Submitted:
21 June 2023
Posted:
21 June 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
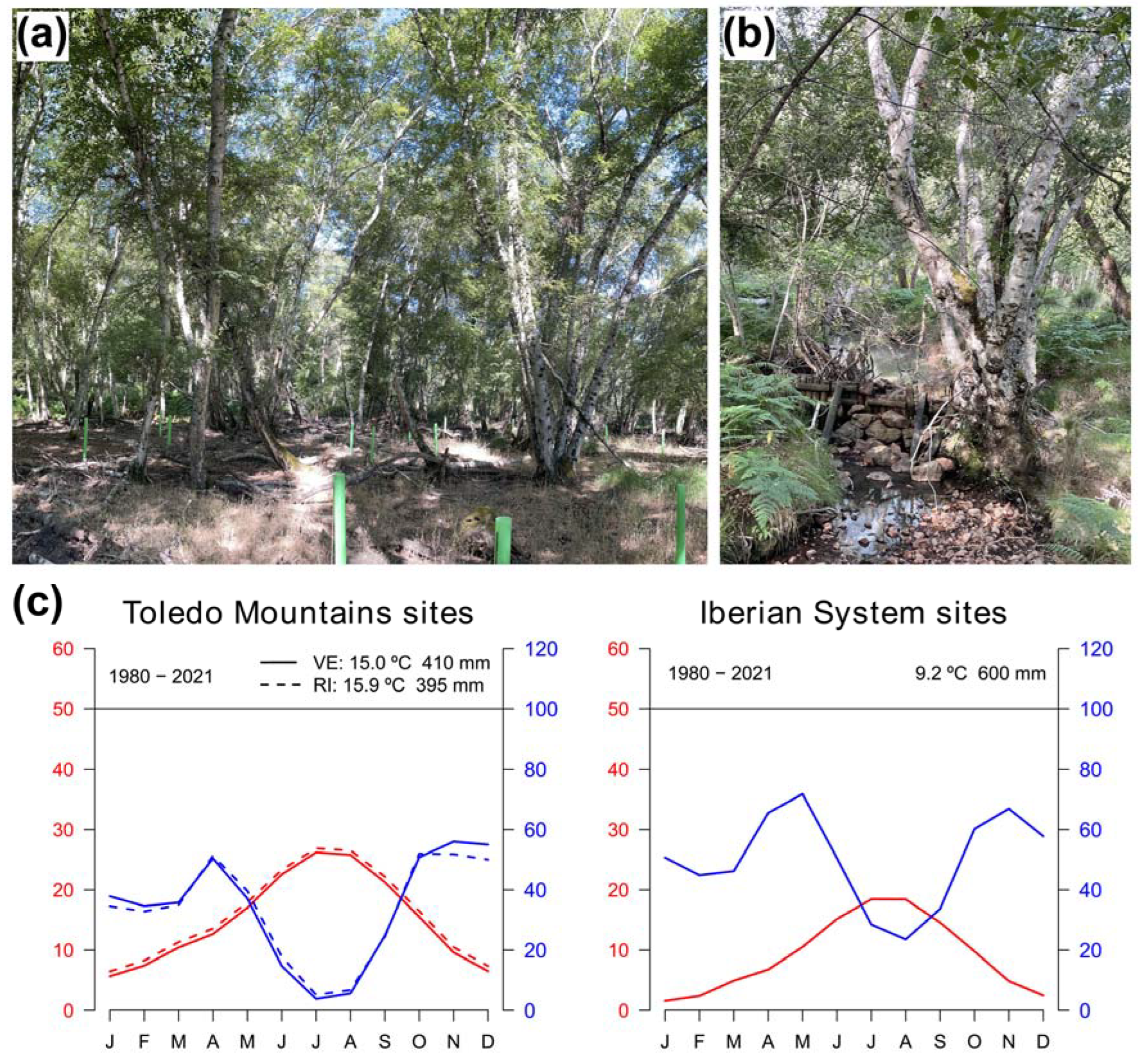
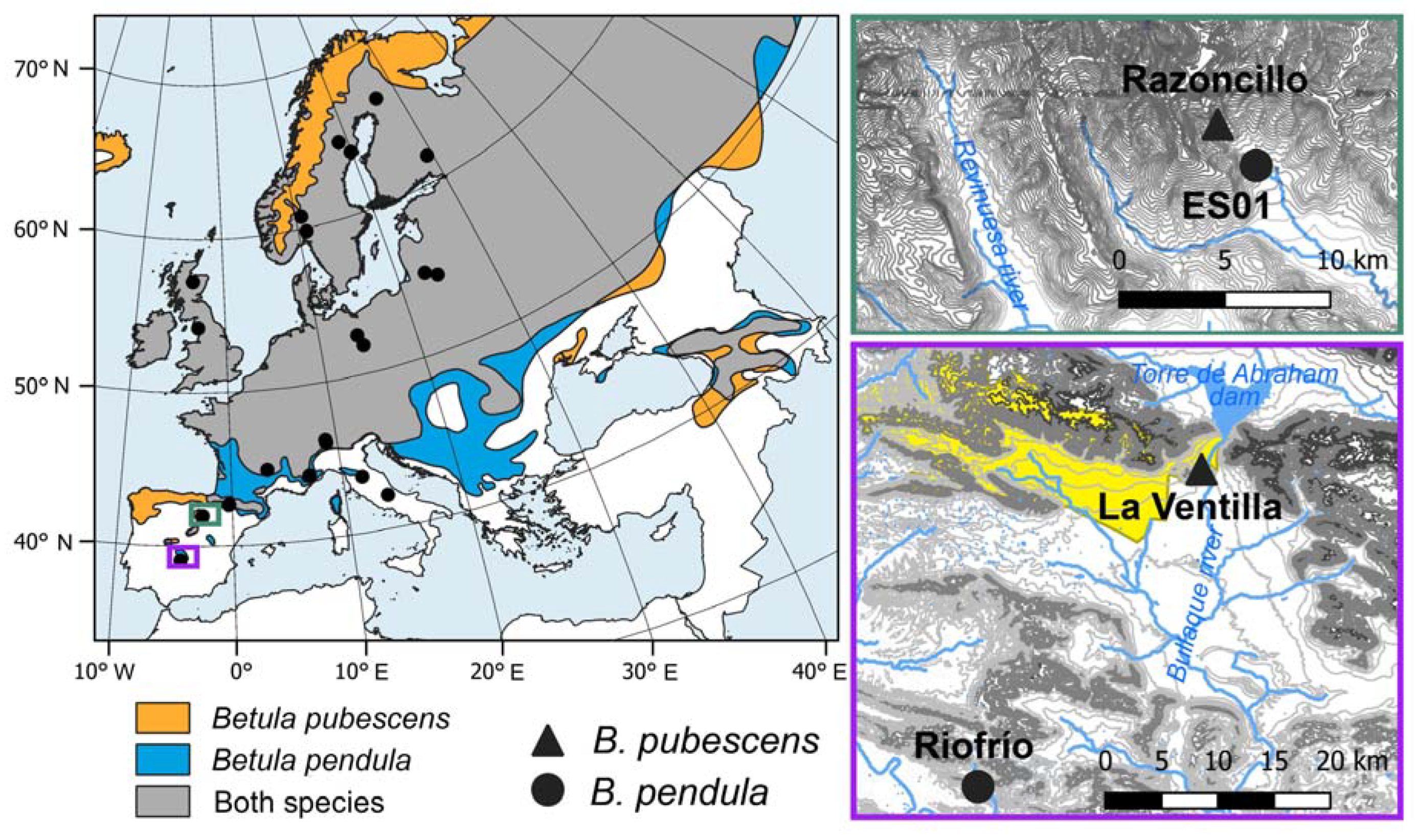
2.1. Study sites and species
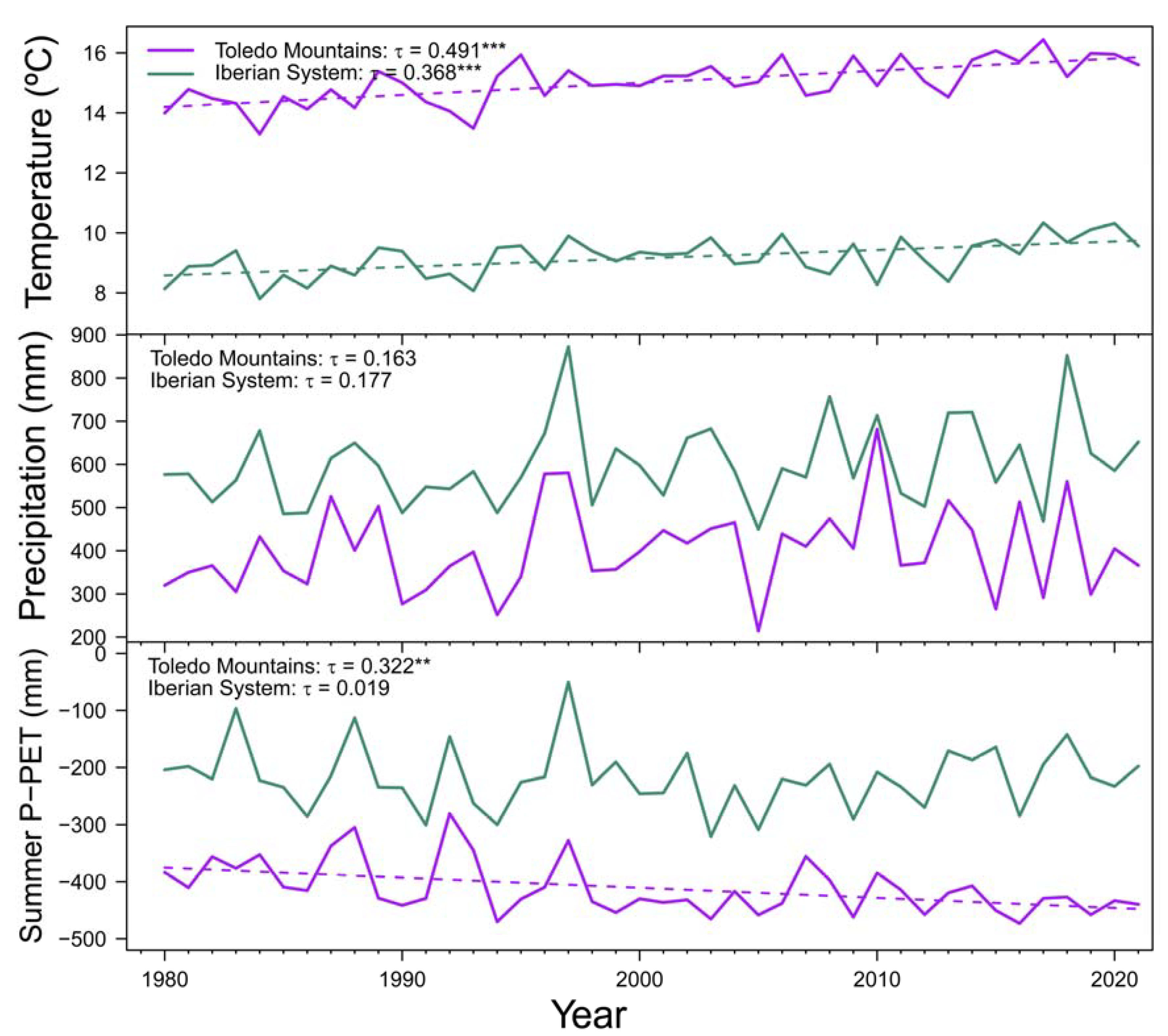
2.2. Climate and river flow data
2.3. Field sampling and dendrochronological methods
2.4. Statistical analyses
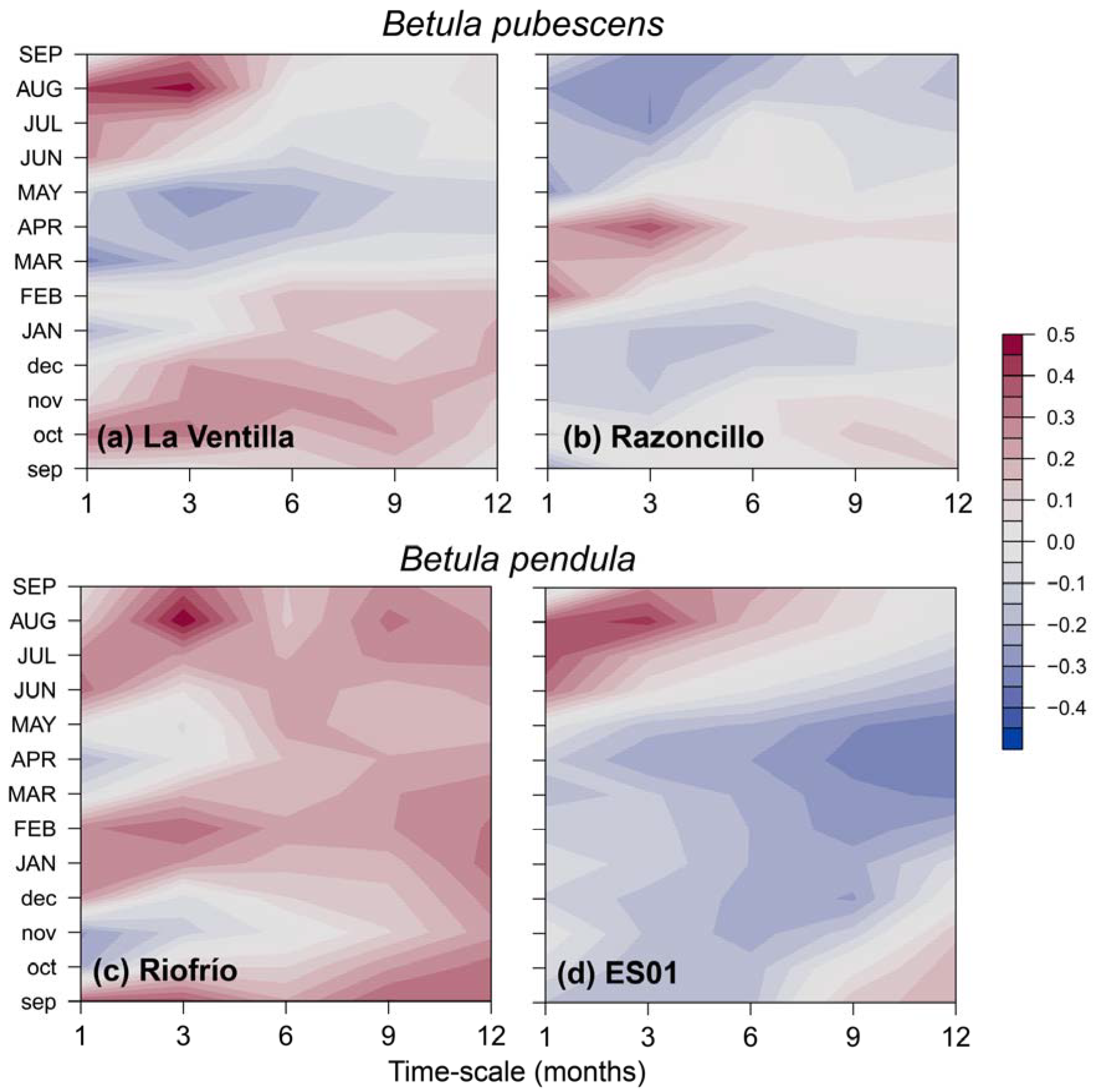
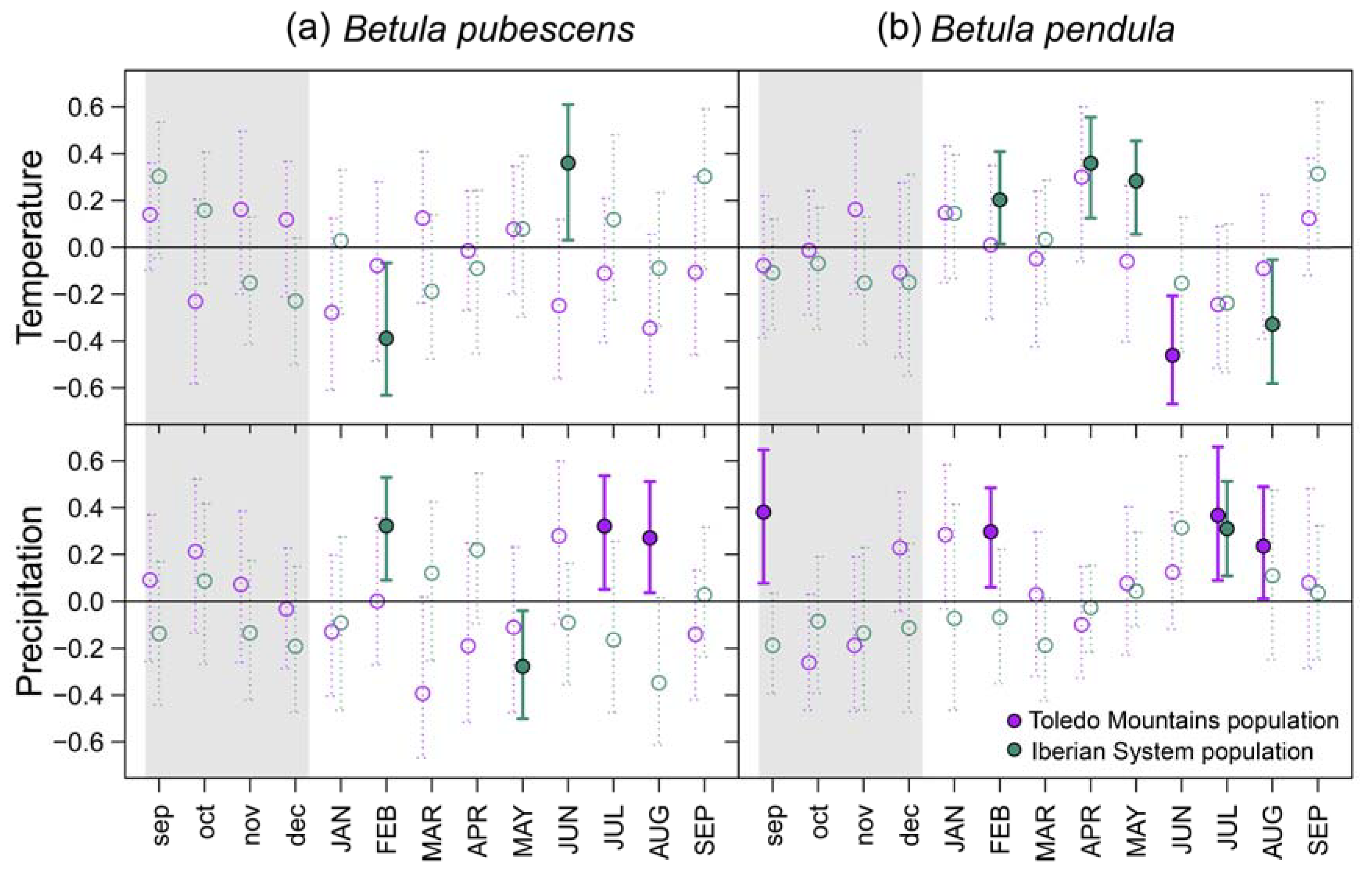
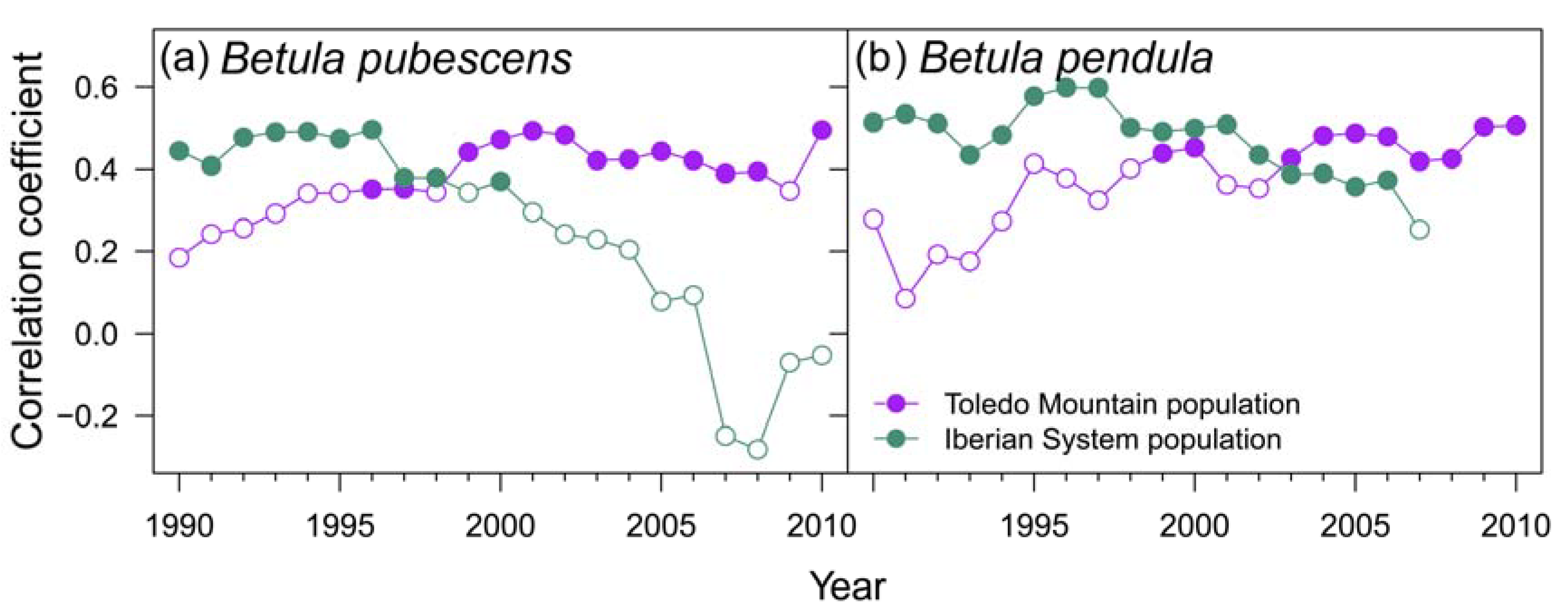
 statistic. Climate – growth relationships were assessed by calculating bootstrapped correlations between site standardized chronologies (RWI) and monthly mean temperature and precipitation sums for the 1980 – 2021 period. The window of analysis spanned from previous September to September of the year of tree-ring formation. We also calculated correlations between RWIs and SPEI, which was calculated at 1-, 3-, 6-, 9- and 12-month time scales, to quantify the relationship between radial growth variability and water deficit. To detect potential instabilities between growth indices and selected climatic data, we computed moving bootstrapped correlations considering moving 20-year long intervals lagged by one year. Pearson correlations were calculated to evaluate the statistical relationship between site RWI series and river flow and reservoir dam data. The aggregate effect of regional climate and local hydrological conditions on radial growth was