1. Introduction
Currently, the attention of scientists around the world is focused on studying the characteristics of nanoparticles of various natures, the synthesis of nanostructured functional materials based on them, as well as the possibility of using them as promising materials for applications [
1,
2,
3,
4]. It is known that due to the high degree of distribution of atoms on the surface and the quantum limitations of nanomaterials, they have unusual unique properties that differ from the properties of their bulk materials [
5].
The study of allotropic forms of carbon, as one of the most dynamically developing areas of modern science, is also associated with the study of fullerenes [
6]. All carbon atoms in fullerene molecules are attractively distributed on their quasi-spheroidal surfaces, which causes unusual features of their behavior in solutions [
7,
8,
9]. Among the currently most studied families of fullerene molecules is the C
70 molecule, the structure of which corresponds to a cellular ring structure resembling a rugby ball. Due to its unique physical and chemical properties, C
70 fullerene has a wide range of applications as the main building blocks in nanotechnologies, electromagnetic devices, solar panels, sensors, pharmaceuticals, tribological materials, coatings, etc. [
10,
11,
12,
13,
14].
To date, many scientific papers have been published on the study of the characteristics of C
70 fullerene in various one- and two component organic solvents [
15,
16,
17,
18,
19]. In this case, C
70 fullerene solutions are mainly considered as dispersed systems, since the C
70 molecule tends to self-organize in solutions. In experiments, the formation of C
70 based complexes in high-concentration solutions in "good" solvents sometimes occurs easily [
20,
21], but often plainly colloidal solutions are formed depending on the preparation method of solution [
22,
23,
24]. Moreover, mixing fullerene solutions in "good" solvents with polar solvents easily leads to the formation of true colloidal systems [
25,
26]. There is evidence that the nature of self-organization of C
70 fullerene is affected not only by the content of the solvent used, but also by the concentration of the fullerene itself [
27,
28,
29,
30].
Understanding the self-organization of C70 molecules in solutions is necessary for the synthesis of nanostructured materials based on them with well-controlled properties. The latter is a very complex experimental problem and requires systematic study, since the phenomena controlling self-organization take place at subnanometer sizes. Especially the study of physical processes occurring at low concentrations of C70 fullerene in two-component solvents is of interest from both fundamental and practical points of view.
This paper presents experimental studies of C70/xylene/tetrahydrofuran solutions at low fullerene concentrations by optical absorption, refractometry, and dynamic light scattering (DLS). The stabilization time of the cluster formation are also discussed in fullerene solution.
2. Materials and Methods
Fullerene C70 with high purity (99.5%) as well as o-xylene (C8H10, hereinafter referred to as xylene) and tetrahydrofuran (C4H8O, hereinafter referred to as THF) organic solvents used in the present study were acquired from Sigma-Aldrich (USA). All chemicals were used as received. THF is good soluble in xylene, their dielectric constant is ~7.52 and ~2.57, respectively.
To prepare C
70 fullerene solutions of various concentrations, a weighed portion of a preliminarily prepared C
70 fullerene crystal was added to a flask with precisely measured amounts of aromatic and non-aromatic solvents (xylene and THF at a volume ratio of 0.9:0.1, respectively). Then the resulting mixture was dissolved in a hermetically sealed glass flask at room temperature for 4-5 hours with mechanical stirring using a programmable laboratory magnetic stirrer "MS-11 H" (WIGO, Poland) with a frequency of 2.5 Hz. The solutions were filtered through a dense filter with a pore size of ~0.22 μm. In the C
70/xylene/THF system, the concentrations of C
70 fullerene used in our experiments are below the solubility limit [
15], and after filtration, almost no substance remains on the filter.
Exact values of refractive indices (n) of C70/xylene/THF systems with two different concentrations (~1.19⋅10-5 and ~2.37⋅10-5 mol⋅L-1) were measured using a high-sensitivity digital refractometer PAL-BX/RI (ATAGO, Japan) at the wavelength of the D1-line of the sodium atom (~589.3 nm). The uncertainty in refractive index measurements of solution was within ±0.01%. The values reported in this work are the average of at least three independent replicated data.
