Submitted:
19 June 2023
Posted:
20 June 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
Patient Population and Cut-off Values
Procedural Data
Laboratory data
Adverse Events and mortality
3. Discussion
4. Materials and Methods
Patients
Laboratory Data
Follow-Up
Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Pettersson, G.B.; Coselli, J.S.; Writing, C.; Pettersson, G.B.; Coselli, J.S.; Hussain, S.T.; Griffin, B.; Blackstone, E.H.; Gordon, S.M.; LeMaire, S.A.; et al. 2016 The American Association for Thoracic Surgery (AATS) consensus guidelines: Surgical treatment of infective endocarditis: Executive summary. J Thorac Cardiovasc Surg 2017, 153, 1241–1258.e1229. [Google Scholar] [CrossRef] [PubMed]
- Pierce, D.; Calkins, B.C.; Thornton, K. Infectious endocarditis: diagnosis and treatment. Am Fam Physician 2012, 85, 981–986. [Google Scholar] [PubMed]
- Habib, G.; Lancellotti, P.; Antunes, M.J.; Bongiorni, M.G.; Casalta, J.P.; Del Zotti, F.; Dulgheru, R.; El Khoury, G.; Erba, P.A.; Iung, B.; et al. 2015 ESC Guidelines for the management of infective endocarditis: The Task Force for the Management of Infective Endocarditis of the European Society of Cardiology (ESC). Endorsed by: European Association for Cardio-Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM). Eur Heart J 2015, 36, 3075–3128. [Google Scholar] [CrossRef]
- Habib, G.; Lancellotti, P.; Iung, B. 2015 ESC Guidelines on the management of infective endocarditis: a big step forward for an old disease. Heart 2016, 102, 992–994. [Google Scholar] [CrossRef]
- Holland, T.L.; Baddour, L.M.; Bayer, A.S.; Hoen, B.; Miro, J.M.; Fowler, V.G., Jr. Infective endocarditis. Nat Rev Dis Primers 2016, 2, 16059. [Google Scholar] [CrossRef] [PubMed]
- Gagneux-Brunon, A.; Pouvaret, A.; Maillard, N.; Berthelot, P.; Lutz, M.F.; Cazorla, C.; Tulane, C.; Fuzellier, J.F.; Verhoeven, P.O.; Fresard, A.; et al. Acute kidney injury in infective endocarditis: A retrospective analysis. Med Mal Infect 2019, 49, 527–533. [Google Scholar] [CrossRef] [PubMed]
