1. INTRODUCTION
A sensor is a detection device that can sense the measured information and convert the sensed information into electrical signals or other required forms of information output according to certain rules to meet the requirements of information transmission, processing, storage, display, recording, and control. Intelligent sensor is equipped with microprocessor and has the ability to collect, process, and exchange information. It is the product of the combination of sensor integration and microprocessor. Compared with general sensor, intelligent sensor has the following three advantages: High-precision information collection can be achieved through software technology; The cost is low; Having certain programming automation capabilities; Diversified functions. Regardless of the type of sensor, the electrical signal output by their conversion elements is generally very weak and accompanied by noise. Therefore, before signal acquisition, a signal conditioning circuit is required to amplify and filter the signal [
1,
2,
3,
4,
5,
6,
7,
8]. In summary, the basic working principle of intelligent sensor is that the sensing element makes the measured signal complete the conversion from the non-electrical domain to the electrical domain. The interface circuit further processes the electrical signal to produce an output consistent with the use of a measurement or control system. Ultimately, the output electrical signal becomes digital signal after analog-to-digital conversion, and the control system resolves the state of the measured quantity from the digital signal and visualizes it through the communication interface or uses it directly for feedback regulation.
In summary, the construction of intelligent sensor requires three elements: sensitive components, interface circuits, and communication interfaces. Since microprocessors are already mentioned in the definition of intelligent sensor, they are not listed here as necessary elements. As the medium of signal type conversion, the selection of sensitive elements is particularly important, which directly affects many performance parameters of the whole sensor, such as signal-to-noise ratio, resolving power, accuracy, etc. Typically, the sensitive element is more dependent on the environment. Changes in temperature, humidity, light intensity and other environmental factors may cause instability of the sensitive element[
9], so most sensors need to be calibrated before working. The self-calibration function of intelligent sensors makes them more widely used than ordinary sensors. It is necessary to emphasize that the calibration of a sensor relies on a known calibration signal and the calibration process will inevitably generate errors that affect sensor accuracy [
10]. Nevertheless, the sensor still needs to be calibrated after a certain period of time.
Interface circuits are often defined as processing circuits for the output signals of sensitive components, which include but are not limited to: impedance conversion circuit, amplifier circuit, current-voltage conversion circuit,bridge circuit, frequency-voltage conversion circuit, charge amplifier circuit,filter circuit, etc. The amplifier circuit and filter circuit are the basic interface circuits for many sensors; the current to voltage conversion circuit is mainly used in current type sensors, and the transconductance amplifier can play a conversion role in this circuit. When selecting a transimpedance amplifier, pay attention to its various performance parameters. Please refer to the amplifier selection manual for details; the frequency-voltage conversion circuit builds a linear relationship between input frequency and output voltage. The main functions of interface circuits are shown in
Table 1.
The current trend in smart sensor design is to digitize the analog signal output from the sensor as soon as possible, and then conduct signal conditioning from the software perspective, such as filtering, linearization, cross sensitivity compensation, etc., which reduces the difficulty of the sensor hardware circuit, enhances the flexibility of signal processing, and greatly avoids signal attenuation and interference in circuit transmission. In practical applications, it is extremely common to replace some functions of hardware circuits with algorithms. This method can achieve rapid interconnection of sensors and intelligent control of systems, which is in line with the rapid development trend of integrated circuits and digital signal processing. Commonly used communication interfaces for smart sensors are Serial Peripheral Interface (SPI), Inter-Integrated Circuit (IIC), Universal Synchronous Asynchronous Receiver Transmitter (USART), etc. In addition to communication interfaces, intelligent sensors should also have high-speed data processing capabilities, visual control terminals, and excellent human-computer interaction interfaces [
11,
12,
13,
14,
15]. Due to the above advantages, smart phones are widely used as terminals for intelligent sensors, effectively developing highly sensitive portable sensor detection systems.
2. CALIBRATION
Calibration is the process of experimentally establishing the correspondence between the output and input of a sensor under specified measurement conditions. If some indicators of the sensor have changed due to the external environment or it has been some time since the last calibration, the sensor needs to be recalibrated. Sensor is typically calibrated once in the factory, and then recalibrated based on user needs. Calibration is a necessary prerequisite for the normal operation of all sensors, as it is the only means for the sensor to obtain calibration equations, and also determines whether the sensor can accurately output analog signals.
2.1. Introduction to calibration methods
The basic method of calibration is to input a known signal to the sensor and obtain the output signal at the same time, thus obtaining a series of curves characterizing the correspondence between the two. The final measured results of the sensor performance indicators are obtained. Of all the calibration results the linear relationship is undoubtedly the simplest and most conducive to sensor calibration. When the calibration result is non-linear, a reasonable data processing method is usually selected to linearize it. When calibrating sensors, the accuracy of the measuring equipment used is usually an order of magnitude higher than that of the sensor to be calibrated for error minimization in calibration. Specifically for a piezoelectric pressure sensor, a piston manometer is used to generate a standard force of known magnitude on the sensor, which will output a corresponding charge signal, which is then measured by a standard detection device of known accuracy to obtain the magnitude of the charge signal, resulting in a set of input-output relationships. The relationship can generally be described by an equation whose parameters are referred to as calibration factors. Such a series of processes is the calibration process for piezoelectric pressure sensors.
Self-calibration means that the output value is traced back to the value reproduced by the standard according to the chain of calibration after the calibration signal has been fed into the sensor. This requires the integration of the corresponding calibration signal source as well as the calibration control circuit or algorithm in the sensing system. If the calibration signal source cannot be integrated into the system, it can only be obtained from an external source, which improves calibration accuracy but is less convenient. The calibration control circuit includes cross-sensitivity compensation, differential sensing, background calibration, etc. In summary, the self calibration of intelligent sensor is actually a process of automatically collaborating with microcontroller and actuator to calibrate sensor components. Although self-calibration cannot completely take the place of an actual calibration, it may reduce the number of calibration points required for a given accuracy condition or extend the time between calibrations. The information storage capacity of intelligent sensor is the basis for successfully accessing the chain of calibration during self-calibration. The intelligent sensor saves calibration data in an Electrically Erasable Programmable Read-Only Memory (EEPROM) during calibration, which can be recalled by the microprocessor at any time when it is needed. EEPROM is generally a separate integrated chip that can be used "plug-and-play" on the same sensor, which means that the relevant calibration data does not need to be updated separately when replacing or recalibrating the same intelligent sensor.
2.2. Self-Calibration by Combining Multiple Sensors
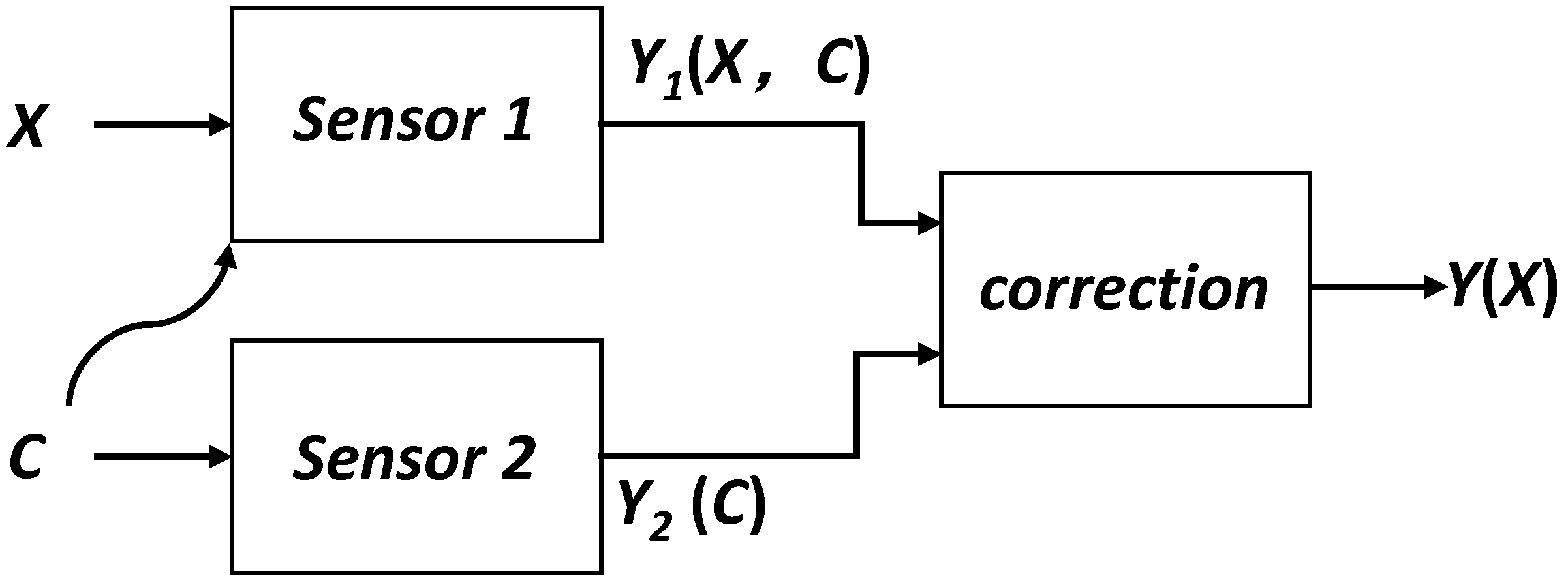
Figure 1 shows the principle of cross-sensitivity compensation, where both the interference signal C and the signal to be measured X can cause a change in the output signal of sensor 1. In order to eliminate the influence of the interference signal C, the cross-sensitivity is compensated for by the detection of the interference signal C by sensor 2. The effectiveness of this method depends on the reproducibility of the cross-sensitivity of sensor 1 to the interfering signal C [
16]. If this cross-sensitivity is highly variable over time, then the improvements that can be obtained may be limited. Where sensors have a defined cross-sensitivity, the addition of sensors can significantly improve overall performance.
