Submitted:
21 June 2023
Posted:
25 June 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Material and Methods
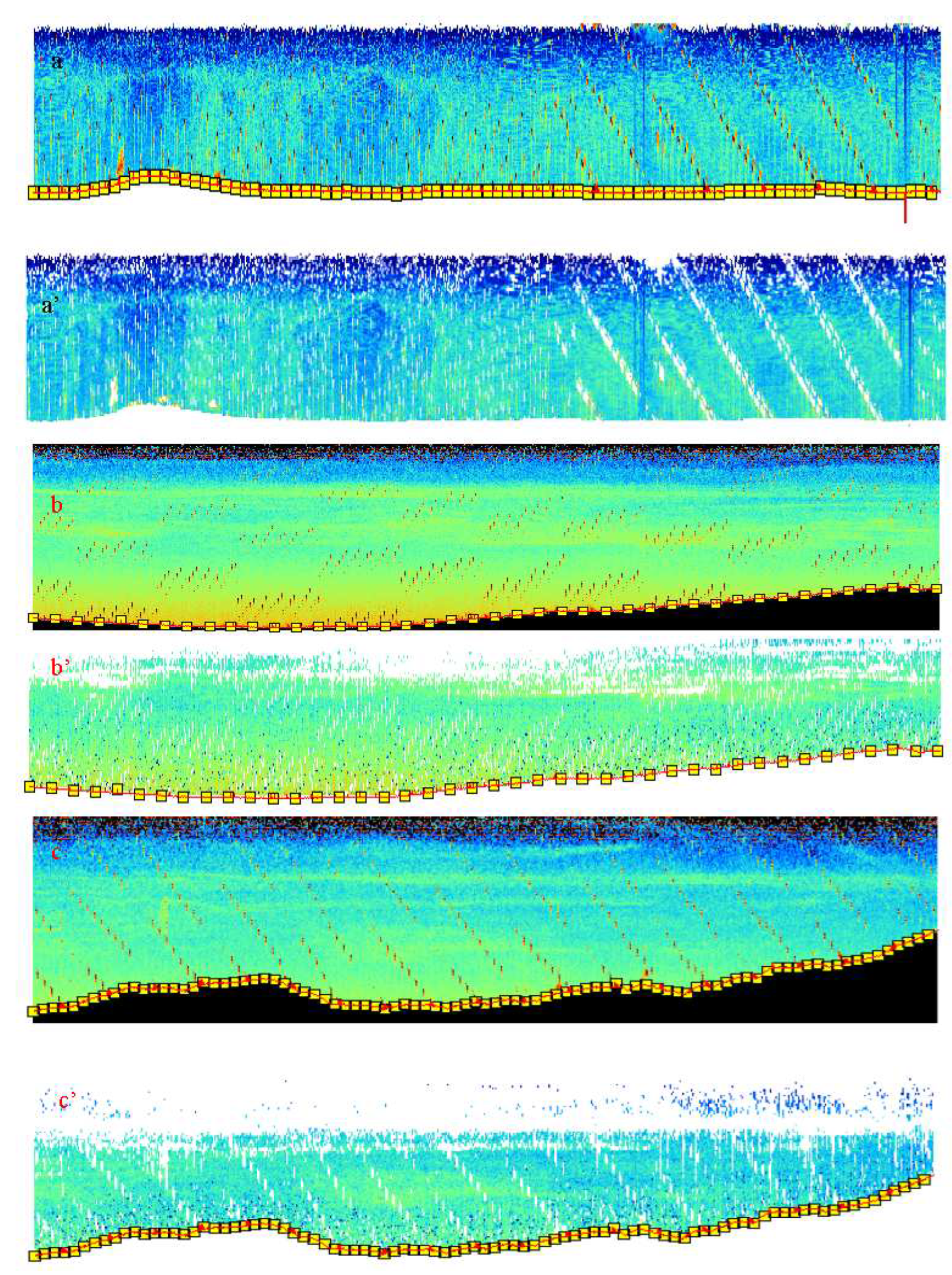
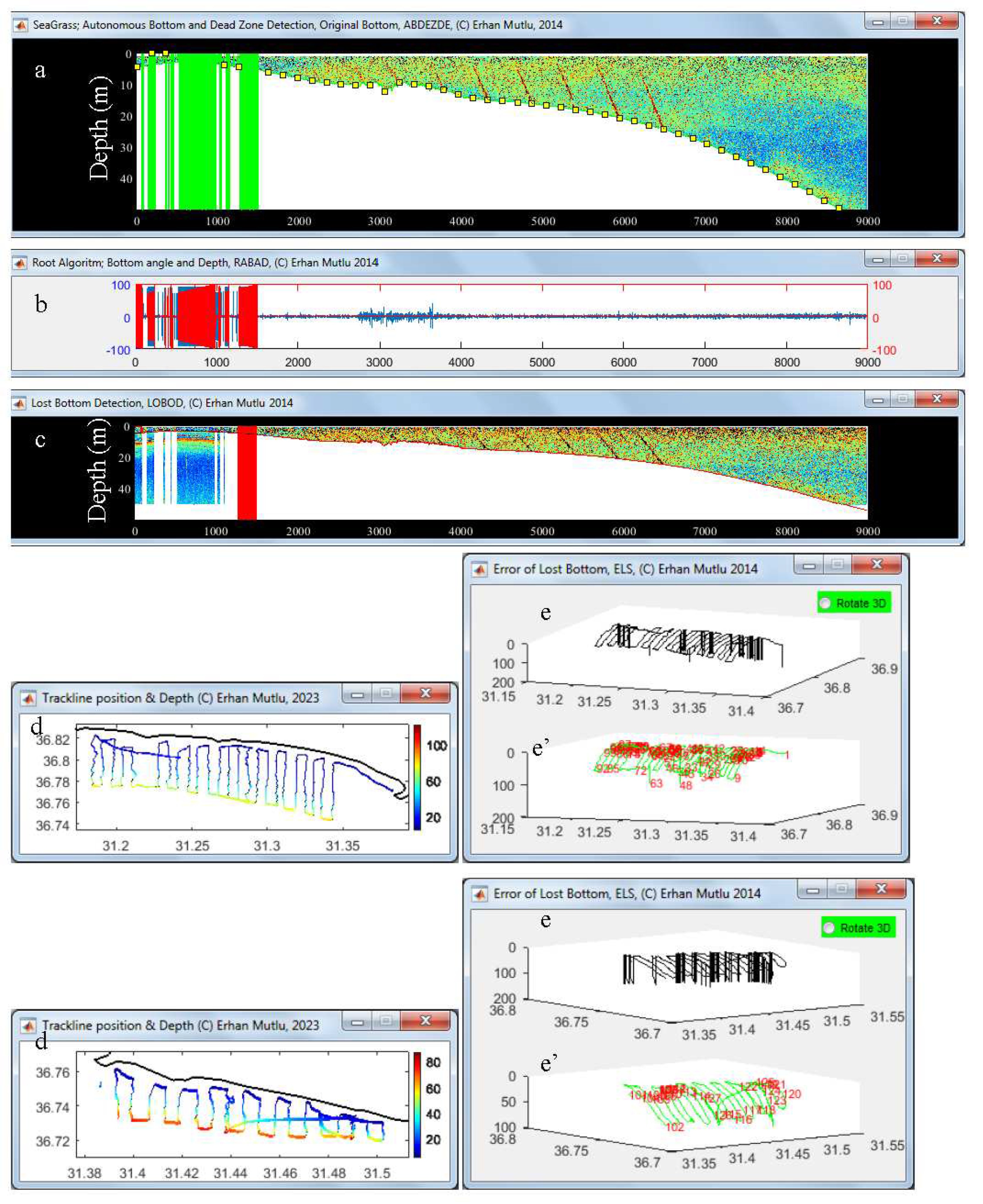
2.1. Algorithm of ‘lost’ bottom (AbdezdeR1)
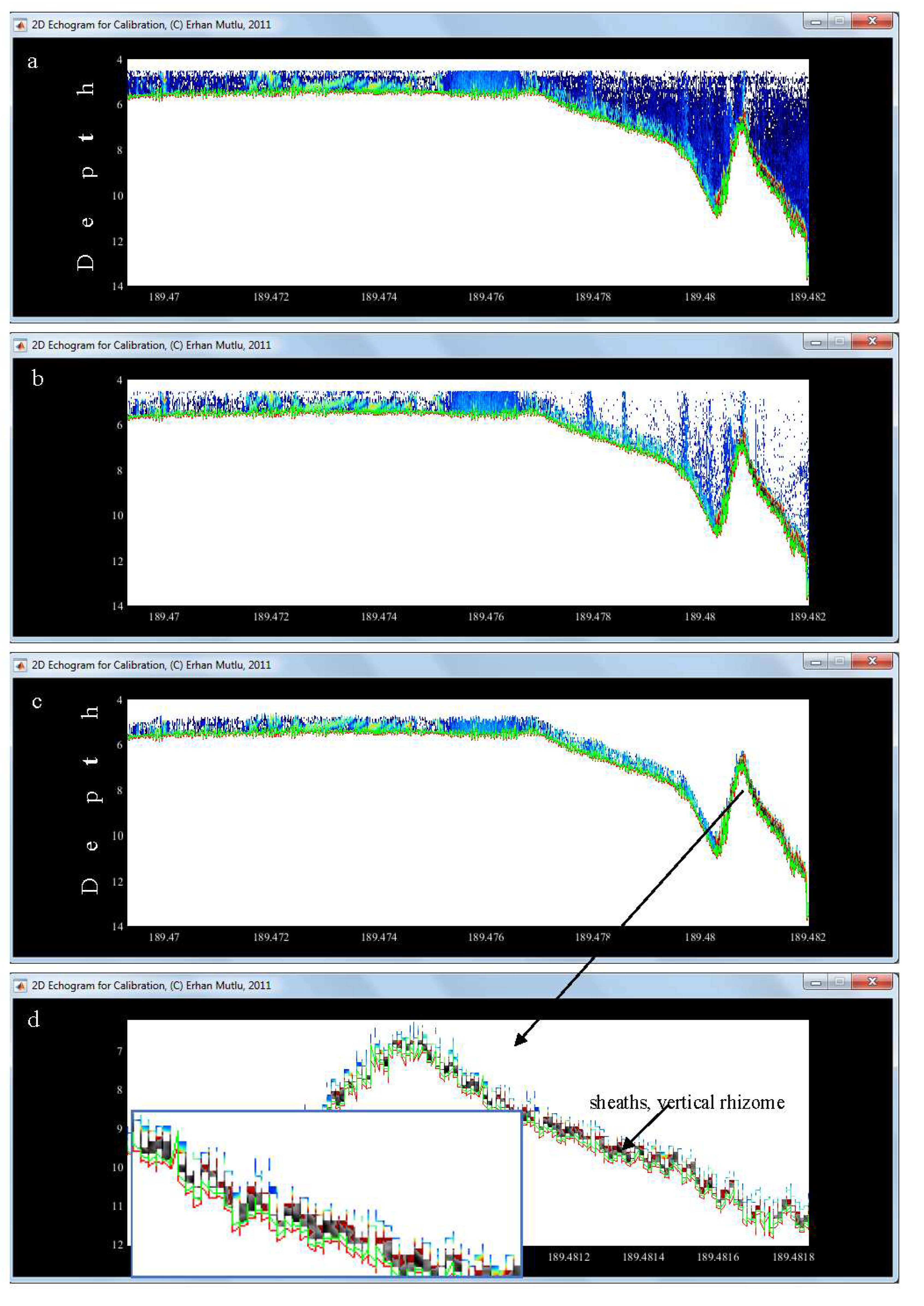
2.1.1. Real bottom recovery
- The chirping of the Sv of the bottom echo has a priority to the next two methods to correct the bottom. This method runs well in cases with clear bottom echo in the misestimated bottom section of the data.
- The running average is automatically called by the processor if there is no clear (weak) bottom echo. This method is functional by correcting the bottom lost between the previous and next corrected bottom depths and angles.