- Peters, E.; Heemskerk, S.; Masereeuw, R.; Pickkers, P. Alkaline phosphatase: a possible treatment for sepsis-associated acute kidney injury in critically ill patients. Am J Kidney Dis 2014, 63, 1038–1048. [Google Scholar] [CrossRef]
- Hummeke-Oppers, F.; Hemelaar, P.; Pickkers, P. Innovative Drugs to Target Renal Inflammation in Sepsis: Alkaline Phosphatase. Front Pharmacol 2019, 10, 919. [Google Scholar] [CrossRef]
- Koyama, I.; Matsunaga, T.; Harada, T.; Hokari, S.; Komoda, T. Alkaline phosphatases reduce toxicity of lipopolysaccharides in vivo and in vitro through dephosphorylation. Clin Biochem 2002, 35, 455–461. [Google Scholar] [CrossRef]
- Poelstra, K.; Bakker, W.W.; Klok, P.A.; Kamps, J.A.; Hardonk, M.J.; Meijer, D.K. Dephosphorylation of endotoxin by alkaline phosphatase in vivo. Am J Pathol 1997, 151, 1163–1169. [Google Scholar]
- Kerner, A.; Avizohar, O.; Sella, R.; Bartha, P.; Zinder, O.; Markiewicz, W.; Levy, Y.; Brook, G.J.; Aronson, D. Association between elevated liver enzymes and C-reactive protein: possible hepatic contribution to systemic inflammation in the metabolic syndrome. Arterioscler Thromb Vasc Biol 2005, 25, 193–197. [Google Scholar] [CrossRef]
- Tung, C.B.; Tung, C.F.; Yang, D.Y.; Hu, W.H.; Hung, D.Z.; Peng, Y.C.; Chang, C.S. Extremely high levels of alkaline phosphatase in adult patients as a manifestation of bacteremia. Hepatogastroenterology 2005, 52, 1347–1350. [Google Scholar]
- Katasako, A.; Sasaki, S.; Raita, Y.; Yamamoto, S.; Tochitani, K.; Murakami, M.; Nishioka, R.; Fujisaki, K. Association between serum alkaline phosphatase and bacteraemia in haemodialysis outpatients: a multicentre retrospective cross-sectional study. BMJ Open 2022, 12, e058666. [Google Scholar] [CrossRef] [PubMed]
