Submitted:
19 June 2023
Posted:
20 June 2023
You are already at the latest version
Abstract
Keywords:
1. History of COVID-19 and Its Variants
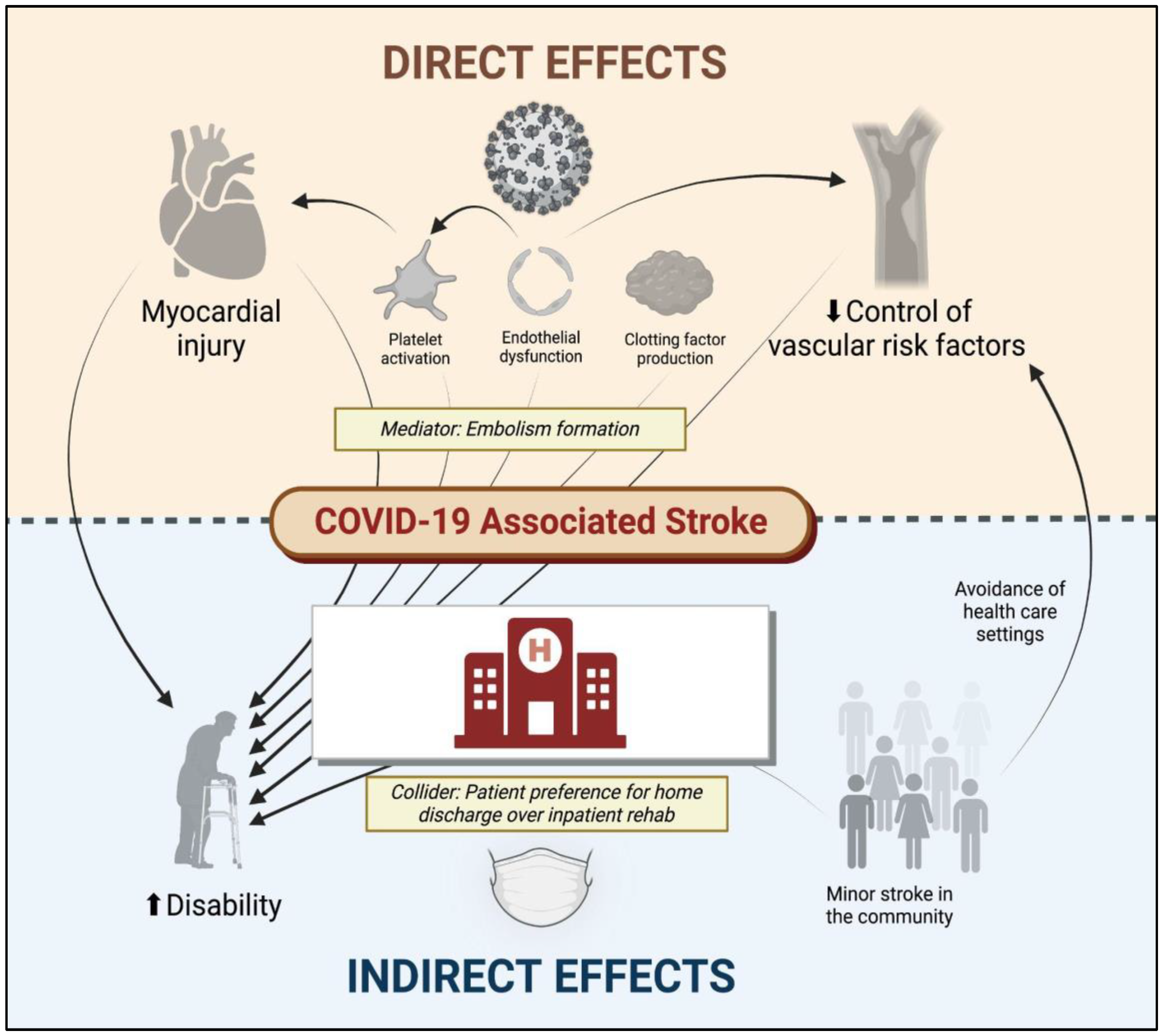
2. Direct and Indirect Relationships between COVID-19 and Cerebrovascular Disease
3. Cerebral Vein Thrombosis
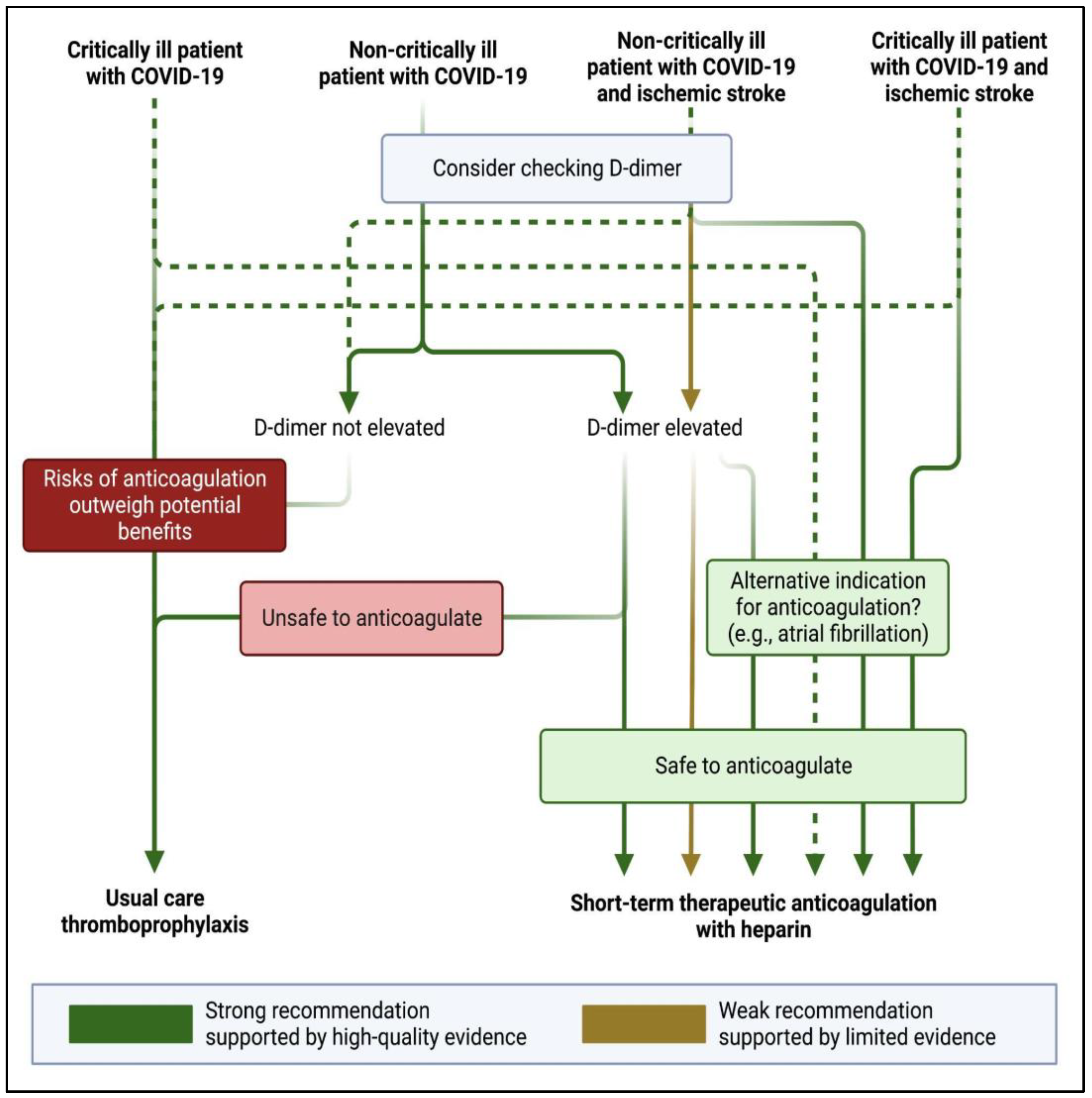
4. Antithrombotic Strategies
5. COVID-19 Impact on Stroke Systems of Care
6. Future Directions
Author Contributions
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegler JE, Abdalkader M, Michel P, Nguyen TN. Therapeutic Trends of Cerebrovascular Disease during the COVID-19 Pandemic and Future Perspectives. J. Stroke. 2022, 24, 179–188. [CrossRef]
- Rana A, Nguyen TN, Siegler JE. Stroke and neurointervention in the COVID-19 pandemic: a narrative review. Expert Rev. Med. Devices 2021, 18, 523–531. [CrossRef] [PubMed]
- Mathieu E, Ritchie H, Rodés-Guirao L, Appel C, Giattino C, Hasell J, Macdonald B, Dattani S, Beltekian D, Ortiz-Ospina E, et al. Coronavirus Pandemic (COVID-19). Our World in Data. 2020 [cited 2023 May 18];Available from: https://ourworldindata.org/covid-cases.
- WHO Coronavirus (COVID-19) dashboard. [cited 2023 Jun 13];Available from: https://covid19.who.int/.
- Nogueira RG, Qureshi MM, Abdalkader M, Martins SO, Yamagami H, Qiu Z, Mansour OY, Sathya A, Czlonkowska A, Tsivgoulis G, et al. Global Impact of COVID-19 on Stroke Care and IV Thrombolysis. Neurology 2021, 96, e2824–e2838.
