Submitted:
20 June 2023
Posted:
21 June 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
- Population: humans and animals;
- Intervention: interleukins polymorphisms in healthy subjects;
- Comparator: interleukins polymorphisms in T1DM patients/animals;
- Outcomes: correlation between interleukins polymorphisms and T1DM.
Eligibility Criteria
Data Extraction
Assessment of Methodological Quality
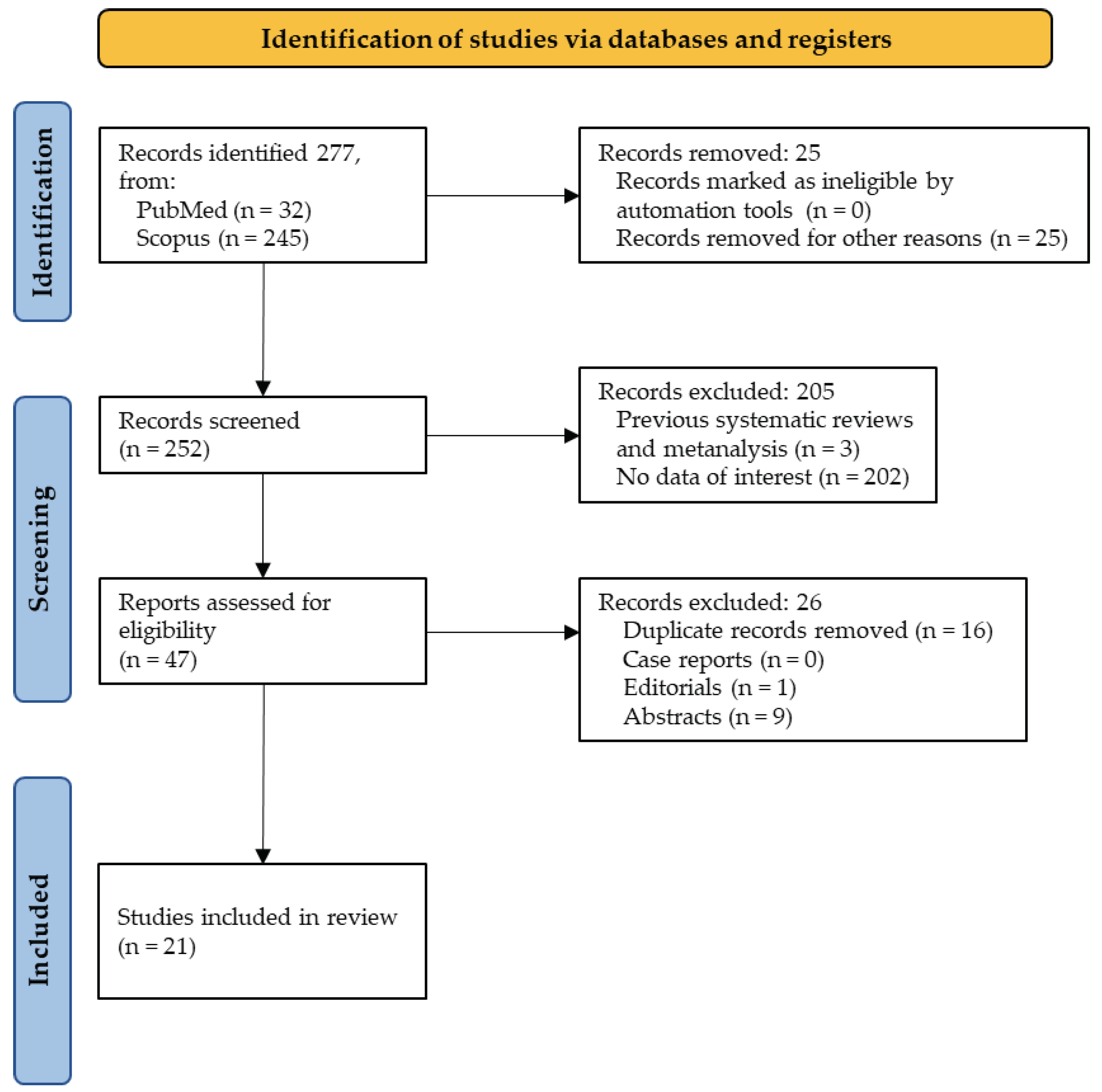
3. Results
3.1. Overall Scenario
3.2. Detailed Results
4. Discussion
Limitations of the Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Authors/Year | Selection | Comparability | Exposure | Total Score |
|---|---|---|---|---|
| Hoffman M. et al. 2022 [13] | 3 | 1 | 2 | 6 |
| Li J. et al. 2022 [14] | 3 | 1 | 2 | 6 |
| Osman A. E. et al. 2022 [15] | 2 | 1 | 2 | 5 |
| Ali Y. et al. 2021 [16] | 2 | 1 | 2 | 5 |
| El Helaly R.M. et al. 2021 [17] | 3 | 1 | 2 | 6 |
| Haghnazari L. et al. 2021 [18] | 3 | 2 | 2 | 7 |
| Hehenkamp P. et al. 2021 [19] | 2 | 1 | 2 | 5 |
| Kumar S. et al. 2021 [20] | 3 | 1 | 2 | 6 |
| Li J. et al. 2021 [21] | 3 | 2 | 2 | 7 |
| Osman A. E. et al. 2021 [22] | 2 | 1 | 2 | 5 |
| Tangjittipokin W. et al. 2021 [23] | 3 | 1 | 2 | 6 |
| Campos L. P. et al. 2020 [24] | 3 | 1 | 2 | 6 |
| Keindl M. et al. 2020 [25] | 3 | 2 | 1 | 6 |
| Sharma C. et al. 2020 [26] | 2 | 2 | 2 | 6 |
| Boechat-Fernandes A. et al. 2019 [27] | 1 | 1 | 1 | 3 |
| Borilova Linhartova P. et al. 2019 [28] | 3 | 1 | 2 | 6 |
| Campos L. P. et al. 2019 [29] | 3 | 1 | 2 | 6 |
| Lundtoft C. et al. 2019 [30] | 3 | 2 | 2 | 7 |
| Borilova Linhartova P. et al. 2018 [9] | 3 | 2 | 2 | 7 |
| Seyfarth J. et al. 2018 [31] | 3 | 1 | 2 | 6 |
| Al-Lahham Y. et al. 2017 [32] | 3 | 1 | 2 | 6 |
| Authors/Year | Conclusions |
|---|---|
| Hoffman M. et al. 2022 [13] | There is higher sIL-7R serum concentrations at T1DM onset and decreasing levels during therapy, whereas IL-7 was only higher in long term patients as compared to controls. |
| Li J. et al. 2022 [14] | Increased serum IL-17A is a risk factor for autoimmune T1D. |
| Osman A. E. et al. 2022 [15] | The SNP associated with low production of IL-4 increases the risk of T1DM in young individuals carrying vulnerable HLA alleles/haplotypes. |
| Ali Y. et al. 2021 [16] | IL-18 gene promoter polymorphisms might be associated with susceptibility to T1D in Egyptian children. |
| El Helaly R.M. et al. 2021 [17] | AA genotype and A allele of IL-10 rs1518111 SNP could be linked to increased risk for T1DM and DN among Egyptian children. |