- Fawley, J.; Gourlay, D.M. Intestinal alkaline phosphatase: a summary of its role in clinical disease. J Surg Res 2016, 202, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Pickkers, P.; Heemskerk, S.; Schouten, J.; Laterre, P.F.; Vincent, J.L.; Beishuizen, A.; Jorens, P.G.; Spapen, H.; Bulitta, M.; Peters, W.H.; et al. Alkaline phosphatase for treatment of sepsis-induced acute kidney injury: a prospective randomized double-blind placebo-controlled trial. Crit Care 2012, 16, R14. [Google Scholar] [CrossRef] [PubMed]
- Al-Fares, A.; Pettenuzzo, T.; Del Sorbo, L. Extracorporeal life support and systemic inflammation. Intensive Care Med Exp 2019, 7, 46. [Google Scholar] [CrossRef] [PubMed]
- Giacinto, O.; Satriano, U.; Nenna, A.; Spadaccio, C.; Lusini, M.; Mastroianni, C.; Nappi, F.; Chello, M. Inflammatory Response and Endothelial Dysfunction Following Cardiopulmonary Bypass: Pathophysiology and Pharmacological Targets. Recent Patents on Inflammation & Allergy Drug Discovery 2019, 13, 158–173. [Google Scholar] [CrossRef]
- Squiccimarro, E.; Labriola, C.; Malvindi, P.G.; Margari, V.; Guida, P.; Visicchio, G.; Kounakis, G.; Favale, A.; Dambruoso, P.; Mastrototaro, G.; et al. Prevalence and Clinical Impact of Systemic Inflammatory Reaction After Cardiac Surgery. Journal of Cardiothoracic and Vascular Anesthesia 2019, 33, 1682–1690. [Google Scholar] [CrossRef]
- Montesinos, M.C.; Takedachi, M.; Thompson, L.F.; Wilder, T.F.; Fernandez, P.; Cronstein, B.N. The antiinflammatory mechanism of methotrexate depends on extracellular conversion of adenine nucleotides to adenosine by ecto-5'-nucleotidase: findings in a study of ecto-5'-nucleotidase gene-deficient mice. Arthritis Rheum 2007, 56, 1440–1445. [Google Scholar] [CrossRef]
- Neuhof, C.; Wendling, J.; Dapper, F.; Bauer, J.; Zickmann, B.; Jochum, M.; Tillmanns, H.; Neuhoft, H. Endotoxemia and cytokine generation in cardiac surgery in relation to flow mode and duration of cardiopulmonary bypass. Shock 2001, 16 (Suppl. 1), 39–43. [Google Scholar] [CrossRef]