- Nguyen TN, Qureshi MM, Klein P, Yamagami H, Mikulik R, Czlonkowska A, Abdalkader M, Sedova P, Sathya A, Lo HC, et al. Global Impact of the COVID-19 Pandemic on Stroke Volumes and Cerebrovascular Events: A 1-Year Follow-up. Neurology 2023, 100, e408–e421. [CrossRef] [PubMed]
- Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [CrossRef]
- Moghimi N, Di Napoli M, Biller J, Siegler JE, Shekhar R, McCullough LD, Harkins MS, Hong E, Alaouieh DA, Mansueto G, et al. The Neurological Manifestations of Post-Acute Sequelae of SARS-CoV-2 infection. Curr. Neurol. Neurosci. Rep. 2021, 21, 44.
- Menni C, Valdes AM, Polidori L, Antonelli M, Penamakuri S, Nogal A, Louca P, May A, Figueiredo JC, Hu C, et al. Symptom prevalence, duration, and risk of hospital admission in individuals infected with SARS-CoV-2 during periods of omicron and delta variant dominance: a prospective observational study from the ZOE COVID Study. Lancet 2022, 399, 1618–1624.
- Kläser K, Molteni E, Graham M, Canas LS, Österdahl MF, Antonelli M, Chen L, Deng J, Murray B, Kerfoot E, et al. COVID-19 due to the B.1.617.2 (Delta) variant compared to B.1.1.7 (Alpha) variant of SARS-CoV-2: a prospective observational cohort study. Sci. Rep. 2022, 12, 1–17.
- Collie S, Champion J, Moultrie H, Bekker L-G, Gray G. Effectiveness of BNT162b2 Vaccine against Omicron Variant in South Africa. N. Engl. J. Med. 2022, 386, 494–496. [CrossRef]
- Grau AJ, Urbanek C, Palm F. Common infections and the risk of stroke. Nat. Rev. Neurol. 2010, 6, 681–694. [CrossRef]
- Lindsberg PJ, Grau AJ. Inflammation and infections as risk factors for ischemic stroke. Stroke 2003, 34, 2518–2532. [CrossRef] [PubMed]
- Rasmussen LD, Engsig FN, Christensen H, Gerstoft J, Kronborg G, Pedersen C, Obel N. Risk of cerebrovascular events in persons with and without HIV: a Danish nationwide population-based cohort study. AIDS 2011, 25, 1637–1646. [CrossRef] [PubMed]
- Boehme AK, Luna J, Kulick ER, Kamel H, Elkind MSV. Influenza-like illness as a trigger for ischemic stroke. Ann. Clin. Transl. Neurol. 2018, 5, 456–463. [CrossRef]
- Merkler AE, Parikh NS, Mir S, Gupta A, Kamel H, Lin E, Lantos J, Schenck EJ, Goyal P, Bruce SS, et al. Risk of Ischemic Stroke in Patients With Coronavirus Disease 2019 (COVID-19) vs Patients With Influenza. JAMA Neurol. 2020. [CrossRef]
- Zhang S, Liu Y, Wang X, Yang L, Li H, Wang Y, Liu M, Zhao X, Xie Y, Yang Y, et al. SARS-CoV-2 binds platelet ACE2 to enhance thrombosis in COVID-19. J. Hematol. Oncol. 2020, 13, 120. [CrossRef]
- Varga Z, Flammer AJ, Steiger P, Haberecker M, Andermatt R, Zinkernagel AS, Mehra MR, Schuepbach RA, Ruschitzka F, Moch H. Endothelial cell infection and endotheliitis in COVID-19. Lancet 2020, 395, 1417–1418.
- Page EM, Ariëns RAS. Mechanisms of thrombosis and cardiovascular complications in COVID-19. Thromb. Res. 2021, 200, 1–8. [CrossRef]
- Sandoval Y, Januzzi JL Jr, Jaffe AS. Cardiac troponin for assessment of myocardial injury in COVID-19: JACC review topic of the week. J. Am. Coll. Cardiol. 2020, 76, 1244–1258. [CrossRef]
- Zhang Y, Xiao M, Zhang S, Xia P, Cao W, Jiang W, Chen H, Ding X, Zhao H, Zhang H, et al. Coagulopathy and Antiphospholipid Antibodies in Patients with Covid-19. N. Engl. J. Med. 2020, 382, e38.
- Rothstein A, Oldridge O, Schwennesen H, Do D, Cucchiara BL. Acute Cerebrovascular Events in Hospitalized COVID-19 Patients. Stroke 2020, 51, e219–e222.
