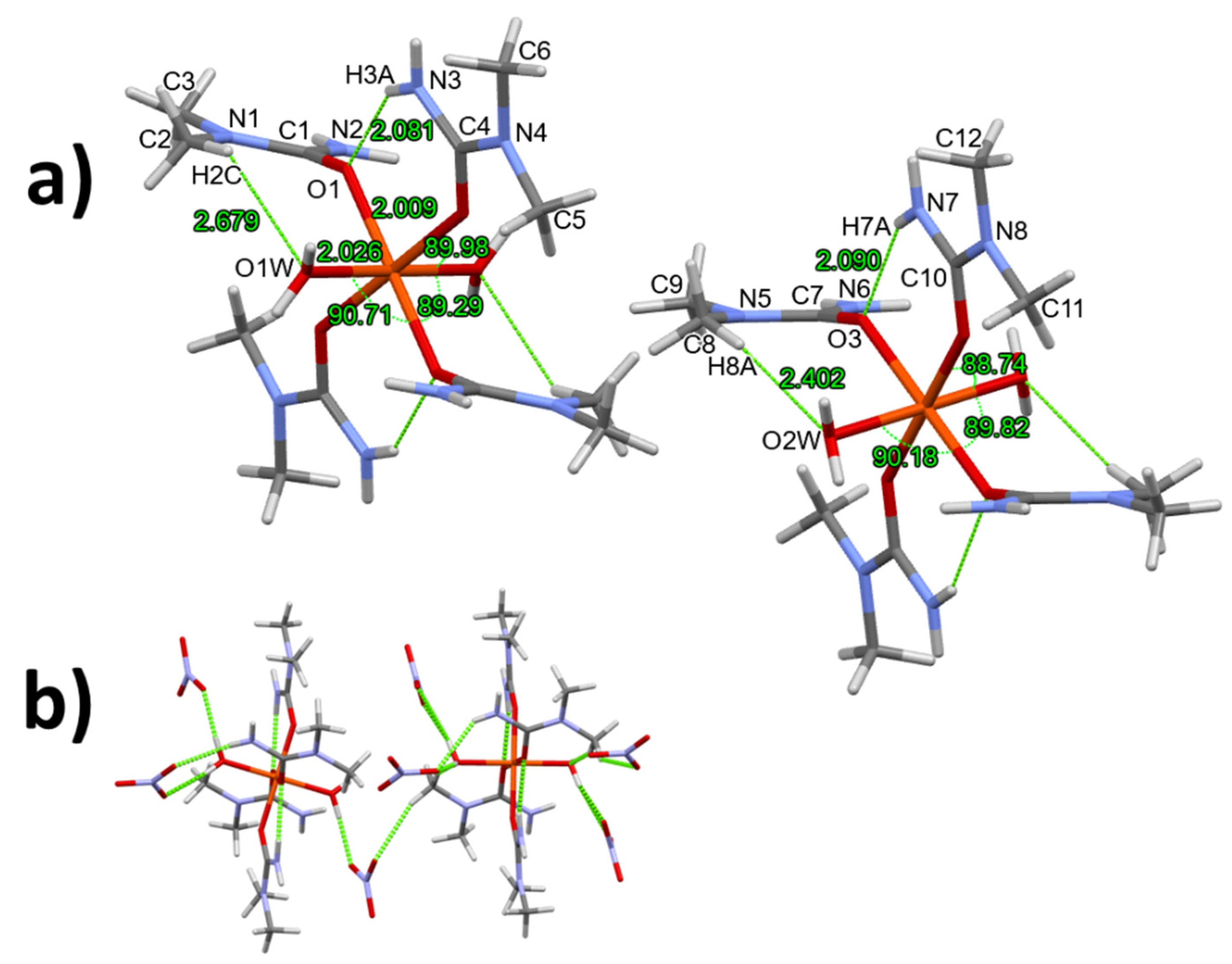
3.1. Single crystal structure of compounds 1-3
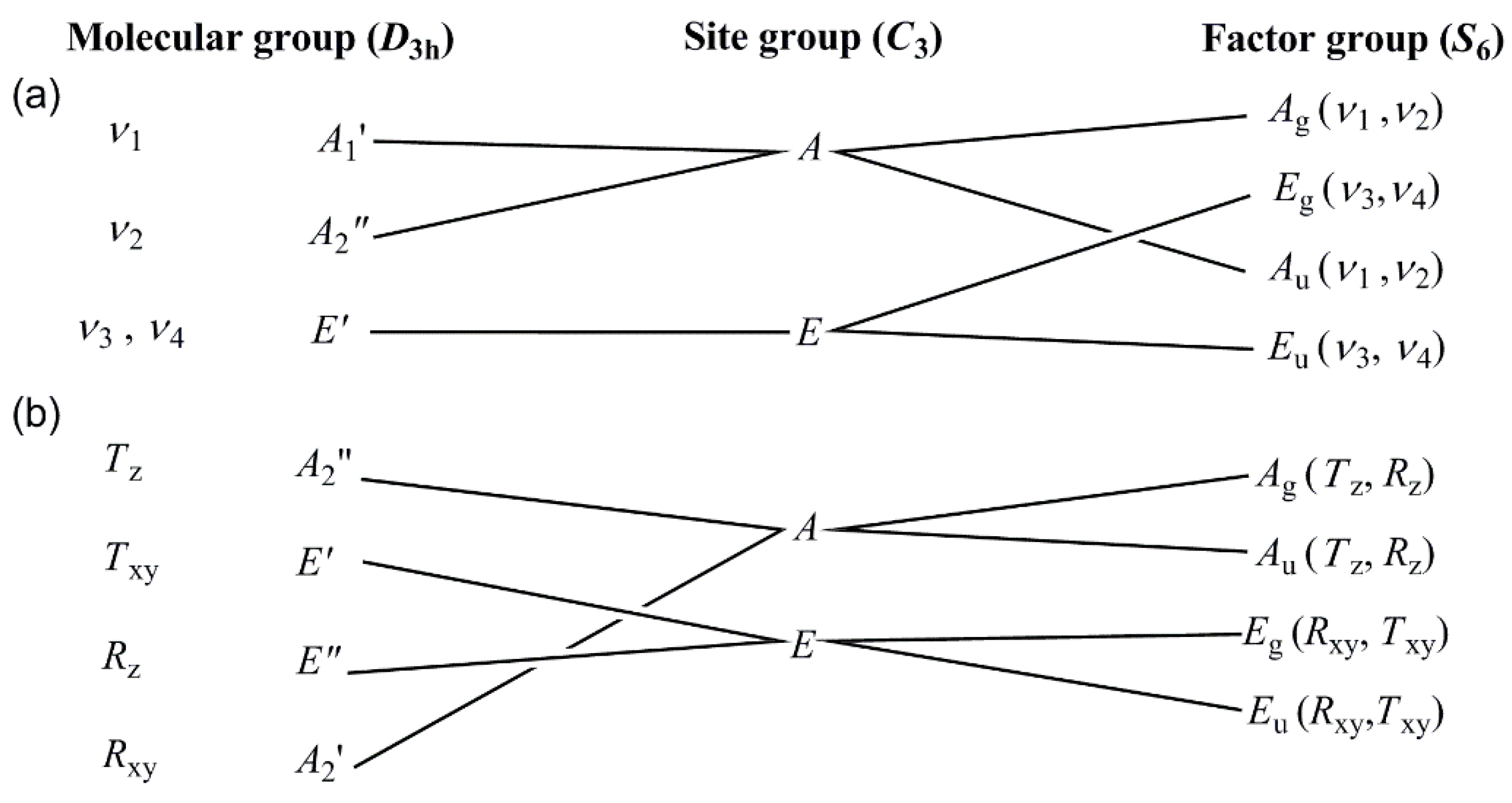
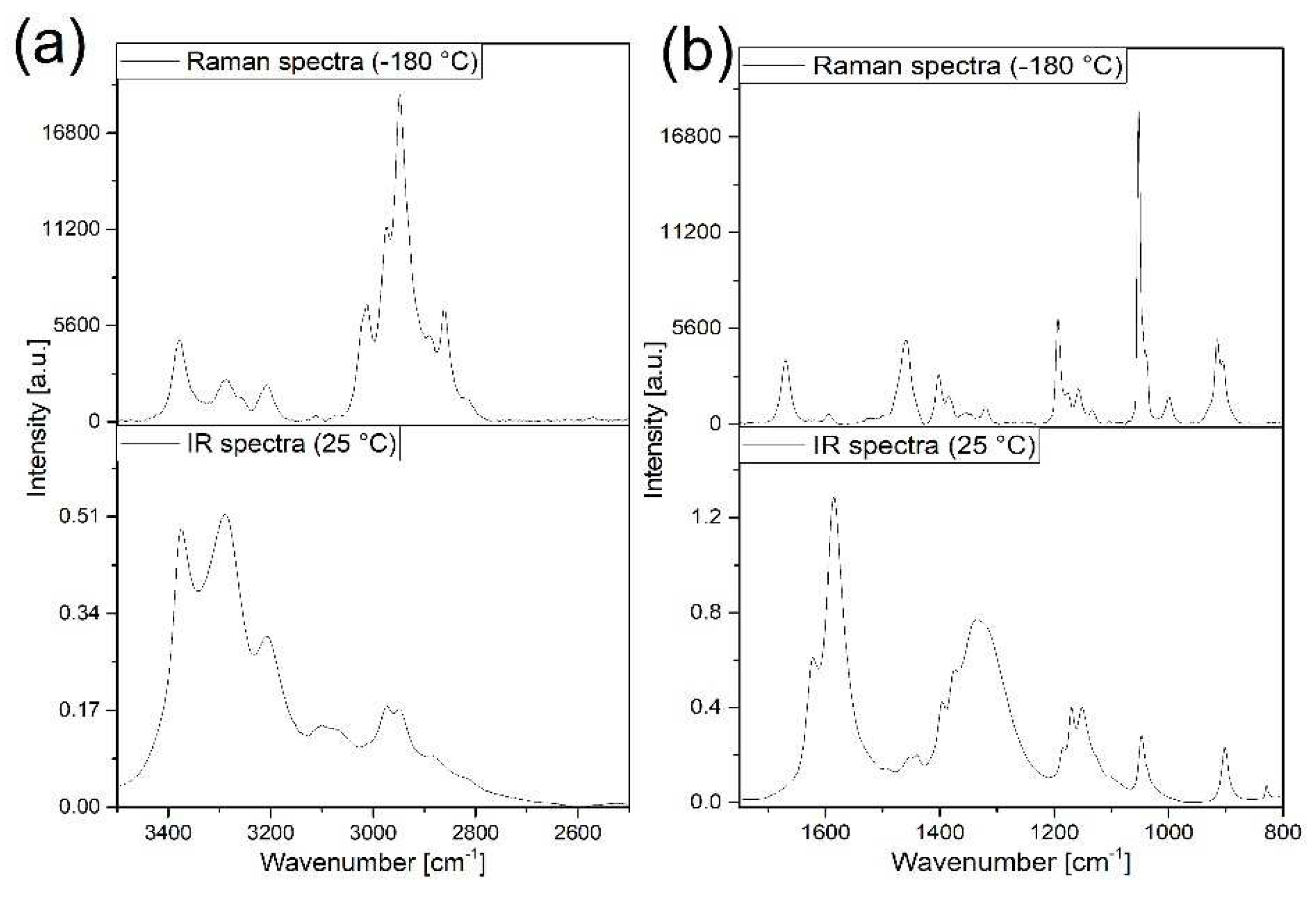
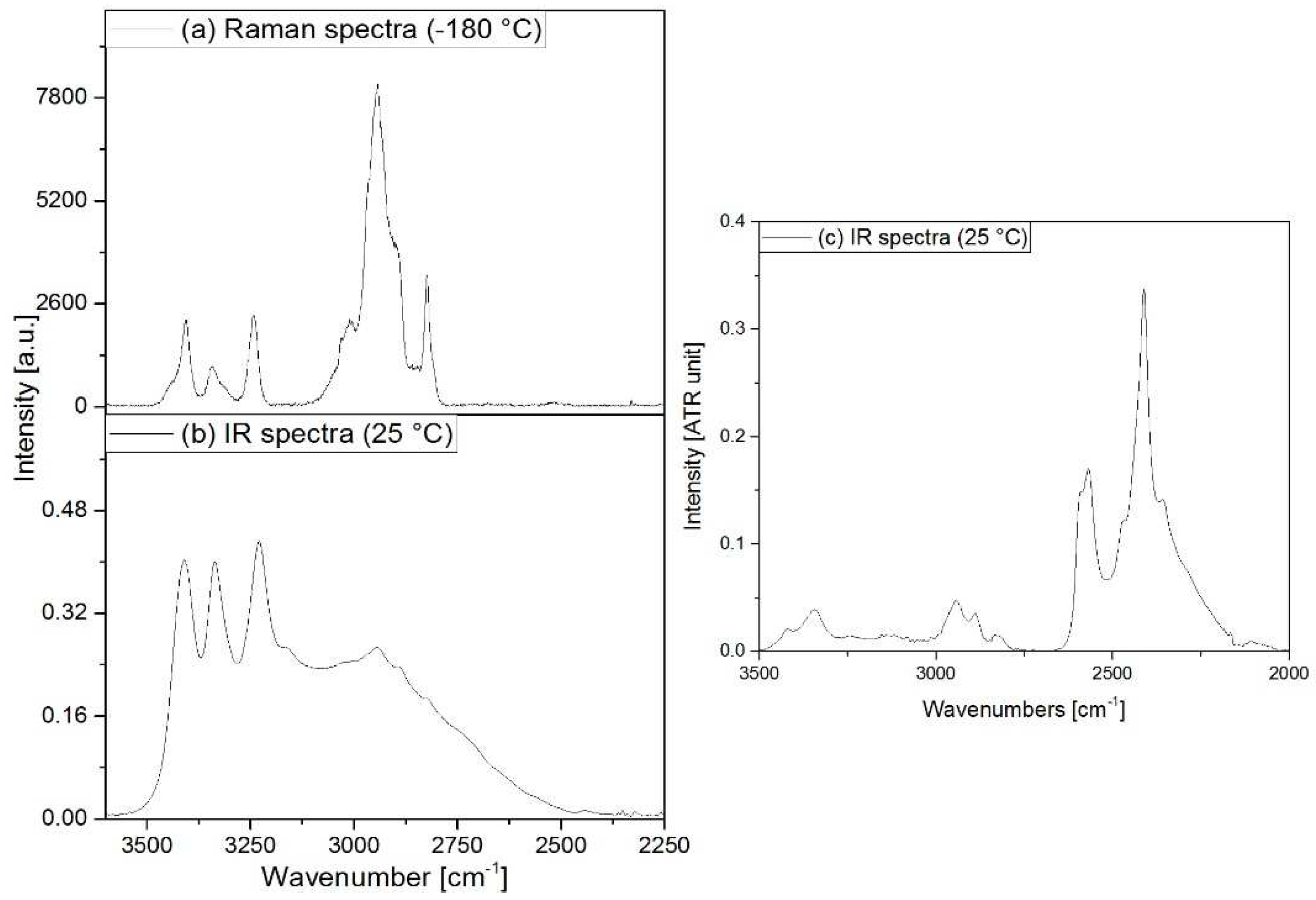
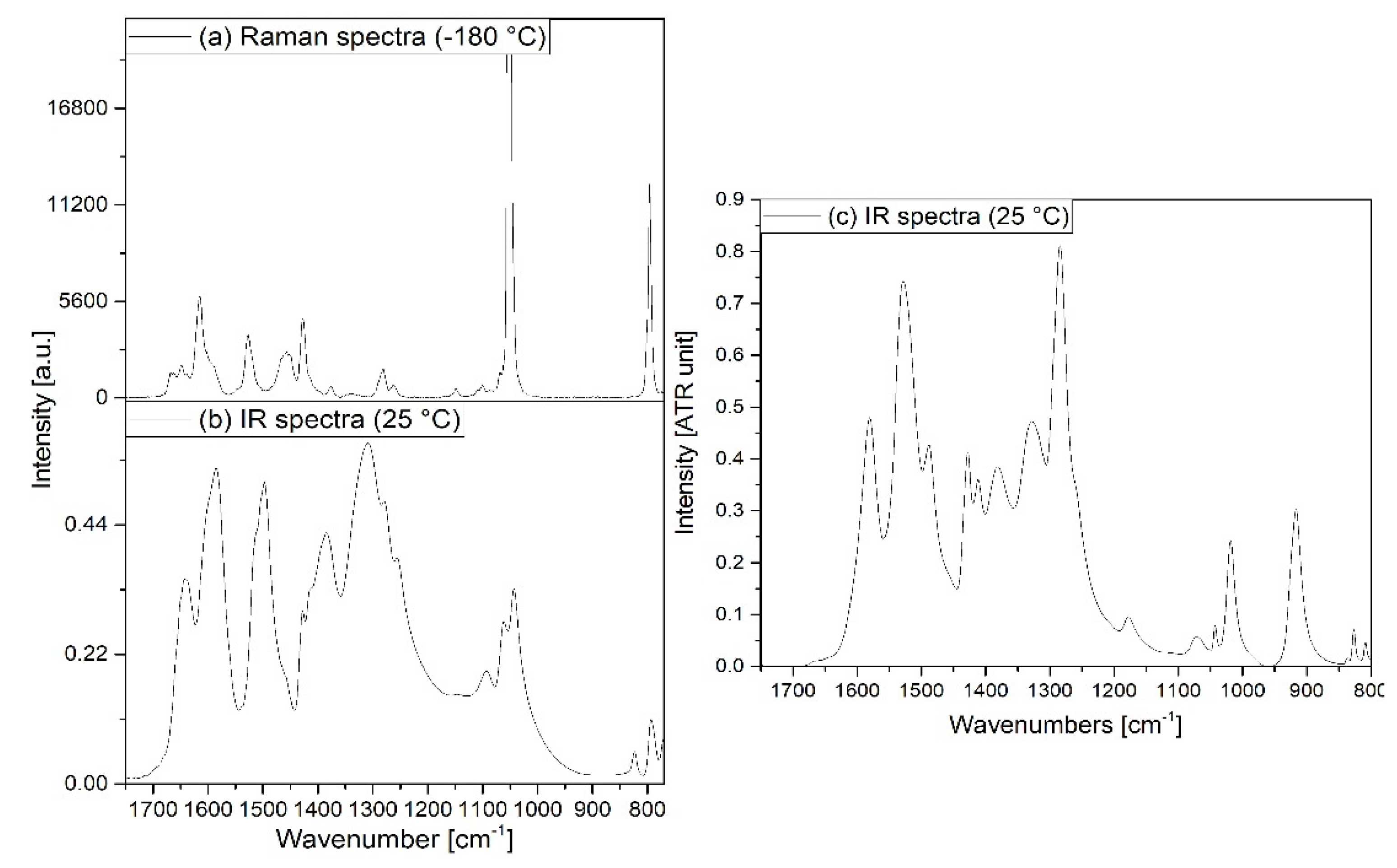
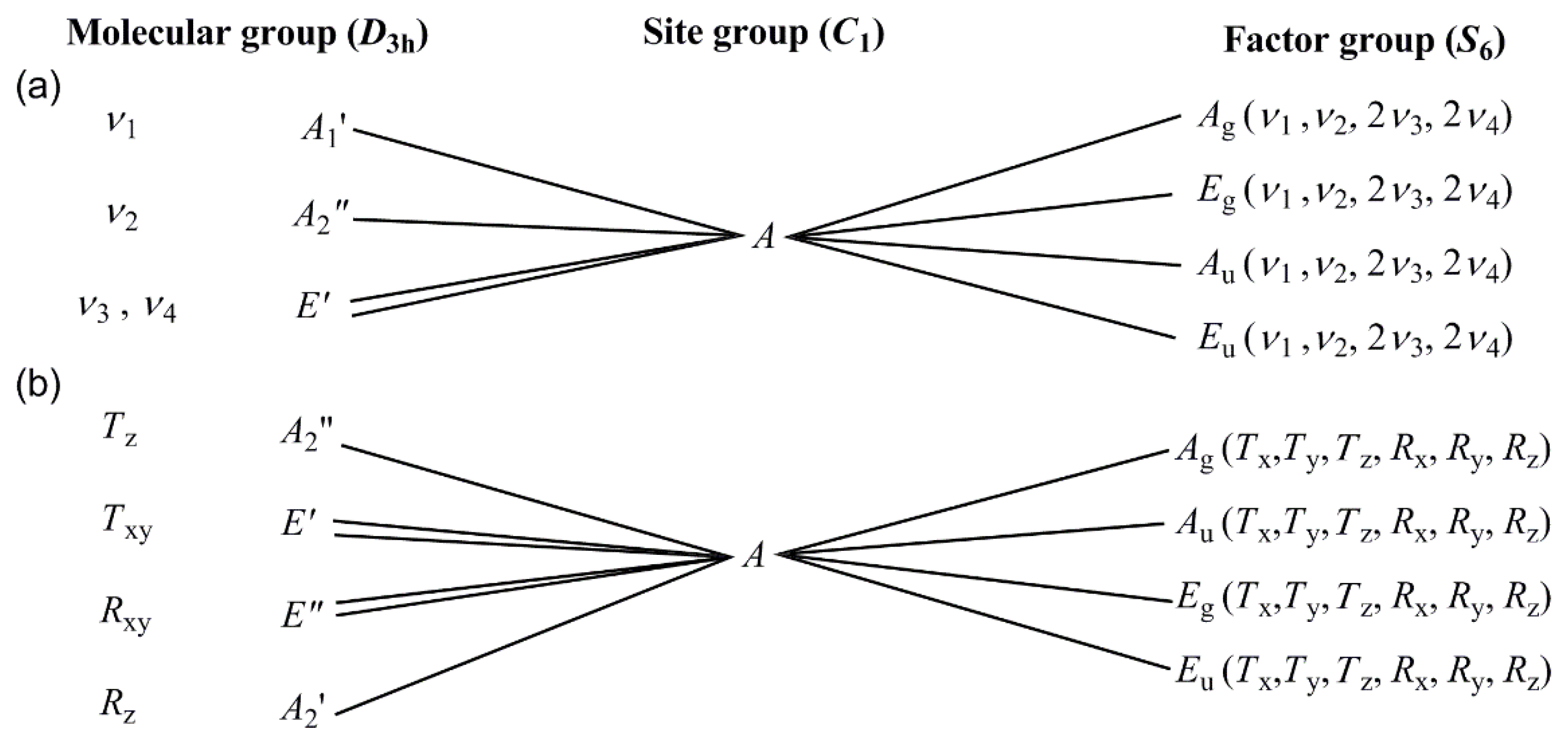
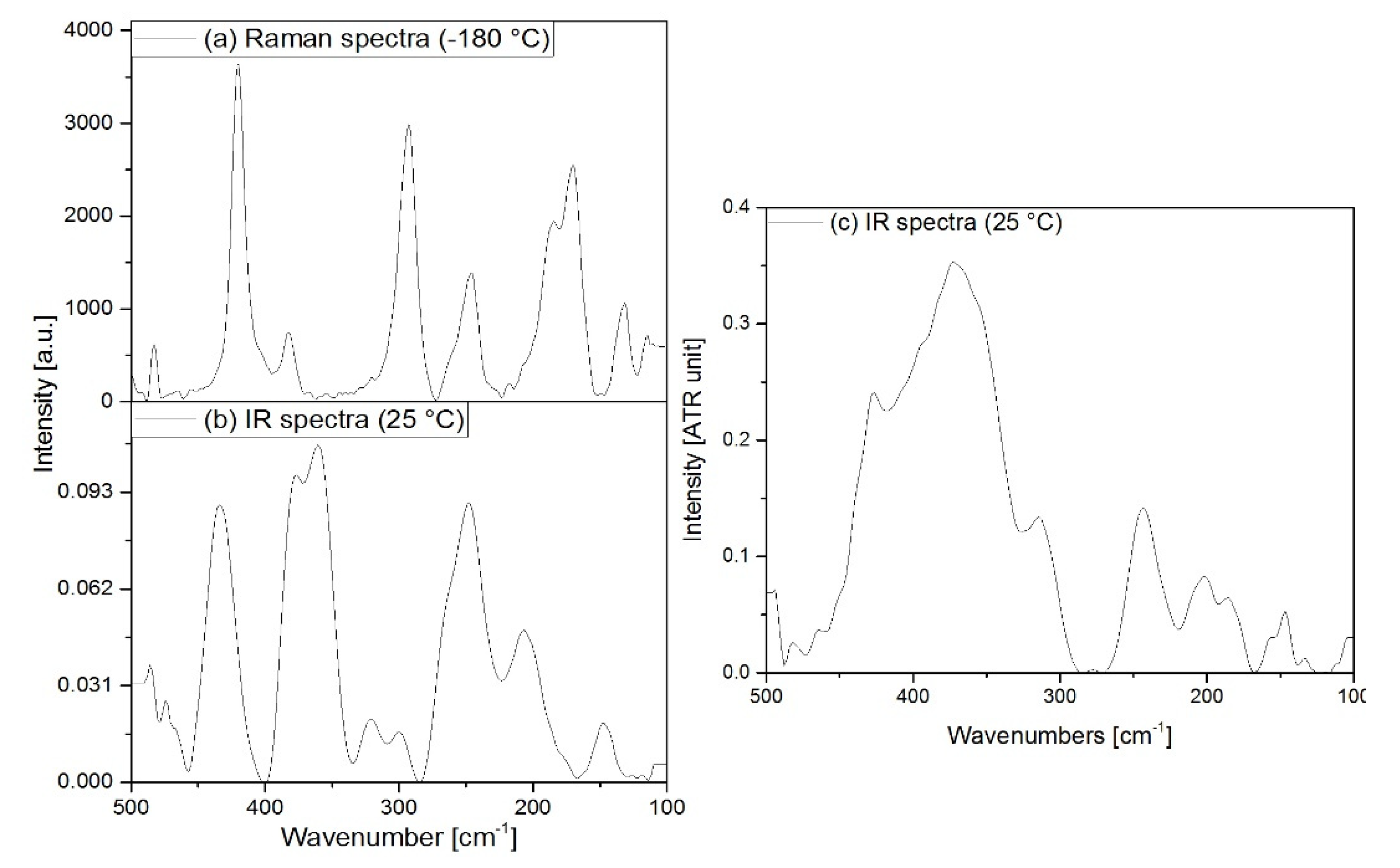
3.2. Vibrational spectrum of compound 1
3.3. Vibrational spectrum of compound 2
3.4. UV spectra of compounds 1 and 2
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Béres, K.A.; Homonnay, Z.; Kvitek, L.; Dürvanger, Z.; Kubikova, M.; Harmat, V.; Szilágyi, F.; Czégény, Z.; Németh, P.; Bereczki, L.; et al. Thermally Induced Solid−Phase Quasi−Intramolecular Redox Reactions of [Hexakis(urea−O)iron(III)] Permanganate: An Easy Reaction Route to Prepare Potential (Fe,Mn)Ox Catalysts for CO2 Hydrogenation. Inorg. Chem. 2022, 61, 14403. [Google Scholar] [CrossRef] [PubMed]
- Béres, K.A.; Homonnay, Z.; Barta Holló, B.; Gracheva, M.; Petruševski, V.M.; Farkas, A.; Dürvanger, Zs.; Kótai, L. Synthesis, structure, and Mössbauer spectroscopic studies on the heat-induced solid-phase redox reactions of hexakis(urea-O)iron(III) peroxodisulfate. J. Mater. Res. 2023, 38, 1102. [Google Scholar] [CrossRef]
- Bereczki, L.; Petruševski, V.M.; Franguelli, F.P.; Béres, K.A.; Farkas, A.; Holló, B.B.; Czégény, Z.; Szilágyi, I.M.; Kótai, L. [Hexaamminecobalt(III)] Dichloride Permanganate—Structural Features and Heat-Induced Transformations into (CoII,MnII)(CoIII,MnIII)2O4 Spinels. Inorganics 2022, 10, 252. [Google Scholar] [CrossRef]
- Franguelli, F.P.; Kováts, É.; Czégény, Z.; Bereczki, L.; Petruševski, V.M.; Barta Holló, B.; Béres, K.A.; Farkas, A.; Szilágyi, I.M.; Kótai, L. Multi-Centered Solid-Phase Quasi-Intramolecular Redox Reactions of [(Chlorido)Pentaamminecobalt(III)] Permanganate—An Easy Route to Prepare Phase Pure CoMn2O4 Spinel. Inorganics 2022, 10, 18. [Google Scholar] [CrossRef]
