Submitted:
21 June 2023
Posted:
21 June 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
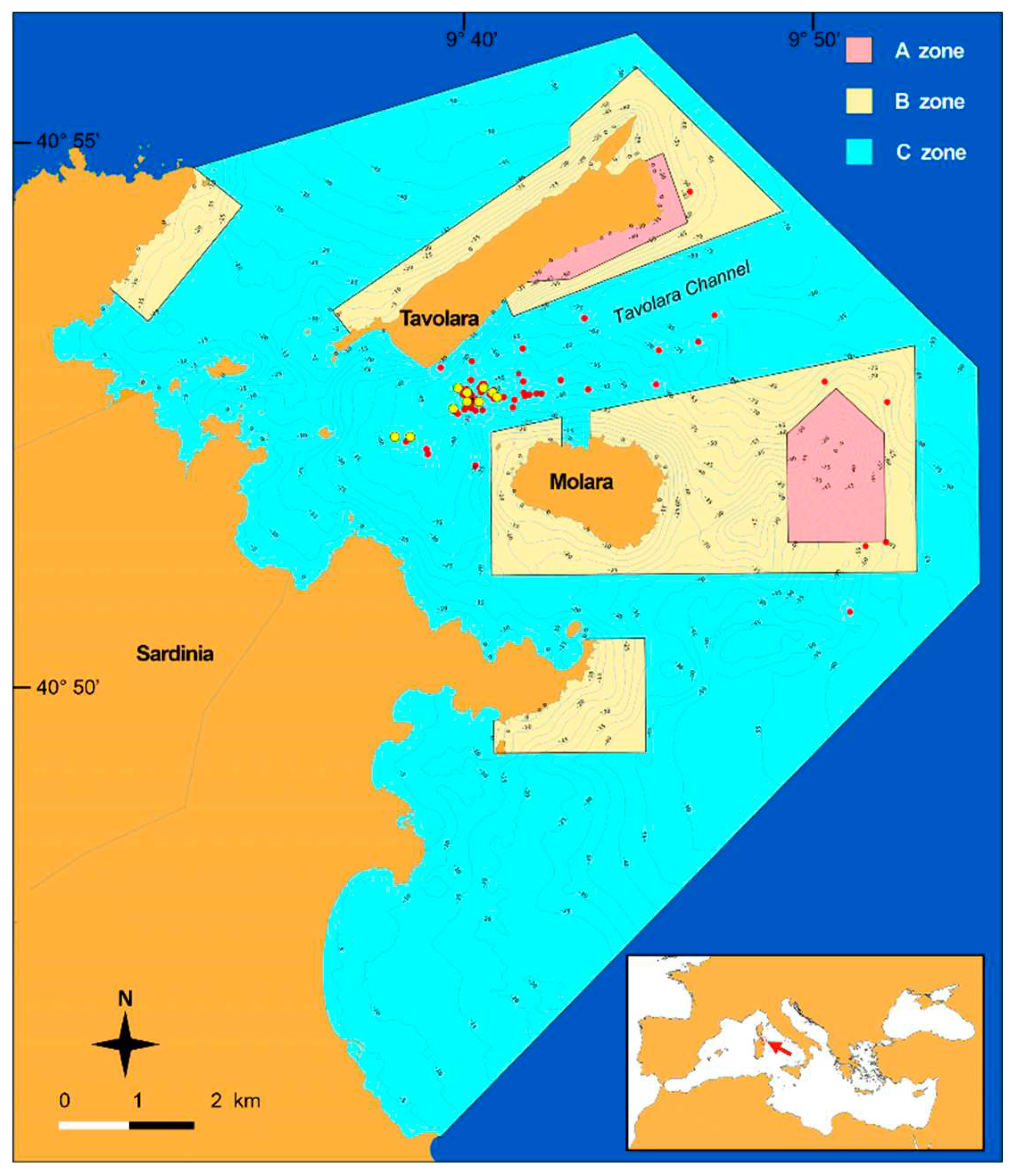
2. Materials and Methods
3. Results
3.1. Paramuricea clavata of the Tavolara Channel granitic shoals
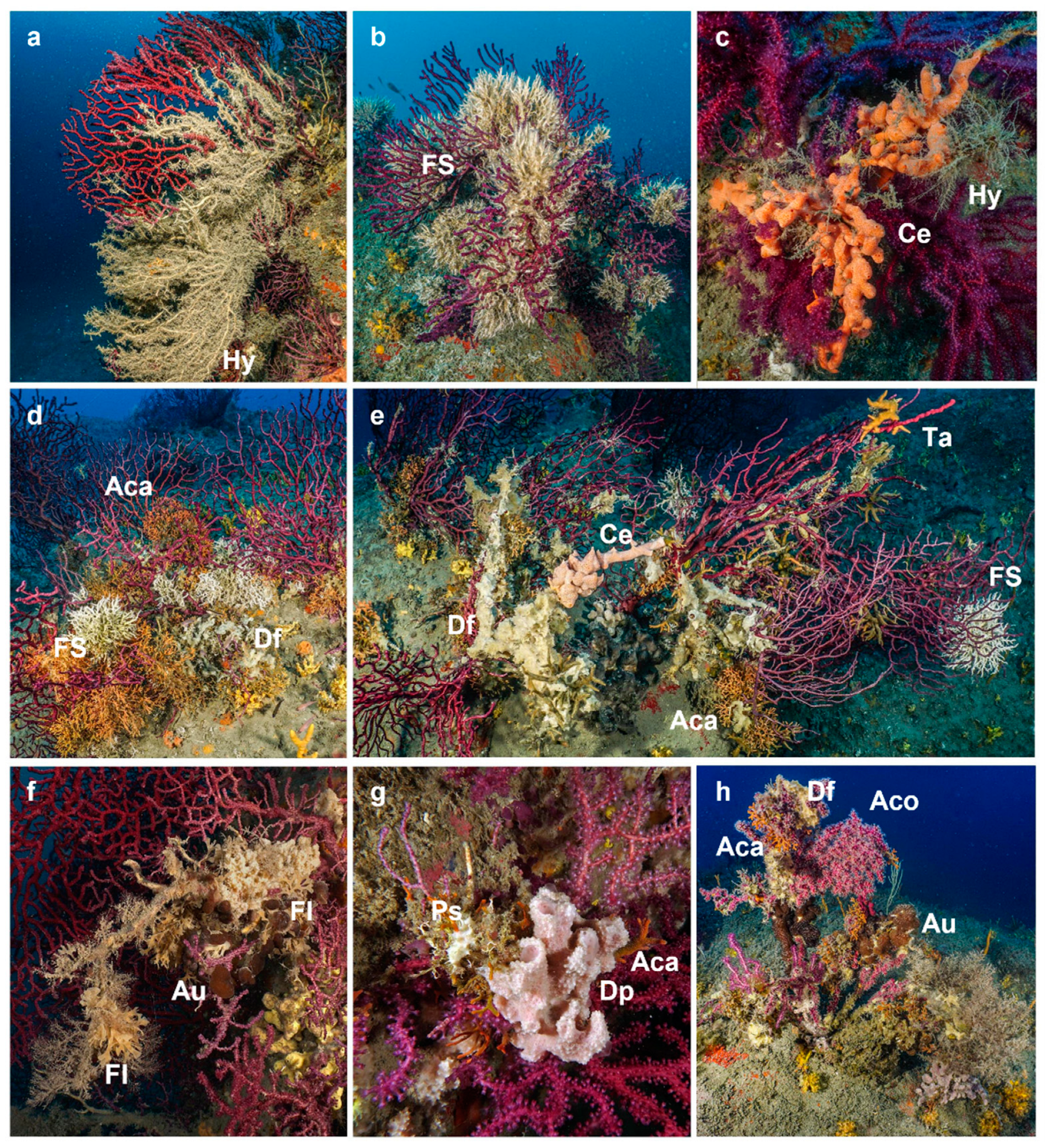
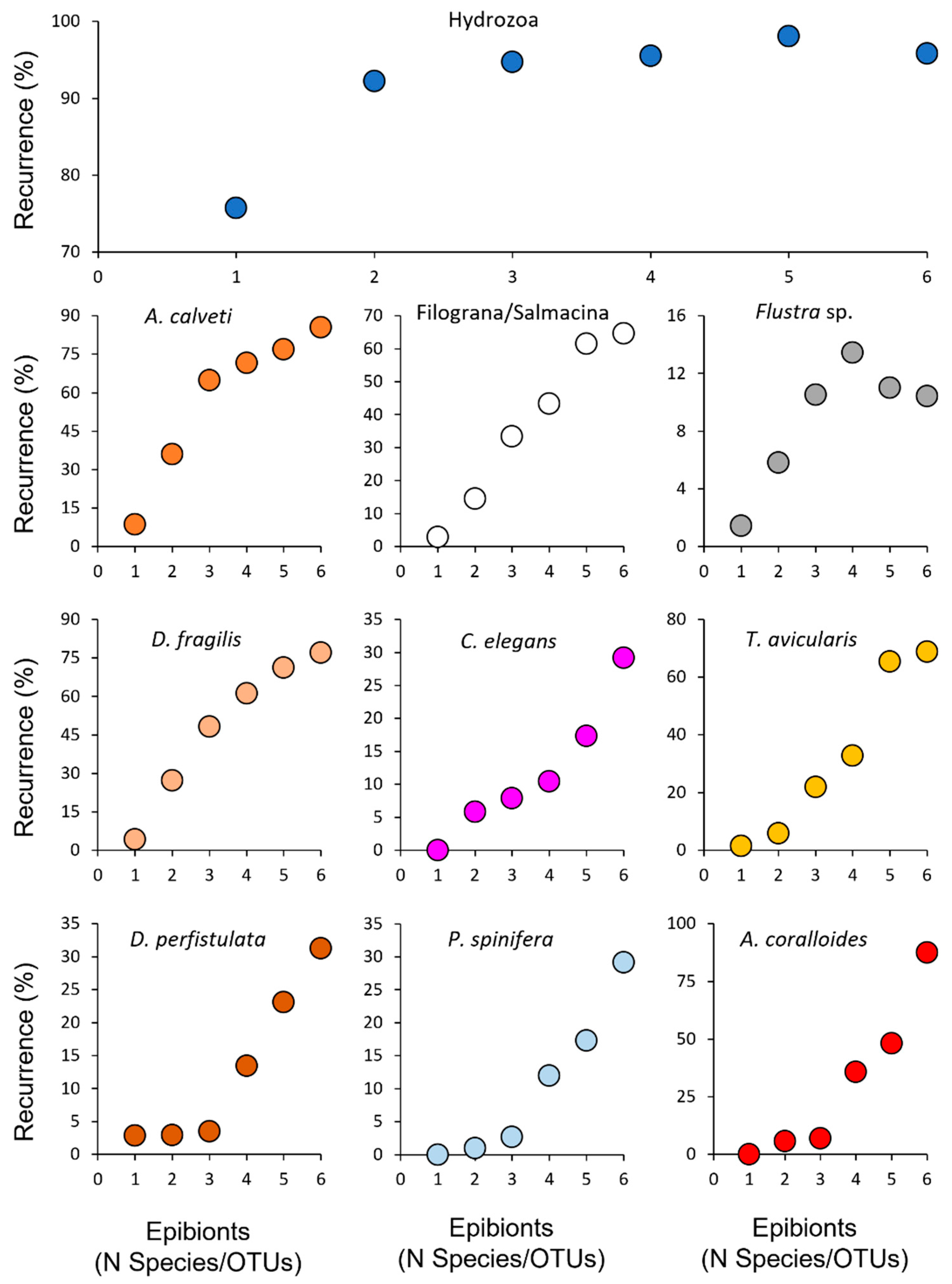
3.2. Epibiosis
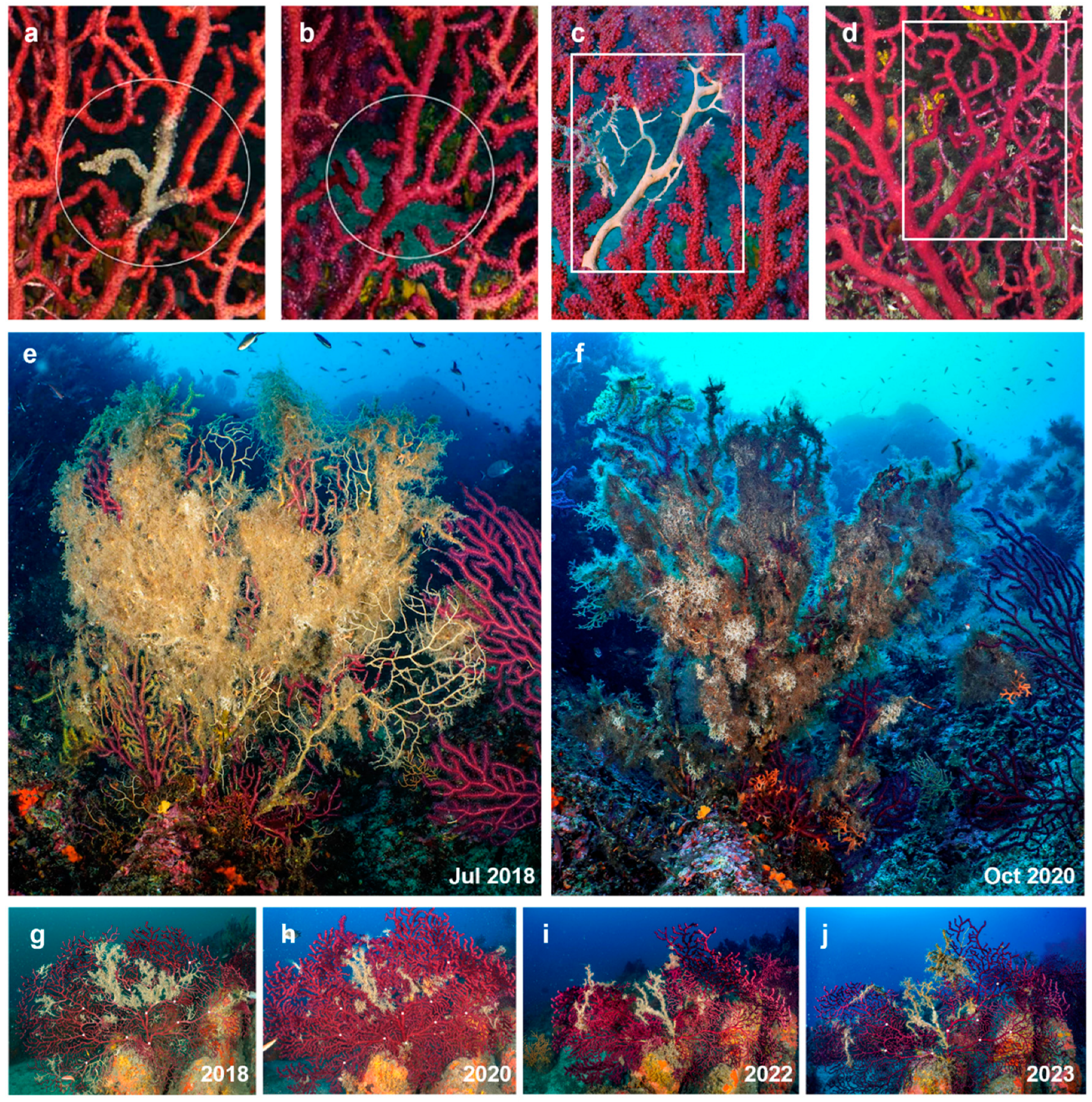
3.3. Observations on single gorgonians through time
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Coelho, M.A. G. , Pearson, G.A., Boavida, J.R.H., Paulo, D., Aurelle, D., Arnaud-Haond, S., Gómez-Gras, D., Bensoussan, N., López-Sendino, P., Cerrano, C., Kipson, S., Bakran-Petricioli, T., Ferretti, E., Linares, C., Garrabou, J., Serrão, E.A., Ledoux, J.