- Harzallah I, Debliquis A, Drénou B. Lupus anticoagulant is frequent in patients with Covid-19. J. Thromb. Haemost. 2020, 18, 2064–2065. [CrossRef] [PubMed]
- Siegler JE, Cardona P, Arenillas JF, Talavera B, Guillen AN, Chavarría-Miranda A, de Lera M, Khandelwal P, Bach I, Patel P, et al. Cerebrovascular events and outcomes in hospitalized patients with COVID-19: The SVIN COVID-19 Multinational Registry. Int. J. Stroke 2021, 16, 437–447. [CrossRef] [PubMed]
- Lakomkin N, Dhamoon M, Carroll K, Singh IP, Tuhrim S, Lee J, Fifi JT, Mocco J. Prevalence of large vessel occlusion in patients presenting with acute ischemic stroke: a 10-year systematic review of the literature. J. Neurointerv. Surg. 2019, 11, 241–245. [CrossRef]
- Ramos-Araque ME, Siegler JE, Ribo M, Requena M, López C, de Lera M, Arenillas JF, Pérez IH, Gómez-Vicente B, Talavera B, et al. Stroke etiologies in patients with COVID-19: the SVIN COVID-19 multinational registry. BMC Neurol. 2021, 21, 43. [CrossRef]
- Siegler JE, Heslin ME, Thau L, Smith A, Jovin TG. Falling stroke rates during COVID-19 pandemic at a comprehensive stroke center. J. Stroke Cerebrovasc. Dis. 2020, 29, 104953. [CrossRef] [PubMed]
- Krist AH, DeVoe JE, Cheng A, Ehrlich T, Jones SM. Redesigning Primary Care to Address the COVID-19 Pandemic in the Midst of the Pandemic. Ann. Fam. Med. 2020, 18, 349–354. [CrossRef]
- Daugherty SE, Guo Y, Heath K, Dasmariñas MC, Jubilo KG, Samranvedhya J, Lipsitch M, Cohen K. Risk of clinical sequelae after the acute phase of SARS-CoV-2 infection: retrospective cohort study. BMJ 2021, 373, n1098.
- Nalbandian A, Sehgal K, Gupta A, Madhavan MV, McGroder C, Stevens JS, Cook JR, Nordvig AS, Shalev D, Sehrawat TS, et al. Post-acute COVID-19 syndrome. Nat. Med. 2021, 27, 601–615.
- Knight R, Walker V, Ip S, Cooper JA, Bolton T, Keene S, Denholm R, Akbari A, Abbasizanjani H, Torabi F, et al. Association of COVID-19 With Major Arterial and Venous Thrombotic Diseases: A Population-Wide Cohort Study of 48 Million Adults in England and Wales. Circulation 2022, 146, 892–906.
- Abdalkader M, Shaikh SP, Siegler JE, Cervantes-Arslanian AM, Tiu C, Radu RA, Tiu VE, Jillella DV, Mansour OY, Vera V, et al. Cerebral Venous Sinus Thrombosis in COVID-19 Patients: A Multicenter Study and Review of Literature. J. Stroke Cerebrovasc. Dis. 2021, 30, 105733. [CrossRef]
- Klein P, Shu L, Nguyen TN, Siegler JE, Omran SS, Simpkins AN, Heldner M, Havenon A de, Aparicio HJ, Abdalkader M, et al. Outcome Prediction in Cerebral Venous Thrombosis: The IN-REvASC Score. J. Stroke Cerebrovasc. Dis. 2022, 24, 404–416.
- Malas MB, Naazie IN, Elsayed N, Mathlouthi A, Marmor R, Clary B. Thromboembolism risk of COVID-19 is high and associated with a higher risk of mortality: A systematic review and meta-analysis. EClinicalMedicine 2020, 29, 100639.
- Otite FO, Patel S, Sharma R, Khandwala P, Desai D, Latorre JG, Akano EO, Anikpezie N, Izzy S, Malik AM, et al. Trends in incidence and epidemiologic characteristics of cerebral venous thrombosis in the United States. Neurology 2020, 95, e2200–e2213. [CrossRef] [PubMed]
- Siegler JE, Klein P, Yaghi S, Vigilante N, Abdalkader M, Coutinho JM, Abdul Khalek F, Nguyen TN. Cerebral Vein Thrombosis With Vaccine-Induced Immune Thrombotic Thrombocytopenia. Stroke 2021, 52, 3045–3053. [CrossRef]
- Dakay K, Cooper J, Bloomfield J, Overby P, Mayer SA, Nuoman R, Sahni R, Gulko E, Kaur G, Santarelli J, et al. Cerebral Venous Sinus Thrombosis in COVID-19 Infection: A Case Series and Review of The Literature. J. Stroke Cerebrovasc. Dis. 2021, 30, 105434. [CrossRef] [PubMed]
- McCullough-Hicks ME, Halterman DJ, Anderson D, Cohen K, Lakshminarayan K. High Incidence and Unique Features of Cerebral Venous Sinus Thrombosis in Hospitalized Patients With COVID-19 Infection. Stroke 2022, 53, e407–e410.
- Baldini T, Asioli GM, Romoli M, Carvalho Dias M, Schulte EC, Hauer L, Aguiar De Sousa D, Sellner J, Zini A. Cerebral venous thrombosis and severe acute respiratory syndrome coronavirus-2 infection: A systematic review and meta-analysis. Eur. J. Neurol. 2021. [CrossRef]
- Hinduja A, Nalleballe K, Onteddu S, Kovvuru S, Hussein O. Impact of cerebral venous sinus thrombosis associated with COVID-19. J. Neurol. Sci. 2021, 425, 117448. [CrossRef]
- Nguyen TN, Qureshi MM, Klein P, Yamagami H, Abdalkader M, Mikulik R, Sathya A, Mansour OY, Czlonkowska A, Lo H, et al. Global Impact of the COVID-19 Pandemic on Cerebral Venous Thrombosis and Mortality. J. Stroke Cerebrovasc. Dis. 2022, 24, 256–265.