- Paparella, D.; Yau, T.M.; Young, E. Cardiopulmonary bypass induced inflammation: pathophysiology and treatment. An update. European Journal of Cardio-Thoracic Surgery: Official Journal of the European Association for Cardio-Thoracic Surgery 2002, 21, 232–244. [Google Scholar] [CrossRef] [PubMed]
- Riddington, D.W.; Venkatesh, B.; Boivin, C.M.; Bonser, R.S.; Elliott, T.S.; Marshall, T.; Mountford, P.J.; Bion, J.F. Intestinal permeability, gastric intramucosal pH, and systemic endotoxemia in patients undergoing cardiopulmonary bypass. JAMA 1996, 275, 1007–1012. [Google Scholar] [CrossRef] [PubMed]
- Davidson, J.; Tong, S.; Hauck, A.; Lawson, D.S.; Jaggers, J.; Kaufman, J.; da Cruz, E. Alkaline phosphatase activity after cardiothoracic surgery in infants and correlation with post-operative support and inflammation: a prospective cohort study. Crit Care 2012, 16, R160. [Google Scholar] [CrossRef]
- Davidson, J.A.; Urban, T.T.; Tong, S.; Maddux, A.; Hill, G.; Frank, B.S.; Watson, J.D.; Jaggers, J.; Simoes, E.A.F.; Wischmeyer, P. Alkaline Phosphatase Activity and Endotoxemia After Infant Cardiothoracic Surgery. Shock 2019, 51, 328–336. [Google Scholar] [CrossRef] [PubMed]
- Poschner, T.; Schaefer, A.K.; Hutschala, D.; Goliasch, G.; Riebandt, J.; Distelmaier, K.; Bernardi, M.H.; Andreas, M.; Brands, R.; Aref, T.; et al. Impact of Venoarterial Extracorporeal Membrane Oxygenation on Alkaline Phosphatase Metabolism after Cardiac Surgery. Biomolecules 2021, 11. [Google Scholar] [CrossRef]
- Schaefer, A.K.; Hutschala, D.; Andreas, M.; Bernardi, M.H.; Brands, R.; Shabanian, S.; Laufer, G.; Wiedemann, D. Decrease in serum alkaline phosphatase and prognostic relevance in adult cardiopulmonary bypass. Interact Cardiovasc Thorac Surg 2020, 31, 383–390. [Google Scholar] [CrossRef]
- Vongpatanasin, W.; Hillis, L.D.; Lange, R.A. Prosthetic heart valves. N Engl J Med 1996, 335, 407–416. [Google Scholar] [CrossRef]
