1. Introduction
As space exploration grows and with commercial space flights having begun, understanding the impact of microgravity on human physiology has become a more urgent question. Space medicine has always been fundamental to human exploration of space1,2. It is now expanding beyond its usual specialty within aerospace medicine, beyond being a discipline that tackles comprehensive health care delivery issues within the challenging environment of space into a discipline that uses that challenging environment for drug discovery and development for both terrestrial and space health applications3–5. The quest for personalized medicine for terrestrial purposes also becomes a quest for personalized medicine for space explorers6,7. Likewise, the current quest for quantifying drug-response at the cellular level in order to expedite terrestrial personalized medicine8 also merges with recent efforts to investigate the impact of microgravity on drug response at the cellular level9. Interestingly, a comprehensive literature review of the impact of microgravity on cancer cells, also highlights the increasing attempts to use simulated microgravity as a tool for drug discovery and development9. Within the broader question of the impact of microgravity on cells, is the more focused question: how do cells, cancer cells in particular, respond to drugs when subjected to microgravity? A comprehensive review of the literature in the last decade showed that little is known regarding how microgravity affects cellular and molecular events that determine physiological and biological responses in cancer cells10. A recapitulation of more recent literature in this regard is appropriate.
It has been shown that microgravity alters effects of chemotherapeutic drugs on cancer cell migration11,12. The alterations were drug-dependent: leukemic cancer cells treated with daunorubicin showed increased chemotactic migration (p < 0.01) following simulated microgravity (µg) compared to normal gravity on earth (1 G). But the same cells treated with doxorubicin showed enhanced migration both in 1 G and following µg11,12. A different group of researchers replicated the major conclusion (namely, alteration of drug-response by microgravity) from these findings11,12 using doxorubicin and gastric cancer cells13. Remarkably, Rembiałkowska et al. found that microgravity increased the chemotherapeutic effect in the case of drug-resistant gastric cancer cells13. In particular, they found that following simulated microgravity, gastric cancer cells showed decreased expression of genes related to drug resistance and increased DNA/RNA-damage marker expression. Moreover, they reported significant reorganization of F-actin fibers in these cells after exposure to microgravity conditions13 and such morphological reorganization might be implicated in the reported altered (here, increased) sensitivity to chemotherapy. Similar cytoskeletal and morphological reorganization induced by microgravity was found to correlate with the reduction of cisplatin resistance in ovarian cancer cells14.
Unsurprisingly, since all these reports11–14 comparing the response of cancer cells to drugs in normal gravity on earth (1 G) versus microgravity conditions are very recent, there is a paucity of such reports in the literature. However, recent literature is replete with findings confirming previously reported changes in single cells including cancer cells, due to microgravity conditions. Our major premise in this work is that those recently confirmed microgravity-induced changes in single cells, may also lead to modulation of drug-response in those cells. A quick survey of recently confirmed microgravity-induced alterations in cancer cells and cancer-related cells follows, in order to place our work within a broader context in space biomedicine.
Macrophages and other immune cells have emerged as major tools and targets in cancer therapy15,16. Macrophages, long known to be altered by microgravity17, have been found to respond to microgravity in the International Space Station (ISS) in a matter of seconds18. Several reports showed that simulated microgravity alters the growth and differentiation of stem cells and cancer stem cells19–23. Recently, human blood-derived stem cells cultured under microgravity conditions in the ISS showed alterations in osteoblastic differentiation24, in line with previous results from simulated microgravity. Grimm et al. provide a review of the literature on the effects of both simulated and real microgravity on the differentiation and growth of stem cells and cancer stem cells25. Cultured aboard the ISS for 5.5 weeks, human induced pluripotent stem cell-derived cardiomyocytes have confirmed microgravity-induced alterations to calcium handling26 in cells. These newly confirmed alterations induced by microgravity in cancer and cancer-related cells have several intersections with currently known hallmarks of cancer27–30 such as sustaining proliferative signaling, resisting cell death, enabling replicative immortality and activating invasion and metastasis. Since anti-cancer drugs use these hallmarks as therapeutic targets, direct or indirect alterations of these hallmarks by microgravity could engender changes in the response of cancer cells to these drugs.
To address the question whether confirmed changes that microgravity produces in cancer cells might cause alterations in the response of such cells to common cancer drugs, we selected two well characterized cancer cell lines: an adherent cell line, T98G31,32 and cancer cells that grow in suspension, K56233,34, to confirm known effects of microgravity via a NASA-developed and widely used rotary cell culture system (RCCS)21,35–37. Following 48 hours of exposure to microgravity, we then treated the leukemic cells with two drugs used in the treatment of leukemia and other cancers, hydroxyurea38, which functions by inhibiting mitosis thereby blocking cell growth, and paclitaxel, which causes mitotic cell cycle arrest by stabilizing microtubules39,40. Using fluorescence-guided morphometry to visualize and quantify the microgravity-induced alterations and implications on drug response, we found an intriguing loss of the significant reduction (p < 0.0l) to the nuclear to cytoplasm ratio of cancer cells treated with hydroxyurea. Changes found with paclitaxel were not statistically significant. Our results accentuate the need for further studies on the impact of microgravity on cancer cell drug responses in particular and cellular drug-response in general, to prime space medicine for space exploration while advancing terrestrial medicine.
2. Materials and Methods
2.1. Cell Culture
We used the glioblastoma multiforme cell line isolated from a 61-year-old patient, T98G (ATCC® CRL-1690™) and the lymphoblastic cell line isolated from the bone marrow of a 53-year-old chronic myelogenous leukemia patient, K562 (ATCC CCL-243) both purchased from the American Type Cell Culture Collection (ATCC, Manassas, VA, USA). T98G cells are adherent cells with a fibroblast morphology. The K562 cells grow in suspension. We followed specific protocols from ATCC in culturing both cell types, as reported in our previous works12,39,41,42. Briefly, we cultured K562 cells using RPMI 1640 (11875093, Life Technologies), supplemented with 10% (v/v) fetal bovine serum (FBS) and 1% penicillin/streptomycin as growth medium. We cultured the T98G cells in Eagle’s Minimum Essential Medium (EMEM, ATCC 30-2003) or Dulbecco’s Modified Eagle Medium (DMEM, Corning 10-013-CMR) supplemented with 10% fetal bovine serum (FBS, Gibco, New York, NY, USA, 10100147) and 1% penicillin–streptomycin (P/S, Sigma Aldrich, St Louis, MI, USA, P4333-100ML). Both cell lines were maintained in an incubator kept at 95% air; 5% CO2 and a temperature of 37 °C. All experiments were performed when cells were in the logarithmic growth phase, usually at a viability of over 95%. Routine viability tests were done using trypan blue (Sigma-Aldrich, St Louis, MI, USA), manual counting with a hemocytometer or Invitrogen Countess II automatic cell counter (Thermo Fisher Scientific, Waltham, MA, USA, AMQAX1000).