- Tu TM, Yi SJ, Koh JS, Saffari SE, Hoe RHM, Chen GJ, Chiew HJ, Tham CH, Seet CYH, Yong MH, et al. Incidence of Cerebral Venous Thrombosis Following SARS-CoV-2 Infection vs mRNA SARS-CoV-2 Vaccination in Singapore. JAMA Netw Open 2022, 5, e222940. [CrossRef] [PubMed]
- Sánchez van Kammen M, Aguiar de Sousa D, Poli S, Cordonnier C, Heldner MR, van de Munckhof A, Krzywicka K, van Haaps T, Ciccone A, Middeldorp S, et al. Characteristics and Outcomes of Patients With Cerebral Venous Sinus Thrombosis in SARS-CoV-2 Vaccine-Induced Immune Thrombotic Thrombocytopenia. JAMA Neurol. 2021, 78, 1314–1323.
- Greinacher A, Thiele T, Warkentin TE, Weisser K, Kyrle PA, Eichinger S. Thrombotic Thrombocytopenia after ChAdOx1 nCov-19 Vaccination. N. Engl. J. Med. 2021. [CrossRef]
- Nicholson M, Goubran H, Chan N, Siegal D. No apparent association between mRNA COVID-19 vaccination and venous thromboembolism. Blood Rev. 2022, 56, 100970. [CrossRef] [PubMed]
- Bikdeli B, Talasaz AH, Rashidi F, Bakhshandeh H, Rafiee F, Rezaeifar P, Baghizadeh E, Matin S, Jamalkhani S, Tahamtan O, et al. Intermediate-Dose versus Standard-Dose Prophylactic Anticoagulation in Patients with COVID-19 Admitted to the Intensive Care Unit: 90-Day Results from the INSPIRATION Randomized Trial. Thromb. Haemost. 2022, 122, 131–141.
- Buijsers B, Yanginlar C, Maciej-Hulme ML, de Mast Q, van der Vlag J. Beneficial non-anticoagulant mechanisms underlying heparin treatment of COVID-19 patients. EBioMedicine 2020, 59, 102969. [CrossRef]
- Sholzberg M, Tang GH, Rahhal H, AlHamzah M, Kreuziger LB, Áinle FN, Alomran F, Alayed K, Alsheef M, AlSumait F, et al. Effectiveness of therapeutic heparin versus prophylactic heparin on death, mechanical ventilation, or intensive care unit admission in moderately ill patients with covid-19 admitted to hospital: RAPID randomised clinical trial. BMJ 2021, 375, n2400.
- Spyropoulos AC, Goldin M, Giannis D, Diab W, Wang J, Khanijo S, Mignatti A, Gianos E, Cohen M, Sharifova G, et al. Efficacy and Safety of Therapeutic-Dose Heparin vs Standard Prophylactic or Intermediate-Dose Heparins for Thromboprophylaxis in High-risk Hospitalized Patients With COVID-19: The HEP-COVID Randomized Clinical Trial. JAMA Intern. Med. 2021, 181, 1612–1620.
- Therapeutic Anticoagulation with Heparin in Noncritically Ill Patients with Covid-19. N. Engl. J. Med. 2021, 385, 790–802. [CrossRef] [PubMed]
- Stone GW, Farkouh ME, Lala A, Tinuoye E, Dressler O, Moreno PR, Palacios IF, Goodman SG, Esper RB, Abizaid A, et al. Randomized Trial of Anticoagulation Strategies for Noncritically Ill Patients Hospitalized With COVID-19. J. Am. Coll. Cardiol. 2023, 81, 1747–1762.
- Sholzberg M, da Costa BR, Tang GH, Rahhal H, AlHamzah M, Baumann Kreuziger L, Ní Áinle F, Almarshoodi MO, James PD, Lillicrap D, et al. Randomized trials of therapeutic heparin for COVID-19: A meta-analysis. Res Pract Thromb Haemost 2021, 5, e12638.
- Simonetto M, Wechsler PM, Merkler AE. Stroke Treatment in the Era of COVID-19: a Review. Curr. Treat. Options Neurol. 2022, 24, 155–171. [CrossRef] [PubMed]
- Cappellari M, Zini A, Sangalli D, Cavallini A, Reggiani M, Sepe FN, Rifino N, Giussani G, Guidetti D, Zedde M, et al. Thrombolysis and bridging therapy in patients with acute ischaemic stroke and Covid-19. Eur. J. Neurol. 2020, 27, 2641–2645. [CrossRef] [PubMed]
- Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J, Wang Y, Song B, Gu X, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 2020, 395, 1054–1062. [CrossRef] [PubMed]
- Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, Wang B, Xiang H, Cheng Z, Xiong Y, et al. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA 2020, 323, 1061–1069. [CrossRef] [PubMed]
- Kleindorfer DO, Towfighi A, Chaturvedi S, Cockroft KM, Gutierrez J, Lombardi-Hill D, Kamel H, Kernan WN, Kittner SJ, Leira EC, et al. 2021 Guideline for the Prevention of Stroke in Patients With Stroke and Transient Ischemic Attack: A Guideline From the American Heart Association/American Stroke Association. Stroke 2021, 52, e364–e467.
