1. Introduction
The Ankaratra forest, one of the biodiversity hotspot in Madagascar, is home to the locally endemic and critically endangered Amphibian
Mantidactylus pauliani [
1,
2]. This species has been recorded at an elevation range above 2000m according to previous research [
3,
4,
5], and specifically lives in the cold rocky stream of the Ankaratra montane. Since
M. pauliani is a typically aquatic species [
6], its distribution is thus restricted. Hence, it highly relies on the streams availability in terms of quantity and quality for breeding and sustenance [
6,
7].
However, these last decades, the forest [
8] and its streams have gradually undergone changes induced by multiple human-led activities including charcoal production, timber harvesting, cattle grazing and wildfire [
5,
9,
10]. Anthropogenic activities increase threats to species habitat including a connectivity disruption of the population [
11] as well as alteration and degradation [
9] which may induce vulnerability as Amphibians are among the highly threatened species in the world [
12].
Given the limited distribution of
M. pauliani and its particular high reliance on streams, it is likely to be sensitive to the change in its habitat [
13] which renders it as an indicator of the environment change. The effect of landscape change regarding to the viability of this locally endemic frog is still understudied thus far [
11], and should be subject to a relatively long-term studies to produce reliable information for conservation action plan. It is therefore crucial to develop an understanding on the impact of habitat change to
M. pauliani population vulnerability.
In this paper, we aim to analyze the viability of
M. pauliani regarding to the landscape change that occurred across different habitat type through spatiotemporal perspective. Based on previous research [
5,
11,
13] and the theoretical considerations, we hypothesized that change in landscape, reflected in habitat degradation, negatively impact
Mantidactylus pauliani population. Specifically, this study will explore the linkage between this species and its environment while determining its distribution and ecological preference once habitat characteristics is identified. This research update information about this critically endangered species in the face of the dynamic of landscape change so as to build an effective conservation action plan.
2. Materials and Methods
2.1. Study area
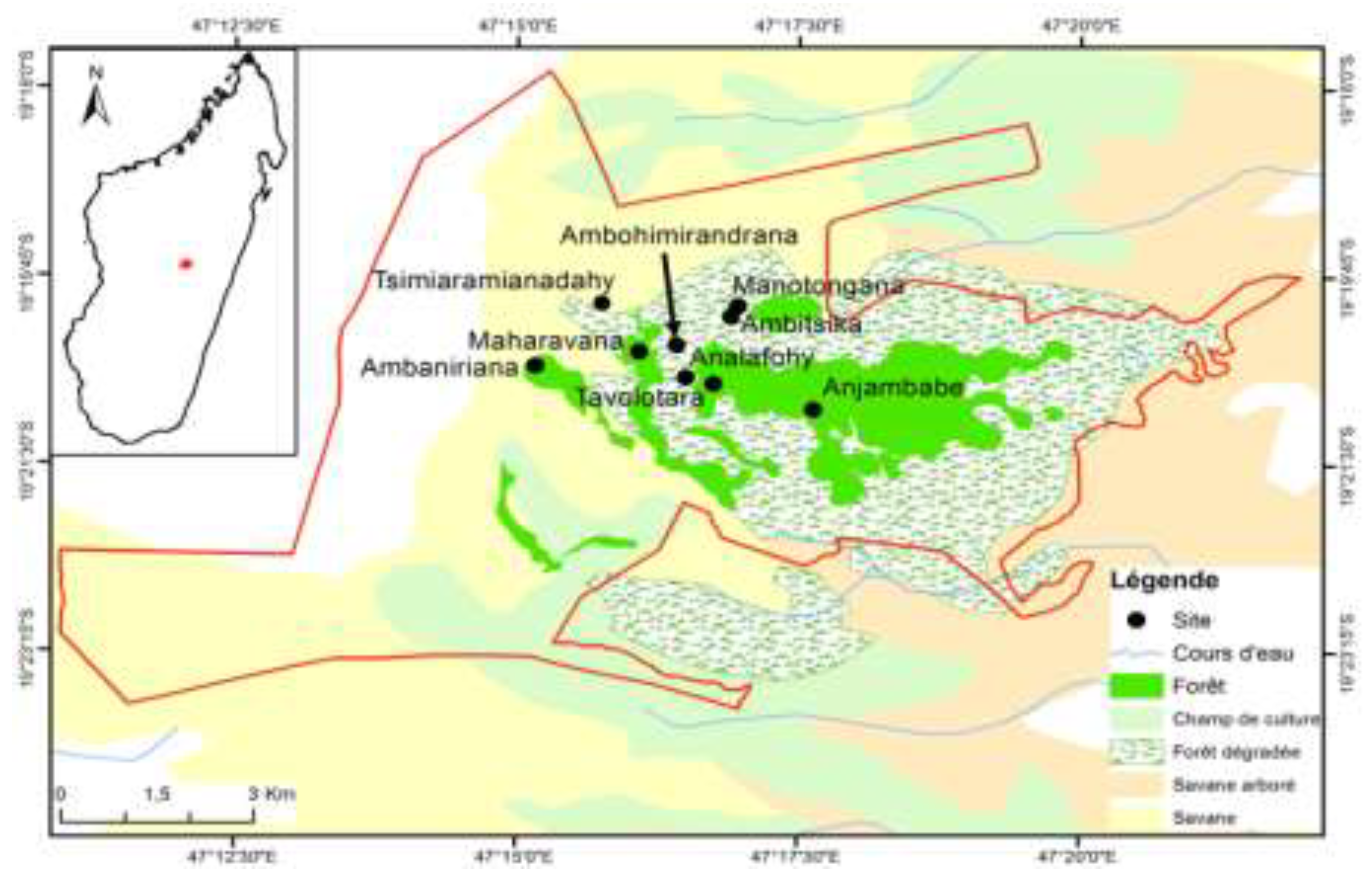
The study was conducted within the New Protected Area (NAP) of Ankaratra, which is one of the remaining forests in the central highlands of Madagascar, covering about 8130 hectares (
Figure 1). It extends over the slopes of the Ankaratra massif, between 19°19' and 19°24' South latitude and 47°14' and 47°22' East longitude. The study area is part of the Manjakatompo forest station, located at 84 km from the city of Antananarivo, via the RN7, and at 17 km west to the city of Ambatolampy.