2.2. Rotary Cell Culture System
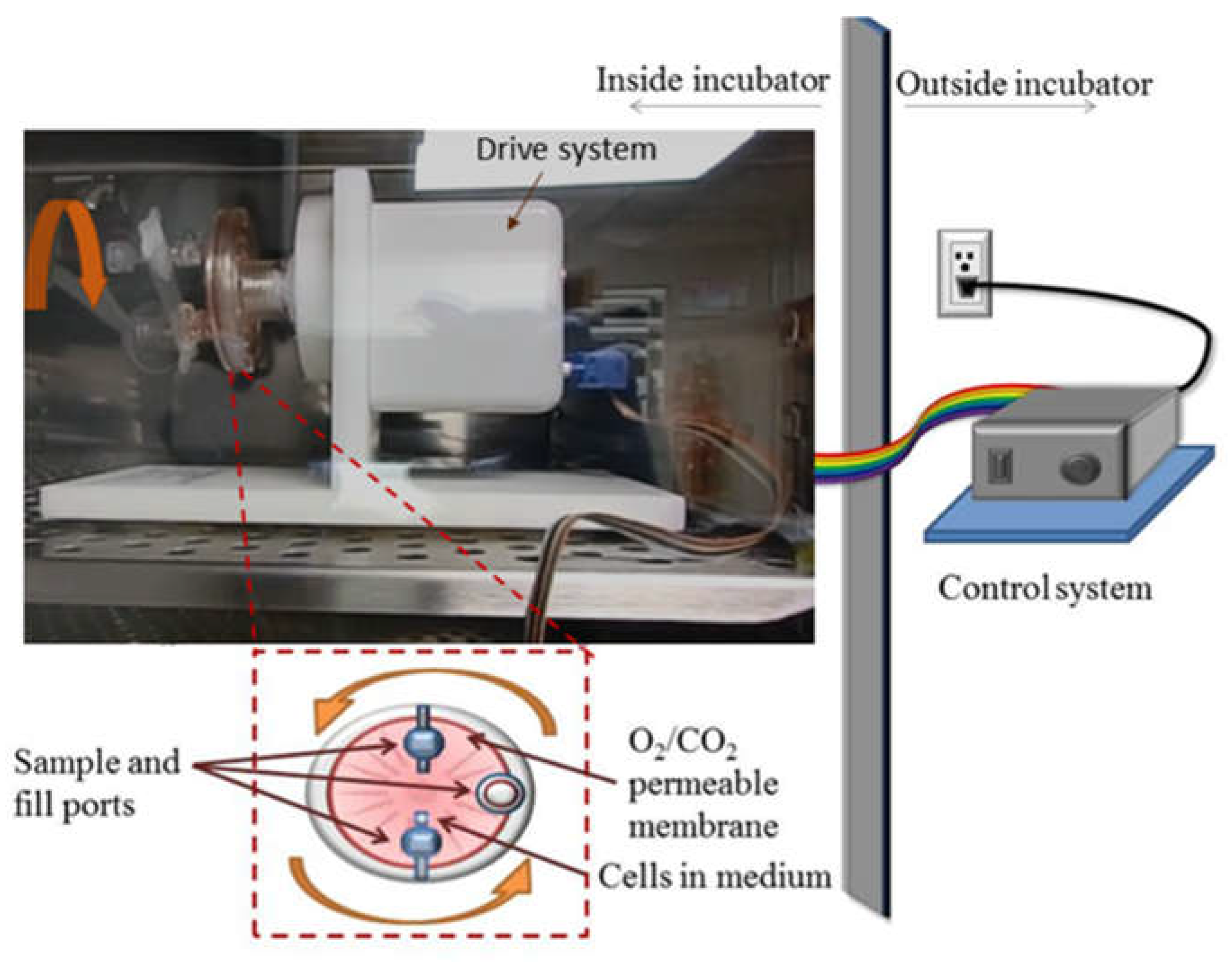
Simulated microgravity conditions were produced using the rotary cell culture system, RCCS™, (
Figure 1). RCCS was developed at the Johnson Space Center by NASA. We purchased a commercially available version from Synthecon
® Inc. (Houston, TX, USA). The RCCS not only simulates microgravity but also serves as a 3D cell culture technology for culturing both suspension and anchorage-dependent cells
13,35,37. The RCCS is a bioreactor equipped with 10 mL or 50 mL disposable high aspect ratio vessel (HARV), with a silicon membrane on one side that provides gas exchange. It is designed to provide a low-shear cell culture system. Via vertical rotation, the RCCS produces rigid-body rotation of the entire HARV and the cell culture medium, thereby randomizing the gravity vector to simulate microgravity
12,22,37 . We seeded T98G and K562 cells at a concentration of 2 × 10
5 cells/mL in T-25 flasks (as static controls at normal gravity, 1
g) and in 10 mL HARVs and rotated at 15 rpm as microgravity condition,
µg. This rotation speed is the reported optimal speed for many cell types
13,36,37 including K562 and T98G cells, in accordance with the manufacturer’s protocols. Cells were maintained in this microgravity condition for 48 hours (K562) or 72 hours (T98G) inside the same incubator (
Figure 1) as the normal gravity or 1 G cells. The T98G cells were used for functional verification of our simulated microgravity conditions while the K562 cells were used to assess drug response.
2.3. Pharmacological Interventions
Following 48 hours of exposure to microgravity µg, the K562 leukemic cells were treated with two chemotherapeutic drugs used against leukemia and other cancers, namely, hydroxyurea and paclitaxel. Hydroxyurea is an antitumor and antileukemic agent which functions by inhibiting mitosis thereby blocking cell growth38. Paclitaxel also functions by blocking cell growth through mitotic cell cycle arrest achieved by stabilizing microtubules39,40. The final concentration of paclitaxel (Sigma-Aldrich 580555) was 5 μM. The final concentration of hydroxyurea (Sigma-Aldrich, H8267) was 100 μM. Cells were incubated for 24 hours following drug treatments, before fluorescence microscopy was carried out, to assist with morphometry.
2.4. Fluorescence Microscopy
Fluorescence microscopy of cells was carried out using a Zeiss Vert.A1 AXIO fluorescence microscope with an inserted USB microscope camera (AmScope MU300), captured using the AmScope (86x) image capture program. The fluorescent dyes, Hoechst 33342 (ThermoFisher Scientific) and Calcein AM (Invitrogen by ThermoFisher Scientific), were used to stain the cells for imaging. Hoechst 33342 is a blue (460-490 nm emission) fluorescent dye used for staining cellular DNA43,44. Calcein AM is an initially nonfluorescent dye which is membrane-permeable, but upon uptake into the cell, is degraded partially, making the dye membrane-impermeable. The degradation also results in the conversion from a nonfluorescent solution to green fluorescence (515 nm peak emission). The dye is thus retained selectively in cells with intact membranes and is often used as a measure of cell viability45, as it stains the whole cytoplasm. Hoechst 3342 was used at a final concentration of 0.1 mM, while Calcein was used at a concentration of 1 µg/mL. Various treatment conditions of both T98G and K562 cells were stained, incubated and imaged as control and also following other experimental conditions such as 48 hours post-microgravity for K562 cells and 78 hours post-microgravity for T98G. The T98G cells were imaged both in the adherent state (1 G) and in the suspended state following microgravity-induced formation of tissue spheroids.