- Yamakawa M, Kuno T, Mikami T, Takagi H, Gronseth G. Clinical Characteristics of Stroke with COVID-19: A Systematic Review and Meta-Analysis. J. Stroke Cerebrovasc. Dis. 2020, 29, 105288. [CrossRef]
- Lang CN, Dettinger JS, Berchtold-Herz M, Utzolino S, Bemtgen X, Zotzmann V, Schmid B, Biever PM, Bode C, Müller-Peltzer K, et al. Correction to: Intracerebral Hemorrhage in COVID-19 Patients with Pulmonary Failure: A Propensity Score-Matched Registry Study. Neurocrit. Care 2021, 34, 1112. [CrossRef]
- Marto JP, Strambo D, Ntaios G, Nguyen TN, Herzig R, Czlonkowska A, Demeestere J, Mansour OY, Salerno A, Wegener S, et al. Safety and Outcome of Revascularization Treatment in Patients With Acute Ischemic Stroke and COVID-19: The Global COVID-19 Stroke Registry. Neurology 2023, 100, e739–e750.
- Sasanejad P, Afshar Hezarkhani L, Arsang-Jang S, Tsivgoulis G, Ghoreishi A, Barlinn K, Rahmig J, Farhoudi M, Sadeghi Hokmabadi E, Borhani-Haghighi A, et al. Safety and Outcomes of Intravenous Thrombolytic Therapy in Ischemic Stroke Patients with COVID-19: CASCADE Initiative. J. Stroke Cerebrovasc. Dis. 2021, 30, 106121.
- Dmytriw AA, Dibas M, Phan K, Efendizade A, Ospel J, Schirmer C, Settecase F, Heran MKS, Kühn AL, Puri AS, et al. Acute ischaemic stroke associated with SARS-CoV-2 infection in North America. J. Neurol. Neurosurg. Psychiatry 2022, 93, 360–368. [CrossRef]
- Nguyen TN, Abdalkader M, Jovin TG, Nogueira RG. Mechanical thrombectomy in the era of the COVID-19 pandemic: emergency preparedness for neuroscience teams: a guidance statement from the Society of vascular …. Stroke Available from: https://www.ahajournals.org/doi/abs/10.1161/STROKEAHA.120.030100. 2020.
- Jabbour P, Dmytriw AA, Sweid A, Piotin M, Bekelis K, Sourour N, Raz E, Linfante I, Dabus G, Kole M, et al. Characteristics of a COVID-19 Cohort With Large Vessel Occlusion: A Multicenter International Study. Neurosurgery 2022, 90, 725–733. [CrossRef]
- Siegler JE, Heslin ME, Thau L, Smith A. Falling stroke rates during COVID-19 pandemic at a Comprehensive Stroke Center: Cover title: Falling stroke rates during COVID-19. J. Stroke Cerebrovasc. Dis. Available from: https://www.sciencedirect.com/science/article/pii/S105230572030361X?casa_token=yXSRnHGlRDsAAAAA:mBl3lMpc1HjgqUiSzc4APwrhkY21fiZjCKEzuNVcWA3s76EPwUjET1RoNRki8hZFBMhxPKhCHuM. 2020.
- SVIN COVID-19 Global SAH Registry. Global impact of the COVID-19 pandemic on subarachnoid haemorrhage hospitalisations, aneurysm treatment and in-hospital mortality: 1-year follow-up. J. Neurol. Neurosurg. Psychiatry Available from: http://dx.doi.org/10.1136/jnnp-2022-329200. 2022. [CrossRef]
- Nguyen TN, Haussen DC, Qureshi MM, Yamagami H, Fujinaka T, Mansour OY, Abdalkader M, Frankel M, Qiu Z, Taylor A, et al. Decline in subarachnoid haemorrhage volumes associated with the first wave of the COVID-19 pandemic. Stroke Vasc Neurol 2021, 6, 542–552. [CrossRef] [PubMed]
- Solomon MD, McNulty EJ, Rana JS, Leong TK, Lee C, Sung S-H, Ambrosy AP, Sidney S, Go AS. The Covid-19 Pandemic and the Incidence of Acute Myocardial Infarction. N. Engl. J. Med. 2020, 383, 691–693.
