1. Introduction
The subterranean species represent an interesting model to study the patterns and rates of evolution due to their restricted dispersal and concerted evolution of drastic adaptations to the underground habitat. For a long time, there has been a question regarding whether chromosomal changes occur differently in subterranean species compared to surface-dwelling mammalian species [
1]). Here we examine the evolutionary dynamics of chromosomal complements within the subfamily of Eurasian moles that mainly live in underground environments.
The family of moles Talpidae is a diverse group of underground insectivores in the order Eulypotyphla. Further, Talpinae is one of the three talpid subfamilies and includes Old World moles, desmans, and shrew moles predominantly found in Eurasia. Talpinae is further classified into five tribes. Notably, two sister tribes within the subfamily Talpinae are a specious and widely distributed tribe Talpini (5 genera, 22 species) and tribe Urotrichini (two monospecific genera
Urotrichus and
Dymecodon) (Table S1). The underground lifestyle of the Talpini results in similar physical traits of moles and makes it difficult to differentiate them, leading to the underestimation of the species diversity [
2]. In Talpini, the genus
Talpa is widely distributed from Western Europe to Asia. The other 4 genera diverged soon after their separation from the common ancestor with
Talpa and have limited ranges in Eastern Asia:
Mogera (5-9 species) and
Euroscaptor (6-8 species), and monospecific
Scaptochirus, Parascaptor, and
Oreoscaptor [
3,
4,
5].
The phylogeny of the Talpini tribe is now well established. Genetic research provided good support for phylogenies based on morphological data [
2,
3,
6]. High genetic differentiation within the most successful genus
Talpa allowed to identify 4 clades with a strict geographical reference for the European and Caucasian group of species, as well as the “davidiana” group, the re-colonization of Europe from Asia was shown [
7]. Nuclear sequence analysis demonstrated the nested position of
T. altaica within
Talpa as a separate Siberian clade. However, previous morphological and mitochondrial data indicated its more isolated position [
8] and even an elevation to a separate genus rank,
Asioscalops altaica [
9]. The genus
Euroscaptor contains two strongly divergent clades: the western one with the type species
E.klossi, including
E. malayana and
E.longirostris sensu lato, and the eastern one with E.parvidens sensu lato and the recently discovered species
E. subanura [
4,
10,
11,
12]. The taxonomy of
E. parvidens and
E. longirostris has not been definitively resolved [
3], so it is proposed to distinguish 2 additional new species within
E.longirostris sensu lato:
E.orlovi and
E.kuznetsovi, and within
E.parvidens a new subspecies of
E. parvidens ngoclinhensis [
4]. Recently Bui et al. [
13] suggested that moles from southern Vietnam (Dalat Plateau) and those from central Vietnam (Kontum Plateau) may represent different species,
E. parvidens and
E. ngoclinhensis, accordingly.
The only island species of
Euroscaptor sensu lato,
E. mizura, claims the status of a separate new genus
Oreoscaptor, being closer in its characteristics to the sister genus
Mogera [
4,
10,
11,
14]. The monophyly of the genus
Mogera is indisputable, now including 9 species, with a widespread continental distribution (
M. robusta and
M.latouchei) and island species with a limited range (
M.wogura, M.imaizumii, M.tokudae, M.etigo, M.uchidai, M.insularis, and
M.kanoana) [
3,
5,
15]. The final status of many
Mogera species has been established recently based on different characters, including karyotypic data. For example,
M.tokudae and
M.etigo represented 2 chromosomal races of the same
M.tokudae species previously, differing by 3 pericentric inversions [
16], at present, they are considered as 2 separate species. At the same time, genetic and morphological data were sufficient to separate
M.insularis and
M.kanoana, which have similar karyotypes [
17]. The taxonomic status of the continental and insular species
M. robusta and
M. wogura were resolved recently [
5].
Karyotypic studies have shown that chromosome sets within the genus
Talpa are sufficiently conservative: the differences are mainly due to a variation in the size of heterochromatin blocks on several pairs of autosomes [
18,
19,
20,
21]. The Asian genera of the tribe Talpini show a higher rate of chromosomal changes affecting the euchromatic part of the genome. For example, within the genus
Mogera, 4 inversions separate the Korean
M. robusta and Japanese populations of
M.wogura [
16]. At the same time, different species of
Mogera may have similar karyotypes as
M. etigo and
M. imaizumii, and the identity between the karyotypes of
M.wogura and
O.mizura [
16] indicates that they have retained the same chromosome set from their common ancestor. Similar karyotypes of the Taiwanese species
M.kanoana and
M.insularis are characterised by a smaller number of chromosomes 2n=32, while in other species of the genus 2n=36 [
17]. The chromosomal evolution of the genus
Euroscaptor is traced in detail by studies of G-banded karyotypes of
E.klossi and
E. malayana and Japanese
Oreoscaptor mizura (formerly treated as
Euroscaptor species). Comparative analysis revealed a reciprocal translocation that separates the karyotypes of the Japanese mountain mole
O. mizura from the Malaysian mole
E. malayana [
22]. A reciprocal translocation followed by a pericentric inversion separates the karyotypes of the Japanese mountain mole and Kloss’ mole [
23].
In the tribe of Japanese shrew moles Urotrichini (which is closely related to Talpini), there are 2 monospecific genera: Dymecodon and Urotrichus, the karyotypic relationships between which have been studied in detail. There are the eastern and western forms of U.talpoides that differ by the pericentric inversion of chromosome pair 14, accompanied by the accumulation of heterochromatin in the eastern form [
24]. According to craniological data, D.pilirostris is considered more primitive, and its karyotype is similar to the karyotype of the western form of U.talpoides [
25].
Molecular cytogenetic analysis in the Talpini tribe was carried out only for two species of the genus
Talpa:
T.europaea (TEUR) and
T.altaica (TALT) [
26,
27]. Using human sorting chromosomes, it was shown that the euchromatin segments of all chromosomes of both species are similar and have the same distribution of segments along the chromosomes, with the exception of the homeologous chromosome TEUR13, which underwent a pericentric inversion and/or centromeric shift. Additional heterochromatic arms changed the morphology of homeologous chromosomes TALT 1/ TEUR 9 and TALT 6/ TEUR 1. Such a high level of chromosome conservation is surprising for early divergent species characterized by high genetic diversity [
7]. Zoo-FISH analysis of data from 7 species representing all three insectivores families: moles, hedgehogs, and shrews revealed conservative blocks and syntenic associations of chromosomes preserved from the putative eutherian common ancestor. Comparing the syntenic associations between insectivoran families allowed us to assess the degree of their evolutionary variability, and it demonstrated that moles’ karyotypes are the most conserved among insectivores [
27].
In this paper, we describe for the first time a GTG-banded karyotype of the small-toothed mole E.parvidens. We trace chromosomal transformations within the genus Euroscaptor by comparing the karyotype of E. parvidens with other karyotypes of the genus. We assess the level of karyotypic transformations within the entire Talpinae subfamily by Zoo-FISH of chromosome-specific painting probes of the Siberian mole T. altaica onto metaphase chromosomes of three representatives of the Talpinae subfamily: the Talpini tribe - E.parvidens and M.imaizumii; and the Urotrichini tribe - Urotrichus talpoides.