| Haghnazari L. et al. 2021 [18] | The G allele of SNP rs1042522 encoding the TP53 gene for IL-6 increases the risk of developing DM in Iranian population. |
| Hehenkamp P. et al. 2021 [19] | T1DM monocytes have impaired IL-7 response and lower IL-7R expression. |
| *Kumar S. et al. 2021 [20] | IL-17A polymorphism was not associated with increased risk for CP in T1DM patients. |
| Li J. et al. 2021 [21] | The concentration of IL-1β in T1DM patients was significantly higher than that in healthy controls. |
| Osman A. E. et al. 2021 [22] | ILs levels in T1DM were higher than controls. |
| Tangjittipokin W. et al. 2021 [23] | IL1B SNPs are associated with T1DM susceptibility. |
| Campos L. P. et al. 2020 [24] | IL-18 polymorphisms were not associated with T1DM onset in children or adults in this population. |
| Keindl M. et al. 2020 [25] | IL2RA gene variants might increase the risk of developing vascular complications in people with T1D. |
| Sharma C. et al. 2020 [26] | IL2-RA gene variants could confer risk alleles for T1D among the Emirati population. |
| Boechat-Fernandes A. et al. 2019 [27] | SNP in IL18 gene could be associated with DM1 age onset. |
| *Borilova Linhartova P. et al. 2019 [28] | Variability in the IL-1B and IL-1RN genes may be one of the factors in the susceptibility to T1DM and CP, although the single variants of these polymorphisms are not crucial for the protein production. |
| Campos L. P. et al. 2019 [29] | IL-6 rs1800795 was not associated with adult-onset T1D. IL-6R rs2228145 was associated with T1D development in adulthood, and carriers of the minor C allele are at increased risk for adult-onset T1D. |
| Lundtoft C. et al. 2019 [30] | IL-7Rα variants may contribute to disease susceptibility against T1D. |
| *Borilova Linhartova P. et al. 2018 [9] | CP does not influence the circulating IL-8 levels. Patients with T1DM+CP had higher circulating IL-8 levels than healthy controls+CP/non-periodontitis. |
| Seyfarth J. et al. 2018 [31] | Only T1D children with the protective haplotype had lower IL-7 serum levels. |
| Al-Lahham Y. et al. 2017 [32] | IL-18 rs187238 was not associated with T1D in Euro-Brazilian population. |
References
- Tang, W.; Cui, D.; Jiang, L.; Zhao, L.; Qian, W.; Long, S.A.; Xu, K. Association of common polymorphisms in the IL 2 RA gene with type 1 diabetes: evidence of 32,646 individuals from 10 independent studies. J. Cell. Mol. Med. 2015, 19, 2481–2488. [Google Scholar] [CrossRef]
