Submitted:
19 June 2023
Posted:
21 June 2023
You are already at the latest version
Abstract
Keywords:
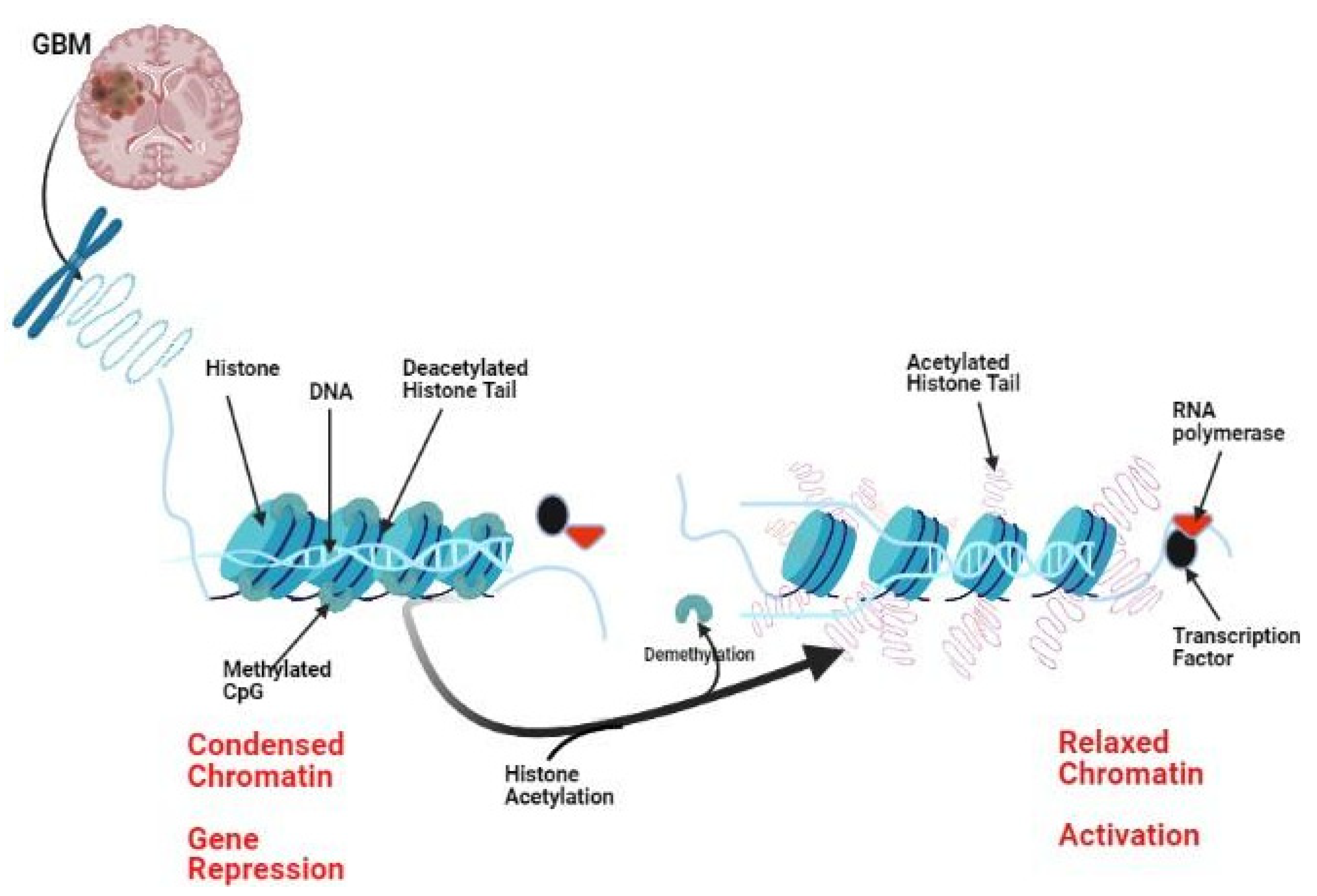
1. Introduction
2. Histone Modifications in Glioma:
2.1. Histone Deacetylation in Glioma:
2.2. Histone Acetylation in Glioma:
2.3. Histone Methylation in Glioma:
2.4. Histone Demethylation in Glioma:
2.5. Histone Ubiquitination in Glioma:
2.6. Histone Sumoylation and Glioma:
2.7. Histone Phosphorylation and Glioma:
2.8. Targeting Histone-Modifying Enzymes in Glioma
3. HDAC inhibitors (HDACi)
3.1. Vorinostat:
3.2. Valproic acid
3.3. Romidepsin (FK228)
3.4. Panobinostat
3.5. Limitations of HDACi in clinical practice:
4. Conclusion and future perspectives:
Abbreviations
| ABTC | The Adult Brain Tumor Consortium |
| EIAEDs | Enzyme-inducing antiepileptic drugs |
| EORTC | European Organization for Research and Treatment of Cancer |
| GBM | Glioblastoma |
| DNA | Deoxynucleic acid |
| EGF | Epidermal Growth Factor |
| HDAC | Histone Deacetylase |
| HDACi | Histone Deacetylase Inhibitors |
| HATs | Histone Acetyltransferases |
| NCIC | National Cancer Institute of Canada |
| USP | Ubiquitin Specific Protease |
| TMZ | Temozolomide |
References
- Sherrod, B.A.; Gamboa, N.T.; Wilkerson, C.; Wilde, H.; Azab, M.A.; Karsy, M.; Jensen, R.L.; Menacho, S.T. Effect of patient age on glioblastoma perioperative treatment costs: a value driven outcome database analysis. J. Neuro-Oncology 2019, 143, 465–473. [Google Scholar] [CrossRef] [PubMed]
- The Cancer Genome Atlas Research Network. Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature 2008, 455, 1061–1068. [Google Scholar] [CrossRef] [PubMed]
- Mclendon, R.E.; Friedman, A.H.; Bigner, D.D.; Van Meir, E.G.; Brat, D.J.; Mastrogianakis, G.M.; et al. Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature 2008, 455, 1061–1068. [Google Scholar] [CrossRef]
- Aparna, S.; Lakshmaiah, K.; Jacob, L.; Lokanatha, D.; Saldanha, S. Epigenetic therapy of cancer with histone deacetylase inhibitors. J. Cancer Res. Ther. 2014, 10, 469–478. [Google Scholar] [CrossRef]
- Stupp, R.; Mason, W.P.; van den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.B.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U.; et al. Radiotherapy plus Concomitant and Adjuvant Temozolomide for Glioblastoma. N. Engl. J. Med. 2005, 352, 987–996. [Google Scholar] [CrossRef]
- Bi, G.; Jiang, G. The molecular mechanism of HDAC inhibitors in anticancer effects. Cell. Mol. Immunol. 2006, 3, 285–290. [Google Scholar] [PubMed]
- Secrist, J.P.; Zhou, X.; Richon, V.M. HDAC inhibitors for the treatment of cancer. Curr. Opin. Investig. drugs (London, Engl. : 2000) 2003, 4. [Google Scholar]
- de Ruijter, A.J.; van Gennip, A.H.; Caron, H.N.; Kemp, S.; van Kuilenburg, A.B. Histone deacetylases (HDACs): characterization of the classical HDAC family. Biochem. J. 2003, 370, 737–749. [Google Scholar] [CrossRef]
- Bezecny, P. Histone deacetylase inhibitors in glioblastoma: pre-clinical and clinical experience. Med Oncol. 2014, 31, 985. [Google Scholar] [CrossRef]
- Adamopoulou, E.; Naumann, U. HDAC inhibitors and their potential applications to glioblastoma therapy. Oncoimmunology 2013, 2, e25219. [Google Scholar] [CrossRef]