Prior to imaging, the dyes were added directly to the culture medium in the concentrations noted previously. Each dye was incubated with the cells for 5-10 minutes. Once absorbed, the cells were washed twice with 2.0 mL of PBS to rinse away excess dye for optimal visual contrast. Cells were imaged while in 1.0 mL of PBS to prevent drying. The Hoechst 33342 DNA stain was imaged under a blue filter, while Calcein was imaged under a green filter to selectively view their emissions.
2.5. Fluorescence-Guided Morphometry
Fluorescence images of cells were analyzed qualitatively and quantitatively using freely available ImageJ software (National Institute of Heath, NIH) and user-developed plugins. ImageJ is widely used in a broad range of applications requiring image analysis. Built-in techniques and plugins have steadily optimized acquisition of image-based measurements, from simple parameters such as length and area, to more complex analyses, some catered to highly specific applications. For our purposes, image analysis was used to gather morphological data and to detect changes in cell characteristics following treatment. Though these effects could be visualized qualitatively, quantitative analysis was carried out using the following parameters:
1. Lacunarity, Λ. Lacunarity, also referred to as texture, inhomogeneity, or “gappiness,” is a measure of heterogeneity in images. As heterogeneity or number and size of gaps in a region of interest increase, so does the lacunarity. This quantity has been used in numerous studies, including our previous work39 as an indicator of granularity or inhomogeneity in cell morphology potentially caused by drug treatments. In this work, lacunarity was used to evaluate the heterogeneity in cell cytoplasm and nuclei following various treatments. Cells were first dyed with Hoechst 33342 to enhance the morphometry. Lacunarity was measured using ImageJ’s FracLac plugin. Cells imaged under 40x magnification were used in the analyses.
In its most basic form, lacunarity is calculated thus:
where
is the lacunarity,
is the caliber or size of the “box” in a grid for counting pixels via the “box counting” method,
g is the position in a grid overlaying the image, and the lacunarity was calculated by dividing the standard deviation
of the pixel values per box by the mean
and squaring the quotient. The lacunarity was thus based on variations in pixel value for set box sizes throughout the image. In addition to presenting the measured values for lacunarity, FracLac has a built-in function which shows the dependence of the lacunarity on the box size. For our work, we used lacunarity to measure the complexity of images of cells, where changes in complexity may have been the direct result of treatment.
2. Circularity. Circularity,
C, is a shape descriptor defined thus:
where
A is the area and
P is the perimeter of the object (cell). The circularity ranges from 0 for infinitely elongated polygon to 1 for a perfect circle. Owing to increased computational power and ease of automatic calculation, circularity is now increasingly used for cell morphometry in diverse biomedical contexts from high-throughput cell sorting for clinical diagnostics using flow cytometry or deformability cytometry
46, to treatment prognosis of sickle cell disease
47.
3. Nuclear to Cytoplasmic Ratio, N/C. Also termed nucleus to cytoplasm ratio, N/C, is simply the ratio of the cross-sectional area of the nucleus divided by that of cytoplasm. The N/C is already widely used as a sensitive diagnostic parameter for malignancy, including the staging of cancer48. This arises from the fact that one of the most prevalent hallmarks of cancerous cells is the enlargement of the nucleus49 engendered by increased amounts of chromatin present within malignant cells.
2.6. Statistics and Error Analyses