- The filling gap is then intervened to correct the bottom. In some case of occurrence of no bottom echo (a gap) due to the occurrence of surface or volume reverberation or intense fish school, the bottom echo disappears. Before filling the gap, the method checks the depths and angles.
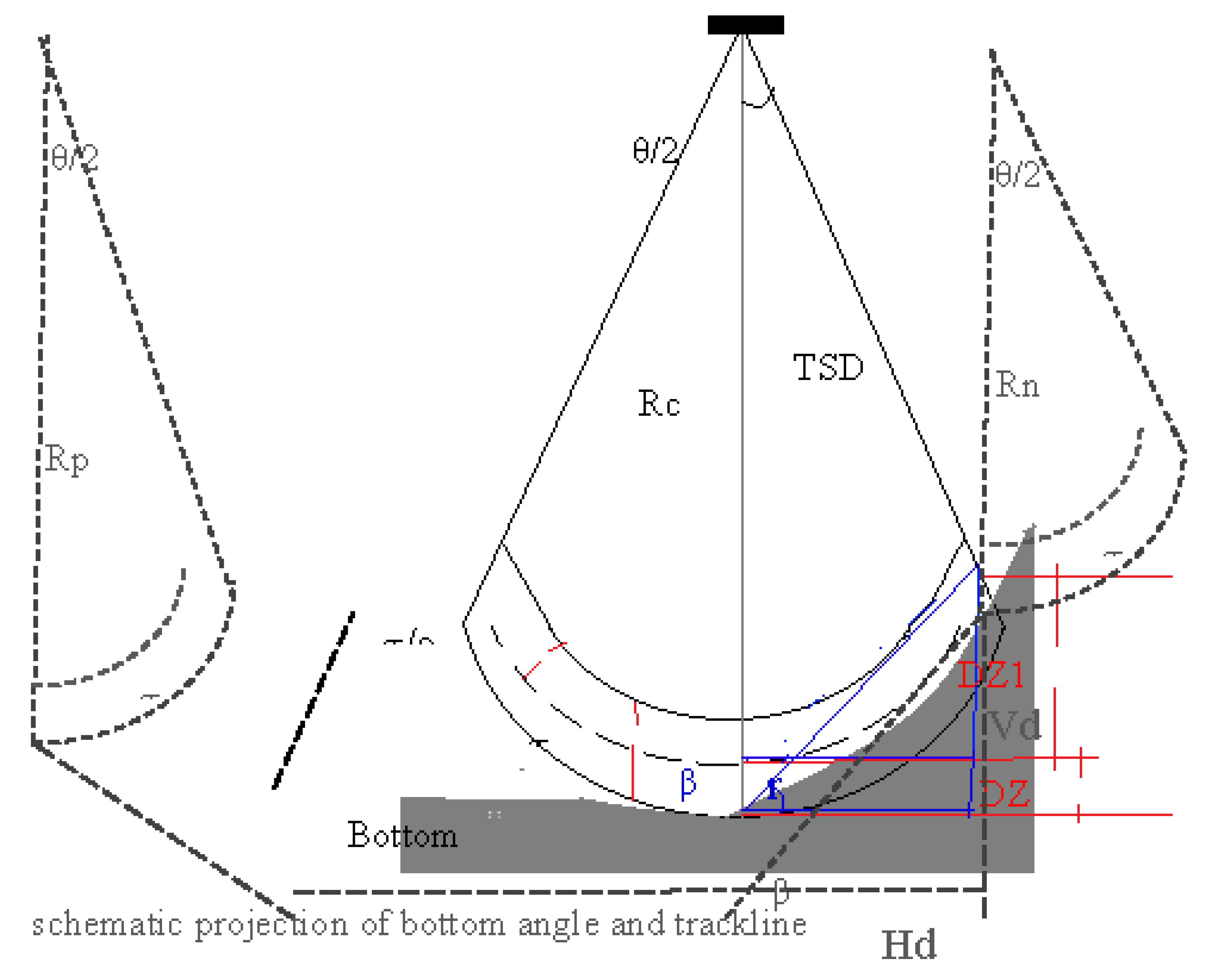
2.1.2. Dead zone estimates
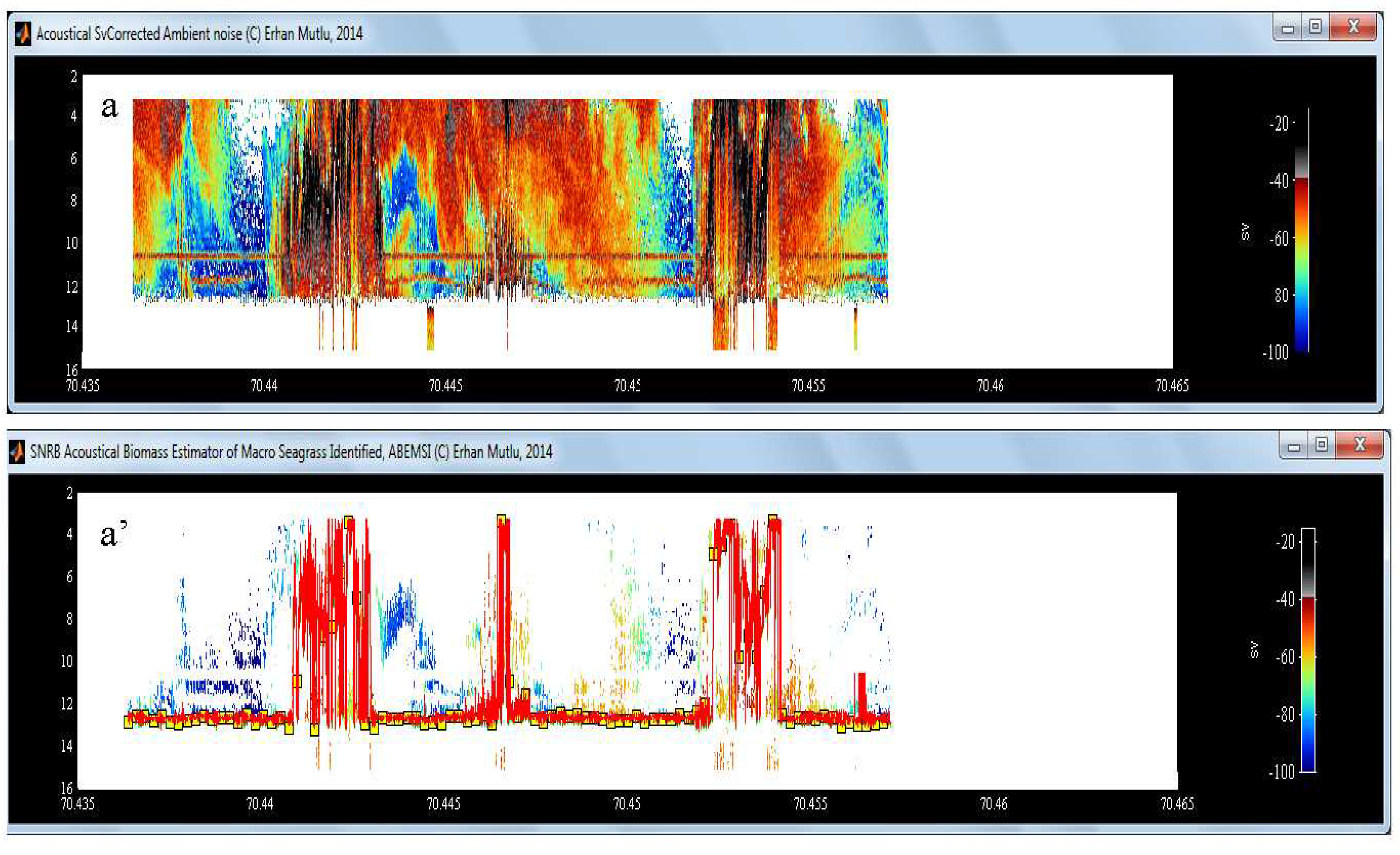
2.2. Algorithm of Noise and Reverberation (AbemsiR1)
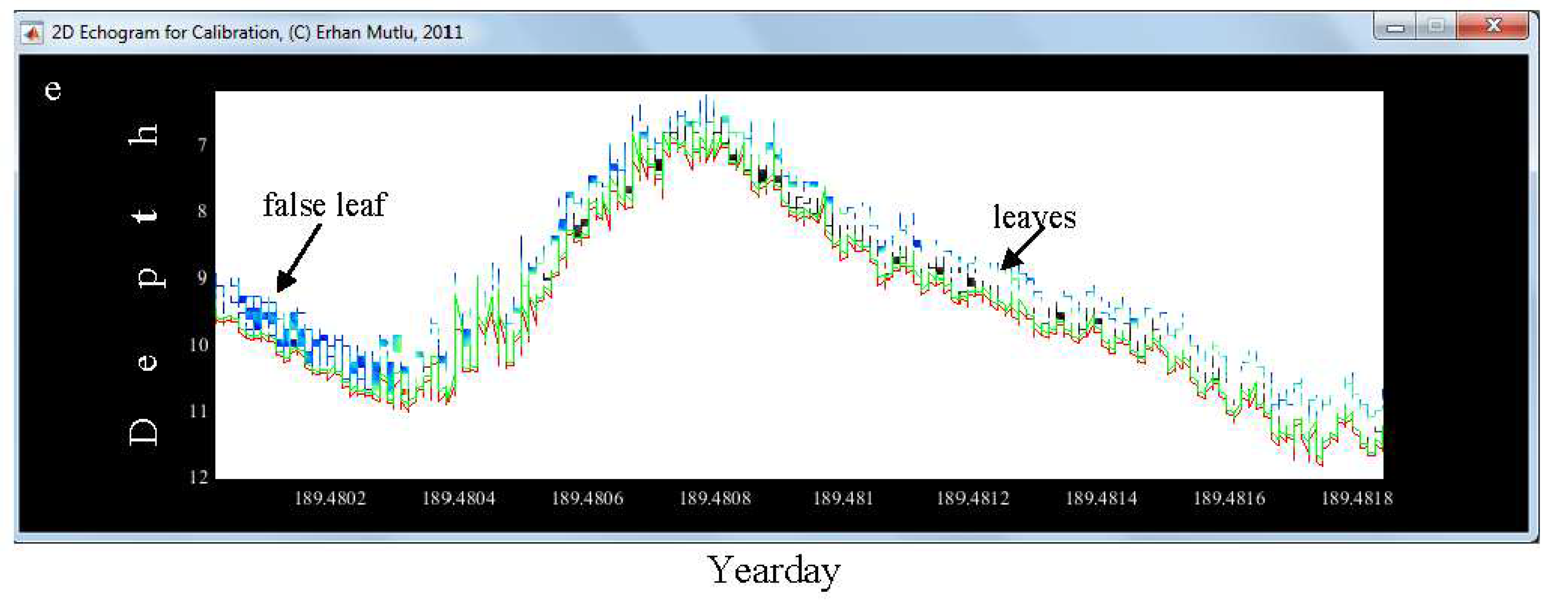
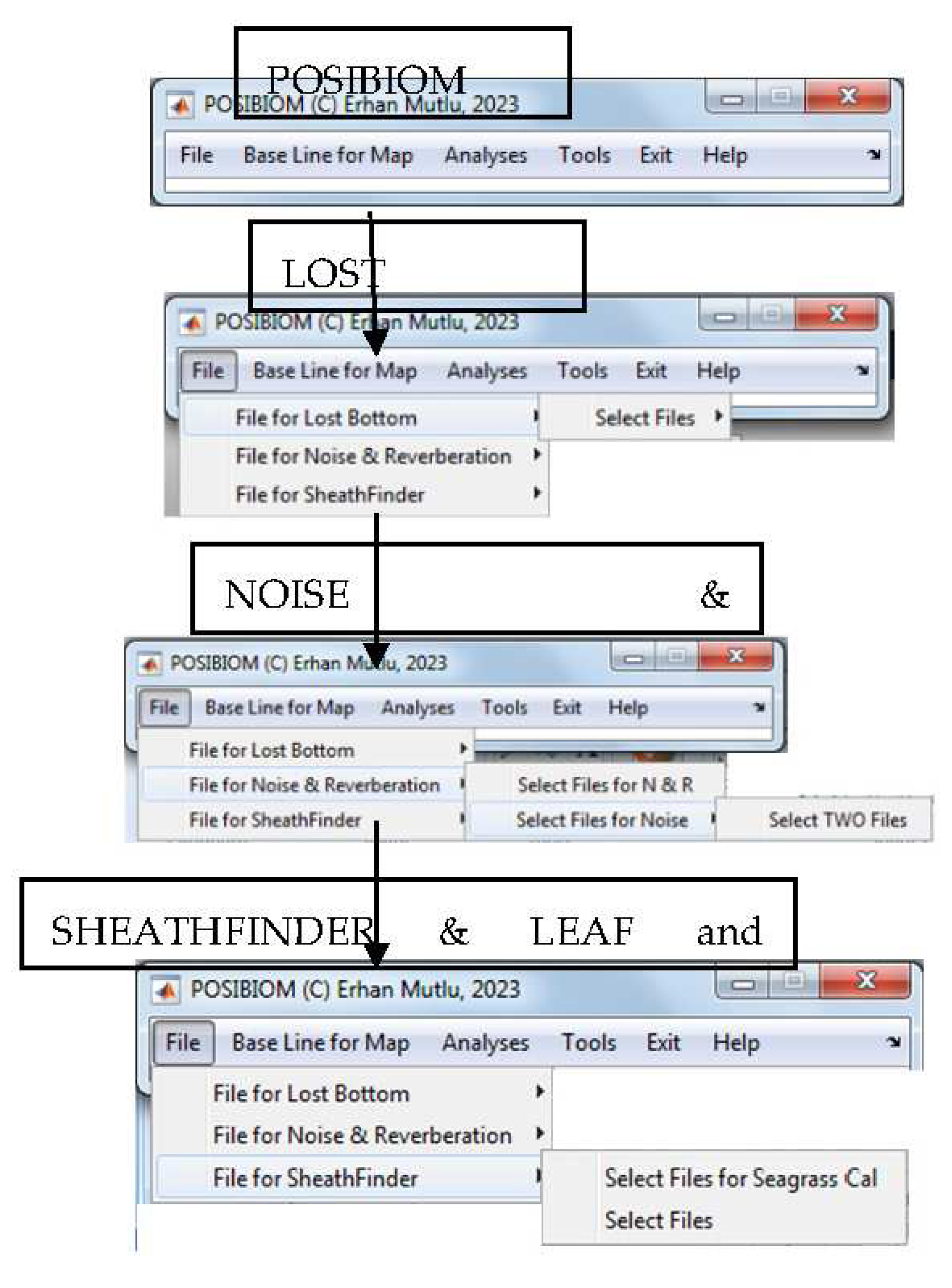
2.3. Algorithm of “SheathFinder” & Leaf length and biomass (Sheathfinder1, and 2)