- Sturm, D.; Bender, S.; Jones, D.T.W.; Lichter, P.; Grill, J.; Becher, O.; Hawkins, C.; Majewski, J.; Jones, C.; Costello, J.F.; et al. Paediatric and adult glioblastoma: multiform (epi)genomic culprits emerge. Nat. Rev. Cancer 2014, 14, 92–107. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.; Hamid, A.; Hussain, A.; Majeed, R.; Qurishi, Y.; Bhat, J.A.; Najar, R.A.; Qazi, A.K.; Zargar, M.A.; Singh, S.K.; et al. Understanding Histone Deacetylases in the Cancer Development and Treatment: An Epigenetic Perspective of Cancer Chemotherapy. DNA Cell Biol. 2012, 31, S62–S71. [Google Scholar] [CrossRef] [PubMed]
- Lucio-Eterovic, A.K.; Cortez, M.A.; Valera, E.T.; Motta, F.J.; Queiroz, R.G.; Machado, H.R.; Carlotti, C.G.; Neder, L.; A Scrideli, C.; Tone, L.G. Differential expression of 12 histone deacetylase (HDAC) genes in astrocytomas and normal brain tissue: class II and IV are hypoexpressed in glioblastomas. BMC Cancer 2008, 8, 243. [Google Scholar] [CrossRef] [PubMed]
- A Glozak, M.; Seto, E. Histone deacetylases and cancer. Oncogene 2007, 26, 5420–5432. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, Y.; Chen, J.; Tan, Q.; Xie, C.; Li, C.; Zhan, W.; Wang, M. Silencing of histone deacetylase 2 suppresses malignancy for proliferation, migration, and invasion of glioblastoma cells and enhances temozolomide sensitivity. Cancer Chemother. Pharmacol. 2016, 78, 1289–1296. [Google Scholar] [CrossRef]
- Zhong, S.; Fan, Y.; Wu, B.; Wang, Y.; Jiang, S.; Ge, J.; Hua, C.; Zhao, G.; Chen, Y.; Xu, H. HDAC3 Expression Correlates with the Prognosis and Grade of Patients with Glioma: A Diversification Analysis Based on Transcriptome and Clinical Evidence. World Neurosurg. 2018, 119, e145–e158. [Google Scholar] [CrossRef]
- Wang, Z.; Hu, P.; Tang, F.; Lian, H.; Chen, X.; Zhang, Y.; He, X.; Liu, W.; Xie, C. HDAC6 promotes cell proliferation and confers resistance to temozolomide in glioblastoma. Cancer Lett. 2016, 379, 134–142. [Google Scholar] [CrossRef]
- Guarente, L. Sirtuins, NAD+, aging, and disease: A retrospective and prospective overview. In Introductory Review on Sirtuins in Biology, Aging, and Disease (pp. 1–6); Elsevier, 2018. [Google Scholar] [CrossRef]
- Feng, J.; Yan, P.-F.; Zhao, H.-Y.; Zhang, F.-C.; Zhao, W.-H.; Feng, M. SIRT6 suppresses glioma cell growth via induction of apoptosis, inhibition of oxidative stress and suppression of JAK2/STAT3 signaling pathway activation. Oncol. Rep. 2016, 35, 1395–1402. [Google Scholar] [CrossRef]
- Chen, H.; Lin, R.; Zhang, Z.; Wei, Q.; Zhong, Z.; Huang, J.; Xu, Y. Sirtuin 1 knockdown inhibits glioma cell proliferation and potentiates temozolomide toxicity via facilitation of reactive oxygen species generation. Oncol. Lett. 2019, 17, 5343–5350. [Google Scholar] [CrossRef]
- Gao, L.; Cueto, M.A.; Asselbergs, F.; Atadja, P. Cloning and Functional Characterization of HDAC11, a Novel Member of the Human Histone Deacetylase Family. J. Biol. Chem. 2002, 277, 25748–25755. [Google Scholar] [CrossRef]
- Sealy, L.; Chalkley, R. DNA associated with hyperacetylated histone is preferentially digested by DNase I. Nucleic Acids Res. 1978, 5, 1863–1876. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Li, L.; Liang, J.; Shi, L.; Yang, J.; Yi, X.; Zhang, D.; Han, X.; Yu, N.; Shang, Y. Histone Acetyltransferase 1 Promotes Homologous Recombination in DNA Repair by Facilitating Histone Turnover. J. Biol. Chem. 2013, 288, 18271–18282. [Google Scholar] [CrossRef]