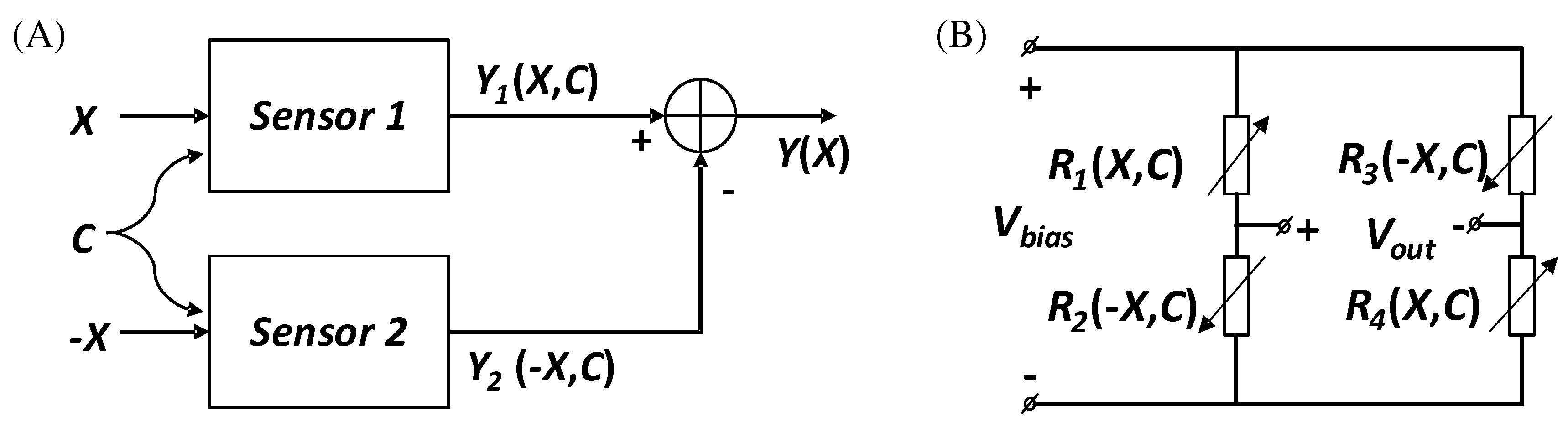
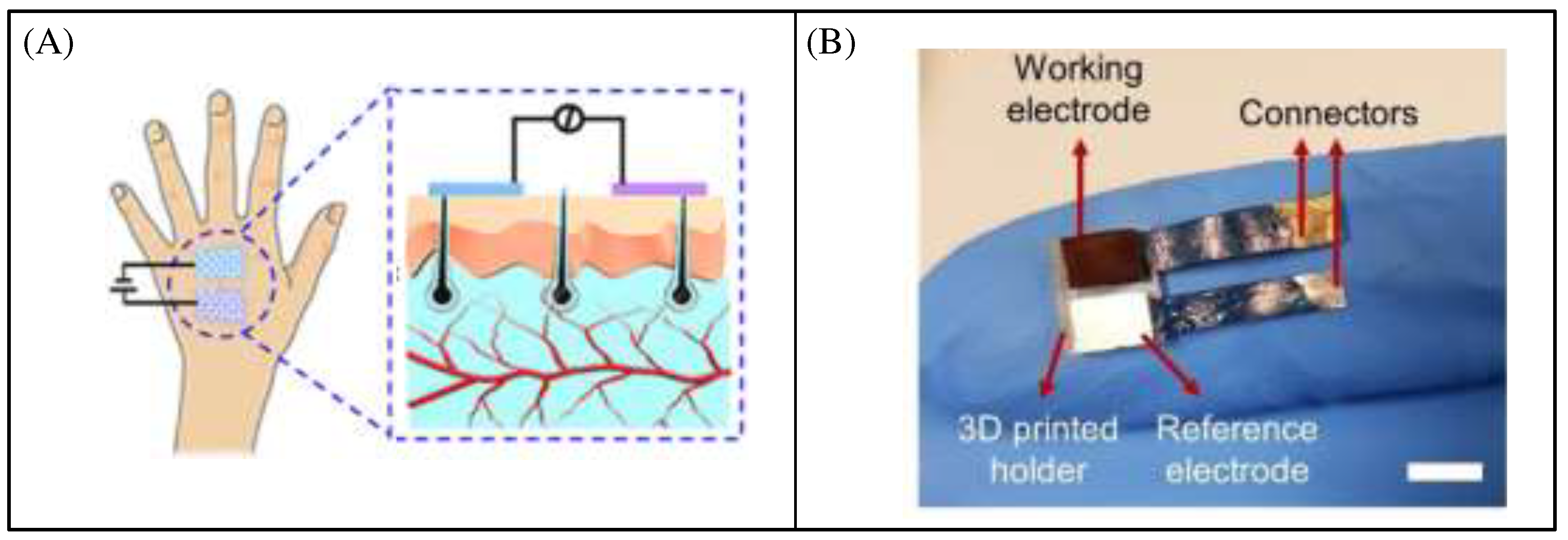
Differential sensing in which two identical sensors are used to measure two signals of the same magnitude and opposite phase that are to be measured (
Figure 2A). As the interfering signal C (e.g. external factors such as temperature) acts on both sensors simultaneously, the resulting errors are cancelled out in the subsequent calculation.
Figure 2B shows an example of a typical differential sensing circuit called the Wheatstone full-bridge [
17]. Where R1 and R2 are shown as a set of strain gauges placed perpendicular to each other, as are R3 and R4. Obviously, the changes caused by temperature on the four strain gauges should be the same and the absolute value of the change in resistance of the four strain gauges should be the same when the object is deformed. A simple calculation shows that the final output voltage Vout is proportional to the absolute value of the change in resistance [
18]. There is no non-linear error and the sensitivity is numerically equal to the supply voltage Vbias.
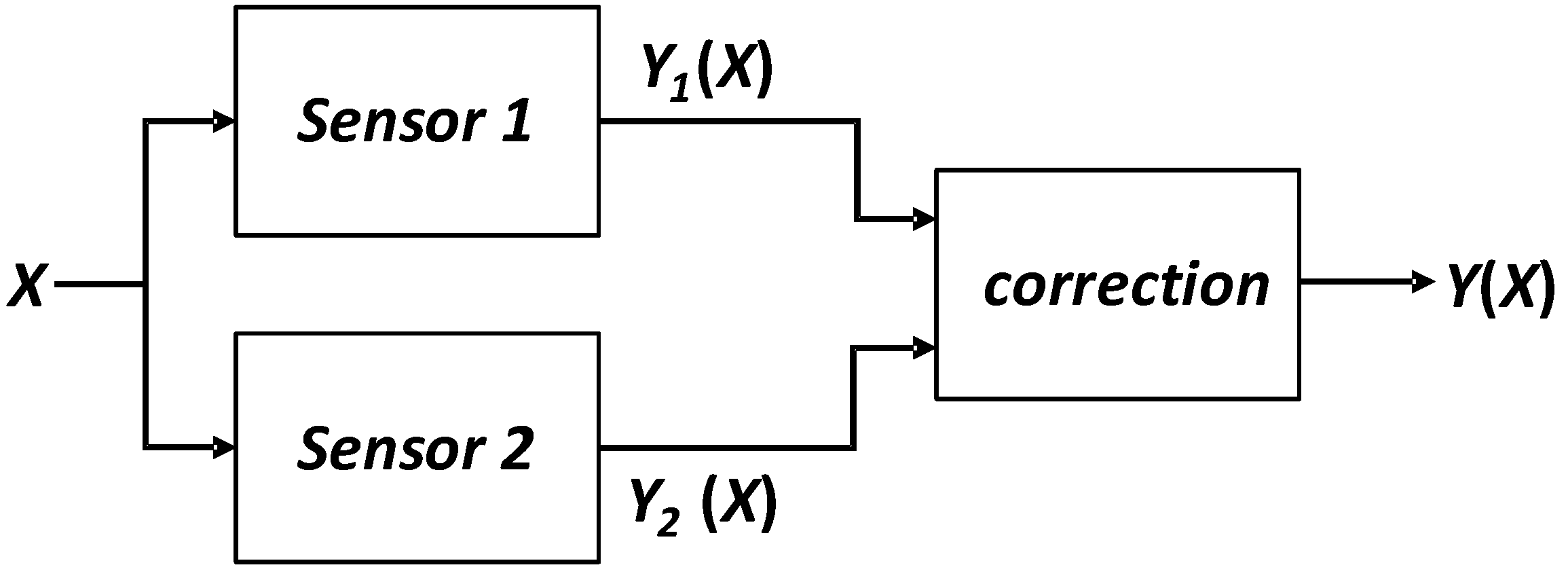
Background calibration is the use of two different sensors to measure the same signal, as shown in
Figure 3 The two different sensors have different characteristics, for example one of them is more accurate but has a slow response time, the other one is not very accurate but has a fast response time. In this case, the system often compares the data from the two sensors and corrects the output of the sensor with the faster response. It is obvious that the combination of these two sensors produces a fast and accurate measurement system.
3. INTELLIGENT PHYSICAL SENSOR
Intelligent physical sensors convert the state of the object being measured into an electrical signal that can be recognized by certain physical effects. This paper focuses on the medical applications of Humidity sensor, Temperature sensor and Strain sensor when used as wearable sensors.
3.1. Humidity sensor
One effective strategy for measuring respiratory humidity is to use ionic conductivity to charactesise changes in humidity. MXene nanosheets can be used as sensing elements in humidity sensors due to their high conductivity, high surface area, and hydrophilicity [
19,
20]. The various functional end groups of MXene nanosheets (such as -OH, -O and -F) make them highly sensitive to the adsorption of water molecules. With the adsorption of water molecules the tunneling distance of MXene nanosheets increases, which ultimately leads to a decrease in ionic conductivity.
