1. Introduction
Ambient air pollution has a large range of negative impacts on human health [
1,
2,
3,
4], mainly increasing the number and severity of respiratory and pulmonary diseases [
5], including infections [
6] and the risk of heart attacks and strokes [
7,
8]. Also, long-term exposure to polluted air can produce perturbations of the immune system [
9]. This air pollution concerns mainly airborne Particulate Matter (PM) with particles smaller than 10 µm (PM
10), and more precisely smaller than 2.5 and 1 µm (PM
2.5 and PM
1).
The possible effect of PM
10 and PM
2.5 pollution on the Covid-19 (SARS-COV-2) pandemic new cases and mortality was intensively studied from less than a year after the pandemic start, mainly based on the situation in the Lombardy region (Italy) where air pollution levels and the mortality were among the highest in Europe. These studies presented a statistical relation between PM levels and Covid-19 morbidity and mortality during the first phase of the pandemic [
10,
11,
12,
13,
14,
15,
16,
17]. These conditions first consisted of respiratory and cardiovascular distresses [
18] and were indeed related to the immune system [
19,
20]. Nevertheless, only few studies have investigated the link between PM and Covid-19 mortality at the European level [
21].
Although a large number of papers support the existence of a relation between air pollution exposure and Covid-19, one can argue that this correlation could be an artefact due for example to weather parameters. The number of Covid-19 cases was found to be lower during periods of high-speed wind, which dissipates the PM pollution [
22,
23] studied the correlation between Covid-19 and weather conditions during the first phase of the pandemic, and concluded that as far as local meteorology is concerned, temperature and humidity are negatively correlated with the number of cases. In winter conditions, PM pollution often increases when temperature decreases, due to heating that produce fine particulates, thus making difficult to decorrelate the various parameters. Nevertheless, the absence of a relation between Covid-19 outbreaks and temperature and UV radiations [
24] could suggest that air pollution is indeed the main contributor to Covid-19 spread. In addition, during a long series of observations conducted in France abatement from rain precipitations was observed for particle larger than 10 µm but not for particles 0.2–10 µm [
25], thus supporting an intervention of fine PM independent of meteorological factors. A proper role of air pollution is further confirmed by studies in which air pollution and Covid-19 morbidity and mortality were statistically related after adjustment for meteorological variables [
18].
All measurements from 32 cities and districts of 6 countries in Western Europe have been combined to show a statistical trend between PM
2.5 levels and Covid-19 mortality for a period longer than all previous studies, from early 2020 to early 2022 [
26]. The authors found an increase of Covid-19 mortality aligned with PM
2.5 mass concentration increases, leading to a factor 5.5±1.0 % increase in mortality when the pollution increases from 5 to 45 µg.m-
3. This corresponds to a mean increase of 10.5±2.5 % of mortality per PM
2.5 1 µg.m-
3. More precisely, the trend, similar for the 6 countries analyzed, depended on the analysis period and decreased with time from the first spread of the pandemic in early 2020 to the vaccinal race after mid-2021. This mean result is close to previous works showing that a PM
2.5 increase of 1 µg.m
-3 can lead to at least an increase of 11% in Covid-19 mortality in the United States [
27]. Similar values were found in England [
28], in the Netherlands [
29], in Northern Italy [
30], in Brazil [
31] and in a meta-analysis on 35 observational studies [
32]. All these studies suggest that the relative effect of PM pollution on Covid-19 mortality is not country-dependent.
A Spearman correlation coefficient of 0.44 was found when analyzing the data in US up to mid-2021 [
33]. The Spearman correlation was between 0.45-0.61 for the most affected part of Italy during the first semester of 2020 [
14]. A positive correlation of 0.29 was also found in Poland in Spring 2020 [
34]. Finally, it has been shown that for the 3 months of 2020 in Wuhan City (China), the Pearson correlation rose to 0.4 considering a lag-time of about 20 days between PM
2.5 spikes and Covid-19 mortality [
35]. These positive correlation values support the hypothesis of a statistical link between PM
2.5 pollution and Covid-19 mortality.
We propose here a detailed data analysis to better understand such relation by considering 16 representative locations in Europe with mean PM2.5 levels from low to high for a total of 81 million people. This work is motivated by the interest first of better evaluating the time-evolution of the relationship between PM2.5 and the Covid-19 mortality at different locations in Europe, and second of providing new results on the possible lag-time between the pollution events and Covid-19 mortality and on the effect of the strength and duration of the events.
2. Materials and methods
We consider here the main period of the pandemics, from early 2020 till the end of 2022. As explained before [
26], the comparison of the temporal evolution of PM
2.5 spikes and Covid-19 mortality needs reliable data in order to minimize the statistical bias that irregularly sampled data may generate (such as missing mortality data for several days followed by a sudden data adjustment).
2.1. PM exposure and Covid-19 data sources
PM
2.5 mass-concentration data were available from different sources. Initial sources were the national air quality monitoring networks, although, because of their operational cost, only few reference stations were available per cities or regions. The other ones were non-official sources with a higher resolution. We consider here the Pollutrack networks in several cities of Europe where measurements are gathered using mobile light optical aerosol counters deployed on rooftops of hundreds of electric vehicles, providing a better spatial coverage of the cities than using just few reference fixed stations [
36]. Thus, we averaged all PM
2.5 daily measurements available in a given region from the air quality networks, and, when available, from the Pollutrack data that provides a better integration of the hyperlocal PM
2.5 variability.
To go further than in previous works [
26], we now consider the results from 16 locations (cities, regions or countries) for a total of almost 81 million people (
Table 1), almost equally representing the most polluted regions in Europe (where reliable Covid-19 data are available) and the regions with medium and low pollution levels, to facilitate direct comparisons. We chose to define “low pollution level” as when PM
2.5 mean mass concentration during the considered period is below 10 µg.m-
3 (twice the new annual mean value recommended by the World Health Organization as of September 2021), “medium pollution level” when the mean value is between 10 and 15 µg.m-
3, and “high pollution level” when the mean value is higher than 15 µg/m
3 (3 times the WHO recommended annual average). Information on the analyzed locations is presented in
Table 1.
For the purpose of this work, Covid-19 mortality data are retrieved from the John Hopkins University datasets (
https://coronavirus.jhu.edu/).
We have chosen to stop the analysis at the end of 2022 because the mean Covid-19 mortality decreased in Europe due to vaccination, herd immunity, and the return of the classical seasonal flu and respiratory diseases. On the other hand, the date of the analysis start depends on the availability of Covid-19 data and on the spread of the pandemic. To be able to compare similar effects of the PM
2.5 level on Covid-19 mortality for the various locations, we have sometimes excluded the period when the pandemic was locally not well managed and where the lockdowns had significantly affected the mortality trend for short time periods. The starting dates of time-period here considered for the 16 locations are given in
Table 1.
2.2. Statistical and epidemiological analyses
All the results and the proposed conclusions in this paper are resulting from different classical statistical analysis. First, the Pearson correlation coefficient was used for the temporal evolution Covid-19 mortality with PM2.5 pollution levels; lag-times between the two sets of data were also considered as a possibility to increase the correlation. Then we introduced the notion of gradients for temporal evolution of PM2.5 and Covid-19 mortally, by calculating at a given time the difference between each value and the value just before, and dividing the results by a temporal window. This calculation was used to establish a relationship between the slope of the time-evolution of the PM2.5 and the Covid-19 mortality. Finally, for all locations, the relationship between these parameters was established by applying linear fits (the data were integrated over dedicated windows to reduce the scatter of the individual values).