Optical absorption spectra of C70 solutions were recorded on a Shimadzu UV-2700 UV-Vis recording spectrophotometer (Shimadzu, Japan) with a spectral resolution of ~0.1 nm in the spectral range of ~185–900 nm.
The nature of the distribution of the dispersed phase of C70 fullerene over the average hydrodynamic radius in solutions was studied by dynamic light scattering (DLS) on a NanoSight LM10 system (Malvern Instruments Ltd., UK).
All measurements on C70 solutions samples were performed at room temperature (T≈24±1 °C). Storage of freshly prepared C70 solutions for different times was carried out in the dark.
3. Results and Discussion
Data on the change in the refractive index (n) of C
70 fullerene solutions provide us with important information about the interaction of solute and solvent molecules. The measured refractive index of the xylene/THF mixture (volume ratio of 0.9:0.1, respectively) is 1.4915. The change in the concentration of C
70 fullerene in the C
70/xylene/THF solution from ~1.19⋅10
-5 to ~2.37⋅10
-5 mol⋅L
-1 led to an increase in the refractive index of the solution medium (see
Table 1). With an increase in the concentration of C
70 in the studied solutions, the intermolecular interactions of molecules are intensified and the processes of self-organization of the fullerene molecule begin. In this case, an increase in the number of bonds between the "C
70-C
70" and "C
70-solvent" molecules leads to a greater interaction of light photons and, consequently, to an increase in the refractive index of solutions. When the solution is stored for up to 3 days, an increase in the refractive index of the solution is observed, however, further storage of the solution (up to 9 days) leads to a decrease in the refractive index of the solution. The decrease in the refractive index of the fullerene solution with an increase in the storage period is possibly due to the enlargement of fullerene nanoclusters in time and, as a consequence, a decrease in their amount in the solution. The value of the refractive index of C