addressed by means of linear mixed effects models (LMM; [51]. One model was adjusted for each site and species including tree-level RWI as response variables and SPEI, temperature, precipitation and river flow (or dam inflow) series as fixed effects. Selected variables were those showing significant correlations in the previous analysis. The presence of multicollinearity among predictors was tested using variance inflation factors (VIF) discarding variables with VIF > 3.
statistic. Climate – growth relationships were assessed by calculating bootstrapped correlations between site standardized chronologies (RWI) and monthly mean temperature and precipitation sums for the 1980 – 2021 period. The window of analysis spanned from previous September to September of the year of tree-ring formation. We also calculated correlations between RWIs and SPEI, which was calculated at 1-, 3-, 6-, 9- and 12-month time scales, to quantify the relationship between radial growth variability and water deficit. To detect potential instabilities between growth indices and selected climatic data, we computed moving bootstrapped correlations considering moving 20-year long intervals lagged by one year. Pearson correlations were calculated to evaluate the statistical relationship between site RWI series and river flow and reservoir dam data. The aggregate effect of regional climate and local hydrological conditions on radial growth was addressed by means of linear mixed effects models (LMM; [51]. One model was adjusted for each site and species including tree-level RWI as response variables and SPEI, temperature, precipitation and river flow (or dam inflow) series as fixed effects. Selected variables were those showing significant correlations in the previous analysis. The presence of multicollinearity among predictors was tested using variance inflation factors (VIF) discarding variables with VIF > 3.3. Results
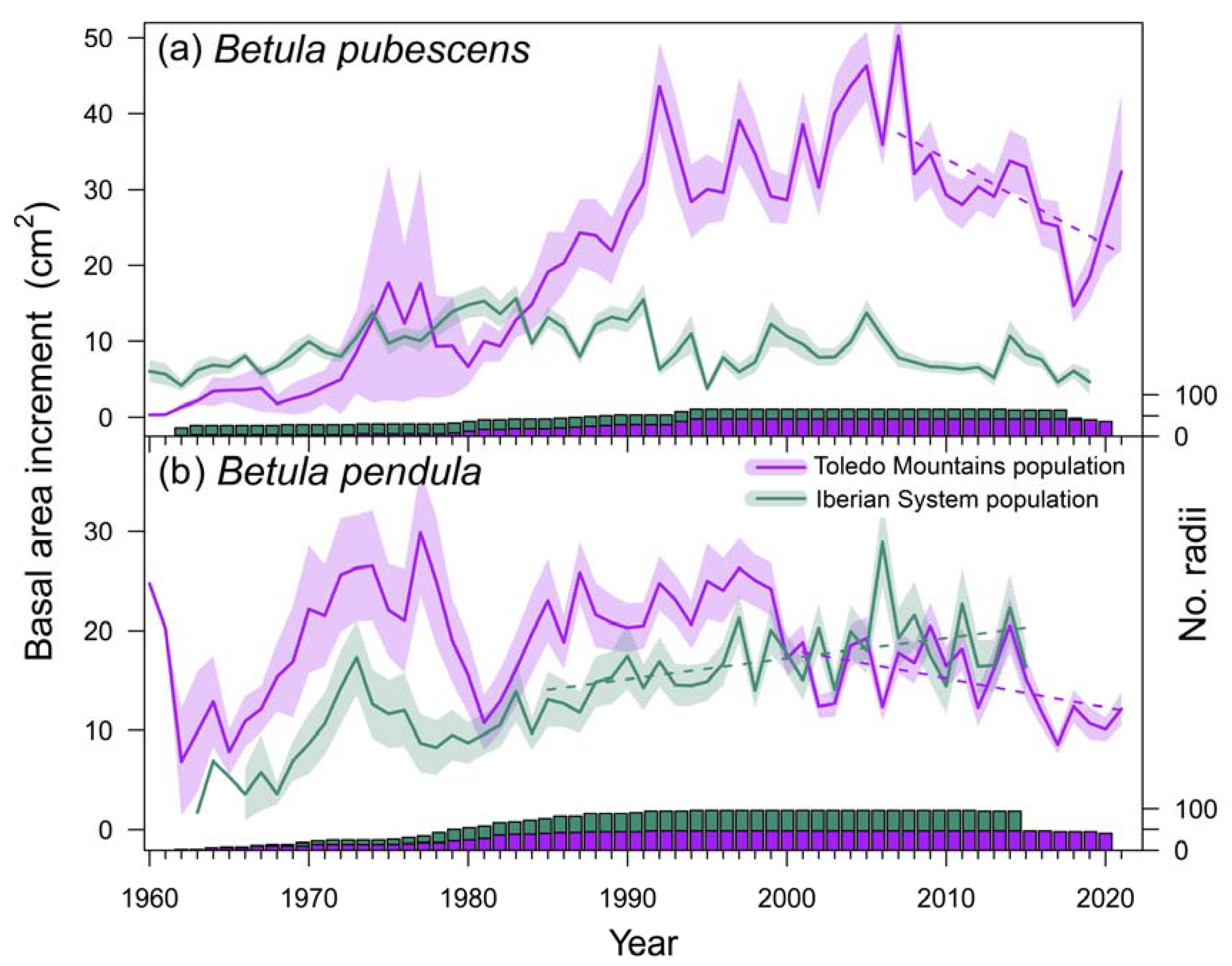
3.1. Growth patterns of birch populations in southern and northern Spain
 = 0.762, p < 0.001; Razoncillo:
= 0.762, p < 0.001; Razoncillo:  = 0.216, p = 0.033; Riofrío:
= 0.216, p = 0.033; Riofrío:  = 0.267, p = 0.016; ES01:
= 0.267, p = 0.016; ES01:  = 0.446, p = 0.004). Afterwards, growth trends became negative at the southern populations with a stepper slope in La Ventilla (
= 0.446, p = 0.004). Afterwards, growth trends became negative at the southern populations with a stepper slope in La Ventilla ( = -0.513, p = 0.017) than in Riofrío (
= -0.513, p = 0.017) than in Riofrío ( = -0.368, p = 0.025), whereas non-significant and positive trends were found at the northern populations (Razoncillo:
= -0.368, p = 0.025), whereas non-significant and positive trends were found at the northern populations (Razoncillo:  = -0.259, p = 0.199; ES01:
= -0.259, p = 0.199; ES01:  = 0.376, p = 0.003) (Figure 2).