To determine the statistical significance of differences found in measured parameters, we carried out statistical and error analyses using analysis of variance (ANOVA) in Origin (OriginLab, Northampton, MA, USA). ANOVA is a well-established parametric method for means-based comparison of data groups. Origin’s ANOVA algorithm provides an attractive benefit in that it minimizes the probability of type-I error in statistical analysis. In type-I error, the null hypothesis is wrongly rejected, leading to the wrong conclusion that results are statistically significant. We performed error analysis using the standard error of the mean (SEM). We carried out at least three independent repeats of every experiment (N = 1, N = 2, N = 3) and report both independent repeats and the averages of the triplicate experiments, as needed, in the main text and in the supplementary information.
3. Results
3.1. Generated 3D tissue spheroids confirm microgravity in RCCS
To confirm that our RCCS does produce simulated microgravity, we trypsinized and suspended T98G cancer cells at a concentration of 2 × 10
5 cells/mL in 10 mL HARVs and rotated these at 15 rpm as already described in the methods section.
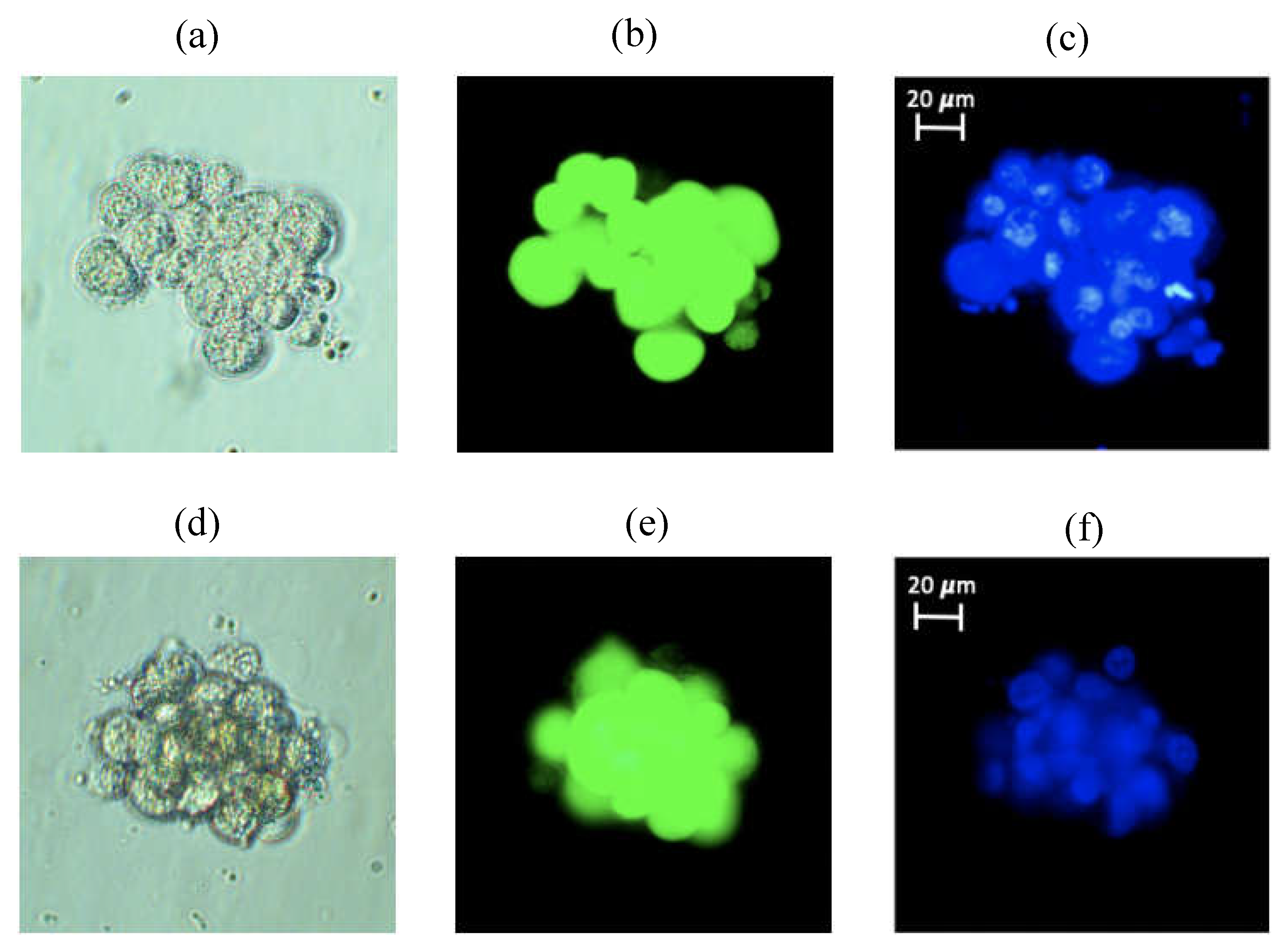
Figure 2 shows two different 3D spheroids formed from about 20 cells (top panel) and about 18 cells (bottom panel). In
Figure 2, the images shown from left to right are (a) and (d) phase contrast, (b) and (e) Calcein-stained (cytoplasm) and (c) and (f) Hoechst-stained (nucleus) images of the same cells in each row. Once brought into suspension, T98G cells usually sink to the bottom of any vessel and adhere to it in less than 6 hours and such adherent cells do not form 3D spheroids but rather 2D monolayer as shown in
Figure S1. Thus, the formation of 3D spheroids inside our RCCS setup, shown in
Figure 2 (and in
Figure S2, to illustrate multiple spheroids), confirms that our setup does generate microgravity, since such 3D spheroid formation is a well-established effect of microgravity
9,25.
3.2. N/C ratio of untreated cells remain unchanged following microgravity
We carried out morphometric analyses on Calcein- and Hoechst-stained images of K562 subjected to 48 hours of microgravity and various pharmacological interventions as previously described in the methods section. Of the three morphometric parameters applied, namely, lacunarity,
Λ, circularity,
C, and nucleus to cytoplasm ratio,
N/C, only the
N/C ratio revealed significant differences in some of the conditions. The morphometric tests were done on 3 to 5 single cells from at least 10 different fields of view taken at 40x, thereby ensuring that over 40 cells were tested for each condition in each experiment, for satisfactory statistical analysis. The lack of significant differences in lacunarity and circularity between different conditions was consistent with our qualitative finding that the entire cytoplasm of the K562 cells did not change much irrespective of the pharmacological intervention. Moreover, the Hoechst stain alone provided sufficient contrast for the extraction of nuclear to cytoplasmic ratio as can be seen in
Figure 2 and
Figure S3.
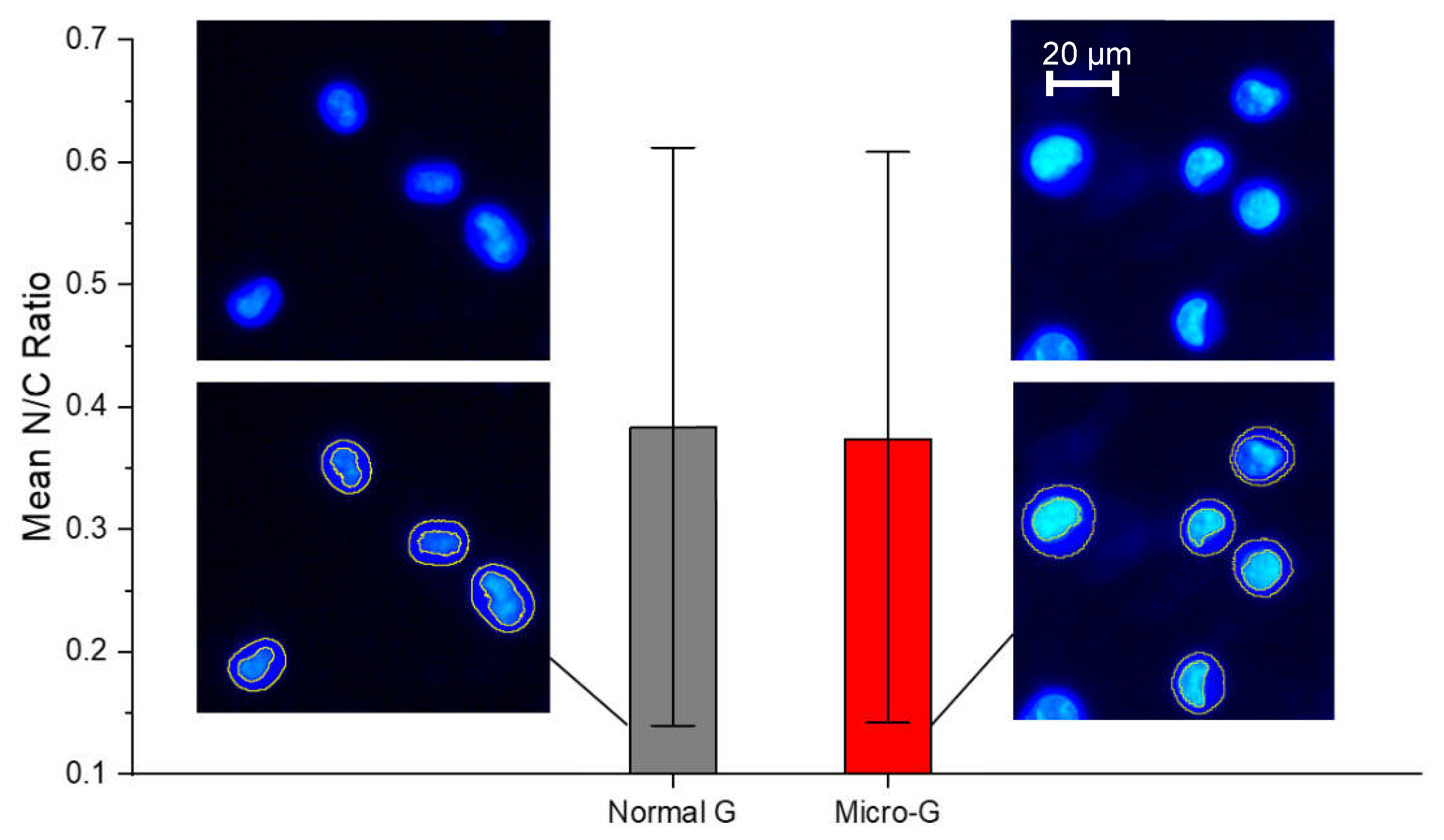
As shown in
Figure 3, the mean
N/C ratio of K562 cells grown in normal gravity (normal G or 1 G) was not significantly different from the
N/C ratio of K562 cells subjected to 48 hours of simulated microgravity (micro-G or
µg). Both conditions show a wide and comparable range of
N/C ratios, consistent with the fact that by coupling DNA content, nuclear size, and cell size according to each cell’s stage in the cell cycle, an unsynchronized cell culture maintains a nearly constant but wide range of
N/C ratios. This is an interesting result that enables any significant differences obtained in
N/C ratios following pharmacological interventions to be attributable to drug response mechanisms.