70/xylene/THF solution after its storage for 9 days remains virtually unchanged. The latter is connected with the stabilization of the process of self-organization of fullerene molecules in solution.
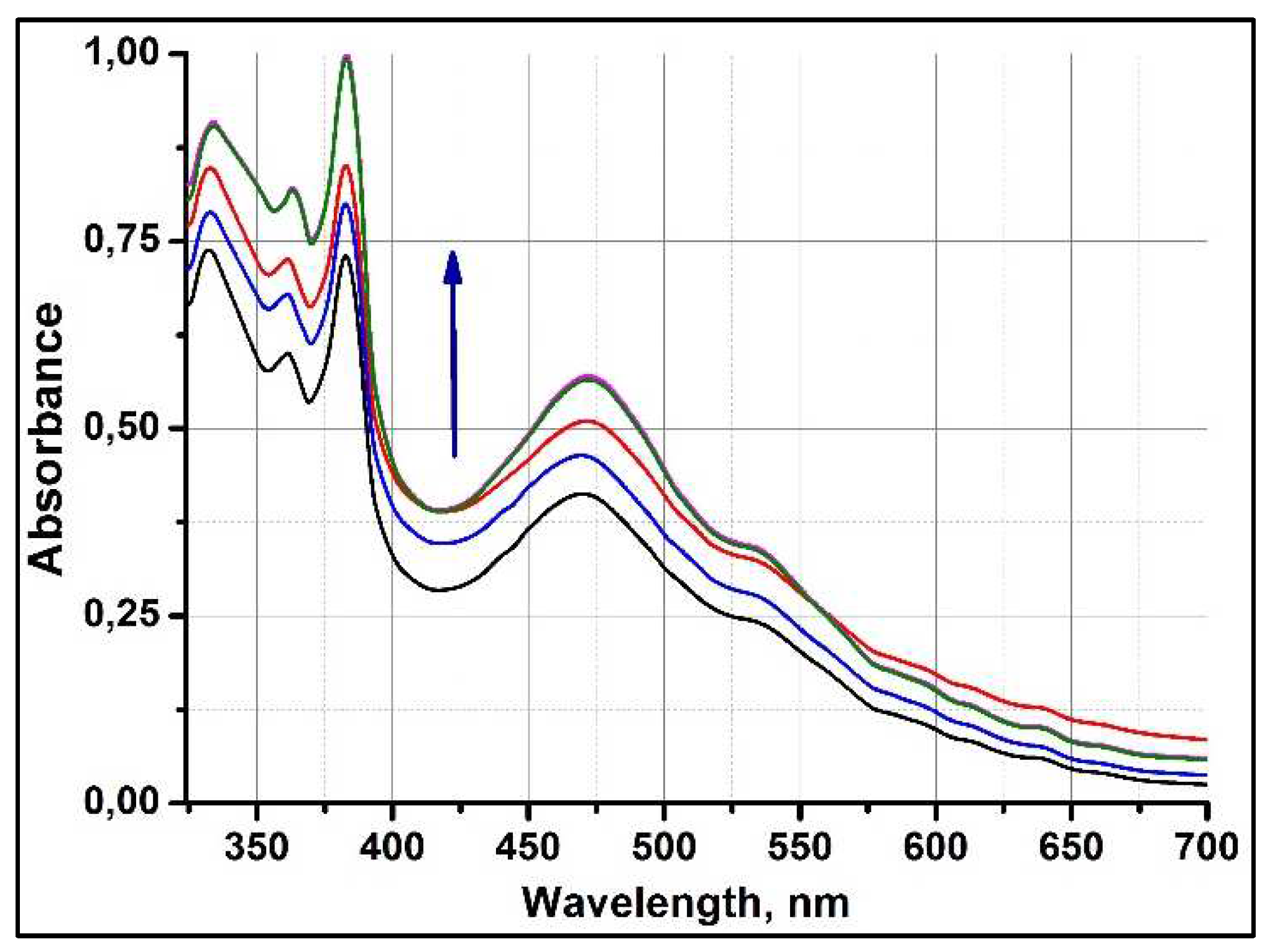
The optical spectrum of C
70/xylene/THF solution was characterized by broad absorption bands in the visible region and relatively intense absorption in the UV region. The change in the absorption spectra in the UV-visible region of the spectrum of a freshly prepared C
70/xylene/THF solution with time is shown in
Figure 1. In this case, the concentration of fullerene in the solution was ~1.19⋅10
-5 mol⋅L
-1. The absorption spectrum of a freshly prepared C
70/xylene/THF solution three pronounced absorption maxima at ~332.2, ~382.7, and ~470.5 nm, as well as two minor maxima at ~361.7 and ~535.4 nm was observed. The behavior of the optical absorption spectrum of the C
70/xylene/THF solution turned out to be sensitive to a certain storage time of the solution (see
Figure 1). It can be seen that after keeping the initial C
70/xylene/THF solution for several days in a dark place, the intensity of the absorption spectrum of the solution fully increases. In addition, there is a red shift of the maxima at ~332.2 nm (by ~1.7 nm), ~361.7 nm (by ~1.5 nm), ~382.7 nm (by ~ 0.5 nm) and ~470.5 nm (by ~2.2 nm). The above effects were caused by an increase in the π-conjugated system of the C
70 cage, which indicates a decrease in the energy gap between S
1 excited and S
0 ground states. In addition, the competition between "C
70-C
70" and "C
70-solvent" intermolecular interactions is dominated by the binding of C
70 molecules in time, forming fullerene associations, which subsequently combine into stable nanoclusters. The results show that the electronic absorption spectra of the C
70/xylene/THF solution after storage for 9 days and 12 days were practically indistinguishable, and this allows us to conclude that the synthesized C
70 fullerene nanoclusters in a mixed solution (xylene/THF) achieve stability.