- Howe, L.; Auston, D.; Grant, P.; John, S.; Cook, R.G.; Workman, J.L.; Pillus, L. Histone H3 specific acetyltransferases are essential for cell cycle progression. Genes Dev. 2001, 15, 3144–3154. [Google Scholar] [CrossRef]
- Sterner, D.E.; Berger, S.L. Acetylation of Histones and Transcription-Related Factors. Microbiol. Mol. Biol. Rev. 2000, 64, 435–459. [Google Scholar] [CrossRef]
- Diao P, -Y.; Li S, -X.; Peng, J.; Yang J, -H.; Pan Y, -C.; Xu X, -P., ... Huang, G.-D. Overexpression of EP300-interacting inhibitor of differentiation 3 predicts poor prognosis in patients with glioblastoma multiforme. International Journal of Clinical and Experimental Pathology 2020, 13, 979–988.
- Lv, D.; Jia, F.; Hou, Y.; Sang, Y.; Alvarez, A.A.; Zhang, W.; Gao, W.-Q.; Hu, B.; Cheng, S.-Y.; Ge, J.; et al. Histone Acetyltransferase KAT6A Upregulates PI3K/AKT Signaling through TRIM24 Binding. Cancer Res 2017, 77, 6190–6201. [Google Scholar] [CrossRef] [PubMed]
- Bedford, M.T.; Richard, S. Arginine methylation an emerging regulator of protein function. Mol. Cell 2005, 18, 263–272. [Google Scholar] [CrossRef]
- Husmann, D.; Gozani, O. Histone lysine methyltransferases in biology and disease. In Nature Structural and Molecular Biology (Vol. 26, issue 10, pp. 880–889); Nature publishing group:2019. [CrossRef]
- Chiang, K.; Zielinska, A.E.; Shaaban, A.M.; Sanchez-Bailon, M.P.; Jarrold, J.; Clarke, T.L.; Zhang, J.; Francis, A.; Jones, L.J.; Smith, S.; et al. PRMT5 Is a Critical Regulator of Breast Cancer Stem Cell Function via Histone Methylation and FOXP1 Expression. Cell Rep. 2017, 21, 3498–3513. [Google Scholar] [CrossRef]
- Xu, K. DNA and Histone Methylation in Prostate Cancer. In Cancer Drug Discovery and Development (Vol. 0, issue 9783319597843, pp. 489–529); Humana press Inc, 2017. [Google Scholar] [CrossRef]
- Casciello, F.; Windloch, K.; Gannon, F.; Lee, J.S. Functional Role of G9a Histone Methyltransferase in Cancer. Front. Immunol. 2015, 6, 487. [Google Scholar] [CrossRef]
- Casciello, F.; Windloch, K.; Gannon, F.; Lee, J.S. Functional Role of G9a Histone Methyltransferase in Cancer. Front. Immunol. 2015, 6, 487. [Google Scholar] [CrossRef]
- Spyropoulou, A.; Gargalionis, A.; Dalagiorgou, G.; Adamopoulos, C.; Papavassiliou, K.A.; Lea, R.W.; Piperi, C.; Papavassiliou, A.G. Role of Histone Lysine Methyltransferases SUV39H1 and SETDB1 in Gliomagenesis: Modulation of Cell Proliferation, Migration, and Colony Formation. NeuroMolecular Med. 2014, 16, 70–82. [Google Scholar] [CrossRef] [PubMed]
- Dong, F.; Li, Q.; Yang, C.; Huo, D.; Wang, X.; Ai, C.; Kong, Y.; Sun, X.; Wang, W.; Zhou, Y.; et al. PRMT2 links histone H3R8 asymmetric dimethylation to oncogenic activation and tumorigenesis of glioblastoma. Nat. Commun. 2018, 9, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Loh, Y.-H.; Zhang, W.; Chen, X.; George, J.; Ng, H.-H. Jmjd1a and Jmjd2c histone H3 Lys 9 demethylases regulate self-renewal in embryonic stem cells. Genes Dev. 2007, 21, 2545–2557. [Google Scholar] [CrossRef] [PubMed]
- D’Oto, A.; Tian, Q.-W.; Davidoff, A.M.; Yang, J. Histone demethylases and their roles in cancer epige-netics. Journal of Medical Oncology and Therapeutics 2016, 1, 34–40.