- Béres, K.A.; Sajó, I.E.; Lendvay, G.; Trif, L.; Petruševski, V.M.; Barta-Holló, B.; Korecz, L.; Franguelli, F.P.; László, K.; Szilágyi, I.M.; Kótai, L. Solid-Phase “Self-Hydrolysis” of [Zn(NH3)4MoO4@2H2O] Involving Enclathrated Water—An Easy Route to a Layered Basic Ammonium Zinc Molybdate Coordination Polymer. Molecules 2021, 26, 4022. [Google Scholar] [CrossRef] [PubMed]
- Fogaca, L.A.; Kováts, É.; Németh, G.; Kamarás, K.; Béres, K.A.; Németh, P.; Petruševski, V.; Bereczki, L.; Holló, B.B.; Sajó, I.E.; Klébert, Sz.; Farkas, A.; Szilágyi, I.M.; Kótai, L. Solid-Phase Quasi-Intramolecular Redox Reaction of [Ag(NH3)2]MnO4 : An Easy Way to Prepare Pure AgMnO2. Inorg Chem 2021, 60, 3749. [Google Scholar] [CrossRef]
- Fogaça, L.A.; Bereczki, L.; Petruševski, V.M.; Barta-Holló, B.; Franguelli, F.P.; Mohai, M.; Béres, K.A.; Sajó, I.E.; Szilágyi, I.M.; Kotai, L. A Quasi-Intramolecular Solid-Phase Redox Reaction of Ammonia Ligands and Perchlorate Anion in Diamminesilver(I) Perchlorate. Inorganics 2021, 9, 38. [Google Scholar] [CrossRef]
- Kovács, G.B.; May, N. v.; Bombicz, P.A.; Klébert, S.; Németh, P.; Menyhárd, A.; Novodárszki, G.; Petrusevski, V.; Franguelli, F.P.; Magyari, J.; Béres, K.; Szilágyi, I.M.; Kótai, L. An Unknown Component of a Selective and Mild Oxidant: Structure and Oxidative Ability of a Double Salt-Type Complex Having κ 1 O-Coordinated Permanganate Anions and Three- and Four-Fold Coordinated Silver Cations. RSC Adv 2019, 9, 28387. [Google Scholar] [CrossRef]
- Solt, H.E.; Németh, P.; Mohai, M.; Sajó, I.E.; Klébert, S.; Franguelli, F.P.; Fogaca, L.A.; Pawar, R.P.; Kótai, L. Temperature-Limited Synthesis of Copper Manganites along the Borderline of the Amorphous/Crystalline State and Their Catalytic Activity in CO Oxidation. ACS Omega 2021, 6, 1523. [Google Scholar] [CrossRef]