As previously proposed [
26], mortality data were divided by the population of the location. Then, both mortality and air pollution data were integrated over one week to limit the scatter of daily pollution values and daily variations in the collecting process of the mortality data. A sliding smoothing procedure was applied on 3 consecutive points to reduce the remaining short-term variations of the data. It was then necessary to adjust the time-scale of the two curves, by researching a better time-resolution of the pollution trend when compared to Covid-19 mortality trend. Tests and trial procedures showed that applying a sliding smoothing procedure on the pollution curve was not enough to increase the correlation between the two curves. Thus, we propose a more complex procedure by using two freedom parameters. The first one assumes that the effect of the pollution on Covid-19 mortality is not direct but requires an integration time; a given exposure time to pollution peaks could be needed to significantly irritate the pulmonary system that will consequently become more sensitive to Covid-19. Mathematically speaking, each value of PM
2.5 pollution was replaced by the mean of the previous PM
2.5 values during a given time-period, acting as an integration procedure, while the Covid-19 mortality values remain unchanged. The second parameter is a possible positive lag-time between this new PM
2.5 curve and the mortality curve, which could be related to the lethality of Covid-19 after invading the pulmonary system. These two parameters are adjusted to search for the higher correlation between the two curves.
As the data could be scattered at a given location mainly because of the mortality-reporting uncertainties, in each location air pollution data and their corresponding mortality data at the corresponding time were averaged with steps of about 0.5 µg.m-3; then the linear fit is applied.
3. Results
3.1. Time evolution of PM2.5 levels and Covid-19 mortality
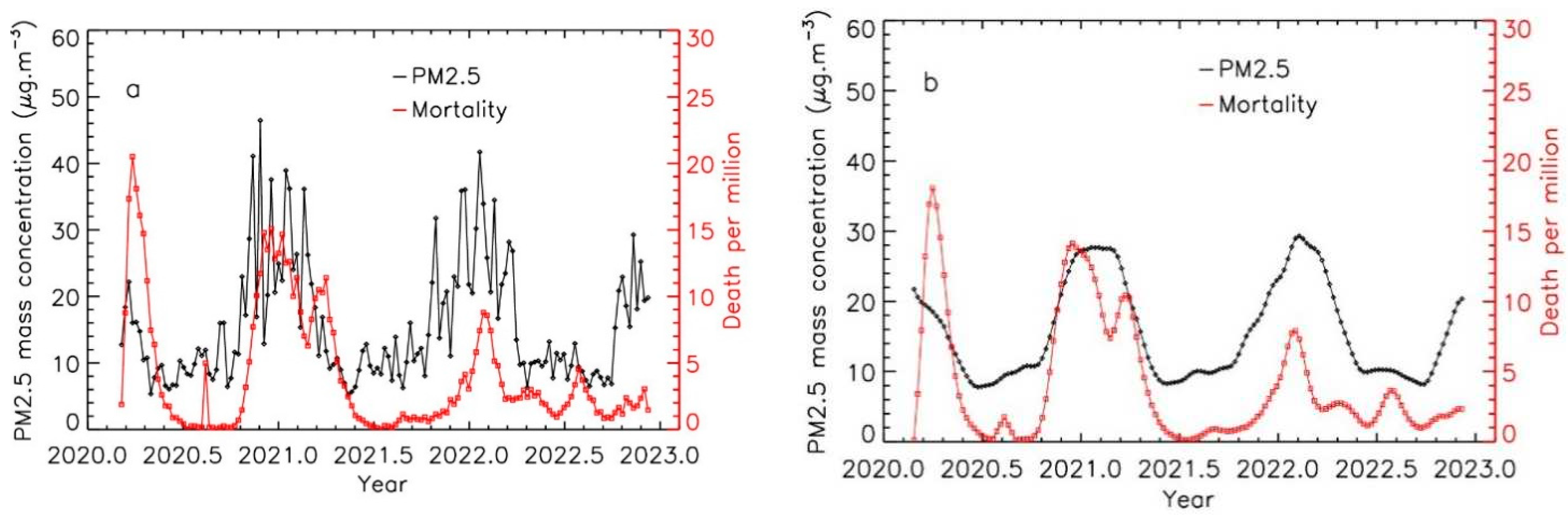
As shown in
Figure 1a and
Figure 2a, PM
2.5 and Covid-19 mortality curves do not present the same behavior, the air pollution curve being more oscillating than the mortality curve. This could be due to the fact that the mortality evolution is a slower phenomenon than the air pollution evolution that is strongly linked to the short time-scale variations of the meteorological parameters (mainly wind speed).
Figure 1b shows time evolution of PM
2.5 levels and Covid-19 mortality for the Emilia-Romagna region, exposed to high levels of pollution using weekly integrated data after applying the integration procedure for PM
2.5 data.
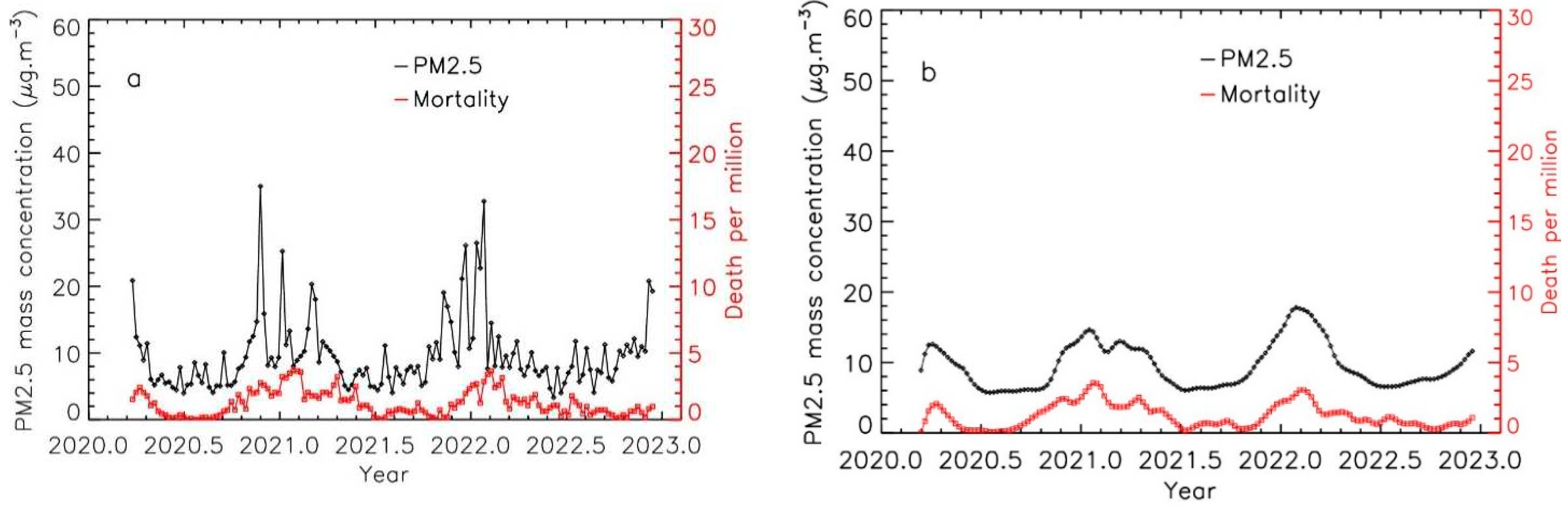
This procedure applied to increase the correlation between the curves even for cities or regions with low pollution levels like Gironde region is shown in
Figure 2b.
