1. Introduction
Highland barley, named Qingke, is widely grown in Qinghai-Tibet Plateau in western China [
1]. Generally, about 98% of highland barley are used as feed or processed into alcoholic products [
2]. Highland barley has a high starch content (more than 90% in dry base), which is suitable for wine production, and thus a lage amount of wine lees are produced in Qinghai-Tibet Plateau. Currently, the wine lees are mainly used as animal feeds, crop fertilizers, cultivation of edible fungi [
3]. The mass fraction of protein in the wine lees from highland barley is more than 15% (in dry base).
It has been accepted there are four kinds of proteins in crops, including prolamin, glutelins, albumins and globulins [
4]. Prolamin is a kind of storage protein in seeds which can provide both carbon and nitrogen elements for seed [
5]. The prolamins have quite high contents of hydrophobic amino acids (such as alanine proline and leucine), which can be easily dispersed in alcoholic solutions The essential amino acids (such as lysine and tryptophan) contents in prolamin is usually quite low, and thus the amino acid in prolamin is not balanced. This indicates the prolamins have little nutritive value. Prolamins can be dissolved in 60%-90% alcohol solutions [
6]. In recent years, it has been widely applied in the field of material field due to its unique self-assembly property, high hydrophobicity and good biocompatibility. Its unique structure and unique amino acid composition render them feasible for preparing differents kinds nanoparticles, fibers, emulsifying agents [
4,
7]. Thus, getting more information on the composition and structure in prolamins from highland barley, more application might be opened.
Based on previous reports, prolamins can be divided into different kinds based on the the ability to form disulfide bonds or solubility [
8]. Taking the zein and kafirin for example, zein can be divided into α-, β-, γ-, and δ-zein. High hydrophobicity can be found in α-zein which accounts for more than 70% of total zein. Similarly, hydrophobic amino acids were abundant in the α-kafirin which accounts for more than 60% of total kafirin [
8]. It can be also observed that prolamins from different cereal seeds have different structure and properites [
8]. For instance, due to the difference of protein conformation and aggregation, zeins, hordeins and gliadins exhibit quite different emulsifying properties, electrospinnabilities and film-forming properties, etc [
5,
9,
10,
11,
12]. However, little is known about the structural and physicochemical properties of prolamins from highland barley, which limits its application.
The object of the study is to investigate the composition, structure and physicochemical properties of prolamins from highland barley. Zein, a widely researched prolamins from corn, is usd for a comparison. To our knowledge, this research is the first report for the prolamins investigations in prolamins from highland barley. The findings will provide the basic data of functional properties of prolamins from highland barley, and be beneficial to the development of prolamin applications in food industry.
2. Results
2.1. Amino acid composition
The amino acid composition determines the structural and physico-chemical properties of proteins. The amino acid composition of prolamins from highland barley is shown in
Table 1. The most abundant amino acid in prolamins from highland barley is glutamic acid (41.12 g/100 g protein), proline (15.89 g/100 g protein), tryptophan (5.62 g/100 g protein) and leucine (5.03 g/100 g protein). Surprisingly, the mass percent of glutamic acid was more than 40%, which was the highest value in cereals. It is generally accepted thatcysteine plays an important role in the structure and functional properties of globular proteins. Cysteine often exists in the form of free sulfhydryl groups, thiolate ion or oxidized into disulfide in proteins [
13]. Compared with zein, prolamins from highland barley have a higher contents of cysteine content, which do not facilitate the the structural stability of prolamins from highland barley by the cysteine cross-linking. The mass percent of hydrophobic and hydrophilic amino acids in prolamins from highland barley was significantly lower than those in zein. The mass ratio of acidic-to-basic amino acids plays a decisive role in the charge of proteins, which also influence the proteins’ solubility [
7]. Therefore, with larger ratio of acidic-to-basic amino acids and less hydrophobic amino acids, prolamins from highland barley might have more hydratability than zein. Relatively, prolamins from highland barley was deficient in lysine (0.58 g/100 protein), but this value was quite larger than zein (0.510 g/100 protein).