- 69. Nogueira RG, Etter K, Nguyen TN, Ikeme S, Wong C, Frankel M, Haussen DC, Del Rio C, McDaniel M, Sachdeva R, et al. Changes in the care of acute cerebrovascular and cardiovascular conditions during the first year of the covid-19 pandemic in 746 hospitals in the USA: retrospective analysis. BMJ Med 2023, 2, e000207.
- Rosenthal MG, Fakhry SM, Morse JL, Wyse RJ, Garland JM, Duane TM, Slivinski A, Wilson NY, Watts DD, Shen Y, et al. Where did all the appendicitis go? Impact of the COVID-19 pandemic on volume, management, and outcomes of acute appendicitis in a nationwide, multicenter analysis. Ann. Surg. Open 2021, 2, e048.
- Gale R, Eberlein S, Fuller G, Khalil C, Almario CV, Spiegel BMR. Public Perspectives on Decisions About Emergency Care Seeking for Care Unrelated to COVID-19 During the COVID-19 Pandemic. JAMA Netw Open 2021, 4, e2120940. [CrossRef]
- Zhao J, Li H, Kung D, Fisher M, Shen Y, Liu R. Impact of the COVID-19 Epidemic on Stroke Care and Potential Solutions. Stroke Available from: https://www.ahajournals.org/doi/abs/10.1161/STROKEAHA.120.030225. 2020.
- Czeisler MÉ, Marynak K, Clarke KEN, Salah Z, Shakya I, Thierry JM, Ali N, McMillan H, Wiley JF, Weaver MD, et al. Delay or Avoidance of Medical Care Because of COVID-19-Related Concerns - United States, June 2020. MMWR Morb. Mortal. Wkly. Rep. 2020, 69, 1250–1257. [CrossRef] [PubMed]
- Olsen SJ, Winn AK, Budd AP, Prill MM, Steel J, Midgley CM, Kniss K, Burns E, Rowe T, Foust A, et al. Changes in Influenza and Other Respiratory Virus Activity During the COVID-19 Pandemic - United States, 2020-2021. MMWR Morb. Mortal. Wkly. Rep. 2021, 70, 1013–1019. [CrossRef] [PubMed]
- Cassell K, Zipfel CM, Bansal S, Weinberger DM. Trends in non-COVID-19 hospitalizations prior to and during the COVID-19 pandemic period, United States, 2017–2021. Nat. Commun. 2022, 13, 1–8.
- Hartnett KP, Kite-Powell A, DeVies J, Coletta MA, Boehmer TK, Adjemian J, Gundlapalli AV, National Syndromic Surveillance Program Community of Practice. Impact of the COVID-19 Pandemic on Emergency Department Visits - United States, January 1, 2019-May 30, 2020. MMWR Morb. Mortal. Wkly. Rep. 2020, 69, 699–704.
- Siegler JE, Zha AM, Czap AL, Ortega-Gutierrez S, Farooqui M, Liebeskind DS, Desai SM, Hassan AE, Starosciak AK, Linfante I, et al. Influence of the COVID-19 Pandemic on Treatment Times for Acute Ischemic Stroke: The Society of Vascular and Interventional Neurology Multicenter Collaboration. Stroke 2020, STROKEAHA120032789.
- Alonso de Leciñana M, Castellanos M, Ayo-Martín Ó, Morales A, Stroke Group - Spanish Society of Neurology. Stroke care during the COVID-19 outbreak in Spain: the experience of Spanish stroke units. Stroke Vasc Neurol 2021, 6, 267–273. [CrossRef] [PubMed]
- Rivera R, Amudio C, Cruz JP, Brunetti E, Catalan P, Sordo JG, Echeverria D, Badilla L, Chamorro A, Gonzalez C, et al. The impact of a two-year long COVID-19 public health restriction program on mechanical thrombectomy outcomes in a stroke network. J. Stroke Cerebrovasc. Dis. 2023, 32, 107138. [CrossRef] [PubMed]
- Jillella DV, Nahab F, Nguyen TN, Abdalkader M, Liebeskind DS, Vora N, Rai V, Haussen DC, Nogueira RG, Desai S, et al. Delays in thrombolysis during COVID-19 are associated with worse neurological outcomes: the Society of Vascular and Interventional Neurology Multicenter Collaboration. J. Neurol. 2022, 269, 603–608.
- Khandelwal P, Al-Mufti F, Tiwari A, Singla A, Dmytriw AA, Piano M, Quilici L, Pero G, Renieri L, Limbucci N, et al. Incidence, Characteristics and Outcomes of Large Vessel Stroke in COVID-19 Cohort: An International Multicenter Study. Neurosurgery 2021, 89, E35–E41. [CrossRef]
- Czap AL, Zha AM, Sebaugh J, Hassan AE, Shulman JG, Abdalkader M, Nguyen TN, Linfante I, Starosciak AK, Ortega-Gutierrez S, et al. Endovascular thrombectomy time metrics in the era of COVID-19: observations from the Society of Vascular and Interventional Neurology Multicenter Collaboration. J. Neurointerv. Surg. 2022, 14, neurintsurg–2020–017205.
