Submitted:
02 June 2023
Posted:
20 June 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and methods
2.1. Pal
2.2. Animal Experimental Design
2.3. Growth Performance
2.4. Sample Collection
2.5. Microbiome Sequencing and Bioinformatics Analysis
2.6. Statistical Analysis
3. Results
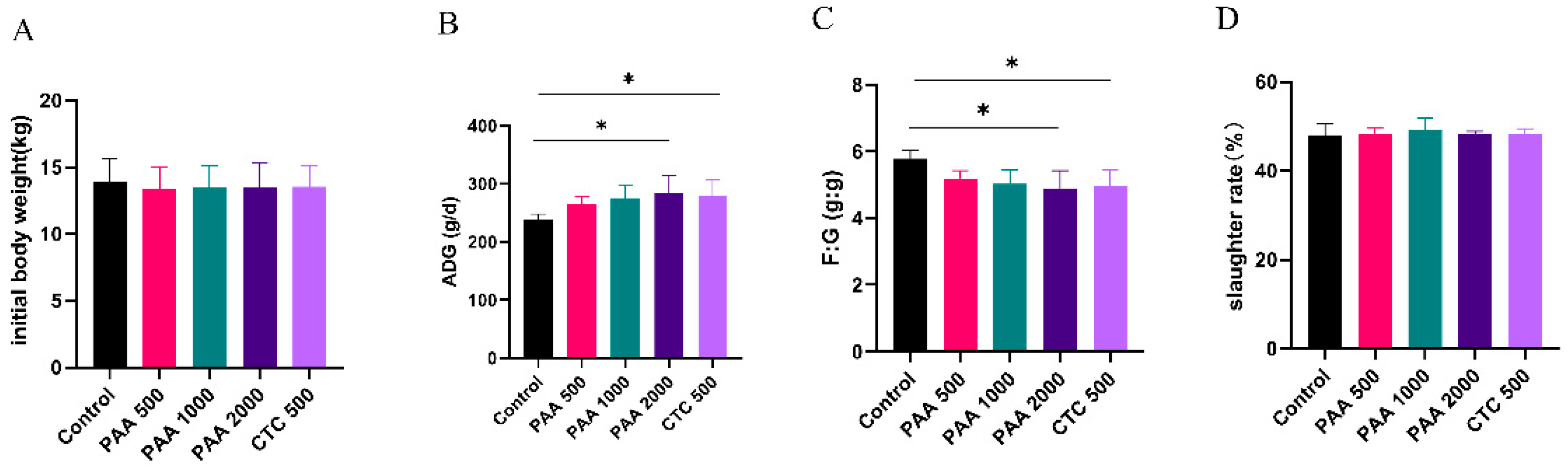
3.1. Animal Growth Performance
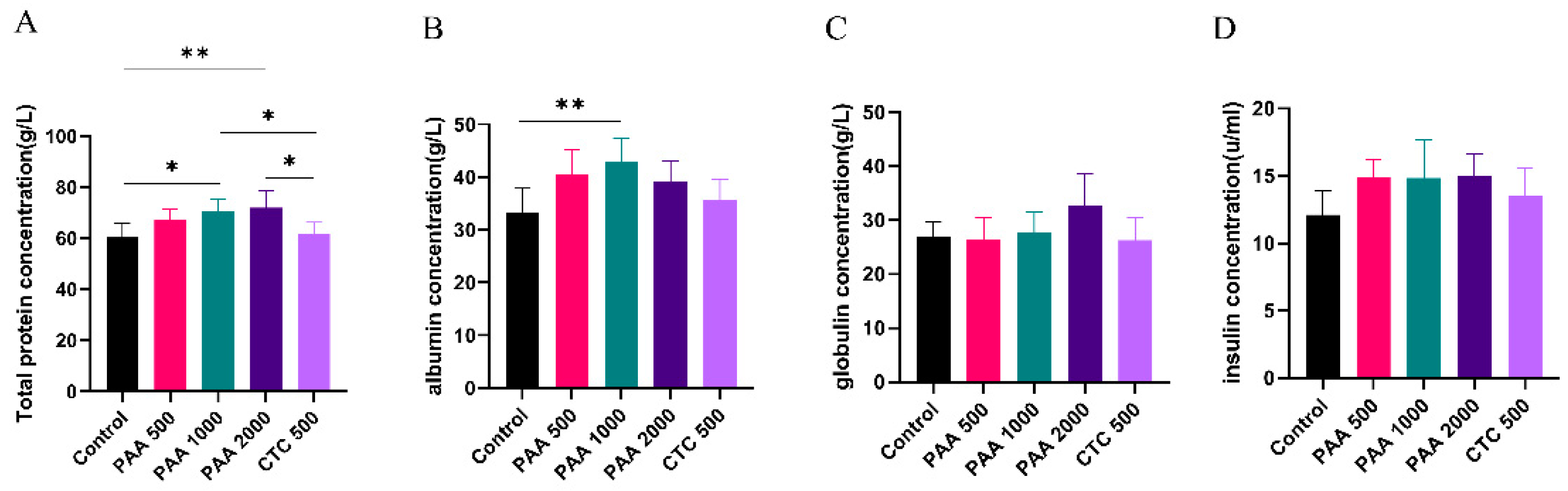
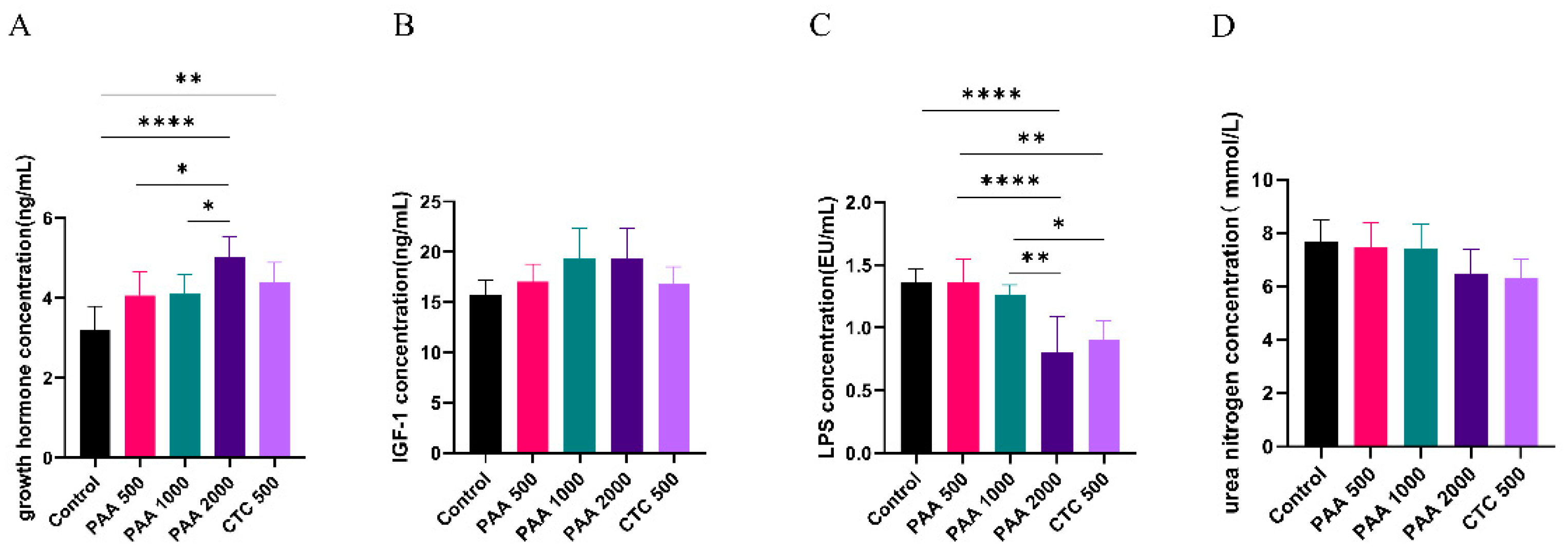
3.2. Blood Parameters
3.3. Rumen Fermentation Parameters
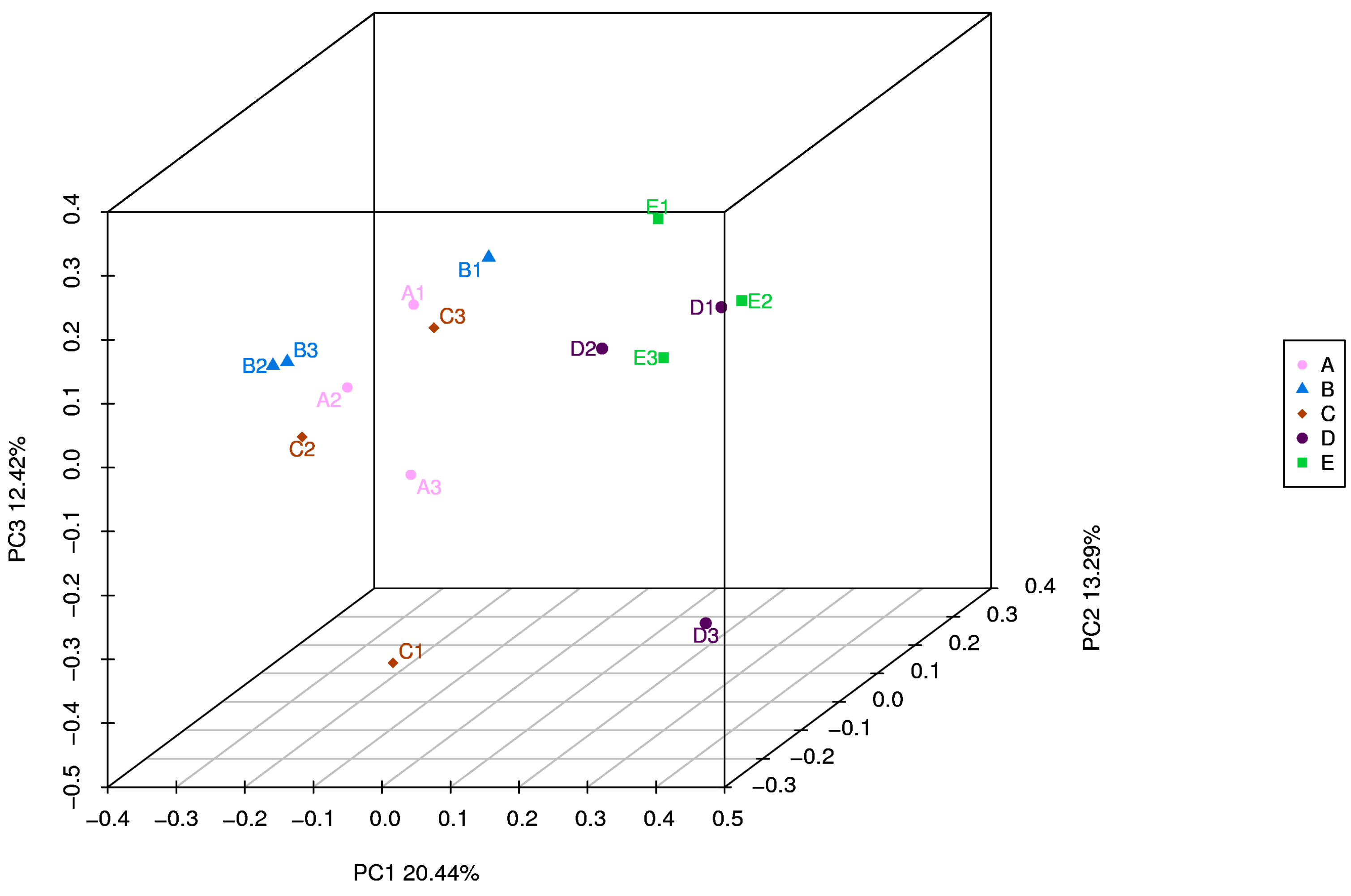
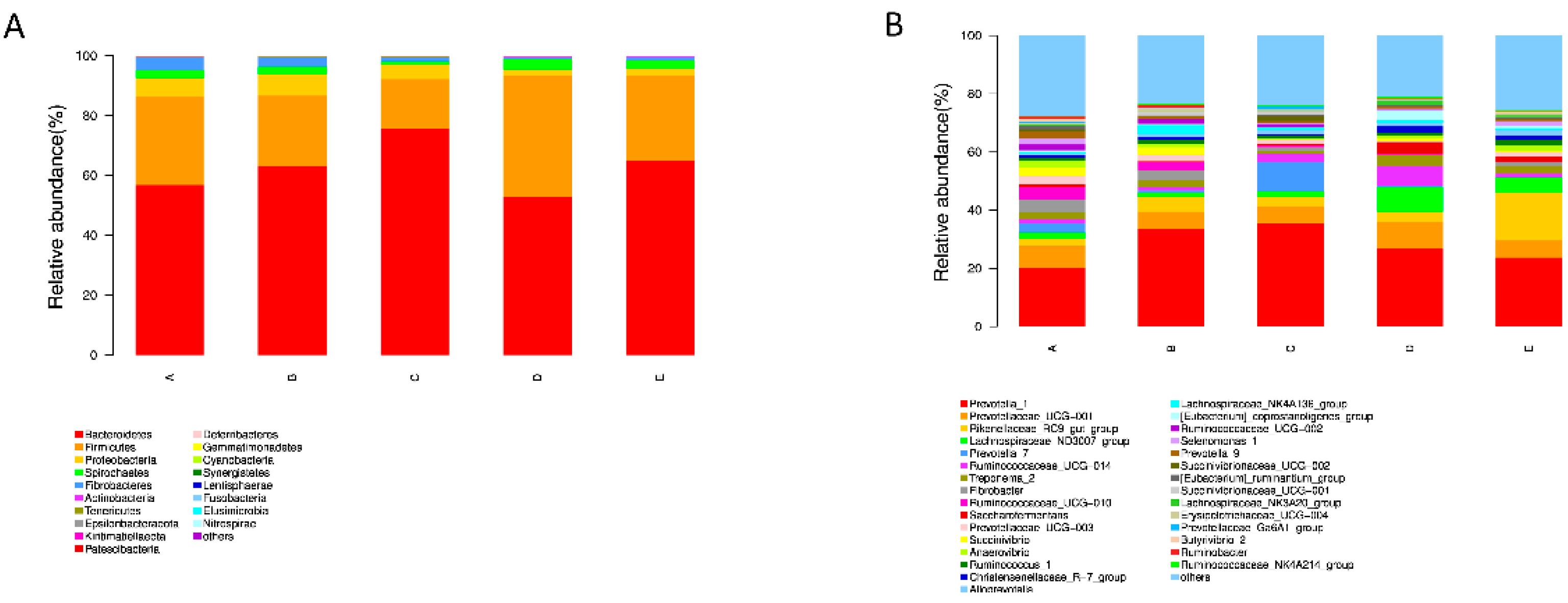
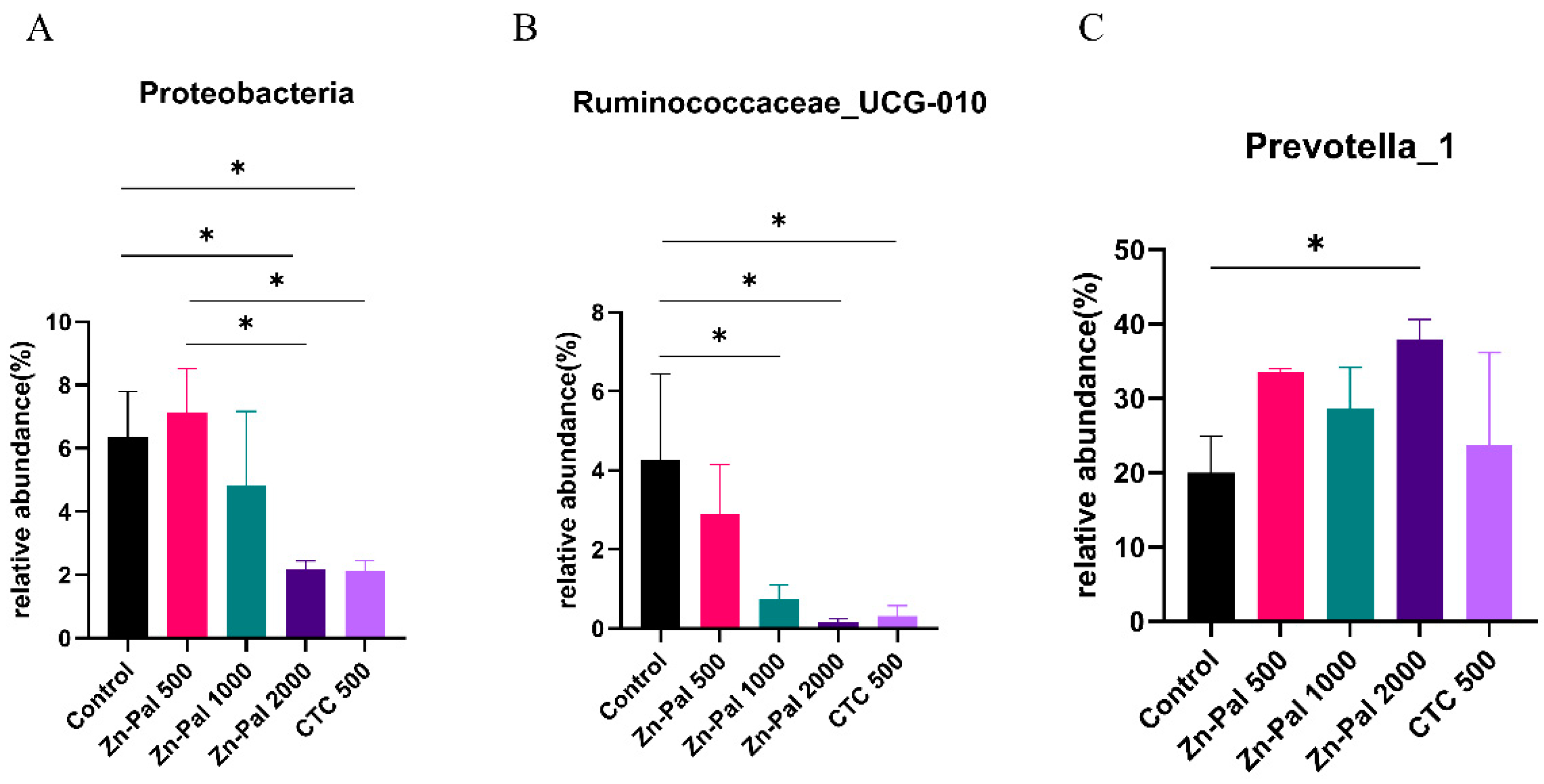
3.4. Microbiome Structure
4. Discussion
Supplementary Data
Author Contributions
Acknowledgments
Conflicts of Interest Statement