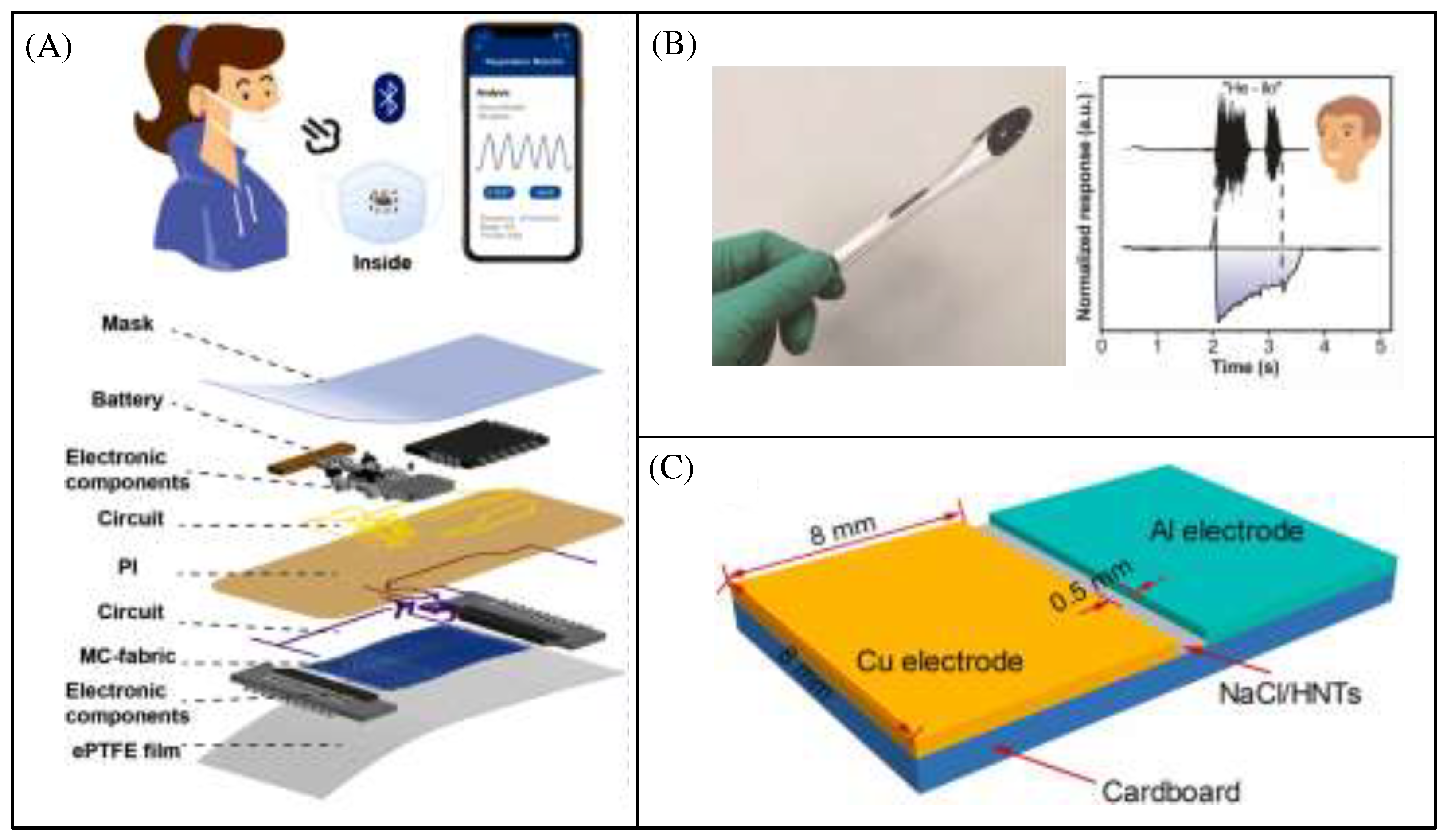
Figure 4A shows the MXene based humidity sensor being integrated into the breathing mask, which transmits data to the cell phone side via Bluetooth[
21]. This humidity sensor can clearly identify different breathing patterns, including normal breathing (12 to 20 breaths/minute), rapid breathing (>24 breaths/minute), slow deep breathing (<12 breaths/minute), and apnea.
Figure 4B depicts a humidity sensor based on SA-MXene composite material, which has enhanced resistance oxidation and sensitivity[
22]. In addition, SA-MXene is made into ink and has been proven suitable for screen printing, which is extremely convenient for the manufacture of flexible sensors.
Figure 4C shows a humidity sensor based on NaCl/HNT, which uses cardboard with high thickness and stability as the substrate to form a planar electrolytic cell structure[
23]. The characterization and humidity sensing test results show that the sensor has a wide humidity sensing range and high response.
3.2. Temperature sensor
The sensitive element used in temperature sensors is the thermistor, which is a resistor whose resistivity changes significantly with temperature and is divided into positive temperature coefficient (PTC) thermistors and negative temperature coefficient (NTC) thermistors. NTC thermistors are widely used because of their own advantages such as high measurement accuracy, interchangeability and reliability. Conductive polymer composites (CPCs) are ideal for making flexible temperature sensors due to their flexibility, good processability and lightweight. However, manufacturing a flexible CPC-based temperature sensor with a linear NTC effect remains a challenge, as CPCs typically exhibit non-monotonicity between resistance and temperature. Most critically, flexible materials can cause temperature measurement errors when they undergo bending deformation.
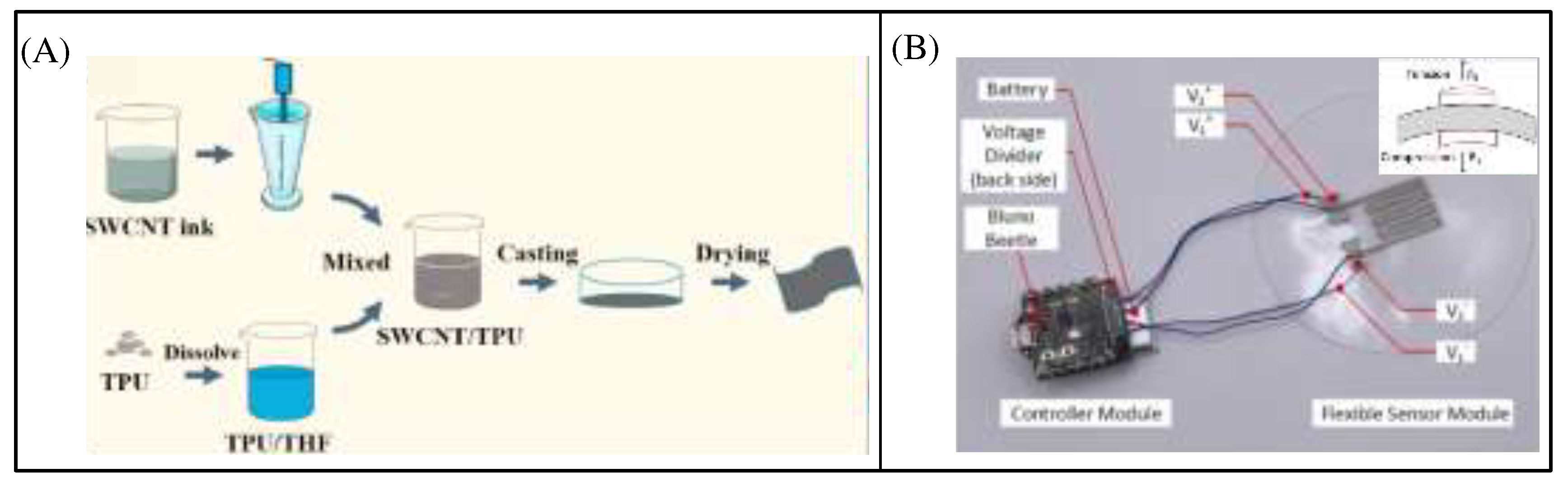
Zhu et al. prepared the flexible thermoplastic polyurethane (TPU)/single-walled carbon nanotubes (SWCNTs) composites that exhibit a monotonic and linear NTC effect over a temperature range of 30-100 °C (
Figure 5A), which can be designed as highly flexible and sensitive temperature sensors. However, measurement errors due to deformation of flexible materials cannot be avoided[
24].
Figure 5B shows a sensing system that uses two identical resistive temperature sensors using the principle of differential sensing to compensate for changes in resistance due to deformation and measure temperature with minimal error[
25]. When the sensor is bent on a curved surface, the resistance of R1 increases due to tension and the resistance of R2 decreases due to compression. The sum of resistance can reduce the effect of bending and the remaining errors can be minimized by calibration.
3.3. Strain sensor
Most conventional strain sensors are based on metal and semiconductor materials, which are less portable, flexible and wearable. The current mainstream strain sensors are based on flexible materials to construct flexible strain sensors, such as polydimethylsiloxane (PDMS), polyethylene terephthalate (PET), polyimide (PI), polyethylene (PE) and polyurethane (PU). The development of dielectric materials with higher dielectric constants, lower leakage currents, higher dipole densities, higher current densities, higher energy densities and fast discharges as well as lower losses, and thus the preparation of these materials as dielectric layers for flexible electronic devices has become a hot research topic. Flexible strain sensors are widely used in many fields such as electronic skins, wearable electronic devices and soft robotics as a flexible, stretchable electronic device, which efficiently convert deformations caused by external forces into electrical signals for motion detection and health monitoring purposes.
As shown in
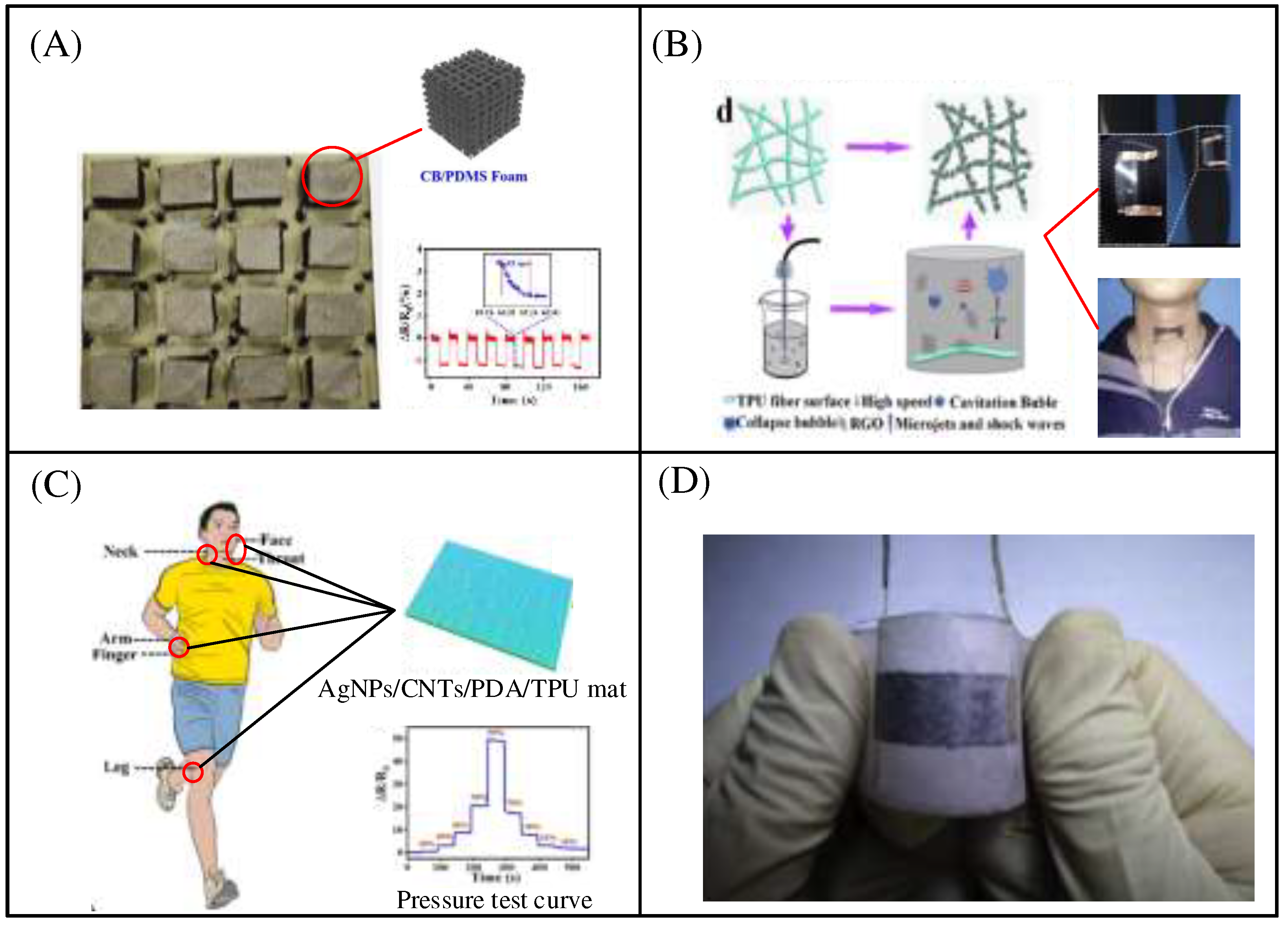
Figure 6A, Zhai et al. reported a flexible sensor based on a CB/DMS foam sensor with a high durability of over 15,000 cycles[
26]. Chen et al. encapsulated the sensor with a polymethyl methacrylate (PMMA) layer approximately 5 µm thick to improve durability [
27]. Kweon et al. reported a flexible strain sensor based on polyvinylidene fluoride (PVDF) copolymer that operated stably over 10,000 strain cycles [
28]. Wang et al. reported a flexible strain sensor consisting of a TPU fibre pad and reduced graphene oxide (RGO) (
Figure 6B), which exhibits good durability due to the strong bond between the TPU fibres and the RGO [
29].
