Introduction
The dimorphic intracellular protozoan parasite Leishmania is transmitted to vertebrates via the bite of the phlebotomine sandfly vector. Leishmaniasis is a parasitic disease spread by vectors that is caused by hemoflagellate parasites of the genus Leishmania. Most infected individuals and animals do not display symptoms, but when they do, they can present in one of three clinical forms: cutaneous leishmaniasis (CL), mucocutaneous leishmaniasis(ML), or visceral leishmaniasis(VL) in humans, and only cutaneous or visceral leishmaniasis in animals [
1]. According to estimates from the World Health Organisation (WHO), 92 nations and 83 territories, respectively, were thought to be endemic for CL and VL, or had previously reported cases of these diseases. Today, almost 1 billion individuals are at risk of contracting leishmaniasis. Annually, there are more than 1 million new cases of CL and 30 000 new cases of VL, respectively [
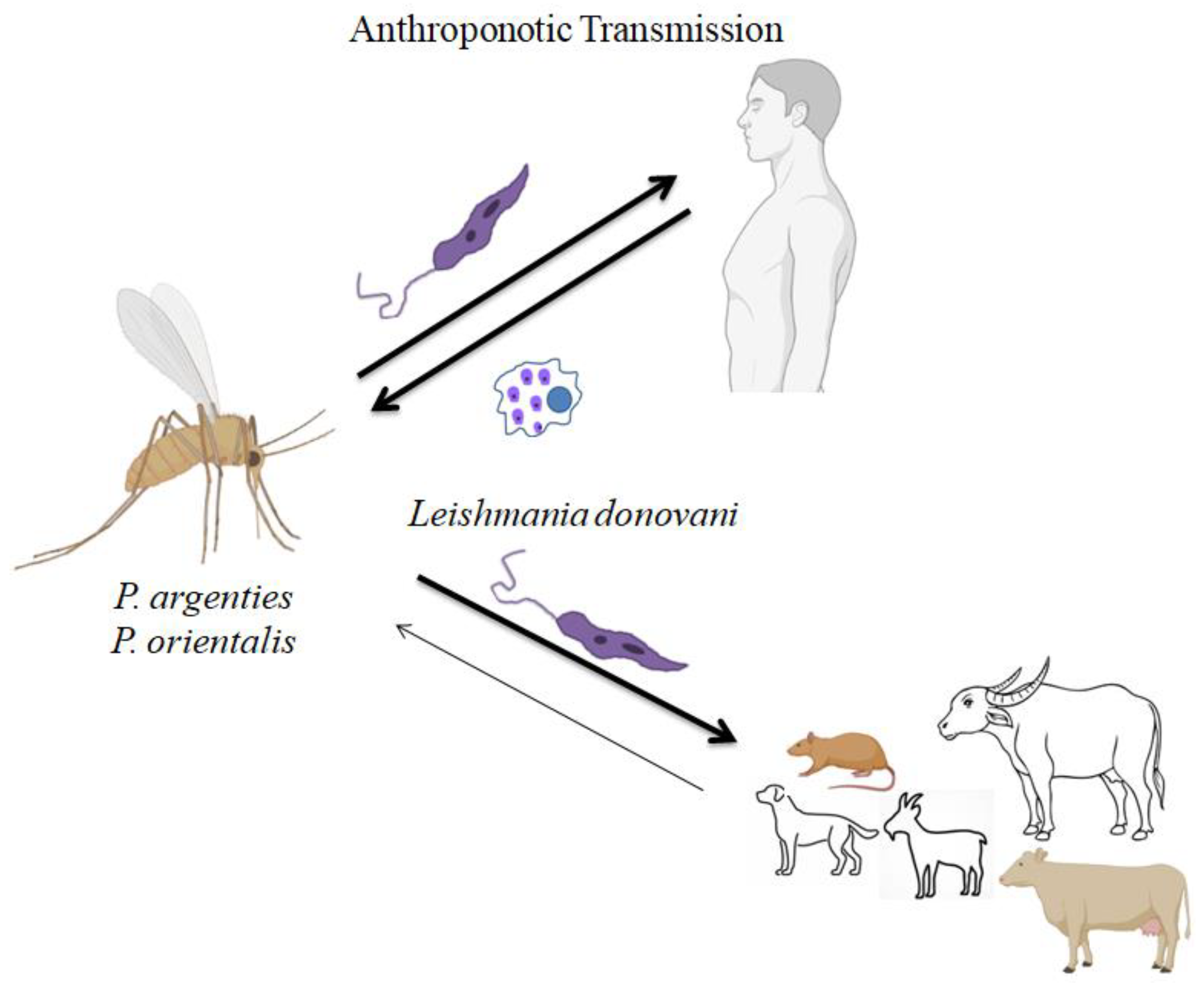
2]. There are two epidemiological patterns for kala-azar, or visceral leishmaniasis (VL). The first type of VL is called Anthroponotic visceral leishmaniasis (AVL) (
Figure 1), and it spreads mainly among humans but can also happen between animals and human beings. The second type of VL is zoonotic visceral leishmaniasis (ZVL) (
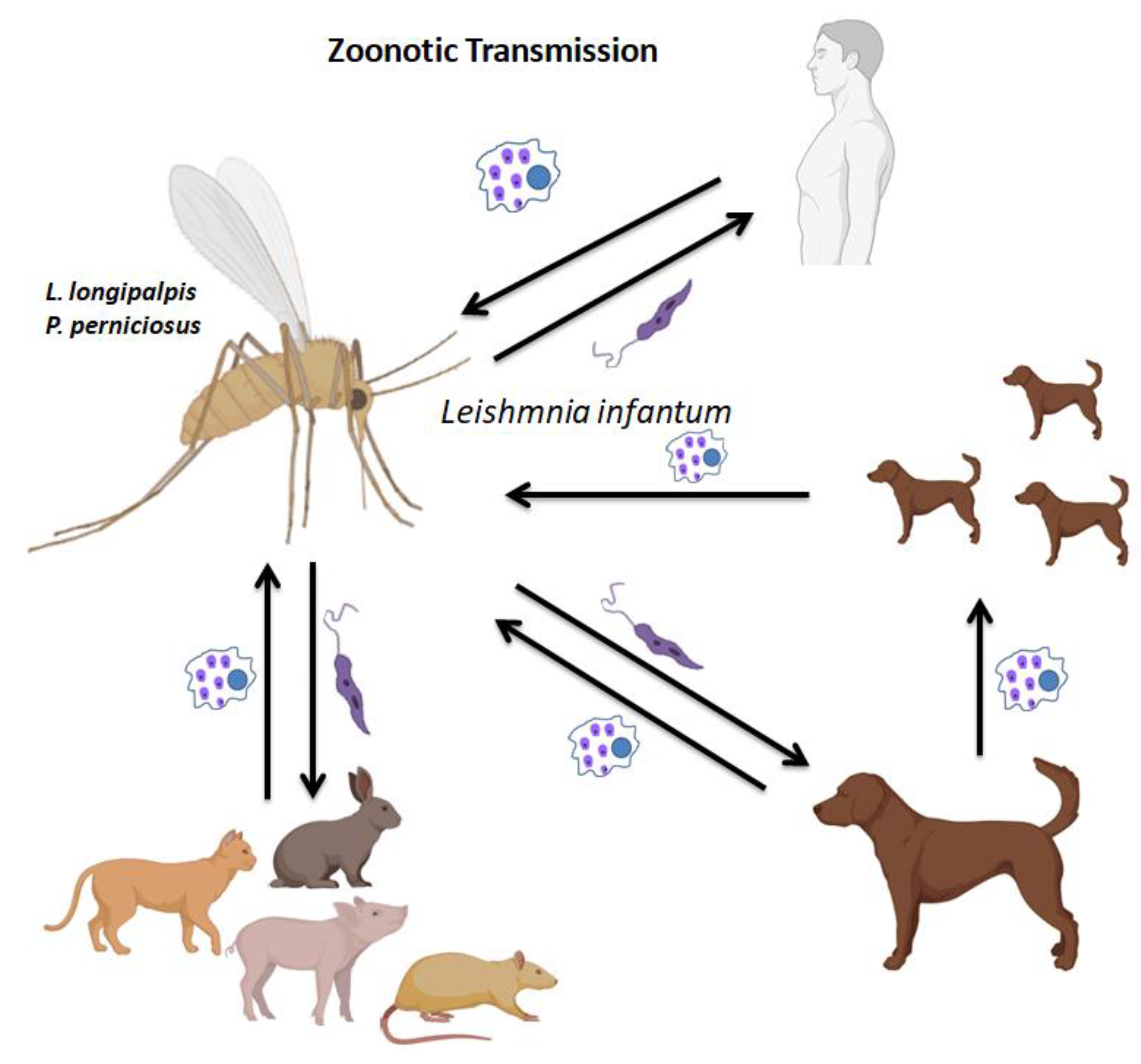
Figure 2), which is spread from infected animals to people with domestic dogs serving as the main reservoir host, both manifests epidemiologically as VL.