References
- Dibner, J.; Richards, J.D. Antibiotic growth promoters in agriculture: History and mode of action. Poultry science 2005, 84, 634–643. [Google Scholar] [CrossRef]
- Durso, L.M.; Cook, K.L. Impacts of antibiotic use in agriculture: What are the benefits and risks? Current opinion in microbiology 2014, 19, 37–44. [Google Scholar] [CrossRef]
- Bacanlı, M.; Başaran, N. Importance of antibiotic residues in animal food. Food and Chemical Toxicology 2019, 125, 462–466. [Google Scholar] [CrossRef]
- Gothwal, R.; Shashidhar, T. Antibiotic pollution in the environment: A review. Clean–Soil, Air, Water 2015, 43, 479–489. [Google Scholar] [CrossRef]
- Cervantes, H.M. Antibiotic-free poultry production: Is it sustainable? Journal of Applied Poultry Research 2015, 24, 91–97. [Google Scholar] [CrossRef]
- Adhikari, P.; Kiess, A.; Adhikari, R.; Jha, R. An approach to alternative strategies to control avian coccidiosis and necrotic enteritis. Journal of applied poultry research 2020, 29, 515–534. [Google Scholar] [CrossRef]
- Fang, J.; Gong, G.; Yuan, J.; Sun, X. Antibiotic use in pig farming and its associated factors in l county in yunnan, china. Veterinary medicine and science 2021, 7, 440–454. [Google Scholar] [CrossRef]
- Murray, H.H. Traditional and new applications for kaolin, smectite, and palygorskite: A general overview. Applied clay science 2000, 17, 207–221. [Google Scholar] [CrossRef]
- Liu, J.H.; Cai, W.K.; Khatoon, N.; Yu, W.H.; Zhou, C.H. On how montmorillonite as an ingredient in animal feed functions. Applied Clay Science 2021, 202, 105963. [Google Scholar] [CrossRef]
- Yan, R.; Hui, A.; Kang, Y.; Zhou, Y.; Wang, A. Effects of palygorskite composites on growth performance and antioxidant status in broiler chickens. Poultry science 2019, 98, 2781–2789. [Google Scholar] [CrossRef]
- Zeng, H.F.; Lin, L.J.; Xi, Y.M.; Han, Z.Y. Effects of raw and heated palygorskite on rumen fermentation in vitro. Applied Clay Science 2017, 138, 125–130. [Google Scholar] [CrossRef]
- SUNWen-kai; LIUQiang; LIUYang. Inhibition effect of zinc-loaded palygorskite on thre especies of common swine pathogenic bacteria in vitro. Journal of Anhui Agricultural Sciences 2019, 47, 85–88.