3.3. Hydroxyurea treated cells have significantly altered N/C ratio in normal G
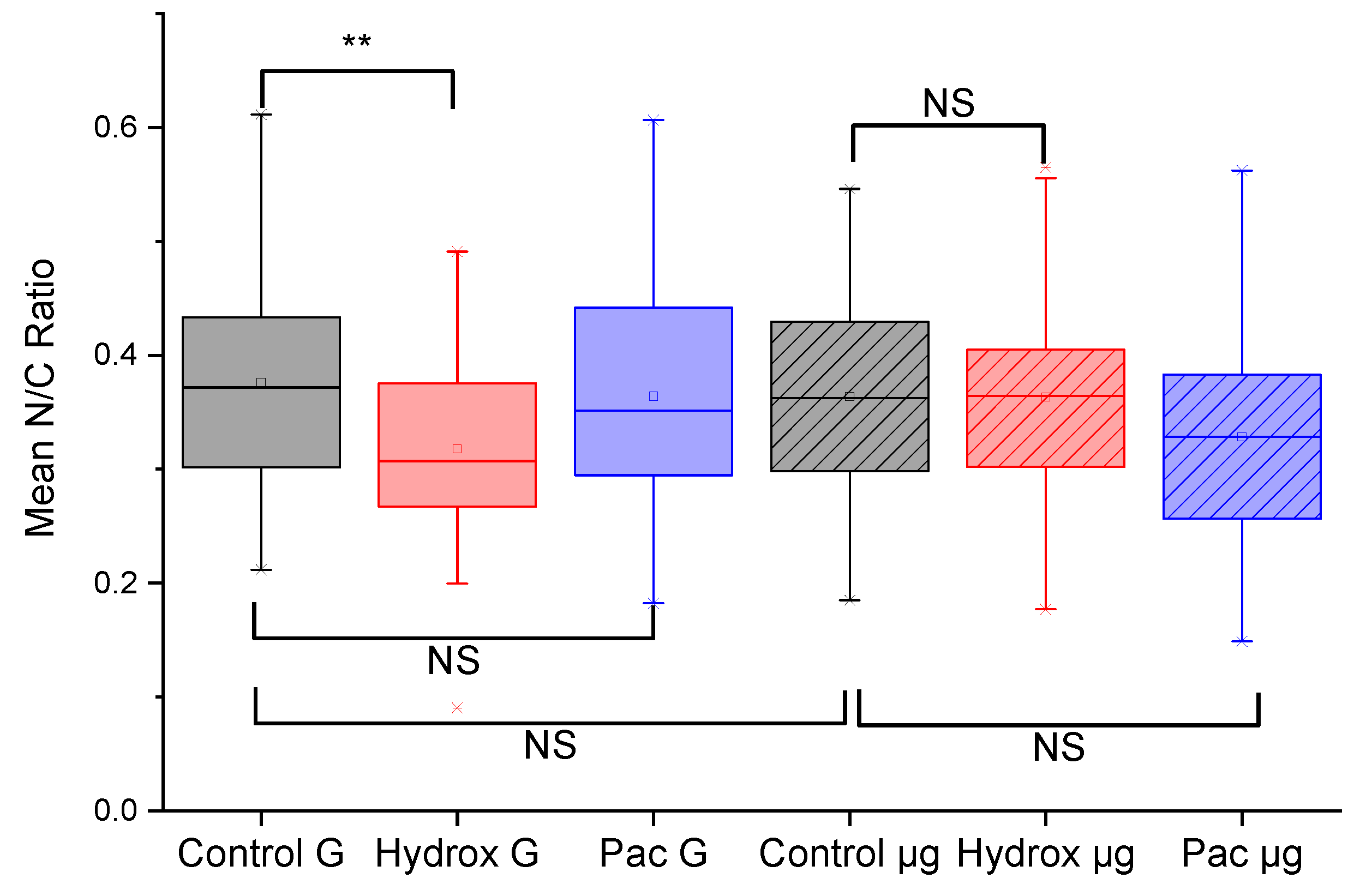
We found a significant decrease (p < 0.01) in
N/C ratio between untreated K562 cells in normal G compared to K562 cells treated for 24 hours with hydroxyurea also in normal G, as shown in
Figure 4. Since hydroxyurea is an antitumor and antileukemic agent which functions by inhibiting mitosis thereby blocking cell growth
38, the significant decrease in
N/C ratio caused by hydroxyurea treatment can be interpreted as a consequence of reduction in nuclear size. This interpretation seems plausible since hydroxyurea arrests cells in the S-phase
50, thereby impacting nuclear size distribution in an unsynchronized cell culture. Arresting cells in S-phase is so characteristic of hydroxyurea that there is widespread use of it as a cell-cycle synchronizing agent in cell biological experiments
50,51. Any loss of this response would therefore be a significant indication of a change in this specific drug response.
3.4. Microgravity eliminates loss of altered N/C ratio Hydroxyurea treated cells
To answer the main question raised in this study, namely, whether microgravity might cause alterations in the response of cancer cells to common cancer drugs, we subjected K562 cells to 48 hours of simulated microgravity using the RCCS as described in the methods section. As shown in
Figure 4, microgravity-subjected K562 cells treated with hydroxyurea (Hydrox µg) had a non-significant change in
N/C ratio compared to untreated microgravity-subjected K562 cells. This was an intriguing result, considering the significant reduction in
N/C ratio in the case of K562 cells in normal G. Moreover, the paclitaxel treated cells showed non-significant changes in
N/C ratio whether in normal G or in microgravity. Since the reduction in
N/C ratio with hydroxyurea treated cells in normal G was rather easy to explain based on the well-known drug mechanism, we further explored evidence for the action of paclitaxel.