- Bereczki, L.; Fogaça, L.A.; Dürvanger, Z.; Harmat, V.; Kamarás, K.; Németh, G.; Holló, B.B.; Petruševski, V.M.; Bódis, E.; Farkas, A.; Szilágyi, I.M.; Kótai, L. Dynamic Disorder in the High-Temperature Polymorph of Bis[Diamminesilver(I)]Sulfate—Reasons and Consequences of Simultaneous Ammonia Release from Two Different Polymorphs. J. Coord. Chem. 2021, 74, 2144. [Google Scholar] [CrossRef]
- Franguelli, F.P.; Barta-Holló, B.; Petruševski, V.M.; Sajó, I.E.; Klébert, S.; Farkas, A.; Bódis, E.; Szilágyi, I.M.; Pawar, R.P.; Kótai, L. Thermal Decomposition and Spectral Characterization of Di[Carbonatotetraamminecobalt(III)] Sulfate Trihydrate and the Nature of Its Thermal Decomposition Products. J. Therm. Anal. Calorim. 2021, 145, 2907. [Google Scholar] [CrossRef]
- Trif, L.; Franguelli, F.P.; Lendvay, G.; Majzik, E.; Béres, K.; Bereczki, L.; Szilágyi, I.M.; Pawar, R.P.; Kótai, L. Thermal Analysis of Solvatomorphic Decakis (Dimethylammonium) Dihydrogendodecatungstate Hydrates. J. Therm. Anal. Calorim. 2021, 144, 81. [Google Scholar] [CrossRef]
- Petrushevski, V.M.; Béres, K.A.; Bombicz, P.; Farkas, A.; Kótai, L.; Bereczki, L. Structural and Raman Spectroscopic Characterization of Tetrapyridinesilver(I) Perrhenate, [Agpy4]ReO4. Macedonian Journal of Chemistry and Chemical Engineering 2022, 41, 37. [Google Scholar] [CrossRef]
- May, N.V.; Bayat, N.; Béres, K.A.; Bombicz, P.; Petruševski, V.M.; Lendvay, G.; Farkas, A.; Kótai, L. Structure and Vibrational Spectra of Pyridine Solvated Solid Bis(Pyridine)Silver(I) Perchlorate, [Agpy2ClO4]·0.5py. Inorganics 2022, 10, 123. [Google Scholar] [CrossRef]
- Mehrotra, R.N. Review on the chemistry of [M(NH3)n](XO4)m (M=transition metal, X=Mn, Tc or Re, n=1-6, m=1-3) ammine complexes, Inorganics, 2023, accepted for publication.
- Hu, B.; Frueh, S.; Garces, H.F.; Zhang, L.; Aindow, M.; Brooks, C.; Kreidler, E.; Suib, S.L. Selective Hydrogenation of CO2 and CO to Useful Light Olefins over Octahedral Molecular Sieve Manganese Oxide Supported Iron Catalysts. Appl Catal B 2013, 132–133, 54. [Google Scholar] [CrossRef]