- qing, W.a. Preparation of chitooli}osaccharide/zn0/attapul}ite nanocomposite antibacterial agent. CN107320488A, 2017-11-07, 2017.
- HUI, A.P.; MA, M.T.; fang, Y.F.; Yuru, K.; qing, W.A. Antibacterial activity of quaternized chitosan modified zn0/attapulgite nanocomposites. materials reports 2022, 36, 42–48. [Google Scholar]
- Zha, P.; Chen, Y.; Wang, S.; Wang, A.; Zhou, Y. Dietary palygorskite-based antibacterial agent supplementation as an alternative to antibiotic improves growth performance, intestinal mucosal barrier function, and immunity in broiler chickens. Poultry science 2022, 101, 101640. [Google Scholar] [CrossRef] [PubMed]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for illumina sequence data. Bioinformatics (Oxford, England) 2014, 30, 2114–2120. [Google Scholar] [CrossRef] [PubMed]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Peña, A.G.; Goodrich, J.K.; Gordon, J.I. , et al. Qiime allows analysis of high-throughput community sequencing data. Nature methods 2010, 7, 335–336. [Google Scholar] [CrossRef]
- Rognes, T.; Flouri, T.; Nichols, B.; Quince, C.; Mahé, F. Vsearch: A versatile open source tool for metagenomics. PeerJ 2016, 4, e2584. [Google Scholar] [CrossRef]
- Yu, L.; Liu, J.; Mao, J.; Peng, Z.; Zhong, Z.; Wang, H.; Dong, L. Dietary palygorskite clay-adsorbed nano-zno supplementation improves the intestinal barrier function of weanling pigs. Frontiers in nutrition 2022, 9, 857898. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, B.; Liu, Q.; Fan, C.; Li, J.; Zhou, Y.; Zhuang, S. Palygorskite supplementation improves growth performance, oxidative status, and intestinal barrier function in cherry valley ducks. The journal of poultry science 2019, 56, 186–194. [Google Scholar] [CrossRef]
- Tang, Z.G.; Wen, C.; Wang, L.C.; Wang, T.; Zhou, Y.M. Effects of zinc-bearing clinoptilolite on growth performance, cecal microflora and intestinal mucosal function of broiler chickens. Animal Feed Science and Technology 2014, 189, 98–106. [Google Scholar] [CrossRef]
- Kanoulas, V.; Papadopoulos, G.A.; Tassis, P.; Koutouzidou, G.; Arsenos, G.; Fortomaris, P. Dietary supplementation of attapulgite improves growth performance in pigs from weaning to slaughter. Veterinary sciences 2022, 9. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Chen, J.F.; Tang, S.G.; Guo, S.C.; He, C.Q.; Qu, X.Y. Effects of essential oil/palygorskite composite on performance, egg quality, plasma biochemistry, oxidation status, immune response and intestinal morphology of laying hens. Poultry science 2022, 101, 101632. [Google Scholar] [CrossRef] [PubMed]
- Sirelkhatim, A.; Mahmud, S.; Seeni, A.; Kaus, N.H.M.; Ann, L.C.; Bakhori, S.K.M.; Hasan, H.; Mohamad, D. Review on zinc oxide nanoparticles: Antibacterial activity and toxicity mechanism. Nano-Micro Letters 2015, 7, 219–242. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Cheng, Y.; Wang, W.; Wang, A.; Zhou, Y. Protective effects of dietary supplementation with a silicate clay mineral (palygorskite) in lipopolysaccharide-challenged broiler chickens at an early age. Animal Feed Science and Technology 2020, 263, 114459. [Google Scholar] [CrossRef]
- Chen, Y.; Cheng, Y.; Yang, W.; Li, X.; Wen, C.; Wang, W.; Wang, A.; Zhou, Y. An evaluation of palygorskite inclusion on the growth performance and digestive function of broilers. Applied Clay Science 2016, 129, 1–6. [Google Scholar] [CrossRef]
- Liu, S.; Song, M.; Yun, W.; Lee, J.; Lee, C.; Kwak, W.; Han, N.; Kim, H.; Cho, J. Effects of oral administration of different dosages of carvacrol essential oils on intestinal barrier function in broilers. Journal of Animal Physiology and Animal Nutrition 2018, 102, 1257–1265. [Google Scholar] [CrossRef]
- Yang, X.; Liu, Y.; Yan, F.; Yang, C.; Yang, X. Effects of encapsulated organic acids and essential oils on intestinal barrier, microbial count, and bacterial metabolites in broiler chickens. Poultry science 2019, 98, 2858–2865. [Google Scholar] [CrossRef]