= 0.376, p = 0.003) (Figure 2).3.2. Effect of climate and local hydrlogy on growth of Spanish birch stands
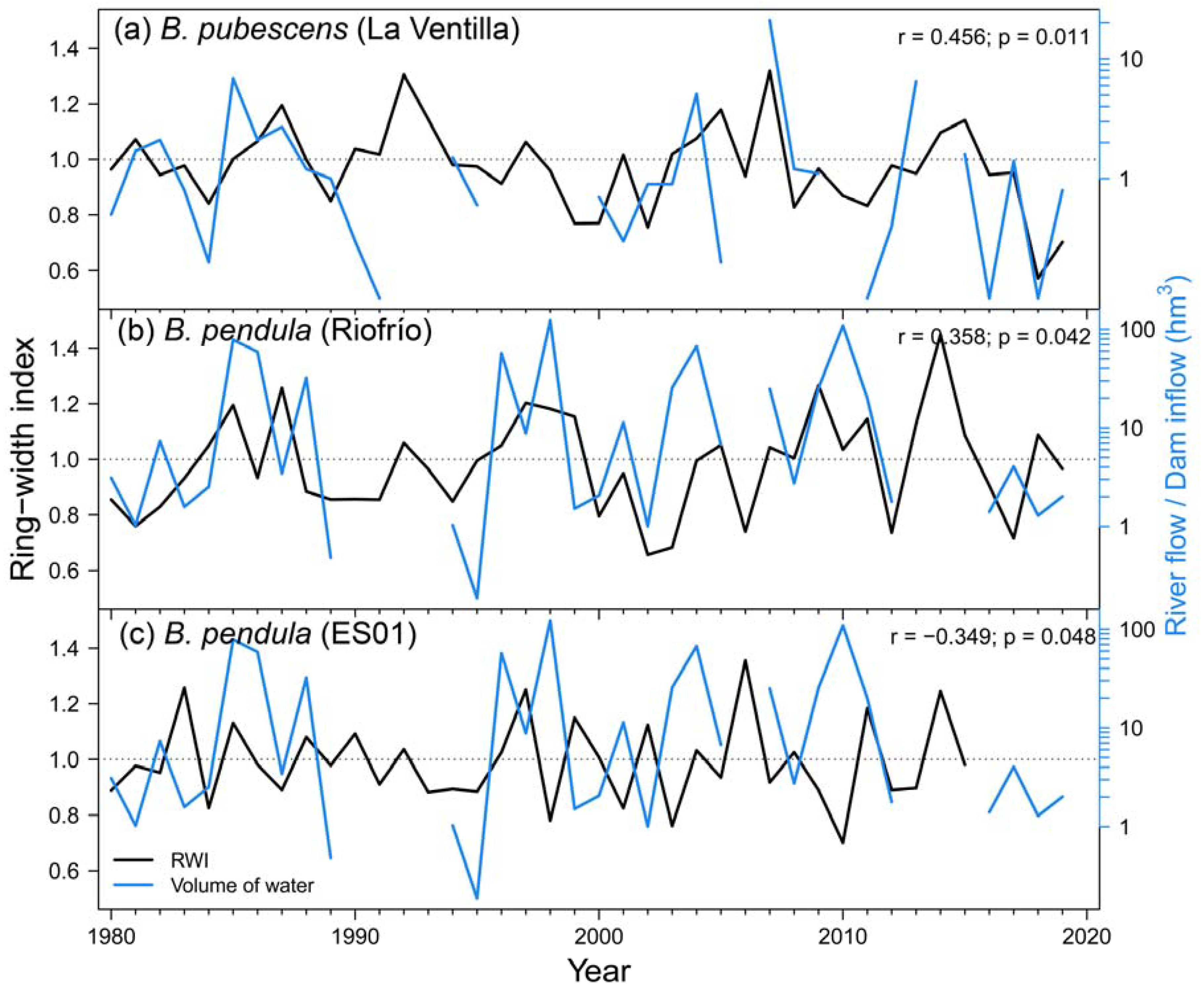
 = -0.275, p = 0.011), which is not found in Revinuesa river (
= -0.275, p = 0.011), which is not found in Revinuesa river ( = -0.149, p = 0.114).
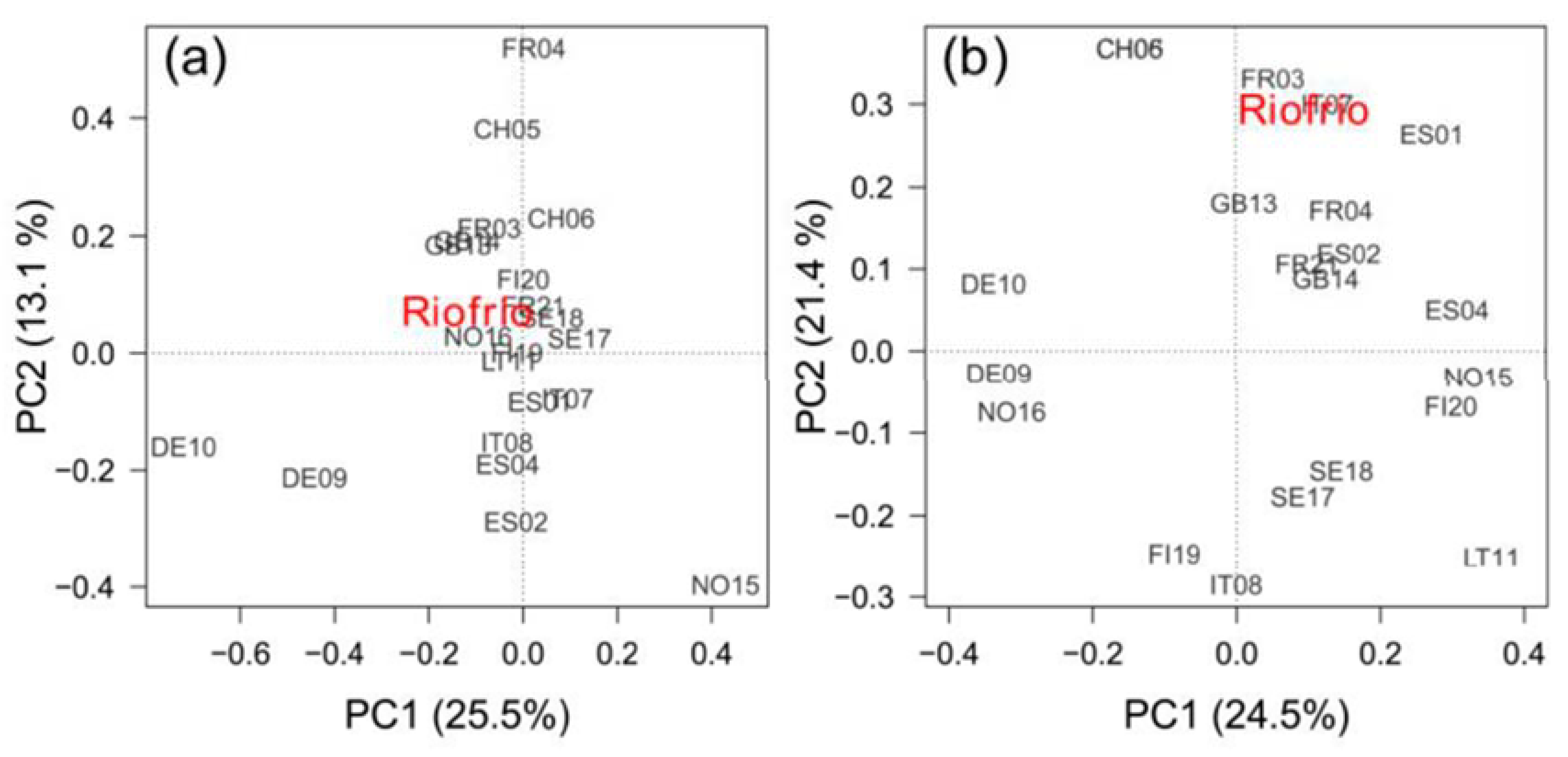
= -0.149, p = 0.114).3.2. Assessment of silver birch growth variability across Europe
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| ID | Country | Latitude (ºN) | Longitude (º E) | Elevation (m a.s.l.) | MAT (ºC) | MAP (mm) | P-PET (mm) | DBH (cm) | TRW (mm) | Tree age (years) | Rbar | EPS |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| FI19 | Finland | 66.37 | 26.73 | 127.13 | 0.79 | 585 | 52 | 19.94 | 1.82 | 48.2 | 0.30 | 0.923 |
| SE17 | Sweden | 64.76 | 18.70 | 278.12 | 1.66 | 578 | 45 | 22.67 | 2.39 | 40.5 | 0.32 | 0.93 |
| SE18 | Sweden | 63.92 | 19.89 | 150.95 | 3.29 | 672 | 123 | 24.11 | 2.42 | 43.5 | 0.25 | 0.887 |
| FI20 | Finland | 61.66 | 29.29 | 89.71 | 4.04 | 614 | 50 | 21.17 | 2.16 | 45.6 | 0.25 | 0.858 |
| NO16 | Norway | 60.80 | 10.75 | 187.28 | 4.56 | 667 | 105 | 25.76 | 3.81 | 27.2 | 0.32 | 0.922 |
| NO15 | Norway | 59.82 | 11.03 | 261.29 | 5.35 | 937 | 369 | 26.06 | 0.75 | 70.6 | 0.22 | 0.893 |
| GB14 | United Kingdom | 57.23 | -3.67 | 279.87 | 7.21 | 903 | 318 | 23.71 | 1.99 | 49.5 | 0.12 | 0.804 |
| LT11 | Lithuania | 55.03 | 23.01 | 56.21 | 7.42 | 632 | 15 | 22.17 | 2.12 | 25.5 | 0.32 | 0.911 |
| LT12 | Lithuania | 54.62 | 24.23 | 112.78 | 7.18 | 637 | 28 | 21.82 | 4.70 | 17.7 | 0.48 | 0.953 |
| GB13 | United Kingdom | 54.23 | -3.03 | 15.37 | 9.13 | 1450 | 825 | 30.24 | 1.87 | 62.4 | 0.15 | 0.827 |
| DE10 | Germany | 52.54 | 14.05 | 57.91 | 9.41 | 564 | -95 | 18.81 | 2.21 | 47.8 | 0.36 | 0.942 |
| DE09 | Germany | 51.84 | 14.44 | 61.95 | 9.75 | 565 | -101 | 30.00 | 2.62 | 61.2 | 0.14 | 0.809 |
| CH05 | Switzerland | 46.32 | 8.96 | 772.51 | 8.56 | 1452 | 843 | 32.28 | 2.92 | 51.1 | 0.19 | 0.842 |
| CH06 | Switzerland | 46.14 | 8.99 | 990.73 | 11.05 | 1487 | 801 | 28.33 | 2.48 | 41.4 | 0.08 | 0.691 |
| FR04 | France | 44.84 | 3.32 | 995.65 | 8.14 | 875 | 288 | 31.53 | 2.54 | 55.0 | 0.24 | 0.895 |
| FR21 | France | 44.21 | 7.10 | 1690.52 | 4.86 | 984 | 489 | 18.61 | 1.43 | 49.1 | 0.09 | 0.51 |
| FR03 | France | 44.20 | 7.07 | 1417.37 | 8.25 | 874 | 271 | 20.73 | 3.44 | 31.3 | 0.23 | 0.923 |
| IT07 | Italy | 43.61 | 11.71 | 1051.84 | 11.92 | 1150 | 458 | 26.13 | 2.01 | 56.1 | 0.05 | 0.497 |
| ES04 | Spain | 42.69 | -0.28 | 1790.00 | 6.56 | 1251 | 716 | 29.80 | 3.44 | 40.0 | 0.30 | 0.628 |
| ES02 | Spain | 42.67 | -0.32 | 1063.39 | 8.19 | 1165 | 579 | 20.74 | 2.69 | 34.9 | 0.20 | 0.845 |
| IT08 | Italy | 42.16 | 13.62 | 1552.42 | 9.81 | 1089 | 459 | 16.72 | 1.03 | 54.7 | 0.16 | 0.741 |
| ES01 | Spain | 41.97 | -2.62 | 1287.29 | 9.22 | 601 | -11 | 29.83 | 3.98 | 40.2 | 0.26 | 0.921 |
| Riofrío | Spain | 39.08 | -4.50 | 648.00 | 15.95 | 415 | -450 | 40.77 | 3.10 | 45.7 | 0.23 | 0.93 |
References
- Ashburner, K.; McAllister, H.A. The genus Betula: a taxonomic revision of birches; Kew Publishing: London, 2016. [Google Scholar]
- Atkinson, M.D. Betula pendula Roth (B. verrucosa Ehrh.) and B. pubescens Ehrh. J. Ecol. 1992, 80, 837–870. [Google Scholar] [CrossRef]
- Hynynen, J.; Niemistö, P.; Viherä-Aarnio, A.; Brunner, A.; Hein, S.; Velling, P. Silviculture of birch (Betula pendula Roth and Betula pubescens Ehrh.) in Northern Europe. Forestry 2010, 83, 103–119. [Google Scholar] [CrossRef]
- Jonczak, J.; Jankiewicz, U.; Kondras, M.; Kruczkowska, B.; Oktaba, L.; Oktaba, J.; Olejniczak, I.; Pawłowicz, E.; Polláková, N.; Raab, T.; et al. The influence of birch trees (Betula spp.) on soil environment – A review. For. Ecol. Manage. 2020, 477. [Google Scholar] [CrossRef]
- Nieuwenhuis, M.; Barrett, F. The growth potential of downy birch (Betula pubescens (Ehrh.)) in Ireland. Forestry 2002, 75, 75–87. [Google Scholar] [CrossRef]
- Kund, M.; Vares, A.; Sims, A. Early growth and development of silver birch (Betula pendula Roth.) plantations on abandoned agricultural land. 2010, 679–688. [Google Scholar] [CrossRef]
- Dubois, H.; Verkasalo, E.; Claessens, H. Potential of birch (Betula pendula Roth and B. pubescens Ehrh.) for forestry and forest-based industry sector within the changing climatic and socio-economic context of western Europe. Forests 2020, 11, 1–26. [Google Scholar] [CrossRef]
- Woodcock, B.A.; Leather, S.R.; Watt, A. Changing management in Scottish birch woodlands: A potential threat to local invertebrate biodiversity. Bull. Entomol. Res. 2003, 93, 159–167. [Google Scholar] [CrossRef]
- Felton, A.; Andersson, E.; Ventorp, D.; Lindbladh, M. A comparison of avian diversity in spruce monocultures and spruce-birch polycultures in Southern Sweden. Silva Fenn. 2011, 45, 1143–1150. [Google Scholar] [CrossRef]
- Wannebo-Nilsen, K.; Bjerke, J.W.; Beck, P.S.A.; Tømmervik, H. Epiphytic macrolichens in spruce plantations and native birch forests along a coast-inland gradient in North Norway. Boreal Environ. Res. 2010, 15, 43–57. [Google Scholar]
