Submitted:
07 July 2023
Posted:
10 July 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Fish
2.2. Virus culture
2.3. Vaccine preparation
2.4. Immunization, sampling, and challenge test
2.5. Immune gene expression by RT-qPCR
2.6. Measurement of IgM antibody levels
2.7. Statistical analysis
3. Results
3.1. Vaccine efficacy
3.2. Immunoglobulin gene expression
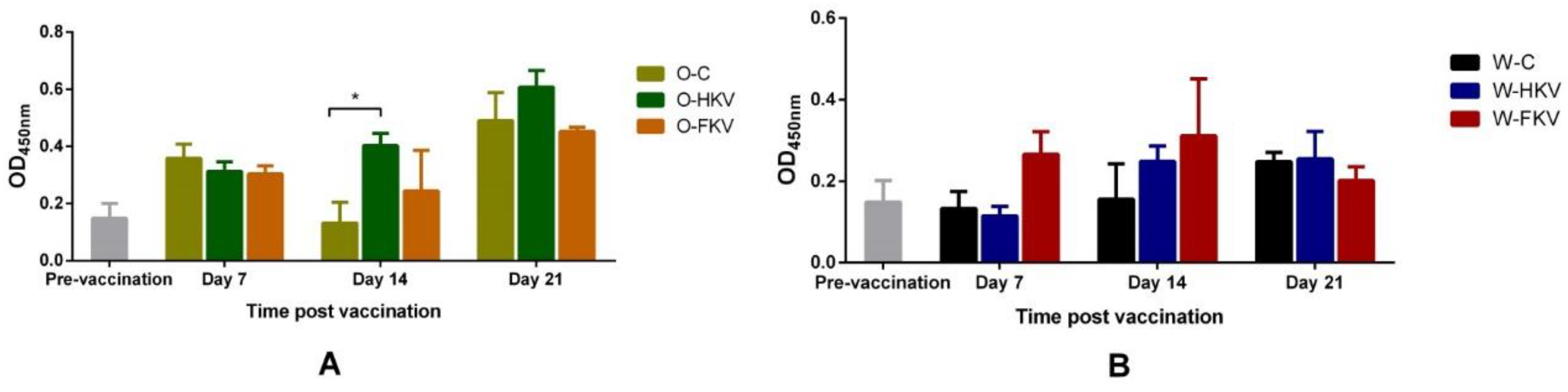
3.3. IgM antibody levels
4. Discussion
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO. In Brief to The State of World Fisheries and Aquaculture 2022. 2022. Available online: https://www.fao.org/3/cc0461en/cc0461en.pdf.
- Prabu, E.; Rajagopalsamy, C.; Ahilan, B.; Jeevagan, I.; Renuhadevi, M. Tilapia—An excellent candidate species for world aquaculture: A review. Annu. Res. Rev. Biol. 2019, 31, 1–14. [Google Scholar] [CrossRef]
- Kayansamruaj, P.; Areechon, N.; Unajak, S. Development of fish vaccine in Southeast Asia: A challenge for the sustainability of SE Asia aquaculture. Fish Shellfish. Immunol. 2020, 103, 73–87. [Google Scholar] [CrossRef]
- Tang, K.F.J.; Bondad-Reantaso, M.G.; Surachetpong, W.; Dong, H.T.; Fejzic, N.; Wang, Q.; Wajsbrot, N.; Hao, B. FAO Fisheries and Aquaculture Circular NFIM / C1220 ( En ) TILAPIA LAKE VIRUS Fisheries and. vol. 1220. 2021.
- Eyngor, M.; Zamostiano, R.; Tsofack, J.E.K.; Berkowitz, A.; Bercovier, H.; Tinman, S.; Lev, M.; Hurvitz, A.; Galeotti, M.; Bacharach, E.; et al. Identification of a Novel RNA Virus Lethal to Tilapia. J. Clin. Microbiol. 2014, 52, 4137–4146. [Google Scholar] [CrossRef]
- Ferguson, H.W.; Kabuusu, R.; Beltran, S.; Reyes, E.; A Lince, J.; del Pozo, J. Syncytial hepatitis of farmed tilapia, Oreochromis niloticus (L.): a case report. J. Fish Dis. 2013, 37, 583–589. [Google Scholar] [CrossRef]
- Dong, H.T.; Senapin, S.; Gangnonngiw, W.; Nguyen, V.V.; Rodkhum, C.; Debnath, P.P.; Delamare-Deboutteville, J.; Mohan, C.V. Experimental infection reveals transmission of tilapia lake virus (TiLV) from tilapia broodstock to their reproductive organs and fertilized eggs. Aquaculture 2020, 515, 734541. [Google Scholar] [CrossRef]
- Yamkasem, J.; Tattiyapong, P.; Kamlangdee, A.; Surachetpong, W. Evidence of potential vertical transmission of tilapia lake virus. J. Fish Dis. 2019, 42, 1293–1300. [Google Scholar] [CrossRef]
- Secombes, C.J.; Belmonte, R. Overview of the Fish Adaptive Immune System. Birkhauser Adv Infect Dis 2016, 35–52. [Google Scholar] [CrossRef]
- Bacharach, E.; Eldar, A. (2016). Tilapia lake virus vaccines. US Patent Application Publication no.US2016/0354458A1. Available online: https://patents.google.com/paten t/US201 60354 458A1/en.