- Ibrahim, D.; Abdelfattah-Hassan, A.; Badawi, M.; Ismail, T.A.; Bendary, M.M.; Abdelaziz, A.M.; Mosbah, R.A.; Mohamed, D.I.; Arisha, A.H.; El-Hamid, M.I.A. Thymol nanoemulsion promoted broiler chicken's growth, gastrointestinal barrier and bacterial community and conferred protection against salmonella typhimurium. Scientific reports 2021, 11, 7742. [Google Scholar] [CrossRef]
- Zhong, H.; Mu, B.; Yan, P.; Jing, Y.; Hui, A.; Wang, A. A comparative study on surface/interface mechanism and antibacterial properties of different hybrid materials prepared with essential oils active ingredients and palygorskite. Colloids and Surfaces A: Physicochemical and Engineering Aspects 2021, 618, 126455. [Google Scholar] [CrossRef]
- Wang, H.; Yu, Z.; Gao, Z.; Li, Q.; Qiu, X.; Wu, F.; Guan, T.; Cao, B.; Su, H. Effects of compound probiotics on growth performance, rumen fermentation, blood parameters, and health status of neonatal holstein calves. Journal of dairy science 2022, 105, 2190–2200. [Google Scholar] [CrossRef]
- Nemati, M.; Amanlou, H.; Khorvash, M.; Moshiri, B.; Mirzaei, M.; Khan, M.A.; Ghaffari, M.H. Rumen fermentation, blood metabolites, and growth performance of calves during transition from liquid to solid feed: Effects of dietary level and particle size of alfalfa hay. Journal of dairy science 2015, 98, 7131–7141. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Ma, J.Y.; Zhou, J.; Wu, J.D.; Li, J.H.; Alugongo, G.M.; Xiao, J.X.; Wang, J.J.; Wang, Y.J.; Wang, W. , et al. Tributyrin supplementation in pasteurized waste milk: Effects on growth performance, health, and blood parameters of dairy calves. Journal of dairy science 2021, 104, 12496–12507. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.W.; Woyengo, T.A. Growth performance, organ weights, and blood parameters of nursery pigs fed diets containing increasing levels of cold-pressed canola cake. Journal of animal science 2018, 96, 4704–4712. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, F.; Ebrahimnezhad, Y.; Sis, N.M.; Ghiasi, J. The effects of zinc oxide nanoparticles on performance, digestive organs and serum lipid concentrations in broiler chickens during starter period. Int J Biosci 2013, 3, 23–29. [Google Scholar] [CrossRef]
- Yang, L.; Wenkai, S.; Qiang, L.; Guihua, Y.; Hengtao, X.; Su, Z. Effects of zinc-bearing palygorskite on growth performance, organ index, biochemical indexes and anti-oxidative capacity in weaned piglets. Journal of Fujian Agriculture and Forestry University 2020, 49, 505–511. [Google Scholar]
- Shiqi, W.; Haoran, Z.; Mingfang, D.; Yueping, C.; Chao, W.; Yanmin, Z. Effects of crystal-bundles disaggregated performance, serum biochemical indices palygorskite on growth antioxidant function and meat quality of broilers. Chinese Journal of Animal Nutrition 2022, 34, 3570–3582. [Google Scholar]
- Szczepkowska, A.; Wójcik, M.; Tomaszewska-Zaremba, D.; Antushevich, H.; Krawczyńska, A.; Wiechetek, W.; Skipor, J.; Herman, A.P. Acute effect of caffeine on the synthesis of pro-inflammatory cytokines in the hypothalamus and choroid plexus during endotoxin-induced inflammation in a female sheep model. International journal of molecular sciences 2021, 22, 13237. [Google Scholar] [CrossRef]
- Bittar, I.P.; Neves, C.A.; Araújo, C.T.; Oliveira, Y.V.R.; Silva, S.L.; Borges, N.C.; Franco, L.G. Dose-finding in the development of an lps-induced model of synovitis in sheep. Comparative medicine 2021, 71, 141–147. [Google Scholar] [CrossRef]
- Danli, J.; Li, L.; Fang, C.; Congli, W.; Zhendan, S. Effects of lps on growth performance and immune function in meat geese. China Poultry 2011, 33, 10–15. [Google Scholar]
- Yilong, J.; Lu, L.; Yueping, C.; Yanmin, Z.; Su, Z. Effects of modified attapulgite on growth performance, antioxidant capacity, immune performance and intestinal barrier function of hy-line brown laying hens before laying. Chinese Journal of Animal Nutrition 2022, 34, 7024–7037. [Google Scholar]