3. Results
3.1. Description of the Karyotype of the Small-Toothed Mole from Vietnam, Euroscaptor Parvidens:
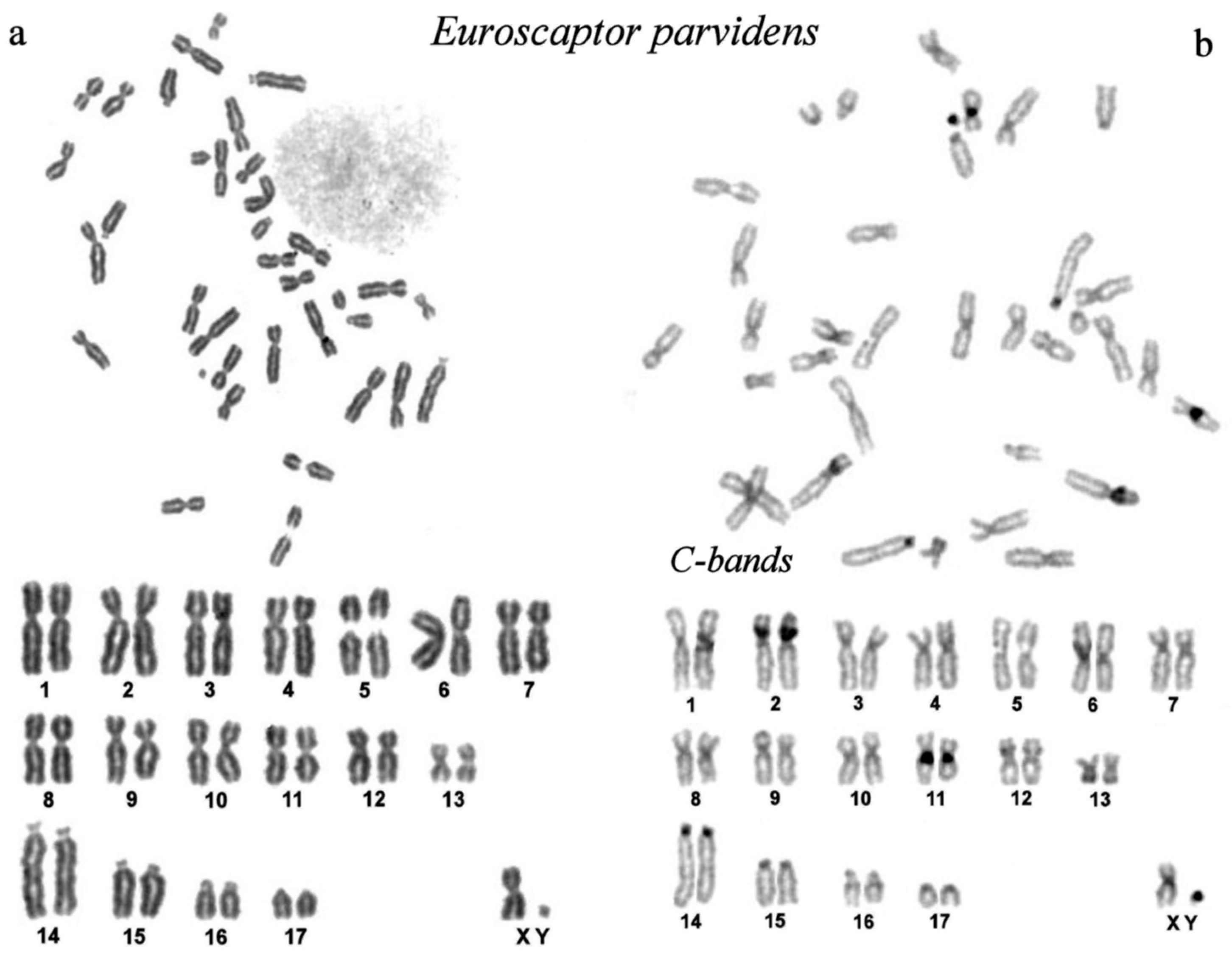
The karyotype of the small-toothed mole (2n=36) consists of 17 pairs of autosomes: 13 pairs of metacentrics, 3 pairs of subtelocentrics and 1 acrocentric, a metacentric X chromosome and the smallest chromosome in the complement is Y chromosome (
Figure 1a).
Large heterochromatin blocks are located in the pericentromeric regions of the short arm of chromosome 2 and the long arm of chromosome 11. Small blocks of C-heterochromatin are located in the pericentromeric regions of the large acrocentric chromosome 14 and the tiny Y chromosome (
Figure 1b).
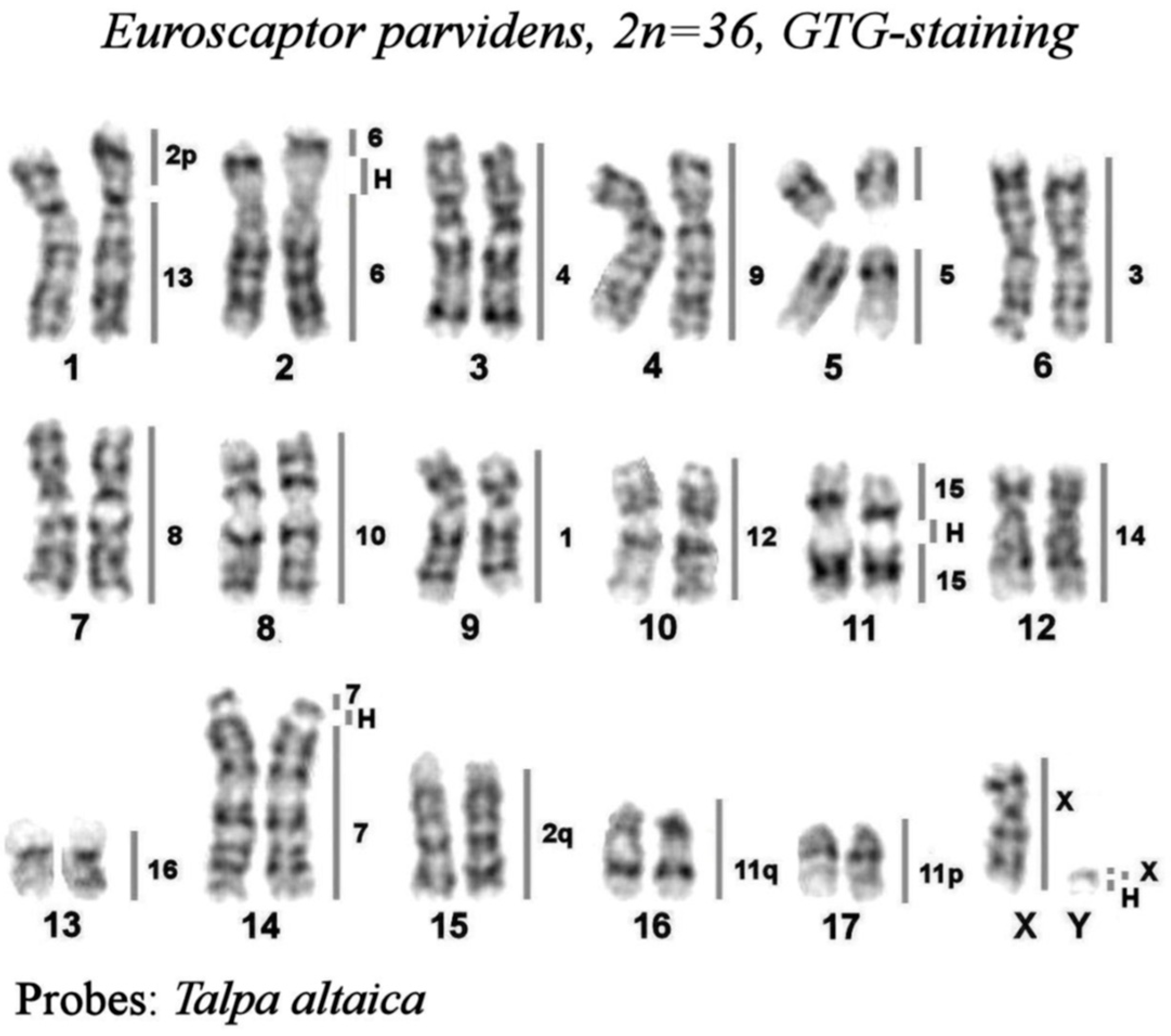
Figure 2 shows a GTG-banded karyotype of the small-toothed mole.