- IPCC Summary for Policymakers. In Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Masson-Delmotte, V., Zhai, P., Pirani, A., Connors, S.L., Péan, C., Berger, S., Caud, N., Chen, Y., Goldfarb, L., Gomis, M.I., Huang, M., Leitzell, K., Lonnoy, E., Matthews, J.B.R., Maycock, T.K., Waterfield, T., Yelekçi, O., Yu, R., Zhou, B., Eds., Eds.; Cambridge University Press, 2021; pp. 3–32. [Google Scholar]
- Seidl, R.; Thom, D.; Kautz, M.; Martin-benito, D.; Peltoniemi, M.; Vacchiano, G.; Wild, J.; Ascoli, D.; Petr, M.; Honkaniemi, J.; et al. Forest disturbances under climate change. Nat. Clim. Chang. 2017, 7, 395–402. [Google Scholar] [CrossRef]
- Allen, C.D.; Breshears, D.D.; McDowell, N.G. On underestimation of global vulnerability to tree mortality and forest die-off from hotter drought in the Anthropocene. Ecosphere 2015, 6, 1–55. [Google Scholar] [CrossRef]
- Hammond, W.M.; Williams, A.P.; Abatzoglou, J.T.; Adams, H.D.; Klein, T.; López, R.; Sáenz-Romero, C.; Hartmann, H.; Breshears, D.D.; Allen, C.D. Global field observations of tree die-off reveal hotter-drought fingerprint for Earth’s forests. Nat. Commun. 2022, 13. [Google Scholar] [CrossRef]
- Kharuk, V.I.; Ranson, K.J.; Oskorbin, P.A.; Im, S.T.; Dvinskaya, M.L. Climate induced birch mortality in Trans-Baikal lake region, Siberia. For. Ecol. Manage. 2013, 289, 385–392. [Google Scholar] [CrossRef]
- Tiwari, A.; Fan, Z.X.; Jump, A.S.; Zhou, Z.K. Warming induced growth decline of Himalayan birch at its lower range edge in a semi-arid region of Trans-Himalaya, central Nepal. Plant Ecol. 2017, 218, 621–633. [Google Scholar] [CrossRef]
- Beck, P.; Caudullo, G.; De Rigo, D.; Tinner, W. Betula pendula, Betula pubescens and other birches in Europe: distribution, habitat, usage and threats. In European Atlas of Forest Tree Species; San-Miguel-Ayanz, J., de Rigo, D., Caudullo, G., Houston Durrant, T., Mauri, A., Eds.; Publication Office of the European Union: Luxembourg, 2016; pp. 70–73. [Google Scholar]
- Thórsson, Æ.T.; Salmela, E.; Anamthawat-Jónsson, K. Morphological, cytogenetic, and molecular evidence for introgressive hybridization in birch. J. Hered. 2001, 62, 404–408. [Google Scholar] [CrossRef] [PubMed]
- Levanič, T.; Eggertsson, O. Climatic effects on birch (Betula pubescens Ehrh.) growth in Fnjoskadalur valley, northern Iceland. Dendrochronologia 2008, 25, 135–143. [Google Scholar] [CrossRef]
- Harr, L.; Esper, J.; Kirchhefer, J.A.; Zhou, W.; Hartl, C. Growth response of Betula pubescens Ehrh. to varying disturbance factors in northern Norway. Trees - Struct. Funct. 2021, 35, 421–431. [Google Scholar] [CrossRef]
- Matisons, R.; Elferts, D.; Schneck, V.; Kowalczyk, J. Silver birch shows nonlinear responses to moisture availability and temperature in the eastern Baltic Sea region. Dendrochronologia 2022, 76, 126003. [Google Scholar] [CrossRef]
- Augustaitis, A.; Augustaitienė, I.; Baugarten, M.; Bičenkienė, S.; Girgždienė, R.; Kulbokas, G.; Linkevičius, E.; Marozas, V.; Mikalajūnas, M.; Mordas, G.; et al. Tree-ring formation as an indicator of forest capacity to adapt to the main threats of environmental changes in Lithuania. Sci. Total Environ. 2018, 615, 1247–1261. [Google Scholar] [CrossRef]
- Schmitt, U.; Jalkanen, R.; Eckstein, D. Cambium dynamics of Pinus sylvestris and Betula spp. in the northern boreal forest in Finland. Silva Fenn. 2004, 38, 167–178. [Google Scholar] [CrossRef]
- Hemery, G.E.; Clark, J.R.; Aldinger, E.; Claessens, H.; Malvolti, M.E.; O’connor, E.; Raftoyannis, Y.; Savill, P.S.; Brus, R. Growing scattered broadleaved tree species in Europe in a changing climate: a review of risks and opportunities. Forestry 2010, 83, 65–81. [Google Scholar] [CrossRef]
- Possen, B.J.H.M.; Oksanen, E.; Rousi, M.; Ruhanen, H.; Ahonen, V.; Tervahauta, A.; Heinonen, J.; Heiskanen, J.; Kärenlampi, S.; Vapaavuori, E. Adaptability of birch (Betula pendula Roth) and aspen (Populus tremula L.) genotypes to different soil moisture conditions. For. Ecol. Manage. 2011, 262, 1387–1399. [Google Scholar] [CrossRef]
- Way, D. a; Oren, R. Differential responses to changes in growth temperature between trees from different functional groups and biomes: a review and synthesis of data. Tree Physiol. 2010, 30, 669–88. [Google Scholar] [CrossRef] [PubMed]
- Hofgaard, A.; Ols, C.; Drobyshev, I.; Kirchhefer, A.J.; Sandberg, S.; Söderström, L. Non-stationary response of tree growth to climate trends along the Arctic Margin. Ecosystems 2019, 22, 434–451. [Google Scholar] [CrossRef]
- Martín, C.; Parra, T.; Clemente-muñoz, M.; Hernandez-bermejo, E. Genetic diversity and structure of the endangered Betula pendula subsp. fontqueri populations in the south of Spain. Silva 2008, 42, 487–498. [Google Scholar]
- Sánchez de Álamo, C.; Sardinero, S.; Bouso, V.; Hernández Palacios, G.; Pérez-Badia, R.; Fernández-González, F. Los abedulares del Parque Nacional De Cabañeros : sistemática, demografía, biología reproductiva y estrategias de conservación. In Proyectos de investigación en parques nacionales: 2006-2009; Organismo Autónomo de Parques Nacionales, Ministerio de Medio Ambiente: Madrid, 2010; pp. 275–309. [Google Scholar]