- Jiang, J.; Wen, C.; Tian, Z.; Wang, Y.; Zhai, Y.; Chen, L.; Li, Y.; Liu, Q.; Wang, C.; Ma, L. Manganese-Promoted Fe 3O4 Microsphere for Efficient Conversion of CO2 to Light Olefins. Ind. Eng. Chem. Res. 2020, 59, 2155. [Google Scholar] [CrossRef]
- Liu, B.; Geng, S.; Zheng, J.; Jia, X.; Jiang, F.; Liu, X. Unravelling the New Roles of Na and Mn Promoter in CO 2 Hydrogenation over Fe3O4 -Based Catalysts for Enhanced Selectivity to Light α-Olefins. Chem.Cat.Chem. 2018, 10, 4718. [Google Scholar]
- Liu, Y.; Chen, J.-F.; Bao, J.; Zhang, Y. Manganese-Modified Fe3O4 Microsphere Catalyst with Effective Active Phase of Forming Light Olefins from Syngas. ACS Catal. 2015, 5, 3905. [Google Scholar] [CrossRef]
- Okaya, Y.; Pepinsky, R.; Takeuchi, Y.; Kuroya, H.; Shimida, A.; Gallitelli, P.; Stemple, N.; Beevers, A. X-ray analyses of some complex-ion structures. Acta Crystallogr. 1957, 10, 798. [Google Scholar]
- Kuz’mina, N.E.; Palkina, K.K.; Savinkina, E.V.; Kozlova, I.A. Products of Reactions of Manganese(II) and Iron(II) Iodides with Urea: Comparison of Structures and Properties. Zh.Neorg.Khim. 2000, 45, 332. [Google Scholar]
- Galeazzi, G.; Russo, U.; Valle, G.; Calogero, S. Mössbauer study of some iron(III) complexes with urea type ligands and the crystal structure of hexakisdimethylureairon(III) perchlorate. Transition Met. Chem. 1981, 6, 325. [Google Scholar] [CrossRef]
- Russo, U.; Calogero, S.; Pra, A.D. Characterization of some high-spin iron(III) complexes with urea derivaties. Crystal structure of diaquatetrakis(perhydropyrimidin-2-one)iron trichloride dihydrate and of perhydropyrimidin-2-one. J. Chem.Soc., DaltonTrans. 1980; 646. [Google Scholar]
- Carp, O.; Patron, L.; Diamandescu, L.; Reller, A. Thermal decomposition study of the coordination compound [Fe(urea)6](NO3)3. Thermochim. Acta 2002, 390, 169. [Google Scholar] [CrossRef]
- Zhao, S.; Sin, A. ; Synthesis of iron(III)-urea complex in organic solvent and its thermal decomposition. Huaxue Shiji 2010, 32, 108. [Google Scholar]