2.2. Data collection
During this study, four 15-days field trips were conducted in September 2018, March 2019, February 2020, and July 2021. These four trips allowed for optimal monitoring of variations in the population size of
Mantidactylus pauliani according to the season (dry and wet) and the type of existing formation. The selection of the study sites took into account different types of environments, the presence of permanent rocky streams, and elevation levels. The sites cover the entire mountain range (crest, valley, and slope), and differ in terms of habitat characteristics and degree of degradation. Six sites were selected since 2018 and 2019, and three additional sites were included in 2020 and 2021, namely Maharavana, Analafohy, and Ambohimirandrana (
Table 1).
On each site, a 100-meter transect was established with varying widths depending on the structure of the stream. The transect served to collect data through direct observation and examination of shelters [
14]. It consists of a 100-meter line along the stream, marked every 10 meters with a "flag". Amphibian abundance is the number of individuals of the species encountered by direct capture without release.
For each individual counted, biometric measurements were taken using a caliper followed by weighing using a Pesola balance. The measurement follows the method of Halliday et al. [
15], which consists of measuring the SVL (Snout Vent Length), which is the length from the tip of the snout to the anal opening; the HW (Head Width), which is the width of the head, and the TL (Tail Length), which is the length from the tip of the snout to the end of the hind leg. Ecological information is a necessary parameter during fieldwork to explain species abundance and distribution.
Additionally, to determine the characteristics of the stream, measurements of water speed and depth were made using the same techniques as [
16]. Speed is measured by timing a floating object (usually a cork) over a distance of one meter. To measure depth, a vertically held straight stick is used between the outer surface and the bottom of a stream at a buried and stable rock. Three samples are taken where the current speed was timed using "flags" for reference. Measurements of water speed and depth are taken at each descent in these same locations [
16].
2.3. Data analysis
For the data analysis, the Chi-square test (χ2) is performed to confirm whether the vertical distribution of M. pauliani for each developmental stage is significantly different according to the study season. It is also performed to determine if the numbers of this species by developmental stage vary by study season. In addition, a multivariate analysis was carried out, using correspondence factor analysis which is a method of data analysis when the variables to be studied are quantitative in nature. This aims to evaluate the ecological preference of the individuals for each developmental stage according to the physical parameters of the streams, and the characteristics of the habitats. It consists in projecting on the factorial plan the individuals (adult, juveniles and tadpoles), and the variables such as water speed ranges ([0.5-1 m/s[ , and [1 -1.5 m/s[ , water depth ranges ([0-20 cm[, [20-40 cm[ and [40-60 cm[), stream width ([0-1 m[, [1-2 m[ and [2-3 m[), slopes (flow slope, medium slope, and steep slope), microhabitat (still water, bank, and rock), stream substrates (muddy, rocky, and sandy) which can influence the distribution of M. pauliani.