Leishmania donovani, which is present in East Africa, Bangladesh, India, and Nepal, is known to cause the first kind of VL [
3].
The most severe form of leishmaniasis, known as visceral leishmaniasis (VL, sometimes called kala-azar), can be fatal if left untreated. Without any known animal reservoirs, the
Leishmania donovani complex of parasites that cause VL in the Indian subcontinent (ISC) transfer from person to person via the sand fly
Phlebotomus argentipes [
4]. The reservoir hosts play a crucial role in the life cycle of the Leishmanial parasite. The primary VL vector in India gathers in and around animal habitation, which provides a favorable, stable microclimate close to a blood meal and may change disease dynamics by changing the rates of vector/host contact [
5]. The complicated interaction between the pathogen vector and reservoir shows geographical and temporal variation [
6]. According to several studies, leishmaniasis can infect a variety of wild, domestic, and synanthropic species, including dogs, cats, rats, foxes, pigs, and rock hyraxes[
7,
8,
9,
10]. A reservoir species is defined as one or more populations with a strong epidemiological bond where a disease can establish a chronic infection and from which an infecting organism is transmitted to the target population. Significant increases in human susceptibility to leishmaniasis are a result of human activities such as migration, deforestation, uncontrolled urbanisation, or changes in infection vulnerability brought on by immunosuppression and malnutrition [
3,
11,
12].The aim of this Systemic research was to investigate and identify possible domestic animal reservoirs for VL infection in India.
Materials and methods
A deliberate, systematic search was carried out on PubMed, Science Direct, and Google Scholar Using specific phrases like Leishmania donovani, zoonotic visceral leishmaniasis, and wild animal reservoir for leishmania donovani. Results that weren't matched were ignored. To identify any specific information pertaining to the study's goal, the results were qualitatively summarized
For the meta-analysis study, research that primarily examined animals infected with visceral leishmaniasis in Indian region was chosen. These studies comprised reports from both experimental and observational research, and the initial screenings were concentrated on those disregarded because of their titles or other title-related issues that rendered them unsuitable for the review. For additional screening, the entire manuscript was needed. Verify that none of the included examinations duplicated clinical records from other sources or examined a particular group of patients. Prior to the review, each approach had been verified twice.