3.2. Comparison of G-Banded Chromosomes of Euroscaptor Species
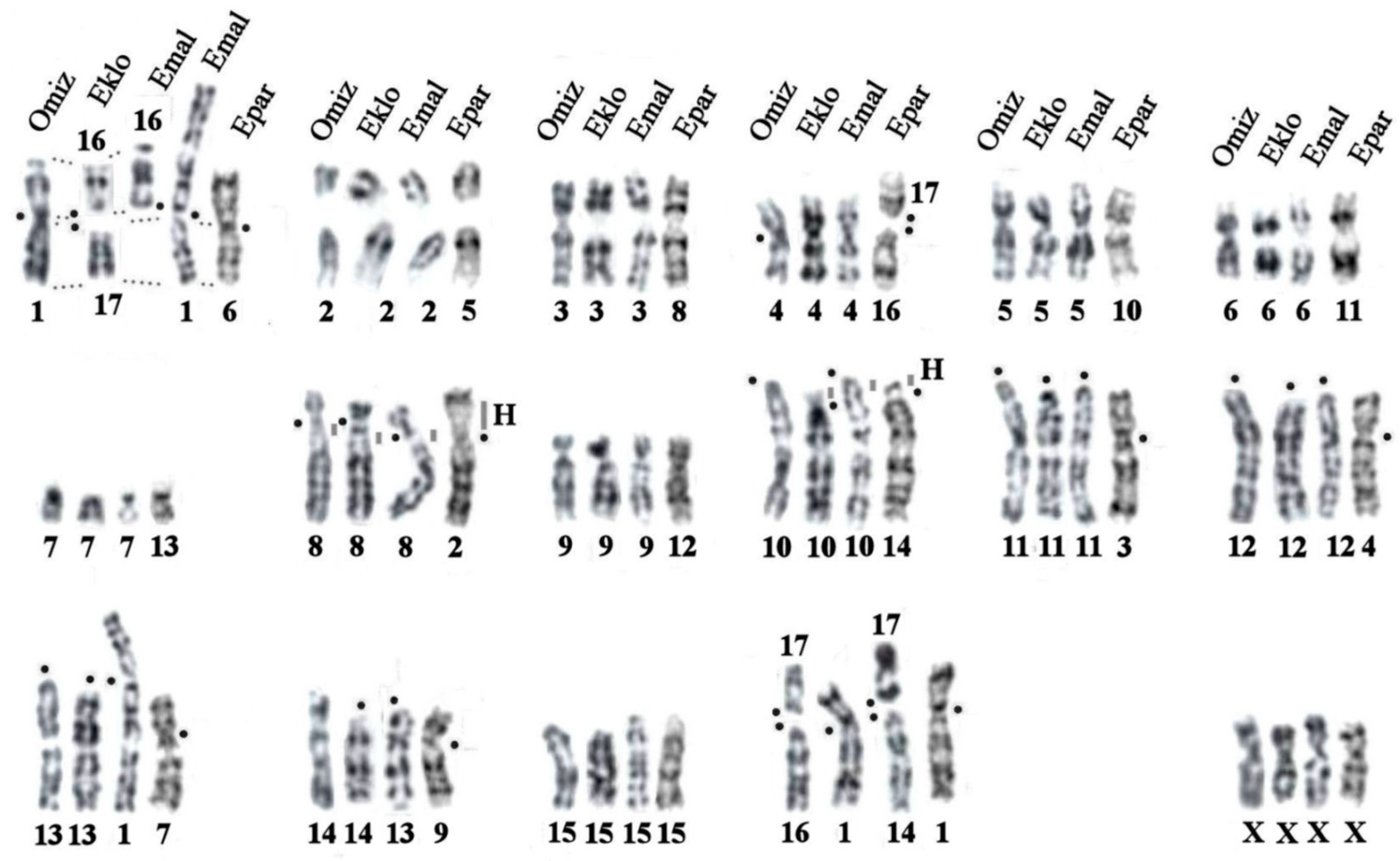
A comparative chromosomal analysis of E. parvidens with available G-banded karyotypes from Euroscaptor (E. klossi, E. malayana) and Japanese mountain mole, Oreoscaptor mizura [
16,
23,
35] was performed (
Figure 3).
The chromosomes of these species were arranged relative to the Japanese mountain mole O. mizura nomenclature (
Table 1). Chromosomal analysis showed the following:
-7 autosomal pairs of the small-toothed mole (chromosomes 5, 8, 10-13, and 15) are homologous to chromosomes 2-7, 10, and 15, respectively, in the all compared species, as well as their X chromosomes;
- accumulation of repeats in the short arm changed the morphology of the submetacentric chromosome 2 of E. parvidens compared to the homologous chromosome 8 of other species;
- 4 metacentric chromosomes of the small-toothed mole (chromosomes 3, 4, 7, and 9) underwent pericentric inversions in contrast to the homologous acrocentric 11, 12, 13, and 14, respectively, of other species;
- a small inversion of the pericentromeric heterochromatin distinguishes the subtelocentric chromosomes 14 of E.parvidens, 10 of E.klossi (EKLO) and the acrocentric chromosomes 10 of both O.mizura (OMIZ) and E.malayana (EMAL), respectively;
- centric fusions/fissions separate chromosomes/chromosomal arms:
- (a)
OMIZ 1 and EPAR 6 with EKLO 16+17 and EMAL 1p+16
- (b)
OMIZ 4, EKLO 4, and EMAL 4 with EPAR 16+17;
- (c)
OMIZ 17+16 and EMIC 17+14 with EKLO 1 and EPAR 1;
The results of the analysis are summarized in
Table 1.
3.3. Chromosome Painting
The whole chromosome set of the Siberian mole (T. altaica, TALT) painting probes was hybridized onto chromosomes of three moles from Southeast Asia: the small-toothed mole (E. parvidens, EPAR) and small Japanese mole (M. imaizumii, MIMI) from tribe Talpini, and the Japanese shrew mole (U. talpoides, UTAL) from tribe Urotrichini.
The chromosome painting delimited homologous chromosomal segments between species from different tribes and provided correspondence between the conserved chromosomal segments within the subfamily Talpinae. Additional rearrangements within the found segments, including pericentric inversions and centromeric shifts, were revealed using a comparative analysis of GTG-banded chromosomes.
1. Assignment of the peaks of the flow-sorted karyotype
The 17 chromosomes of a T. altaica female were resolved into 14 peaks (Figure S1). 11 peaks each contained a single chromosome (TALT 1, 2, 3, 5 8, 10-12, 14-16), three peaks included two chromosomes each (TALT 4+7, 6+9, 13+X). Therefore, the whole set of painting probes contains all 16 pairs of autosomes and the X chromosome.