- Luelmo-Lautenschlaeger, R.; Pérez-Díaz, S.; Alba-Sánchez, F.; Abel-Schaad, D.; López-Sáez, J.A. Vegetation history in the Toledo Mountains (Central Iberia): Human impact during the last 1300 years. Sustainability 2018, 10, 2575. [Google Scholar] [CrossRef]
- Morales-Molino, C.; Tinner, W.; Perea, R.; Carrión, J.S.; Colombaroli, D.; Valbuena-Carabaña, M.; Zafra, E.; Gil, L. Unprecedented herbivory threatens rear-edge populations of Betula in southwestern Eurasia. Ecology 2019, 100, 1–15. [Google Scholar] [CrossRef]
- Hampe, A.; Petit, R.J. Conserving biodiversity under climate change: The rear edge matters. Ecol. Lett. 2005, 8, 461–467. [Google Scholar] [CrossRef] [PubMed]
- Aitken, S.N.; Bemmels, J.B. Time to get moving: Assisted gene flow of forest trees. Evol. Appl. 2016, 9, 271–290. [Google Scholar] [CrossRef]
- Borovics, A.; Mátyás, C. Decline of genetic diversity of sessile oak at the retracting (xeric) limits. Ann. For. Sci. 2013, 70, 835–844. [Google Scholar] [CrossRef]
- Fréjaville, T.; Vizcaíno-Palomar, N.; Fady, B.; Kremer, A.; Benito Garzón, M. Range margin populations show high climate adaptation lags in European trees. Glob. Chang. Biol. 2020, 26, 484–495. [Google Scholar] [CrossRef] [PubMed]
- Camarero, J.J.; Gazol, A.; Sangüesa-Barreda, G.; Vergarechea, M.; Alfaro-Sánchez, R.; Cattaneo, N.; Vicente-Serrano, S.M. Tree growth is more limited by drought in rear-edge forests most of the times. For. Ecosyst. 2021, 8, 1–15. [Google Scholar] [CrossRef]
- Dyderski, M.K.; Paź, S.; Frelich, L.E.; Jagodziński, A.M. How much does climate change threaten European forest tree species distributions? Glob. Chang. Biol. 2018, 24, 1150–1163. [Google Scholar] [CrossRef] [PubMed]
- Fritts, H.C. Tree Rings and Climate; Blackburn Press: Caldwell, USA, 2001. [Google Scholar]
- Xu, K.; Wang, X.; Liang, P.; An, H.; Sun, H.; Han, W.; Li, Q. Tree-ring widths are good proxies of annual variation in forest productivity in temperate forests. Sci. Rep. 2017, 7, 1–8. [Google Scholar] [CrossRef]
- López-Sáez, J.A.; García-Río, R.; Alba-Sánchez, F.; García-Gómez, E.; Pérez-Díaz, S. Peatlands in the Toledo Mountains (central Spain): Characterisation and conservation status. Mires Peat 2014, 15, 1–23. [Google Scholar]
- Martínez-Sancho, E.; Slámová, L.; Morganti, S.; Grefen, C.; Carvalho, B.; Dauphin, B.; Rellstab, C.; Gugerli, F.; Opgenoorth, L.; Heer, K.; et al. The GenTree Dendroecological Collection, tree-ring and wood density data from seven tree species across Europe. Sci. Data 2020, 7, 1–7. [Google Scholar] [CrossRef]
- de Rigo, D.; Caudullo, G.; Durrant, T.H.; San-Miguel-Ayanz, J. The European Atlas of Forest Tree Species: Modelling, data and information on forest tree species. In The European Atlas of Forest Tree Species; Publication Office of the European Union: Luxembourg, 2016; pp. 40–45. [Google Scholar]
- Cornes, R.; van der Schrier, G.; van den Besselaar, E.J.M.; Jones, P.D. An ensemble version of the E-OBS temperature and precipitation datasets. J. Geophys. Res. Atmos. 2018, 123, 9391–9409. [Google Scholar] [CrossRef]
- Vicente-Serrano, S.M.; Beguería, S.; López-Moreno, J.I. A multiscalar drought index sensitive to global warming: The standardized precipitation evapotranspiration index. J. Clim. 2010, 23, 1696–1718. [Google Scholar] [CrossRef]
- Beguería, S.; Vicente-Serrano, S.M. SPEI: Calculation of the Standardised Precipitation-Evapotranspiration Index. R package version 1.7. 2017.
- R core Team R: A language and environment for statistical computing. R foundation for statistical computing. 2023.
- Luelmo-lautenschlaeger, R.; Morales-molino, C.; Blarquez, O.; Pérez-díaz, S.; Sabariego-ruiz, S.; Ochando, J.; Carrión, J.S.; Perea, R.; Fernández-gonzález, F.; López-sáez, J.A. Long-term vegetation history of a relict birch forest (Betula pubescens subsp. celtiberica (Rothm. & Vasc.) Rivas Mart.) in the Toledo Mountains (central Iberia). Conservation implications. Rev. Palaeobot. Palynol. 2023, 315, 104906. [Google Scholar]
- Larsson, L.A.; Larsson, P.O. CDendro and CooRecorder (v. 9.3.1) 2018. 2018.
- Holmes, R.L. Computer-assisted quality control in tree-ring dating and measurement. Tree-Ring Bull. 1983, 43, 69–78. [Google Scholar]
- Biondi, F.; Qeadan, F. A theory-driven approach to tree-ring standardization: Defining the biological trend from expected basal area increment. Tree-Ring Res. 2008, 64, 81–96. [Google Scholar] [CrossRef]
- Pinheiro, J.C.; Bates, D.M. Mixed-effects models in S and S-PLUS.; Springer-Verlag: New York, 2000. [Google Scholar]
- Wood, S.N. Generalized Additive Models. An Introduction with R; Chapman and Hall/CRC: Boca Raton, USA, 2017. [Google Scholar]
- Wood, S.N. Thin-plate regression splines. J. R. Stat. Soc. Ser. B 2003, 65, 95–114. [Google Scholar] [CrossRef]
- Burnham, K.P.; Anderson, D.R. Model Selection and Multimodel Inference: a Practical Information-theoretic Approach; 2nd ed.; Springer-Verlag: New York, 2002. [Google Scholar]
- Bunn, A.; Korpela, M.; Biondi, F.; Campelo, F.; Mérian, P.; Qeadan, F.; Zang, C. dplR: Dendrochronology Program Library in R. R package version 1.7.1. 2020.
- McLeod, A.I. Kendall: Kendall rank correlation and Mann-Kendall trend test. R package version 2.2. 2011.