2.2. SDS-PAGE analysis
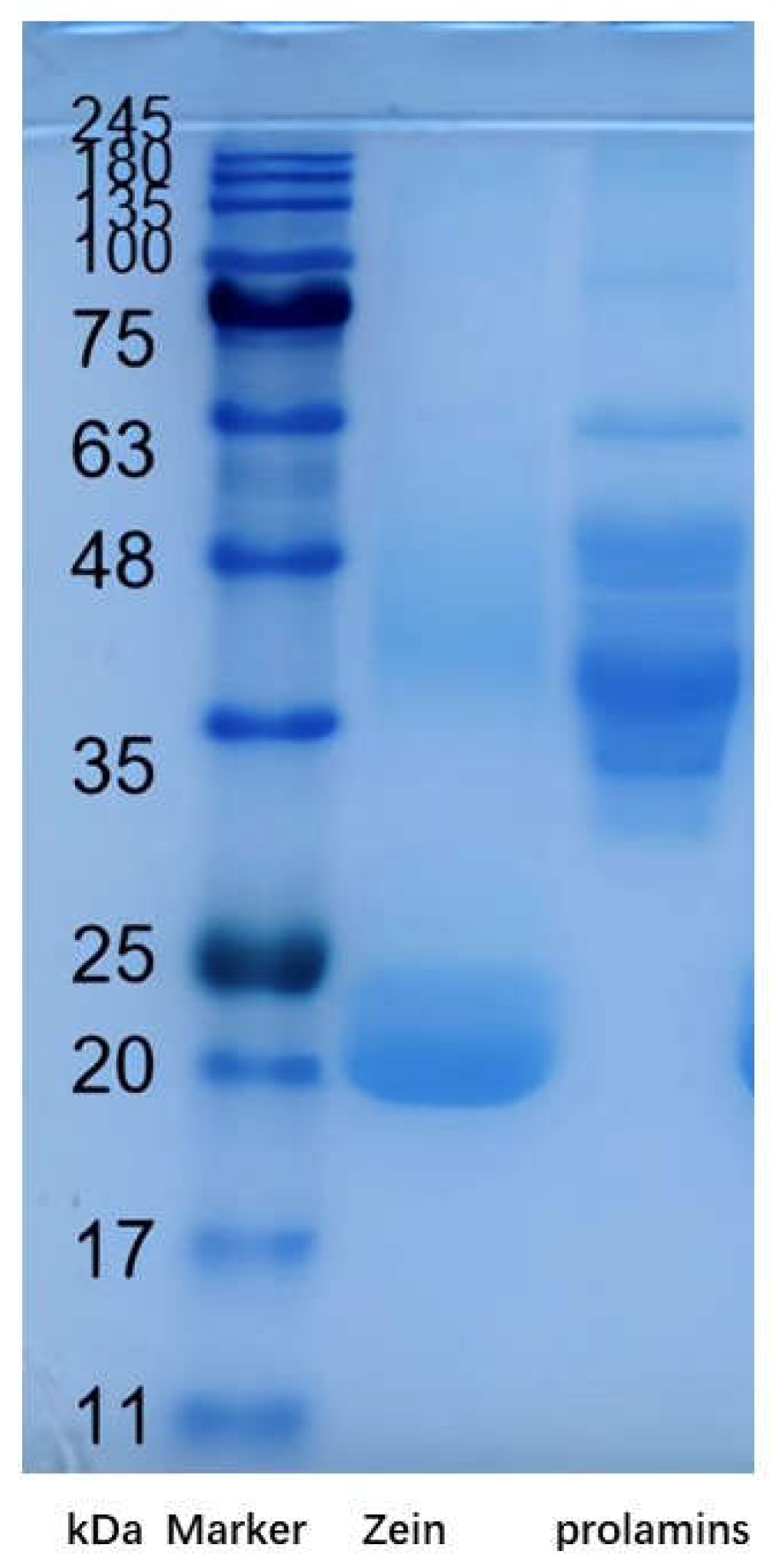
The The SDS-PAGE profiles of prolamins from highland barley and zein are presented in
Figure 1. Two intense bands with molecular weights of 20 and 24 kDa, in zein were observed. A slight band with 46 kDa can be also observed in zein. This is consist with our previous research [
14]. However, bands with molecular weights of 30, 45, 48 and 63 kDa in prolamins from highland barley, can be observed. This indicated the molecular weights of prolamins from highland barley are quite larger than zein. Almost all physico-chemical properties of proteins, including viscosity, emulsifying property, gel property, filming forming properties and so on are closely related to the molecular weights [
15,
16]. This indicates prolamins from highland barley might exhibit quite different functional properties from zein.