Pressure is analyzed from the calculated value of the sum of the disturbance parameters of each threats; called index of pressure IP (duration, intensity and magnitude of the pressure) according to [
17]. If IP ≤4, the pressure is minor, IP =]4-7[, the pressure is medium, if IP≥ 7 the pressure is major. On the other hand, threats by site are analyzed and interpreted according to the degrees of threat (Dm), where (Dm) is the ratio between IP and Pp (Pp: sum of IP). It varies from 0 to 1. If 0.8 < IP ≤ 1, the threat is very high; 0.7< IP ≤ 0.8, the threat is high; 0.5 < IP ≤ 0.7, the threat is medium; 0.3 < IP ≤ 0.5, the threat is low and 0 < IP ≤ 0.3, the pressure is very low.
3. Results
3.1. Species distribution and abundance
Actually
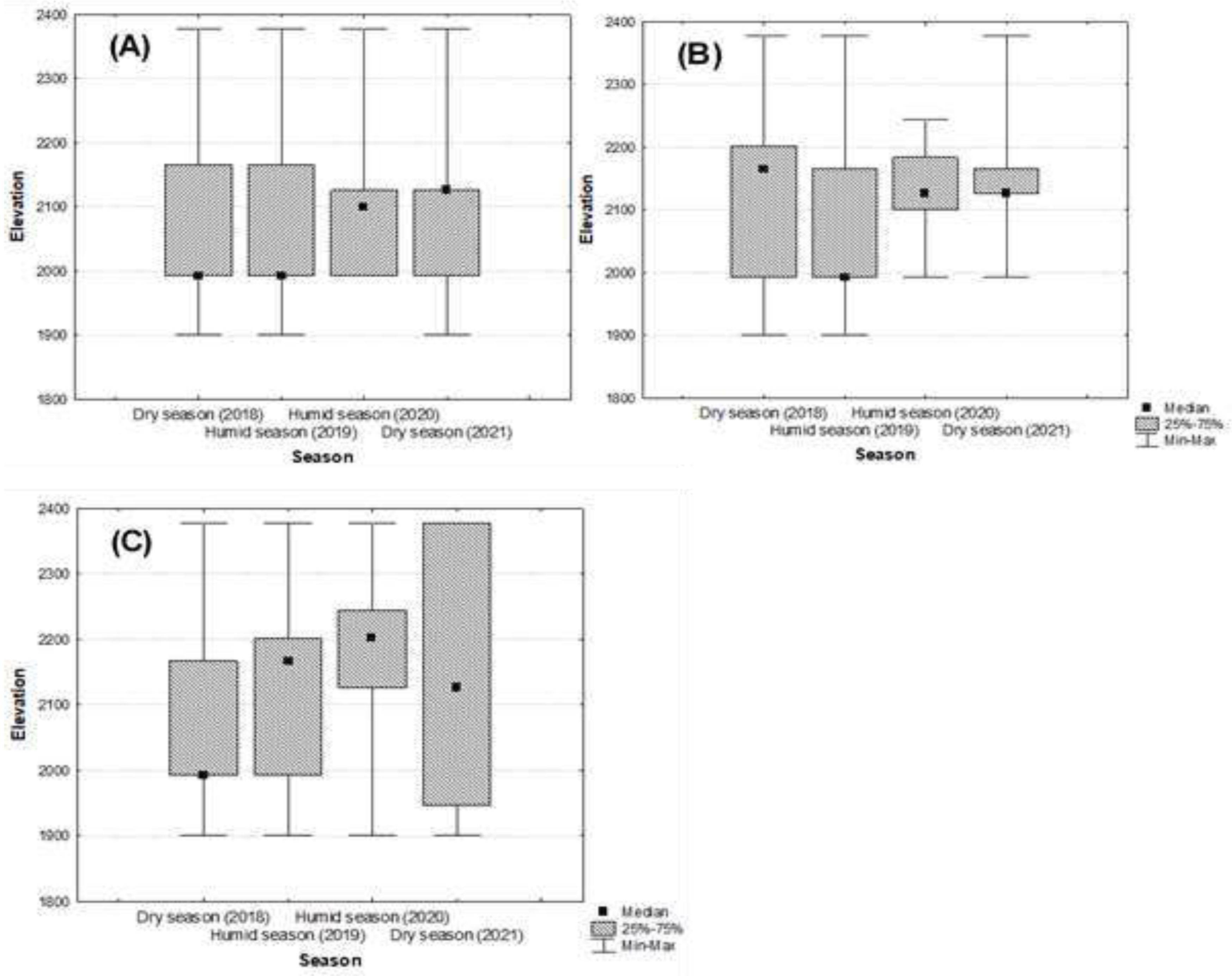
Mantidactylus pauliani is recorded between 1900 m to 2373 m elevation (
Figure 2), but almost of the individuals observed concentrated between 1993 to 2200 m asl.. Generally, this slope distribution along the montane stream varies significantly for all stages of the development according the field survey seasons (Adult stage: χ2 = 222.26, p-value < 0.0001, df = 24; Juvenile stage: χ2 = 152.52, p-value< 0.0001, df = 24; Tadpole stage: χ2 = 382.48, p-value < 0.0001, df = 24).
For adult individuals, they remain stable between 1993-2166 m in September 2018 (dry season), and in March 2019 (wet season) with optimum habitat at 1993 m in both seasons. However, most of them found at 2100 m elevation during the wet season (February 2020), and above 2100 m during the dry season (July 2021). For juvenile individuals, most of them are inventoried starting at 2150 m elevation in September 2018 (dry season) and at 1993 m elevation during the wet season (March 2019). During the wet season (February 2020), and the dry season (July 2021), their distribution increases from 2100 m elevation. For tadpoles, the large of them are found at 1993 m elevation during the September 2018 (dry season), and this elevation rises from 2150 m during the wet season (March 2019) and 2200 m in February 2020, then down from 2100 m during the dry season in July 2021 (
Figure 2).
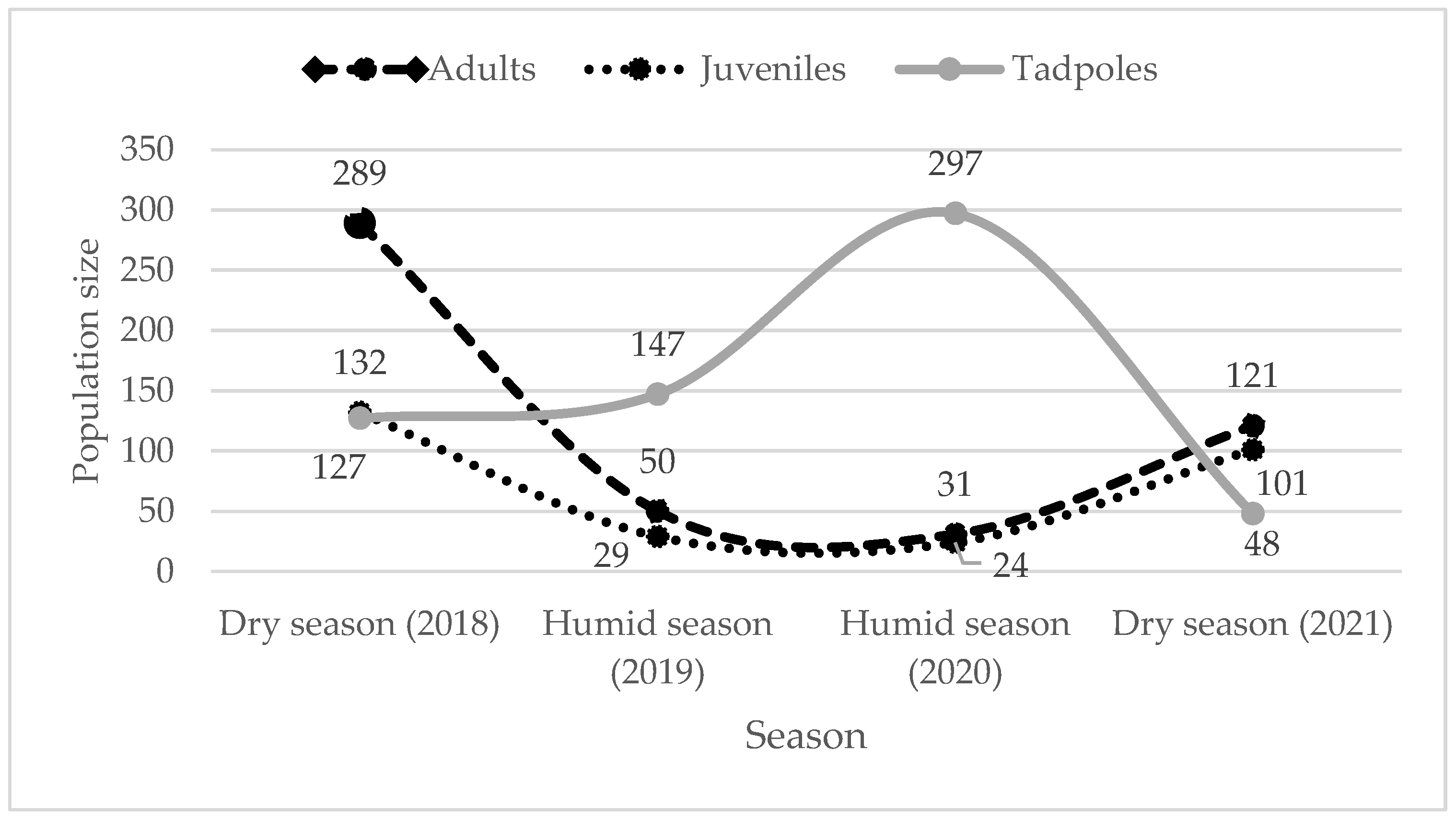
The great variation of the number of the individuals were observed during the four years (2018, 2019, 2020 and 2021,
Figure 3) for all stages of the development depending the season (χ2 = 462.89, P < 0.0001, df = 6). Adults are more abundant than juveniles and tadpoles during the dry seasons (2018, 2021), and tadpoles during the humid seasons (2019, 2020), but we observed more juveniles than tadpoles in dry season 2021 (101
vs 48).