2. Painting the E. parvidens genome with T. altaica probes
The hybridization results are presented as a map, where all T. altaica probes are assigned to the G-banded karyotype of E. parvidens (
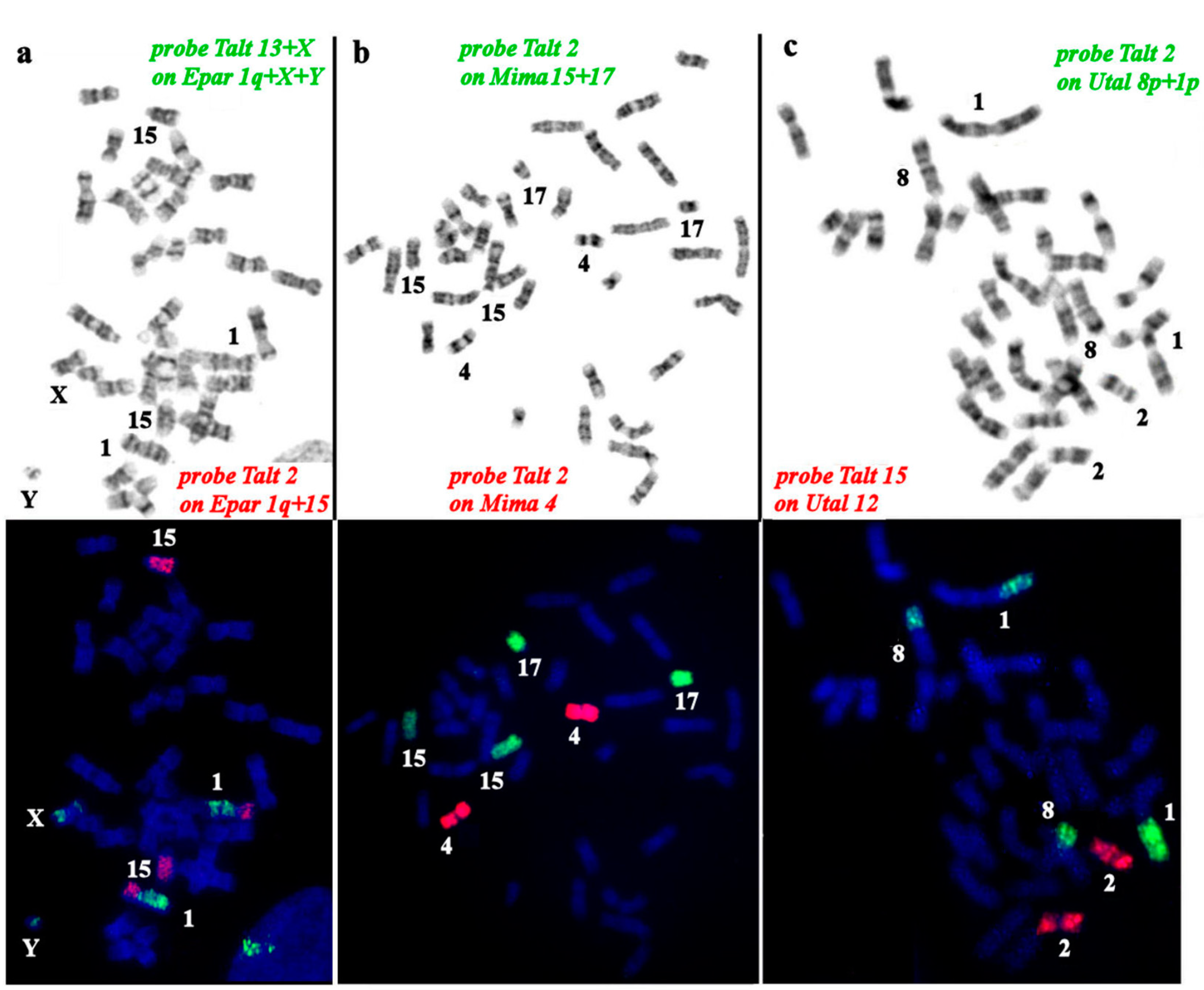
Figure 2). The Siberian mole painting probes delineated 20 homologous segments in the small-toothed mole karyotype. 14 Siberian mole chromosomes (TALT 1, 3-10, 12, 14-16, and X) were conserved in the karyotype of the small-toothed mole in toto. The metacentric chromosome TALT 11 underwent centromeric fission, and its arms are represented as acrocentrics EPAR 16 and EPAR 17. The large metacentric TALT 2 also split into two segments, and the smallest segment then fused with TALT 13, forming the largest chromosome EPAR 1. The largest part of TALT 2 formed the acrocentric EPAR 15 (
Figure 4a). The weak signal on EPAR 5p can be explained by the underrepresentation of the homologous chromosome of TALT5 in the painting probe. The presence of additional signals produced by both TALT1 and TALT6 on the EPAR 2p is explained by the presence in these areas of heterochromatin blocks of similar composition.
3. Painting the Mogera imaizumii genome with T. altaica probes
The hybridization results are presented as a map on the G-banded karyotype of the small Japanese mole (Mogera imaizumii) (
Figure 5). The Siberian mole painting probes delineated 18 homologous segments in the small Japanese mole karyotype. Almost all Siberian mole chromosomes (TALT 1, 3-16, and X) were entirely conserved in the karyotype of M.imaizumii; only one metacentric TALT 2 underwent fission into two segments which produced acrocentrics MIMI 15 and MIMI 17 (
Figure 4b).
4. Painting the Urotrichus talpoides genome with T. altaica probes.
The hybridization results are presented as a map on the G-banded karyotype of the Japanese shrew mole Urotrichus talpoides (
Figure 6). The Siberian mole painting probes delineated 19 homologous segments in the Japanese shrew mole karyotype. Fifteen Siberian mole chromosomes (TALT 1, 3-9, 11-16, and X) were conserved in toto in the karyotype of U. talpoides. Two Siberian mole metacentrics - TALT 2 (
Figure 4c) and TALT 10 - were divided into two segments, which fused with other elements producing the largest metacentric UTAL 1, medium-size metacentric UTAL 8, and acrocentric UTAL 15.
3.4. Comparison of Karyotypes of 4 Species of the Talpinae Subfamily
The results of painting with sorted chromosomes of the Siberian mole allowed us to compare species from the other genera of the Talpinae subfamily: mole species from four genera of the Talpini tribe: Euroscaptor, Oreoscaptor, Mogera, and Talpa with the Japanese shrew mole (Urotrichus talpoides) from the Urotrichini tribe.
Figure 7 shows a comparative analysis of the chromosomes of E.parvidens (EPAR), M.imaizumii (MIMI), and U.talpoides (UTAL) ordered relative to the karyotype of the Siberian mole T.altaica (TALT):
- 6 pairs of autosomes homologous to chromosomes 3, 5, 12, 14-16 of the Siberian mole remained conserved, as did the X chromosomes in all species;
- amplification of additional heterochromatin occurred in the p-arm of EPAR 2. A small block of centromeric heterochromatin is present in homologs from all compared species;
- 5 pairs of autosomes homologous to chromosomes 4, 7-9, and 13 of the Siberian mole differ by pericentric inversions and/or centromeric shifts;
- 4 pairs of autosomes homologous to chromosomes 1, 2, 10, and 11 of the Siberian mole are involved in chromosome fusion/fission.
All obtained data are summarized in Table S2.
3.5. Comparison of Karyotypes of 8 Species from the Subfamily Talpinae
We expanded the number of analyzed species to 8 by supplementing the above-mentioned species with the karyotype of the European mole (Talpa europaea), previously studied by comparative painting with human probes. Thus, comparing the karyotypes of 7 species of the tribe Talpini and one species of the tribe Urotrichini, we tried to find their conserved elements and chromosome rearrangements that accompanied the divergence of these species. Based on the data obtained, we can assume the following (
Table 2,
Figure 8):
- 5 autosomal pairs homologous to chromosomes 5, 12, 14 -16 and X-chromosome of the Siberian mole remained unchanged in all eight mole species;
- chromosomal pairs homologous to TALT 6 are characterized by the amplification of heterochromatic blocks in the centromeric regions of chromosomes TEUR 1p and EPAR 2p (
Figure 8a). Small blocks of heterochromatin are present in the centromeric region of all studied species;
- whole arm homology is generally preserved:
- (a)
UTAL 1p/8p, OMIZ 15/17, MIMI 15/17, EKLO 1p/15 EMAL 15/17 and EPAR 1p/15 to TALT 2/TEUR 2 (
Figure 8b);
- (b)
EKLO 16/17 and EMAL 1p+16 are homologous to TALT 3 (
Figure 8c);
- (c)
UTAL 8q/UTAL15 is homologous to TALT 10 (
Figure 8d);
- (d)
EPAR 16/EPAR 17 is homologous to TALT 11 (
Figure 8e).
- inversions/centromeric shifts occurred on 6 chromosomes:
(a) The chromosomal arms TALT 1q, TEUR 9q, and UTAL 1q are homologous to the acrocentrics EKLO 14, EMAL 13, MIMA 14, and OMIZ 14, respectively. Pericentric inversions of the proximal part of the q-arms produced submetacentric chromosomes EPAR 9 and MIMI 14. The short arms of TALT 1 and TEUR 9 are composed of heterochromatic blocks. UTAL1 resulted from a centric fusion of two ancestral acrocentrics (
Figure 8f);
(b) Chromosomes TALT 4, TEUR 3, and UTAL 3 are homologous; pericentric inversions of their p-arms led to the appearance of acrocentric chromosomes 11 in EKLO, OMIZ, and EMAL. A subsequent pericentric inversion of the subcentromeric region led to the appearance of the submetacentric chromosome MIMA 11. A centromeric shift probably led to the appearance of the submetacentric chromosome EPAR 3 (
Figure 8g);
(c) Chromosomes TALT 7, TEUR 4, and UTAL 4 are homologous. Pericentric inversions of the p-arms led to the appearance of acrocentric chromosomes OMIZ 10, EMAL10, and MIMA 10. The appearance of additional heterochromatic arms on EKLO 10 and EPAR 14 explains their subtelocentric morphology (
Figure 8h).