-B. (2023). Not out of the Mediterranean: Atlantic populations of the gorgonian Paramuricea clavata are a separate sister species under further lineage diversification. Ecology and Evolution 2023, 13, e9740. [Google Scholar] [CrossRef] [PubMed]
- Otero, M.M., Numa, C., Bo, M., Orejas, C., Garrabou, J., Cerrano, C., KružicÅL, P., Antoniadou, C., Aguilar, R., Kipson, S., Linares, C., Terr.n-Sigler, A., Brossard, J., Kersting, D., Casado-Amez.a, P., Garc.a, S., Goffredo, S., Ocana, O., Caroselli, E., Maldonado, M., Bavestrello, G., Cattaneo-Vietti, R. and .zalp, B. (2017). Overview of the Conservation Status of Mediterranean Anthozoans; IUCN: Málaga, Spain, 2017, pp. 1–73. Available online: http://hdl.handle.net/10508/11253ISBN 978-2-8317-1845-3. [CrossRef]
- Harmelin, J.G. , Marinopoulos, J. Population structure and partial mortality of the gorgonian Paramuricea clavata (Risso) in the north-western Mediterranean (France, Port-Cros Island). Marine life 1994, 4, 5–13. [Google Scholar]
- Bavestrello, G., Cerrano, C., Zanzi, D., Cattaneo-Vietti, R. Damage by fishing activities to the Gorgonian coral Paramuricea clavata in the Ligurian Sea. Aquatic Conservation: Marine and Freshwater Ecosystems 1997, 7, 253-262.