3.2. Sex-ratio
Based on the various environments encountered during the period of descent, the sex ratio is calculated. Females are more common in open areas than males, with the exception of the dry season (July 2021), when males are more prevalent. Males outnumber females in forest environments. With levels of 2.33 and 3.50, respectively, during the wet season in March 2019 and February 2020, the sex ratio is exceptionally high, especially in forest habitats. In contrast, the sex ratio in savannah ecosystems is only at its highest in the dry season (July 2021) and at its lowest point (0.33) in the wet season (February 2020) (
Table 2).
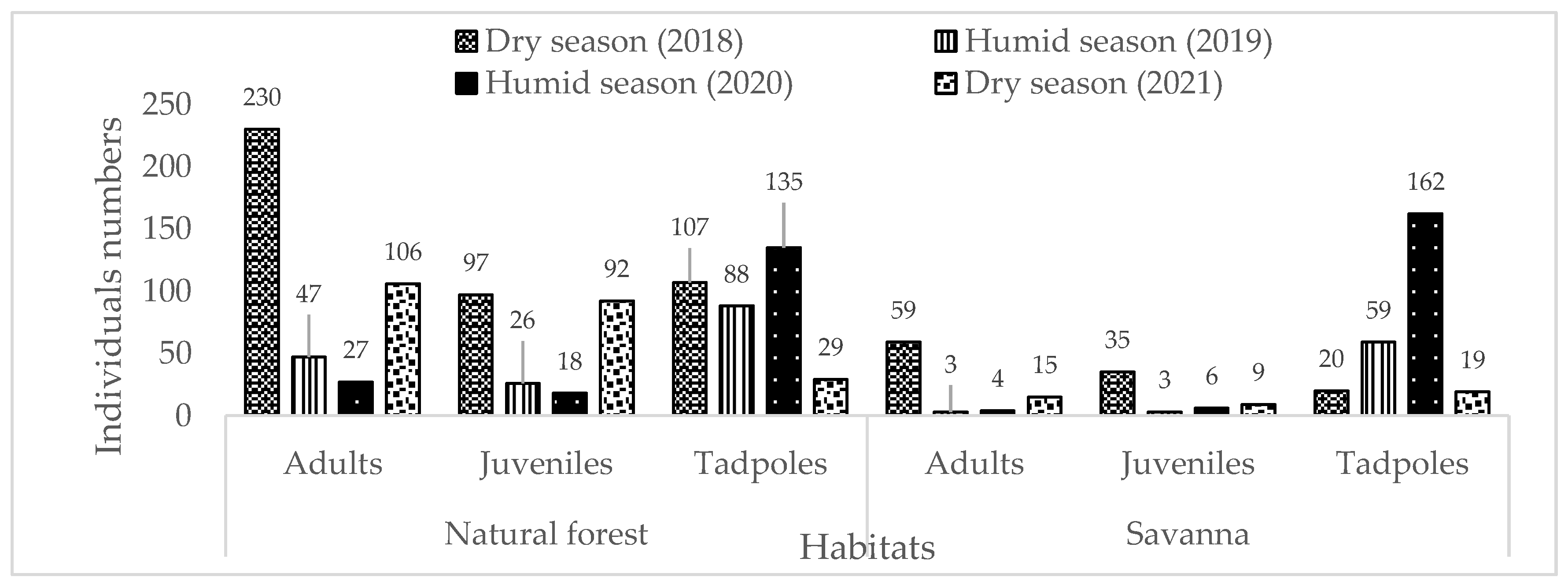
3.3. Habitat preference
Regarding ecological niche, adult species preferred forest habitat than savannah environment (dry season 2018: 230
vs 59 individuals; wet season 2019: 47
vs 3 individuals: wet season: 27
vs 4 individuals and dry season 2021: 106 vs 15). However, for tadpoles, they are more abundant in the savannah habitat than forest during the wet season in February 2020 (162
vs 135 individuals) (
Figure 4).
In addition, a spatial vicariate coloration was noticed among the individual sampled. That means a species-based in open area at high elevation has a lighter skin compared to those encountered in closed environments at lower altitudes (
Figure 5).