3.5. Paclitaxel treated cells show no alterations in N/C ratio in normal G and in microgravity
In normal G, the paclitaxel treated cells showed no significant change in
N/C ratio compared to untreated cells (
Figure 4). Likewise, following 48 hours of microgravity, the paclitaxel treated cells also showed no significant change in
N/C ratio compared to untreated cells (
Figure 4). Since paclitaxel causes mitotic cell cycle arrest by stabilizing microtubules
39,40, one would expect the classic formation of micronuclei due to mitotic catastrophe in paclitaxel treated cells. That is exactly what we found in our paclitaxel treated K562 cells (24 hours post-treatment), as shown in
Figure 5. Nuclear fragmentation was found both in the normal G set of experiments as well as in the microgravity set of experiments. Of course, nuclear fragmentation does not mean reduction in nuclear size but rather several pieces of the nuclear material instead of one nucleus. Taken together,
Figure 4 and
Figure 5 indicate that microgravity significantly alters K562 response to hydroxyurea, but not the response to paclitaxel. Some of the implications of this finding and the need for further work are discussed next.
4. Discussion and Conclusions
We have quantified microgravity-induced changes to drug-response in K562 cancer cells using fluorescence microscopy and morphometry. Morphometric parameters used included lacunarity, circularity and nuclear to cytoplasmic ratio, N/C. The N/C ratio was the parameter that showed the most significant changes between experimental conditions. Our findings have broad implications for reported work in the literature and ongoing research while giving impetus to new research directions. Firstly, the generated 3D tissue spheroids of T98G cells confirm microgravity in RCCS at our selected 15 RPM, since 3D spheroid formation is a well-ascertained effect of microgravity on adherent cells that have been brought into the suspended state under microgravity conditions9,25. Secondly, N/C ratio of untreated cells remain unchanged following microgravity. Although this result enabled us to attribute significant differences obtained in N/C ratios following pharmacological interventions to drug-response mechanisms in the cells, it will still be important to reconcile this “non-change” with the microgravity-induced genomic and proteomic differences reported in the literature52,53. Thirdly, hydroxyurea treated cells have significantly (p < 0.01) reduced N/C ratio in normal G. Microgravity apparently induces a loss of this reduction. This finding of ours supports the increasing attempts to use simulated microgravity as a tool for drug discovery and development9,10. Our fourth and fifth findings that microgravity eliminates the significant reduction of N/C ratio in hydroxyurea treated cells while the paclitaxel treated cells show no such alterations in N/C ratio in both normal G and in microgravity, extend recent findings that microgravity alters effects of chemotherapeutic drugs on cancer cell migration11,12 in a drug-dependent manner. Thus, a lot more work needs to be done on various cell types, using many different drugs before we can have reliable speculations on the molecular mechanisms by which microgravity induces changes to drug-responses in cells.
Overall, our results indicate a clear but tentative answer to the overarching question addressed in this work, namely, whether microgravity induces alterations to drug response in cancer cells. The clear and tentative answer is a caveated affirmative, that microgravity induces changes to drug response in cancer cells in a drug-dependent manner. Of course, other cancer cell types need to be tested similarly and many other drugs need to be used. Furthermore, detailed molecular mechanisms will emerge after more parameters are interrogated beyond morphometric characteristics. Our work has set the stage for a varied and variegated exploration of drug-response in microgravity conditions, in view of personalized space medicine and in view of earth-based biomedical applications.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org, Figure S1: Images of adherent T98G cells; Figure S2: Microgravity-induced spheroid formation in T98G cells; Figure S3: Steps in morphometry of T98G cells using ImageJ.
Author Contributions
Conceptualization, A.E.; methodology, S.M., A.T., C.P., and A.E.; software, S.M., A.T., C.P.; validation, S.M., A.T., C.P., and A.E.; formal analysis, S.M., A.T., C.P.; investigation, S.M., A.T., C.P., M.J., G.K., Y.W. and A.E.; resources, A.E., M.J., and Y.W.; data curation, S.M., A.T., C.P., and A.E.; writing—original draft preparation, S.M., A.T., C.P., and A.E.; writing—review and editing, A.E.; visualization, S.M., A.T., C.P., Y.W. and A.E.; supervision, A.E.; project administration, A.E.; funding acquisition, A.E. and M.J. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding. This work was received funding support from the Creighton University Startup Grant to 240133 (to AEE) and the Creighton University Center for Undergraduate Research (CURAS).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article and within supplementary material.
Acknowledgments
Authors acknowledge the collegiality and support of other members of the Translational Biomedical Physics research group especially Ashley Abrahams and Virdi Sukhman whose sub-projects occasioned the common use and culture of cell lines reported here.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Hodkinson, P.; Anderton, R.; Posselt, B.; Fong, K. An overview of space medicine. Br. J. Anaesth. 2017, 119, i143–i153. [Google Scholar] [CrossRef]
- Roberts, D.R.; Albrecht, M.H.; Collins, H.R.; Asemani, D.; Chatterjee, A.R.; Spampinato, M.V.; Zhu, X.; Chimowitz, M.I.; Antonucci, M.U. Effects of Spaceflight on Astronaut Brain Structure as Indicated on MRI. New Engl. J. Med. 2017, 377, 1746–1753. [Google Scholar] [CrossRef] [PubMed]
- Greener, M. Drug discovery and development: the final frontier. Prescriber 2020, 31, 18–22. [Google Scholar] [CrossRef]