- Theophanides, T.; Harvey, P.D. Structural and spectroscopic properties of metal-urea complexes. Coord. Chem. Rev. 1987, 76, 237. [Google Scholar] [CrossRef]
- Drakopoulou, L.; Papatriantafyllopoulou, C.; Terzis, A.; Perlepes, S.P.; Manessi-Zoupa, E.; Papaefstathiou, G.S. Synthesis, X-Ray Structure, and Characterization of a Complex Containing the Hexakis(urea)cobalt(II) Cation and Lattice Urea Molecule. Bioinorg. Chem. Appl. 2007; 51567. [Google Scholar]
- Bala, R.; Sachdeva, D.; Kumar, M.; Parakash, V. Advances in coordination chemistry of hexaurea complexes of chromium(III). J. Coord. Chem. 2020, 73, 2801. [Google Scholar] [CrossRef]
- Aghabozorg, H.; Palenik, G.J.; Stoufer, R.C.; Summers, J. Dynamic Jahn-Teller effect in a manganese(III) complex. Synthesis and structure of hexakis(urea)manganese(III) perchlorate. Inorg. Chem. 1982, 21, 3903. [Google Scholar] [CrossRef]
- Figgis, B.N.; Wadley, L.G.B.; Graham, J. Crystal structure of hexaurea salts of trivalent metals. I. Ti(urea)6(ClO4)3 at room temperature. Acta Cryst. 1972; B28, 187. [Google Scholar]
- Russo, U.; Calogero, S.; Burriesci, N.; Petrera, M. Mössbauer characterization of some new high-spin iron complexes with urea and thiourea derivatives. J. Inorg. Nucl. Chem. 1979, 41, 25. [Google Scholar] [CrossRef]
- Yamauchi, S.; Sakai, Y.; Tominaga, T. Paramagnetic relaxation effects on Mössbauer spectra of hexakis/alkylurea/iron/III/ complexes. J. Radioanal. Nucl. Chem. 1987, 119, 283. [Google Scholar] [CrossRef]
- Yamauchi, S.; Sakai, Y.; Nishioji, H.; Tominaga, T. Mössbauer spectroscopic study of the magnetic relaxation in tris(β-diketonato)iron(III) complexes. Int. J. Appl. Radiat. Isot. 1983, 34, 977. [Google Scholar] [CrossRef]