3. Results
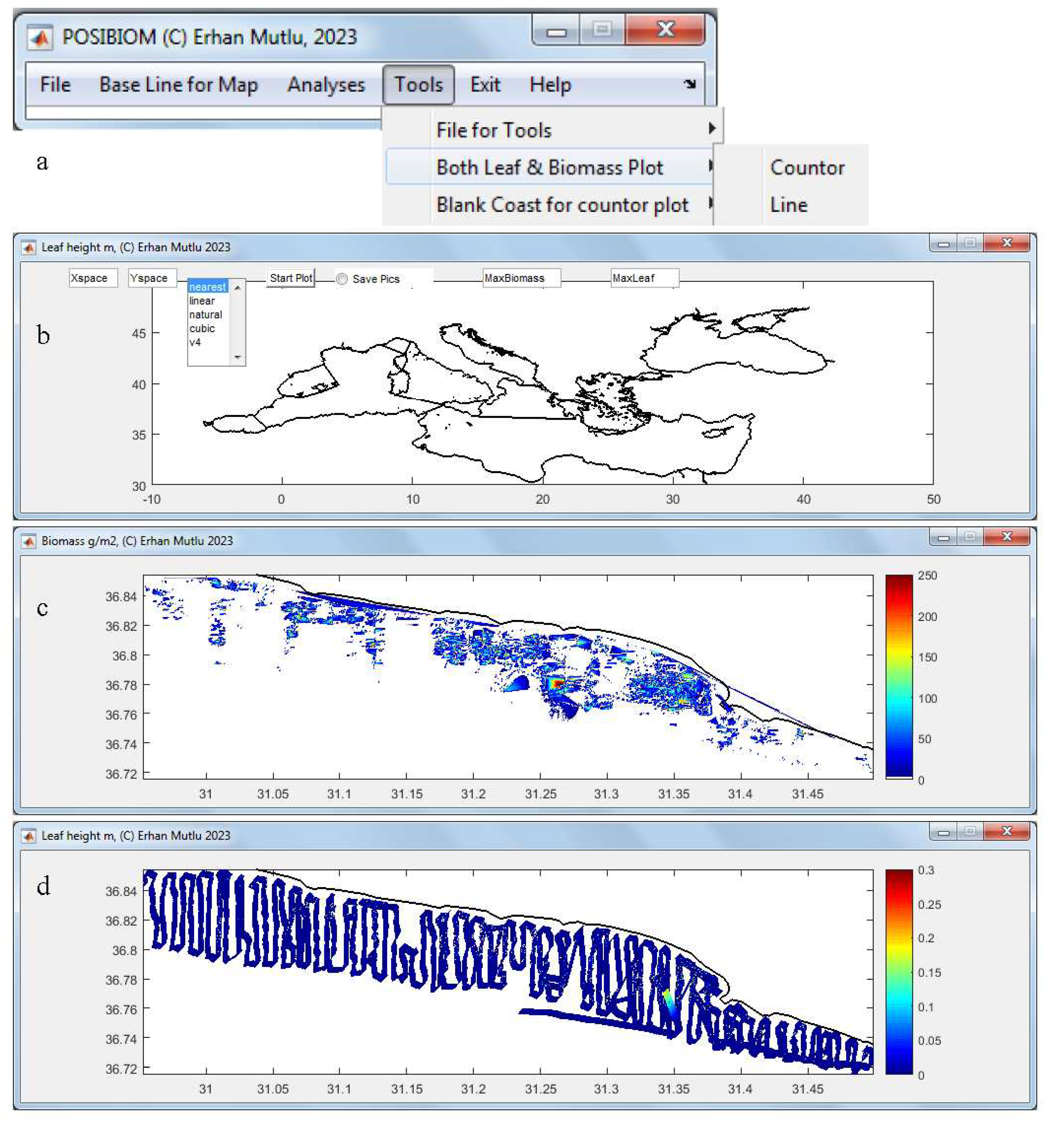
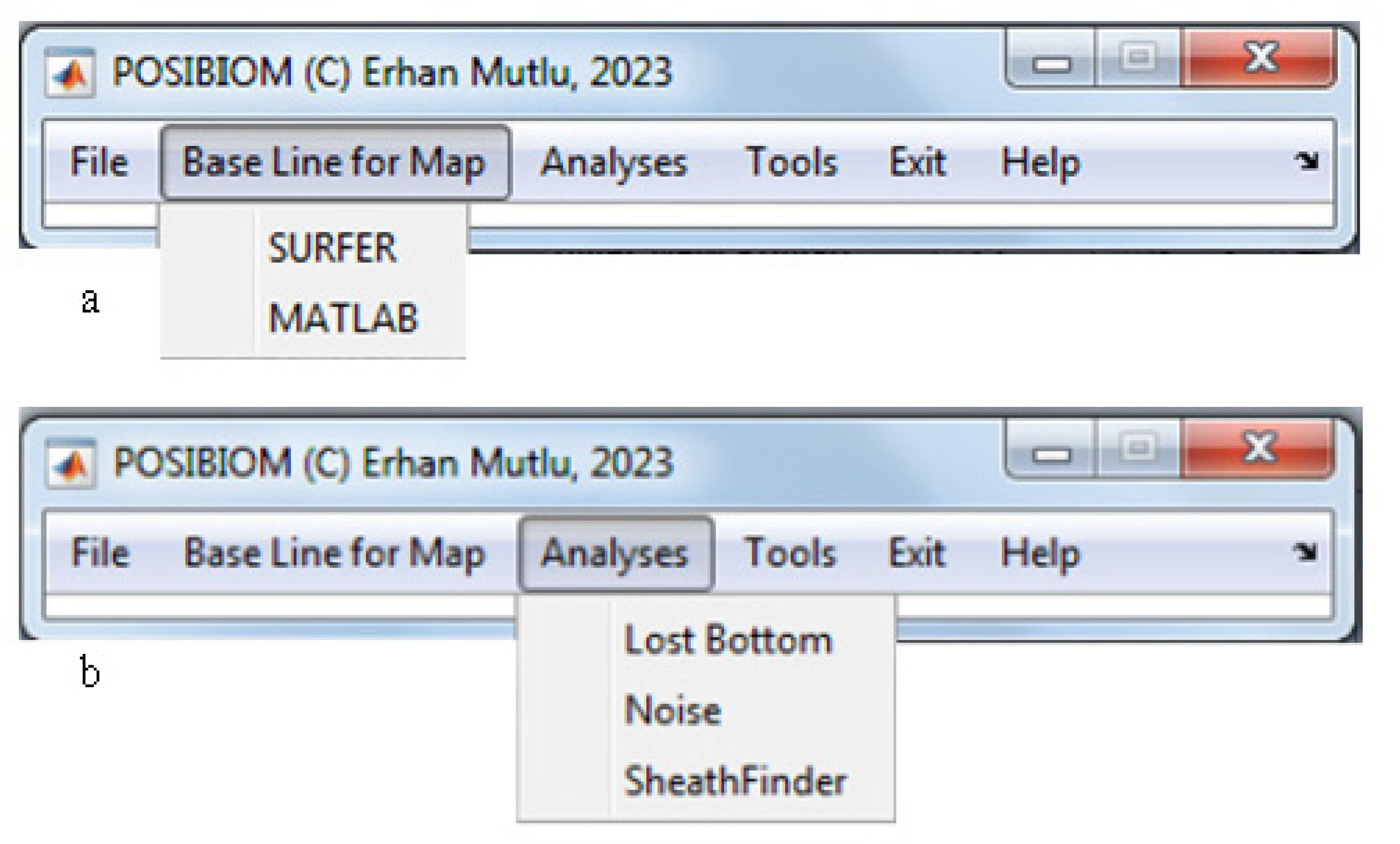
3.1. Flowchart of POSIBIOM
3.2. Lost Bottom and Dead Zone
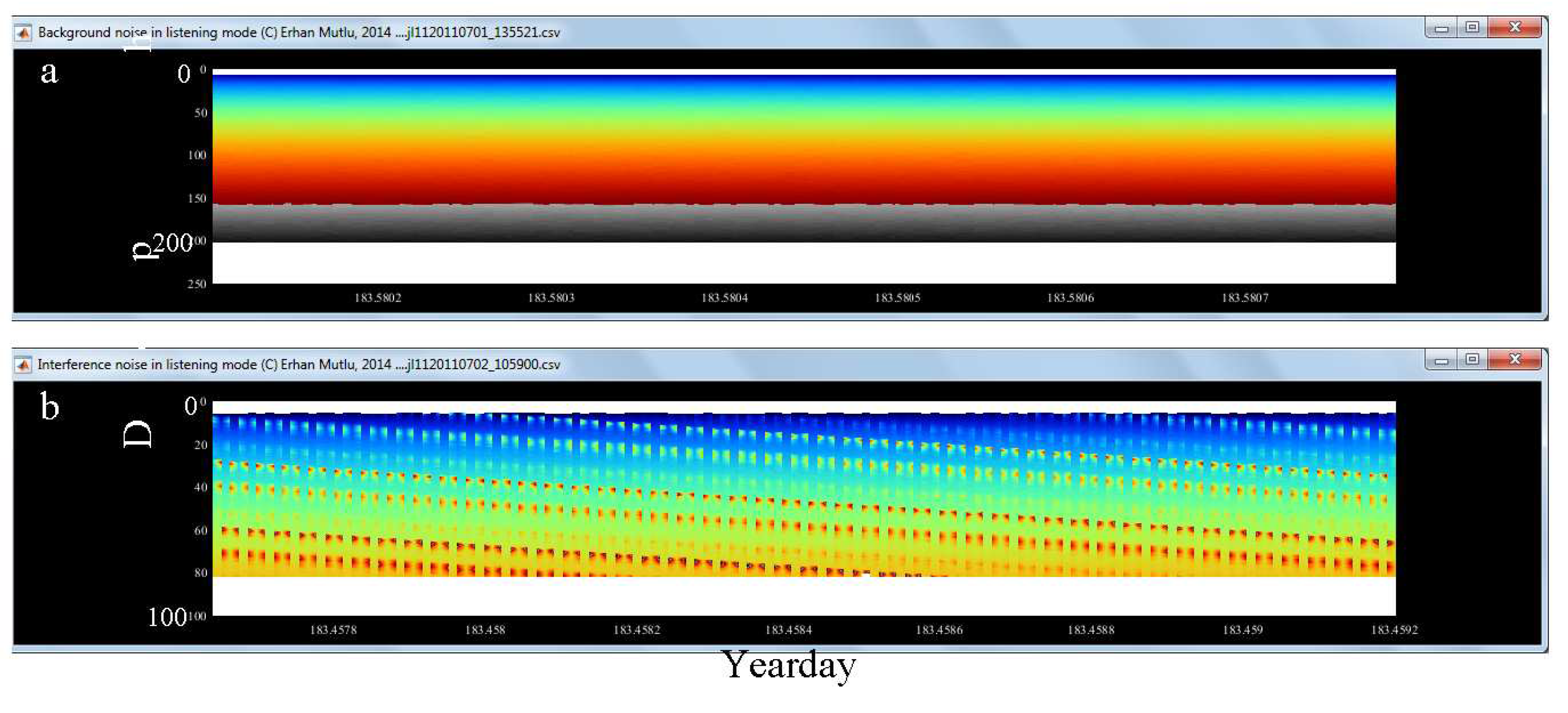
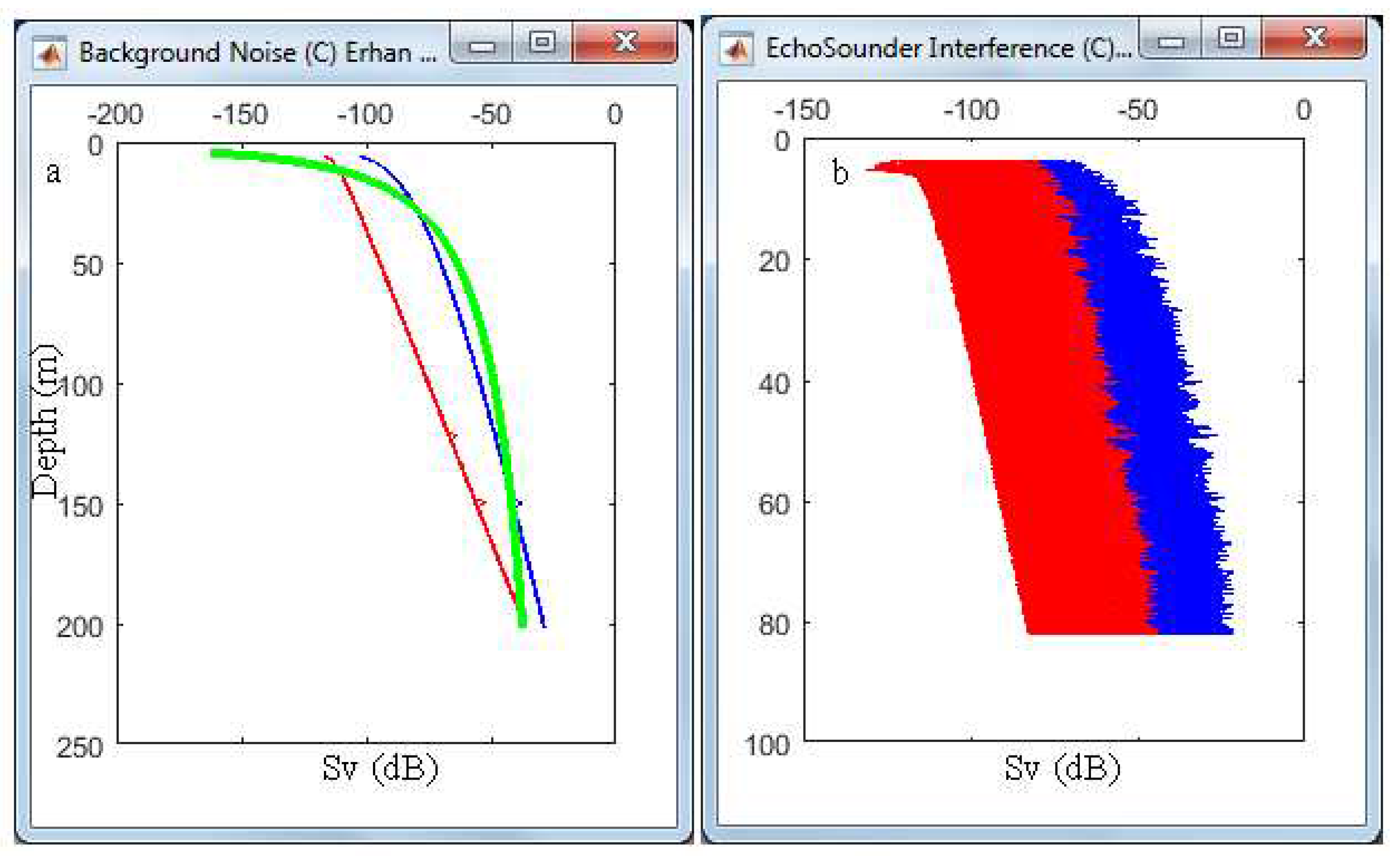
3.3. Noise, Reverberation and Interference
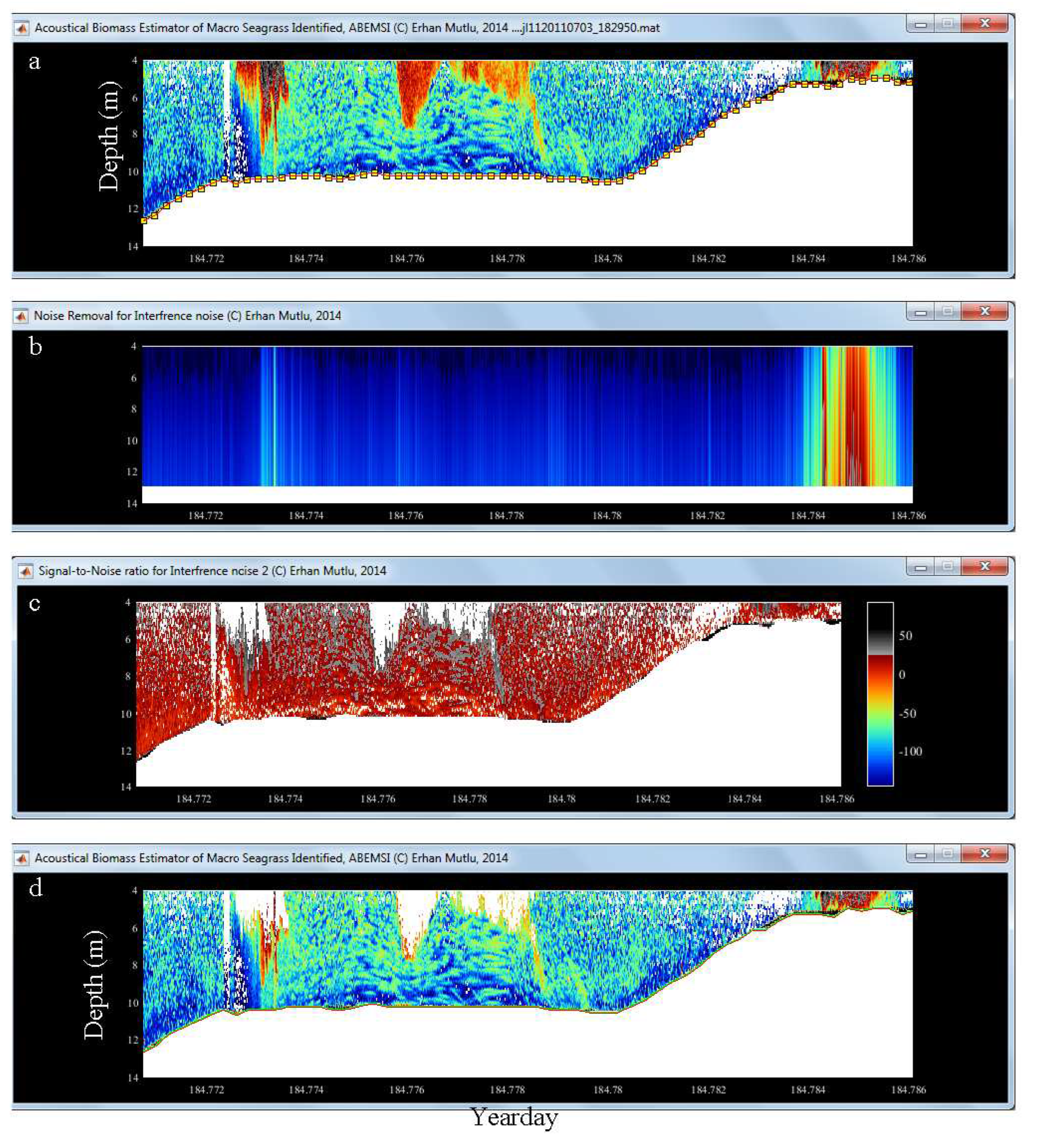
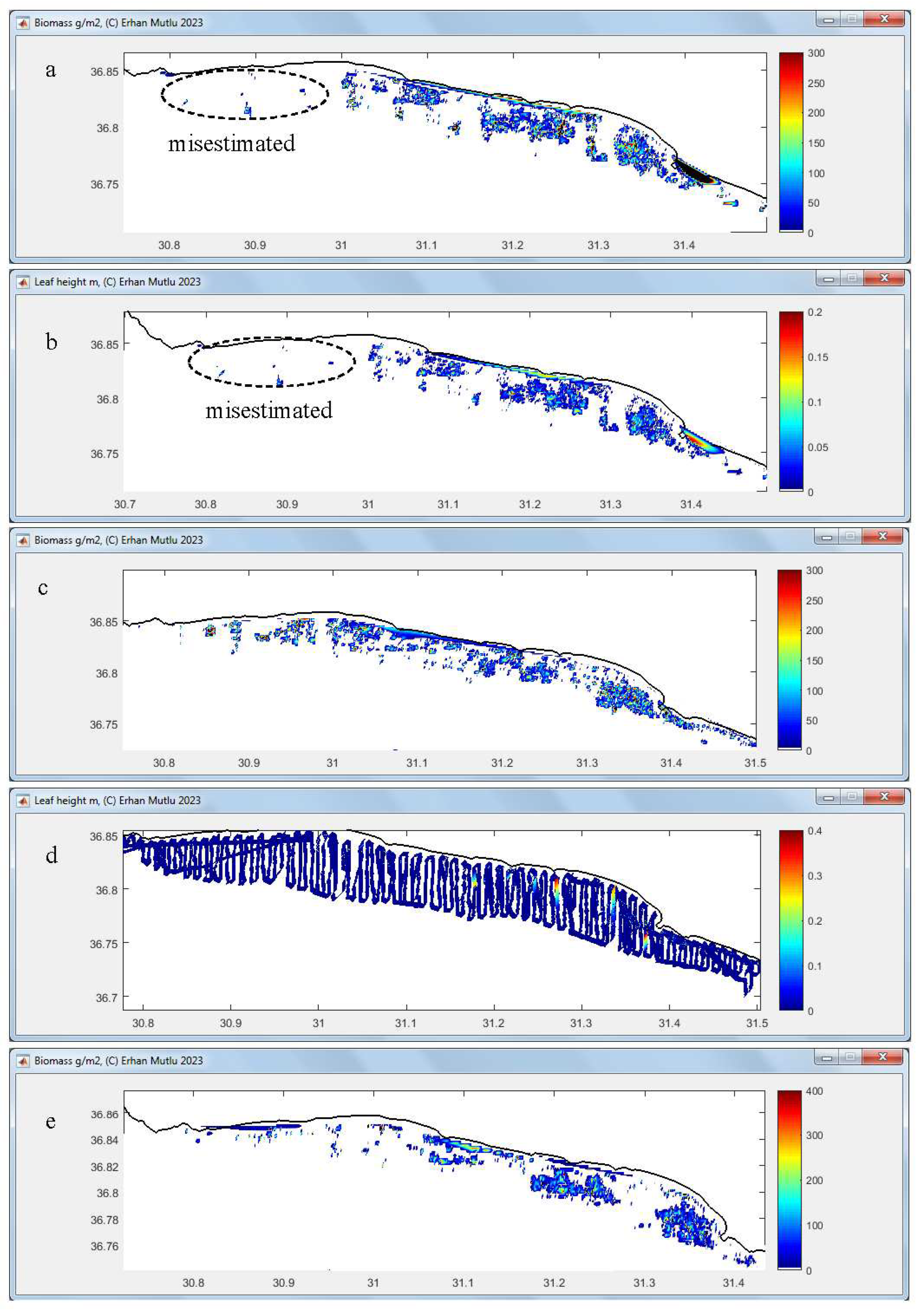
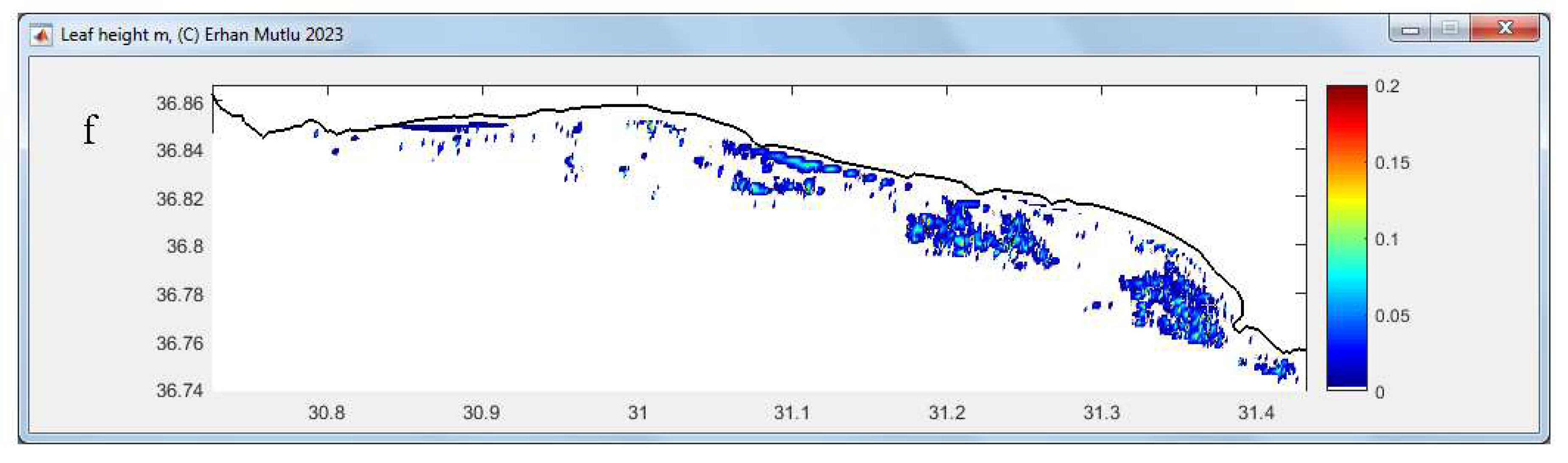
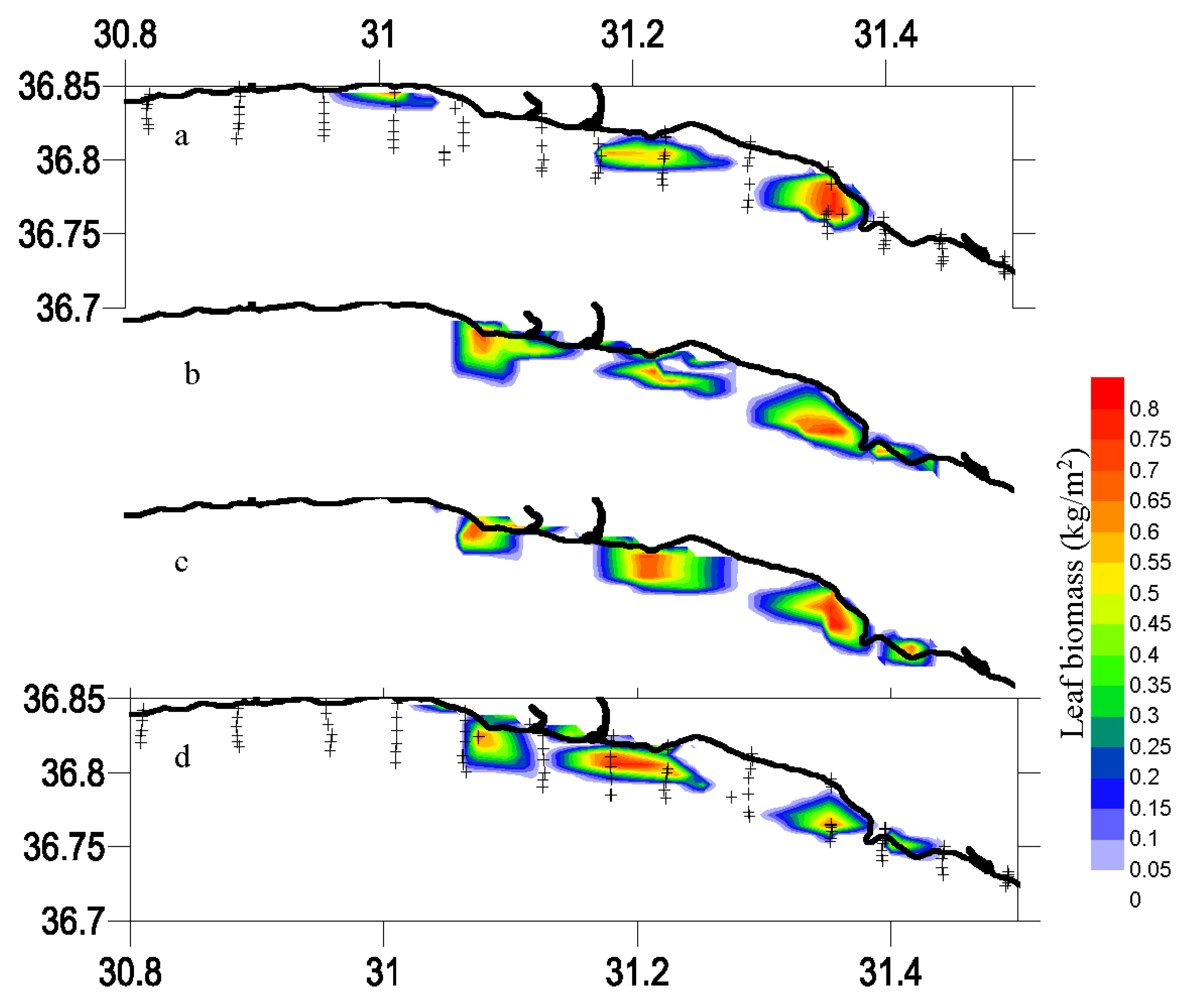
3.4. Leaf and Biomass Estimation
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
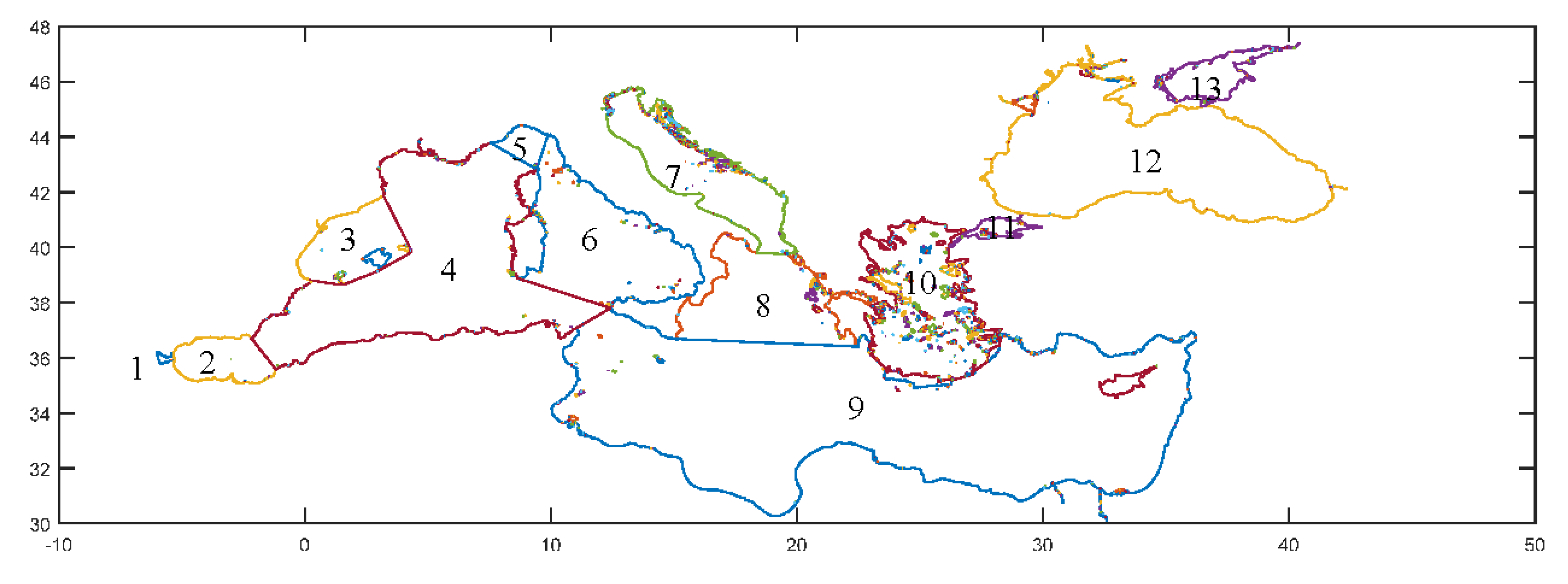
- Gibraltar Straits
- Alboran Sea
- Balearic Sea
- West Mediterranean Sea