3.4. Microhabitat preference
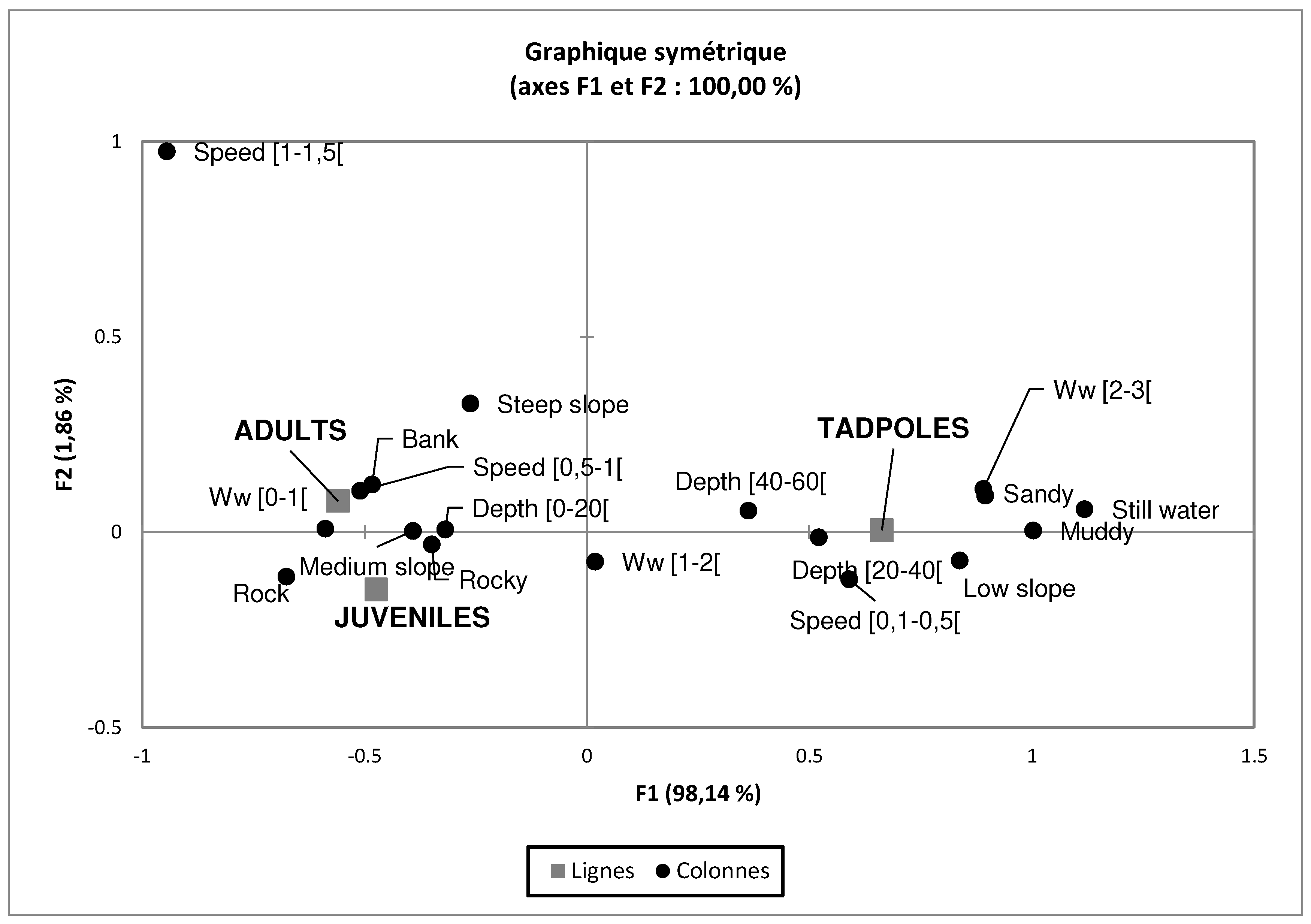
The
Figure 6 presents the projection all of the individual stages associated with habitat variable preferences. The first factorial explains 100% of the total variability, i.e. 98.17% for axis 1 and 1.83% for axis 2. Thus, the both factors can explain easily the distribution of the individuals relative to ecological guilds. Adult individuals are more numerous in streams that have a width between 0 to 1 m, a velocity between 0.5 to 1.5 m/s, and a depth between 0 to 20 cm. They observed close to the riverbank with abrupt slope. Juvenile individuals found under or hidden behind the rocky with medium slope. The tadpoles have a preference on deep still-watered (40 to 60 cm depth) with a wide watercourse (2 to 3 m), and it is also observed in watercourses with sandy substrates.
3.5. Vulnerability analysis
Throughout this study, six types of threats, most of which are related to human activities, were frequently observed: bush fires, charcoal burning, logging, grazing fires, cattle roaming and trampling of waterways, and the expansion of agricultural areas on slopes. These different types of threats lead directly or indirectly to the degradation of the habitat of this species.
Nevertheless, the intensity of these different pressures varies according to the type of formation and their proximity to the watercourse. The intensity of the threat is highest in the open area where the pressure index is high, IP ≥ 0.5 (principally livestock grazing) (
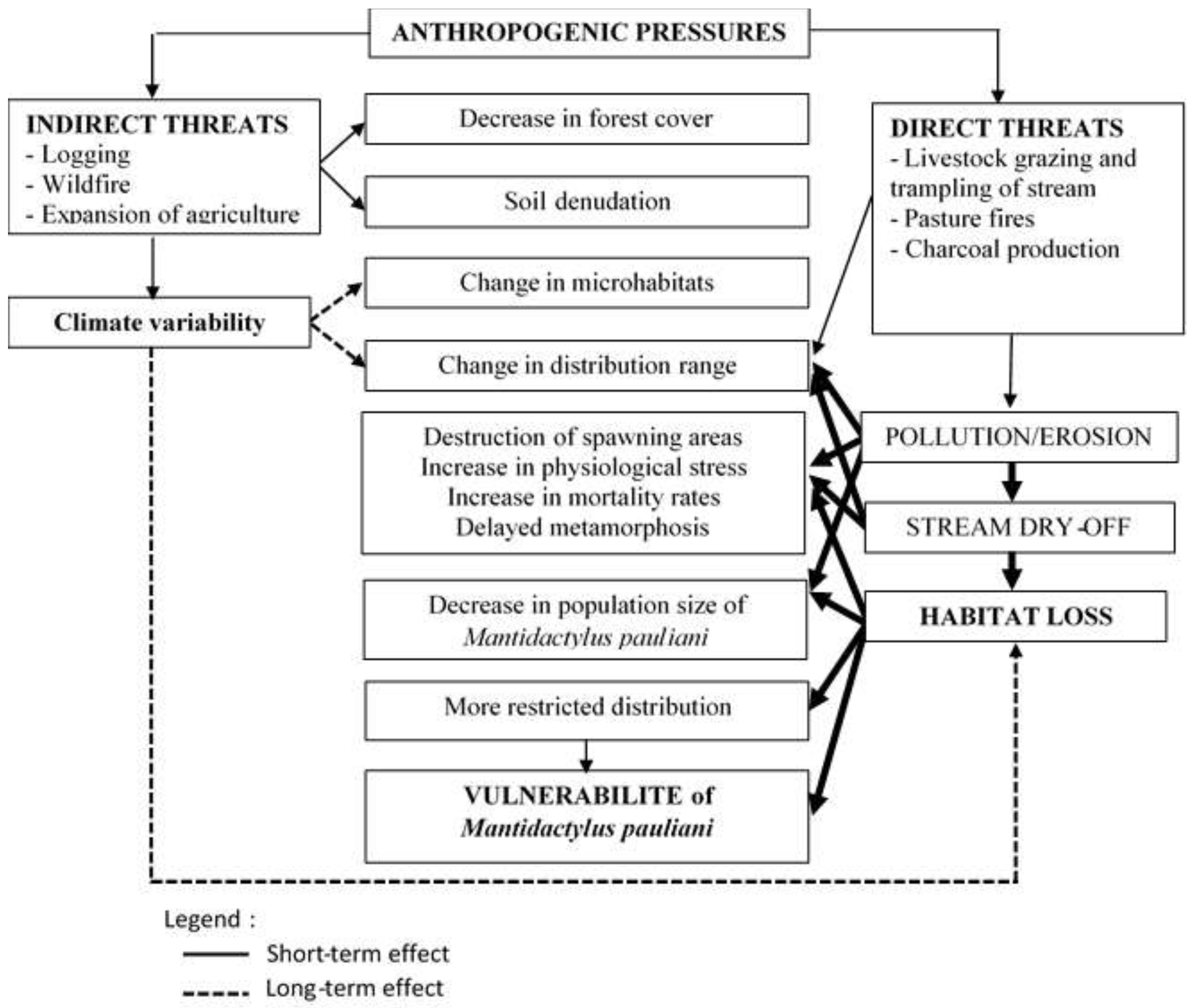
Table 3). For the forested area, only anthropogenic activities near streams represent a high threat to the species, e.g case of the edge for Manotongana, IP=0.63 (logging and charcoal). The following diagram is established to demonstrate the existing relationship between the viability of the species and changes in its habitat caused by anthropogenic pressures (
Figure 7).
According to this diagram and threats analysis (
Figure 7), the IP highest can affect species directly or indirectly according the distance relative to stream. On the one hand, generally indirect threats lead to decrease the forest cover, the soil denudation, and driving climate variability. In the long-term, this climatic variability leads to change the microhabitats and of course the species distribution. On the other hand, the direct threats cause erosion and which leads pollution, and finally drying up. As a final consequence the population decreasing (case of Manotongana population). For tadpoles, the stress caused rapid metamorphosis and as a result the vulnerability of the adult population due to habitat erosion.
4. Discussion
4.1. Distribution
Mantidactylus pauliani is a critically endangered highland species with a permanent decline [
18]. Available data suggest a reduced distribution range for this species between 2000-2300 m elevation [
6,
10,
19], but our study shows that this range extends from 1900-2392 m. We suggest two hypotheses to explain this. The first one is that the research effort increased because we added a new transect along the slope from 1900 m to 2400 m. The second one is that our study encompasses all seasons (dry and rainy) in comparison with the other authors who focused lonely on the wet season (breeding period). According to [