- Martiz, A.; Károly, Z.; Domján, A.; Mohai, M.; Bereczki, L.; Trif, L.; Farkas, A.; László, K.; Menyhárd, A.; Kótai, L. Nano-ZrO2@C, Nano-(ZrC, ZrO2)@C and Nano-ZrC@C Composites Prepared by Plasma-Assisted Carbonization of Zr-Loaded Iminodiacetate-Functionalized Styrene-Divinylbenzene Copolymers. Inorganics 2022, 10, 77. [Google Scholar] [CrossRef]
- Martiz, A.; Károly, Z.; Trif, L.; Mohai, M.; Bereczki, L.; Németh, P.; Molnár, Z.; Menyhárd, A.; Pawar, R.P.; Tekale, S.; Kótai, L. Plasma-assisted preparation of nano-(ZrC, ZrO2)@ carbon composites from Zr-loaded sulfonated styrene–divinylbenzene copolymers. J. Therm. Anal. Calorim. 2022; 1. [Google Scholar]
- Sheldrick, G.M. SHELXT– Integrated space-group and crystal-structure determination. Acta Crystallogr. A 2015, 71, 3. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. C Struct. Chem. 2015, 71, 3. [Google Scholar] [CrossRef] [PubMed]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: a complete structure solution, refinement and analysis program. J. Appl. Cryst. 2009, 42, 339. [Google Scholar] [CrossRef]
- Macrae, C.F.; Sovago, I.; Cottrell, S.J.; Galek, P.T.A.; McCabe, P.; Pidcock, E.; Platings, M.; Shields, G.P.; Stevens, J.S.; Towler, M.; Wood, P.A. Mercury 4.0: from visualization to analysis, design and prediction. J. Appl. Crystallogr. 2020, 53, 226. [Google Scholar] [CrossRef]
- Kocsis, T.; Magyari, J.; Sajó, I.E.; Pasinszki, T.; Homonnay, Z.; Szilágyi, I.M.; Farkas, A.; May, Z.; Effenberger, H.; Szakáll, S.; Pawar, R.P.; Kótai, L. Evidence of quasi-intramolecular redox reactions during thermal decomposition of ammonium hydroxodisulfitoferriate(III), (NH4)2[Fe(OH)(SO3)2]·H2O. J. Therm. Anal. Calorim. 2018, 132, 493. [Google Scholar] [CrossRef]
- Smeets, S.; Lutz, M. Hexakis(urea-κO)zinc(II) dinitrate at 110 and 250 K: uniaxial negative thermal expansion. Acta Crystallogr. C Struct. Chem. 2011, 67, 50. [Google Scholar] [CrossRef] [PubMed]
- Kuz'mina, N.E.; Palkina, K.K.; Savinkina, E.V.; Kuznetsova, N.T.; Kozlova, I.A. Syntheses and Crystal Structures of Nickel(II) and Cobalt(II) Urea Diiodoiodates [Ni(CON2H4)6][I3]2·2(CON2H4) and [Co(CON2H4)6][I3]2 · 2(CON2H4). Zh.Neorg.Khim. 2000, 45, 780. [Google Scholar]