2.3. Secondary Structures
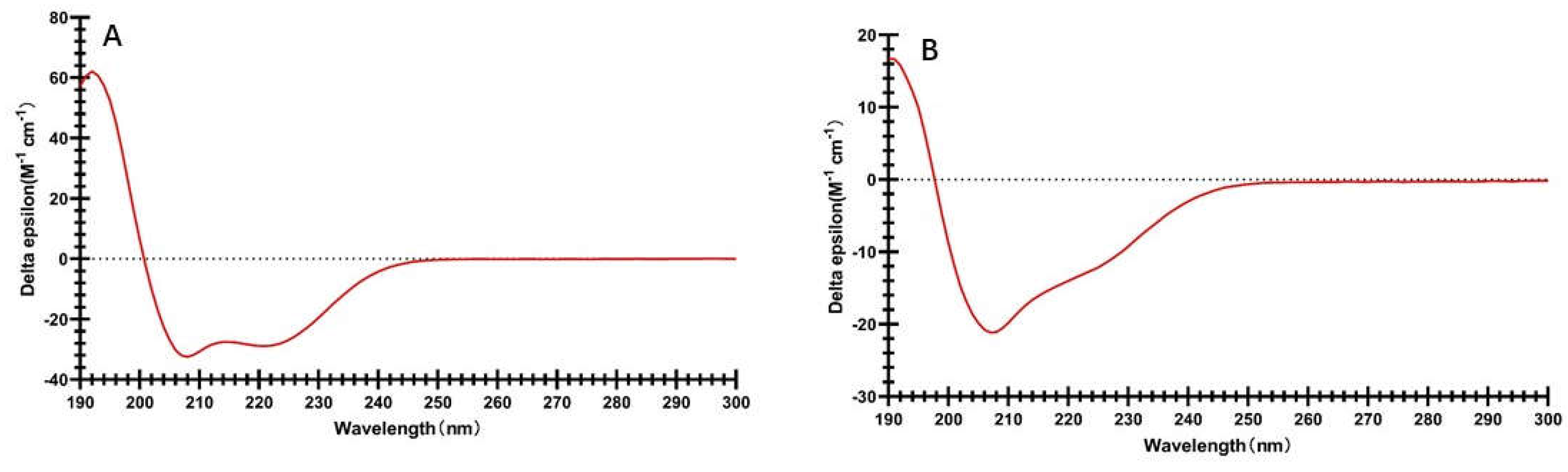
Circular dichroism has been widely used to analyze the secondary structural characterics of proteins [
17,
18]. The circular dichroism spectra of zein and prolamins from highland barley are shown in
Figure 2. It can be observed that prolamins from highland barley and zein exhibited quite different spectra, indicating they have different secondary structures, although both of them belong to the prolamin family. Circular dichroism spectra of zein has a negative peak at 222 and 208 nm, while has a positive peak at about 192 nm. This indicates the α-helical structure is the main secondary structure in zein [
9]. A helical wheel structure in zein was concluded in which nine repeating subunits are in an antiparallel shape [
19,
20]. Circular dichroism spectra of prolamins from highland barley has a negative peak at 208 nm, while the negative peak at 222 nm disappeared. This suggested prolamins from highland barley has less α-helical structure and more β-turn helices structure, which might be due to the high proportion of proline in prolamins from highland barley, inhibiting the formation of α-helical structure in proteins secondary structures. β-turn helices favor the formation of compact and globular protein structure. Therefore, it can be speculated that prolamins from highland barley might have a more compact structure, leading to less exposure of more amino acid residue on their structure. This might endow the prolamins from highland barley more stability than zein [
21,
22].
2.4. Physicochemical Properties
2.4.1. Thermal Properties
DSC is generally applied to characterize the thermal stability of proteins [
23]. It is widely accepted that a higher peak temperature of denaturation (T
p) means higher stability of proteins. Enthalpy of denaturation (ΔH) is the energy that denatures the proteins [
13,
24].
Table 2 shows the starting denaturation temperature (T
0), peak temperature of denaturation (T
p) and enthalpy of denaturation (ΔH). The T
p of prolamins from highland barley is 112.41±2.45 ℃, which is quite lager than zein. This indicates the thermal stability of prolamins from highland barley is quite higher than zein. The enthalpy of denaturation of prolamins from highland barley is also larger than zein. After being treated, the structure of a protein changes from its native state to a denatured state, which also accompanies by the unfolding of the structure. The higher T
p and ΔH of prolamins from highland barley than zein might be closely related to the prolamins from highland barley has a more compact secondary structure, which is consist with the result of secondary structures.
2.4.2. Surface hydrophobicity
The surface hydrophobicity of proteins was evaluated with a fluorescent probe (sodium 8-anilino-1-naphthalenesulfonate), which specifically bind to the hydrophobic areas on proteins, and thus could reflect the surface hydrophobic properties of proteins [
25]. It can be observed that the surface hydrophobicity index of prolamins from highland barley was quite lower than zein (
Table 2). This indicated the prolamins from highland barley is less hydrophobic than zein. This is largely related to the lower contents of hydrophobic amino acids in prolamins from highland barley and less exposed hydrophobic binding sites [
13].