- Maddaloni, E.; Moretti, C.; Mignogna, C.; Buzzetti, R. Adult-onset autoimmune diabetes in 2020: An update. Maturitas 2020, 137, 37–44. [Google Scholar] [CrossRef]
- Hober, D.; Sauter, P. Pathogenesis of type 1 diabetes mellitus: interplay between enterovirus and host. Nat. Rev. Endocrinol. 2010, 6, 279–289. [Google Scholar] [CrossRef]
- A Gregory, G.; Robinson, T.I.G.; E Linklater, S.; Wang, F.; Colagiuri, S.; de Beaufort, C.; Donaghue, K.C.; International Diabetes Federation Diabetes Atlas Type 1 Diabetes in Adults Special Interest Group; Magliano, D.J.; Maniam, J.; et al. Global incidence, prevalence, and mortality of type 1 diabetes in 2021 with projection to 2040: a modelling study. Lancet Diabetes Endocrinol. 2022, 10, 741–760. [Google Scholar] [CrossRef]
- Khdair, S.I.; Jarrar, W.; Jarrar, Y.B.; Bataineh, S.; Al-Khaldi, O. Association of HLA-DRB1 and -DQ Alleles and Haplotypes with Type 1 Diabetes in Jordanians. Endocrine, Metab. Immune Disord. - Drug Targets 2020, 20, 895–902. [Google Scholar] [CrossRef]
- Luotola, K.; Alanne, M.; Lanki, T.; Moilanen, L.; Surakka, I.; Pietilä, A.; Nieminen, M.S.; Peters, A.; Jula, A.; Perola, M.; et al. Association of Variation in the Interleukin-1 Gene Family with Diabetes and Glucose Homeostasis. J. Clin. Endocrinol. Metab. 2009, 94, 4575–4583. [Google Scholar] [CrossRef]
- Löe, H. Periodontal Disease: The sixth complication of diabetes mellitus. Diabetes Care 1993, 16, 329–334. [Google Scholar] [CrossRef]
- Giannobile, W.; Braun, T.; Caplis, A.; Doucette-Stamm, L.; Duff, G.; Kornman, K. Patient Stratification for Preventive Care in Dentistry. J. Dent. Res. 2013, 92, 694–701. [Google Scholar] [CrossRef]
- Linhartova, P.B.; Kavrikova, D.; Tomandlova, M.; Poskerova, H.; Rehka, V.; Dušek, L.; Holla, L.I. Differences in Interleukin-8 Plasma Levels between Diabetic Patients and Healthy Individuals Independently on Their Periodontal Status. Int. J. Mol. Sci. 2018, 19, 3214. [Google Scholar] [CrossRef]
- Linhartova, P.B.; Kastovsky, J.; Lucanova, S.; Bartova, J.; Poskerova, H.; Vokurka, J.; Fassmann, A.; Kankova, K.; Holla, L.I. Interleukin-17AGene Variability in Patients with Type 1 Diabetes Mellitus and Chronic Periodontitis: Its Correlation with IL-17 Levels and the Occurrence of Periodontopathic Bacteria. Mediat. Inflamm. 2016, 2016, 1–9. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Syst. Rev. 2021, 89, 105906. [Google Scholar]
- PROSPERO. Available online: https://www.crd.york.ac.uk/prospero/#aboutpage (accessed on 18 June 2022).