- Palkina, K.K.; Kuz'mina, N.E.; Orlova, V.; Kondokova, I. Coordination Compounds of Some Transition Metal, Magnesium, and Calcium Nitrates with Dimethylurea. Zh.Neorg.Khim 1999, 44, 963. [Google Scholar]
- Figgis, B.N.; Skelton, B.W.; White, A.H. Crystal structures of hexa(N-methylurea)cobalt(II) sulfate and thiosulfate. Aust. J. Chem. 1980, 33, 425. [Google Scholar] [CrossRef]
- Masłowska, J.; Cedzyńska, K. The structure of iron(III) complexes with carbamide derivatives. J. Mol. Struct. 1973, 19, 521. [Google Scholar] [CrossRef]
- Mido, Y.; Murata, H. Infrared Absorption Spectra of Urea-formaldehyde Initial Condensation Products. II. Dimethylurea. Bull. Chem. Soc. Jpn. 1969, 42, 3372. [Google Scholar] [CrossRef]
- Penland, R.B.; Lane, T.J.; Quagliano, J.V. Infrared Absorption Spectra of Inorganic Coordination Complexes. VII. Structural Isomerism of Nitro- and Nitritopentamminecobalt(III) Chlorides. J. Am. Chem. Soc. 1956, 78, 887. [Google Scholar] [CrossRef]
- Cotton, S.A.; Gibson, J.F. Electron paramagnetic resonance and vibrational spectra of some iron(III) complexes with oxygen-containing ligands. J. Chem. Soc. A 1971, 0, 1690. [Google Scholar] [CrossRef]
- Butorc, V.; Simeon, V.; Tomisic, V. Effect of temperature on the UV spectra of concentrated NaNO3 aqueous solutions. Croat. Chem. Acta 2007, 80, 533. [Google Scholar]
| Label | Compound 1 | Compound 2 | Compound 3 |
|---|---|---|---|
| Compound | [Fe(N,N’-DMU)6](NO3)3 | [Fe(N,N-DMU)4(H2O)2](NO3)3 | [Fe(N,N-DMU)6](NO3)3.3H2O |
| Empirical formula | C18H48FeN15O15 | C12H36FeN11O15 | C18H54FeN15O18 |
| Formula weight | 770.56 | 630.37 | 824.61 |
| Temperature | 100.00(10) | 113(2) | 105(2) K |
| Radiation and wavelength | Cu-Kα, λ=1.54184Å | Mo-Kα, λ=0.71073Å | Cu-Kα, λ=1.54184 Å |
| Crystal system | Trigonal | Triclinic | Trigonal |