(d) Chromosomes TALT 8, TEUR 7, and UTAL 7 are homologous, pericentric inversions of the p-arm lead to the appearance of acrocentric chromosomes MIMA 13, OMIZ 13, EKLO 13, and q-arm EMAL1. The appearance of the submetacentric EPAR 7 can be explained by the centromeric shift followed by an inversion. (
Figure 8i).
(e) Chromosomes TALT 9, TEUR 6, and UTAL 6 are homologous, pericentric inversions of the p-arms led to the appearance of acrocentric chromosomes MIMA 12, OMIZ 12, EKLO12, and EMAL12. A subsequent pericentric inversion of the proximal part of an ancestral acrocentric led to the appearance of a submetacentric EPAR 4 (
Figure 8j).
(f) The most confusing scenario of rearrangements relates to TALT 13, TEUR 13, UTAL 13, MIMA 16, and EPAR 1, the homology of these elements was shown only by the TALT 13 painting probes, and the difference in the GTG pattern of these chromosomes can be explained by a series of inversions. For example, the chromosomes TALT 13 and TEUR 13, according to human painting probe localisation and GTG-banding, differ by a pericentric inversion on TEUR 13q and the proximal part of TALT 13q. In Euroscaptor species, chromosomes EPAR 1 and EKLO 1 are similar, and both have resulted from fusions of ancestral acrocentrics, whereas their q-arms are homologous to TALT 13 and OMIZ 16. EMAL 1 is also a result of the ancestral centric fusion of two acrocentrics. Thus, TALT 13 homologs in all species have undergone multiple inversions and fusions.
4. Discussion
Comparative analysis of differentially banded chromosomes of different species reflects the patterns of evolutionary changes in the taxon and can serve, together with craniological and genetic characteristics, as a basis for species status ranking. The use of painting probes expands the possibilities of interspecific comparison, including widely diverged species from different genera and higher taxa. Chromosomal rearrangements characteristic for a particular taxon can serve as markers of evolutionary alterations, providing additional support in phylogenetic reconstructions. Here we traced evolutionary changes of chromosomal complements in a diverse group of talpine moles.
4.1. Chromosomal Rearrangements in the Genus Euroscaptor Sensu Lato
Differentially banded karyotypes were available only for 3 species of this group: two species from the western clade -
E. klossi and
E. malayana (the latter was previously called
E. micrura malayana) and the island species
Oreoscaptor mizura (formerly known as
E. mizura) [
16,
23,
35]. By incorporating
E.parvidens into the comparative analysis, not only did the number of species of the genus being studied increased, but the representativeness of the analysis also expanded to include a member of the eastern clade. We have shown that each stage of species divergence within the genus is marked by certain rearrangements in half of the autosomes, which have undergone changes in contrast to the rest of the autosomes (homologs EPAR 5, 8, 10-15), which were conserved (
Table 1,
Figure 3). The Japanese mountain mole,
Oreoscaptor mizura, formerly treated as
E. mizura, was suggested to be elevated to genus level [
2,
4,
10,
11,
36]. Its karyotype differs from the
Euroscaptor by the absence of evolutionary rearrangements, which gives reason to assume its chromosome set is the most conservative and close to the ancestral karyotype of the genus. Moreover, its similarity to the karyotype of
M.wogura, a species from another genus [
16], suggests that these species retained a chromosomal set of an ancestor common to both genera. Comparing the karyotypes of
O. mizura and
E. malayana, Kawada [
35] explained the differences by a reciprocal translocation in the Malaysian mole. Our analysis of a larger number of species showed that the interchromosomal rearrangements, such as fissions and fusions occurred successively.
O.mizura, similarly to
E. parvidens and other representatives of the genera
Talpa and
Urotrichus, preserved the ancestral variant of chromosome 1 (
Table 1 and
Table 2,
Figure 3 and
Figure 8).
E. malayana, as well as another representative of the Western clade -
E. klossi, underwent a fission of the ancestral variant of chromosome 1, which can be considered a karyotypic signature of the western clade (EKLO 16-17/EMAL 1p-16). Further fusion of one of the formed segments with a large acrocentric in
E. malayana led to the appearance of the largest submetacentric EMAL 1, which is the distinctive characteristic of the Malaysian mole.
The karyotype of the small-toothed mole turned out to be the most rearranged relative to the other species of the genus. The rearrangements include pericentric inversions of 4 pairs of chromosomes (EPAR 3, 4, 7 and 9), fission that led to the formation of two chromosomes (EPAR 16 and 17), and amplification of heterochromatin on the short arm of EPAR 2. Chromosomal analysis of the remaining species/subspecies of the clade will demonstrate if these rearrangements are characteristic of the entire eastern clade. According to Zemlemerova et al. [
4], there is a large difference in genetic and morphological data between geographically distant populations of
E.parvidens, in contrast to the closely related species
E.subanura with low genetic and morphological variability, despite its widespread distribution associated with its recent colonization.
A chromosomal rearrangement, which occurred independently in some species of the western and eastern clades (homoplasy), may be a fusion that led to the formation of chromosome 1 in E.klossi and E.parvidens, but it does not exist in the Malaysian mole, or in the Japanese mountain mole. Further karyotypic analysis with the involvement of a larger number of species will help to resolve this issue.
The analysis of the genus Euroscaptor karyotypes has shown that pericentric inversions, fissions, fusions, and additional heterochromatin play a major role in the evolution of the genus. The chromosome set of the Japanese mountain mole (O.mizura) can be considered the closest to the ancestral karyotype of the genus. Significantly, all stages of divergence of the genus carry chromosomal markers for each species and group of species. The inclusion of a larger number of species in the analysis will help to reconstruct the karyotypic history of the genus in more detail.
4.2. Chromosomal Rearrangements in the Talpini and Urotrichini Tribes
We expanded the comparative analysis of karyotypes to the level of a subfamily, by including eight species representing not only the genera
Euroscaptor and
Oreoscaptor, but also the genera
Mogera and
Talpa from the Talpini tribe, and used
Urotrichus talpoides from the Urotrichini tribe as an outgroup. For comparison, the chromosomal painting was performed with sorted chromosomes of the Siberian mole (
T.altaica) on chromosomes of
E.parvidens, Mogera imaizumii, and
U.talpoides. The comparison was supplemented by the considered above species of the genus
Euroscaptor and the European mole
T.europaea described earlier [
26,
27].
The analysis showed that autosomes, homologous to the large NOR-bearing chromosome (TALT 5) and most of the small autosomes (TALT 12, 14-16), as well as the X chromosome, remained unchanged in all eight mole species (
Table 2,
Figure 8). Variations in the amount of heterochromatin on TALT 1 and TEUR 9 homologs are found only in species of
Talpa, including
T.romana and
T.occidentalis [
21,
37], as is the TALT 2p+2q/TEUR 2p+2q fusion. Both of these characteristics can serve as cytogenetic signatures of the genus
Talpa.