The Pearson correlation coefficients before and after applying the first sliding smoothing and the 2-parameters adjustments are given in
Table 2. The mean value for the integration is 8.8 ± 2.5 weeks, and the mean value for the shift (lag-time) is 0.7 ± 0.8 weeks. These results could indicate that an exposure of about 2 months to significant pollution peaks is needed to irritate sufficiently the pulmonary system before impacting Covid-19 mortality. These deleterious effects are reversible and are similar for the negative trends (decrease of mortality with pollution dispersion). On the other hand, about one-week shift may indicate the swiftness of virus lethality.
The proposed procedure strongly improved the Pearson correlations for most cases, reaching correlations up to 0.8. Considering the strength of PM2.5 spikes and Covid-19 mortality rates that differ from one county to another, the convergence to similar values seems to indicate that these correlations are real and that the proposed procedure can reveal the time effect of pollution on Covid-19 mortality.
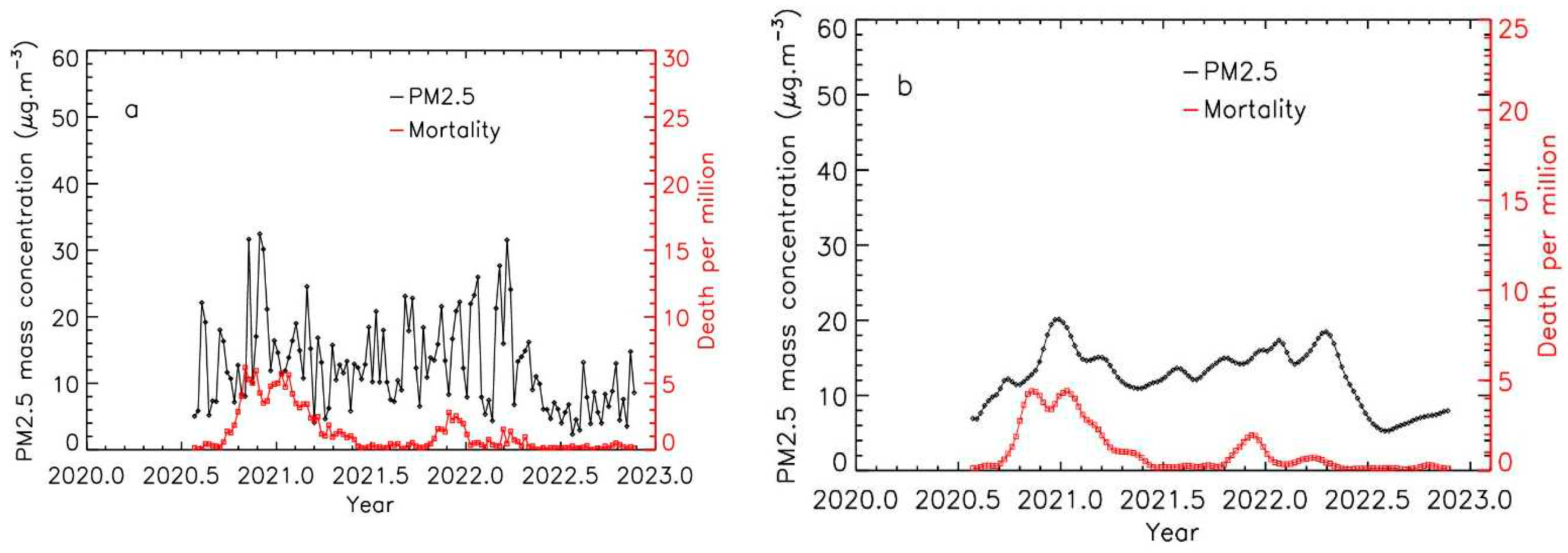
Nevertheless, the correlations remained close or lower than 0.5 for 4 locations (Nordrhein-Westfalen, Yorkshire and the Humber, London and Zuid-Holland). The first two ones are coming from statistical uncertainty due to the low level of air pollution and of mortality, as considered in the previous analysis [
26]. Taking in consideration these cases of low pollution levels is of importance to provide some control samples to be compared to higher pollution levels. The two other cases correspond to medium pollution levels, but compared to the other cities, PM levels remained relatively constant with time, with no well-defined periods of pollution peaks, as shown for Zuid-Holland (mainly Rotterdam city) in
Figure 3.
3.2. Linear relation between PM2.5 levels and Covid-19 mortality
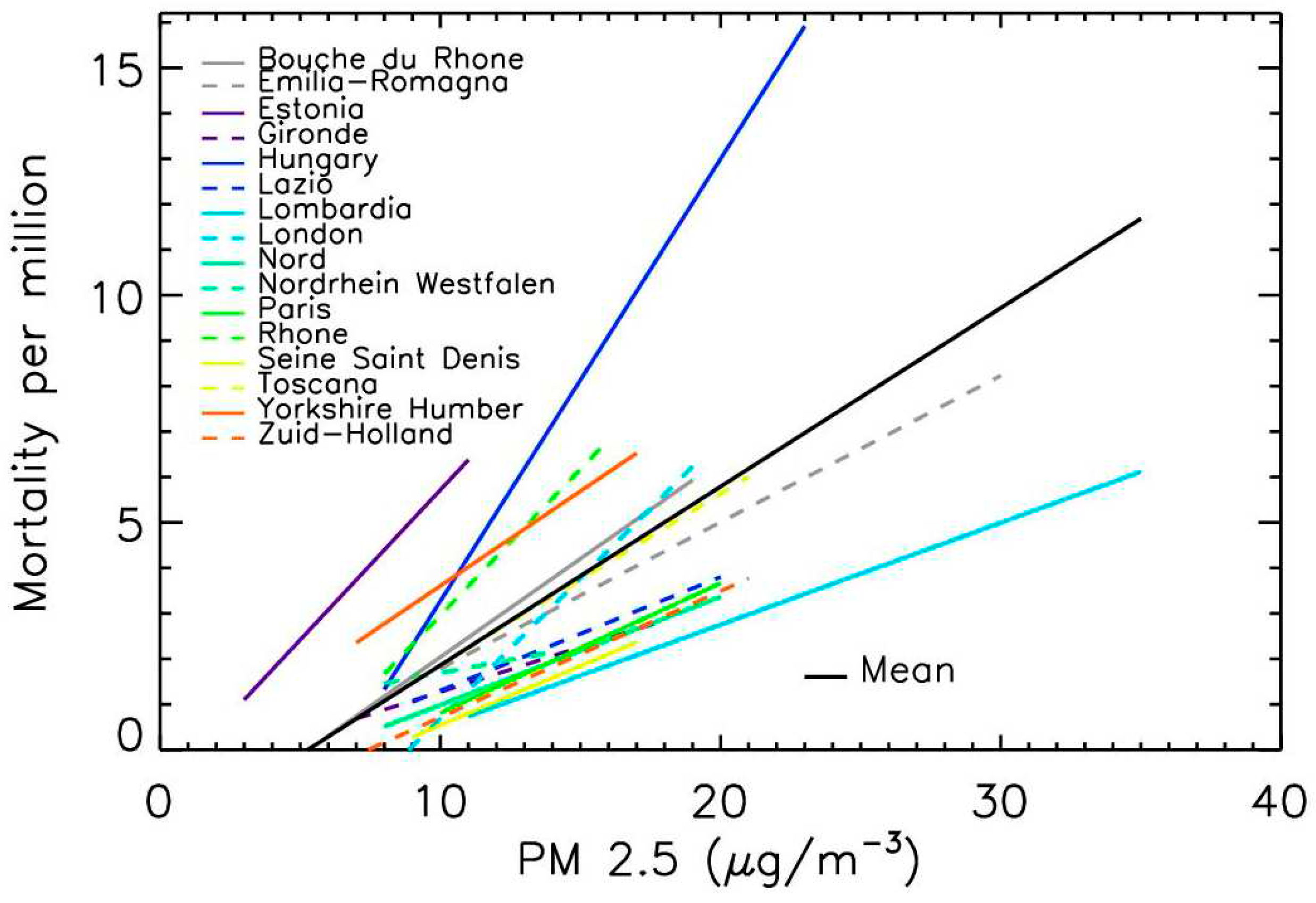
The relationship between Covid-19 mortality and PM
2.5 exposure was established by applying a linear fit for all locations. Individual measurements were scattered from one location to another, because of the possible heterogeneity of the population density (for example variations between large towns and rural zones), the health status of the populations that can influence the mortality and the local management of the pandemic [
26].