- Hoffmann, M.; Enczmann, J.; Balz, V.; Kummer, S.; Reinauer, C.; Döing, C.; Förtsch, K.; Welters, A.; Vasconcelos, M.K.; Mayatepek, E.; et al. Interleukin-7 and soluble Interleukin-7 receptor levels in type 1 diabetes – Impact of IL7RA polymorphisms, HLA risk genotypes and clinical features. Clin. Immunol. 2022, 235, 108928. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Xu, L.; Zhao, W.; Pan, J.; Lu, J.; Lu, H.; Yan, J.; Weng, J.; Liu, F. Serum IL-17A concentration and a IL17RA single nucleotide polymorphism contribute to the risk of autoimmune type 1 diabetes. Diabetes/Metabolism Res. Rev. 2022, 38, e3547. [Google Scholar] [CrossRef] [PubMed]
- E Osman, A.; Brema, I.; AlQurashi, A.; Al-Jurayyan, A.; Bradley, B.; A Hamza, M. Single nucleotide polymorphism rs 2070874 at Interleukin-4 is associated with increased risk of type 1 diabetes mellitus independently of human leukocyte antigens. Int. J. Immunopathol. Pharmacol. 2022, 36. [Google Scholar] [CrossRef]
- Ali, Y.B.M.; El-Gahel, H.E.; Abdel-Hakem, N.E.; Gadalla, M.E.; El-Hefnawy, M.H.; El-Shahat, M. Association between IL-18 and IL-6 gene polymorphisms and the risk of T1D in Egyptian children. J. Diabetes Metab. Disord. 2021, 20, 439–446. [Google Scholar] [CrossRef]
- El Helaly, R.M.; Elzehery, R.R.; El-Emam, O.A.; El Domiaty, H.A.; Elbohy, W.R.; Aboelenin, H.M.; Salem, N.A. Genetic association between interleukin-10 gene rs1518111 and rs3021094 polymorphisms and risk of type 1 diabetes and diabetic nephropathy in Egyptian children and adolescents. Pediatr. Diabetes 2021, 22, 567–576. [Google Scholar] [CrossRef]
- Haghnazari, L.; Sabzi, R. Relationship between TP53 and interleukin-6 gene variants and the risk of types 1 and 2 diabetes mellitus development in the Kermanshah province. J. Med. Life. 2021, 14, 37–44. [Google Scholar] [CrossRef]
- Hehenkamp, P.; Hoffmann, M.; Kummer, S.; Reinauer, C.; Döing, C.; Förtsch, K.; Enczmann, J.; Balz, V.; Mayatepek, E.; Meissner, T.; et al. Interleukin-7-dependent nonclassical monocytes and CD40 expression are affected in children with type 1 diabetes. Eur. J. Immunol. 2021, 51, 3214–3227. [Google Scholar] [CrossRef]
- Pks, S.K.; Varghese, S.; S, T.K.; N, J.D.; G, L.D. Association of IL-17A Polymorphism with Chronic Periodontitis in Type 1 Diabetes Patients. J. Dent. 2021, 22, 180–186. [Google Scholar] [CrossRef]
- Li, J.; Sun, X.; Luo, S.; Lin, J.; Xiao, Y.; Yu, H.; Huang, G.; Li, X.; Xie, Z.; Zhou, Z. The Positivity Rate of IA-2A and ZnT8A in the Chinese Han Population With Type 1 Diabetes Mellitus: Association With rs1143627 and rs1143643 Polymorphisms in the IL1B Gene. Front. Pharmacol. 2021, 12. [Google Scholar] [CrossRef]