- Falkenberg, K.J.; Johnstone, R.W. Histone deacetylases and their inhibitors in cancer, neurological diseases and immune disorders. Nat. Rev. Drug Discov. 2014, 13, 673–691. [Google Scholar] [CrossRef]
- Banelli, B.; Carra, E.; Barbieri, F.; Wurth, R.; Parodi, F.; Pattarozzi, A.; Carosio, R.; Forlani, A.; Allemanni, G.; Marubbi, D.; Florio, T.; Daga, A.; Romani, M. The histone demethylase KDM5A is a key factor for the resistance to temozolomide in 38- glioblastoma. Cell Cycle. 2015, 14, 3418–3429. [Google Scholar] [CrossRef]
- Sareddy, G.R.; Nair, B.C.; Krishnan, S.K.; Gonugunta, V.K.; Zhang, Q.-G.; Suzuki, T.; Miyata, N.; Brenner, A.J.; Brann, D.W.; Vadlamudi, R.K. KDM1 is a novel therapeutic target for the treatment of gliomas. Oncotarget 2013, 4, 18–28. [Google Scholar] [CrossRef]
- Shou, T.; Yang, H.; Lv, J.; Liu, D.; Sun, X. MicroRNA-3666 suppresses the growth and migration of glioblastoma cells by targeting KDM2A. Mol. Med. Rep. 2019, 19, 1049–1055. [Google Scholar] [CrossRef]
- Staberg, M.; Rasmussen, R.D.; Michaelsen, S.R.; Pedersen, H.; Jensen, K.E.; Villingshøj, M.; Skjoth-Rasmussen, J.; Brennum, J.; Vitting-Seerup, K.; Poulsen, H.S.; et al. Targeting glioma stem-like cell survival and chemoresistance through inhibition of lysine-specific histone demethylase KDM2B. Mol. Oncol. 2018, 12, 406–420. [Google Scholar] [CrossRef]
- Wang, B.; Fan, X.; Ma, C.; Lei, H.; Long, Q.; Chai, Y. Downregulation of KDM4A Suppresses the Survival of Glioma Cells by Promoting Autophagy. J. Mol. Neurosci. 2016, 60, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Romani, M.; Daga, A.; Forlani, A.; Pistillo, M.P.; Banelli, B. Targeting of Histone Demethylases KDM5A and KDM6B Inhibits the Proliferation of Temozolomide-Resistant Glioblastoma Cells. Cancers 2019, 11, 878. [Google Scholar] [CrossRef] [PubMed]
- Komander, D.; Rape, M. The Ubiquitin Code. Annu. Rev. Biochem. 2012, 81, 203–229. [Google Scholar] [CrossRef] [PubMed]
- Jeusset, L.M.-P.; McManus, K.J. Developing Targeted Therapies That Exploit Aberrant Histone Ubiquitination in Cancer. Cells 2019, 8, 165. [Google Scholar] [CrossRef]
- Fang, X.; Zhou, W.; Wu, Q.; Huang, Z.; Shi, Y.; Yang, K.; Chen, C.; Xie, Q.; Mack, S.C.; Wang, X.; et al. Deubiquitinase USP13 maintains glioblastoma stem cells by antagonizing FBXL14-mediated Myc ubiquitination. J. Exp. Med. 2017, 214, 245–267. [Google Scholar] [CrossRef]
- Oikonomaki, M.; Bady, P.; Hegi, M.E. Ubiquitin Specific Peptidase 15 (USP15) suppresses glioblastoma cell growth via stabilization of HECTD1 E3 ligase attenuating WNT pathway activity. Oncotarget 2017, 8, 110490–110502. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Liang, P.; Ji, W.; Yu, Z.; Chen, H.; Jiang, L. Ubiquitin-specific protease 4 promotes glioblastoma multiforme via activating ERK pathway. OncoTargets Ther. 2019, ume 12, 1825–1839. [Google Scholar] [CrossRef]
- Park, H.J.; Yun, D.-J. New Insights into the Role of the Small Ubiquitin-like Modifier (SUMO) in Plants. Int Rev Cell Mol Biol. 2013, 300, 161–209. [Google Scholar] [CrossRef]
- Celen, A.B.; Sahin, U. Sumoylation on its 25th anniversary: mechanisms, pathology, and emerging concepts. FEBS J. 2020, 287, 3110–3140. [Google Scholar] [CrossRef]
- Han, Z.-J.; Feng, Y.-H.; Gu, B.-H.; Li, Y.-M.; Chen, H. The post-translational modification, SUMOylation, and cancer (Review). Int. J. Oncol. 2018, 52, 1081–1094. [Google Scholar] [CrossRef]
- Yang, Y.; He, Y.; Wang, X.; Liang, Z.; He, G.; Zhang, P.; Zhu, H.; Xu, N.; Liang, S. Protein SUMOylation modification and its associations with disease. Open Biol. 2017, 7. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Ji, S. Inhibition of Ubc9-Induced CRMP2 SUMOylation Disrupts Glioblastoma Cell Proliferation. J. Mol. Neurosci. 2019, 69, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Liang, Z.; Xia, Z.; Wang, X.; Ma, Y.; Sheng, Z.; Gu, Q.; Shen, G.; Zhou, L.; Zhu, H.; et al. SAE1 promotes human glioma progression through activating AKT SUMOylation-mediated signaling pathways. Cell Commun. Signal. 2019, 17, 1–14. [Google Scholar] [CrossRef]
- Rossetto, D.; Avvakumov, N.; Côté, J. Histone phosphorylation: A chromatin modification involved in diverse nuclear events. Epigenetics 2012, 7, 1098–1108. [Google Scholar] [CrossRef] [PubMed]