- Zhang, J.; Lv, Y.; Tang, C.; Wang, X. Effects of dietary supplementation with palygorskite on intestinal integrity in weaned piglets. Applied clay science 2013, 86, 185–189. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, Y.; Wang, X.; Li, F.; Zhang, D.; Li, X.; Zhao, Y.; Zhao, L.; Xu, D.; Cheng, J. , et al. Association between rumen microbiota and marbling grade in hu sheep. Frontiers in microbiology 2022, 13, 978263. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zeng, H.; Wang, C.; Han, Z. Effect of methionine hydroxy analog feed supplements: Significant alteration and enrichment of rumen microbiota and metabolome in hu sheep. Frontiers in veterinary science 2022, 9, 999726. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.K.; Zhang, X.X.; Li, F.D.; Li, C.; Li, G.Z.; Zhang, D.Y.; Song, Q.Z.; Li, X.L.; Zhao, Y.; Wang, W.M. Characterization of the rumen microbiota and its relationship with residual feed intake in sheep. Animal: An international journal of animal bioscience 2021, 15, 100161. [Google Scholar] [CrossRef]
- Wang, X.; Xu, T.; Zhang, X.; Zhao, N.; Hu, L.; Liu, H.; Zhang, Q.; Geng, Y.; Kang, S.; Xu, S. The response of ruminal microbiota and metabolites to different dietary protein levels in tibetan sheep on the qinghai-tibetan plateau. Frontiers in veterinary science 2022, 9, 922817. [Google Scholar] [CrossRef]
- Zeng, H.; Lin, L.; Xi, Y.; Han, Z. Effects of raw and heated palygorskite on rumen fermentation in vitro. Applied Clay Science 2017, 138, 125–130. [Google Scholar] [CrossRef]
- Chen, M.; Tuo, S.; Xi, Y.; Zhang, L.; Zeng, H.; Han, Z. Effect of zinc-bearing palygorskite on rumen bacterial diversity in vitro. Acta Microbiologica Sinica 2018, 58, 346–358. [Google Scholar]
- Lijuan, L. Effect of attapulgite on rumen fermentation and lactation performance of mid-lactation cows. master, NanJing agricutural university, 2014.
- Xie, X.; Yang, C.; Guan, L.L.; Wang, J.; Xue, M.; Liu, J.X. Persistence of cellulolytic bacteria fibrobacter and treponema after short-term corn stover-based dietary intervention reveals the potential to improve rumen fibrolytic function. Frontiers in microbiology 2018, 9, 1363. [Google Scholar] [CrossRef]
- Weimer, P.J. Redundancy, resilience, and host specificity of the ruminal microbiota: Implications for engineering improved ruminal fermentations. Frontiers in microbiology 2015, 6, 296. [Google Scholar] [CrossRef]
- Jin, R.; Chen, Y.; Kang, Y.; Gu, Y.; Wen, C.; Wang, A.; Zhou, Y. Effects of dietary palygorskite supplementation on cecal microbial community structure and the abundance of antibiotic-resistant genes in broiler chickens fed with chlortetracycline. Clays and Clay Minerals 2021, 69, 205–216. [Google Scholar] [CrossRef]
- Chalvatzi, S.; Kalamaki, M.S.; Arsenos, G.; Fortomaris, P. Dietary supplementation with the clay mineral palygorskite affects performance and beneficially modulates caecal microbiota in laying pullets. Journal of applied microbiology 2016, 120, 1033–1040. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wang, C.; Gu, X.; Zhao, J.; Nie, C.; Zhang, W.; Ma, X. Dietary montmorillonite improves the intestinal mucosal barrier and optimizes the intestinal microbial community of weaned piglets. Frontiers in microbiology 2020, 11, 593056. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Zhong, Z.; Ma, H.; Lin, L.; Xie, F.; Mao, S.; Irwin, D.M.; Wang, Z.; Zhang, S. Mucosal microbiota and metabolome in the ileum of hu sheep offered a low-grain, pelleted or non-pelleted high-grain diet. Frontiers in microbiology 2021, 12, 718884. [Google Scholar] [CrossRef]
- Yan, B.; Jia, T.; Wang, Z.; Zhu, W. Comparative research of intestinal microbiota diversity and body mass regulation in eothenomys miletus from different areas of hengduan mountain regions. Frontiers in microbiology 2022, 13, 1026841. [Google Scholar] [CrossRef]