- Zeng, W.; Wang, Y.; Chen, X.; Wang, Q.; Bergmann, S.M.; Yang, Y.; Wang, Y.; Li, B.; Lv, Y.; Li, H.; et al. Potency and efficacy of VP20-based vaccine against tilapia lake virus using different prime-boost vaccination regimens in tilapia. Aquaculture 2021, 539, 736654. [Google Scholar] [CrossRef]
- Zeng, W.; Wang, Y.; Hu, H.; Wang, Q.; Bergmann, S.; Wang, Y.; Li, B.; Lv, Y.; Li, H.; Yin, J.; et al. Cell Culture-Derived Tilapia Lake Virus-Inactivated Vaccine Containing Montanide Adjuvant Provides High Protection against Viral Challenge for Tilapia. Vaccines 2021, 9, 86. [Google Scholar] [CrossRef]
- Mai, T.T.; Kayansamruaj, P.; Taengphu, S.; Senapin, S.; Costa, J.Z.; Del-Pozo, J.; Thompson, K.D.; Rodkhum, C.; Dong, H.T. Efficacy of heat-killed and formalin-killed vaccines against Tilapia tilapinevirus in juvenile Nile tilapia ( Oreochromis niloticus ). J. Fish Dis. 2021, 44, 2097–2109. [Google Scholar] [CrossRef]
- Mai, T.T.; Kayansamruaj, P.; Soontara, C.; Kerddee, P.; Nguyen, D.-H.; Senapin, S.; Costa, J.Z.; Del-Pozo, J.; Thompson, K.D.; Rodkhum, C.; et al. Immunization of Nile Tilapia (Oreochromis niloticus) Broodstock with Tilapia Lake Virus (TiLV) Inactivated Vaccines Elicits Protective Antibody and Passive Maternal Antibody Transfer. Vaccines 2022, 10, 167. [Google Scholar] [CrossRef] [PubMed]
- Tattiyapong, P.; Kitiyodom, S.; Yata, T.; Jantharadej, K.; Adamek, M.; Surachetpong, W. Chitosan nanoparticle immersion vaccine offers protection against tilapia lake virus in laboratory and field studies. Fish Shellfish. Immunol. 2022, 131, 972–979. [Google Scholar] [CrossRef] [PubMed]
- Bonam, S.R.; Partidos, C.D.; Halmuthur, S.K.M.; Muller, S. An Overview of Novel Adjuvants Designed for Improving Vaccine Efficacy. Trends Pharmacol. Sci. 2017, 38, 771–793. [Google Scholar] [CrossRef] [PubMed]
- Tafalla, C.; Bøgwald, J.; Dalmo, R.A. Adjuvants and immunostimulants in fish vaccines: Current knowledge and future perspectives. Fish Shellfish. Immunol. 2013, 35, 1740–1750. [Google Scholar] [CrossRef]
- Jazayeri, S.D.; Lim, H.X.; Shameli, K.; Yeap, S.K.; Poh, C.L. Nano and Microparticles as Potential Oral Vaccine Carriers and Adjuvants Against Infectious Diseases. Front. Pharmacol. 2021, 12, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Hoare, R.; Jung, S.-J.; Ngo, T.; Bartie, K.; Bailey, J.; Thompson, K.; Adams, A. Efficacy and safety of a non-mineral oil adjuvanted injectable vaccine for the protection of Atlantic salmon (Salmo salar L.) against Flavobacterium psychrophilum. Fish Shellfish. Immunol. 2019, 85, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Veenstra, K.A.; Wang, T.; Russell, K.S.; Tubbs, L.; Ben Arous, J.; Secombes, C.J. Montanide™ ISA 763A VG and ISA 761 VG induce different immune pathway responses in rainbow trout (Oncorhynchus mykiss) when used as adjuvant for an Aeromonas salmonicida bacterin. Fish Shellfish. Immunol. 2021, 114, 171–183. [Google Scholar] [CrossRef]
- Arous, J.B. Vaccine oil adjuvants for the development of aquaculture. Veterinary Science Today 2016;4:62-65.