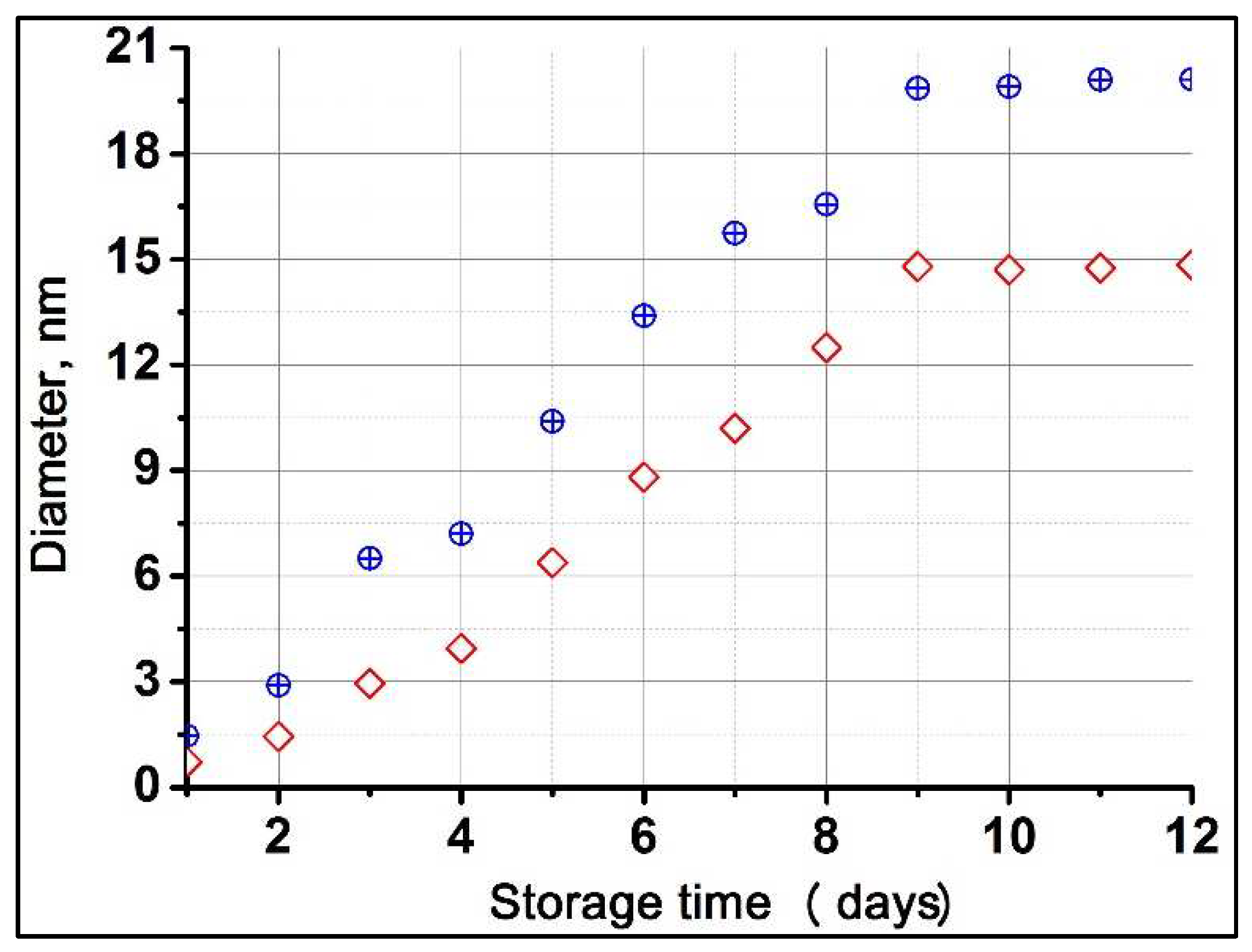
To assess the degree of self-organization of C
70 molecules in a C
70/xylene/THF solution over time, we measured the hydrodynamic diameter of C
70 nanoclusters by the DLS method. The change in the intensity distribution of the maximum size of synthesized C
70 nanoclusters in the C
70/xylene/THF system with time at two fixed concentrations of fullerene C
70 (~1.19⋅10
-5 and ~2.37⋅10
-5 mol⋅L
-1) is shown in
Figure 2. It was observed that the beginning of the formation of C
70 nanoclusters depends on the initial fullerene concentration. At a C
70 concentration of ~1.19⋅10
-5 mol⋅L
-1 from the 3rd day and at a C
70 concentration of ~2.37⋅10
-5 mol⋅L
-1 from the 2nd day, the synthesis of nanoclusters began. The nanocluster diameter increases almost linearly up to 9 days of storage of the C
70/xylene/THF solution, and then the self-organization process proceeds slowly and their size remains unchanged up to 12 days. Thus, we can confirm that there is indeed a self-organization of fullerene molecules in weakly concentrated C
70/xylene/THF solution, leading to the formation of small nanoclusters, and that it is a time-dependent physical process.