A systematic review of the titles and abstracts was done to confirm the full evaluation of significant publications. The chosen full-text papers received an independent assessment utilizing the previously mentioned determination study selection criteria. Only a small number of research mentioned zoonotic leishmaniasis from different regions of India. The research papers that are chosen includes certain animals that have been diagnosed with leishmaniasis. The data about the outcomes, including disease severity, disease prevention and the overall finding from each publication were explained.
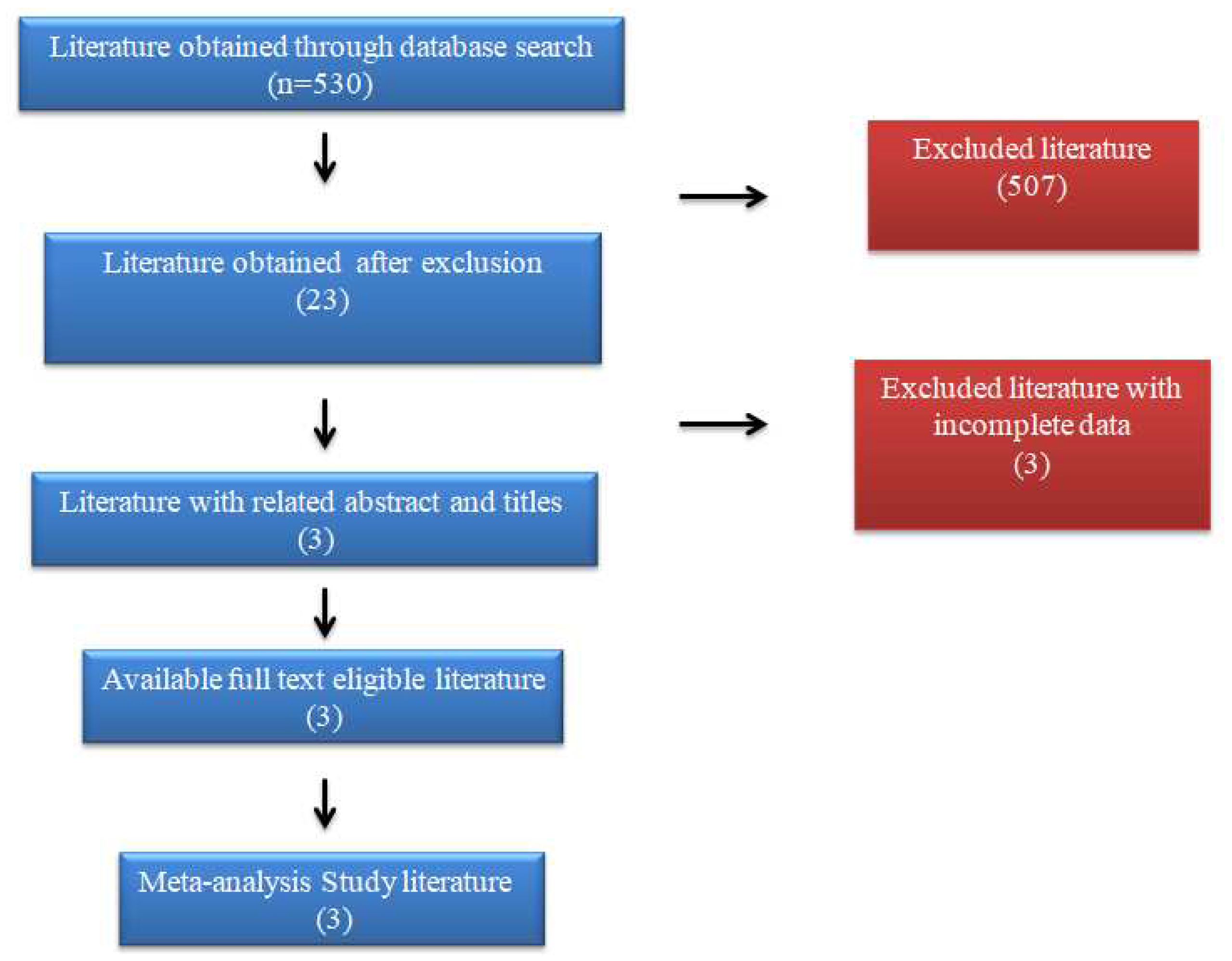
A flowchart for conducting literature searches is presented in FIGURE 2. Implementing the search technique provided 530 potentially important references, of which 507 were prohibited because of copy avoidance, irrelevant titles, research publications from countries other than India, or modified compositions. Later, the remaining 20 investigations were rejected for having unmatched data during the full-text review. According to the set parameters, 3 review studies were considered for this meta-analysis. The combined data of all the animals that showed the ability as the reservoirs for the leishmaniasis has been given in
table 1
During 1983 in India, epidemic of the disease, possible animal reservoirs of Indian kala-azar were investigated, including dogs, Rattus rattus, and Bandicoota bengalensis. Five out of 226 B. bengalensis samples underwent microscopic and serological investigations, and antileishmanial antibodies were discovered in these samples.
In Bihar, India, SINGH ET AL [
13] found antibodies to a recombinant kinetoplast antigen (rK39 antigen) in amastigotes of the visceralizing Leishmania species,
L. donovani complex, were examined in serum samples from 1,220 animals from seven districts with endemic human VL. Additionally, samples identified as positive by rK39 antigen serology were examined using PCR. Of 1,220 animals, antibodies to rK39 suggestive of VL were found in 33. According to rK39 serology, 31 of 867 goats, 1 of 161 cattle, and 1 of 54 wild rats tested positive. By rK39 serology, none of the 106 chickens, 26 sheep, 3 water buffaloes, or 3 canines tested positive. In 20 rK39 positive goat blood samples and 1 positive cow blood sample,
Leishmania donovani DNA was found by PCR.
In other study it was confirmed that dogs serve as
L. donovani parasite reservoirs in the Kani tribe communities in the Thiruvananthapuram district of Kerala, India's Thiruvananthapuram Tribe villages, which are situated in the southernmost portion of the Western Ghats, where peripheral blood samples from 47 domestic dogs and 25 wild rats, were tested for Leishmania infection. It was concluded that Leishmaniasis cases with cutaneous symptoms and a high sandfly population are common in this region. In order to guard against the predatory behavior of wild animals, the tribes domesticate dogs. Using PCR-RFLP analysis of the UTR region of the heat shock protein 70 (hsp70) gene and the diagnostic kinetoplast mini-circle DNA,
Leishmania donovani parasite DNA was only found in 3 of the blood samples taken from domestic dogs. None of the rat blood samples that were taken had any positive results.