- Cao, T.T.; Tsai, M.-A.; Yang, C.-D.; Wang, P.-C.; Kuo, T.-Y.; Chen, H.-C.G.; Chen, S.-C. Vaccine efficacy of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) from Edwardsiella ictaluri against E. tarda in tilapia. J. Gen. Appl. Microbiol. 2014, 60, 241–250. [Google Scholar] [CrossRef]
- Xu, W.; Jiao, C.; Bao, P.; Liu, Q.; Wang, P.; Zhang, R.; Liu, X.; Zhang, Y. Efficacy of Montanide™ ISA 763 A VG as aquatic adjuvant administrated with an inactivated Vibrio harveyi vaccine in turbot (Scophthalmus maximus L.). Fish Shellfish. Immunol. 2018, 84, 56–61. [Google Scholar] [CrossRef]
- Chamtim, P.; Suwan, E.; Dong, H.T.; Sirisuay, S.; Areechon, N.; Wangkahart, E.; Hirono, I.; Mavichak, R.; Unajak, S. Combining segments 9 and 10 in DNA and recombinant protein vaccines conferred superior protection against tilapia lake virus in hybrid red tilapia (oreochromis sp.) compared to single segment vaccines. Front. Immunol. 2022, 13, 935480. [Google Scholar] [CrossRef]
- Taengphu, S.; Kayansamruaj, P.; Kawato, Y.; Delamare-Deboutteville, J.; Mohan, C.V.; Dong, H.T.; Senapin, S. Concentration and quantification of Tilapia tilapinevirus from water using a simple iron flocculation coupled with probe-based RT-qPCR. PeerJ 2022, 10, e13157. [Google Scholar] [CrossRef]
- Reed, L. .; Muench, H. A simple method of estimating fifty per cent endpoints. Am. J. Hyg. 1938, 27, 493–497. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Soonthonsrima, T.; Wangman, P.; Chaivisuthangkura, P.; Pengsuk, C.; Sithigorngul, P.; Longyant, S. Generation of mouse monoclonal antibodies specific to tilapia immunoglobulin using fish immunoglobulin/BSA complex for monitoring of the immune response in Nile tilapia Oreochromis niloticus. Aquac. Res. 2018, 50, 277–283. [Google Scholar] [CrossRef]
- Wangkahart, E.; Thongsrisuk, A.; Vialle, R.; Pholchamat, S.; Sunthamala, P.; Phudkliang, J.; Srisapoome, P.; Wang, T.; Secombes, C.J. Comparative study of the effects of Montanide™ ISA 763A VG and ISA 763B VG adjuvants on the immune response against Streptococcus agalactiae in Nile tilapia (Oreochromis niloticus). Fish Shellfish. Immunol. 2023, 134, 108563. [Google Scholar] [CrossRef] [PubMed]
- Smith, N.C.; Rise, M.L.; Christian, S.L. A Comparison of the Innate and Adaptive Immune Systems in Cartilaginous Fish, Ray-Finned Fish, and Lobe-Finned Fish. Front. Immunol. 2019, 10, 2292. [Google Scholar] [CrossRef] [PubMed]
- Secombes, C.; Wang, T. The innate and adaptive immune system of fish. Woodhead Publishing Limited; 2012. [CrossRef]
- Somamoto, T.; Nakanishi, T.; Okamoto, N. Role of Specific Cell-Mediated Cytotoxicity in Protecting Fish from Viral Infections. Virology 2002, 297, 120–127. [Google Scholar] [CrossRef]