- Raymaekers V, Demeestere J, Bellante F, De Blauwe S, De Raedt S, Dusart A, Jodaitis L, Lemmens R, Loos C, Noémie L, et al. The impact of COVID-19 on acute stroke care in Belgium. Acta Neurol. Belg. 2021, 121, 1251–1258.
- Stroke Council Leadership A. Temporary Emergency Guidance to US Stroke Centers During the Coronavirus Disease 2019 (COVID-19) Pandemic: On Behalf of the American Heart Association …. Stroke. 2020;Available from: https://www.ahajournals.org/doi/abs/10.1161/STROKEAHA.120.030023?casa_token=-gS7ZSAmrEQAAAAA:gLTFfX0gdo0YH3A2aoiUnMsrSBsKplk3HPGqYOTpgcBfEpc2akSNLyDjW6Sw884P5IWhFU3SzxjJbnY. g: from: https://www.ahajournals.org/doi/abs/10.1161/STROKEAHA.120.030023?casa_token=-gS7ZSAmrEQAAAAA, 1161.
- Nguyen TN, Jadhav AP, Dasenbrock HH, Nogueira RG, Abdalkader M, Ma A, Cervantes-Arslanian AM, Greer DM, Daneshmand A, Yavagal DR, et al. Subarachnoid hemorrhage guidance in the era of the COVID-19 pandemic - An opinion to mitigate exposure and conserve personal protective equipment. J. Stroke Cerebrovasc. Dis. 2020, 29, 105010.
- Abdalkader M, Sathya A, Malek AM, Fifi JT, Norbash AM, Wakhloo AK, Nguyen TN. Roadmap for Resuming Elective Neuroendovascular Procedures Following the First COVID-19 Surge. J. Stroke Cerebrovasc. Dis. 2020, 29, 105177. [CrossRef] [PubMed]
- Nguyen TN, Qureshi MM, Klein P, Yamagami H, Mikulik R, Etminan N, Abdalkader M, Mansour OY, Czlonkowska A, Lo H, et al. Global impact of the COVID-19 pandemic on subarachnoid haemorrhage hospitalisations, aneurysm treatment and in-hospital mortality: 1-year follow-up. J. Neurol. Neurosurg. Psychiatry Available from: https://scholarworks.bwise.kr/skku/handle/2021.sw.skku/98710. 2022.
- Thau L, Siegal T, Heslin ME, Rana A, Yu S, Kamen S, Chen A, Vigilante N, Gallagher S, Wegner K, et al. Decline in Rehab Transfers Among Rehab-Eligible Stroke Patients During the COVID-19 Pandemic. J. Stroke Cerebrovasc. Dis. 2021, 30, 105857. [CrossRef] [PubMed]
- Ortega-Gutierrez S, Farooqui M, Zha A, Czap A, Sebaugh J, Desai S, Jadhav A, Vora N, Rai V, Jovin TG, et al. Decline in mild stroke presentations and intravenous thrombolysis during the COVID-19 pandemic: the Society of vascular and Interventional Neurology multicenter collaboration. Clin. Neurol. Neurosurg. 2021, 201, 106436.
- Roberts P, Wertheimer J, Park E, Nuño M, Riggs R. Identification of Functional Limitations and Discharge Destination in Patients With COVID-19. Arch. Phys. Med. Rehabil. 2021, 102, 351–358. [CrossRef]
- Maltser S, Trovato E, Fusco HN, Sison CP, Ambrose AF, Herrera J, Murphy S, Kirshblum S, Bartels MN, Bagay L, et al. Challenges and Lessons Learned for Acute Inpatient Rehabilitation of Persons With COVID-19: Clinical Presentation, Assessment, Needs, and Services Utilization. Am. J. Phys. Med. Rehabil. 2021, 100, 1115–1123.
- Spanko, A. Nursing home industry projects $34B in revenue losses, 1,800 closures or mergers due to COVID. Skilled Nursing News. 2021 [cited 2023 May 1];Available from: https://skillednursingnews.com/2021/02/nursing-home-industry-projects-34b-in-revenue-losses-1800-closures-or-mergers-due-to-covid/. 1 May.
- Hubert GJ, Corea F, Schlachetzki F. The role of telemedicine in acute stroke treatment in times of pandemic. Curr. Opin. Neurol. 2021, 34, 22–26. [CrossRef]
- Miller A, Segan S, Rehmani R, Shabsigh R, Rahme R. Letter: Dismantling the Apocalypse Narrative: The Myth of the COVID-19 Stroke. Neurosurgery 2020, 87, E703–E704. [CrossRef]
- Chavda VP, Chen Y, Dave J, Chen Z-S, Chauhan SC, Yallapu MM, Uversky VN, Bezbaruah R, Patel S, Apostolopoulos V. COVID-19 and vaccination: myths vs science. Expert Rev. Vaccines 2022, 21, 1603–1620.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