- Ligurian Sea
- Tyrrhenian Sea
- Adriatic Sea
- Ionian Sea
- East Mediterranean Sea
- Aegean Sea
- Sea of Marmara
- Black Sea
- Azov Sea
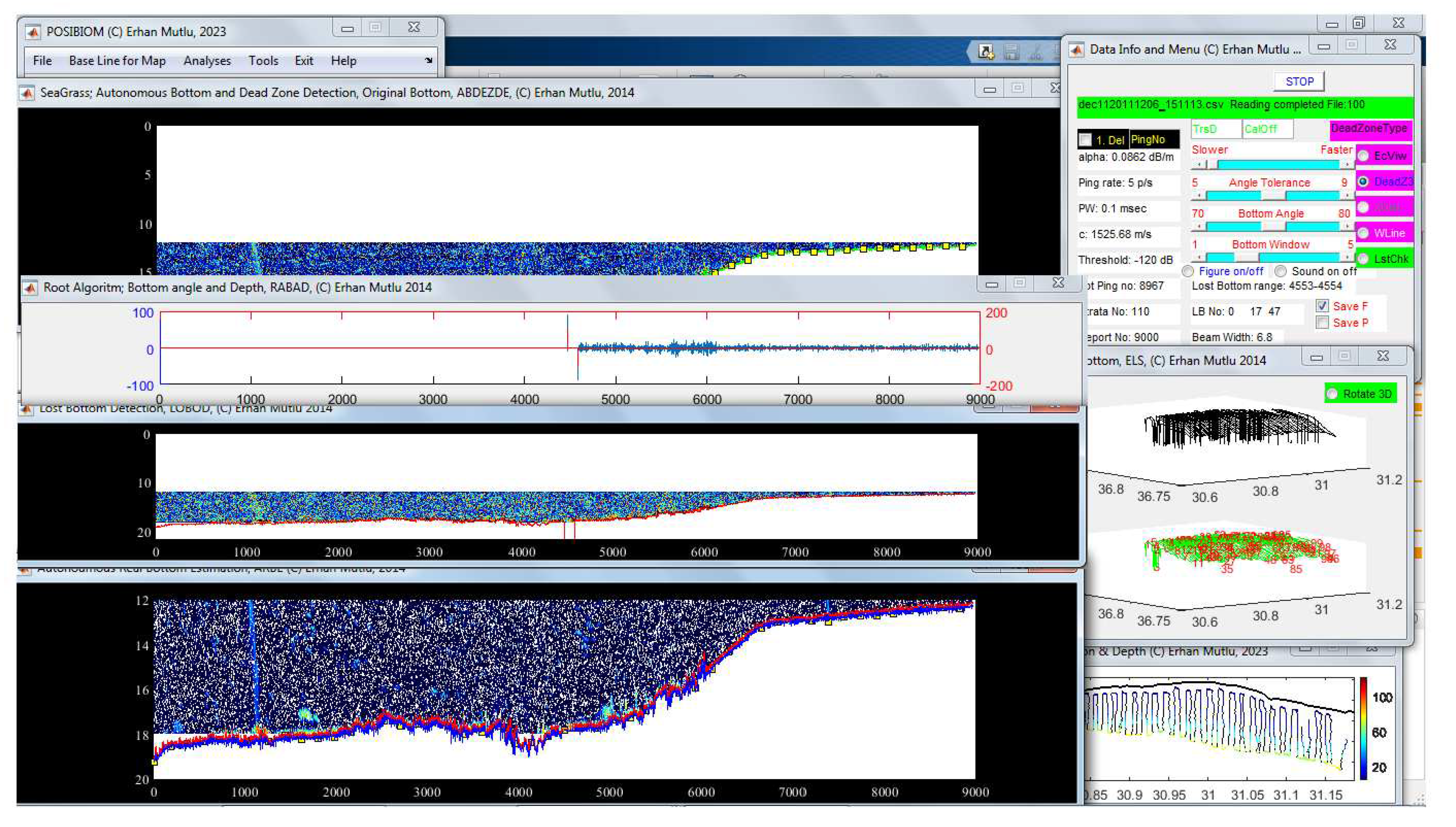
| Info variables | Description |
|---|---|
| alpha | Absorption coefficient |
| Ping rate | Pulse rate per second |
| PW | Pulse width |
| c | Sound speed |
| Threshold | Minimum data collection threshold |
| Tot ping no* | Total number of pings |
| Strata* | Number of stratum (vertical resolution) |
| Report No* | Number of reporting data (horizontal resolution) |
| Beam width | Angle of main lobe of beam |
References