L. donovani infection in dogs was proven via sequencing [
15].
The CL symptoms result in secondary bacterial and fungal infections in addition to the lesions at the locations of sandfly bites. There have been reports of damaging and disfiguring sores on exposed skin. Even after they have healed, the visible scars cause affected people to experience psychosocial issues [
16]. CL thus becomes a significant barrier to socioeconomic advancement. For the purpose of implementing control measures or monitoring programmes, it is crucial to understand the function of wild animals as ideal hosts or reservoirs of Leishmania zoonotic species. In India, the kala-azar virus is thought to be anthroponotic, and earlier efforts to find an animal reservoir host although employing insensitive techniques have failed. India has recorded a small number of instances of visceral leishmaniasis in several animal species, but none have been determined to be zoonotic VL. The extent to which L. donovani infection of domestic animals promotes transmission to other animals or humans in India is not recognized. Evidence from prior vector-borne disease eradication methods showed that domestic species' developing diseases hampered elimination [
17,
18,
20,
21,
22]. public health and veterinary researchers have neglected the role that few animals role in the maintenance and transmission of VL, and that this aspect of the epizootiology of VL needs to be critically examined to update prevention and treatment programmes in kala-azar endemic areas.
Conclusion
According to the evaluations, although there is less data, CVL is endemic in several regions of India and goats are both the disease's primary host and possible reservoir. Goats have higher CVL infection rates than other animals, which raises the possibility that people could contract the disease from them. In order to stop the spread of the disease, it is also important to assess the condition of the sandflies that feed on the blood from this diseased reservoir—Hosts. It is strongly advised that thorough measures be put into place to prevent and control this disease, especially in endemic areas, due to the rising trend of CVL in India. The mammals' infectious epidemiology still needs to be widely recognized. The public health and veterinary researchers have neglected the role of few animals role in the maintenance and transmission of VL, and that this aspect of the epizootiology of VL needs to be critically examined to update prevention and treatment programmes in kala-azar endemic areas.
Author Contributions
All authors contributed to writing-original draft preparation, review and editing.
Funding
This research was funded by VtR Inc-CGU (SCRPD1L0221); DOXABIO-CGU (SCRPD1K0131), and CGU grant (UZRPD1L0011, UZRPD1M0081).
Institutional Review Board Statement
“Not applicable”
Informed Consent Statement
Not applicable
Data Availability Statement
Not applicable
Conflicts of Interest
The authors declare no conflict of interest.
References
- G. B. Ogden and P. C. Melby, “Leishmania,” Encycl. Microbiol., pp. 663–673, Jan. 2009. [CrossRef]
- “Leishmaniasis”. https://www.who.int/health-topics/leishmaniasis#tab=tab_1 (accessed Jun. 13, 2023).
- Desjeux, P. Leishmaniasis: current situation and new perspectives. Comp. Immunol. Microbiol. Infect. Dis. 2004, 27, 305–318. [Google Scholar] [CrossRef] [PubMed]
- Singh, O.P.; Singh, B.; Chakravarty, J.; Sundar, S. Current challenges in treatment options for visceral leishmaniasis in India: a public health perspective. Infect. Dis. Poverty 2016, 5, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Keeling, M. Spatial Models of Interacting Populations. Infect. Dis. Poverty, 1999; 99. [Google Scholar] [CrossRef]
- Raymond, R.W.; McHugh, C.P.; Witt, L.R.; Kerr, S.F. Temporal and spatial distribution of Leishmania mexicana infections in a population of Neotoma micropus. . 2003, 98, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Palatnik-De-Sousa, C.B.; Day, M.J. One Health: The global challenge of epidemic and endemic leishmaniasis. Parasites Vectors 2011, 4, 197–197. [Google Scholar] [CrossRef] [PubMed]