2.4.3. Emulsifying properties
The emulsifying properties is important processing functionalities for edible food, which refer to the protein’s hydrophile-lipophile balance. The emulsifying activity index refers to the ability of a protein to coat onto the oil-water interface, while the emulsifying stability index refers to the ability of a proten to stabilize the oil-in water emulsions [
26]. It can be observed that the emulsifying activity index of prolamins from highland barley is significant larger than zein (
Table 2), indicating the former is easier to absorb onto the oil-water interfaces. The emulsifying stability index of prolamins from highland barley was also larger than zein, suggesting it can stablize oil droplets better than zein. Therefore, it can be concluded that the prolamins from highland barley exhibited better emulsifying properties than zein.
2.5. Morphology of electrospun fibers
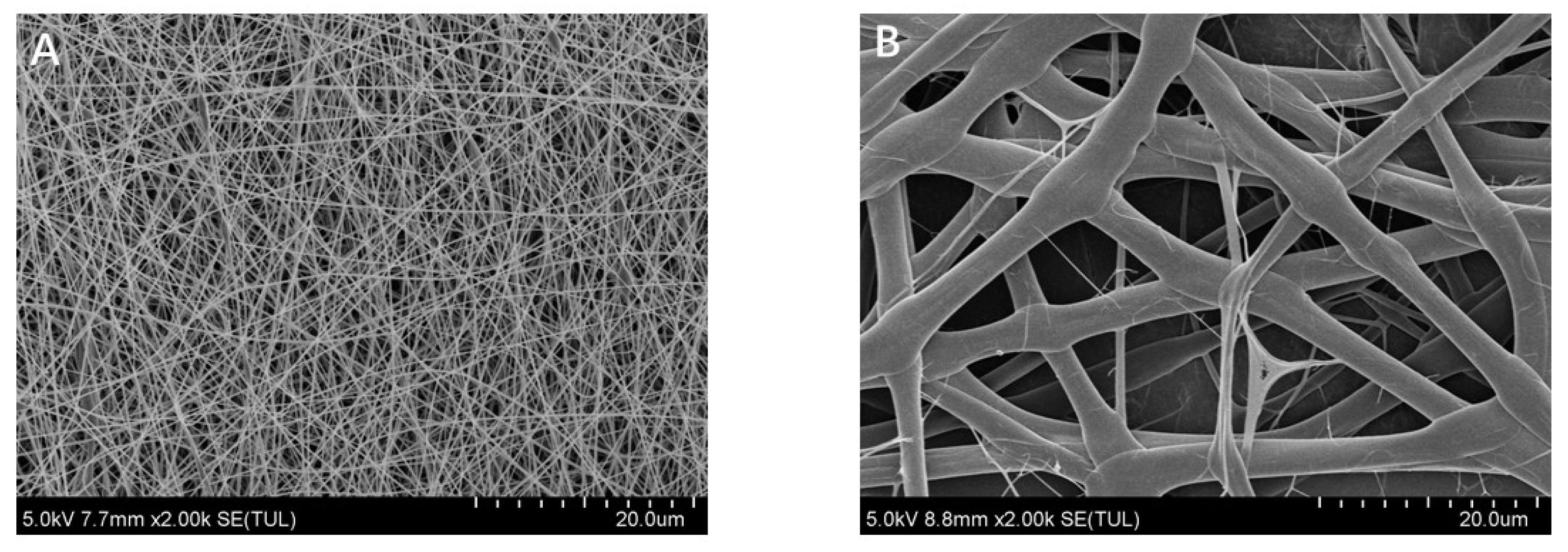
The morphology of electrospun fibrous prepared with zein and prolamins from highland barley are shown in
Figure 3. Surprisingly, the diameter of fibers prepared with prolamins from highland barley was almost 6 times that of zein (2600 nm vs 400 nm). This might be related to the higher molecular weight of prolamins from highland barley than zein, causing to the higher viscosity of prolamin dispersions than that of zein (1.042 Pa·S vs 0.283 Pa·S) [
27]. It can be also observed that ribbon structures rather than fibers were formed by prolamins from highland barley, while round fibers were formed by zein. It is considered that ribbon structure might be related to the fast evaporation of the solvent, which leads to the formation of a skin in formed jets [
28]. Therefore, we can speculate that the acetic acid evaporates slower in the presence of prolamins from highland barley than zein.