Figure 4 presents the linear fits for the 16 location (mean correlation of 0.9 per fit). The same trend is systematically observed, an increase of mortality with increasing PM
2.5 level, even for locations with low air pollution levels. The difference of the slope and the value at origin (PM
2.5 value for zero-mortality) slightly differed from one location to another because of the population heterogeneity and pandemic management variations. The mean value of the slope was 0.39±0.22, meaning a mortality increase of about 40±20% per 1 µg.m
-3 PM
2.5 increase.
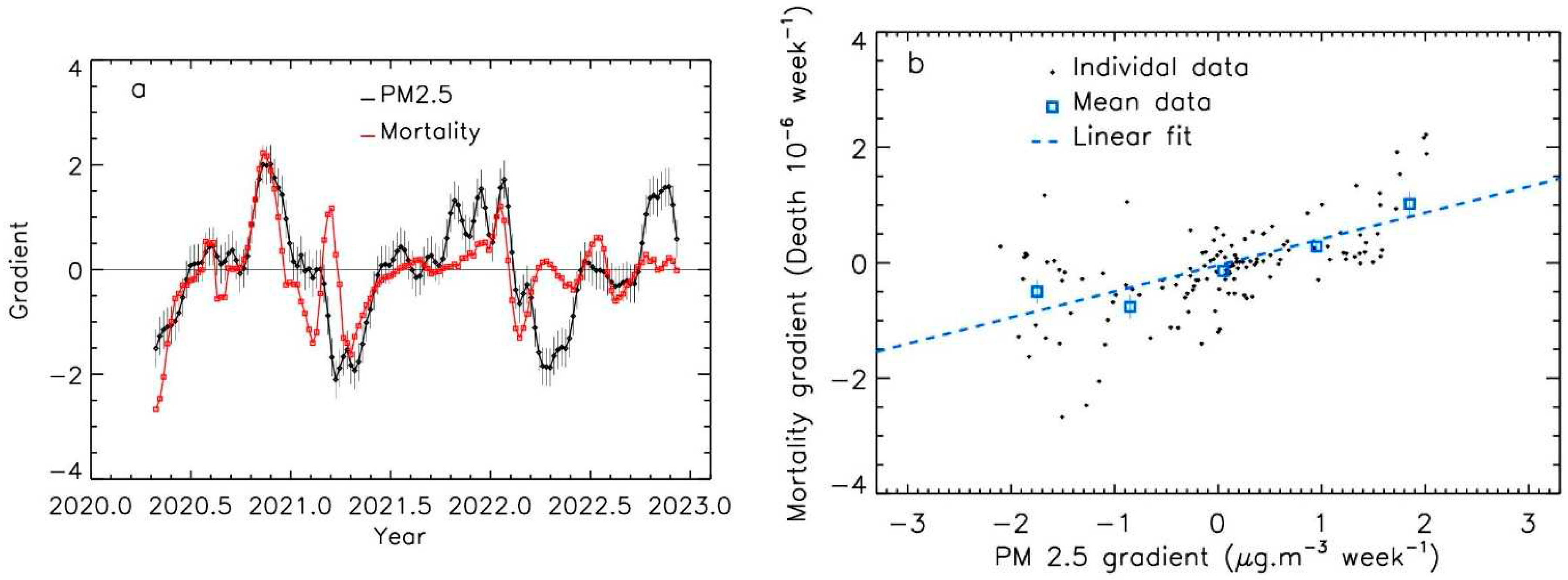
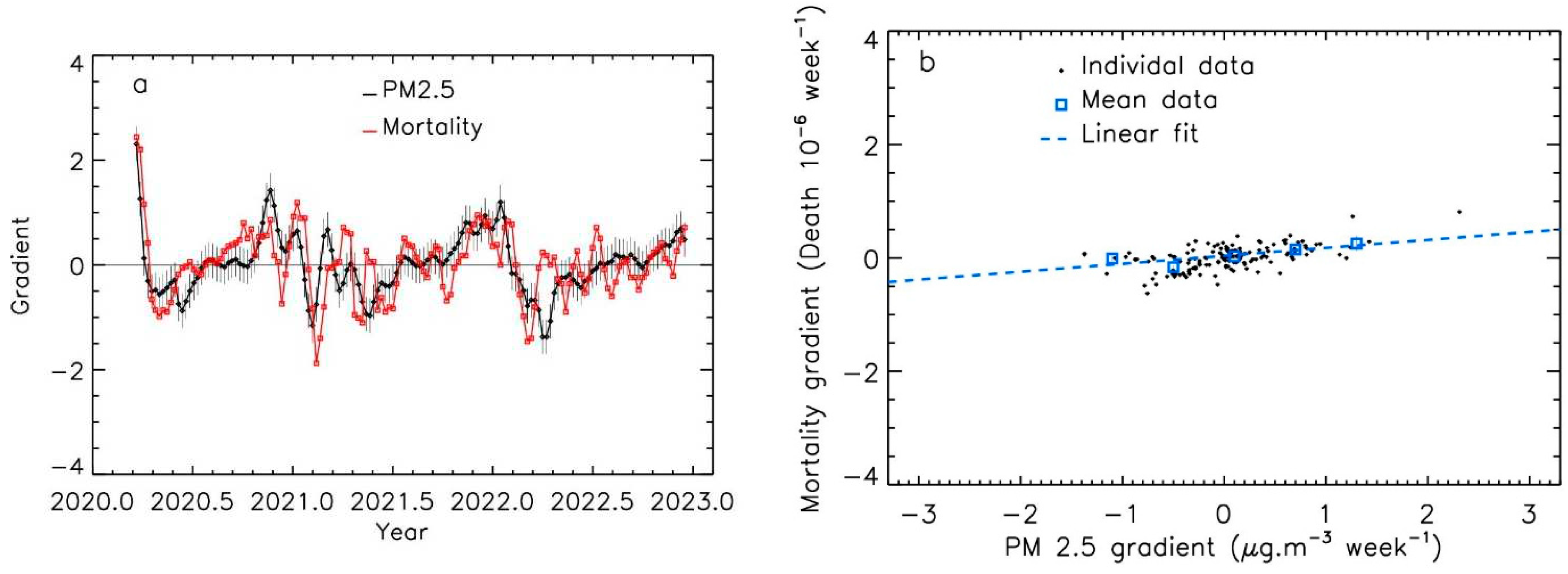
The shape of the curves for the time-evolution of PM
2.5 levels and Covid-19 mortality suggests that a relationship existed between the slope of the curves. The gradients values had similar magnitude for Covid-19 mortality and PM
2.5 spikes.