- Bo, M. , Bava, S., Canese, S., Angiolillo, M., Cattaneo-Vietti, R., Bavestrello, G. Fishing impact on deep Mediterranean rocky habitats as revealed by ROV investigation. Biol. Conserv. 2014, 171, 167–176. [Google Scholar] [CrossRef]
- Angiolillo, M.; di Lorenzo, B.; Farcomeni, A.; Bo, M.; Bavestrello, G.; Santangelo, G.; Cau, A.; Mastascusa, V.; Cau, A.; Sacco, F.; et al. Distribution and assessment of marine debris in the deep Tyrrhenian Sea (NW Mediterranean Sea, Italy). Mar. Pollut. Bull. 2015, 92, 149–159. [Google Scholar] [CrossRef]
- Bavestrello, G. , Bertone, S., Cattaneo-Vietti, R., Cerrano, C., Gaino, E., Zanzi, D. Mass mortality of Paramuricea clavata (Anthozoa, Cnidaria) on Portofino Promontory cliffs, Ligurian Sea, Mediterranean Sea. Mar. Life 1994, 4, 15–19. [Google Scholar]
- Cerrano, C. , Bavestrello, G., Bianchi, C. N., Cattaneo-Vietti, R. Bava, S. Morganti, C. Morri, C., Picco, P. Sara, G., Schiaparelli, S., Siccardi, A., Sponga, F. A catastrophic mass-mortality episode of gorgonians and other organisms in the Ligurian Sea (NW Mediterranean), summer. Ecol. Letters 2000, 3, 284–293. [Google Scholar] [CrossRef]
- Perez, T. , Garrabou, J., Sartoretto, S., Harmelin, J.G., Francour, P., Vacelet, J. Massive mortality of marine invertebrates: an unprecedented event in northwestern Mediterranean. Comptes rendus de l'Academie des sciences. Serie III, Sciences de la vie 2000, 323, 853–865. [Google Scholar] [CrossRef]
- Coma, R. , Pola, E., Ribes, M., Zabala, M. Long-term assessment of temperate octocoral mortality patterns, protected vs. unprotected areas. Ecological Applications 2004, 14, 1466–1478. [Google Scholar] [CrossRef]
- Coma R, Ribes M, Serrano E, Jiménez E, Salat J, Pascual J (2009) Global warming-enhanced stratification and mass mortality events in the Mediterranean. Proc Natl Acad Sci USA 106:6176–6181. [CrossRef]
- Schiaparelli, S. , Castellano, M., Povero, P., Sartoni, G., Cattaneo-Vietti, R. A benthic mucilage event in North-Western Mediterranean Sea and its possible relationships with the summer 2003 European heatwave: short term effects on littoral rocky assemblages. Mar. Ecol. 2007, 28, 341–353. [Google Scholar] [CrossRef]
- Garrabou, J. , Coma, R., Bensoussan, N., Bally, M., Chevaldonne, P. et al. Mass mortality in Northwestern Mediterranean rocky benthic communities: effects of the 2003 heat wave. Global Change Biology 2009, 15, 1090–1103. [Google Scholar] [CrossRef]
- Garrabou, J. , Gómez-Gras, D., Ledoux, J. B., Linares, C., Bensoussan, N., López-Sendino, P., et al. Collaborative database to track mass mortality events in the Mediterranean Sea. Front. Mar. Sci. 2019, 707. [Google Scholar] [CrossRef]
- Garrabou, J. , Gómez-Gras, D., Medrano, A., Cerrano, C., Ponti, M., Schlegel, R., et al. Marine heatwaves drive recurrent mass mortalities in the Mediterranean Sea. Global Change Biology 2022, 28, 5708–5725. [Google Scholar] [CrossRef]
- Huete-Stauffer, C. , Vielmini, I., Palma, M., Navone, A., Panzalis, P., Vezzulli, L., Misic, C., Cerrano, C. Paramuricea clavata (Anthozoa, Octocorallia) loss in the Marine Protected Area of Tavolara (Sardinia, Italy) due to a mass mortality event. Mar. Ecol. 2011, 32, 107–116. [Google Scholar] [CrossRef]
- Teixido, N. , Casas, E., Cebrian, E., Linares, C., Garrabou, J. Impacts on coralligenous outcrop biodiversity of a dramatic coastal storm. PloS one 2013, 8, e53742. [Google Scholar] [CrossRef] [PubMed]
- Turicchia, E. , Abbiati, M., Sweet, M., Ponti, M. Mass mortality hits gorgonian forests at Montecristo Island. Diseases of Aquatic Organisms 2018, 131, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Betti, F. , Bavestrello, G., Bo, M., Ravanetti, G., Enrichetti, F., Coppari, M., Cappanera, V., Venturini, S., Cattaneo-Vietti, R. Evidences of fishing impact on the coastal gorgonian forests inside the Portofino MPA (NW Mediterranean Sea). Oc. Coast. Manag. 2020, 187, 105105. [Google Scholar] [CrossRef]
- Casoli, E. , Ventura, D., Mancini, G., Cardone, S., Farina, F., Donnini, L., Pace, D.S., Shaul, R., Belluscio, A., Ardizzone, G. Rehabilitation of Mediterranean animal forests using gorgonians from fisheries by-catch. Restor. Ecol. 2022, 30, e13465. [Google Scholar] [CrossRef]