Figure 6C shows a tensile strain sensor based on AgNPs/CNTs/PDA/TPU pad (ACTM) composites[
30]. Using a TPU fiber pad as a stretchable substrate, PDA is anchored to the surface of the TPU, improving the interface between the substrate and nano fillers. Finally, CNT and AgNPs are modified on the surface of the PDA/TPU, and the introduction of PDA effectively prevents the oxidation of AgNPs.
Figure 6D shows a wearable strain sensor based on nylon fabric encapsulated graphene film (GF/NF). The flexible and high conductivity graphene films prepared by thermal expansion compression molding process have good electrical properties and stability[
31]. In addition, a GF/NF strain sensor was used to detect multiple joints in the human body. The data results show that the GF/NF strain sensor has high sensitivity and good repeatability. The strain sensor has a wide detection range, high sensitivity, stable response, and long-term durability, and can be used for human motion detection. The sensitivity of flexible sensors varies depending on their active material, structure and sensing mechanism. The ideal flexible strain sensor should have high sensitivity, wide strain sensing range, satisfactory repeatability, sample preparation process and versatility, allowing the sensor to monitor the human body faster, easier, more accurately and more comprehensively.
4. INTELLIGENT ELECTROCHEMICAL SENSOR
Physical sensors monitor only physical signals of the human body, such as electrophysiological signals. For further monitoring of human health, such as health monitoring through body fluids and other human metabolites that contain many disease markers, Chemical biosensors are needed. As the need for personal health monitoring continues to grow, it has been necessary to develop a sensor that can provide diverse and dynamic detection of analytes[
32]. Electrochemical wearable sensors are an increasingly attractive solution because of the advantages mentioned above. Modern chemistry sensors are mostly based on the principle of electrochemical reactions and use integrated electronic sensors for non-invasive measurements of biomarkers, and can therefore be called electrochemical sensors[
33,
34]. These sensors can collect and analyze biological fluids including sweat, tears, saliva and interstitial fluid (ISF). Promoted by the material innovation from traditional metal and semiconductor materials to flexible/stretchable 2D material, polymer and biomaterials, wearable chemical sensors have shown great prospects in applications of healthcare and environmental monitoring by interfacing with different signal transmission technologies such as Bluetooth, Wi-Fi, RF.
4.1. Sweat sensor
Sweat is particularly popular because of easy acquisition from the large skin surface and contains abundant markers such as metabolites, proteins and hormones [
35]. Wearable sweat sensors combine the benefits of non-invasive sweat sampling and wearable real-time measurement to provide a powerful platform for monitoring the rich biochemical composition of sweat in relation to physiological conditions [
36].
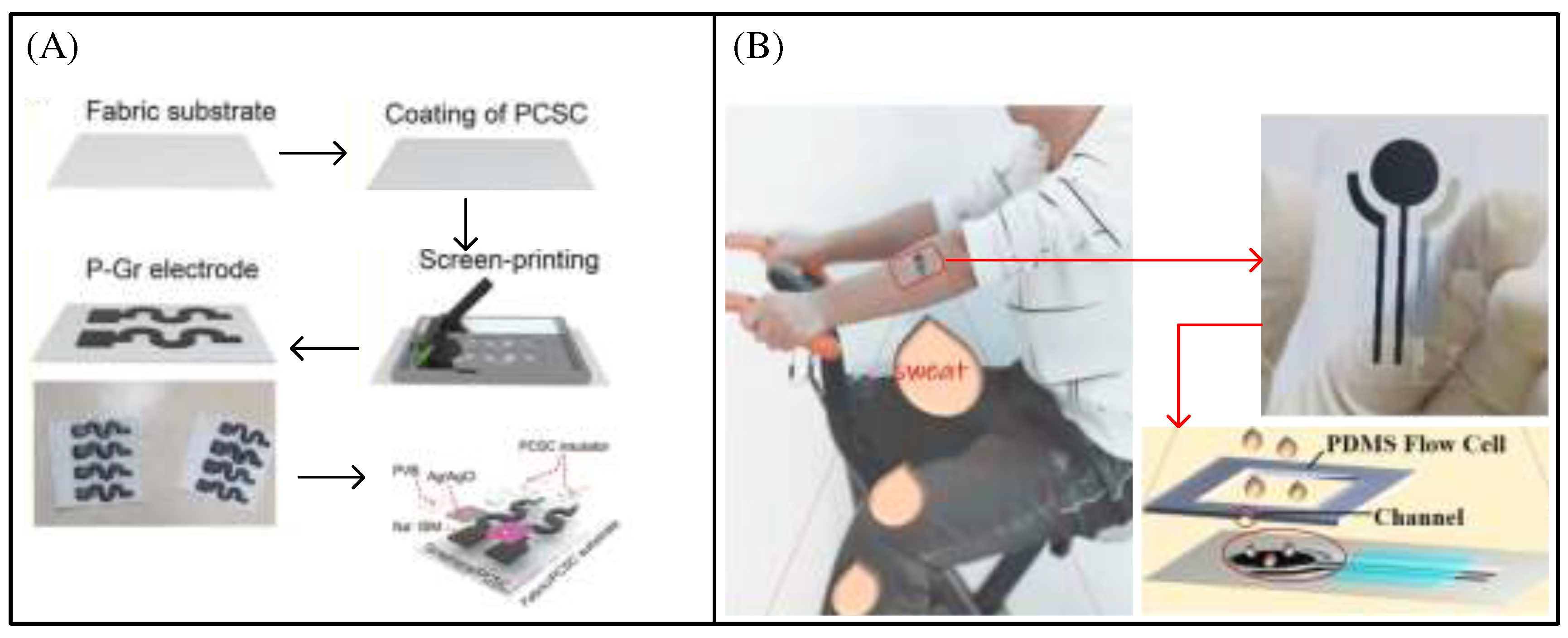
Figure 7A shows a self-repairing, robust, and electrically conductive ink by combining graphene (Gr) with poly(1,4-cyclohexanedimethanol succinate-co-citrate)(PCSC). The results showed that the resulting PCSC/Gr (P-Gr) conductor had a high conductivity of 1243 Sm
-1 and maintained a tensile strain of 213%. The electrode printed with this ink has almost no resistance change under 200% tensile strain[
37]. The electrode is used to make a sweat sensor to detect sodium ions and by integrating the sensor with a wireless electronic module, a wearable portable sweat c is formed. Jia et al. prepared a skin tattoo biosensor consisting of conductive inks directly attached on the wearer's skin for electrochemical lactate sensor[
38]. The sensor has good selectivity for lactic acid and still shows a good linear response at lactic acid concentrations up to 20 mM, enabling continuous monitoring of lactic acid content in human sweat.
Figure 7B shows a flexible electrochemical biosensor based on Ag nanowires (AgNWs) and MIP for monitoring lactic acid in sweat. The lactate sensor was applied to the epidermis of volunteers to monitor lactic acid in vivo based on differential pulse voltammetry (DPV) response measurements[
39]. The results showed that the calibration curve had good linearity (correlation coefficient of 0.995) and specificity within the set concentration range, and the detection limit (LOD) of lactic acid detection in PBS was estimated to be as low as 0.22 μ M (S/N = 3).