- Zeileis, A.; Kleiber, C.; Walter, K.; Hornik, K. Testing and dating of structural changes in practice. Comput. Stat. Data Anal. 2003, 44, 109–123. [Google Scholar] [CrossRef]
- Zang, C.; Biondi, F. Treeclim: An R package for the numerical calibration of proxy-climate relationships. Ecography (Cop.). 2015, 38, 431–436. [Google Scholar]
- Bates, D.; Maechler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar]
- Kuznetsova, A.; Brockhoff, P.B.; Christensen, R.H.B. lmerTest package: Tests in linear mixed effects models. J. Stat. Software2 2017, 82, 1–26. [Google Scholar] [CrossRef]
- Wood, S.N. Fast stable restricted maximum likelihood and marginal likelihood estimation of semiparametric generalized linear models. J. R. Stat. Soc. Ser. B 2011, 73, 3–36. [Google Scholar] [CrossRef]
- Castells, X.; Gutiérrez, E. Phenology and stem growth dynamics of Betula pendula Roth. in the Spanish Pyrenees. Res. Sq. 2022. [Google Scholar]
- Marchand, L.J.; Dox, I.; Gricar, J.; Prislan, P.; Van den Bulcke, J.; Fonti, P.; Campioli, M. Timing of spring xylogenesis in temperate deciduous tree species relates to tree growth characteristics and previous autumn phenology. Tree Physiol. 2020, 41, 1161–1170. [Google Scholar] [CrossRef] [PubMed]
- Pudas, E.; Leppälä, M.; Tolvanen, A.; Poikolainen, J.; Venäläinen, A.; Kubin, E. Trends in phenology of Betula pubescens across the boreal zone in Finland. Int. J. Biometeorol. 2008, 52, 251–259. [Google Scholar] [CrossRef]
- Fernández-Martínez, J.; Fransi, M.A.; Fleck, I. Ecophysiological responses of Betula pendula, Pinus uncinata and Rhododendron ferrugineum in the Catalan Pyrenees to low summer rainfall. Tree Physiol. 2016, 36, 1520–1535. [Google Scholar] [CrossRef] [PubMed]
- Aspelmeier, S.; Leuschner, C. Genotypic variation in drought response of silver birch (Betula pendula Roth): Leaf and root morphology and carbon partitioning. Trees - Struct. Funct. 2006, 20, 42–52. [Google Scholar]
- Aspelmeier, S.; Leuschner, C. Genotypic variation in drought response of silver birch (Betula pendula): Leaf water status and carbon gain. Tree Physiol. 2004, 24, 517–528. [Google Scholar]
- Hacket-Pain, A.J.; Ascoli, D.; Vacchiano, G.; Biondi, F.; Cavin, L.; Conedera, M.; Drobyshev, I.; Liñán, I.D.; Friend, A.D.; Grabner, M.; et al. Climatically controlled reproduction drives interannual growth variability in a temperate tree species. Ecol. Lett. 2018, 21, 1833–1844. [Google Scholar] [CrossRef] [PubMed]
- Davi, H.; Cailleret, M.; Restoux, G.; Amm, A.; Pichot, C.; Fady, B. Disentangling the factors driving tree reproduction. Ecosphere 2016, 7, 1–16. [Google Scholar]
- Cailleret, M.; Jansen, S.; Robert, E.M.R.; Desoto, L.; Aakala, T.; Antos, J.A.; Beikircher, B.; Bigler, C.; Bugmann, H.; Caccianiga, M.; et al. A synthesis of radial growth patterns preceding tree mortality. Glob. Chang. Biol. 2017, 23, 1675–1690. [Google Scholar]
- Camarero, J.J.; Gazol, A.; Sangüesa-Barreda, G.; Cantero, A.; Sánchez-Salguero, R.; Sánchez-Miranda, A.; Granda, E.; Serra-Maluquer, X.; Ibáñez, R. Forest growth responses to drought at short- and long-term scales in Spain: Squeezing the stress memory from tree rings. Front. Ecol. Evol. 2018, 6, 1–11. [Google Scholar]
- Hannus, S.; Hirons, A.; Baxter, T.; McAllister, H.A.; Wiström, B.; Sjöman, H. Intraspecific drought tolerance of Betula pendula genotypes: an evaluation using leaf turgor loss in a botanical collection. Trees - Struct. Funct. 2021, 35, 569–581. [Google Scholar] [CrossRef]
- Ranney, T.G.; Bir, R.E.; Skroch, W.A. Comparative drought resistance among six species of birch (Betula): influence of mild water stress on water relations and leaf gas exchange. Tree Physiol. 1991, 8, 351–360. [Google Scholar]