- Chimienti, G. , De Padova, D., Adamo, M., Mossa, M., Bottalico, A., Lisco, A., Ungaro, N., Mastrototaro, F. Effects of global warming on Mediterranean coral forests. Sci. Rep. 2021, 11, 20703. [Google Scholar] [CrossRef]
- Chimienti, G. , Maiorca, M., Digenis, M., Poursanidis, D. Conservation status of upper-mesophotic octocoral habitats at Sporades Archipelago (Aegean Sea). Mar. Pollut. Bull. 2023, 190, 114868. [Google Scholar] [CrossRef]
- Angiolillo, M. , Fortibuoni, T. Impacts of marine litter on Mediterranean reef systems: from shallow to deep waters. Front. Mar. Sci. 2020, 7, 581966. [Google Scholar] [CrossRef]
- Sini, M. , Kipson, S., Linares, C., Koutsoubas, D., Garrabou, J. The yellow gorgonian Eunicella cavolini: demography and disturbance levels across the Mediterranean Sea. PloS one 2015, 10, e0126253. [Google Scholar] [CrossRef]
- Linares, C. , Coma, R., Diaz, D., Zabala, M., Hereu, B., Dantart, L. Immediate and delayed effects of a mass mortality event on gorgonian population dynamics and benthic community structure in the NW Mediterranean Sea. Mar. Ecol. Prog. Ser. 2005, 305, 127–137. [Google Scholar] [CrossRef]
- Canessa, M. , Bavestrello, G., Bo, M., Trainito, E., Panzalis, P., Navone, A., Caragnano, A., Betti, F., Cattaneo-Vietti, R. Coralligenous assemblages differ between limestone and granite: a case study at the Tavolara-Punta Coda Cavallo Marine Protected Area (NE Sardinia, Mediterranean Sea). Reg. Stud. Mar. Sci. 2020, 35, 101159. [Google Scholar] [CrossRef]
- Canessa, M. , Bavestrello, G., Trainito, E., Bianchi, C. N., Morri, C., Navone, A., Cattaneo-Vietti, R. (2021). A large and erected sponge assemblage on granite outcrops in a Mediterranean Marine Protected Area (NE Sardinia). Reg. Stud. Mar. Sci. 2021, 44, 101734. [Google Scholar] [CrossRef]
- Calvisi, G. , Trainito, E., Pais, M., Franci, G., Schiaparelli, S. Prima segnalazione di un episodio di mortalità di Gorgonacei lungo la costa dell’isola di Tavolara (Sardegna settentrionale). Biol. Mar. Mediterr. 2003, 10, 506–508. [Google Scholar]
- Canessa, M. , Bavestrello, G. Valutazione sperimentale dello stato di conservazione del coralligeno dell'AMP Tavolara - Punta Coda Cavallo nell’ambito della Strategia Marina. Relazione finale. Genova, 28 Novembre 2018.
- Amedeo, I. , Canessa, M., Panzalis, P., Trainito, E. The need for change in a changing world: fishing impact within the Tavolara-Punta Coda Cavallo MPA. In 4th Mediterranean Symposium on the Conservation of the Coralligenous and other calcareous bio-concretions Genoa, 2022, (p. 11).
- Pititto, F.; Trainito, E.; Macic, V.; Rais, C.; Torchia, G. The resolution in benthic carthography: A detailed mapping technique and a multiscale GIS approach with applications to coralligenous assemblages. In Proceedings of the Conference: 2° Mediterranean Symposium on the Conservation of Coralligenous & Other Calcareous Bio-Concretions, Portorož, Slovenia, 29–30 October 2014. [Google Scholar] [CrossRef]
- Rasband, W.S. ImageJ: Image processing and analysis in Java. Astrophysics Source Code Library, 2012, record ascl:1206.013. Bibcode:2012ascl.soft06013R.
- Tsounis, G.; Martinez, L.; Bramanti, L.; Viladrich, N.; Gili, J.M. , Martinez, Á.; Rossi, S. Anthropogenic effects on reproductive effort and allocation of energy reserves in the Mediterranean octocoral Paramuricea clavata. Mar. Ecol. Prog. Ser. 2012, 449, 161–172. [Google Scholar] [CrossRef]
- Kipson, S. , Linares, C., Čižmek, H., Cebrián, E., Ballesteros, E., Bakran-Petricioli, T., Garrabou, J. Population structure and conservation status of the red gorgonian Paramuricea clavata (Risso, 1826) in the Eastern Adriatic Sea. Mar. Ecol. 2015, 36, 982–993. [Google Scholar] [CrossRef]
- Arnoux A., J.G. Harmelin, J.L. Monod, L.A. Romana, H. Zibrowius - Altérations des peuplements benthiques de roches profondes en Méditerranée nord-occidentale : quelques aspects biologiques et molysmologiques. Comptes rendus de l'Académie des sciences. Série 3, Sciences de la vie. 1992, Vol 314, Num 5, pp 219-225. Available online: http://pascal-francis.inist.fr/vibad/index.php?action=getRecordDetail&idt=5153892.