- Colantoni, P.; Gallignani, P.; Fresi, E.; Cinelli, F. Patterns of Posidonia oceanica (L.) DELILE Beds around the Island of Ischia (Gulf of Naples) and in adjacent waters. PSZNI Mar. Ecol. 1982; 3(1), 53–74. [Google Scholar] [CrossRef]
- Brown, C.J.; Smith, S.J.; Lawton, P.; Anderson, J.T. Benthic habitat mapping: A review of progress towards improved understanding of the spatial ecology of the seafloor using acoustic techniques. Estuar. Coast. Shelf Sci. 2011, 92, 502–520. [Google Scholar] [CrossRef]
- Pal, D.; Hogland, W. An overview and assessment of the existing technological options for management and resource recovery from beach wrack and dredged sediments: An environmental and economic perspective. J. Environ. Manag. 2022, 302, 113971. [Google Scholar] [CrossRef] [PubMed]
- Boudouresque, C.F.; Meinesz, A. Découverte de l’herbier de Posidonies. Cahier Parc Nation Port-Cros. 1982, 4, 1–79. [Google Scholar]
- Mutlu, E.; Olguner, C.; Gökoğlu, M.; Özvarol, Y. Seasonal growth dynamics of Posidonia oceanica in a pristine Mediterranean gulf. Ocean. Sci. J. 2022, 57, 381–397. [Google Scholar] [CrossRef]
- Vacchi, M.; De Falco, G.; Simeone, S.; Montefalcone, M.; Morri, C.; Ferrar, M.; Bianchi, C.N. Biogeomorphology of the Mediterranean Posidonia oceanica seagrass meadows. Earth Surf. Proc. Land. 2017, 42, 42–54. [Google Scholar] [CrossRef]