Figure 5 and
Figure 6 present the examples of the Emilia Romagna and the Gironde regions. The mean features are well correlated between the two curves; nevertheless, the agreement between the two curves seems lower after 2022, which could be due to the effect of the vaccine that decreases the amplitude of the mortality, and to the progress of herd immunity.
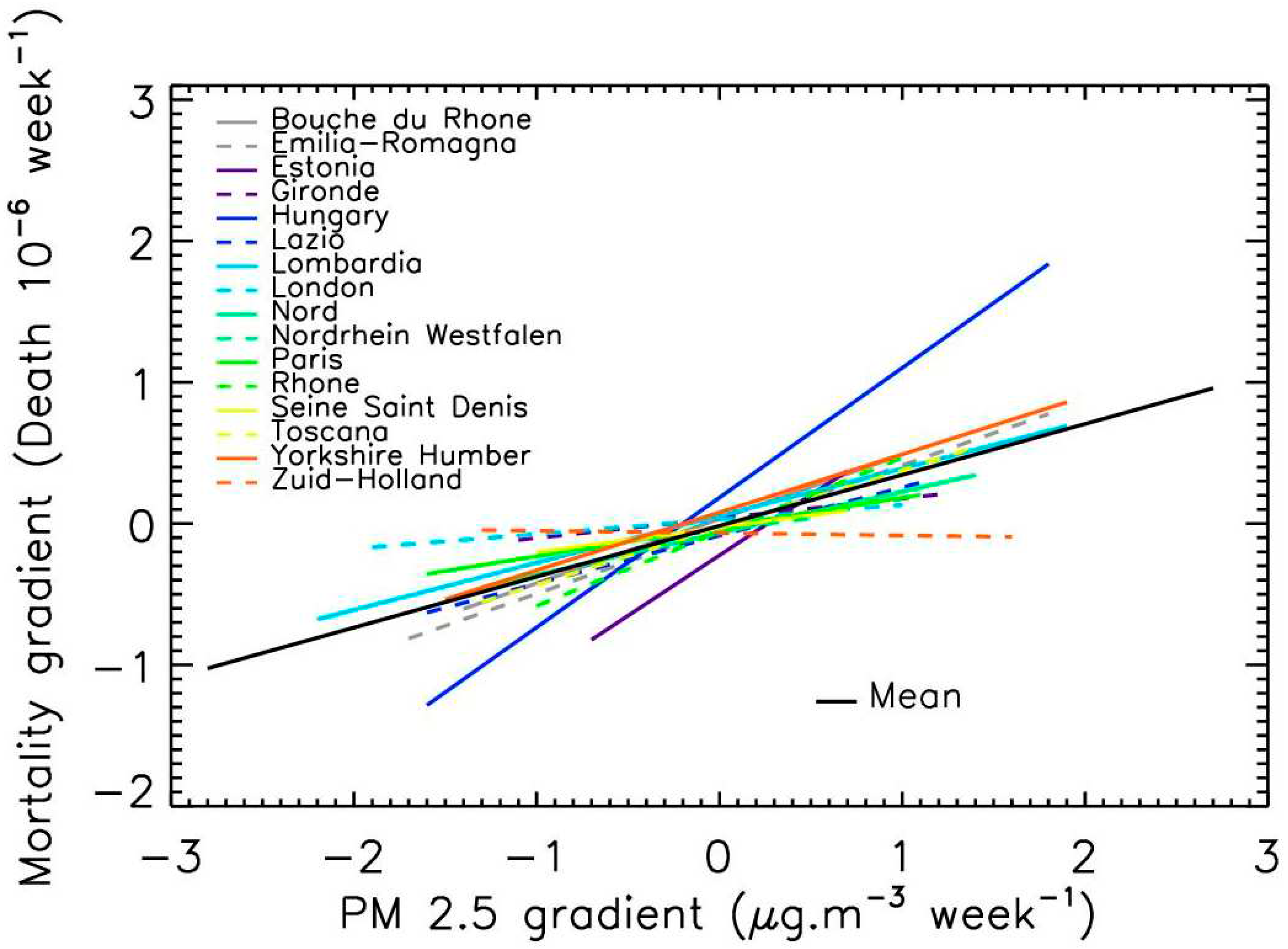
The synthesis of the linear fits for the gradients at the 16 locations is presented in
Figure 7 (mean correlation of 0.9 per fit). Globally, Covid-19 mortality gradients also linearly increased with increasing PM
2.5 gradients; the mean slope being 0.36±0.25. These results indicate that the strength of the PM
2.5 peak is also an increasing factor contributing to Covid-19 mortality.
In fact, 3 groups can be itemized: The locations presenting a strong slope (Hungary and Estonia), the locations with close-to-zero slope while having a medium mean level of pollution (Zuid-Holland, London), and the other cities. The difference between the first and the third group could once again be due to varying managements of the pandemic and population densities. Although only two cases have been spotted, the second group could indicate that cities with almost constant pollution levels, or without successive relatively short-lasting peaks of PM2.5 and low values in-between, could limit the pulmonary inflammation. Thus, steep variations of PM2.5 exposure rather than permanent exposure to medium pollution levels could be an aggravating factor of Covid-19 mortality.
4. Discussion
This study went more in depth on the investigation of the time-relation between PM
2.5 levels and Covid-19 mortality. Using data from 16 European representative locations, we found a Covid-19 mortality increase of about 40±20% per each 1 µg.m
-3 PM
2.5 increase. This value is strongly higher than the 10% value previously reported [
26,
28,
29,
30,
31,
32] and can be explained by two different reasons. The first reason is that the mean value is dominated by the locations with high values as for Hungary, although most of the location remain in the 10-40% increase range. The second reason is that the method of data analysis we have proposed reduces the scatter of the data, increases the correlation between PM2.5 levels and Covid-19 mortality time-evolution, and thus increases the trend.
Furthermore, an exposure to several PM peaks during a 2-month period constituted the main factor for mortality increase, rather than a permanent exposition to (medium) pollution levels. The stronger the positive gradient of the pollution peak, the stronger the positive gradient of Covid-19 mortality; the effect is reversible, with a faster decrease of Covid-19 mortality being observed during faster decrease of PM2.5 peaks. Thus, the lag-time combined with the strength and the duration of the pollution peaks should be considered by health policies for the management of the new and seasonal pandemics.
To explain the observed relation between PM
2.5 levels and Covid-19 mortality, it have proposed [
18] that fine PM and overall ultra-fine-particles (UFP) increase the airway permeability by reducing tight junctions proteins, which facilitates virus penetration, as well as the fact that PM boosts the action of the angiotensin-converting enzyme 2 (ACE2). This explanation was confirmed in a review of the process of lung inflammation resulting from PM exposure and thus the impact on Covid-19 mortality following a cytokinic storm [
37]. In particular, the authors studied the role of the angiotensin-converting enzyme 2 (ACE2), a receptor that is involved in the entry of the virus into pulmonary cells. Indeed, ACE-2 is a receptor for coronaviruses including the severe acute respiratory syndrome coronavirus 1 and 2 (SARS-CoV), and ACE-2 is overexpressed under chronic exposure to air pollution such as NO
2 and PM
2.5. ACE-2 is overexpressed in the case of medical comorbidities that contribute to the development of severe Covid-19.
Different natures of PM are present in ambient air [
20]. Apart from dust episodes, in Europe mainly coming from Sahara [
38], the main PM peaks are due to anthropogenic activities. The peaks occur during periods of anticyclonic conditions when pollutants accumulate and cannot be dispersed by winds [
36,
39]. The primary and secondary PM originate from vehicular traffic throughout the year (mainly carbonaceous particles), from industrials activities (all kinds of particles), from heating in winter (mainly carbonaceous particles), and from agricultural activities in autumn and spring (mainly ammonium). Other kinds of particles can be also present, like plastic, mineral and metal particles, coming from tyres, car and train brakes. In fact, no seasonal effect linked to the origin and thus the main nature of the particles was scrutinized during our analysis. The PM composition is complex; thus, it is difficult to estimate on which kind of particles the population was exposed. Consequently, we cannot conclude if Covid-19 mortality is due to all kinds of particles or to specific ones.