5. Conclusions
We have presented the experimental results of studying the interactions and self-organization processes of C70 fullerene molecules in a two-component solvent system. Our experimental results obtained using high resolution methods confirm the formation of nanoclusters of C70 molecules in the binary (xylene/THF) solvent system. It was found that the degree of intermolecular interaction in the C70/xylene/THF solution depends on the initial concentration of fullerene and the time of keeping the solution.
It was established by refractometry that with a grow in the concentration of C70 in the C70/xylene/THF solution, the enhance in the number of bonds between the "C70-C70" and "C70-solvent" molecules leads to a greater interaction of light photons and, as a consequence, an increase in the refractive index of solutions. When storing C70/xylene/THF solution of a fixed concentration at room temperature, an increase in the refractive index of the solution is observed from the beginning, but at later periods of storage of the solution (in the period of 3-9 days), its decrease was established. The latter is associated with an increase in the size of the synthesized C70 nanoclusters over time and, as a result, the decrease in their amount in solution. Using optical absorption spectroscopy, the sensitivity of the behavior of the electronic absorption of a C70/xylene/THF solution to a certain storage time was determined, which is manifested by an increase in the intensity of the absorption spectrum and a red shift of the characteristic maxima (from ~0.5 nm to ~2.2 nm). This is caused by a decrease in the energy gap between the excited S1 and ground S0 states of the fullerene; ultimately, the competition of intermolecular interactions "C70-C70" and "C70-solvent" is dominated by the binding of C70 molecules in time, forming nanoclusters. In addition, it was found that the electronic absorption spectra of the solution remained unchanged after storage for 9 days, which indicates the achievement of stability in the process of self-organization of C70 molecules. Using the DLS method, it was determined that the time of the beginning of the formation of C70 nanoclusters and their final size depend on the initial concentration of fullerene and the time of keeping the solution. The obtained scientific results can be used to evaluate the formation of different nanostructures in binary solutions of fullerenes and similar nanoparticles.
Author Contributions
Conceptualization, U.K.M.; methodology, U.K.M.; data measurements and investigation, Sh.A.E., D.T.S., K.N.M., T.A.Ch. and B.A.A.; formal analysis, U.K.M., Sh.A.E., D.T.S., K.N.M., T.A.Ch. and B.A.A.; writing—original draft preparation, U.K.M. and Sh.A.E.; writing—review and editing, U.K.M. All authors have read and agreed to the published version of the manuscript.
Funding
The research was financially supported by the Fund for Basic Research of the Academy of Sciences of Uzbekistan: "Investigation of the physical regularities of the self-organization processes of organic nanoscale materials in liquid systems".
Conflicts of Interest
The authors declare no conflict of interest.