4.2. Tear sensor
Tears contain a variety of biomarkers, and as a more readily available biological fluid than blood, the concentrations of many metabolites in tears reflect true blood levels [
40]. Wearable tear-based sensing systems have been reported to detect a wide range of analytes including glucose and lactate [
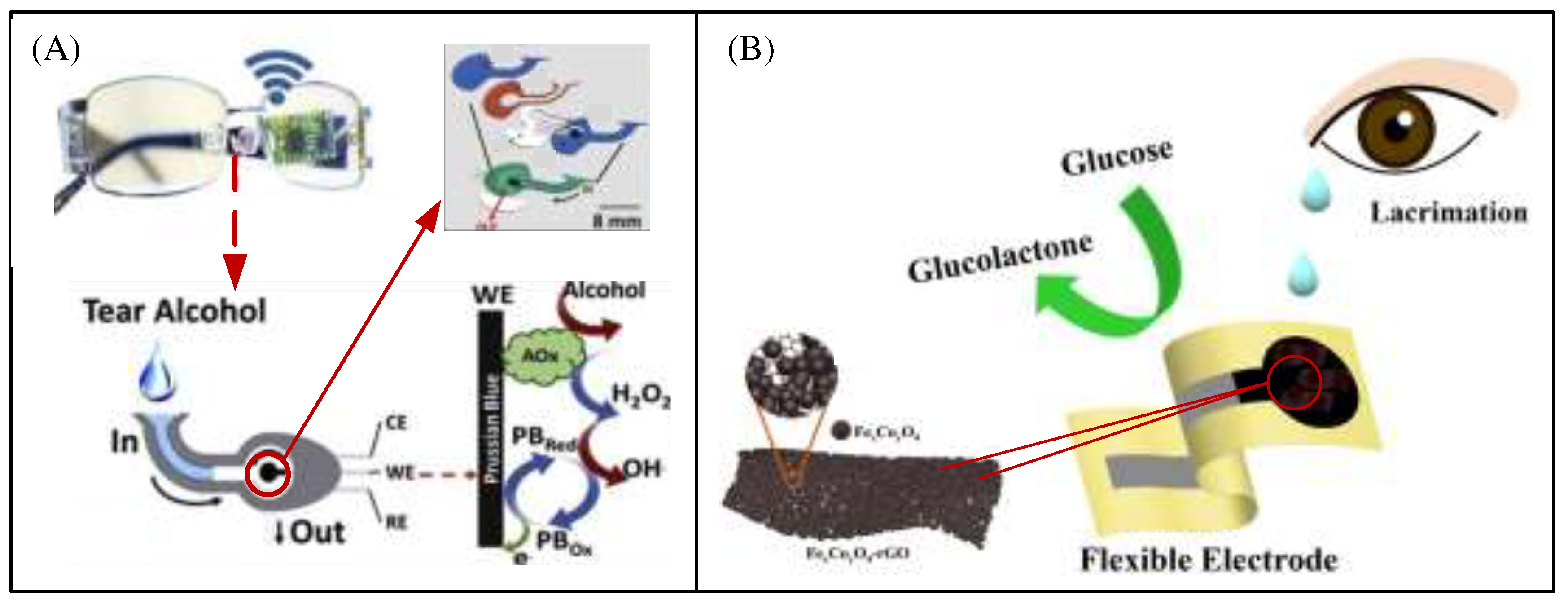
41]. Most of these systems integrate electrochemical sensors into an eyewear-based platform that has integrated electronics and is commonly used for daily monitoring. R. Sempionatto et al. mounted a line fluid device on the nose bridge pad of an eyeglass thereby enabling a non-invasive wearable tear biosensor system. This system requires stimulation of the eye before tears can be collected for real-time monitoring of different target analytes such as alcohol (
Figure 8A), vitamins and glucose in tears [
42].
Figure 8B shows that an electrochemical sensor for analyzing glucose in tears, where a suspension consisting of Fe, Co bimetallic oxides and reduced graphene oxide was added dropwise to the electrode surface, and Nafion solution was added dropwise after the suspension dried to prevent the material from falling off [
43]. This non-enzymatic glucose detection platform is characterized by high sensitivity, high selectivity and low detection limits, and can be used for dynamic analysis of glucose content in tear fluid.
4.3. Saliva sensor
Saliva is a biofluid that is more readily available non-invasively than sweat or tears, which has an abundance of components such as electrolytes, metabolites and protein biomarkers that provide a timely picture of the oral cavity [
44]. However, compared to sweat sensors, saliva sensors have significant limitations in terms of where they can be worn. In addition, it is challenging to fix the sensor on the teeth and effectively prevent sensor wear.
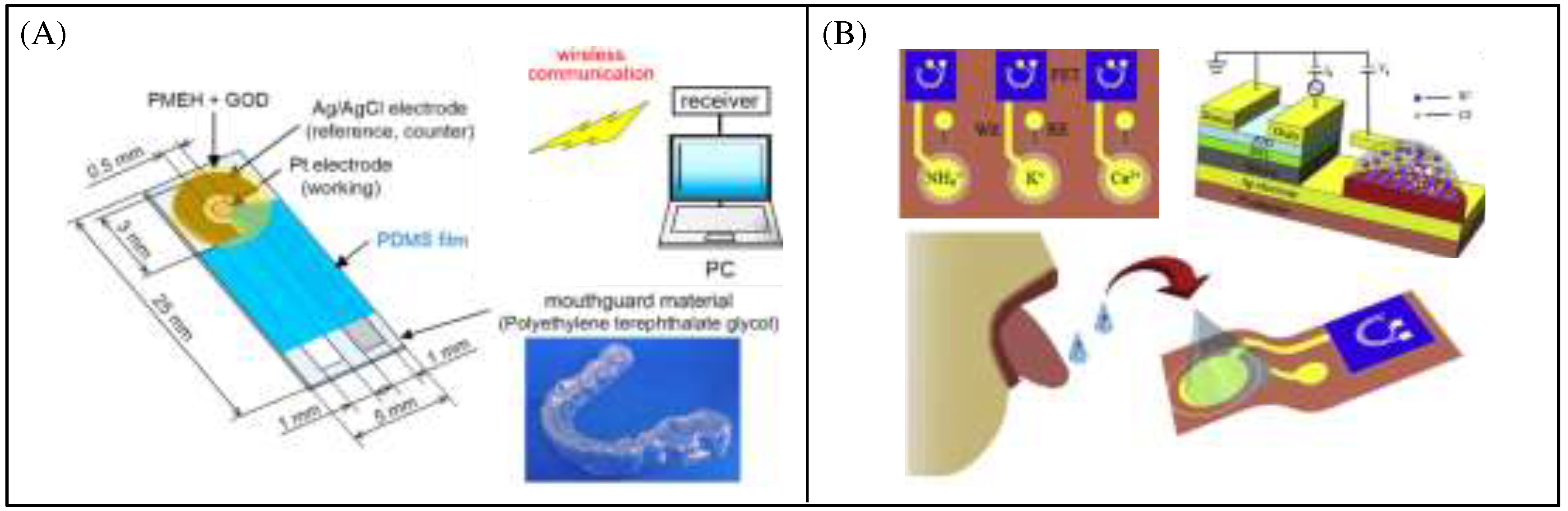
Figure 9A shows a saliva sensor used to detect glucose, which can achieve long-term real-time non-invasive monitoring through wireless communication modules. The sensor combines Pt and Ag/AgCl electrodes onto a tooth guard bracket with an enzyme film. The electrode is formed on the polyethylene terephthalate (PETG) surface of the tooth guard[
45]. The Pt working electrode is coated with a glucose oxidase (GOD) film. The results show that the glucose sensor can perform high sensitivity detection in the glucose range of 5-1000 µ mol/L, which covers the range of glucose concentrations found in human saliva. Joseph wang's group reported a tooth-care lactate biosensor based on a three-electrode system, with the specific scheme of modifying lactate oxidase on the sensor to catalyze lactate in saliva. The high repeatability and selectivity of the sensor create favorable conditions for continuous monitoring of the oral cavity with a Bluetooth transceiver for communication [
46]. Bao et al. used a 3D printing method to directly connect a field effect transistor (FET) and an ion selective electrode (ISE) to produce a flexible ion selective field effect transistor (ISFET) (
Figure 9B). Then, the sensitivity and selectivity of mixed ISFETs were further studied. Finally, the possibility of ISFET detecting NH
4+,K
+,Ca
2+ in artificial saliva from interfering ion mixtures was demonstrated, expanding the application of ISFET as the next generation electronic tongue in the field of non-invasive real-time health monitoring[
47]. M. S. Mannoor et al. reported a wireless graphene bacterial sensor mounted on tooth enamel. The self-assembled antimicrobial peptide is immobilized on graphene as an artificial receptor, and the conductivity of the graphene film changes when the immobilized peptide binds to a specific bacterial target. Finally, the signal is read by an RF reading device [
48]. P. Tseng et al. reported a RF-Trilayer saliva sensor [
49],which is consist of an active layer sandwiched between two reverse-facing split ring resonators where the dielectric sensing signal is wirelessly coupled to the reader to achieve food consumption analysis such as pH, salinity, biomolecules, and alcohol.
4.4. Interstitial fluid sensor
Interstitial fluid exists between cells and capillaries, produced by the filtration of blood plasma through capillaries, and its composition is very similar to that of blood plasma. Interstitial fluid lacks some of the proteins present in blood plasma, which is due to the fact that some of the large protein components cannot pass through when blood plasma passes through capillaries for fluid exchange [
50]. Because of the close association of interstitial fluid with plasma, the detection of biomarkers, metabolites and drugs in interstitial fluid is essential[
51,
52,
53,
54]. In order to minimize damage to the patient's skin, techniques such as microneedle[
55] and electroporation[
56] have been developed to obtain interstitial fluid. However, these extraction techniques are not yet suitable for clinical applications due to different equipment and methods, long extraction times and generally low extraction volumes[
57]. The development of wearable devices based on biochemical detection of interstitial fluid by combining electrochemical sensors with microneedles or patches[
58,
59] for extracting interstitial fluid is still the focus in the field of health monitoring.
Yao et al. proposed a noninvasive glucose sensor that can extract interstitial fluid (
Figure 10A) and perform sensitive detection of glucose by the current applied between electrodes with a graphene/carbon nanotube/glucose oxidase composite textile as the working electrode[
60]. The sensor was verified to be capable of continuous noninvasive detection by comparison with the detection results of a glucose meter.
Figure 10B shows that Muamer Dervisevicet al. developed a high-density polymeric MNA (PMNA)-based sensing patch that uses microneedle technology to monitor the skin ISF pH[
61]. The sensing patch was tested to detect pH 4.00-8.60 with a sensitivity of 62.9 mV/pH and an accuracy of ±0.036 pH units.
5. INTELLIGENT BIOSENSOR
Biosensing technology is a high technology that grows from the interpenetration of various disciplines such as biology, chemistry, physics, medicine, and electronics. A biosensor is an analytical system consisting of immobilized biosensitive materials, appropriate physicochemical transducers (such as oxygen electrodes, photosensitive tubes, field-effect tubes, piezoelectric crystals, etc.) and signal amplification devices [
62]. Due to its good selectivity, high sensitivity, fast analysis, low cost, the possibility of online continuous monitoring in complex systems, and especially its high degree of automation, miniaturization and integration [
63], the biosensor has gained a vigorous and rapid development in recent decades. In 1967, S.J. Updik et al. produced the first generation of glucose biosensor by containing glucose oxidase in a polyacrylamide colloid and immobilizing this colloidal film on the tip of a septum oxygen electrode. In clinical medicine, biosensors using enzymes as sensitive materials have been successfully applied to the detection of blood glucose, lactate, vitamin C, uric acid, urea, glutamate, transaminases[
64], etc. In addition, immunosensors, genosensors and aptasensors are all biosensors with their own characteristics and applications.