- Blue, R.S.; Bayuse, T.M.; Daniels, V.R.; Wotring, V.E.; Suresh, R.; Mulcahy, R.A.; Antonsen, E.L. Supplying a pharmacy for NASA exploration spaceflight: challenges and current understanding. npj Microgravity 2019, 5, 1–12. [Google Scholar] [CrossRef]
- Eyal, S.; Derendorf, H. Medications in Space: In Search of a Pharmacologist’s Guide to the Galaxy. Pharm. Res. 2019, 36, 148. [Google Scholar] [CrossRef] [PubMed]
- Loriè, E.P.; Baatout, S.; Choukér, A.; Buchheim, J.-I.; Baselet, B.; Russo, C.D.; Wotring, V.; Monici, M.; Morbidelli, L.; Gagliardi, D.; et al. The Future of Personalized Medicine in Space: From Observations to Countermeasures. Front. Bioeng. Biotechnol. 2021, 9, 739747. [Google Scholar] [CrossRef]
- Crucian, B.E. Countermeasures-based Improvements in Stress, Immune System Dysregulation and Latent Herpesvirus Reactivation onboard the International Space Station—Relevance for Deep Space Missions and Terrestrial Medicine. Neurosci. Biobehav. Rev. 2020, 115, 68–76. [Google Scholar]
- Lei, W.; Yuan, M.; Long, M.; Zhang, T.; Huang, Y.-E.; Liu, H.; Jiang, W. scDR: Predicting Drug Response at Single-Cell Resolution. Genes 2023, 14, 268. [Google Scholar] [CrossRef]
- Grimm, D.; Schulz, H.; Krüger, M.; Cortés-Sánchez, J.L.; Egli, M.; Kraus, A.; Sahana, J.; Corydon, T.J.; Hemmersbach, R.; Wise, P.M.; et al. The Fight against Cancer by Microgravity: The Multicellular Spheroid as a Metastasis Model. Int. J. Mol. Sci. 2022, 23, 3073. [Google Scholar] [CrossRef]
- Jhala, D.V.; Kale, R.K.; Singh, R.P. Microgravity Alters Cancer Growth and Progression. Curr. Cancer Drug Targets 2014, 14, 394–406. [Google Scholar] [CrossRef]
- Prasanth, D.; Suresh, S.; Mimlitz, M.; Zetocha, N.; Ekpenyong, A.E. Microgravity Modulates Drug-Induced Enhancement of Cancer Cell Migration. Biophys. J. 2017, 112, 311a. [Google Scholar] [CrossRef]
- Prasanth, D.; Suresh, S.; Prathivadhi-Bhayankaram, S.; Mimlitz, M.; Zetocha, N.; Lee, B.; Ekpenyong, A. Microgravity Modulates Effects of Chemotherapeutic Drugs on Cancer Cell Migration. Life 2020, 10, 162. [Google Scholar] [CrossRef] [PubMed]
- Rembiałkowska, N.; Baczyńska, D.; Dubińska-Magiera, M.; Choromańska, A.; Bieżuńska-Kusiak, K.; Gajewska-Naryniecka, A.; Novickij, V.; Saczko, J.; Przystupski, D.; Kulbacka, J. RCCS Bioreactor-Based Modeled Microgravity Affects Gastric Cancer Cells and Improves the Chemotherapeutic Effect. Membranes 2022, 12, 448. [Google Scholar] [CrossRef] [PubMed]
- Przystupski, D.; Górska, A.; Szewczyk, A.; Drąg-Zalesińska, M.; Kulbacka, J. 3D Clinorotation Affects Drug Sensitivity of Human Ovarian Cancer Cells. Microgravity Sci. Technol. 2021, 33, 1–12. [Google Scholar] [CrossRef]
- Mantovani, A.; Allavena, P.; Marchesi, F.; Garlanda, C. Macrophages as tools and targets in cancer therapy. Nat. Rev. Drug Discov. 2022, 21, 799–820. [Google Scholar] [CrossRef]
- Andersen, J.K.; Miletic, H.; Hossain, J.A. Tumor-Associated Macrophages in Gliomas—Basic Insights and Treatment Opportunities. Cancers 2022, 14, 1319. [Google Scholar] [CrossRef]
- Tauber, S.; Lauber, B.A.; Paulsen, K.; Layer, L.E.; Lehmann, M.; Hauschild, S.; Shepherd, N.R.; Polzer, J.; Segerer, J.; Thiel, C.S.; et al. Cytoskeletal stability and metabolic alterations in primary human macrophages in long-term microgravity. PLoS ONE 2017, 12, e0175599. [Google Scholar] [CrossRef]
- Thiel, C.S.; de Zélicourt, D.; Tauber, S.; Adrian, A.; Franz, M.; Simmet, D.M.; Schoppmann, K.; Hauschild, S.; Krammer, S.; Christen, M.; et al. Rapid adaptation to microgravity in mammalian macrophage cells. Sci. Rep. 2017, 7, 43. [Google Scholar] [CrossRef]
- Zayzafoon, M.; Gathings, W.E.; McDonald, J.M. Modeled Microgravity Inhibits Osteogenic Differentiation of Human Mesenchymal Stem Cells and Increases Adipogenesis. Endocrinology 2004, 145, 2421–2432. [Google Scholar] [CrossRef]
- Mitsuhara, T.; Takeda, M.; Yamaguchi, S.; Manabe, T.; Matsumoto, M.; Kawahara, Y.; Yuge, L.; Kurisu, K. Simulated microgravity facilitates cell migration and neuroprotection after bone marrow stromal cell transplantation in spinal cord injury. Stem Cell Res. Ther. 2013, 4, 35. [Google Scholar] [CrossRef]
- Costantini, D.; Overi, D.; Casadei, L.; Cardinale, V.; Nevi, L.; Carpino, G.; Di Matteo, S.; Safarikia, S.; Valerio, M.; Melandro, F.; et al. Simulated microgravity promotes the formation of tridimensional cultures and stimulates pluripotency and a glycolytic metabolism in human hepatic and biliary tree stem/progenitor cells. Sci. Rep. 2019, 9, 5559. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Y.; Zhang, S.; Peng, G.; Liu, T.; Li, Y.; Xiang, D.; Wassler, M.J.; Shelat, H.S.; Geng, Y.; et al. Rotating Microgravity-Bioreactor Cultivation Enhances the Hepatic Differentiation of Mouse Embryonic Stem Cells on Biodegradable Polymer Scaffolds. Tissue Eng. Part A 2012, 18, 2376–2385. [Google Scholar] [CrossRef]
- Ran, F.; An, L.; Fan, Y.; Hang, H.; Wang, S. Simulated microgravity potentiates generation of reactive oxygen species in cells. Biophys. Rep. 2016, 2, 100–105. [Google Scholar] [CrossRef] [PubMed]
- Gambacurta, A.; Merlini, G.; Ruggiero, C.; Diedenhofen, G.; Battista, N.; Bari, M.; Balsamo, M.; Piccirillo, S.; Valentini, G.; Mascetti, G.; et al. Human osteogenic differentiation in Space: proteomic and epigenetic clues to better understand osteoporosis. Sci. Rep. 2019, 9, 1–10. [Google Scholar] [CrossRef]
- Grimm, D.; Wehland, M.; Corydon, T.J.; Richter, P.; Prasad, B.; Bauer, J.; Egli, M.; Kopp, S.; Lebert, M.; Krüger, M. The effects of microgravity on differentiation and cell growth in stem cells and cancer stem cells. STEM CELLS Transl. Med. 2020, 9, 882–894. [Google Scholar] [CrossRef] [PubMed]
- Wnorowski, A.; Sharma, A.; Chen, H.; Wu, H.; Shao, N.-Y.; Sayed, N.; Liu, C.; Countryman, S.; Stodieck, L.S.; Rubins, K.H.; et al. Effects of Spaceflight on Human Induced Pluripotent Stem Cell-Derived Cardiomyocyte Structure and Function. Stem Cell Rep. 2019, 13, 960–969. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. The hallmarks of cancer. Cell 2000, 100, 57–70. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Fouad, Y.A.; Aanei, C. Revisiting the hallmarks of cancer. Am. J. Cancer Res. 2017, 7, 1016–1036. [Google Scholar] [PubMed]