References
- Li, Z.; Wang, L.; Li, Y.; Feng, Y.; Feng, W. Carbon-based functional nanomaterials: Preparation, properties and applications. Compos. Sci. Technol. 2019, 179, 10–40. [Google Scholar] [CrossRef]
- Khan, I.; Saeed, Kh.; Khan, I. Nanoparticles: Properties, applications and toxicities. Arab. J. Chem. 2019, 12, 908–931. [Google Scholar] [CrossRef]
- Fabbiani, M.; Cesano, F.; Pellegrino, F.; Negri, Ch. Design, Characterization and Applications of Functional Nanomaterials. Molecules 2021, 26, 7097. [Google Scholar] [CrossRef] [PubMed]
- Joudeh, N.; Linke, D. Nanoparticle classification, physicochemical properties, characterization, and applications: a comprehensive review for biologists. J. Nanobiotechnol. 2022, 20, 1–29. [Google Scholar] [CrossRef]
- Roduner, E. Size matters: why nanomaterials are different. Chem. Soc. Rev. 2006, 35, 583–592. [Google Scholar] [CrossRef]
- Georgakilas, V.; Perman, J.A.; Tucek, J.; Zboril, R. Broad Family of Carbon Nanoallotropes: Classification, Chemistry, and Applications of Fullerenes, Carbon Dots, Nanotubes, Graphene, Nanodiamonds, and Combined Superstructures. Chem. Rev. 2015, 115, 4744–4822. [Google Scholar] [CrossRef]
- Kokhkharov, A.M.; Bakhramov, S.A.; Makhmanov, U.K.; Kokhkharov, R.A.; Zakhidov, E.A. Self-induced polarization rotation of laser beam in fullerene (C70) solutions. Opt. Commun. 2012, 285, 2947–2951. [Google Scholar] [CrossRef]
- Saraswati, T.E.; Setiawan, U.H.; Ihsan, M.R.; Isnaeni, I.; Herbani, Y. The Study of the Optical Properties of C60 Fullerene in Different Organic Solvents. Open Chem. 2019, 17, 1198–1212. [Google Scholar] [CrossRef]
- Yablonskaya, O.; Buravleva, E.; Novikov, K.; Voeikov, V. Peculiarities of the Physicochemical Properties of Hydrated C60 Fullerene Solutions in a Wide Range of Dilutions. Front. Phys. 2021, 9, 1–17. [Google Scholar] [CrossRef]
- Speranza, G. Carbon Nanomaterials: Synthesis, Functionalization and Sensing Applications. J. Nanomater. 2021, 11, 967. [Google Scholar] [CrossRef]
- Sachdeva, Sh.; Singh, D.; Tripathi, S.K. Optical and electrical properties of fullerene C70 for solar cell applications. Opt. Mater. 2020, 101, 109717. [Google Scholar] [CrossRef]
- Li, W.; Zhao, T. Hydroxyurea anticancer drug adsorption on the pristine and doped C70 fullerene as potential carriers for drug delivery. J. Mol. Liq. 2021, 340, 117226. [Google Scholar] [CrossRef]
- Upadhyay, R.K.; Kumar. A. A novel approach to minimize dry sliding friction and wear behavior of epoxy by infusing fullerene C70 and multiwalled carbon nanotubes. Tribol. Int. 2018, 120, 455–464. [Google Scholar] [CrossRef]
- Benzigar, M.R.; Joseph, S.; Baskar, A.V.; Park, D.H.; Chandra, G.; Umapathy, S.; Talapaneni, S.N.; Vinu, A. Ordered Mesoporous C70 with Highly Crystalline Pore Walls for Energy Applications. Adv. Funct. Mater. 2018, 28, 1803701. [Google Scholar] [CrossRef]
- Deguchi, Sh.; Alargova, R.G.; Tsujii, K. Stable Dispersions of Fullerenes, C60 and C70, in Water. Preparation and Characterization. Langmuir 2001, 17, 6013–6017. [Google Scholar] [CrossRef]
- Kokhkharov, A.M.; Zakhidov, E.A.; Gofurov, Sh.P.; Bakhramov, S.A.; Makhmanov, U.K. Clusterization of fullerene C70 molecules in solutions and its influence to optical and nonlinear optical properties of solutions. Int. J. Nanotechnol. 2013, 12, 1350027. [Google Scholar] [CrossRef]