5.1. Immunosensor
Owing to their high affinity and specificity, antibodies are one of the most effective detection elements used in biosensors[
65], and biosensors utilizing antibodies are referred to as immunosensors. Lee's group presented a portable, integrated magnetic-electrochemical assay with eight-channels for rapid EV screening (
Figure 11A). EVs were first captured and labeled by magnetic beads and then assessed via electrochemical immunoassay[
66]. The device simplifies vesicle isolationand demonstrated rapid extracellular vesicle profiling in clinical samples. M.O.Shaikh et al. fabricated a simple disposable electrochemical immunosensor for the point of care testing of microalbuminuria[
67].The immunosensor was performed on the surface of carbon interdigitated microelectrodes (IMEs) printed on flexible polyethylene terephthalate (PET) sheets (
Figure 11B).
5.2. Genosensor
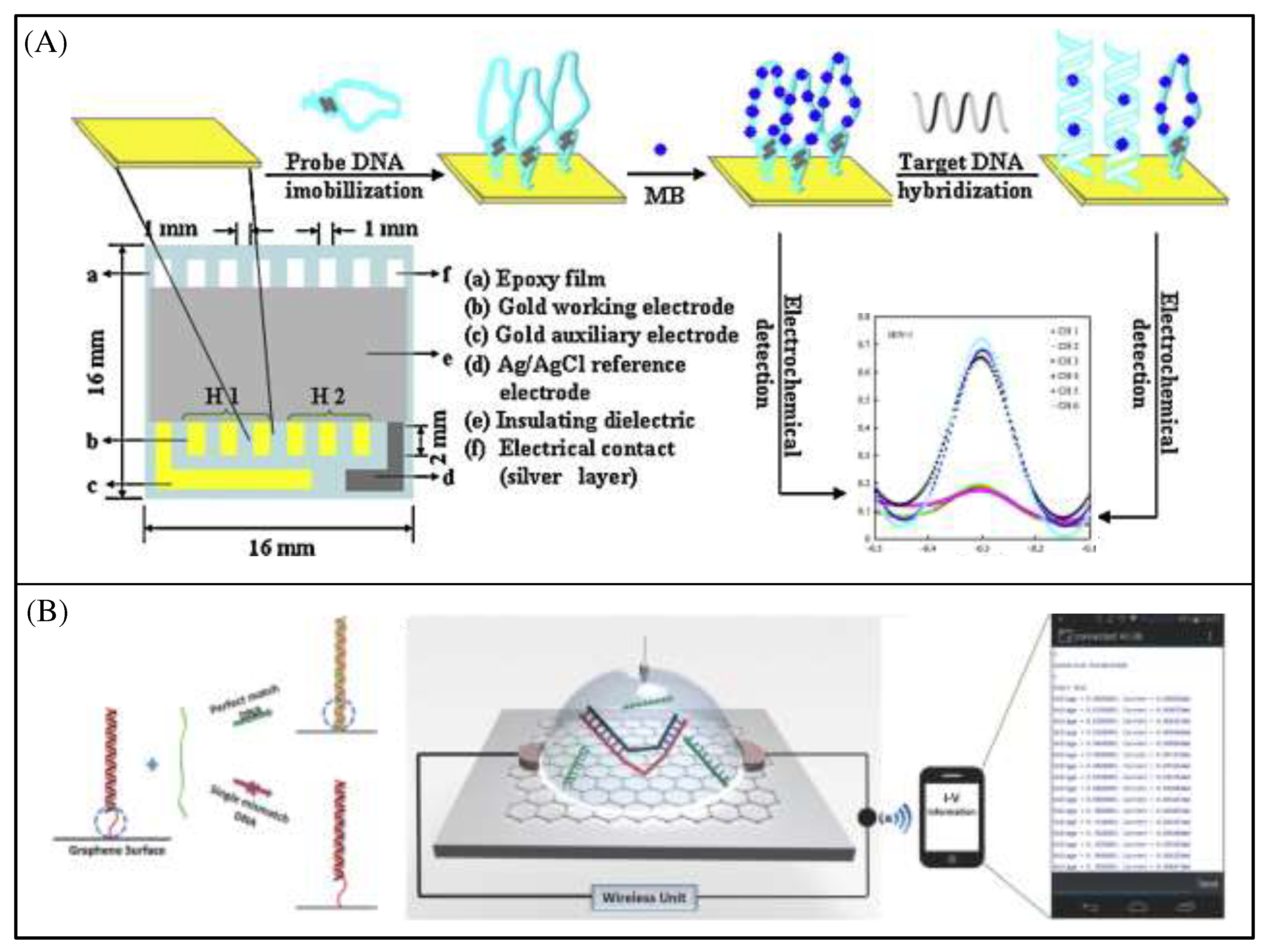
Genosensors are a widely reported class of biosensors [
68]. The application in clinical disease diagnosis is the greatest advantage of genosensors, which can help physicians understand disease processes at the level of DNA, RNA, proteins and their interactions. D. Zhang et al. developed a label-free DNA biosensor array[
69] consisting of six gold working electrodes, a gold auxiliary electrode and an Ag/AgCl reference electrode (
Figure 12A). The six gold working electrodes were equally divided into two groups, each with a kind of thiolated hairpin-DNA probe modified on the surface for the simultaneous detection of the human immunodeficiency virus (HIV) oligonucleotide sequences HIV-1 and HIV-2. The biosensor array showed a good specificity without the obvious cross-interference. M. T. Hwang et al. developed a Wireless label-free graphene-based FET genotyping platform [
70] which able to discriminate single nucleotide polymorphism with high sensitivity, based on the immobilization of DNA nanotweezers onto the graphene surface (
Figure 12B). DNA strand displacement in the presence of the target DNA and the subsequent DNA nanotweezers opening causes the switching of different involved strands which is ultimately translated as a charge difference before and after.
5.3. Aptasensor
Among the wide variety of biomolecular recognition elements (BRE), aptamers are widely used due to remarkable affinity, specificity and their ease of synthesis [
71]. In general, an aptamer is a specific structure formed by folding a single-stranded RNA or DNA molecule that binds to a specific target molecule [
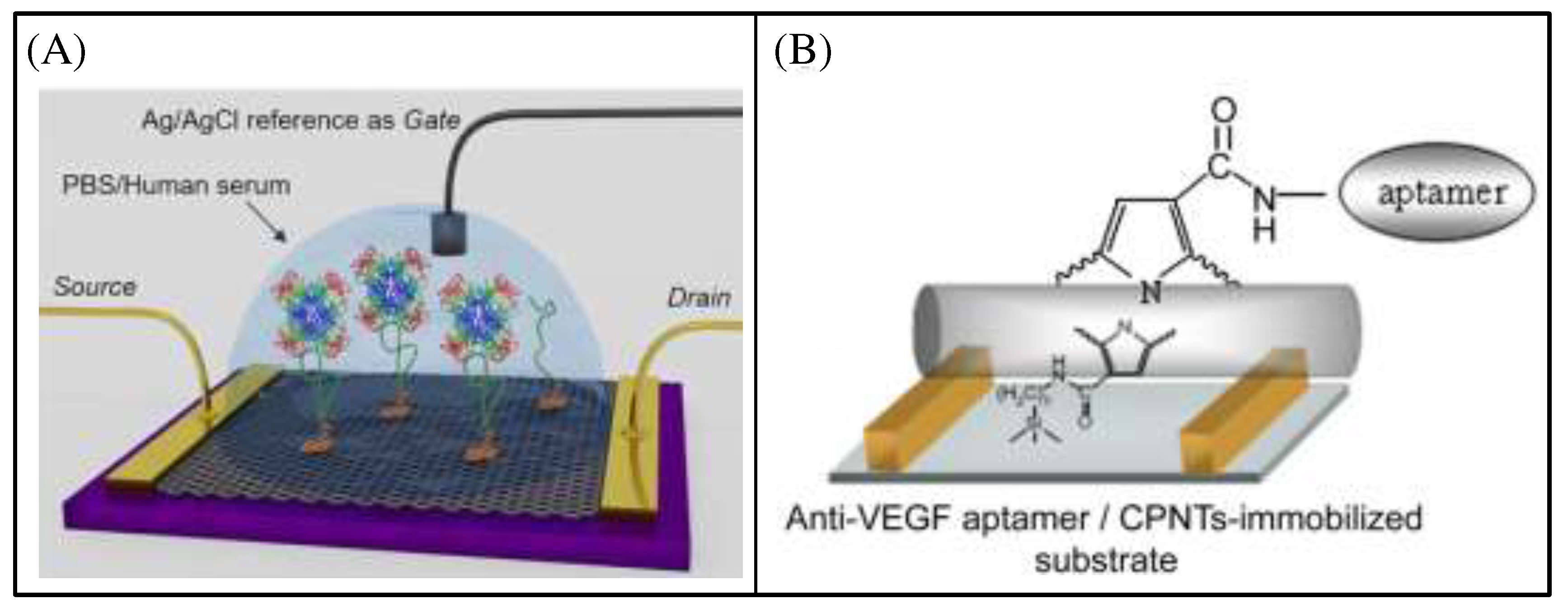
72]. Aptamer-based BRE together with other biosensing components provide a specific, selective, sensitive, easy-to-operate, fast, stable and compact analytical platform, the aptasensor. G. Figueroa-Miranda et al. presented system-integrated (
Figure 13A) reduced graphene oxide (rGO) based 2DBioFETs [
73] modified with PfLDH-specific 2008s aptameras an optimal PoC solution for quantitative screening of malarial parasitemia. O. S. Kwon et al. demonstrated the high-performance FET sensor(
Figure 13B), which could detect ca.400 fM of VEGF concentration, based on anti-VEGF RNA aptamer conjugated carboxylated polypyrrole nanotubes (CPNTs) [
74]. Moreover, the CPNTs-aptamer FET sensors can be repeatedly used for various concentrations of the target molecule (VEGFs) through the washing and rinsing processes.