3. Materials and Methods
3.1. Materials
Zein was purchased from Sigma-Aldrich (St. Louis, MO, USA). Sodium dodecyl sulfate (SDS), 1-anilino-8-naphthalene sulfonate (ANS), dithiothreitol (DTT), hydrochloric acid (HCl) and sodium hydroxide (NaOH) were obtained from Aladdin Biochemical Technology Co., Ltd. (Shanghai, China). Ethanol was purchased from Yongda Chemical Reagent Co., LTD (Tianjin, China). Water was purified to 18.2 MΩ with a purifier (Sartorius Cor-poration, Göttingen, Germany). Wine lees were obtained from the local highland barley wine factory.
3.2. Preparation of prolamins from highland barley
The wine lees were dried to constant weight at 70℃ in an electrothermal blowing dry box (DHG-9070B, Oulaibo, Jinan, China). The dried samples were smashed and passed through the 40 mesh sieve.
One hundred grams of the powder were added in 1,000 mL 75% ethanol aqueous solution and mixed with a magnetic stirrer for 5 min [
29,
30]. Then the samples were incubated for 120 min at 40 °C in a water bath (HH-S6, Oulaibo, Jinan, China). After centrifuged for 15 min at 5,000 g (H3-18K, Kecheng, Hunan, China), the supernatant was poured into a 20 L stainless steel stock pot. 8,000 mL water was added into the pot to precipitate the proteins. The mixture was centrifuged for 15 min at 5,000 g and the sediment was collected and lyophilized to constant weight (CTFD-12S, Yonghechuangxin, Qingdao, China).
3.3. Sodium dodecyl sulfate polyacrylamide gel electrophoresis
The 12% separating gel and 4% stacking gel were used for the sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) analyses [
14]. Ten microliters of samples (1.5 mg/mL) were loaded onto the gels and the protein markers including 11–180 kDa was set as a reference. Electrophoresis was performed with a Mini-PROTEAN 3 Cell electrophoresis system (Bio-Rad Laboratories, CA, USA) at 100 V. The protein band density was analyzed with Image J software (MD, USA.)
3.4. Amino acid composition analyses
Twenty milligrams of samples were added into the hydrolysis tube, and 15 mL of 6 mol/L hydrochloric acid solution was added to it, following by adding 3-4 drops of phenol. The hydrolysis tube was placed in the refrigerant and frozen for 5 min. Then hydrolysis tube was connected to the suction pipe of the vacuum pump, vacuumed, and filled with nitrogen. After repeated vacuuming-filling nitrogen for 3 times, the tube was sealed in a nitrogen-filled state. The sealed hydrolysis tube was hydrolyzed in electrothermal blast incubator or hydrolysis furnace at 110 °C ± 1 °C for 22 h, and then the tube was taken out and cooled to room temperature. The hydrolysate was filtered to a 50 mL volumetric flask through an open hydrolysis tube. The hydrolysis tube was rinsed multiple times with a small amount of water, and the rinse solution was collected in the same 50 mL volumetric flask, which was finally filled to the mark with water and shaked properly to mix the solution evenly. A precise volume of 1.0 mL of the filtrate was transferred into a 25 mL test tube. A concentrator or parallel evaporator were carried out to dry the test tube under reduced pressure at 40 ℃ to 50 ℃. After drying, the residue was dissolved in 1 mL to 2 mL of water and dried again under reduced pressure until completely dry. The dried residue was dissolved into 2.0 mL of pH 2.2 sodium citrate buffer solution. After mixing thoroughly by shaking, the solution was then filtered through a 0.22 μm filter membrane and transferred to an instrument sample vial to prepare the sample detection solution for instrument measurement. The amino acids were measured using an automatic amino acid analyzer (Hitachi L-8900, Tokyo, Japan). The amino acid composition of proteins was reported as g/100 g protein [
31].
3.5. Circular dichroism spectroscopy
The protein was dispersed in 75% ethanol solution with a final concentration of 50 μg/mL. The Far-UV circular dichroism spectrum (190~250 nm) of the sample was measured by circular dichroism spectrometer (MOS-450, BioLogic Inc) at 25 ℃ [
32].
3.6. Differential scanning calorimetry
Differential scanning calorimetry (Shimadzu, Kyoto, Japan) was applied for the thermal analysis of proteins. Briefly, 10.0 mg of the powder was added into aluminum plate, crimped. The samples were heated from 25 °C to 300 °C at 10 °C/min and protected with nitrogen at 200 mL/min. An empty aluminum plate was used as a control [
33].