- Lau, A.T.; Lee, S.-Y.; Xu, Y.-M.; Zheng, D.; Cho, Y.-Y.; Zhu, F.; Kim, H.-G.; Li, S.-Q.; Zhang, Z.; Bode, A.M.; et al. Phosphorylation of Histone H2B Serine 32 Is Linked to Cell Transformation. J. Biol. Chem. 2011, 286, 26628–26637. [Google Scholar] [CrossRef]
- Choi, H.S.; Choi, B.Y.; Cho, Y.-Y.; Mizuno, H.; Kang, B.S.; Bode, A.M.; Dong, Z. Phosphorylation of Histone H3 at Serine 10 Is Indispensable for Neoplastic Cell Transformation. Cancer Res 2005, 65, 5818–5827. [Google Scholar] [CrossRef] [PubMed]
- Pacaud, R.; Cheray, M.; Nadaradjane, A.; Vallette, F.M.; Cartron, P.-F. Histone H3 Phosphorylation in GBM: a New Rational to Guide the Use of Kinase Inhibitors in anti-GBM Therapy. Theranostics 2015, 5, 12–22. [Google Scholar] [CrossRef]
- Weller, M.; Wick, W.; Aldape, K.; Brada, M.; Berger, M.; Pfister, S.M.; Nishikawa, R.; Rosenthal, M.; Wen, P.Y.; Stupp, R.; et al. Glioma. In Nature Reviews Disease Primers. Nat Rev Dis Primers. 2015, 1, 15017. [Google Scholar] [CrossRef]
- A Azab, M.; Alomari, A.; Azzam, A.Y. Featuring how calcium channels and calmodulin affect glioblastoma behavior. A review article. Cancer Treat. Res. Commun. 2020, 25, 100255. [Google Scholar] [CrossRef]
- Li, A.; Walling, J.; Kotliarov, Y.; Center, A.; Steed, M.E.; Ahn, S.J.; Rosenblum, M.; Mikkelsen, T.; Zenklusen, J.C.; Fine, H.A. Genomic Changes and Gene Expression Profiles Reveal That Established Glioma Cell Lines Are Poorly Representative of Primary Human Gliomas. Mol. Cancer Res. 2008, 6, 21–30. [Google Scholar] [CrossRef]
- Mann, B.S.; Johnson, J.R.; Cohen, M.H.; Justice, R.; Pazdur, R. FDA Approval Summary: Vorinostat for Treatment of Advanced Primary Cutaneous T-Cell Lymphoma. Oncologist 2007, 12, 1247–1252. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, R.; Braga, C.; Santos, G.; Bronze, M.R.; Perry, M.J.; Moreira, R.; Brites, D.; Falcão, A.S. Targeting Gliomas: Can a New Alkylating Hybrid Compound Make a Difference? ACS Chem. Neurosci. 2017, 8, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Ciesielski, M.; Ramakrishnan, S.; Miles, K.M.; Ellis, L.; Sotomayor, P.; Shrikant, P.; Fenstermaker, R.; Pili, R. Class I Histone Deacetylase Inhibitor Entinostat Suppresses Regulatory T Cells and Enhances Immunotherapies in Renal and Prostate Cancer Models. PLOS ONE 2012, 7, e30815. [Google Scholar] [CrossRef]
- Ferrara, N.; Hillan, K.J.; Novotny, W. Bevacizumab (Avastin), a humanized anti-VEGF monoclonal antibody for cancer therapy. Biochem. Biophys. Res. Commun. 2005, 333, 328–335. [Google Scholar] [CrossRef]
- Carew, J.S.; Giles, F.J.; Nawrocki, S.T. Histone deacetylase inhibitors: Mechanisms of cell death and promise in combination cancer therapy. Cancer Lett. 2008, 269, 7–17. [Google Scholar] [CrossRef]
- Namdar, M.; Perez, G.; Ngo, L.; Marks, P.A. Selective inhibition of histone deacetylase 6 (HDAC6) induces DNA damage and sensitizes transformed cells to anticancer agents. Proc. Natl. Acad. Sci. 2010, 107, 20003–20008. [Google Scholar] [CrossRef]
- Ungerstedt, J.S.; Sowa, Y.; Xu, W.-S.; Shao, Y.; Dokmanovic, M.; Perez, G.; Ngo, L.; Holmgren, A.; Jiang, X.; Marks, P.A. Role of thioredoxin in the response of normal and transformed cells to histone deacetylase inhibitors. Proc. Natl. Acad. Sci. 2005, 102, 673–678. [Google Scholar] [CrossRef]
- Siegel, D.; Hussein, M.; Belani, C.; Robert, F.; Galanis, E.; Richon, V.M.; Garcia-Vargas, J.; Sanz-Rodriguez, C.; Rizvi, S. Vorinostat in solid and hematologic malignancies. J. Hematol. Oncol. 2009, 2, 31–31. [Google Scholar] [CrossRef]
- Modesitt, S.C.; Sill, M.; Hoffman, J.S.; Bender, D.P. A phase II study of vorinostat in the treatment of persistent or recurrent epithelial ovarian or primary peritoneal carcinoma: A Gynecologic Oncology Group study. Gynecol. Oncol. 2008, 109, 182–186. [Google Scholar] [CrossRef] [PubMed]
- Galanis, E.; Jaeckle, K.A.; Maurer, M.J.; Reid, J.M.; Ames, M.M.; Hardwick, J.S.; Reilly, J.F.; Loboda, A.; Nebozhyn, M.; Fantin, V.R.; et al. Phase II Trial of Vorinostat in Recurrent Glioblastoma Multiforme: A North Central Cancer Treatment Group Study. J. Clin. Oncol. 2009, 27, 2052–2058. [Google Scholar] [CrossRef]
- Galanis, E.; Anderson, S.K.; Lafky, J.M.; Uhm, J.H.; Giannini, C.; Kumar, S.K.; Kimlinger, T.K.; Northfelt, D.W.; Flynn, P.J.; Jaeckle, K.A.; et al. Phase II Study of Bevacizumab in Combination with Sorafenib in Recurrent Glioblastoma (N0776): A North Central Cancer Treatment Group Trial. Clin. Cancer Res. 2013, 19, 4816–4823. [Google Scholar] [CrossRef] [PubMed]