6. CONCLUSION AND PERSPECTIVE
This paper provides an overview of intelligent sensing technology and its applications in medicine. Intelligent medical sensing detection, as a comprehensive science and technology, is an important branch of sensing technology. Its design and application must take full account of ergonomics, the specificity and complexity of biosignals as well as the biocompatibility, reliability and safety of intelligent medical sensors. In recent years, wearable sensors have become particularly prominent due to their lightness, flexibility, and excellent mechanical properties, and most importantly, their ability to enable real-time, non-invasive and continuous monitoring of biomarkers in various biofluids, which have also become major targets for wearable sensor development. In addition, wearable sensors can use smartphones as control terminals, which offers great opportunities for personalized, digital healthcare. In the latest non-invasive wearable sensor designs, it is desirable for the sensors to be able to detect multiple biomarkers through self-calibration to improve the reliability of wearable sensing as well as the ease of calibration. In addition, the accuracy and sensitivity of wearable sensors need to be improved in order to be able to detect some trace but important biomarkers. Based on such existing problems, the performance of wearable sensors should be improved by developing new materials. Combined with big data analytics and machine learning, advances in intelligent medical sensing technology bode well for the integration of wearable health systems. The real-time, continuous monitoring capabilities of wearable sensors provide a high-quality source of information for big data analysis, and successful integration will provide the means for patient-centered chronic disease management, reduce the frequency of clinical visits, and provide personalized, on-demand interventions.
Declaration of Interests
The authors declare no competing interests.
Author Contributions
Jie Fu: Literature research and collation, Conceptualization, Methodology, Data curation, Writing – original draft; Qiya Gao: Information access, Writing – revising; Shuang Li: Structural design, Writing – review & editing, Supervision.
Acknowledgment
This work was supported by the National Key Research and Development Program of China (Grant No. 2022YFF1202700), the National Natural Science Foundation of China (Grant No. 82001922), the HongKong Scholars Program (Grant No. XJ2021034).
References
- G. Hu, M. Wang, Survey on Biomedical Signal Processing, Journal of Data Acquisition & Processing, 30(2015) 915-32.
- P.J. Quintana Barcia, J. García, Signal conditioning II: analog filters, in: J. García (Ed.) Encyclopedia of Electrical and Electronic Power Engineering, Elsevier, Oxford, 2023, pp. 57-69.
- X. Dao, M. Gao, Z. Zhang, C. Li, Y. Wang, Design of multi-parameter composite modulated signal for anti-deceptive jamming, AEU - International Journal of Electronics and Communications, 132(2021) 153646. [CrossRef]
- Fernandez-Vazquez, G. Jovanovic Dolecek, Generalized Chebyshev Filters for the Design of IIR Filters and Filter Banks, Circuits Systems and Signal Processing, 33(2014) 2237-50. [CrossRef]
- M. Nedelchev, D. Dobrev, Low sensitivity symmetrical response microwave filters, Microelectronics Journal, 37(2006) 546-53.
- S.C.D. Roy, A New Chebyshev-like Low-pass Filter Approximation, Circuits Systems and Signal Processing, 29(2010) 629-36. [CrossRef]
- M. Sengul, Ieee, Transitional Butterworth-Chebyshev Filters, 18th Mediterranean Microwave Symposium (MMS), Istanbul, TURKEY, 2018, pp. 157-9.
- L. Zhen, S. Si, Research of Chebyshev Equivalent Ripple Approximation Filter Based on DSP, International Conference on Mechatronics and Materials Engineering (ICMME 2011), Qiqihar, PEOPLES R CHINA, 2011, pp. 446-+.
- M. Nankali, N.M. Nouri, M. Navidbakhsh, N.G. Malek, M.A. Amindehghan, A.M. Shahtoori, et al., Highly stretchable and sensitive strain sensors based on carbon nanotube-elastomer nanocomposites: the effect of environmental factors on strain sensing performance, Journal of Materials Chemistry C, 8(2020) 6185-95. [CrossRef]
- T. Sun, Z.-h. Yu, Moving target localization in distributed MIMO radar systems with sensor position errors in the presence of a calibration object, Digital Signal Processing, 131(2022) 103751. [CrossRef]
- H. Ma, J. Chen, J. Tian, X. Chen, Design of Personal Health Monitoring System Based on Body Sensor Network, Measurement & Control Technology, 34(2015) 24-7.
- V. Paruchuri, S. Chellappan, Context Aware Identity Management using Smart Phones, 8th IEEE International Conference on Broadband, Wireless Computing, Communication and Applications (BWCCA), Univ Technol Compiegne, Compiegne, FRANCE, 2013, pp. 184-90. [CrossRef]
- S. Wang, H. Shen, C. Luo, Temperature Measurement with Bluetooth under Android Platform, Zhongguo yi liao qi xie za zhi = Chinese journal of medical instrumentation, 39(2015) 181-96.
- C. Wen, H. Yuan, Y. Gao, J. Li, Ieee, The Abnormal Behavior Recognition Based on The Smart Mobile Sensors, 9th International Symposium on Computational Intelligence and Design (ISCID), Hangzhou, PEOPLES R CHINA, 2016, pp. 390-3.
- X. Yin, W. Shen, J. Samarabandu, X. Wang, Human Activity Detection Based on Multiple Smart Phone Sensors and Machine Learning Algorithms, IEEE 19th International Conference on Computer Supported Cooperative Work in Design (CSCWD), Calabria, ITALY, 2015, pp. 582-7. [CrossRef]
- F. Becker, O. Paul, Efficient cross-sensitivity compensation in multisensor systems by half-blind calibration, Sensors and Actuators A: Physical, 257(2017) 154-64. [CrossRef]
- E. Sifuentes, O. Casas, F. Reverter, R. Pallas-Areny, Ieee, Improved direct interface circuit for resistive full- and half-bridge sensors, 4th International Conference on Electrical and Electronics Engineering, Mexico City, MEXICO, 2007, pp. 100-3. [CrossRef]
- G. de Graaf, R.F. Wolffenbuttel, Circuit for readout and linearisation of sensor bridges, 30th European Solid-State Circuits Conference (ESSCIRC 2004), Leuven, BELGIUM, 2004, pp. 451-4.
- Y.-U. Haq, R. Ullah, S. Mazhar, R. Khattak, A.A. Qarni, Z.-U. Haq, et al., Synthesis and characterization of 2D MXene: Device fabrication for humidity sensing, Journal of Science: Advanced Materials and Devices, 7(2022) 100390. [CrossRef]
- G. Jia, A. Zheng, X. Wang, L. Zhang, L. Li, C. Li, et al., Flexible, biocompatible and highly conductive MXene-graphene oxide film for smart actuator and humidity sensor, Sensors and Actuators B: Chemical, 346(2021) 130507. [CrossRef]
- H. Xing, X. Li, Y. Lu, Y. Wu, Y. He, Q. Chen, et al., MXene/MWCNT electronic fabric with enhanced mechanical robustness on humidity sensing for real-time respiration monitoring, Sensors and Actuators B: Chemical, 361(2022). [CrossRef]
- M.-y. Yang, M.-l. Huang, Y.-z. Li, Z.-s. Feng, Y. Huang, H.-j. Chen, et al., Printing assembly of flexible devices with oxidation stable MXene for high performance humidity sensing applications, Sensors and Actuators B: Chemical, 364(2022) 131867. [CrossRef]
- Y. Jiang, Z. Duan, Z. Fan, P. Yao, Z. Yuan, Y. Jiang, et al., Power generation humidity sensor based on NaCl/halloysite nanotubes for respiratory patterns monitoring, Sensors and Actuators B: Chemical, 380(2023) 133396. [CrossRef]
- G. Zhu, F. Wang, L. Chen, C. Wang, Y. Xu, J. Chen, et al., Highly flexible TPU/SWCNTs composite-based temperature sensors with linear negative temperature coefficient effect and photo-thermal effect, Composites Science and Technology, 217(2022). [CrossRef]
- M. Usman, N. Jamhour, J. Hettinger, W. Xue, Smart Wearable Flexible Temperature Sensor with Compensation against Bending and Stretching Effects, Sensors and Actuators A: Physical, (2023). [CrossRef]
- W. Zhai, Q. Xia, K. Zhou, X. Yue, M. Ren, G. Zheng, et al., Multifunctional flexible carbon black/polydimethylsiloxane piezoresistive sensor with ultrahigh linear range, excellent durability and oil/water separation capability, Chemical Engineering Journal, 372(2019) 373-82. [CrossRef]
- Z. Chen, T. Ming, M.M. Goulamaly, H. Yao, D. Nezich, M. Hempel, et al., Enhancing the Sensitivity of Percolative Graphene Films for Flexible and Transparent Pressure Sensor Arrays, Advanced Functional Materials, 26(2016) 5061-7. [CrossRef]
- Kweon, S.J. Lee, J.H. Oh, Wearable high-performance pressure sensors based on three-dimensional electrospun conductive nanofibers, NPG Asia Materials, 10(2018) 540-51. [CrossRef]
- Y. Wang, J. Hao, Z. Huang, G. Zheng, K. Dai, C. Liu, et al., Flexible electrically resistive-type strain sensors based on reduced graphene oxide-decorated electrospun polymer fibrous mats for human motion monitoring, Carbon, 126(2018) 360-71. [CrossRef]
- P. Zhan, Y. Jia, W. Zhai, G. Zheng, K. Dai, C. Liu, et al., A fibrous flexible strain sensor with Ag nanoparticles and carbon nanotubes for synergetic high sensitivity and large response range, Composites Part A: Applied Science and Manufacturing, 167(2023) 107431. [CrossRef]
- S. Lu, S. Wang, G. Wang, J. Ma, X. Wang, H. Tang, et al., Wearable graphene film strain sensors encapsulated with nylon fabric for human motion monitoring, Sensors and Actuators A: Physical, 295(2019) 200-9. [CrossRef]
- G.M. Maddocks, M.A. Daniele, Chemical Sensors: Wearable Sensors, in: R. Narayan (Ed.) Encyclopedia of Sensors and Biosensors (First Edition), Elsevier, Oxford, 2023, pp. 260-80.