20], one biological response to climate change is the upward shift of the species distribution due to temperature upped. This was observed during our study. Even though
M. pauliani observed at 1900 m asl, it tends towards higher during the four-year descents.
There is a difference in the number of individuals for each developmental stage for different periods. During the dry season in 2018 (pre-reproduction period), we observed more adults than juveniles and tadpoles (289 vs 132, 127 individuals), but there are more tadpoles during the reproduction period (humid season 2019, 2020) than the other periods (dry season 2018, 2021). This indicating that the juveniles could reached maturity that means adults (121 adults
vs 101 juveniles; dry season 2021). We noticed that the number of the tadpoles decreased quickly between humid season (297/2020) and dry season (48/2021), we can explain this through the fast-flowing streams between both periods and impact the speed of tadpole metamorphosis by big strees [
21].
The majority of recorded individuals are found at mid-slope (1900 m and 2200 m) where the forests are stable and the microhabitat in the form of a variety of rocks (fine sand and gravel) is available as a refuge [
16]. In addition, tadpoles are more abundant in open habitats than in closed habitats because sunlight is strong and facilitates their hatching and development [
22,
23].
4.2. Sex-ratio
Concerning sex ratio according habitat and period, we noticed a high ratio in forested areas at low-elevation than in open and savannah habitats at high altitude, this observation confirm the previous study by [
24]. Therefore, females attract males to lay their eggs and we documented that Mantellinae rarely lay their eggs in water, but sometimes just overhanging the water surface smoothing sperm fertilization by male [
3,
25].
In all savannah environments, the habitat is open, so sunlight is strong despite a decrease in ambient temperature. Regarding seasonal distribution,
M. pauliani is more abundant during the cold and dry season and decreases during the rainy season. This observation is the same as that observed by [
20]. Furthermore, high-elevation species reproduce throughout the year unlike species at medium and low altitudes which are inactive during the cold and dry season [
26]. This explains the abundance of
Mantidactylus pauliani along the year. However, certain author as [
27] explained that changes in abundance correlated with short-term and long-term habitat loss. The case of the Manotongana transect at 2167 m elevation asl, the habitat there was fragmented and degraded due to the silting erosion leading it drying up. This is why this site has a very low individual, only one adult male was recorded in September 2018 and no individual has been reported in this site since February 2020.
4.3. Habitat preference and adaptation
According to [
16], the numerous of rock dimensions, and shapes were the favorable habitats because they balanced the speed water, this assumption is the same as the habitat of
M. pauliani where their presence, and abundance correlate with the rock presence and also the water depth, degree of slope, water velocity, and stream-slept.