Another parameter to be considered is the size distribution of particles. We have used here the mass-concentration of PM
2.5, which corresponds to all particles smaller than 2.5 µm, although the largest ones mainly contribute to the values. Obviously, the smaller the particles, the deeper they penetrate in the human body. It has reported [
4] that PM
0.1 (particles smaller than 100 nm) are those that cause more pulmonary inflammation. Thus, it should be better to consider the PM size-distribution rather than the integrated mass-concentrations, since the size-distribution depends on the origin of the pollution events [
38]. The lower limit of scientific optical aerosol counters is of about 200nm (and of 300nm for Pollutrack sensors here used), thus they cannot detect such ultrafine particles. Expensive instruments could be used, using the Scanning Mobility Particle Sizer systems [
40], but they are not operated in routine by air monitoring networks; they are mainly used during dedicated field campaigns. Thus, one solution for future works should be to use the smallest size classes of Pollutrack sensors in cities where they are already deployed (32 European capitals and major cities to date), to tentatively improve Covid-19 mortality analysis (or other respiratory illnesses) in function of the number concentrations of particles of a few hundred nm in size.
5. Conclusion
To better understand the relation between the time-evolution curves of PM2.5 spikes and Covid-19 mortality, we have proposed to consider the historic population exposure to pollution peaks rather than instantaneous measurements, in 16 representative European cities. The related increase of Covid-19 mortality and PM2.5 levels was linear both for increasing and decreasing periods. Probably due to vaccination campaigns and the collective immunity progression, the correlation decreased at the end of 2022 in Europe. As an example, in France, flu and bronchiolitis mortalities increased at the end of 2022 to the detriment of Covid-19 (although still present), occurring a few weeks after a strong PM2.5 pollution peak.
The 2-month exposure period to PM2.5 we have suggested is probably just a first step and should be adjusted through a more complex equation, for example by considering a decreasing memory effect on PM exposure with time. The first analysis presented here have shown the interest of considering PM2.5 peaks for Covid-19 mortality rather than the permanent exposure to a mean level. It will be of interest to see if this approach is specific to this Coronavirus or could be also applied to other respiratory diseases like the flu. Finally, other data than PM2.5 mass-concentrations must be considered, in particular the number-concentration and size-distribution of particles smaller than 1 µm obtained with counters used during research campaigns and/or operated by private networks searching way beyond WHO recommendations.
Acknowledgements
The authors thank the Pollutrack team for the deployment of hundreds of mobile PM sensors in Paris then across Europe, the ENEDIS and DPD groups for offering their fleets of electric vehicles and financing the sensors, and Scott Stonham for his most efficient proofreading.
References
- Seaton, A.; Godden, D.; MacNee, W.; Donaldson, K. Particulate air pollution and acute health effects. Lancet 1995, 345(8943), 176–178. [Google Scholar] [CrossRef] [PubMed]
- Beelen, R.; Raaschou-Nielsen, O.; Stafoggia, M.; et al. Effects of long-term exposure to air pollution on natural-cause mortality: an analysis of 22 European cohorts within the multicentre ESCAPE project. Lancet 2014, 383(9919), 785–795. [Google Scholar] [CrossRef] [PubMed]
- WHO (World Health Organization). Ambient air pollution: A global assessment of exposure and burden of disease. Report 2016, WHO Library Cataloguing-in-Publication Data.
- Schraufnagel, D.E.; Balmes, J.R.; Cowl, C.T.; De Matteis, S.; Jung, S.H.; Mortimer, K.; Perez-Padilla, R.; Rice, M.B.; Riojas-Rodriguez, H.; Sood, A.; Thurston, G.D. Air Pollution and Noncommunicable Diseases A Review by the Forum of International Respiratory Societies’ Environmental Committee, Part 1: The Damaging Effects of Air Pollution. CHEST 2019, 155, 409–416. [Google Scholar] [CrossRef] [PubMed]
- Thurston, G. D.; Kipen, H.; Annesi-Maesano, I.; Balmes, J.; Brook, R. D.; Cromar, K.; De Matteis, S.; Forastiere, F.; Forsberg, B.; Frampton, M. W.; Grigg, J.; Heederik, D.; Kelly, F. J.; Kuenzli, N.; Laumbach, R. Peters, A.; Rajagopalan, S. T.; Rich, D.; Ritz, B.; Samet, J. M.; Sandstrom, T.; Sigsgaard, T.; Sunyer, J.; Brunekreef, B. A joint ERS/ATS policy statement: what constitutes an adverse health effect of air pollution? An analytical framework. Eur Respir J. 2017, 49, 1600419. [Google Scholar] [CrossRef]
- Horne, B. D.; Joy E., A.; Hofmann, M. G.; Gesteland, P. H.; Cannon J., B.; Lefler, J. S.; Blagev, D. P.; Korgenski, E. K.; Torosyan, N.; Hansen, G. I.; Kartchner, D.; Pope III, C. A. Short-Term Elevation of Fine Particulate Matter Air Pollution and Acute Lower Respiratory Infection. Amer. J. Respir. Crit. Care Med. 2018, 198(6), 759–766. [Google Scholar] [CrossRef] [PubMed]
- Robertson, S.; Miller, M. R. Ambient air pollution and thrombosis. Part. Fib. Toxicol. 2018, 15, 1. [Google Scholar] [CrossRef] [PubMed]
- Miller, M. R. Oxidative stress and the cardiovascular effects of air pollution. Free Rad. Bio. Med. 2020, 151, 69–87. [Google Scholar] [CrossRef]
- Glencross, D. A.; Ho, T.-R.; Camiña, N.; Hawrylowicz, C. M; Pfeffer, P. E. Air pollution and its effects on the immune system. Free Rad. Biol. Med. 2020, 151, 56–68. [Google Scholar] [CrossRef]
- Coccia, M. Factors determining the diffusion of Covid-19 and suggested strategy to prevent future accelerated viral infectivity similar to COVID. Sci. Total. Environ. 2020, 729, 138474. [Google Scholar] [CrossRef]
- Conticini, E.; Frediani, B.; Caro, D. Can atmospheric pollution be considered a co-factor in extremely high level of SARS-CoV-2 lethality in Northern Italy? Environ. Pol. 2020, 261, 114465. [Google Scholar] [CrossRef]
- Frontera, A.; Cianfanelli, L.; Vlachos, K.; Landoni, G.; Cremona, G. Severe air pollution links to higher mortality in Covid-19 patients: The “double-hit” hypothesis. J. Infect. 2020, 81(2), 255–259. [Google Scholar] [CrossRef] [PubMed]
- Fronza, R.; Lusic, M.; Schmidt, M.; Lucic, B. Spatial–Temporal Variations in Atmospheric Factors Contribute to SARS-CoV-2 Outbreak. Viruses 2020, 12, 588. [Google Scholar] [CrossRef]