- Ghiaseddin, A.; Reardon, D.; Massey, W.; Mannerino, A.; Lipp, E.S.; Herndon, J.E.; McSherry, F.; Desjardins, A.; Randazzo, D.; Friedman, H.S.; et al. Phase II Study of Bevacizumab and Vorinostat for Patients with Recurrent World Health Organization Grade 4 Malignant Glioma. Oncol. 2018, 23, 157–e21. [Google Scholar] [CrossRef] [PubMed]
- Peters, K.B.; Lipp, E.S.; Miller, E.; Herndon, J.E.; McSherry, F.; Desjardins, A.; Reardon, D.A.; Friedman, H.S. Phase I/II trial of vorinostat, bevacizumab, and daily temozolomide for recurrent malignant gliomas. J. Neuro-Oncol. 2018, 137, 349–356. [Google Scholar] [CrossRef]
- Lee, E.Q.; Puduvalli, V.K.; Reid, J.M.; Kuhn, J.G.; Lamborn, K.R.; Cloughesy, T.F.; Chang, S.M.; Drappatz, J.; Yung, W.K.A.; Gilbert, M.R.; et al. Phase I Study of Vorinostat in Combination with Temozolomide in Patients with High-Grade Gliomas: North American Brain Tumor Consortium Study 04-03. Clin. Cancer Res. 2012, 18, 6032–6039. [Google Scholar] [CrossRef]
- Chinnaiyan, P.; Chowdhary, S.; Potthast, L.; Prabhu, A.; Tsai, Y.-Y.; Sarcar, B.; Kahali, S.; Brem, S.; Yu, H.M.; Rojiani, A.; et al. Phase I trial of vorinostat combined with bevacizumab and CPT-11 in recurrent glioblastoma. Neuro-Oncology 2012, 14, 93–100. [Google Scholar] [CrossRef]
- Qian, D.Z.; Wang, X.; Kachhap, S.K.; Kato, Y.; Wei, Y.; Zhang, L.; Atadja, P.; Pili, R. The Histone Deacetylase Inhibitor NVP-LAQ824 Inhibits Angiogenesis and Has a Greater Antitumor Effect in Combination with the Vascular Endothelial Growth Factor Receptor Tyrosine Kinase Inhibitor PTK787/ZK222584. Cancer Res 2004, 64, 6626–6634. [Google Scholar] [CrossRef] [PubMed]
- Sarcar, B.; Kahali, S.; Chinnaiyan, P. Vorinostat enhances the cytotoxic effects of the topoisomerase I inhibitor SN38 in glioblastoma cell lines. J Neurooncol. 2010, 99, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Friday, B.B.; Anderson, S.K.; Buckner, J.; Yu, C.; Giannini, C.; Geoffroy, F.; Schwerkoske, J.; Mazurczak, M.; Gross, H.; Pajon, E.; et al. Phase II trial of vorinostat in combination with bortezomib in recurrent glioblastoma: a north central cancer treatment group study. Neuro-Oncology 2012, 14, 215–221. [Google Scholar] [CrossRef]
- Weller, M.; Gorlia, T.; Cairncross, J.G.; Bent, M.J.v.D.; Mason, W.; Belanger, K.; Brandes, A.A.; Bogdahn, U.; Macdonald, D.R.; Forsyth, P.; et al. Prolonged survival with valproic acid use in the EORTC/NCIC temozolomide trial for glioblastoma. Neurology 2011, 77, 1156–1164. [Google Scholar] [CrossRef]
- Göttlicher, M.; Minucci, S.; Zhu, P.; Krämer, O.H.; Schimpf, A.; Giavara, S.; Sleeman, J.P.; Lo Coco, F.; Nervi, C.; Pelicci, P.G.; et al. Valproic acid defines a novel class of HDAC inhibitors inducing differentiation of transformed cells. EMBO J. 2001, 20, 6969–6978. [Google Scholar] [CrossRef]
- Wen, P.Y.; Schiff, D. Valproic acid as the AED of choice for patients with glioblastoma?: The jury is out. Neurology 2011, 77, 1114–1115. [Google Scholar] [CrossRef] [PubMed]
- Valiyaveetti, D.; Malik, M.; Joseph, D.M.; Ahmed, S.F.; Kothwal, S.A.; Vijayasaradhi, M. Effect of valproic acid on survival in glioblastoma: A prospective single-arm study. South Asian J. Cancer 2018, 07, 159–162. [Google Scholar] [CrossRef] [PubMed]
- Iwamoto, F.M.; Lamborn, K.R.; Kuhn, J.G.; Wen, P.Y.; Yung, W.K.A.; Gilbert, M.R.; Chang, S.M.; Lieberman, F.S.; Prados, M.D.; Fine, H.A. A phase I/II trial of the histone deacetylase inhibitor romidepsin for adults with recurrent malignant glioma: North American Brain Tumor Consortium Study 03-03. Neuro-Oncology 2011, 13, 509–516. [Google Scholar] [CrossRef]
- Hirata, Y.; Sasaki, T.; Kanki, H.; Choong, C.-J.; Nishiyama, K.; Kubo, G.; Hotei, A.; Taniguchi, M.; Mochizuki, H.; Uesato, S. New 5-Aryl-Substituted 2-Aminobenzamide-Type HDAC Inhibitors with a Diketopiperazine Group and Their Ameliorating Effects on Ischemia-Induced Neuronal Cell Death. Sci. Rep. 2018, 8, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Jordan, J.T.; Wen, P.Y. Novel Chemotherapeutic Approaches in Adult High-Grade Gliomas. In Cancer Treatment and Research. Cancer Treat Res. 2015, 163, 117–142. [Google Scholar] [CrossRef]