- Bramanti, L. , Magagnini, G., De Maio, L., Santangelo, G. Recruitment, early survival and growth of the Mediterranean Red Coral Corallium rubrum 1758, a four-year study. J. Exp. Mar. Biol. Ecol 2005, 314, 69–78. [Google Scholar] [CrossRef]
- Cupido, R. , Cocito, S., Sgorbini, S., Bordone, A., Santangelo, G. Response of a gorgonian (Paramuricea clavata) population to mortality events: recovery or loss. In Aquatic conservation marine and freshwater ecosystems; John Wiley & Sons, Ltd., 2008; Volume 18, pp. 984–992. ISSN 1052-7613. [Google Scholar]
- Enrichetti, F. , Bavestrello, G., Betti, F., Rindi, F., Tregrosso, A., Bo, M. Fate of lost fishing gears: Experimental evidence of biofouling colonization patterns from the northwestern Mediterranean Sea. Environ. Pollut. 2021, 268, 115746. [Google Scholar] [CrossRef]
- Asoh, K. , Yoshikawa, T., Kosaki, R., Marschall, E.A. Damage to cauliflower coral by monofilament fishing lines in Hawaii. Conserv. Biol. 2004, 18, 1645–1650. [Google Scholar] [CrossRef]
- Yoshikawa, T. , Asoh, K. Entanglement of monofilament fishing lines and coral death. Biol. Conserv. 2004, 117, 557–560. [Google Scholar] [CrossRef]
- Chiappone, M. , Dienes, H., Swanson, D.W., Miller, S.L. Impacts of lost fishing gear on coral reef sessile invertebrates in the Florida Keys National Marine Sanctuary. Biol. Conserv. 2005, 121, 221–230. [Google Scholar] [CrossRef]
- Hinz, H. Impact of bottom fishing on animal forests: science, conservation, and fisheries management. Marine Animal Forests: The Ecology of Benthic Biodiversity Hotspots; Rossi, S., Ed, 2017, 1041-1059.
- Rovere, A.; Ferraris, F.; Parravicini, V.; Navone, A.; Morri, C.; Bianchi, C.N. Characterization and evaluation of a marine protected area: ‘Tavolara–Punta Coda Cavallo’ (Sardinia, NW Mediterranean). J. Maps 2013, 9, 279–288. [Google Scholar] [CrossRef]
- Cupido, R. , Cocito, S., Barsanti, M., Sgorbini, S., Peirano, A., Santangelo, G. Unexpected long-term population dynamics in a canopy-forming gorgonian coral following mass mortality. Mar. Ecol. Prog. Ser. 2009, 394, 195–200. [Google Scholar] [CrossRef]
- Lindsay, S.M. Frequency of injury and the ecology of regeneration in marine benthic invertebrates. Integr. Comparat. Biol. 2010, 50, 479–493. [Google Scholar] [CrossRef]
- Wahle, C.M. Regeneration of injuries among Jamaican gorgonians: the roles of colony physiology and environment. Biol. Bull. 1983, 165, 778–790. [Google Scholar] [CrossRef]
- Castanaro, J. , Lasker, H.R. Colony growth responses of the Caribbean octocoral, Pseudopterogorgia elisabethae, to harvesting. Invertebrate Biology 2003, 122, 299–307. [Google Scholar] [CrossRef]
- Cerrano, C. , Bavestrello, G. Medium-term effects of die-off of rocky benthos in the Ligurian Sea. What can we learn from gorgonians? Chem. Ecol. 2008, 24(S1), 73–82. [Google Scholar] [CrossRef]
- Sánchez, J.A. , Lasker, H.R. Do multi–branched colonial organisms exceed normal growth after partial mortality? Proceedings of the Royal Society of London. Series B: Biological Sciences 2004, 271 (suppl. 3), S117–S120. [Google Scholar] [CrossRef]
- Loya, Y. Skeletal regeneration in a Red Sea scleractinian coral population. Nature 1976, 261, 490–491. [Google Scholar] [CrossRef]
- Riegl, B. , Riegl, A. Studies on coral community structure and damage as a basis for zoning marine reserves. Biol. Conserv. 1996, 77, 269–277. [Google Scholar] [CrossRef]
- Cerrano, C.; Arillo, A.; Azzini, F.; Calcinai, B.; Castellano, L.; Muti, C.; Valisano, L.; Zega, G.; Bavestrello, G. Gorgonian population recovery after a mass mortality event. Aquat. Conserv. 2005, 15, 147–157. [Google Scholar] [CrossRef]
- Ros, J.D., Romero, J., Ballesteros, E, Gili, J.M. Diving in blue water. The benthos. Western Mediterranean. R. Margalef, ed., Pergamon Press, Oxford, 1985, pp: 233-295.
- Hrs-Brenko, M. , Legac, M. Inter-and intra-species relationships of sessile bivalves on the eastern coast of the Adriatic Sea. Natura Croatica 2006, 15, 203. [Google Scholar]
- Basile, G. , Cerrano, C., Radjasa, O., Povero, P., Zocchi, E. ADP-ribosyl cyclase and abscisic acid are involved in the seasonal growth and in post-traumatic tissue regeneration of Mediterranean sponges. J. Exp. Mar. Biol. Ecol. 2009, 381, 10–17. [Google Scholar] [CrossRef]
- Chimienti, G. Vulnerable forests of the pink sea fan Eunicella verrucosa in the Mediterranean Sea. Diversity 2000, 12, 176. [Google Scholar] [CrossRef]
- McFadden, C.S. Genetic and taxonomic relationships among Northeastern Atlantic and Mediterranean populations of the soft coral Alcyonium coralloides. Mar. Biol. 1999, 133, 171–184. [Google Scholar] [CrossRef]
- McFadden, C.S. , Donahue, R., Hadland, B.K., Weston, R. A molecular phylogenetic analysis of reproductive trait evolution in the soft coral genus Alcyonium. Evolution 2001, 55, 54–67. [Google Scholar] [CrossRef]
- Quintanilla, E. , Gili, J. M., López-González, P. J., Tsounis, G., Madurell, T., Fiorillo, I., Rossi, S. Sexual reproductive cycle of the epibiotic soft coral Alcyonium coralloides (Octocorallia, Alcyonacea). Aquat. Biol. 2013, 18, 113–124. [Google Scholar] [CrossRef]
- Ten Hove, H.A. Different causes of mass occurrence in serpulids. Biology and systematics of colonial organisms 1979, 11, 281–298. [Google Scholar]
- Ten Hove, H.A., Van den Hurk, P. A review of Recent and fossil serpulid'reefs'; actuopalaeontology and'Upper Malm'serpulid limestones in NW Germany. Geologie en Mijnbouw 1993, (1). Netherlands Journal of Geosciences. Kluwer/Cambridge University Press: Den Haag, Cambridge. ISSN 0016-7746.