- Tattiyapong, P.; Dechavichitlead, W.; Waltzek, T.B.; Surachetpong, W. Tilapia develop protective immunity including a humoral response following exposure to tilapia lake virus. Fish Shellfish. Immunol. 2020, 106, 666–674. [Google Scholar] [CrossRef]
- Velázquez, J.; Acosta, J.; Lugo, J.M.; Reyes, E.; Herrera, F.; González, O.; Morales, A.; Carpio, Y.; Estrada, M.P. Discovery of immunoglobulin T in Nile tilapia (Oreochromis niloticus): A potential molecular marker to understand mucosal immunity in this species. Dev. Comp. Immunol. 2018, 88, 124–136. [Google Scholar] [CrossRef]
- Wang, B.; Wang, P.; Wu, Z.-H.; Lu, Y.-S.; Wang, Z.-L.; Jian, J.-C. Molecular Cloning and Expression Analysis of IgD in Nile Tilapia (Oreochromis niloticus) in Response to Streptococcus agalactiae Stimulus. Int. J. Mol. Sci. 2016, 17, 348. [Google Scholar] [CrossRef]

| Treatment | Abbreviation | Number of fish challenged | Number of total dead fish at 15 dpc | RPS (%) 15 dpc |
|---|---|---|---|---|
| Water based (W) | ||||
| Control (Cell culture supernatant, L15) | W-C | 31 | 5 | |
| Heat-killed vaccine | W-HKV | 27 | 2 | 54.6 |
| Formalin-killed vaccine | W-FKV | 31 | 2 | 59.7 |
| Oil-based (O) (MontanideTM ISA 763A VG) | ||||
| Control (L15 + Oil adjuvant) | O-C | 32 | 8 | |
| Heat-killed vaccine | O-HKV | 28 | 5 | 32.0 |
| Formalin-killed vaccine | O-FKV | 24 | 9 | 0 |
| Gene | Oligo sequences | Annealing temperature (oC) | Product size (bp) | Reference |
|---|---|---|---|---|
| EF-1α | F-5’-CTACAGCCAGGCTCGTTTCG-3’ R-5’-CTTGTCACTGGTCTCCAGCA-3’ |
56 | 139 | [34] |
| IgM | F-5’-GGATGACGAGGAAGCAGACT-3’ R-5’-CATCATCCCTTTGCCACTGG-3’ |
53 | 122 | [34] |
| IgT | F-5’-TGACCAGAAATGGCGAAGTCTG-3’ R-5’-GTTATAGTCACATTCTTTAGAATTACC-3’ |
53 | 163 | [34] |
| IgD | F-5′- AACACCACCCTGTCCCTGAAT- 3′ R-5’-GGGTGAAAACCACATTCCAAC- 3’ |
61 | 127 | [35] |
| CD4 | F- 5′- GCTCCAGTGTGACGTGAAA- 3′ R- 5′- TACAGGTTTGAGTTGAGCTG- 3′ |
61 | 106 | [13] |
| CD8 | F- 5′- GCTGGTAGCTCTGGCCTTT- 3′ R- 5′- TGTGATGGTGTGGGCATCTC- 3′ |
49.5 | 91 | [13] |
| Time point | Treatment groups | Head kidney | ||||
|---|---|---|---|---|---|---|
| IgM | IgT | IgD | CD4 | CD8 | ||
| D7 | O-C | 1.07 ± 0.46 | 1.02 ± 0.20 | 1.07 ± 0.44 | 1.09 ± 0.56 | 1.08 ± 0.51 |
| O-HKV | 1.75 ± 0.78 | 3.14 ± 0.73↑ | 2.93 ± 1.24↑ | 8.53 ± 4.97↑ | 0.79 ± 0.11 | |
| O-FKV | 1.53 ± 0.47 | 1.61 ± 0.99 | 1.89 ± 0.11↑ | 2.22 ± 0.46 | 0.68 ± 0.46 | |
| W-C | 1.55 ± 1.77 | 1.48 ± 1.61 | 1.07 ± 0.44 | 1.05 ± 0.40 | 1.03 ± 0.31 | |
| W-HKV | 0.96 ± 0.74 | 0.55 ± 0.32 | 1.19 ± 0.86 | 0.33 ± 0.05 | 0.20 ± 0.01 | |
| W-FKV | 6.00 ± 2.81↑ | 1.43 ± 0.85 | 3.80 ± 1.81↑ | 3.51 ± 2.55 | 2.26 ± 2.22 | |