- Wang, A.; Athan, E.; Pappas, P.A.; Fowler, V.G., Jr.; Olaison, L.; Pare, C.; Almirante, B.; Munoz, P.; Rizzi, M.; Naber, C.; et al. Contemporary clinical profile and outcome of prosthetic valve endocarditis. JAMA 2007, 297, 1354–1361. [Google Scholar] [CrossRef]
- Doenst, T.; Borger, M.A.; Weisel, R.D.; Yau, T.M.; Maganti, M.; Rao, V. Relation between aortic cross-clamp time and mortality--not as straightforward as expected. Eur J Cardiothorac Surg 2008, 33, 660–665. [Google Scholar] [CrossRef]
- Nissinen, J.; Biancari, F.; Wistbacka, J.O.; Peltola, T.; Loponen, P.; Tarkiainen, P.; Virkkila, M.; Tarkka, M. Safe time limits of aortic cross-clamping and cardiopulmonary bypass in adult cardiac surgery. Perfusion 2009, 24, 297–305. [Google Scholar] [CrossRef]
- Coleman, J.E. Structure and mechanism of alkaline phosphatase. Annu Rev Biophys Biomol Struct 1992, 21, 441–483. [Google Scholar] [CrossRef] [PubMed]
- Davidson, J.A.; Urban, T.T.; Baird, C.; Tong, S.; Woodruff, A.; Twite, M.; Jaggers, J.; Simoes, E.A.F.; Wischmeyer, P. Alkaline Phosphatase in Infant Cardiopulmonary Bypass: Kinetics and Relationship to Organ Injury and Major Cardiovascular Events. J Pediatr 2017, 190, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Coux, G.; Trumper, L.; Elias, M.M. Renal function and cortical (Na(+)+K(+))-ATPase activity, abundance and distribution after ischaemia-reperfusion in rats. Biochim Biophys Acta 2002, 1586, 71–80. [Google Scholar] [CrossRef]
- Khundmiri, S.J.; Asghar, M.; Khan, F.; Salim, S.; Yusufi, A.N. Effect of reversible and irreversible ischemia on marker enzymes of BBM from renal cortical PT subpopulations. Am J Physiol 1997, 273, F849–F856. [Google Scholar] [CrossRef]
- Corredor, C.; Thomson, R.; Al-Subaie, N. Long-Term Consequences of Acute Kidney Injury After Cardiac Surgery: A Systematic Review and Meta-Analysis. J Cardiothorac Vasc Anesth 2016, 30, 69–75. [Google Scholar] [CrossRef]