- Cavin, L.; Jump, A.S. Highest drought sensitivity and lowest resistance to growth suppression are found in the range core of the tree Fagus sylvatica L. not the equatorial range edge. Glob. Chang. Biol. 2017, 23, 362–379. [Google Scholar] [CrossRef] [PubMed]
- Ranno, V.; Blandino, C.; Giusso Del Galdo, G. A comparative study on temperature and water potential thresholds for the germination of Betula pendula and two Mediterranean endemic birches, Betula aetnensis and Betula fontqueri. Seed Sci. Res. 2020, 30, 249–261. [Google Scholar]
- Vivero, J.L. Betula pendula var. fontqueri (Rothm.) G.Moreno & Peinado. In The IUCN Red List of Threatened Species 1998. 1998. [Google Scholar]
- Luelmo-Lautenschlaeger, R.; Pérez-Díaz, S.; Blarquez, O.; Morales-Molino, C.; López-Sáez, J.A. The Toledo Mountains: A resilient landscape and a landscape for resilience? hazards and strategies in a mid-elevation mountain region in central Spain. Quaternary 2019, 2. [Google Scholar]
- Luelmo-Lautenschlaeger, R.; Blarquez, O.; Pérez-Díaz, S.; Morales-Molino, C.; López-Sáez, J.A. The Iberian Peninsula’s burning heart — Long-term fire history in the Toledo Mountains (Central Spain). Fire 2019, 2. [Google Scholar]
- Luelmo Lautenschlaeger, R.; Pérez Díaz, S.; López Sáez, J.A. Paisajes relictos en los Montes de Toledo (España). Los humedales como refugio. In Conservación, Gestión y Restauración de la Biodiversidad. XI Congreso Español y I Congreso Iberoamericano de Biogeografía. Santander (España), 22-25 de junio; Carracedo, V., García-Codron, J.C., Garmendia, C., Rivas, V., Eds.; Asociación de Geógrafos Españoles (AGE): Santander, 2020; pp. 591–600. [Google Scholar]
- Morales-Molino, C.; Colombaroli, D.; Tinner, W.; Perea, R.; Valbuena-Carabaña, M.; Carrión, J.S.; Gil, L. Vegetation and fire dynamics during the last 4000 years in the Cabañeros National Park (central Spain). Rev. Palaeobot. Palynol. 2018, 253, 110–122. [Google Scholar] [CrossRef]
| Betula pubescens | Betula pendula | |||
|---|---|---|---|---|
| Site ID | La Ventilla | Razoncillo | Riofrío | ES01 |
| Latitude (º N) | 39º 20’ 25” | 41º 59‘ 12“ | 39º 05’ 00” | 41 58‘ 08“ |
| Longitude (º W) | 4º 16’ 40” | 2º 38‘ 38“ | 4º 30’ 10” | 2º 37‘ 19“ |
| Elevation (m a.s.l.) | 638 | 1555 | 648 | 1290 |
| DBH (cm) | 49.9 ± 3.1 | 35.0 ± 1.3 | 40.8 ± 1.6 | 29.8 ± 1.8 |
| Tree age (years) | 38 ± 2 | 67 ± 4 | 46 ± 2 | 40 ± 2 |
| No. sampled trees (No. radii) | 21 (44) | 12 (24) | 23 (46) | 25 (50) |
| TRW (mm) 1 | 5.01 ± 0.27 | 1.43 ± 0.11 | 3.10 ± 0.22 | 3.46 ± 0.28 |
| AC1 | 0.71 ± 0.03 | 0.62 ± 0.04 | 0.73 ± 0.02 | 0.52 ± 0.06 |
| Rbar1 | 0.278 | 0.360 | 0.296 | 0.239 |
| MS1 | 0.297 | 0.369 | 0.288 | 0.318 |
| EPS1 | 0.864 | 0.871 | 0.906 | 0.863 |
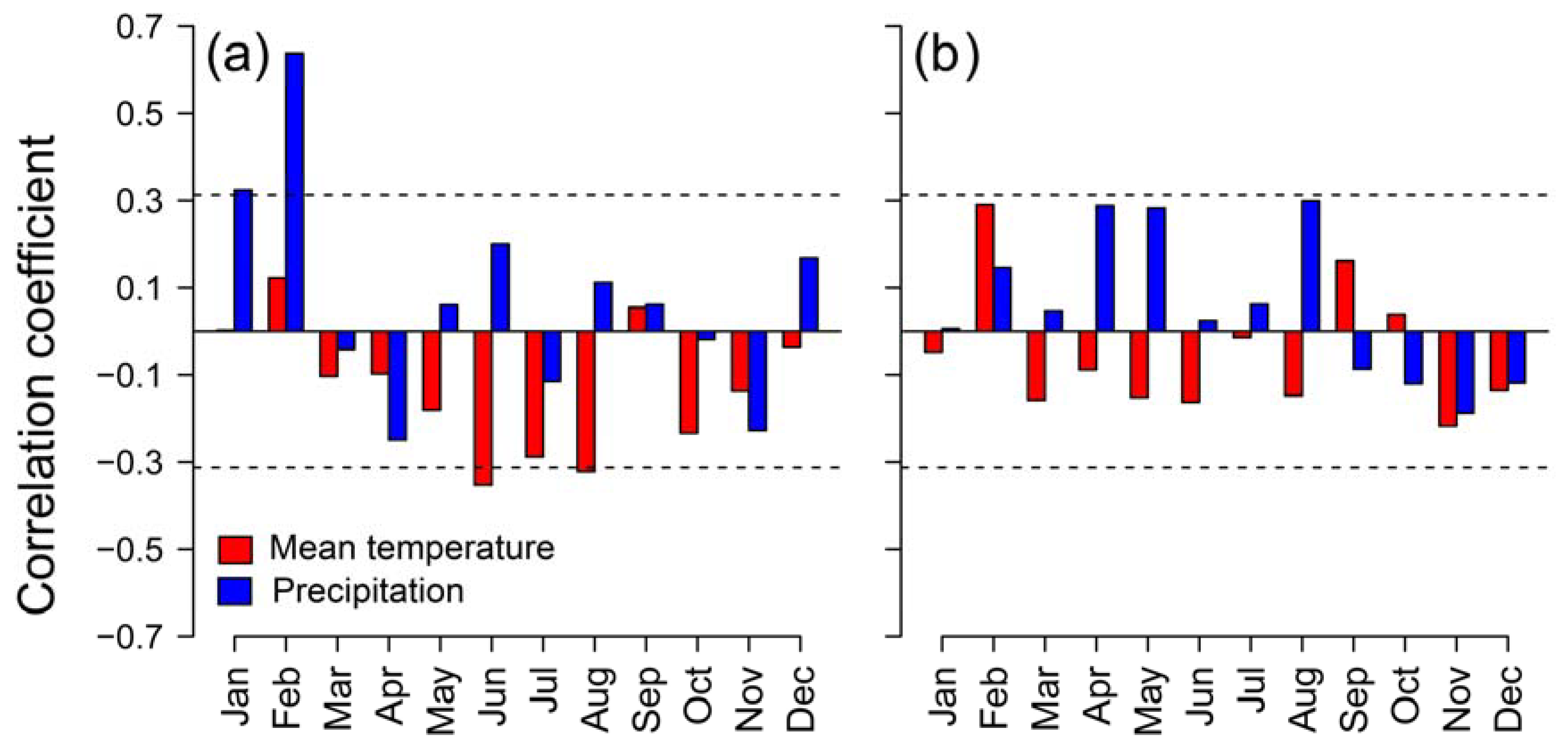
| Species | Site | Variable | F |
|---|---|---|---|
| Betula pubescens | La Ventilla | Prec. Jul | 2.161 |
| Prec. aug | 0.448 | ||
| SPEI3 August | 9.317** | ||
| Dam inflow Nov-1 | 15.335*** | ||
| Razoncillo | Temp. Feb | 1.164 | |
| Temp. Jun | 10.660** | ||
| Prec. Feb | 0.216 | ||
| Prec. May | 2.780+ | ||
| SPEI3 April | 6.610* | ||
| Betula pendula | Riofrío | Prec. Sep-1 | 3.897* |
| Prec. Feb | 2.472 | ||
| Prec. Jul | 0.539 | ||
| SPEI3 August | 7.357** | ||
| River flow Feb | 7.983** | ||
| ES01 | Temp. Feb | 2.981+ | |
| Temp. Apr | 0.317 | ||
| Temp. May | 25.944*** | ||
| Temp. Aug | 29.684*** | ||
| Prec. Jul | 1.760 | ||
| SPEI3 August | 4.019* | ||
| River flow Sep-1 | 3.942* |
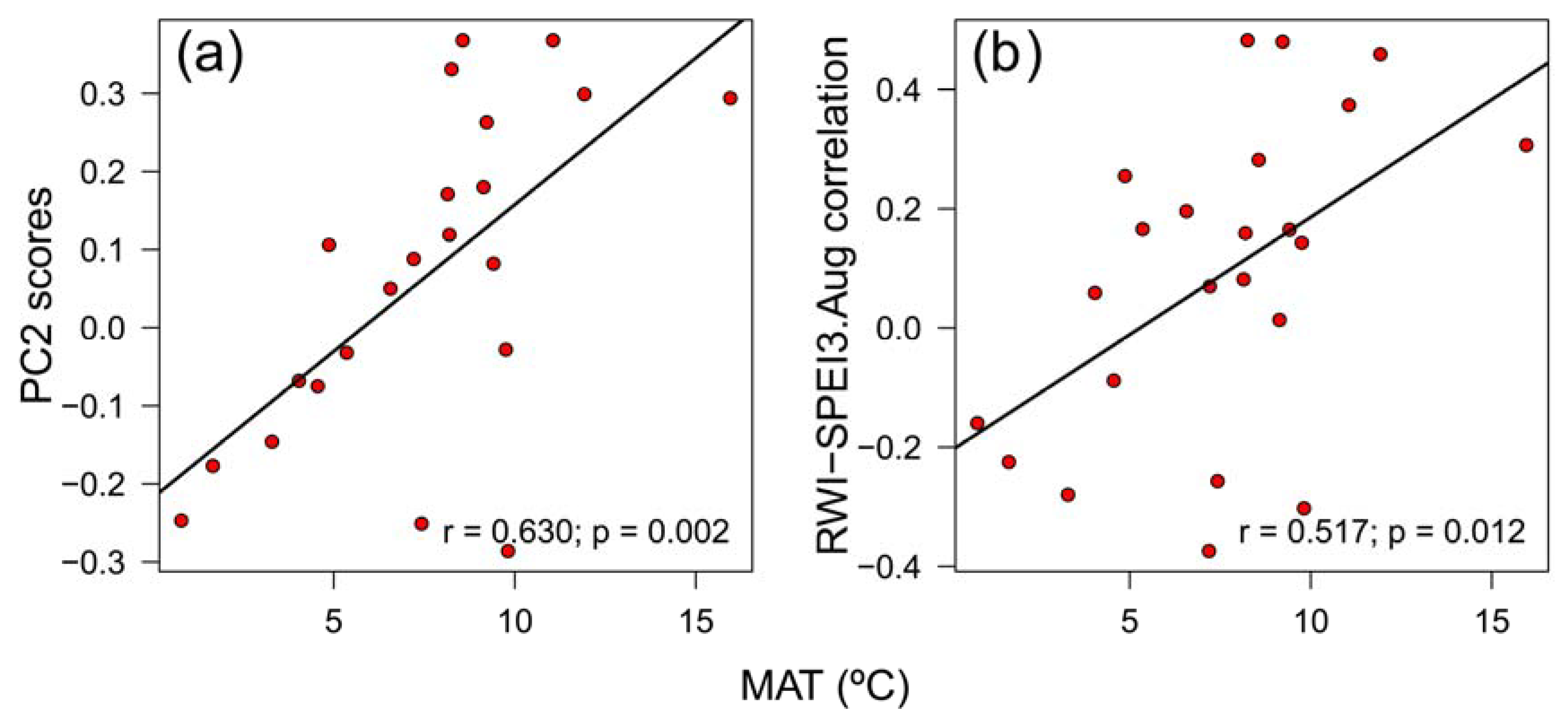
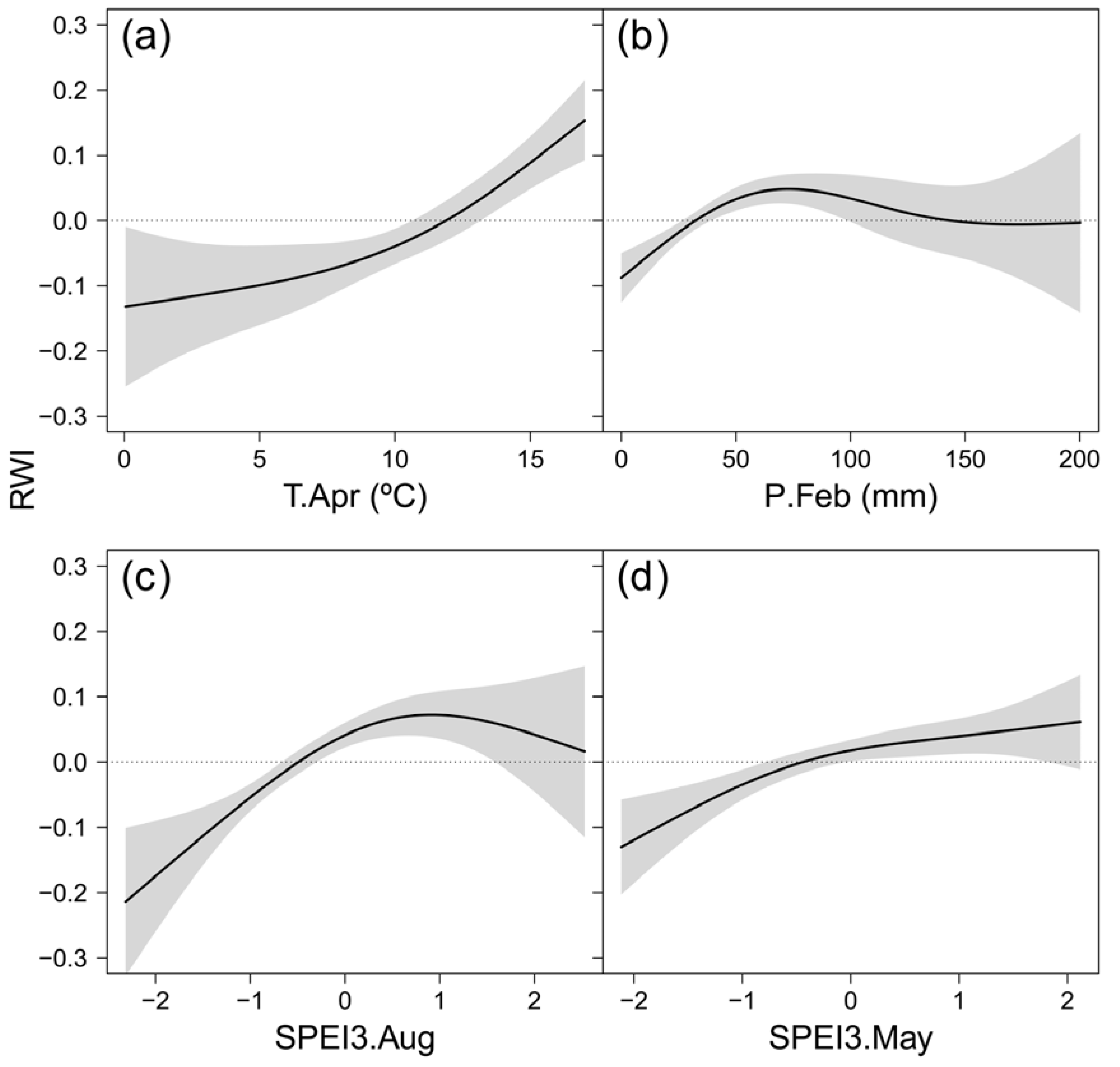
| Variable | Effective degree of freedom | F-statistic | p-value |
|---|---|---|---|
| Temp. Apr | 2.034 | 8.912 | 0.002 |
| Prec. Feb | 2.626 | 5.636 | <0.001 |
| SPEI3 August | 2.442 | 14.307 | <0.001 |
| SPEI3 May | 2.060 | 5.869 | 0.001 |
| R2m | 0.143 | ||
| R2c | 0.303 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