The rate of chromosomal rearrangements is much higher in the Asian mole genera. Chromosomal analysis revealed the pericentric inversions of 4 pairs of chromosomes homologous to the chromosomes of the Siberian mole 4, 7, 8, and 9 are common to two close genera of
Mogera and
Euroscaptor (
Table 2,
Figure 7 and
Figure 8). The presence of common chromosomal characteristics is consistent with morphological and genetic data on their close relationship. Interestingly, further rearrangements of these chromosomes are observed in representatives of both genera. Thus the homologs of the TALT 4 chromosome subsequently underwent an inversion in
M.imaizimii (chromosome 11) and a centromeric shift in
E. parvidens (chromosome 3). It should be noted that it is this inversion that separates the karyotypes of
M.imaizimii and
M.etigo from the karyotype of
M.wogura [
16], and perhaps it is an apomorphic trait of
M.imaizimii karyotype, while the other species of
Mogera preserved a common variant of chromosome 11 for both genera. TALT 7 homologs in
E.parvidens (chromosome 14) and in
E.klossi (chromosome 10) developed small heterochromatic short arms. According to Kawada [
16], 4 inversions on homologs of TALT 7, TALT 8, TALT 13, and TALT 2p separate the Korean
M. robusta and Japanese populations of
M.wogura. TALT 8 homologs in
E.parvidens (chromosome 7) and
E. malayana (chromosome 1) underwent a centromeric shift and a fusion, respectively. The TALT 9 homolog has undergone another inversion in
E.parvidens (chromosome 7). In general, for each species in the genus
Euroscaptor, there is one characteristic feature associated with further chromosome transformations homologous to one of these 4 chromosomes. The exception is
E.parvidens, in which all 4 homologous chromosomes underwent further rearrangements and additional fission, which led to the formation of chromosomes EPAR 16 and EPAR17. At the moment, the karyotype of the small-toothed mole is the most derived among all the mole species studied so far.
It can be assumed that the karyotype of the western form of
U.talpoides, used here, is the closest to the ancestral karyotype of the Urotrichini tribe. According to our data, the phylogenetic features of the genus
Urotrichus include two fusions that led to the formation of UTAL 1 and UTAL 8 and one fission that led to the formation of UTAL 15 and UTAL 8q (
Table 2,
Figure 8). It is noteworthy that the accumulation of heterochromatin is characteristic of UTAL 14 homologs, which is not shown for the western form, but is observed together with a pericentric inversion in the eastern form of the species.
4.3. Reconstruction of the Ancestral Karyotype of the Talpini and Urotrichini Tribes
Our analysis allowed us to propose a possible ancestral karyotype of the tribe and, based on it, to trace the features of chromosomal rearrangements accompanying the divergence of moles. The existing phylogenetic trees of moles differ in the position of the Japanese mountain mole
Oreoscaptor mizura relative to representatives of the genera
Mogera and
Euroscaptor. Some researchers place it basal to these genera [
3,
4,
10,
11], others place it in a separate clade together with
Mogera, different from the branch leading to
Euroscaptor,
Parascaptor, and
Scaptorchirus [
2,
7].
During the analysis of the numbers of chromosomal rearrangements on the phylogenetic tree, we observed different rates of karyotypic transformations (
Figure 9). Two rearrangements, including one fusion and amplification of heterochromatin, took place in about 10 million years of the shared history of the Siberian and European moles. Over the next 5 million years after this divergence,
T.europaea underwent a pericentric inversion in the chromosome 13 and amplification of heterochromatin in the short chromosome 1, and
T. altaica only acquired an amplified heterochromatic block in the short arm of chromosome 1. The data obtained confirm chromosomal conservation within the genus
Talpa, which also demonstrates morphological stability as opposed to high genetic variability [
7]. According to our results, only the karyotype of Urotrichus talpoides from the close Urotrichini tribe is more stable, with three rearrangements over 35 million years.
Karyotypes of the Asian species were actively rearranged after divergence from the genus
Talpa, undergoing 4 inversions over 5 million years; then the rate of karyotypic divergence became heterogeneous in different lineages. Exceptional chromosome conservation is noted in the Japanese mountain mole
O.mizura, which is consistent with its basal position relative to the rest of the Asian moles (
Euroscaptor, Mogera). The karyotype of the Japanese
M.wogura is no less stable, whereas the Korean population of
M. robusta underwent 4 inversions in a fairly short time after divergence [
16]. Karyotypes of representatives of the western clade of
Euroscaptor (E.klossi and E.malayana) underwent only a few rearrangements, while further divergence of eastern clade (
E.parvidens) was accompanied by 5 rearrangements over about 7 million years.
The data obtained here by karyotype analysis of the species from the Talpini and Urotrichini tribes allowed us to estimate the rates of chromosomal transformations within the Talpinae subfamily. A high level of chromosomal conservation in the genus Talpa was confirmed, and cytogenetic signatures in each of the species groups were determined. It is shown that the Asian species of the tribe are characterized by pericentric inversions (and other transformations) of 4 pairs of autosomes. The karyotype of the Japanese mountain mole O.mizura seems to be the most conserved among the Asian moles. The most frequently occurring types of chromosomal rearrangements in moles are the pericentric inversions and amplification of heterochromatin. The inclusion of a larger number of species in the comparative analysis will allow us to more accurately reconstruct the chromosomal history of moles more accurately.
Figure 1.
The karyotype of small-toothed mole from Vietnam, Euroscaptor parvidens, 2n=36. a. Routine Giemsa chromosome staining: top - complete metaphase plate, bottom - the karyotype. Note the secondary constriction on chromosome 5, a likely site of nuclear organizing region (NOR). b. Constitutive heterochromatin by CBG staining: top - complete metaphase plate, bottom - the karyotype. Note prominent heterochromatin bands on chromosomes 2, 11, 14, and Y.
Figure 1.
The karyotype of small-toothed mole from Vietnam, Euroscaptor parvidens, 2n=36. a. Routine Giemsa chromosome staining: top - complete metaphase plate, bottom - the karyotype. Note the secondary constriction on chromosome 5, a likely site of nuclear organizing region (NOR). b. Constitutive heterochromatin by CBG staining: top - complete metaphase plate, bottom - the karyotype. Note prominent heterochromatin bands on chromosomes 2, 11, 14, and Y.
Figure 2.
The karyotype of small-toothed mole from Vietnam, Euroscaptor parvidens, 2n=36, GTG-staining. The lines and numbers on the right delineate homologous chromosome segments revealed by mapping the set of Talpa altaica chromosome painting probes by FISH. “H” designates the heterochromatic regions.
Figure 2.
The karyotype of small-toothed mole from Vietnam, Euroscaptor parvidens, 2n=36, GTG-staining. The lines and numbers on the right delineate homologous chromosome segments revealed by mapping the set of Talpa altaica chromosome painting probes by FISH. “H” designates the heterochromatic regions.
Figure 3.
Comparative analysis tracing chromosome homologies between G-banded karyotypes of four asian mole species from Euroscaptor and Oreoscaptor genera: E. parvidens (Epar, this paper), E. klossi (Eklo), E. malayana (Emal), and Japanese mountain mole, Oreoscaptor mizura (Omiz) [
16,
23,
35]. “H” - designates constitutive heterochromatin blocks. Black dot designates a centromere position. Karyotypes differ by heterochromatin accumulation, centromere position, pericentric inversions and centric fusions. The karyotype of E. parvidens is diverged and distinguished by five chromosome changes from two other Euroscaptor species.
Figure 3.
Comparative analysis tracing chromosome homologies between G-banded karyotypes of four asian mole species from Euroscaptor and Oreoscaptor genera: E. parvidens (Epar, this paper), E. klossi (Eklo), E. malayana (Emal), and Japanese mountain mole, Oreoscaptor mizura (Omiz) [
16,
23,
35]. “H” - designates constitutive heterochromatin blocks. Black dot designates a centromere position. Karyotypes differ by heterochromatin accumulation, centromere position, pericentric inversions and centric fusions. The karyotype of E. parvidens is diverged and distinguished by five chromosome changes from two other Euroscaptor species.
Figure 4.
Comparative chromosome painting using a Talpa altaica (Talt) set of chromosome painting probes on chromosomes of several talpin mole species: a. Euroscaptor parvidens (Epar), b. Mogera imaizumii (Mima), c. Urotrichus talpoides (Utal).
Figure 4.
Comparative chromosome painting using a Talpa altaica (Talt) set of chromosome painting probes on chromosomes of several talpin mole species: a. Euroscaptor parvidens (Epar), b. Mogera imaizumii (Mima), c. Urotrichus talpoides (Utal).