- Zhang, C.Y.; Zhang, H.H.; Zhao, S.L.; Wu, H.Y.; Li, H.N.; Li, W.; Yang, J. Clinical value of alkaline phosphatase on the surface membrane of neutrophils for prediction of bacteremia in patients with systemic inflammatory response syndrome. Diagn Microbiol Infect Dis 2021, 100, 114105. [Google Scholar] [CrossRef] [PubMed]
- Hamarneh, S.R.; Mohamed, M.M.; Economopoulos, K.P.; Morrison, S.A.; Phupitakphol, T.; Tantillo, T.J.; Gul, S.S.; Gharedaghi, M.H.; Tao, Q.; Kaliannan, K.; et al. A novel approach to maintain gut mucosal integrity using an oral enzyme supplement. Ann Surg 2014, 260, 706–714. [Google Scholar] [CrossRef]
- Biancari, F.; Dalen, M.; Fiore, A.; Ruggieri, V.G.; Saeed, D.; Jonsson, K.; Gatti, G.; Zipfel, S.; Perrotti, A.; Bounader, K.; et al. Multicenter study on postcardiotomy venoarterial extracorporeal membrane oxygenation. J Thorac Cardiovasc Surg 2020, 159, 1844–1854. [Google Scholar] [CrossRef]
- Khorsandi, M.; Dougherty, S.; Bouamra, O.; Pai, V.; Curry, P.; Tsui, S.; Clark, S.; Westaby, S.; Al-Attar, N.; Zamvar, V. Extra-corporeal membrane oxygenation for refractory cardiogenic shock after adult cardiac surgery: a systematic review and meta-analysis. J Cardiothorac Surg 2017, 12, 55. [Google Scholar] [CrossRef]
- Li, J.S.; Sexton, D.J.; Mick, N.; Nettles, R.; Fowler, V.G., Jr.; Ryan, T.; Bashore, T.; Corey, G.R. Proposed modifications to the Duke criteria for the diagnosis of infective endocarditis. Clin Infect Dis 2000, 30, 633–638. [Google Scholar] [CrossRef]
| Overall n=314 |
AP drop < 30% n=135 |
AP drop ≥ 30% n=179 |
p-valueª | ||
|---|---|---|---|---|---|
| Age | 62 (70;48) | 59 (69;45) | 62 (70;49) | 0.230 | |
| Female | 85 (27.1) | 37 (27.4) | 48 (26.8) | 0.907 | |
| BMI | 25.3 (28.9;22.8) | 25.1 (28.9;22.9) | 25.6 (28.7;22.5) † | 0.703 | |
| EuroScore II | 11.7 (26.4;5.5) | 9.1 (15.9;3.5) | 16.1 (34.3;6.4) | 0.000* | |
| NYHA III | 78 (24.8) | 34 (25.2) | 44 (24.6) | 0.902 | |