- Mayo, M.W.; Denlinger, C.E.; Broad, R.M.; Yeung, F.; Reilly, E.T.; Shi, Y.; Jones, D.R. Ineffectiveness of Histone Deacetylase Inhibitors to Induce Apoptosis Involves the Transcriptional Activation of NF-κB through the Akt Pathway. J. Biol. Chem. 2003, 278, 18980–18989. [Google Scholar] [CrossRef]
- Seo, Y.J.; Kang, Y.; Muench, L.; Reid, A.; Caesar, S.; Jean, L., ... Kim, S.W.(2014). Image guided synthesis reveals potent blood-brain barrier permeable histone deacetylase inhibitors. ACS Chemical Neuroscience 5(7), 588–596. [CrossRef]
- Seo, Y.J.; Muench, L.; Reid, A.; Chen, J.; Kang, Y.; Hooker, J.M.; Volkow, N.D.; Fowler, J.S.; Kim, S.W. Radionuclide labeling and evaluation of candidate radioligands for PET imaging of histone deacetylase in the brain. Bioorganic Med. Chem. Lett. 2013, 23, 6700–6705. [Google Scholar] [CrossRef]
- Hooker, J.M.; Kim, S.W.; Alexoff, D.; Xu, Y.; Shea, C.; Reid, A.; Volkow, N.; Fowler, J.S. Histone Deacetylase Inhibitor MS-275 Exhibits Poor Brain Penetration: Pharmacokinetic Studies of [11C]MS-275 using Positron Emission Tomography. ACS Chem. Neurosci. 2010, 1, 65–73. [Google Scholar] [CrossRef]
- Saidi, D.; Cheray, M.; Osman, A.M.; Stratoulias, V.; Lindberg, O.R.; Shen, X.; Blomgren, K.; Joseph, B. Glioma-induced SIRT1-dependent activation of hMOF histone H4 lysine 16 acetyltransferase in microglia promotes a tumor supporting phenotype. Oncoimmunology 2018, 7, e1382790. [Google Scholar] [CrossRef]
- Heddleston, J.M.; Wu, Q.; Rivera, M.; Minhas, S.; Lathia, J.D.; E Sloan, A.; Iliopoulos, O.; Hjelmeland, A.B.; Rich, J.N. Hypoxia-induced mixed-lineage leukemia 1 regulates glioma stem cell tumorigenic potential. Cell Death Differ. 2012, 19, 428–439. [Google Scholar] [CrossRef] [PubMed]
- Fontebasso, A.M.; Schwartzentruber, J.; Khuong-Quang, D.-A.; Liu, X.-Y.; Sturm, D.; Korshunov, A.; Jones, D.T.W.; Witt, H.; Kool, M.; Albrecht, S.; et al. Mutations in SETD2 and genes affecting histone H3K36 methylation target hemispheric high-grade gliomas. Acta Neuropathol. 2013, 125, 659–669. [Google Scholar] [CrossRef]
- Bozek, D.; Wang, A.; Hao, X.; Johnston, M.; Luchman, H.A.; Weiss, S. STEM-28. DOT1L EPIGENETICALLY REGULATES GBM BRAIN TUMOR STEM CELLS. Neuro-Oncology 2017, 19, vi231–vi232. [Google Scholar] [CrossRef]
- Wang, S.; Tan, X.; Yang, B.; Yin, B.; Yuan, J.; Qiang, B.; Peng, X. The role of protein arginine-methyltransferase 1 in gliomagenesis. BMB Rep. 2012, 45, 470–475. [Google Scholar] [CrossRef] [PubMed]
- Cheng, T.; Xu, Y. Effects of Enhancer of Zeste Homolog 2 (EZH2) Expression on Brain Glioma Cell Proliferation and Tumorigenesis. Experiment 2018, 24, 7249–7255. [Google Scholar] [CrossRef]
- Yan, F.; Alinari, L.; Lustberg, M.E.; Martin, L.K.; Cordero-Nieves, H.M.; Banasavadi-Siddegowda, Y.; Virk, S.; Barnholtz-Sloan, J.; Bell, E.H.; Wojton, J.; et al. Genetic Validation of the Protein Arginine Methyltransferase PRMT5 as a Candidate Therapeutic Target in Glioblastoma. Cancer Res 2014, 74, 1752–1765. [Google Scholar] [CrossRef]
- Kong, Y.; Ai, C.; Dong, F.; Xia, X.; Zhao, X.; Yang, C.; Kang, C.; Zhou, Y.; Zhao, Q.; Sun, X.; et al. Targeting of BMI-1 with PTC-209 inhibits glioblastoma development. Cell Cycle 2018, 17, 1199–1211. [Google Scholar] [CrossRef]
- Fan, L.; Chen, Z.; Wu, X.; Cai, X.; Feng, S.; Lu, J.; Wang, H.; Liu, N. Ubiquitin-Specific Protease 3 Promotes Glioblastoma Cell Invasion and Epithelial–Mesenchymal Transition via Stabilizing Snail. Mol. Cancer Res. 2019, 17, 1975–1984. [Google Scholar] [CrossRef]
- Grunda, J.M.; Nabors, L.B.; Palmer, C.A.; Chhieng, D.C.; Steg, A.; Mikkelsen, T.; Diasio, R.B.; Zhang, K.; Allison, D.; Grizzle, W.E.; et al. Increased Expression of Thymidylate Synthetase (TS), Ubiquitin Specific Protease 10 (USP10) and Survivin is Associated with Poor Survival in Glioblastoma Multiforme (GBM). J. Neuro-Oncology 2006, 80, 261–274. [Google Scholar] [CrossRef]
- Wang, Z.; Song, Q.; Xue, J.; Zhao, Y.; Qin, S. Ubiquitin-specific protease 28 is overexpressed in human glioblastomas and contributes to glioma tumorigenicity by regulating MYC expression. Exp. Biol. Med. 2016, 241, 255–264. [Google Scholar] [CrossRef]