3.7. Surface hydrophobicity index
Sodium 8-anilino-1-naphthalenesulfonate (10 mM) solution was prepared with 10 mM phosphate buffer (pH 8.0) and stored in dark [
13]. The protein dispersion with concentrations of 1, 0.8, 0.6, 0.4, 0.2, 0.1 and 0.05 mg/mL was prepared with 75% aqueous ethanol. During the determination, 10 μL sodium 8-anilino-1-naphthalenesulfonate solution was added to 1 mL protein dispersion, vortexed and mixed for 30 s, and reacted for 5 min. The fluorescence intensity was measured using a multifunctional microplate detector (RF-5301PC, Shimadzu, Kyoto, Japan). The excitation wavelength was 360 nm and the emission wavelength was 460 nm. The surface hydrophobicity index of the corresponding hydrolysate is the slope obtained by plotting the concentration with fluorescence intensity.
3.8. Emulsifying properties
The sample solution with a concentration of 0.3% (w/w) was prepared with 10 mM phosphate buffer (pH 7.0). 15 g solution was put into a 50 mL centrifuge tube, and 5 g medium-chain triglyceride (MCT) was added. The sample solution was treated by a high-speed disperser at 25000 rpm for 1 min. 50 μL sample was taken from the bottom of the test tube at 0 min and 10 min after stirring, respectively. The sample solution was diluted 100 times with 0.1% (w/v) SDS solution, and the absorbance at 500 nm was measured. SDS solution was used as blank. Emulsifying activity index (EAI) and emulsifying stability index (ESI) are calculated as follows:
DF is the dilution factor, DF=100; c is protein concentration, 0.003 g/mL; φ is the optical path, φ = 0.01 m; θ is the proportion of oil phase in the emulsion, θ = 0.25; a0 and A10 were the absorbance at 0 min and 10 min, respectively.
3.9. Preparation and characterization of the electrospun fibers
Dispersions containing proteins and glycerol were prepared by dissolving protein powder and glycerol into glacial acetic acid, assisted with magnetic stirring (500 rpm) at room temperature for 30 min. The final concentration of protein and glycerol were 25% and 2.5% (w/w), respectively. The fibers were prepared on an electrospinning apparatus (HZ-11, Huizhidianfang, Qingdao, China). Electrospinning processing was performed at room temperature. The voltage was 17 kV and tip-to-collector distance was kept at 13 cm. The morphologies of fibers were observed with SU8020 Scanning electron microscopy (Hitachi, Tokyo, Japan).
3.10. Statistical analysis
All experiments were carried out in triplicate and the results were expressd as mean ± standard deviation. Statistical analysis used single-factor analysis of variance (ANOVA) and Duncan's multiple range test.
4. Conclusions
The prolamins from highland barley have quite different composition, structure and functionalities with the widely used zein. With more charged and hydrophilic amino ac-ids and molecular weight, prolamins from highland barley have higher surface charge, water holding capacity, thermal stability, emulsifying capacity and stability, while with lower oil absorption capacity. The diameter of fibers prepared with prolamins from highland barley was almost 6 times that of zein, while ribbon structures rather than fibers were formed by prolamins from highland barley. The results would provide the guidance for ap-plication of prolamins from highland barley.
Author Contributions
Conceptualization, J.X. and W.Z.; methodology, J.X.; software, J.X.; validation, J.X., W.Z. and Z.L.; formal analysis, Z.L. and P.W.; investigation, W.Z.; resources, W.Z.; data curation, W.Z.; writing—original draft preparation, J.X.; writing—review and editing, J.X.; visualization, J.X.; supervision, W.Z; project administration, W.Z; funding acquisition, W.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Tibet barley processing test station, grant number CARS-05-13B.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
We would like to acknowledge Dr. Pengjie Wang (China Agricultural University) for the help of fiber preparation.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the prolamins are available from the authors.