| Space group | R-3 | P-1 | R-3 |
| Unit cell dimensions | a = 12.1232(2)Å | a = 9.8655(6)Å | a = 22.0051(3) Å |
| b = 12.1232(2)Å | b = 10.5568(7)Å | b = 22.0051(3) Å | |
| c =22.5665(4)Å | c = 15.5569(10)Å | c = 13.9132(2) Å | |
| α = 90° | α = 77.658(5)° | α = 90° | |
| β = 90° | β = 76.538(5)° | β = 90° | |
| γ = 120° | γ = 62.239(4)° | γ = 120° | |
| Volume | 2872.30(11)Å3 | 1383.86(16)Å3 | 5834.51(18) Å3 |
| Z | 3 | 2 | 6 |
| Density (calculated) | 1.337 Mg/m3 | 1.513 Mg/m3 | 1.408 Mg/m3 |
| Absorption coefficient, μ | 3.844 mm-1 | 0.629 mm-1 | 3.887 mm-1 |
| F(000) | 1221 | 662 | 2622 |
| Crystal colour | orange | orange | orange |
| Crystal description | block | prism | rod |
| Crystal size | 0.39 x 0.37 x 0.29 mm | 0.37 x 0.33 x 0.32 mm3 | 0.197 x 0.066 x 0.049 mm3 |
| Absorption correction | gaussian | numerical | Gaussian |
| Max. and min. transmission | 0.2790.328 | 0.9070.962 | 1.0000.569 |
| Θ-range for data collection | 4.645 ≤ Θ ≤ 75.409° | 5.116 ≤ Θ ≤ 25.349° | 3.934 ≤ Θ ≤ 75.583° |
| Index ranges | -15≤h≤ 15;-15≤k≤14;-28 ≤l≤28 | -11≤h≤11;-12≤k≤12;-18≤l≤18 | -27≤h≤27;-27≤k≤27; -17≤l≤17 |
| Reflections collected | 13522 | 22946 | 27109 |
| Completeness to 2Θ | 1.000 | 0.990 | 100.0 % |
| Independent reflections | 1324 [R(int) =0.0395] | 5030 [R(int) =0.0547] | 2672 [R(int) = 0.0466] |
| Refinement method | full-matrix least-squares on F2 | full-matrix least-squares on F2 | full-matrix least-squares on F2 |
| Data / restraints / parameters | 1321 /20 /96 | 5030 /1 /363 | 2672 / 0 / 164 |
| Goodness-of-fit on F2 | 1.113 | 1.114 | 1.088 |
| Final R indices [I>2σ(I)] | R1 =0.0577, wR2 =0.1672 | R1 =0.0504, wR2 =0.1032 | R1 = 0.0417, wR2 = 0.1233 |
| R indices (all data) | R1 =0.0577, wR2 =0.1673 | R1 =0.0660, wR2 =0.1089 | R1 = 0.0427, wR2 = 0.1242 |
| Largest diff. peak and hole | 0.978;-0.803 e.Å-3 | 0.445;-0.326 e.Å-3 | 0.522 and -0.376 e.Å-3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