- Osman, A.E.; Brema, I.; AlQurashi, A.; Al-Jurayyan, A.; Bradley, B.; Hamza, M.A. Association of single-nucleotide polymorphisms in tumour necrosis factor and human leukocyte antigens genes with type 1 diabetes. Int. J. Immunogenetics 2021, 48, 326–335. [Google Scholar] [CrossRef]
- Tangjittipokin, W.; Umjai, P.; Khemaprasit, K.; Charoentawornpanich, P.; Chanprasert, C.; Teerawattanapong, N.; Narkdontri, T.; Santiprabhob, J. Vitamin D pathway gene polymorphisms, vitamin D level, and cytokines in children with type 1 diabetes. Gene 2021, 791, 145691. [Google Scholar] [CrossRef]
- Campos, L.; Graciolo, V.; Welter, M.; Lopes, M.; Nesi-França, S.; Picheth, G.; Rego, F. Research Article The IL18 rs1946518 and PTPN22 rs2476601 polymorphisms are not associated with adult- and childhood-onset type 1 diabetes mellitus. Genet. Mol. Res. 2020, 19. [Google Scholar] [CrossRef]
- Keindl, M.; Fedotkina, O.; du Plessis, E.; Jain, R.; Bergum, B.; Jensen, T.M.; Møller, C.L.; Falhammar, H.; Nyström, T.; Catrina, S.-B.; et al. Increased Plasma Soluble Interleukin-2 Receptor Alpha Levels in Patients With Long-Term Type 1 Diabetes With Vascular Complications Associated With IL2RA and PTPN2 Gene Polymorphisms. Front. Endocrinol. 2020, 11, 575469. [Google Scholar] [CrossRef]
- Sharma, C.; Ali, B.R.; Osman, W.; Afandi, B.; Aburawi, E.H.; A Beshyah, S.; Al-Mahayri, Z.; Al-Rifai, R.H.; Al Yafei, Z.; ElGhazali, G.; et al. Association of variants in PTPN22, CTLA-4, IL2-RA, and INS genes with type 1 diabetes in Emiratis. Ann. Hum. Genet. 2021, 85, 48–57. [Google Scholar] [CrossRef]
- Boëchat-Fernandes, A.; Réa, R.R.; Romanzini, N.B.; Gomes, M.B.; Furtado-Alle, L.; Souza, R.L.R. IL18 Gene Polymorphism Influences Age of Onset of DM1 in African Ancestry Brazilians. J. Pediatr. Genet. 2019, 08, 038–040. [Google Scholar] [CrossRef]
- Linhartova, P.B.; Poskerova, H.; Tomandlova, M.; Bartova, J.; Kankova, K.; Fassmann, A.; Holla, L.I. Interleukin-1 Gene Variability and Plasma Levels in Czech Patients with Chronic Periodontitis and Diabetes Mellitus. Int. J. Dent. 2019, 2019, 1–10. [Google Scholar] [CrossRef]
- Campos, L.; Graciolo, V.; Sousa, M.; Martins, B.; Souza, S.; Alberton, D.; Picheth, G.; Rego, F. Research Article Polymorphisms rs1800795 of interleukin-6 and rs2228145 of interleukin-6 receptor genes in Euro-Brazilians with adult-onset type 1 diabetes mellitus. Genet. Mol. Res. 2019, 18. [Google Scholar] [CrossRef]
- Lundtoft, C.; Seyfarth, J.; Oberstrass, S.; Rosenbauer, J.; Baechle, C.; Roden, M.; Holl, R.W.; Mayatepek, E.; Kummer, S.; Meissner, T.; et al. Autoimmunity risk- and protection-associated IL7RA genetic variants differentially affect soluble and membrane IL-7Rα expression. J. Autoimmun. 2019, 97, 40–47. [Google Scholar] [CrossRef]