References
- Lyu, Y.; Ma, S.; Liu, J.; Wang, X. A systematic review of highland barley: Ingredients, health functions and applications. Grain & Oil Science and Technology 2021, 5, 35–43. [Google Scholar] [CrossRef]
- Li, Y.; Li, T.; Liu, R.H. Bioactive compounds of highland barley and their health benefits. J. Cereal Sci. 2021, 103, 103366. [Google Scholar] [CrossRef]
- Long, R.; Dong, S.; Wei, X.; Pu, X. The effect of supplementary feeds on the bodyweight of yaks in cold season. Livest. Prod. Sci. 2005, 93, 197–204. [Google Scholar] [CrossRef]
- Tapia-Hernández, J.A.; Del-Toro-Sánchez, C.L.; Cinco-Moroyoqui, F.J.; Juárez-Onofre, J.E.; Ruiz-Cruz, S.; Carvajal-Millan, E.; López-Ahumada, G.A.; Castro-Enriquez, D.D.; Barreras-Urbina, C.G.; Rodríguez-Felix, F. Prolamins from cereal by-products: Classification, extraction, characterization and its applications in micro- and nanofabrication. Trends Food Sci. Technol. 2019, 90, 111–132. [Google Scholar] [CrossRef]
- Li, C.; Hu, Q.; Liang, Y.; Wei, Y.; Wang, J. Physicochemical properties and application in film preparation of prolamin from distiller's grains. International Journal of Food Science & Technology 2022, 57, 5206–5215. [Google Scholar] [CrossRef]
- Tan, H.; Zhou, H.; Guo, T.; Li, J.; Zhang, C.; Wang, S.; Zhang, Y.; Ma, L. Zein structure and its hidden zearalenone: Effect of zein extraction methods. Food Chem. 2021, 374, 131563. [Google Scholar] [CrossRef]
- Abdullah; Fang, J.; Liu, X.; Javed, H.U.; Cai, J.; Zhou, Q.; Huang, Q.; Xiao, J. Recent advances in self-assembly behaviors of prolamins and their applications as functional delivery vehicles. Crit. Rev. Food Sci. Nutr. 2022, 1–28. [Google Scholar] [CrossRef]
- Yang, Y.; He, S.; Zhang, Y.; Li, X.; Liu, H.; Li, Q.; Cao, X.; Ye, Y.; Sun, H. Comparison of crude prolamins from seven kidney beans (Phaseolus vulgaris L.) based on composition, structure and functionality. Food Chem. 2021, 357, 129748. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, L. Electrospinning of Prolamin Proteins in Acetic Acid: The Effects of Protein Conformation and Aggregation in Solution. Macromol. Mater. Eng. 2012, 297, 902–913. [Google Scholar] [CrossRef]
- Qazanfarzadeh, Z.; Kadivar, M.; Shekarchizadeh, H.; Porta, R. Functional Properties of Rye Prolamin (Secalin) and Their Improvement by Protein Lipophilization through Capric Acid Covalent Binding. Foods 2021, 10, 515. [Google Scholar] [CrossRef]
- Zhang, Y.; Jing, X.; Chen, Z.; Wang, X. Effects of moderate-intensity pulsed electric field on the structure and physicochemical properties of foxtail millet ( Setaria italica ) prolamin. Cereal Chem. 2022, 100, 360–370. [Google Scholar] [CrossRef]
- Liu, X.; Yang, Y.-J.; Wang, Z.-W. Structure characteristics of Coix seeds prolamins and physicochemical and mechanical properties of their films. J. Cereal Sci. 2018, 79, 233–239. [Google Scholar] [CrossRef]
- Zhang, F.; Fu, Y.; Liu, Z.; Shen, Q. Comparison of the characteristics of prolamins among foxtail millet varieties with different palatability: Structural, morphological, and physicochemical properties. Int. J. Biol. Macromol. 2021, 186, 194–205. [Google Scholar] [CrossRef]
- Chen, H.; Li, Y.; Liu, A.; Wu, L.; Yan, W.; Tong, Y.; Wang, P. Circular Extraction: Innovative Use of a Switchable Composite Extractant for Prolamin Extraction from Grain Byproducts. ACS Food Sci. Technol. 2022, 2, 630–637. [Google Scholar] [CrossRef]
- Yolandani; Ma, H.; Li, Y.; Liu, D.; Zhou, H.; Liu, X.; Wan, Y.; Zhao, X. Ultrasound-assisted limited enzymatic hydrolysis of high concentrated soy protein isolate: Alterations on the functional properties and its relation with hydrophobicity and molecular weight. Ultrasonics Sonochemistry 2023, 95, 106414. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Y.; Wang, C.; He, J.; Mo, H. Antioxidant activity, functional properties, and cytoprotective effects on HepG2 cells of tree Peony (Paeonia suffruticosa Andr.) seed protein hydrolysate as influenced by molecular weights fractionation. Foods 2022, 11, 2592. [Google Scholar] [CrossRef] [PubMed]