| D14 | O-C | 1.58 ± 1.21 | 1.09 ± 0.48 | 1.01 ± 0.17 | 1.20 ± 0.73 | 1.40 ± 1.42 |
| O-HKV | 4.94 ± 4.11 | 0.77 ± 0.45 | 1.46 ± 0.92 | 2.89 ± 3.02 | 0.95 ± 1.00 | |
| O-FKV | 1.84 ± 2.25 | 0.37 ± 0.36 | 1.08 ±1.14 | 0.91 ± 0.42 | 1.40 ±1.03 | |
| W-C | 1.44 ± 1.05 | 0.96 ± 0.80 | 1.53 ± 1.70 | 1.02 ± 0.28 | 1.47 ± 1.59 | |
| W-HKV | 1.81 ± 0.64 | 1.09 ± 0.53 | 1.35 ± 0.45 | 2.25 ± 0.80 | 2.04 ± 1.37 | |
| W-FKV | 7.61 ± 8.34 | 2.93 ± 2.19 | 3.46 ± 2.27 | 5.65 ± 2.31↑ | 1.40 ± 1.15 | |
| D21 | O-C | 1.16 ± 0.80 | 1.02 ± 0.22 | 1.04 ± 0.33 | 1.80 ± 0.45 | 1.02 ± 0.23 |
| O-HKV | 0.91 ± 0.42 | 1.44 ± 0.40 | 3.22 ± 0.44↑↑ | 2.33 ± 0.71 | 2.31 ± 2.30 | |
| O-FKV | 1.91 ± 0.26 | 0.90 ± 0.13 | 2.09 ± 0.45↑ | 0.96 ± 0.43 | 1.00 ± 0.75 | |
| W-C | 1.36 ±1.30 | 1.29 ± 0.93 | 1.17 ± 0.82 | 1.22 ± 0.73 | 2.28 ± 3.21 | |
| W-HKV | 1.25 ± 0.94 | 1.20 ± 1.30 | 2.01 ±1.45 | 0.82 ± 0.50 | 5.29 ± 2.81 | |
| W-FKV | 0.83 ±0.66 | 0.66 ± 0.53 | 0.93 ± 0.61 | 0.46 ± 0.43 | 1.48 ± 1.66 | |
| Time point | Treatment groupsgroups | Spleen | ||||
|---|---|---|---|---|---|---|
| IgM | IgT | IgD | CD4 | CD8 | ||
| D7 | O-C | 1.51 ± 1.60 | 1.20 ± 0.75 | 1.05 ± 0.38 | 1.13 ± 0.72 | 1.08 ± 0.52 |
| O-HKV | 1.14 ± 0.45 | 0.90 ± 0.49 | 1.00 ± 0.78 | 2.80 ± 1.62↑↑ | 0.58 ± 0.73 | |
| O-FKV | 1.51 ± 0.94 | 0.93 ± 0.75 | 2.04 ± 1.34 | 1.93 ± 0.82 | 0.75 ± 0.49 | |
| W-C | 1.72 ± 1.97 | 1.79 ± 2.09 | 1.24 ± 0.86 | 1.44 ± 1.33 | 1.18 ± 0.71 | |
| W-HKV | 1.04 ±1.02 | 1.09 ± 0.96 | 1.23 ± 0.86 | 1.01 ± 0.69 | 0.50 ± 0.27 | |
| W-FKV | 0.31 ± 0.12 | 0.38 ± 0.22 | 0.42 ± 0.16 | 0.51 ± 0.21 | 0.19 ± 0.16 | |
| D14 | O-C | 1.19 ± 0.70 | 1.67 ± 1.72 | 1.20 ± 0.89 | 1.25 ± 1.01 | 2.60 ±2.90 |
| O-HKV | 1.09 ± 0.80 | 1.00 ± 0.67 | 1.22 ± 1.04 | 2.87 ± 1.96 | 1.50 ± 1.63 | |
| O-FKV | 1.81 ± 0.87 | 1.11 ± 0.32 | 2.71 ± 1.71 | 4.29 ± 1.00↑ | 6.75 ± 5.60 | |
| W-C | 1.43 ± 1.19 | 2.31 ± 2.80 | 1.66 ± 1.88 | 1.50 ± 1.11 | 1.06 ± 0.39 | |
| W-HKV | 6.91 ± 3.32↑ | 6.07 ± 4.82 | 4.12 ± 1.40 | 3.12 ± 1.03 | 6.14 ± 4.74 | |
| W-FKV | 4.30 ± 3.22 | 5.89 ± 0.41↑ | 5.69 ± 1.15↑ | 2.31 ± 0.51 | 3.44 ± 2.36 | |
| D21 | O-C | 1.02 ± 0.25 | 1.21 ± 0.94 | 1.02 ± 0.25 | 1.10 ± 0.51 | 1.08 ± 0.53 |
| O-HKV | 0.70 ± 0.24 | 1.15 ± 0.32 | 2.12 ± 0.56↑ | 0.83 ± 0.38 | 0.92 ± 0.75 | |
| O-FKV | 1.60 ± 2.38 | 0.43 ± 0.52 | 2.20 ± 3.05 | 0.61 ± 0.87 | 0.98 ± 1.36 | |
| W-C | 1.10 ± 0.55 | 1.70 ± 1.82 | 1.11 ± 0.65 | 1.83 ± 1.65 | 1.03 ± 0.29 | |
| W-HKV | 1.82 ± 1.79 | 2.76 ± 2.03 | 2.23 ± 2.55 | 1.58 ± 1.37 | 2.82 ± 1.55↑ | |
| W-FKV | 0.37 ± 0.10 | 0.79 ± 0.59 | 0.57 ± 0.19 | 0.33 ± 0.24 | 0.46 ± 0.38 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