- Accarino, G.; Lorenzetti, S.; Aloisio, G. Assessing correlations between short-term exposure to atmospheric pollutants and Covid-19 spread in all Italian territorial areas. Environmental Pollution 2021, 268(A), 115714. [Google Scholar] [CrossRef] [PubMed]
- Rohrer, M.; Flahault, A.; Stoffel, M. Peaks of fine particulate matter may modulate the spreading and virulence of Covid-19. Earth Syst. Environ. 2020, 4, 789–796. [Google Scholar] [CrossRef]
- Gupta, A.; Bherwani, H.; Gautam, S.; Anjum, S.; Musugu, K.; Kumar, N.; Anshul, A. , Kumar, R. Air pollution aggravating Covid-19 lethality? Exploration in Asian cities using statistical models. Environ. Develop. Sustain. 2021, 23, 6408–6417. [Google Scholar] [CrossRef]
- Sidell, M. A.; Chen, Z.; Huang, B. Z.; Chow, T.; Eckel, S. P.; Martinez, M. P.; Lurmann, F.; Thomas, D. C.; Gilliland, F. D.; Xiang, A. H. Ambient air pollution and Covid-19 incidence during four 2020–2021 case surges. Environ. Res. 2022, 208, 112758. [Google Scholar] [CrossRef]
- Bourdrel, T.; Annesi-Maesano, I.; Alahmad, B.; Maesano, C. N.; Bind, M.-D. The impact of outdoor air pollution on Covid-19: a review of evidence from in vitro, animal, and human studies. Europ. Resp. Rev. 2021, 30, 200242. [Google Scholar] [CrossRef] [PubMed]
- Mehmood, K.; Saifullah, I.M.; Abzar, M.M. Can exposure to PM2.5 particles increase the incidence of coronavirus disease 2019? Sc. Total Environ. 2020, 741, 140441. [Google Scholar] [CrossRef] [PubMed]
- Zoran, M. A.; Savastru, R. S.; Savastru, D. M.; Tautan, M. N. Assessing the relationship between surface levels of PM2.5 and PM10 particulate matter impact on Covid-19 in Milan, Italy. Sci. Total Envir. 2020, 738, 139825. [Google Scholar] [CrossRef]
- Annesi-Maesano, I.; Maesano, C. N.; Dessimond, B.; Prud’homme, J.; Colette, A.; Banerjee, S. Has the Spring 2020 lockdown modified the relationship between air pollution and Covid-19 mortality in Europe? Allergy 2020, 77, 1620–1622. [Google Scholar] [CrossRef]
- Coccia, M. How (un)sustainable Environments are Related to the Diffusion of Covid-19: The Relation between Coronavirus Disease 2019, Air Pollution, Wind Resource and Energy. Sustainability 2020, 12, 9709. [Google Scholar] [CrossRef]
- Srivastava, A. Covid-19 and air pollution and meteorology-an intricate relationship: A review. Chemosphere 2021, 263, 128297. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Pan, J.; Liu, Z.; Meng, X.; Wang, W.; Kan, H.; Wang, W. No association of Covid-19 transmission with temperature or UV radiation in Chinese cities. Europ. Respir. J. 2020, 55, 2000517. [Google Scholar] [CrossRef] [PubMed]
- McMullen, N.; Annesi-Maesano, I.; Renard, J.-B. Impact of rain precipitation on urban atmospheric particle matter measured at three locations in France between 2013 and 2019. Atmosphere 2021, 12, 769. [Google Scholar] [CrossRef]
- Renard, J.-B.; Surcin, J.; Annesi-Maesano, I.; Delaunay, G.; Poincelet, E.; Dixsaut, G. Relation between PM2.5 pollution and Covid-19 mortality in Western Europe for the 2020-2022 period. Sci. Total Envir. 2022, 848, 157579. [Google Scholar] [CrossRef]
- Wu, X.; Nethery, R. C.; Sabath, B. M.; Braun, D.; Dominici, F. Exposure to Air Pollution and Covid-19 Mortality in the United States. Sci. Advan. 2020, 6(45), eabd4049. [Google Scholar] [CrossRef]
- Travaglio, M.; Yu, Y.; Popovic, R.; Selley, L.; Leal, N.S.; Martins, L.M. Links between air pollution and Covid-19 in England. Environ. Pollut. 2021, 268, 115859. [Google Scholar] [CrossRef]
- Cole, M.; Ozgen, C.; Strobl, E. Air pollution exposure and Covid-19. Environ. Res. Econo. 2020, 76, 581–610. [Google Scholar] [CrossRef]
- Coker, E.S; Cavalli, L.; Fabrizi, E.; Guastella, G.; Lippo, E.; Parisi, M.L.; Pontarollo, N.; Rizzati, M.; Varacca, A.; Vergalli, S. The Effects of Air Pollution on COVID-19 Related Mortality in Northern Italy. Environ Resour Econ (Dordr) 2020, 76(4), 611–634. [Google Scholar] [CrossRef]
- Damasceno, R. M.; Cicerelli, R. E.; de Almeida, T.; Requia W., J. Air pollution and Covid-19 mortality in Brazil. Atmosphere 2022, 14(1), 5. [Google Scholar] [CrossRef]
- Zang, S.T.; Luan, J.; Li, L.; Yu, H.X.; Wu, Q.J.; Chang, Q.; Zhao, Y.H. Ambient air pollution and COVID-19 risk: Evidence from 35 observational studies. Environ Res. 2022, 204(Pt B), 112065. [Google Scholar] [CrossRef]
- Bossak, B. H.; Andritsch, S. Covid-19 and Air Pollution: A spatial analysis of particulate matter concentration and pandemic-associated mortality in the US. Int. J. Environ. Res. Public Health 2022, 2022 19, 729. [Google Scholar] [CrossRef]
- Semczuk-Kaczmarek, K.; Rys-Czaporowska, A.; Sierdzinski, J.; Kaczmarek, L. D.; Szymanski, F. M.; Platek, A. E. Association between air pollution and Covid-19 mortality and morbidity. Intern Emerg Med. 2022, 17(2), 467–473. [Google Scholar] [CrossRef] [PubMed]
- Shao, L.; Cao, Y.; Jones, T.; Santosh, M.; Silva, L. F. O.; Ge, S.; da Boit, K.; Feng, X.; Zhang, M.; BéruBé, K. Covid-19 mortality and exposure to airborne PM2.5: A lag time correlation, Science of The Total Environment. Sci. Total Envir. 2022, 806, 151286. [Google Scholar] [CrossRef]
- Renard, J.-B.; Marchand, C. High Resolution Mapping of PM2.5 Concentrations in Paris (France) Using Mobile Pollutrack Sensors Network in 2020. Atmosphere 2021, 12, 529. [Google Scholar] [CrossRef]
- Comunian, S.; Dongo, D.; Milani, C.; Palestini, P. Air Pollution and Covid-19: The Role of particulate matter in the spread and increase of Covid-19’s morbidity and mortality. Int. J. Environ. Res. Pub. Health 2020, 17, 4487. [Google Scholar] [CrossRef]
- Renard, J.-B.; Dulac, F.; Durand, P.; Bourgeois, Q.; Denjean, C.; Vignelles, D.; Couté, B.; Jeannot, M.; Verdier, N.; Mallet, M. In situ measurements of desert dust particles above the western Mediterranean Sea with the balloon-borne Light Optical Aerosol Counter/sizer (LOAC) during the ChArMEx campaign of summer 2013. Atmos. Chem. Phys. 2018, 18, 3677–3699. [Google Scholar] [CrossRef]
- Renard, J.-B.; Michoud, V.; Giacomoni, J. Vertical Profiles of Pollution Particle Concentrations in the Boundary Layer above Paris (France) from the Optical Aerosol Counter LOAC Onboard a Touristic Balloon. Sensors 2020, 20, 1111. [Google Scholar] [CrossRef]
- Stolzenburg, M. R.; McMurry, P. H. . Method to assess performance of scanning mobility particle sizer (SMPS) instruments and software. Aeros. Sci. Technol. 2018, 52, 609–613. [Google Scholar] [CrossRef]
Figure 1.