The variation in the color of adult individuals' skin between in low-elevation forest (black one) and open high-elevation environments (lighter one) could be explained by adaptation to physical conditions such as temperature variations as suggested by [
28]. This study discussed that the blackish color in montane amphibian could be a response to mitigate ultraviolet or even though to escape predators as a camouflage-like [
29], although further researches are needed in this shape behavior. Although forest degradation represents a threat to Ankaratra's biodiversity, our study demonstrated that both savannah and natural forest represent an important environment for this species to ensure their viability, this finding is similar to those previously advanced by [
5]. However, the impact of forest degradation need in-depth analyses, till now it has not and does not have immediate impact on the population of this species as suggested similar studies [
30], but a long-term effect is to be analyzed. Ankaratra massif is vulnerable to various anthropogenic activities that can have negative impacts on species survival. Erosion, livestock wandering and trampling of watercourses remain the most significant threats, especially at high altitudes, which cause the destruction of watercourses. According to [
1], high altitudes are refugees for several species with restricted distribution and are also more vulnerable. Given that fragmentation is one of the major threats to the disappearance of amphibians [
31].
Our recommendation to assure Ankaratra and M. pauliani serenity consists to strengthen a patrol monitoring though Forest Ranger, especially in open breeding areas (savannah habitat). The establishment of a fire break system around the forest is also essential to minimize damage caused by bush fires. Environmental education, awareness-raising and consciousness-raising within the village community must also be undertaken. This will allow them to understand the importance of the forest and make them aware that biodiversity conservation will benefit them in the long term. The last but not least consists to implement and ameliorate livelihoods of local population through services from water.
5. Conclusion
Overall, our study highlights the effect of landscape change on Mantidactylus pauliani population and its vulnerability as this study highlight that this species shows an overall abundance in natural forest compared to those in degraded and open area while tadpoles shows preference in savannah area. The findings also reveal that species abundance differ following the season across the four years of observation. Moreover, the results also demonstrate that species was found from 1900 m and above and can reach 2073 m a.s.l elevation. The reproduction occurs throughout the year but top during the humid season, and take place especially in savannah habitat which is an area that have the ecological guiled that tadpoles need for their development
As the aquatic, Mantidactylus pauliani has a restricted distribution ranging from 1900m and above, its viability depends on viability of the watercourse in top montane, which is its reproduction place, and the availability of the habitat of adults (natural forest). Our study serves as support for decision making in the elaboration of conservation strategy and in determining the focus area for monitoring (reproduction site) with proactive measures to prevent the irreversible loss of the one of the emblematic species of Ankaratra massif.
Supplementary Materials
The following supporting information can be downloaded at:
Preprints.org, Figure S1: photos of pressure and threats in Ankaratra massif. Photo of
Mantidactylus pauliani and its habitat.
Author Contributions
Conceptualization, N.R. and H.O.R.; validation, N.R., B.A. and H.O.R.; writing—original draft preparation, H.O.R.; writing—review and editing, H.O.R.; supervision, N.R. and B.A. All authors have read and agreed to the published version of the manuscript.” Please turn to the CRediT taxonomy for the term explanation.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
Acknowledgments
We thank the “Direction Générale des Forêts de Madagascar” who gave us the research permits, and the community-based at Ankaratra villages who contributed to smooth the realization of the fieldwork (Guides, cooking, camp assistance). We thank all of the staff of V.I.F, the former Ankaratra NPA manager in supporting us.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Andreone, F.; Cox, N.A.; Glaw, F.; Köhler, J.; Rabibisoa, N.H.C.; Randriamahazo, H.; Randrianasolo, H.; Raxworthy, C.J.; Stuart, S.N.; Vallan, D.; et al. Update of the Global Amphibian Assessment for Madagascar in Light of Species Discoveries, Nomenclature Changes, and New Field Information. Monogr. Mus. Reg. Sci. Nat. Torino 2008, XLV, 419–438. [Google Scholar]
- Andreone, F.; Carpenter, A.I.; Cox, N.; du Preez, L.; Freeman, K.; Furrer, S.; Garcia, G.; Glaw, F.; Glos, J.; Knox, D.; et al. The Challenge of Conserving Amphibian Megadiversity in Madagascar. PLoS Biol. 2008, 6, e118. [Google Scholar] [CrossRef] [PubMed]
- Glaw, F.; Vences, M. A Field Guide to the Amphibians and Reptiles of Madagascar; 3. ed.; Vences & Glaw: Germany, 2007; ISBN 978-3-929449-03-7. [Google Scholar]
- Rakotonoely, S.A.X. Biologie et Écologie de Deux Espèces d’amphibiens Boophis Williamsi (GUIBE, 1974) et Mantidactylus Pauliani GUIBE, 1974) Critiquement En Danger Du Massif de l’Ankaratra, Antananarivo: Madagascar, 2012.
- Rosa, S.C.F. Is There a Future for the Amphibians of the Ankaratra Massif Reserve? Understanding the Role of Landscape Change, Porto: Portugal, 2017.
- Vences, M.; Andreone, F.; Glaw, F.; Raminosoa, N.; Randrianirina, J.E.; Vieites, D.R. Amphibians and Reptiles of the Ankaratra Massif: Reproductive Diversity, Biogeography and Conservation of a Montane Fauna in Madagascar. Ital. J. Zool. 2002, 69, 263–284. [Google Scholar] [CrossRef]
- Andreone, F.; Cadle, J.E.; Cox, N.; Glaw, F.; Nussbaum, R.A.; Raxworthy, C.J.; Stuart, S.N.; Vallan, D.; Vences, M. Species Review of Amphibian Extinction Risks in Madagascar: Conclusions from the Global Amphibian Assessment. Conserv. Biol. 2005, 19, 1790–1802. [Google Scholar] [CrossRef]
- Suzzi-Simmons, A. Status of Deforestation of Madagascar. Glob. Ecol. Conserv. 2023, 42, e02389. [Google Scholar] [CrossRef]
- Bletz, M.C.; Rosa, G.M.; Andreone, F.; Courtois, E.A.; Schmeller, D.S.; Rabibisoa, N.H.C.; Rabemananjara, F.C.E.; Raharivololoniaina, L.; Vences, M.; Weldon, C.; et al. Widespread Presence of the Pathogenic Fungus Batrachochytrium Dendrobatidis in Wild Amphibian Communities in Madagascar. Sci. Rep. 2015, 5, 8633. [Google Scholar] [CrossRef]
- Rabemananjara, F.; Randriamahazo, H.; Rahantamalala, J.; Rahantalisoa, H.; Rakotoarisoa, J.M.; Rabibisoa, N.H.C.; Andreone, F. The Conservation Effort for Two Critically Endangered Amphibian Species of the Ankaratra Massif, Boophis Williamsi and Mantidactylus Pauliani.