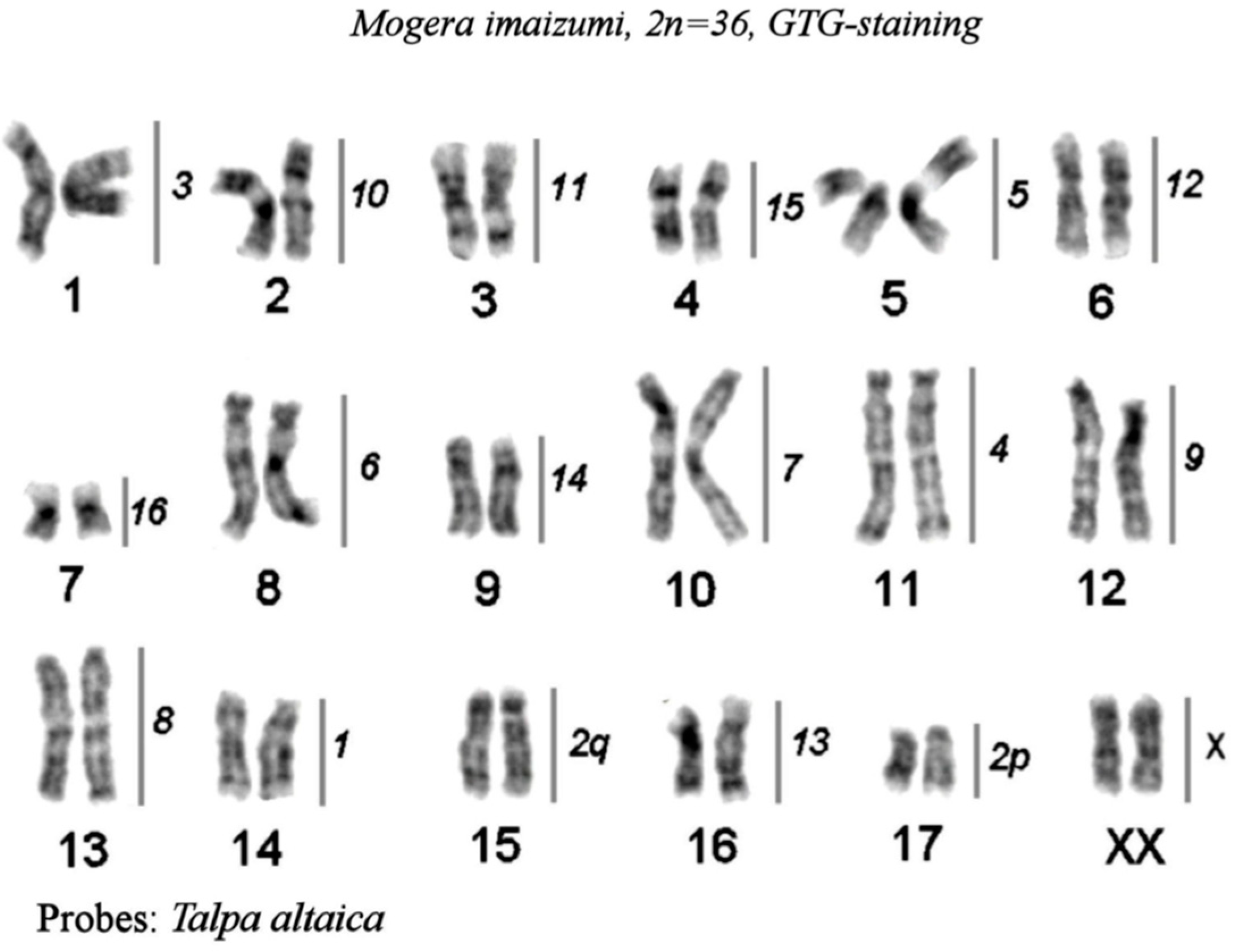
Figure 5.
Comparative chromosome map of M. imaizumii and T. altaica. The karyotype of small Japanese mole, Mogera imaizumi, 2n=36, GTG-staining. The lines and numbers on the right delineate homologous chromosome segments revealed by mapping the set of Talpa altaica chromosome painting probes by FISH. T. altaica chromosome 2 is fissioned in M. imaizumii.
Figure 5.
Comparative chromosome map of M. imaizumii and T. altaica. The karyotype of small Japanese mole, Mogera imaizumi, 2n=36, GTG-staining. The lines and numbers on the right delineate homologous chromosome segments revealed by mapping the set of Talpa altaica chromosome painting probes by FISH. T. altaica chromosome 2 is fissioned in M. imaizumii.
Figure 6.
Comparative chromosome map of Japanese shrew mole, Urotrichus talpoides (2n=34) and T. altaica. The lines and numbers on the right delineate homologous chromosome segments revealed by mapping the set of T. altaica chromosome painting probes by FISH on GTG-stained chromosomes of U. talpoides. T. altaica chromosomes 2 and 10 are fissioned in U. talpoides; the T. altaica chromosome segments 2p and 10q are fused.
Figure 6.
Comparative chromosome map of Japanese shrew mole, Urotrichus talpoides (2n=34) and T. altaica. The lines and numbers on the right delineate homologous chromosome segments revealed by mapping the set of T. altaica chromosome painting probes by FISH on GTG-stained chromosomes of U. talpoides. T. altaica chromosomes 2 and 10 are fissioned in U. talpoides; the T. altaica chromosome segments 2p and 10q are fused.
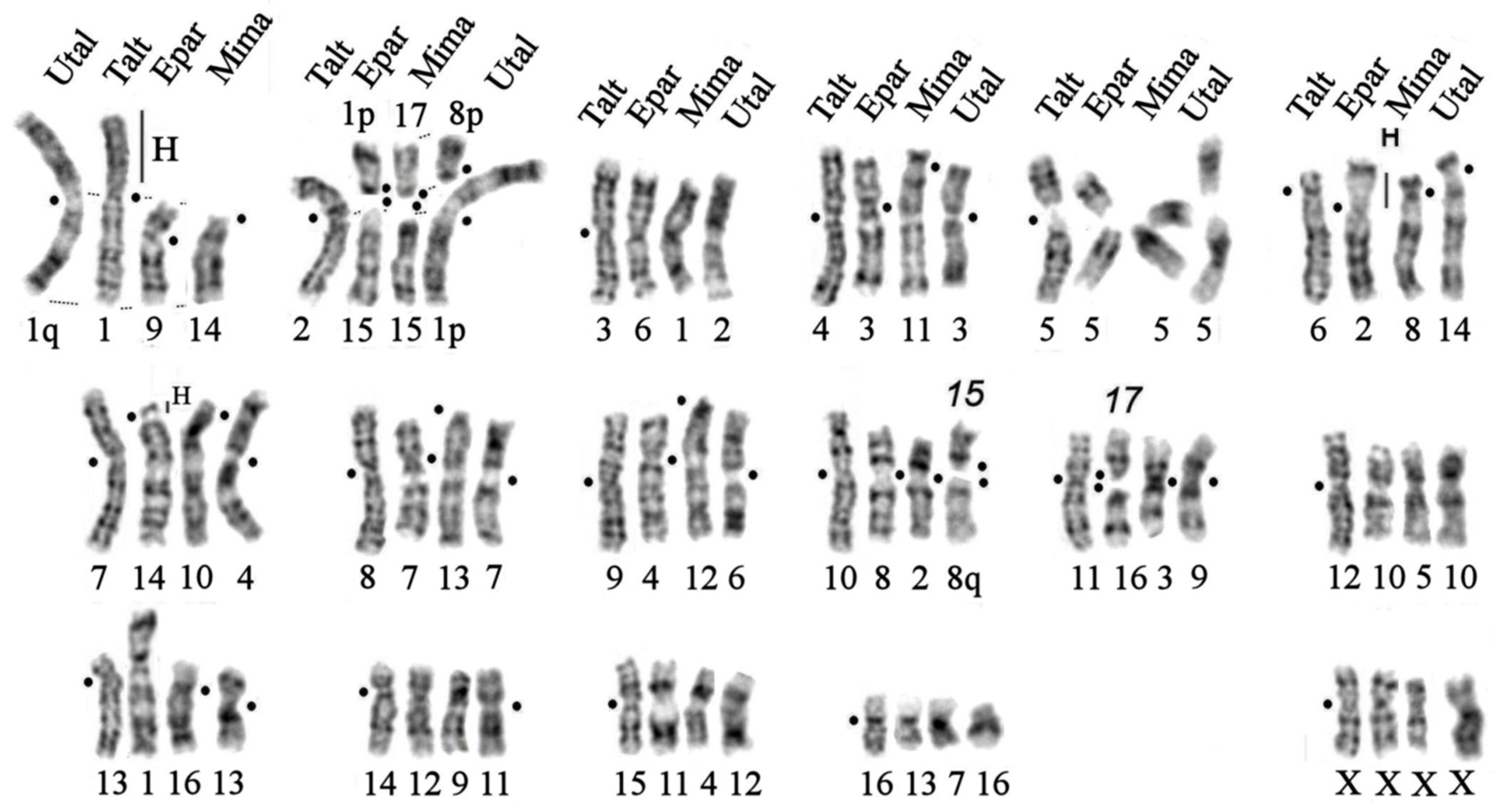
Figure 7.