| NYHA IV | 89 (28.3) | 35 (25.9) | 54 (30.2) | 0.409 | |
| LVEF | 60 (60;55) | 60 (60;55) | 60 (60;55) | 0.042* | |
| Hypertension | 200 (63.7) | 79 (58.5) | 121 (67.6) | 0.098 | |
| Atrial fibrillation | 90 (28.7) | 38 (28.1) | 52 (29.1) | 0.861 | |
| IDDM | 15 (4.8) | 6 (4.4) | 9 (5.0) | 0.810 | |
| Preoperative dialysis | 32 (10.2) | 8 (5.9) | 24 (13.4) | 0.030* | |
| Cancer | 19 (6.1) | 10 (7.4) | 9 (5.0) | 0.381 | |
| h/o stroke | 123 (39.2) | 55 (40.7) | 68 (38.0) | 0.621 | |
| Modified Duke Criteria | |||||
| Major criteria | Positive BC | 259 (82.5) | 107 (79.3) | 152 (84.9) | 0.192 |
| Vegetation | 266 (84.7) | 114 (84.4) | 152 (84.9) | 0.908 | |
| Annular abscess | 131 (41.7) | 55 (40.7) | 76 (42.5) | 0.760 | |
| Minor criteria | IV drug abuse | 24 (7.6) | 11 (8.1) | 13 (7.3) | 0.770 |
| Fever > 38°C | 201 (64.0) | 81 (60) | 120 (67.0) | 0.198 | |
| Vascular phenomena | 161 (51.3) | 67 (49.6) | 94 (52.5) | 0.613 | |
| Immunologic phenomena | 19 (6.1) | 11 (8.1) | 8 (4.5) | 0.176 | |
| PVE | 99 (31.5) | 27 (20.0) | 72 (40.2) | 0.000* | |
| Preoperative ventilation | 142 (45.2) | 64 (47.4) | 78 (43.6) | 0.499 | |
| Preoperative inotropic support | 88 (28.0) | 32 (23.7) | 56 (31.3) | 0.139 | |
| CPR | 13 (4.1) | 5 (3.7) | 8 (4.5) | 0.736 | |
| Lactate value | 0.9 (1.3;0.7) | 0.8 (1.2;0.7) | 1.0 (1.3;0.7) | 0.013* |
| Overall n=314 |
AP drop < 30% n=135 |
AP drop ≥ 30% n=179 |
p-valueª | |
|---|---|---|---|---|
| Urgent operation | 262 (83.4) | 118 (87.4) | 144 (80.4) | 0.100 |
| Emergency operation | 49 (15.6) | 16 (11.9) | 33 (18.4) | 0.111 |
| Salvage operation | 3 (1.0) | 1 (0.7) | 2 (1.1) | 0.734 |
| Full sternotomy | 303 (96.5) | 128 (94.8) | 175 (97.8) | 0.159 |
| Re-sternotomy | 102 (32.5) | 29 (21.5) | 73 (40.8) | 0.000* |
| Isolated AVR | 168 (53.5) | 84 (62.2) | 84 (46.9) | 0.007* |
| Isolated MVR | 93 (29.6) | 39 (28.9) | 54 (30.2) | 0.806 |
| Double valve replacement | 52 (16.9) | 12 (8.9) | 41 (22.9) | 0.001* |