Time evolution of PM2.5 levels and Covid-19 mortality for the Emilia-Romagna region, exposed to high levels of pollution; a: weekly integrated data; b: weekly integrated data after applying the integration procedure for PM2.5 pollution data.
Figure 1.
Time evolution of PM2.5 levels and Covid-19 mortality for the Emilia-Romagna region, exposed to high levels of pollution; a: weekly integrated data; b: weekly integrated data after applying the integration procedure for PM2.5 pollution data.
Figure 2.
Time evolution of PM2.5 levels and Covid-19 mortality for the Gironde region, exposed to low levels of pollution; a: weekly integrated data; b: weekly integrated data after applying the integration procedure for PM2.5 pollution data.
Figure 2.
Time evolution of PM2.5 levels and Covid-19 mortality for the Gironde region, exposed to low levels of pollution; a: weekly integrated data; b: weekly integrated data after applying the integration procedure for PM2.5 pollution data.
Figure 3.
Time evolution of PM2.5 levels and Covid-19 mortality for the Zuid-Holland region, exposed to low levels of pollution; a: weekly integrated data; b: weekly integrated data after applying the integration procedure for PM2.5 data.
Figure 3.
Time evolution of PM2.5 levels and Covid-19 mortality for the Zuid-Holland region, exposed to low levels of pollution; a: weekly integrated data; b: weekly integrated data after applying the integration procedure for PM2.5 data.
Figure 4.
Trend evolution of Covid-19 mortality per million inhabitants vs. PM2.5 for the 16 locations. The lines represent the linear fits in the domain range where PM2.5 and mortality values are available.
Figure 4.
Trend evolution of Covid-19 mortality per million inhabitants vs. PM2.5 for the 16 locations. The lines represent the linear fits in the domain range where PM2.5 and mortality values are available.
Figure 5.
Gradient evolution of PM2.5 levels and of Covid-19 mortality per million inhabitants, for the Emilia-Romagna region; a: time evolution; b: data and linear fit.
Figure 5.
Gradient evolution of PM2.5 levels and of Covid-19 mortality per million inhabitants, for the Emilia-Romagna region; a: time evolution; b: data and linear fit.
Figure 6.
Gradient evolution of PM2.5 levels and of Covid-19 mortality per million inhabitants, for the Gironde region; a: time evolution; b: data and linear fit.
Figure 6.
Gradient evolution of PM2.5 levels and of Covid-19 mortality per million inhabitants, for the Gironde region; a: time evolution; b: data and linear fit.
Figure 7.
Trend evolution of the gradients of Covid-19 mortality per million inhabitants vs. PM2.5 levels, for the 16 locations. The lines represent the linear fits in the domain range where PM 2.5 gradients and mortality gradients are available.
Figure 7.
Trend evolution of the gradients of Covid-19 mortality per million inhabitants vs. PM2.5 levels, for the 16 locations. The lines represent the linear fits in the domain range where PM 2.5 gradients and mortality gradients are available.
Table 1.
Regions, departments or studied cities, population, source of PM pollution data and level of pollution peaks, time-period considered.
Table 1.
Regions, departments or studied cities, population, source of PM pollution data and level of pollution peaks, time-period considered.
| Location Name (Country) |
Population (106 Inhabitants) |
Source of PM2.5 Data |
Mean PM2.5 Level |
Starting Date |
| Bouche du Rhone (FR) |
2.0 |
Pollutrack |
Medium |
Mid-Dec 2020 |
| Emilia-Romagna (IT) |
4.5 |
Air quality network |
High |
Mid-April 2020 |
| Estonia (EE) |
1.3 |
Air quality network |
Low |
Mid-Dec. 2020 |
| Gironde (FR) |
1.6 |
Air quality network |
Low |
Mid-March 2020 |
| Hungary (HU) |
9.7 |
Air quality network |
Medium |
Mid-April 2020 |
| Lazio (IT) |
5.9 |
Air quality network |
Medium |
Mid-Dec. 2020 |
| Lombardy (IT) |
10.1 |
Air quality network |
High |
Mid-Dec. 2020 |
| London (GB) |
9.0 |
Pollutrack |
Medium |
End May 2020 |
| Nord (FR) |
2.6 |
Pollutrack |
Medium |
Early Jan. 2021 |
|
Nordrhein-Westfalen (DE)
|
17.9 |
Air quality network |
Low |
Mid-April 2020 |
| Paris (FR) |
2.2 |
Pollutrack |
High |
Mid-Dec. 2020 |
| Rhone (FR) |
1.9 |
Air quality network |
Medium |
Mid-Dec. 2020 |
| Seine Saint-Denis (FR) |
1.7 |
Air quality network |
Medium |
Mid-Dec. 2020 |
| Toscana (IT) |
3.7 |
Air quality network |
Medium |
Mid-Dec. 2020 |
| Yorkshire and the Humber (GB) |
5.5 |
Air quality network |
Low |
Early July 2020 |
| Zuid-Holland (NL) |
3.7 |
Pollutrack |
Medium |
End July 2020 |
Table 2.
Regions, departments or cities studied, population, length of the integration (in week) for PM2.5 levels data, and shift (in week) of the Covid19 mortality curve.
Table 2.
Regions, departments or cities studied, population, length of the integration (in week) for PM2.5 levels data, and shift (in week) of the Covid19 mortality curve.
| Location Name (Country) |
Integration Width (Weeks) |
Shift (Weeks) |
Correlation before |
Correlation after |
| Bouche du Rhone (FR) |
7 |
2 |
0.56 |
0.82 |
| Emilia-Romagna (IT) |
9 |
0 |
0.52 |
0.76 |
| Estonia (EE) |
14 |
0 |
0.28 |
0.60 |
| Gironde (FR) |
11 |
0 |
0.36 |
0.81 |
| Hungary (HU) |
7 |
1 |
0.56 |
0.83 |
| Lazio (IT) |
9 |
2 |
0.37 |
0.79 |
| Lombardia (IT) |
9 |
0 |
0.47 |
0.78 |
| London (GB) |
8 |
0 |
0.25 |
0.45 |
| Nord (FR) |
14 |
1 |
0.41 |
0.78 |
| Nordrhein-Westfalen (DE) |
7 |
0 |
0.17 |
0.42 |
| Paris (FR) |
8 |
1 |
0.33 |
0.62 |
| Rhone (FR) |
7 |
1 |
0.40 |
0.73 |
| Seine Saint-Denis (FR) |
10 |
0 |
0.41 |
0.64 |
| Toscana (IT) |
8 |
3 |
0.53 |
0.80 |
| Yorkshire and the Humber (GB) |
5 |
0 |
0.25 |
0.41 |
| Zuid-Holland (NL) |
8 |
0 |
0.29 |
0.51 |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).