Cross-species chromosome comparison of four talpine species based on chromosome painting of T. altaica probes: Japanese shrew mole (Urotrichus talpoides, Utal, 2n=34), Siberean mole (Talpa altaica, Talt, 2n=34), small-toothed mole (Euroscaptor parvidens, Epar, 2n=36), and small Japanese mole (Mogera imaizumi, 2n=36, Mima). “H” - designates constitutive heterochromatin blocks. Black dot designates a centromere position.
Figure 7.
Cross-species chromosome comparison of four talpine species based on chromosome painting of T. altaica probes: Japanese shrew mole (Urotrichus talpoides, Utal, 2n=34), Siberean mole (Talpa altaica, Talt, 2n=34), small-toothed mole (Euroscaptor parvidens, Epar, 2n=36), and small Japanese mole (Mogera imaizumi, 2n=36, Mima). “H” - designates constitutive heterochromatin blocks. Black dot designates a centromere position.
Figure 8.
Evolutionary chromosome changes (a-f) in the complex of eight species of subfamily Talpinae revealed by comparative chromosome painting and G-banding analysis: Siberean mole (Talpa altaica, Talt, 2n=34), European mole (Talpa europaea, Teur, 2n=34), Japanese shrew mole (Urotrichus talpoides, Utal, 2n=34), small Japanese mole (Mogera imaizumi, 2n=36, Mima), Japanese mountain mole (Oreoscaptor mizura, Omiz, 2n=36), Kloss’s mole (Euroscaptor klossi, Eklo, 2n=36), Malaysian mole (Euroscaptor malayana, Emal, 2n=36), and small-toothed mole (Euroscaptor parvidens, Epar, 2n=36). “H” - designates constitutive heterochromatin blocks. Black dot designates a centromere position.
Figure 8.
Evolutionary chromosome changes (a-f) in the complex of eight species of subfamily Talpinae revealed by comparative chromosome painting and G-banding analysis: Siberean mole (Talpa altaica, Talt, 2n=34), European mole (Talpa europaea, Teur, 2n=34), Japanese shrew mole (Urotrichus talpoides, Utal, 2n=34), small Japanese mole (Mogera imaizumi, 2n=36, Mima), Japanese mountain mole (Oreoscaptor mizura, Omiz, 2n=36), Kloss’s mole (Euroscaptor klossi, Eklo, 2n=36), Malaysian mole (Euroscaptor malayana, Emal, 2n=36), and small-toothed mole (Euroscaptor parvidens, Epar, 2n=36). “H” - designates constitutive heterochromatin blocks. Black dot designates a centromere position.
Figure 9.
The phylogenetic tree built based on cladistic analysis of ancestral chromosome syntenies in a complex of eight Talpinae species from genera
Euroscaptor, Oreoscaptor, Mogera,
Talpa, and
Urotrichus. inv - inversion, cs - centromeric shift, ‘+’ - fusion, ‘-’ - fission, “H” - additional heterochromatic block, AKT - Ancestral Karyotype of Talpinae. MYA - million years ago. Divergence dates are from He et al.[
2].
Figure 9.
The phylogenetic tree built based on cladistic analysis of ancestral chromosome syntenies in a complex of eight Talpinae species from genera
Euroscaptor, Oreoscaptor, Mogera,
Talpa, and
Urotrichus. inv - inversion, cs - centromeric shift, ‘+’ - fusion, ‘-’ - fission, “H” - additional heterochromatic block, AKT - Ancestral Karyotype of Talpinae. MYA - million years ago. Divergence dates are from He et al.[
2].
Table 1.
Chromosomes of the Euroscaptor species arranged according to the Oreoscaptor mizura chromosome.
Table 1.
Chromosomes of the Euroscaptor species arranged according to the Oreoscaptor mizura chromosome.
| O.mizura |
E.parvidens |
E.klossi |
E.malayana |
| 1 |
6 |
16+17 |
1p+16 |
| 2 |
5 |
2 |
2 |
| 3 |
8 |
3 |
3 |
| 4 |
16+17 |
4 |
4 |
| 5 |
10 |
5 |
5 |
| 6 q г/х |
11+q г/х |
6+ г/х |
6 + г/х |
| 7 |
13 |
7 |
7 |
| 8 |
2 + р г/х |
8 |
8 |
| 9 |
12 |
9 |
9 |
| 10 q г/х |
14+ р г/х |
10+ р г/х |
10+ q г/х |
| 11 |
3 inv |
11 |
11 |
| 12 |
4 inv |
12 |
12 |
| 13 |
7 cs |
13 |
1q |
| 14 |
9inv |
14 |
13 |
| 15 |
15 |
15 |
15 |
| 16 |
1q |
1q |
14 |
| 17 |
1p |
1p |
17 |
Table 2.
The chromosomes of the Talpinae species arranged according to the T.altaica chromosomes.
Table 2.
The chromosomes of the Talpinae species arranged according to the T.altaica chromosomes.
| T. altaica |
T. europaea |
U. talpoides |
O. mizura |
M. imaizumii |
E. klossi |
E. malayana |
E. parvidens |
| 1q (+рг/х) |
9q (+р г/х) |
1q |
14 |
14 |
14 |
13 |
9 inv |
| 2 |
2 |
1p+8p |
17+15 |
15+17 |
1р+15 |
17+15 |
1p+15 |
| 3 |
5 |
2 |
1 |
1 |
16+17 |
1p+16 |
6 |
| 4 |
3 |
3 |
11 inv |
11 inv+ inv |
11 inv |
11 inv |
3 inv +cs |
| 5 |
8 |
5 |
2 |
5 |
2 |
2 |
5 |
| 6q |
1+р г/х |
14 |
8q |
8q |
8q |
8q |
2+р г/х |
| 7 |
4 |
4 |
10 inv |
10 inv |
10 inv+ р/гх |
10 inv |
14 inv+р/гх |
| 8 |
7 |
7 |
13 inv |
13 inv |
13 inv |
1q inv (+1р) |
7 inv +cs |
| 9 |
6 |
6 |
12 inv |
12 inv |
12 inv |
12 inv |
4 inv + inv |
| 10 |
10 |
8q+15 |
3 |
2 |
3 |
3 |
8 |
| 11 |
12 |
9 |
4 |
3 |
4 |
4 |
16+17 |
| 12 |
11 |
10 |
5 |
5 |
5 |
5 |
10 |
| 13 |
13 inv |
13 |
16 |
16 |
1q |
14 |
1q |
| 14 |
14 |
11 |
9 |
9 |
9 |
9 |
12 |
| 15 |
15 |
12 |
6 |
4 |
6 |
6 |
11 |
| 16 |
16 |
16 |
7 |
7 |
7 |
7 |
13 |