| Surgery time | 276 (390;220) | 240 (300;195) | 348 (455;255) | 0.000* |
| CPB | 139 (204;104) | 110 (155;90) | 162 (237;117) | 0.000* |
| ACC | 100 (144;70) | 81 (113;62) | 111 (159;79) | 0.000* |
| Overall n=314 |
AP drop < 30% n=135 |
AP drop ≥ 30% n=179 |
p-valueª | |
|---|---|---|---|---|
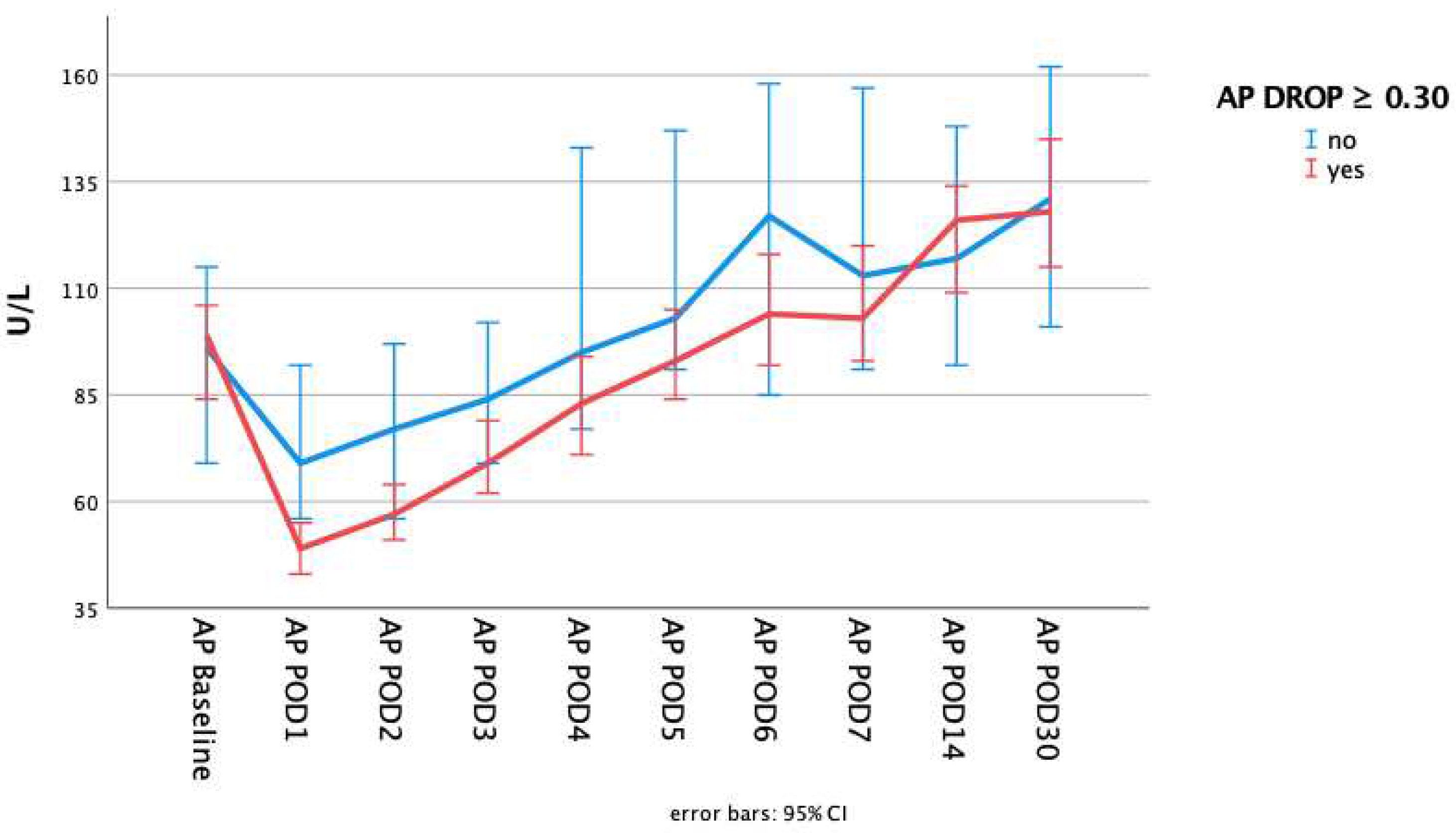
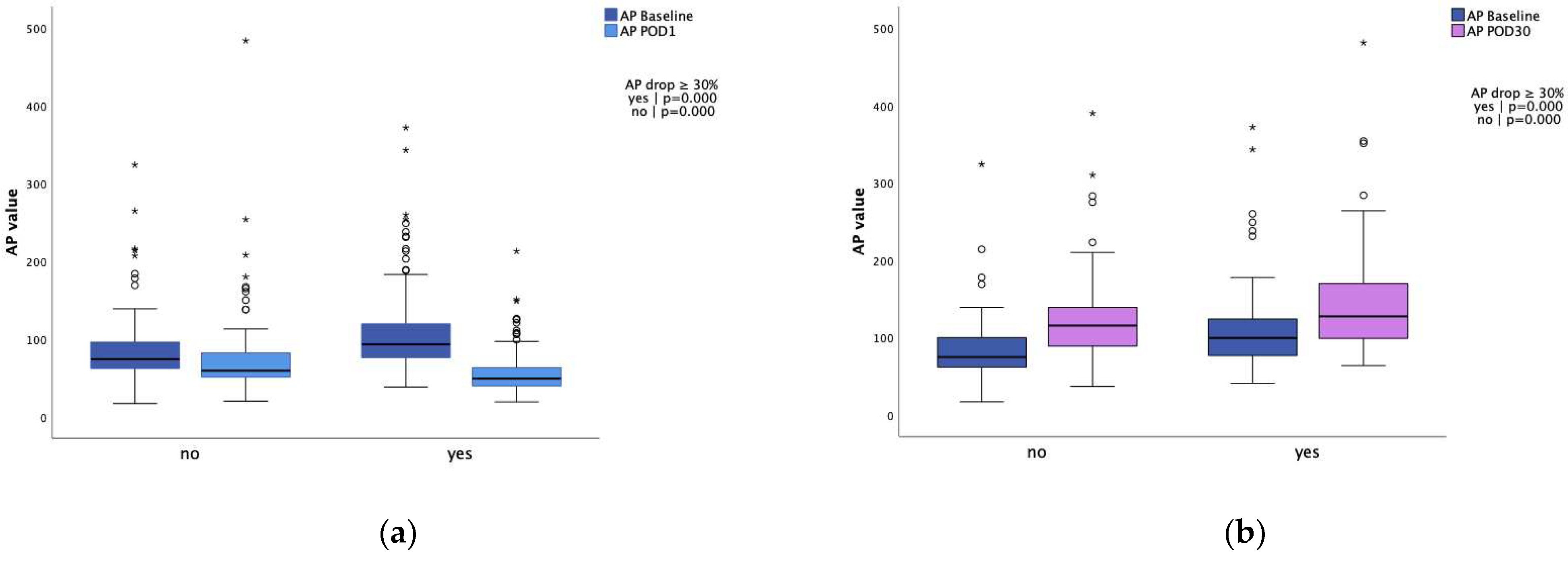
| Baseline AP value | 85 (112;69) | 74 (96;62) | 93 (120;76) | 0.000* |
| Baseline CRP value | 4.2 (10.7;1.6) | 3.9 (8.3;1.7) | 4.5 (11.7;1.5) | 0.456 |
| AP Drop | 32.5 (45.2;23.0) | 21.4 (25.8;11.5) | 42.7 (54.7;36.4) | 0.000* |
| First day baseline AP value surpassedå | 5 (7;4) | 4 (6;3) | 6 (7;4) | 0.000* |
| Baseline within 3 days | 118 (37.6) | 59 (43.7) | 59 (33.0) | 0.052 |
| Baseline within 5 days | 208 (66.2) | 100 (74.1) | 108 (60.3) | 0.011* |
| 30day AP value∫ | 121 (157;95) | 116 (141;89) | 128 (172;99) | 0.089 |
| 30day CRP valueç | 3.4 (7.8;1.1) | 2.1 (5.7;0.7) | 4.6 (10.6;1.8) | 0.001* |
| Overall n=314 |
AP drop < 30% n=135 |
AP drop ≥ 30% n=179 |
p-valueª | ||
|---|---|---|---|---|---|
| Bleeding revision | 38 (12.1) | 10 (7.4) | 28 (15.6) | 0.027* | |
| Need for any renal replacement therapy |
49 (15.6) | 10 (7.4) | 39 (21.8) | 0.001* | |
| Need for any renal replacement therapy without preoperative | 37 (11.8) | 9 (6.7) | 28 (15.6) | 0.015* | |
| Need for ECMO | 37 (11.8) | 3 (2.2) | 34 (19.0) | 0.000* | |
| Prolonged intubation | 107 (34.1) | 38 (28.1) | 69 (38.5) | 0.054 | |
| Prolonged ICU stay | 129 (41.1) | 43 (31.9) | 86 (48.0) | 0.004* | |
| Prolonged hospital stay | 62 (19.7) | 22 (16.3) | 40 (22.3) | 0.182 | |
| Mortality | |||||
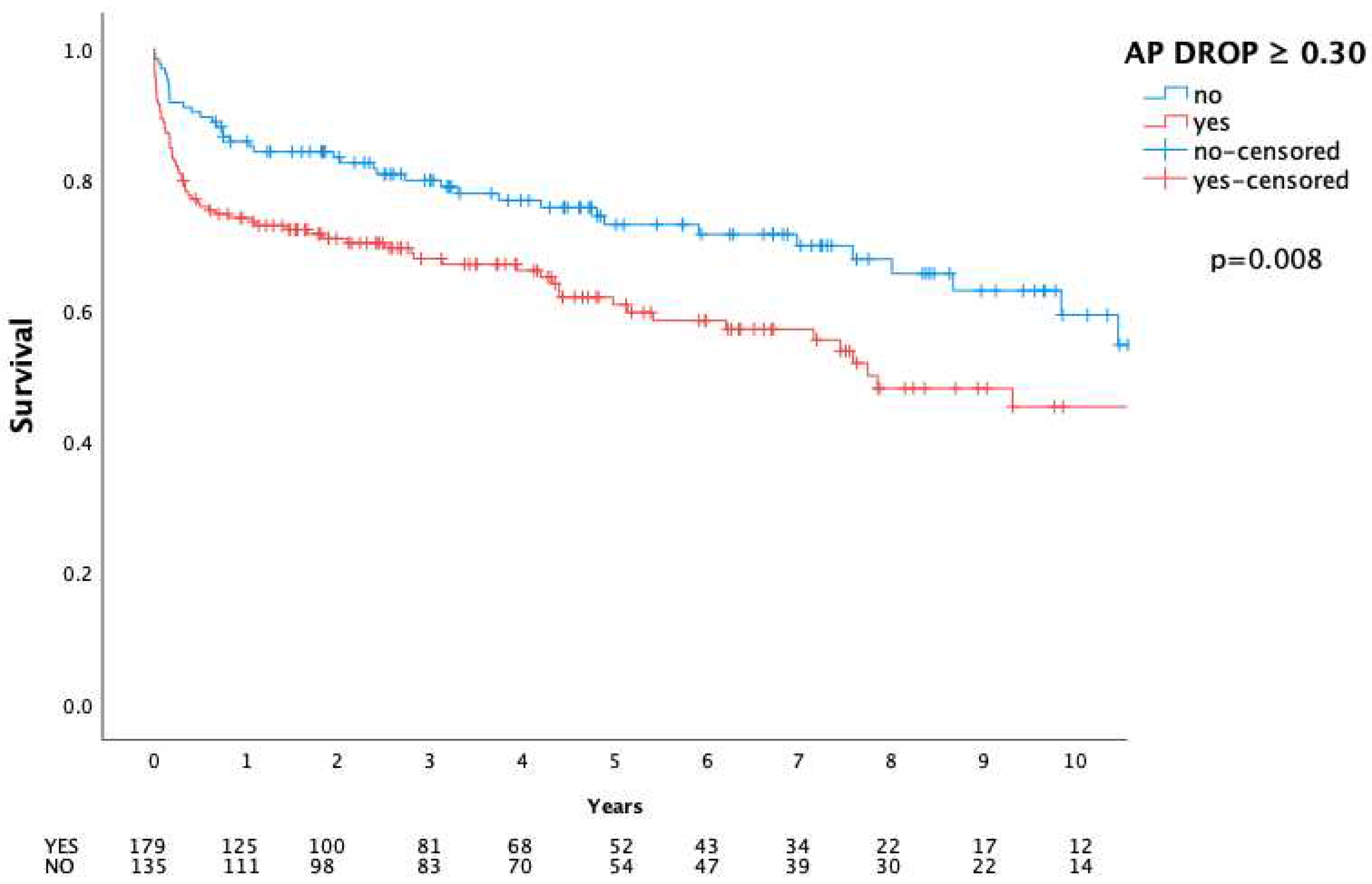
| 30 day | 23 (7.3) | 4 (3.0) | 19 (10.6) | 0.010* | |
| In-hospital | 39 (12.4) | 8 (5.9) | 31 (17.3) | 0.002* | |
| 1 year | 65 (20.7) | 19 (14.1) | 46 (25.7) | 0.012* |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