- J. Zhao, H. Guo, J. Li, A.J. Bandodkar, J.A. Rogers, Body-Interfaced Chemical Sensors for Noninvasive Monitoring and Analysis of Biofluids, Trends in Chemistry, 1(2019) 559-71. [CrossRef]
- N. Promphet, S. Ummartyotin, W. Ngeontae, P. Puthongkham, N. Rodthongkum, Non-invasive wearable chemical sensors in real-life applications, Analytica Chimica Acta, 1179(2021). [CrossRef]
- C. Legner, U. Kalwa, V. Patel, A. Chesmore, S. Pandey, Sweat sensing in the smart wearables era: Towards integrative, multifunctional and body-compliant perspiration analysis, Sensors and Actuators A: Physical, 296(2019) 200-21. [CrossRef]
- A.M.V. Mohan, V. Rajendran, R.K. Mishra, M. Jayaraman, Recent advances and perspectives in sweat based wearable electrochemical sensors, TrAC Trends in Analytical Chemistry, 131(2020) 116024. [CrossRef]
- S. Gyu Son, H. Jun Park, S.-M. Kim, S. Jin Kim, M. Sik Kil, J.-M. Jeong, et al., Ultra-fast self-healable stretchable bio-based elastomer/graphene ink using fluid dynamics process for printed wearable sweat-monitoring sensor, Chemical Engineering Journal, 454(2023) 140443. [CrossRef]
- W. Jia, A.J. Bandodkar, G. Valdes-Ramirez, J.R. Windmiller, Z. Yang, J. Ramirez, et al., Electrochemical tattoo biosensors for real-time noninvasive lactate monitoring in human perspiration, Anal Chem, 85(2013) 6553-60. [CrossRef]
- Q. Zhang, D. Jiang, C. Xu, Y. Ge, X. Liu, Q. Wei, et al., Wearable electrochemical biosensor based on molecularly imprinted Ag nanowires for noninvasive monitoring lactate in human sweat, Sensors and Actuators B: Chemical, 320(2020) 128325. [CrossRef]
- R. Moreddu, J.S. Wolffsohn, D. Vigolo, A.K. Yetisen, Laser-inscribed contact lens sensors for the detection of analytes in the tear fluid, Sensors and Actuators B: Chemical, 317(2020) 128183. [CrossRef]
- D. Pankratov, E. González-Arribas, Z. Blum, S. Shleev, Tear Based Bioelectronics, Electroanalysis, 28(2016) 1250-66.
- J.R. Sempionatto, L.C. Brazaca, L. García-Carmona, G. Bolat, A.S. Campbell, A. Martin, et al., Eyeglasses-based tear biosensing system: Non-invasive detection of alcohol, vitamins and glucose, Biosensors and Bioelectronics, 137(2019) 161-70.
- F. Zhou, H. Zhao, K. Chen, S. Cao, Z. Shi, M. Lan, Flexible electrochemical sensor with Fe/Co bimetallic oxides for sensitive analysis of glucose in human tears, Analytica Chimica Acta, 1243(2023) 340781. [CrossRef]
- P. Swetha, U. Balijapalli, S.-P. Feng, Wireless accessing of salivary biomarkers based wearable electrochemical sensors: A mini-review, Electrochemistry Communications, 140(2022) 107314. [CrossRef]
- T. Arakawa, Y. Kuroki, H. Nitta, P. Chouhan, K. Toma, S.-i. Sawada, et al., Mouthguard biosensor with telemetry system for monitoring of saliva glucose: A novel cavitas sensor, Biosensors and Bioelectronics, 84(2016) 106-11. [CrossRef]
- J. Kim, G. Valdes-Ramirez, A.J. Bandodkar, W. Jia, A.G. Martinez, J. Ramirez, et al., Non-invasive mouthguard biosensor for continuous salivary monitoring of metabolites, Analyst, 139(2014) 1632-6. doi:10.1039/c3an02359a. [CrossRef]
- C. Bao, M. Kaur, W.S. Kim, Toward a highly selective artificial saliva sensor using printed hybrid field effect transistors, Sensors and Actuators B: Chemical, 285(2019) 186-92. [CrossRef]
- M.S. Mannoor, H. Tao, J.D. Clayton, A. Sengupta, D.L. Kaplan, R.R. Naik, et al., Graphene-based wireless bacteria detection on tooth enamel, Nat Commun, 3(2012) 763. [CrossRef]
- P. Tseng, B. Napier, L. Garbarini, D.L. Kaplan, F.G. Omenetto, Functional, RF-Trilayer Sensors for Tooth-Mounted, Wireless Monitoring of the Oral Cavity and Food Consumption, Adv Mater, 30(2018) e1703257. [CrossRef]
- H. Haslene-Hox, O. Tenstad, H. Wiig, Interstitial fluid—A reflection of the tumor cell microenvironment and secretome, Biochimica et Biophysica Acta (BBA) - Proteins and Proteomics, 1834(2013) 2336-46. [CrossRef]
- H. Chang, M. Zheng, X. Yu, T. Aung, R.Z. Seeni, R. Kang, et al., A Swellable Microneedle Patch to Rapidly Extract Skin Interstitial Fluid for Timely Metabolic Analysis, ADVANCED MATERIALS, 29(2017). [CrossRef]
- J. Zhang, N. Hao, W. Liu, M. Lu, L. Sun, N. Chen, et al., In-depth proteomic analysis of tissue interstitial fluid for hepatocellular carcinoma serum biomarker discovery, BRITISH JOURNAL OF CANCER, 117(2017) 1676-84. [CrossRef]
- J. Hadrevi, B. Ghafouri, A. Sjors, H. Antti, B. Larsson, A.G. Crenshaw, et al., Comparative metabolomics of muscle interstitium fluid in human trapezius myalgia: an in vivo microdialysis study, EUROPEAN JOURNAL OF APPLIED PHYSIOLOGY, 113(2013) 2977-89. [CrossRef]
- T.K.L. Kiang, V. Schmitt, M.H.H. Ensom, B. Chua, U.O. Haefeli, Therapeutic drug monitoring in interstitial fluid: A feasibility study using a comprehensive panel of drugs, JOURNAL OF PHARMACEUTICAL SCIENCES, 101(2012) 4642-52. [CrossRef]
- J. Madden, C. O'Mahony, M. Thompson, A. O'Riordan, P. Galvin, Biosensing in dermal interstitial fluid using microneedle based electrochemical devices, Sensing and Bio-Sensing Research, 29(2020). [CrossRef]
- R. Zhao, C. Wang, F. Lu, L. Du, Z. Fang, X. Guo, et al., A Flexible Interdigital Electrode Used in Skin Penetration Promotion and Evaluation with Electroporation and Reverse Iontophoresis Synergistically, SENSORS, 18(2018). [CrossRef]
- P.R. Miller, R.M. Taylor, T. Bao Quoc, G. Boyd, T. Glaros, V.H. Chavez, et al., Extraction and biomolecular analysis of dermal interstitial fluid collected with hollow microneedlesis, Communications Biology, 1(2018).
- E. Vranic, A. Tucak, M. Sirbubalo, O. Rahic, A. Elezovic, J. Hadziabdic, Microneedle-Based Sensor Systems for Real-Time Continuous Transdermal Monitoring of Analytes in Body Fluids, PROCEEDINGS OF THE INTERNATIONAL CONFERENCE ON MEDICAL AND BIOLOGICAL ENGINEERING, CMBEBIH 20192020, pp. 167-72.
- P. Bollella, S. Sharma, A.E.G. Cass, R. Antiochia, Minimally-invasive Microneedle-based Biosensor Array for Simultaneous Lactate and Glucose Monitoring in Artificial Interstitial Fluid, ELECTROANALYSIS, 31(2019) 374-82. [CrossRef]
- Y. Yao, J. Chen, Y. Guo, T. Lv, Z. Chen, N. Li, et al., Integration of interstitial fluid extraction and glucose detection in one device for wearable non-invasive blood glucose sensors, Biosensors and Bioelectronics, 179(2021) 113078. [CrossRef]
- M. Dervisevic, E. Dervisevic, L. Esser, C.D. Easton, V.J. Cadarso, N.H. Voelcker, Wearable microneedle array-based sensor for transdermal monitoring of pH levels in interstitial fluid, Biosensors and Bioelectronics, 222(2023) 114955. [CrossRef]
- S. Cajigas, D. Soto, J. Orozco, Biosensors: Biosensors With Signal Amplification, in: R. Narayan (Ed.) Encyclopedia of Sensors and Biosensors (First Edition), Elsevier, Oxford, 2023, pp. 429-57.
- A.A. Hamzah, S. Nadzirah, Biosensor Development, in: R. Narayan (Ed.) Encyclopedia of Sensors and Biosensors (First Edition), Elsevier, Oxford, 2023, pp. 209-17.
- . [CrossRef]
- D. Miura, R. Asano, Biosensors: Immunosensors, in: R. Narayan (Ed.) Encyclopedia of Sensors and Biosensors (First Edition), Elsevier, Oxford, 2023, pp. 298-314.
- S. Jeong, J. Park, D. Pathania, C.M. Castro, R. Weissleder, H. Lee, Integrated Magneto-Electrochemical Sensor for Exosome Analysis, ACS NANO, 10(2016) 1802-9. [CrossRef]
- M.O. Shaikh, P.-Y. Zhu, C.-C. Wang, Y.-C. Du, C.-H. Chuang, Electrochemical immunosensor utilizing electrodeposited Au nanocrystals and dielectrophoretically trapped PS/Ag/ab-HSA nanoprobes for detection of microalbuminuria at point of care, Biosensors and Bioelectronics, 126(2019) 572-80. [CrossRef]
- F. Ansah, F. Krampa, J.K. Donkor, C. Owusu-Appiah, S. Ashitei, V.E. Kornu, et al., Ultrasensitive electrochemical genosensors for species-specific diagnosis of malaria, Electrochimica Acta, 429(2022) 140988. [CrossRef]
- D. Zhang, Y. Peng, H. Qi, Q. Gao, C. Zhang, Label-free electrochemical DNA biosensor array for simultaneous detection of the HIV-1 and HIV-2 oligonucleotides incorporating different hairpin-DNA probes and redox indicator, Biosensors and Bioelectronics, 25(2010) 1088-94. [CrossRef]
- M.T. Hwang, Z. Wang, J. Ping, D.K. Ban, Z.C. Shiah, L. Antonschmidt, et al., DNA Nanotweezers and Graphene Transistor Enable Label-Free Genotyping, Adv Mater, (2018) e1802440. [CrossRef]
- T. Bhardwaj, P. Dalal, A.S. Rathore, S.K. Jha, An aptamer based microfluidic chip for impedimetric detection of Ranibizumab in a bioreactor, Sensors and Actuators B: Chemical, 312(2020) 127941. [CrossRef]
- Q. Zhu, L. Liu, R. Wang, X. Zhou, A split aptamer (SPA)-based sandwich-type biosensor for facile and rapid detection of streptomycin, Journal of Hazardous Materials, 403(2021) 123941. [CrossRef]
- G. Figueroa-Miranda, Y. Liang, M. Suranglikar, M. Stadler, N. Samane, M. Tintelott, et al., Delineating charge and capacitance transduction in system-integrated graphene-based BioFETs used as aptasensors for malaria detection, Biosensors and Bioelectronics, 208(2022) 114219. [CrossRef]
- Kwon, S.J. Park, J. Jang, A high-performance VEGF aptamer functionalized polypyrrole nanotube biosensor, Biomaterials, 31(2010) 4740-7. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).