- Akhoundi, M.; Kuhls, K.; Cannet, A.; Votýpka, J.; Marty, P.; Delaunay, P.; Sereno, D. A Historical Overview of the Classification, Evolution, and Dispersion of Leishmania Parasites and Sandflies. PLOS Neglected Trop. Dis. 2016, 10, e0004349. [Google Scholar] [CrossRef] [PubMed]
- Rohousova, I.; Talmi-Frank, D.; Kostalova, T.; Polanska, N.; Lestinova, T.; Kassahun, A.; Yasur-Landau, D.; Maia, C.; King, R.; Votypka, J.; et al. Exposure to Leishmania spp. and sand flies in domestic animals in northwestern Ethiopia. Parasites Vectors 2015, 8, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Dereure, J.; Boni, M.; Pratlong, F.; Osman, M.H.; Bucheton, B.; El-Safi, S.; Feugier, E.; Musa, M.; Davoust, B.; Dessein, A.; et al. Visceral leishmaniasis in Sudan: first identifications of Leishmania from dogs. Trans. R. Soc. Trop. Med. Hyg. 2000, 94, 154–155. [Google Scholar] [CrossRef] [PubMed]
- Alvar, J.; Aparicio, P.; Aseffa, A.; Den Boer, M.; CañavateC. ; Dedet, J.-P.; Gradoni, L.; Ter Horst, R.; López-VélezR.; Moreno, J. The Relationship between Leishmaniasis and AIDS: the Second 10 Years. Clin. Microbiol. Rev. 2008, 21, 334–359. [Google Scholar] [CrossRef] [PubMed]
- Thakur, C. Socio-economics of visceral leishmaniasis in Bihar (India). Trans. R. Soc. Trop. Med. Hyg. 2000, 94, 156–157. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Mishra, J.; Singh, R.; Singh, S. Animal Reservoirs of Visceral Leishmaniasis in India. J. Parasitol. 2013, 99, 64–67. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, L.; Chakarvarty, A.K. Investigation of possible zoonotic reservoirs of Indian kala-azar. Ann. Trop. Med. Parasitol. 1984, 78, 501–504. [Google Scholar] [CrossRef] [PubMed]
- Jambulingam, P.; Kumar, N.P.; Nandakumar, S.; Paily, K.; Srinivasan, R. Domestic dogs as reservoir hosts for Leishmania donovani in the southernmost Western Ghats in India. Acta Trop. 2017, 171, 64–67. [Google Scholar] [CrossRef] [PubMed]
- Turan, E.; Kandemir, H.; Yeşilova, Y.; Ekinci, S.; Tanrıkulu, O.; Kandemir, S.B.; Gurel, M.S. Assessment of psychiatric morbidity and quality of life in children and adolescents with cutaneous leishmaniasis and their parents. Adv. Dermatol. Allergol. 2015, 32, 344–348. [Google Scholar] [CrossRef] [PubMed]
- Kushwaha, A.K.; Scorza, B.M.; Singh, O.P.; Rowton, E.; Lawyer, P.; Sundar, S.; Petersen, C.A. Domestic mammals as reservoirs for Leishmania donovani on the Indian subcontinent: Possibility and consequences on elimination. Transbound. Emerg. Dis. 2021, 69, 268–277. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury NK, Deepika, Choudhury R, Sonawane GA, Mavinamar S, Lyu X, Pandey RP, Chang CM. Nanoparticles as an effective drug delivery system in COVID-19. Biomed Pharmacother. 2021 Nov;143:112162.
- Ribeiro, G.D.O.; da Costa, A.C.; Gill, D.E.; Ribeiro, E.S.D.; Rego, M.O.d.S.; Monteiro, F.J.C.; Villanova, F.; Nogueira, J.S.; Maeda, A.Y.; de Souza, R.P.; et al. Guapiaçu virus, a new insect-specific flavivirus isolated from two species of Aedes mosquitoes from Brazil. Sci. Rep. 2021, 11, 1–13. [Google Scholar] [CrossRef]
- Pandey, R.P.; Mukherjee, R.; Priyadarshini, A.; Gupta, A.; Vibhuti, A.; Leal, E.; Sengupta, U.; Katoch, V.M.; Sharma, P.; Moore, C.E.; et al. Potential of nanoparticles encapsulated drugs for possible inhibition of the antimicrobial resistance development. Biomed. Pharmacother. 2021, 141, 111943. [Google Scholar] [CrossRef] [PubMed]
- Paul, A.; Raj, V.; Vibhuti, A.; Pandey, R. Larvicidal efficacy of Andrographis paniculata and Tinospora cordifolia against aedes aegypti: A dengue vector. Pharmacogn. Res. 2020, 12, 352. [Google Scholar] [CrossRef]
- Dutt, Y.; Dhiman, R.; Singh, T.; Vibhuti, A.; Gupta, A.; Pandey, R.P.; Raj, V.S.; Chang, C.-M.; Priyadarshini, A. The Association between Biofilm Formation and Antimicrobial Resistance with Possible Ingenious Bio-Remedial Approaches. Antibiotics 2022, 11, 930. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).