- Sarà, M. Competition and cooperation in sponge populations. In Symposium of the Zoological Society of London 1970, Vol. 25, pp. 273-284.
- Denoual, D. Mortalité massive de gorgones rouges (Paramuricea clavata) en méditerranée durant l'été 1999 (Doctoral dissertation). Ecole Nationale Vétérinaire de Toulouse - ENVT, 2004, 86 p.
- Wahl, M. Marine epibiosis. I. Fouling and antifouling: some basic aspects. Mar. Ecol. Prog. Ser. /, 1989; 175–189. Available online: https://www.jstor.org/stable/24842178.


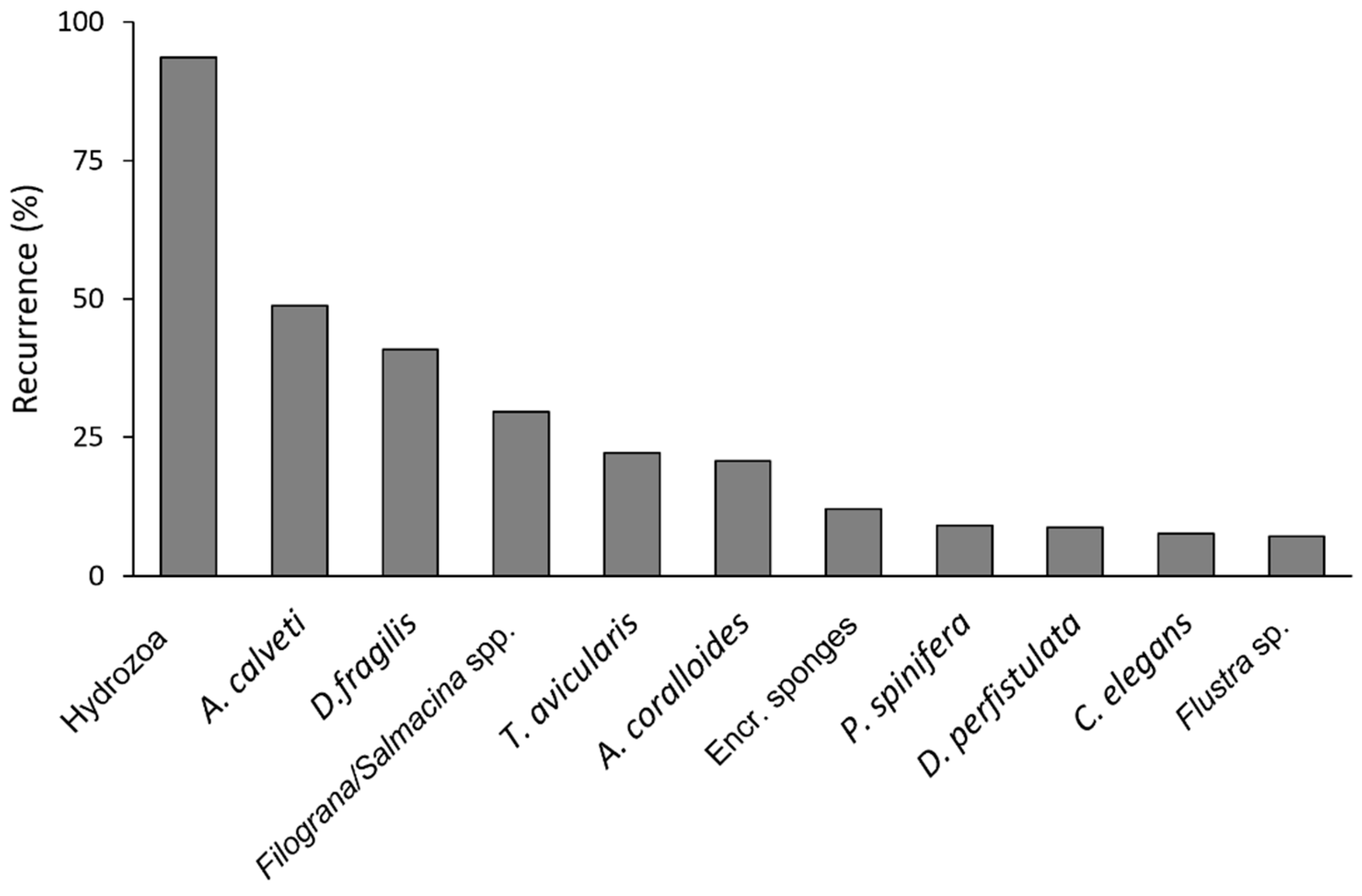
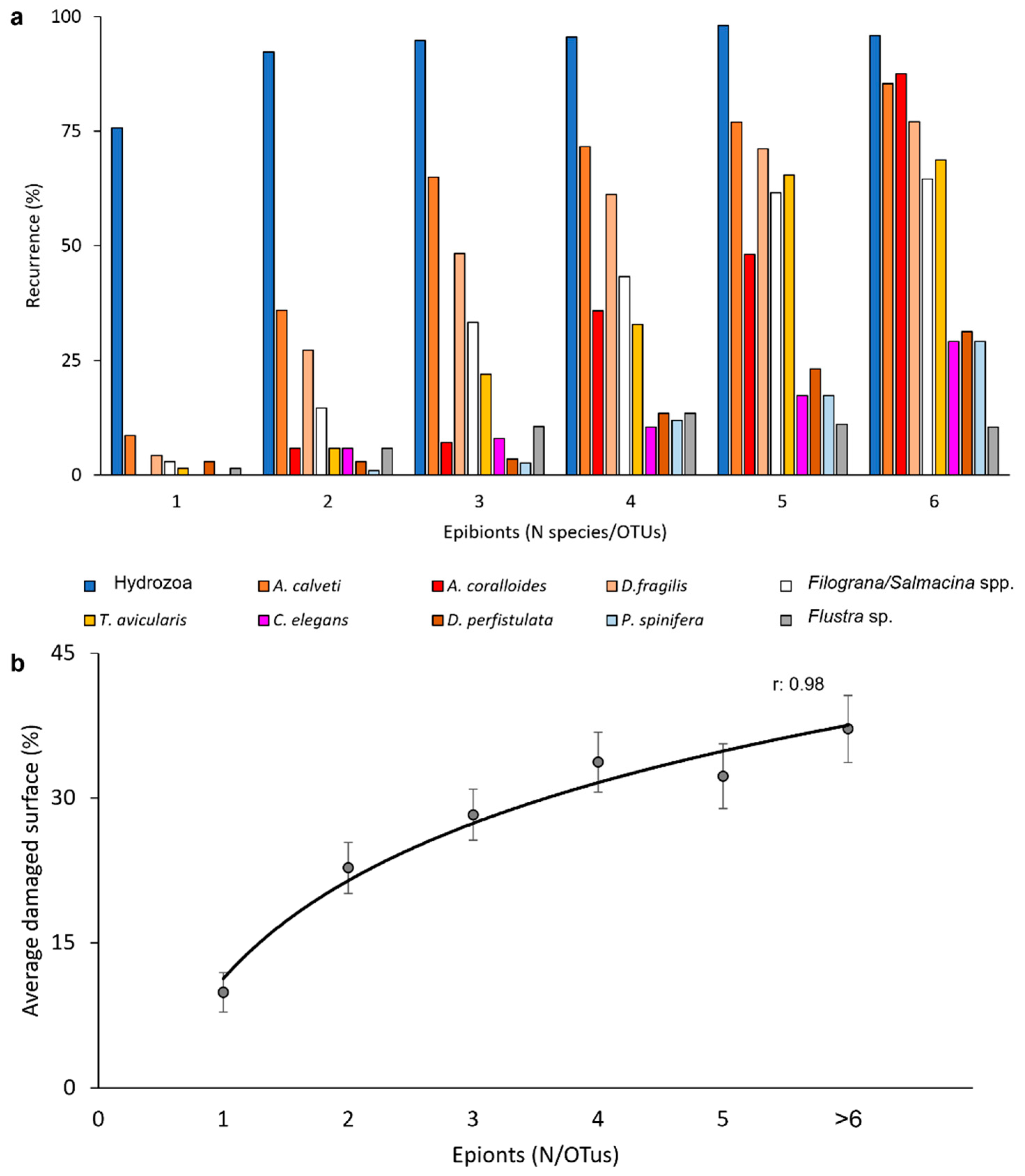

| Species/OTUs | Recurrence (%) |
Species/OTUs | Recurrence (%) |
|---|---|---|---|
| Algae | Anellids | ||
| Crustose Coralline Algae | 2.5 | Filograna/Salmacina spp. | 33.3 |
| Flabellia petiolata | 0.2 | Serpulids | 5.6 |
| Valonia sp. | 0.2 | Bryozoans | |
| Sponges | Adeonella calveti | 52.3 | |
| Anchinoe tenacior | 0.2 | Beania magellanica | 0.9 |