- Jones, C. Circular dichroism of biopharmaceutical proteins in a quality-regulated environment. J. Pharm. Biomed. Anal. 2022, 219, 114945. [Google Scholar] [CrossRef] [PubMed]
- Micsonai, A.; Moussong, É.; Murvai, N.; Tantos, Á.; Toke, O.; Réfrégiers, M.; Wien, F.; Kardos, J. Disordered-ordered protein binary classification by circular dichroism spectroscopy. Biophysical Journal 2023, 122, (3, Supplement 1), 344a. [Google Scholar] [CrossRef]
- Turasan, H.; Kokini, J.L. Advances in Understanding the Molecular Structures and Functionalities of Biodegradable Zein-Based Materials Using Spectroscopic Techniques: A Review. Biomacromolecules 2017, 18, 331–354. [Google Scholar] [CrossRef]
- Selling, G.W.; Hamaker, S.A.H.; Sessa, D.J. Effect of Solvent and Temperature on Secondary and Tertiary Structure of Zein by Circular Dichroism. Cereal Chem. 2007, 84, 265–270. [Google Scholar] [CrossRef]
- Sawyer, N.; Arora, P.S. Hydrogen Bond Surrogate Stabilization of β-Hairpins. ACS Chem. Biol. 2018, 13, 2027–2032. [Google Scholar] [CrossRef] [PubMed]
- Lahiri, P.; Verma, H.; Ravikumar, A.; Chatterjee, J. Protein stabilization by tuning the steric restraint at the reverse turn. Chem. Sci. 2018, 9, 4600–4609. [Google Scholar] [CrossRef]
- Lang, B.E.; Cole, K.D. Differential scanning calorimetry and fluorimetry measurements of monoclonal antibodies and reference proteins: Effect of scanning rate and dye selection. Biotechnol. Prog. 2017, 33, 677–686. [Google Scholar] [CrossRef] [PubMed]
- Lv, X.; Wang, S.; Zhao, Y.; Shan, P. A reinforcement learning based method for protein’s differential scanning calorimetry signal separation. Measurement 2022, 188. [Google Scholar] [CrossRef]
- Zhang, M.; Yang, Y.; Acevedo, N.C. Effects of pre-heating soybean protein isolate and transglutaminase treatments on the properties of egg-soybean protein isolate composite gels. Food Chem. 2020, 318, 126421. [Google Scholar] [CrossRef]
- Mu, T.-H.; Tan, S.-S.; Xue, Y.-L. The amino acid composition, solubility and emulsifying properties of sweet potato protein. Food Chemistry 2009, 112, 1002–1005. [Google Scholar] [CrossRef]
- Vicente, A.C.B.; Medeiros, G.B.; Vieira, D.D.C.; Garcia, F.P.; Nakamura, C.V.; Muniz, E.C.; Corradini, E. Influence of process variables on the yield and diameter of zein-poly(N-isopropylacrylamide) fiber blends obtained by electrospinning. J. Mol. Liq. 2019, 292, 109971. [Google Scholar] [CrossRef]
- Federici, E.; Selling, G.W.; Campanella, O.H.; Jones, O.G. Incorporation of Plasticizers and Co-proteins in Zein Electrospun Fibers. J. Agric. Food Chem. 2020, 68, 14610–14619. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Li, X.; Song, X.; Li, Q.; Zheng, F.; Li, H.; Sun, J.; Huang, M.; Sun, B. Characterization of prolamin recycled from the byproduct of the Baijiu brewing industry (Jiuzao) by SDS-PAGE, multispectral analysis, and morphological analysis. Food Biosci. 2022, 49. [Google Scholar] [CrossRef]
- Constantino, A.B.T.; Garcia-Rojas, E.E. Proteins from pseudocereal seeds: solubility, extraction, and modifications of the physicochemical and techno-functional properties. Journal of the Science of Food and Agriculture 2022, 102, 2630–2639. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, J.; Wang, P.; Tong, Y.; Li, Y.; Chen, H. Functional Properties of Corn Byproduct-Based Emulsifier Prepared by Hydrothermal–Alkaline. Molecules 2023, 28, 665. [Google Scholar] [CrossRef] [PubMed]
- Bing, J.; Xiao, X.; McClements, D.J.; Biao, Y.; Chongjiang, C. Protein corona formation around inorganic nanoparticles: Food plant proteins-TiO2 nanoparticle interactions. Food Hydrocolloids 2021, 115, 106594. [Google Scholar] [CrossRef]
- Miri, M.A.; Najafi, M.B.H.; Movaffagh, J.; Ghorani, B. Encapsulation of Ascorbyl Palmitate in Zein by Electrospinning Technique. J. Polym. Environ. 2020, 29, 1089–1098. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).