- Wen, K.; Liu, L.; Zhao, M.; Geng, T.; Gong, D. The changes in microbiotic composition of different intestinal tracts and the effects of supplemented lactobacillus during the formation of goose fatty liver. Frontiers in microbiology 2022, 13, 906895. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, K.; Lin, Y.; Li, M.; Wang, X.; Yu, Q.; Sun, H.; Cheng, Q.; Xie, Y.; Wang, C. , et al. Effect of cellulase and lactic acid bacteria on the fermentation quality, carbohydrate conversion, and microbial community of ensiling oat with different moisture contents. Frontiers in microbiology 2022, 13, 1013258. [Google Scholar] [CrossRef]
- Hu, C.; Ding, L.; Jiang, C.; Ma, C.; Liu, B.; Li, D.; Degen, A.A. Effects of management, dietary intake, and genotype on rumen morphology, fermentation, and microbiota, and on meat quality in yaks and cattle. Frontiers in nutrition 2021, 8, 755255. [Google Scholar] [CrossRef]
- Pan, L.; Han, P.; Ma, S.; Peng, R.; Wang, C.; Kong, W.; Cong, L.; Fu, J.; Zhang, Z.; Yu, H. , et al. Abnormal metabolism of gut microbiota reveals the possible molecular mechanism of nephropathy induced by hyperuricemia. Acta pharmaceutica Sinica. B 2020, 10, 249–261. [Google Scholar] [CrossRef]
- Biswas, K.; Hoggard, M.; Jain, R.; Taylor, M.W.; Douglas, R.G. The nasal microbiota in health and disease: Variation within and between subjects. Frontiers in microbiology 2015, 9, 134. [Google Scholar] [CrossRef]
- Xiaohan, L.; Yuping, Z.; ZHOU, Y. Zinc desorption and antibacterial activity on e. Coli k88of zinc-bearing palygorskite. Non-Metallic Mines 2015, 38, 9–12. [Google Scholar]
- Wang, L.C.; Zhang, T.T.; Wen, C.; Jiang, Z.Y.; Wang, T.; Zhou, Y.M. Protective effects of zinc-bearing clinoptilolite on broilers challenged with salmonella pullorum. Poultry science 2012, 91, 1838–1845. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Guo, C.; Li, Y.; Han, X.; Luo, X.; Chen, L.; Zhang, T.; Wang, N.; Wang, W. Alginate oligosaccharides ameliorate dss-induced colitis through modulation of ampk/nf-κb pathway and intestinal microbiota. Nutrients 2022, 14, 2864. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Liu, Y.; Huang, K.; Yang, B.; Zhang, Y.; Yu, Z.; Wang, J. Fecal microbiota dynamics and its relationship to diarrhea and health in dairy calves. Journal of animal science and biotechnology 2022, 13, 132. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Ma, M.P.; Diao, Q.Y.; Tu, Y. Saponin-induced shifts in the rumen microbiome and metabolome of young cattle. Frontiers in microbiology 2019, 10, 356. [Google Scholar] [CrossRef]
| Ingredients | Content, % of DM | Chemical composition, % of DM | Content, % of DM |
|---|---|---|---|
| Corn | 30.8 | DM | 87.19 |
| Soybean meal | 5.8 | ME/(MJ/kg) | 16.91 |
| Peanut meal | 3 | CP | 14.61 |
| Bean straw | 24 | NDF | 49.71 |
| Corn germ meal | 15 | ADF | 13.04 |
| Rice husk | 5 | EE | 4.71 |
| Wheat middling | 2 | Ash | 10.06 |
| Molasses | 1 | Ca | 1.08 |
| Malt root | 6 | P | 0.62 |
| Limestone | 2 | ||
| Premix | 5 | ||
| Total | 100 |
| Items | CON | PAA 500 | PAA 1000 | PAA 2000 | CTC 500 | SEM | p-Value |
|---|---|---|---|---|---|---|---|
| pH | 6.43 | 6.53 | 6.46 | 6.43 | 6.44 | 0.02 | 0.67 |
| Total VFA (mmol/L) | 54.08 | 51.19 | 60.74 | 37.74 | 59.55 | 5.04 | 0.643 |
| Acetate | 39.03 | 34.45 | 37.99 | 29.72 | 36.71 | 2.95 | 0.903 |
| Propionate | 14.23 | 10.28 | 14.38 | 7.14 | 12.98 | 1.25 | 0.357 |
| Butyrate | 6.43 | 3.28 | 4.76 | 4.27 | 4.9 | 0.48 | 0.378 |
| Valerate | 0.85 | 0.49 | 0.71 | 0.61 | 0.74 | 0.07 | 0.696 |
| Isobutyrate | 1.57 | 1.01 | 1.12 | 1.44 | 1.46 | 0.12 | 0.604 |
| Isovalerate | 2.76 | 1.65 | 1.76 | 2.08 | 2.68 | 0.23 | 0.438 |
| Acetate/propionate(A:P) | 2.82 | 4.29 | 2.84 | 4.44 | 2.89 | 0.69 | 0.468 |
| Items | CON | PAA 500 | PAA1000 | PAA 2000 | CTC 500 | SEM | p-Value |
|---|---|---|---|---|---|---|---|
| simpson | 0.9816b | 0.9808b | 0.9644b | 0.9373a | 0.9660b | 0.004 | 0.002 |
| Chao1 | 2797.4b | 2831.3b | 2628.8b | 2330.7a | 2577.4ab | 57.8 | 0.014 |
| Observed species | 2214.2b | 2243.8b | 2037.9ab | 1771.1a | 2005.1ab | 55.3 | 0.016 |
| shannon | 7.5b | 7.4b | 6.9b | 6.4a | 7.1b | 0.12 | 0.005 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