- Seyfarth, J.; Lundtoft, C.; Förtsch, K.; Ahlert, H.; Rosenbauer, J.; Baechle, C.; Roden, M.; Holl, R.W.; Mayatepek, E.; Kummer, S.; et al. Interleukin-7 receptor α-chain haplotypes differentially affect soluble IL-7 receptor and IL-7 serum concentrations in children with type 1 diabetes. Pediatr. Diabetes 2018, 19, 955–962. [Google Scholar] [CrossRef]
- Al-Lahham, Y.; Mendes, A.K.B.; Souza, E.M.; Alberton, D.; Rego, F.G.M.; Valdameri, G.; Picheth, G. Research Article Interleukin-18 (rs187238) and glucose transporter 4 (rs5435) polymorphisms in Euro-Brazilians with type 1 diabetes. Genet. Mol. Res. 2017, 16. [Google Scholar] [CrossRef]
- Stang, A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur. J. Epidemiology 2010, 25, 603–605. [Google Scholar] [CrossRef]
| Authors/Year | Population (Age)/Ethnicity | IL/#Polymorphism | Analysis |
|---|---|---|---|
| Hoffman M. et al. 2022 [13] | 349 (Children/adolescents)/German | IL-7/2 | NGS |
| Li J. et al. 2022 [14] | 270 (Adults)/Chinese | IL-6, IL-17(A-F)/7 | PCR |
| Osman A. E. et al. 2022 [15] | 371 (All ages)/Saudi | IL-4, IL-10/5 | PCR |
| Ali Y. et al. 2021 [16] | 218 (Children)/Egyptian | IL-6, IL-18/3 | PCR |
| El Helaly R.M. et al. 2021 [17] | 230 (Children/adolescents)/Egyptian | IL-10/2 | PCR |
| Haghnazari L. et al. 2021 [18] | 136 (Adults)/Iranian | IL-6/1 | Spectrophotometry |
| Hehenkamp P. et al. 2021 [19] | 40 (Children)/German | IL-7/1 | PCR |
| *Kumar S. et al. 2021 [20] | 90 (Adults)/Indian | IL-17A/1 | PCR |
| Li J. et al. 2021 [21] | 1092 (Adolescents)/Chinese | IL-1B/2 | Mass spectometry |
| Osman A. E. et al. 2021 [22] | 328 (All ages)/Saudi | IL-1(A, B), IL-2, IL-12/5 | PCR |
| Tangjittipokin W. et al. 2021 [23] | 200 (Children/Adolescents)/Thai | IL-2, IL-4. IL-6, IL-10, IL-13, IL-17A/6 | PCR |
| Campos L. P. et al. 2020 [24] | 611 (Children/Adults)/Euro-Brazilian | IL-18/1 | PCR |
| Keindl M. et al. 2020 [25] | 79 (Adults)/Scandinavian | sIL-2R/68 | Flow citometry |
| Sharma C. et al. 2020 [26] | 310 (Adolescents, Adults)/Emiratis | IL-2RA/1 | PCR |
| Boechat-Fernandes A. et al. 2019 [27] | 1101 (Adolescents)/Brazilian | IL-12B, IL-18/3 | PCR |
| *Borilova Linhartova P. et al. 2019 [28] | 659 (Adults)/Czech | IL-1/2 | PCR |
| Campos L. P. et al. 2019 [29] | 291 (Adults)/Euro-Brazilian | IL-6, IL-6R/2 | PCR |
| Lundtoft C. et al. 2019 [30] | 301 (Children, Adolescents)/German | IL-7RA/2 | PCR |
| *Borilova Linhartova P. et al. 2018 [9] | 109 (Adults)/Czech | IL-8, IL-8R/2 | PCR |
| Seyfarth J. et al. 2018 [31] | 301 (Adolescents)/German | IL-7RA, sIL-7R/2 | PCR |
| Al-Lahham Y. et al. 2017 [32] | 280 (Adults)/Euro-Brazilian | IL-18/1 | PCR |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