- Welch, D.R.; Hurst, D.R. Defining the Hallmarks of Metastasis. Cancer Res 2019, 79, 3011–3027. [Google Scholar] [CrossRef]
- Stein, G.H. T98G: An anchorage-independent human tumor cell line that exhibits stationary phase G1 arrest in vitro. J. Cell. Physiol. 1979, 99, 43–54. [Google Scholar] [CrossRef]
- Fuster, E.; Candela, H.; Estévez, J.; Vilanova, E.; Sogorb, M.A. A Transcriptomic Analysis of T98G Human Glioblastoma Cells after Exposure to Cadmium-Selenium Quantum Dots Mainly Reveals Alterations in Neuroinflammation Processes and Hypothalamus Regulation. Int. J. Mol. Sci. 2022, 23, 2267. [Google Scholar] [CrossRef] [PubMed]
- Klein, E.; Vánky, F.; Ben-Bassat, H.; Neumann, H.; Ralph, P.; Zeuthen, J.; Polliack, A. Properties of the K562 cell line, derived from a patient with chronic myeloid leukemia. Int. J. Cancer 1976, 18, 421–431. [Google Scholar] [CrossRef]
- Zhou, B.; Ho, S.S.; Greer, S.U.; Zhu, X.; Bell, J.M.; Arthur, J.G.; Spies, N.; Zhang, X.; Byeon, S.; Pattni, R.; et al. Comprehensive, integrated, and phased whole-genome analysis of the primary ENCODE cell line K562. Genome Res. 2019, 29, 472–484. [Google Scholar] [CrossRef]
- Cui, Y.; Yin, Y.; Zou, Y.; Zhao, Y.; Han, J.; Xu, B.; Chen, B.; Xiao, Z.; Song, H.; Shi, Y.; et al. The Rotary Cell Culture System increases NTRK3 expression and promotes neuronal differentiation and migratory ability of neural stem cells cultured on collagen sponge. Stem Cell Res. Ther. 2021, 12, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Yi, Z.-C.; Xia, B.; Xue, M.; Zhang, G.-Y.; Wang, H.; Zhou, H.-M.; Sun, Y.; Zhuang, F.-Y. Simulated microgravity inhibits the proliferation of K562 erythroleukemia cells but does not result in apoptosis. Adv. Space Res. 2009, 44, 233–244. [Google Scholar] [CrossRef]
- Morabito, C.; Steimberg, N.; Mazzoleni, G.; Guarnieri, S.; Fanò-Illic, G.; Mariggiò, M.A. RCCS Bioreactor-Based Modelled Microgravity Induces Significant Changes on In Vitro 3D Neuroglial Cell Cultures. BioMed Res. Int. 2015, 2015, 1–14. [Google Scholar] [CrossRef]
- Madaan, K.; Kaushik, D.; Verma, T. Hydroxyurea: a key player in cancer chemotherapy. Expert Rev. Anticancer. Ther. 2012, 12, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Merrick, M.; Mimlitz, M.J.; Weeder, C.; Akhter, H.; Bray, A.; Walther, A.; Nwakama, C.; Bamesberger, J.; Djam, H.; Abid, K.; et al. In vitro radiotherapy and chemotherapy alter migration of brain cancer cells before cell death. Biochem. Biophys. Rep. 2021, 27, 101071. [Google Scholar] [CrossRef]
- Zhu, L.; Chen, L. Progress in research on paclitaxel and tumor immunotherapy. Cell. Mol. Biol. Lett. 2019, 24, 40. [Google Scholar] [CrossRef]
- Prathivadhi-Bhayankaram, S.V.; Ning, J.; Mimlitz, M.; Taylor, C.; Gross, E.; Nichols, M.; Guck, J.; Ekpenyong, A.E. Chemotherapy impedes in vitro microcirculation and promotes migration of leukemic cells with impact on metastasis. Biochem. Biophys. Res. Commun. 2016, 479, 841–846. [Google Scholar] [CrossRef]
- Walter, Y.; Hubbard, A.; Benoit, A.; Jank, E.; Salas, O.; Jordan, D.; Ekpenyong, A. Development of In Vitro Assays for Advancing Radioimmunotherapy against Brain Tumors. Biomedicines 2022, 10, 1796. [Google Scholar] [CrossRef]
- Hughes-Fulford, M.; Lewis, M. Effects of Microgravity on Osteoblast Growth Activation. Exp. Cell Res. 1996, 224, 103–109. [Google Scholar] [CrossRef]
- Scharenberg, C.W.; Harkey, M.A.; Torok-Storb, B. The ABCG2 transporter is an efficient Hoechst 33342 efflux pump and is preferentially expressed by immature human hematopoietic progenitors. Blood 2002, 99, 507–512. [Google Scholar] [CrossRef] [PubMed]
- Neri, S.; Mariani, E.; Meneghetti, A.; Cattini, L.; Facchini, A. Calcein-Acetyoxymethyl Cytotoxicity Assay: Standardization of a Method Allowing Additional Analyses on Recovered Effector Cells and Supernatants. Clin. Diagn. Lab. Immunol. 2001, 8, 1131–1135. [Google Scholar] [CrossRef] [PubMed]
- Otto, O.; Rosendahl, P.; Mietke, A.; Golfier, S.; Herold, C.; Klaue, D.; Girardo, S.; Pagliara, S.; Ekpenyong, A.; Jacobi, A.; et al. Real-time deformability cytometry: on-the-fly cell mechanical phenotyping. Nat. Methods 2015, 12, 199–202. [Google Scholar] [CrossRef]
- Asuquo, M.I.; Effa, E.; Gbotosho, O.; Otu, A.; Toepfner, N.; Ameh, S.; Bhayankaram, S.-P.; Zetocha, N.; Nwakama, C.; Egbe, W.; et al. Microfluidic Microcirculation Mimetic as a Tool for the Study of Rheological Characteristics of Red Blood Cells in Patients with Sickle Cell Anemia. Appl. Sci. 2022, 12, 4394. [Google Scholar] [CrossRef]
- Moore, M.J.; Sebastian, J.A.; Kolios, M.C. Determination of cell nucleus-to-cytoplasmic ratio using imaging flow cytometry and a combined ultrasound and photoacoustic technique: a comparison study. J. Biomed. Opt. 2019, 24, 106502. [Google Scholar] [CrossRef] [PubMed]
- Singh, I.; Lele, T.P. Nuclear Morphological Abnormalities in Cancer: A Search for Unifying Mechanisms. 2022, 70, 443–467. [CrossRef]
- Musiałek, M.W.; Rybaczek, D. Hydroxyurea—the good, the bad and the ugly. Genes 2021, 12, 1096. [Google Scholar] [CrossRef]
- Koç, A.; Wheeler, L.J.; Mathews, C.K.; Merrill, G.F. Hydroxyurea Arrests DNA Replication by a Mechanism That Preserves Basal dNTP Pools. J. Biol. Chem. 2004, 279, 223–230. [Google Scholar] [CrossRef]
- Nichols, H.L.; Zhang, N.; Wen, X.; Rossi, M.L.; Rubbini, G.; Gioglio, L.; Martini, M.; Fesce, R.; Canella, R.; Leparulo, A.; et al. Proteomics and genomics of microgravity. Physiol. Genom. 2006, 26, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Vidyasekar, P.; Shyamsunder, P.; Arun, R.; Santhakumar, R.; Kapadia, N.K.; Kumar, R.; Verma, R.S. Genome Wide Expression Profiling of Cancer Cell Lines Cultured in Microgravity Reveals Significant Dysregulation of Cell Cycle and MicroRNA Gene Networks. PLoS ONE 2015, 10, e0135958. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).