- Hazell, D. Frog Ecology in Modified Australian Landscapes: A Review. Wildl. Res. 2003, 30, 193. [Google Scholar] [CrossRef]
- Hernández-Ordóñez, O.; Martínez-Ramos, M.; Arroyo-Rodríguez, V.; González-Hernández, A.; González-Zamora, A.; Zárate, D.A.; Reynoso, V.H. Distribution and Conservation Status of Amphibian and Reptile Species in the Lacandona Rainforest, Mexico: An Update after 20 Years of Research. Trop. Conserv. Sci. 2014, 7, 1–25. [Google Scholar] [CrossRef]
- Fu, L.; Wang, X.; Yang, S.; Li, C.; Hu, J. Morphological Variation and Its Environmental Correlates in the Taihangshan Swelled-Vented Frog across the Qinling Mountains. Animals 2022, 12, 2328. [Google Scholar] [CrossRef]
- Raxworthy, C.J.; Nussbaum, R.A. A Rainforest Survey of Amphibians, Reptiles and Small Mammals at Montagne d’Ambre, Madagascar. Biol. Conserv. 1994, 69, 65–73. [Google Scholar] [CrossRef]
- Halliday, T.R.; Verrell, P.A. Body Size and Age in Amphibians and Reptiles. J. Herpetol. 1988, 253–265. [Google Scholar] [CrossRef]
- Moisan, J.; Pelletier, L. Guide de Surveillance Biologique Basée Sur Les Macros Invertébrées Benthiques d’eau Douce Du Québec Cours d’eaux Peu Profondes à Substrats Grossier. Dir. Suivi L’état L’environnement Ministère Dév. Parcs ISBN 2008, 978-550.
- ZICOMA Evaluation de La Faune Aviaire Dans Les Zones Humides Entre Le Parc National de Ranomafana et Celui d’Andringitra. W: Projet d’Appui a la Gestion de l’Environnement, International Resources Group: Washington, DC, 2000.
- IUCN IUCN. 2022. The IUCN Red List of Threatened Species. Version 2022–2.
- Blommers-Schlösser, R.M.A. Biosystematics of the Malagasy Frogs. I. Mantellinae (Ranidae). Beaufortia 1979, 29, 1–77. [Google Scholar]
- Rabibisoa, N.H.C.; Raxworthy, C.J.; Andreone, F. Changement Climatique et Amphibien 2008.
- Haramura, T. Microhabitat Selection by Tadpoles of Buergeria Japonica Inhabiting the Coastal Area. J. Ethol. 2007, 25, 3–7. [Google Scholar] [CrossRef]
- Dittrich, C.; Drakulić, S.; Schellenberg, M.; Thein, J.; Rödel, M.-O. Some like It Hot? Developmental Differences in Yellow-Bellied Toad (Bombina Variegata) Tadpoles from Geographically Close but Different Habitats. Can. J. Zool. 2016, 94, 69–77. [Google Scholar] [CrossRef]
- Brattstrom, B.H. Thermal Control of Aggregation Behavior in Tadpoles. Herpetologica 1962, 18, 38–46. [Google Scholar]
- Edmonds, D.; Rakotoarisoa, J.C.; Rasoanantenaina, S.; Sam, S.S.; Soamiarimampionona, J.; Tsimialomanana, E.; Rainer Dolch, Y.; Rabemananjara, F.; Rabibisoa, N.; Robsomanitrandrasana, E. Captive Husbandry, Reproduction, and Fecundity of the Golden Mantella (Mantella Aurantiaca) at the Mitsinjo Breeding Facility in Madagascar. Salamandra 2015, 51, 315–325. [Google Scholar]
- Porcel, X.; Dubos, N.; Nöel, J.; Lava, H.; Velo, J.H.; Melo, M.; Rosa, G.M.; Andreone, F.; Crottini, A. Male Parental Care in Malagasy Stream-Dwelling Frogs of the Mantidactylus Femoralis Group (Anura: Mantellidae: Ochthomantis): Egg Guarding in Ochthomantis. Herpetol. Notes 2022, 15, 55–61. [Google Scholar]
- Raxworthy, C.J.; Nussbaum, R.A. Montane Amphibian and Reptile Communities in Madagascar. Conserv. Biol. 1996, 10, 750–756. [Google Scholar] [CrossRef]
- Cocca, W.; Andreone, F.; Belluardo, F.; Rosa, G.M.; Randrianirina, J.E.; Glaw, F.; Crottini, A. Resolving a Taxonomic and Nomenclatural Puzzle in Mantellid Frogs: Synonymization of Gephyromantis Azzurrae with G. Corvus, and Description of Gephyromantis Kintana Sp. Nov. from the Isalo Massif, Western Madagascar. ZooKeys 2020, 951, 133. [Google Scholar] [CrossRef]
- Thomas, M.; Raharivololoniaina, L.; Glaw, F.; Vences, M.; Vieites, D.R. Montane Tadpoles in Madagascar: Molecular Identification and Description of the Larval Stages of Mantidactylus Elegans, Mantidactylus Madecassus, and Boophis Laurenti from the Andringitra Massif. Copeia 2005, 2005, 174–183. [Google Scholar] [CrossRef]
- Rakotozafy, L.M.S.; Rahantalisoa, H.; Rabemananjara, F. A Survival Blueprint for the Madagascar Frog, Mantidactylus Pauliani, from Ankaratra Massif, Madagascar. Zool. Soc. Lond. Lond. UK 2019, 26. [Google Scholar]
- Konopik, O.; Steffan-Dewenter, I.; Grafe, T.U. Effects of Logging and Oil Palm Expansion on Stream Frog Communities on Borneo, Southeast Asia. Biotropica 2015, 47, 636–643. [Google Scholar] [CrossRef]
- Tabarelli, M.; Gascon, C. Lessons from Fragmentation Research: Improving Management and Policy Guidelines for Biodiversity Conservation. Conserv. Biol. 2005, 19, 734–739. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).