- Manyakina, O.S.; Semenov, K.N.; Charykov, N.A.; Ivanova, N.M.; Keskinov, V.A.; Sharoyko, V.V.; Letenko, D.G.; Nikitin, V.A.; Klepikov, V.V.; Murin, I.V. Physico-chemical properties of the water-soluble C70-tris-malonic solutions. J. Mol. Liq. 2015, 211, 487–493. [Google Scholar] [CrossRef]
- Kim, J.; Park, Ch.; Choi, H.Ch. Selective Growth of a C70 Crystal in a Mixed Solvent System: From Cube to Tube. Chem. Mater. 2015, 27, 2408–2413. [Google Scholar] [CrossRef]
- Mchedlov-Petrossyan, N.; Marfunin, M.; Klochkov, V.; Radionov, P. Hydrosol of C70 fullerene: synthesis and stability in electrolytic solutions. Ukr. Chem. J. 2021, 87, 63–73. [Google Scholar] [CrossRef]
- Tezuka, N.; Umeyama, T.; Matano, Y.; Shishido, T.; Kawasaki, M.; Nishi, M.; Hirao, K.; Lehtivuori, H.; Tkachenko, N.V.; Lemmetyinen, H.; Honsho, Y.; Seki, Sh.; Imahori, H. Good Solvent Effects of C70 Cluster Formations and Their Electron-Transporting and Photoelectrochemical Properties. Phys. Chem. B 2010, 114, 14287–14297. [Google Scholar] [CrossRef]
- Mchedlov-Petrossyan, N.O. Fullerenes in Liquid Media: An Unsettling Intrusion into the Solution Chemistry. Chem. Rev. 2013, 113, 5149–5193. [Google Scholar] [CrossRef] [PubMed]
- Mikheev, I.V.; Volkov, D.S.; Proskurnin, M.A.; Avramenko, N.V.; Korobov, M.V. Preparation and characterization of a new clustered {C70}n fullerene material. Nanosyst-Phys. Chem. M. 2014, 5, 46–52. [Google Scholar]
- Törpe, A.; Belton, D.J. Improved Spectrophotometric Analysis of Fullerenes C60 and C70 in High-solubility Organic Solvents. Anal. Sci. 2015, 31, 125–130. [Google Scholar] [CrossRef]
- Kyzyma, O.A.; Avdeev, M.V.; Bolshakova, O.I.; Melentev, P.; Sarantseva, S.V.; Ivankov, O.I.; Korobov, M.V.; Mikheev, I.V.; Tropin, T.V.; Kubovcikova, M.; Kopcansky, P.; Korolovych, V.F.; Aksenov, V.L.; Bulavin, L.A. State of aggregation and toxicity of aqueous fullerene solutions. Appl. Surf. Sci. 2019, 483, 69–75. [Google Scholar] [CrossRef]
- Bakhramov, S.A.; Kokhkharov, A.M.; Makhmanov, U.K.; Parpiev, O.R.; Khabibullaev, P.K. Photoinduced optical activity of C70 fullerene in organic solvents. J. Appl. Spectrosc. 2009, 76, 82–89. [Google Scholar] [CrossRef]
- Mchedlov-Petrossyan, N.O.; Marfunin, M.O. Formation, Stability, and Coagulation of Fullerene Organosols: C70 in Acetonitrile−Toluene Solutions and Related Systems. Langmuir 2021, 37, 7156–7166. [Google Scholar] [CrossRef]
- Datta, K.; Mukherjee, A.K. Aggregation of [70] fullerene in presence of acetonitrile: A chemical kinetic experiment. J. Chem. Phys. 2006, 124, 144509. [Google Scholar] [CrossRef]
- Lucian, M.; Marius-Adrian, H.; Baltog, I.; Mihaela, B.; Nicoleta, P.; Velula, T. , Bucur, C. Absorption and luminescence properties of C70 aggregates in solvent mixtures. Rom. Rep. Phys. 2009, 54, 529–538. [Google Scholar]
- Bulavin, L.A.; Nagorna, T.V.; Kyzyma, O.A.; Chudoba, D.; Ivankov, O.I.; Nagornyi, A.V.; Avdeev, M.V. Fullerene Clustering in C70/N-Methyl-2-Pyrrolidone/Toluene liquid System. Ukr. J. Phys. 2018, 63, 116–120. [Google Scholar] [CrossRef]
- Ginzburg, B.M.; Tuichiev, Sh.; Rashidov, D.; Sodikov, F.H.; Tabarov, S.H.; Shepelevskii, A.A. Step-Wise Concentration Influence of Fullerenes C60 and C70 on the Various Parameters of Condensed Systems. Part 1: The Concept of Step-Wise Behavior and its Manifestation in Fullerene Solutions. J. Macromol. Sci., Part B: Phys. 2015, 54, 533–543. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).