| Crella elegans | 10.4 | Cellaria salicornioides | 0.5 |
| Dysidea avara | 0.2 | Flustra sp. | 9.6 |
| Dysidea fragilis | 43.8 | Pentapora fascialis | 1.6 |
| Dysidea perfistulata | 10.2 | Turbicellepora avicularis | 26.9 |
| Haliclona mediterranea | 1.4 | Encr. Bryozoans n.i. | 1.4 |
| Ircinia variabilis | 0.2 | Crustaceans | |
| Oscarella lobularis | 2.5 | Periclimenes scriptum* | 0.5 |
| Pleraplysilla spinifera | 9.9 | Echinoderms | |
| Terpios fugax | 2.8 | Antedon mediterranea* | 0.2 |
| Encr. Sponges | 14.4 | Astrospartus mediterraneus* | 0.2 |
| Cnidarians | Chordata | ||
| Hydrozoa | 91.9 | Aplidium undulatum | 7.9 |
| Alcyonium coralloides | 22.9 | Clavelina dellavallei | 1.6 |
| Epizoanthus sp. | 0.2 | Clavelina oblonga | 0.9 |
| Molluscs | Didemnids | 0.9 | |
| Pteria hirundo | 0.7 | Pycnoclavella communis | 0.7 |
| Calliostoma conulus* | 0.2 | ||
| Flabellina ischitana* | 0.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