- Lee, E.Q.; Reardon, D.A.; Schiff, D.; Drappatz, J.; Muzikansky, A.; Grimm, S.A.; Norden, A.D.; Nayak, L.; Beroukhim, R.; Rinne, M.L.; et al. Phase II study of panobinostat in combination with bevacizumab for recurrent glioblastoma and anaplastic glioma. Neuro-Oncology 2015, 17, 862–867. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Dong, L.; Bao, S.; Wang, M.; Yun, Y.; Zhu, R. FK228 augmented temozolomide sensitivity in human glioma cells by blocking PI3K/AKT/mTOR signal pathways. Biomed. Pharmacother. 2016, 84, 462–469. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.T.T.; Zhang, Y.; Shang, E.; Shu, C.; Quinzii, C.M.; Westhoff, M.-A.; Karpel-Massler, G.; Siegelin, M.D. Inhibition of HDAC1/2 Along with TRAP1 Causes Synthetic Lethality in Glioblastoma Model Systems. Cells 2020, 9, 1661. [Google Scholar] [CrossRef] [PubMed]
| HAT involved | Mechanism involved | Reference |
|---|---|---|
| KAT6A/MYST3 | Glioma cell-induced proliferation through H3K23ac/TRIM24-PI3K/AKT pathway | 27 |
| KAT8 | Manipulation of the H4K16 acetylation level in microglia, using the intrinsic H4K16 acetyltransferase activities, adjusted the microglia's tumor-supporting function. | 92 |
| KAT3B | An inhibitor for KAT3B acetyltransferase is highly expressed in GBM and correlates with a dismal prognosis. | 26 |
| Methyltransferases | ||
|---|---|---|
| KAMTs | PRMTs | |
| Type 1 | Type 2 | |
| SET1 | PRMT1 | PRMT5 |
| SET2 | PRMT2 | PRMT7 |
| SMYD | PRMT3 | PRMT9 |
| SUV4-20 | PRMT6 | |
| SET7/9 | PRMT8 | |
| SUV39 | PRMT4 | |
| HATs | Cell line used | Effect | Reference |
|---|---|---|---|
| KMT1A | Glioma cell lines (GOS-3, 1321N1, T98G, U87MG) | Positive correlation with aggressive tumors | 34 |
| KMT2A | Cell lines isolated from primary human GBM | Glioma stem cells were blunted following silencing of KMT2A | 93 |
| KMT3A | Patient-derived tumor cells | Expressed in High-grade pediatric glioma | 94 |
| KMT4 | Xenograft models | Inhibition of KMT4 reduced stem cell expression of stemness markers | 95 |
| KMT6 | Patient-derived GBM cultures | Reduced expression levels of KMT6 are associated with low expression of oncogenes as c-myc. | 97 |
| PRMT1 | T98G, U87MG, and A172 cell lines and mouse xenografts | Highly expressed in glioma cell lines | 96 |
| PRMT2 | U87 and T98G cell lines | Expressed in high-grade gliomas and associated with poor prognosis. | 35 |
| PRMT5 | U373MG and LN229 cell lines | The expression is high in the high-grade glioma | 98 |
| Ubiquitin specific enzymes | Preclinical study | Reference |
|---|---|---|
| USP1 | USP1 is overexpressed in glioma stem cells. Inhibition of USP1 increased radiosensitivity of GBM cells | 99 |
| USP3 | USP3 is highly expressed in GBM and correlates with poor prognosis. | 100 |
| USP4 | USP4 is highly expressed in GBM cells | 49 |
| USP 10 | USP 10 is overexpressed and linked to poorsurvival in GBM patients | 101 |
| USP 13 | USP13 is highly expressed in GBM and is required by glioma stem cells to maintain its stemness features. | 47 |
| USP 15 | USP15 attenuates the WNT pathway mediated by stabilization of HECTD1, supporting a tumor-suppressing role of USP15 in GBM cells. | 48 |
| USP28 | USP 28 is overexpressed in GBM cell lines andis associated with a high grade of glioma. | 102 |
| HDAC inhibitor | Combination therapy | Tumor type | Result | Sponsor | Reference |
|---|---|---|---|---|---|
| Vorinostat | Temozolomide + Isotretinoin, bortezomib |
Recurrent GBM | Still active | ||
| Bevacizumab | Recurrent GBM | No change in overall survival or progression-free survival compared to bevacizumab therapy. | Duke University Durham | 74 | |
| Temozolomide + Bevacizumab |
Recurrent GBM | Progression-free survival for six months was not affected. | Duke University Durham | 75 | |
| Bevacizumab + Irinotecan, Temsirolimus |
Diffuse intrinsic pontine glioma | Active | |||
| Radiotherapy | High –grade glioma and anaplastic astrocytoma | Active | National Cancer Institute | ||
| Valproic acid | VPA, temozolomide, and radiotherapy | Newly diagnosed GBM in adults | Active | National Cancer Institute |
|
| Romidepsin | Recurrent GBM | Completed and showed that romidepsin is ineffective in the treatment of recurrent GBM. | 85 | ||
| Panobinostat | Pediatric intrinsic pontine glioma |
Active | |||
| Bevacizumab | Recurrent GBM | Adding this agent to bevacizumab did not improve the outcome compared to bevacizumab alone. | 103 | ||
| Convection-enhanced delivery (CED) |
Diffuse pontine glioma |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