- Spalding, M.; Taylor, M.; Ravilious, C.; Short, F.; Green, E. The distribution and status of seagrasses. In: Green, E.P.; Short, F. (Eds.), World Atlas of Seagrasses. University California Press, 2003, pp. 5–26.
- Aires, T.; Marbà, N.; Cunha, R.L.; Kendrick. G.A.; Walker, D.I.; Serrão, E.A.; Duarte, C.M.; Arnaud-Haond, S. Evolutionary history of the seagrass genus Posidonia. Mar. Ecol. Prog. Ser. 2011, 421, 117–130. [Google Scholar] [CrossRef]
- Bianchi, C.N.; Morri, C.; Chiantore, M.; Montefalcone, M.; Parravicini, V.; Rovere, A. Mediterranean Sea biodiversity between the legacy from the past and a future of change. In: Science N (ed) Life in the Mediterranean Sea: a look at habitat changes, Stambler, N.; Publishers, New York, 2012, pp 1–55.
- Planton, S.; Lionello, P.; Artale, V.; Aznar, R.; Carrillo, A.; Colin, J.; Congedi, L.; Dubois, C.; Elizalde, A.; Gualdi, S.; Hertig, E.; Jacobeit, J.; Jorda, G.; Li, L.; Mariotti, A.; Piani, C.; Ruti, P.; Sanchez-Gomez, E.; Sannino, G.; Sevault, F.; Somot, S.; Tsimplis, M. The climate of the Mediterranean region in future climate projections. In: Lionello P (ed) The climate of the Mediterranean region, from the past to the future. Elsevier, Amsterdam, 2012, pp 449–502.
- Pergent, G.; Bazairi, H.; Bianchi, C.N.; Boudouresque, C.F.; Buia, M.C.; Calvo, S.; Clabaut, P.; Harmelin-Vivien, M.; Mateo, M.A.; Montefalcone, M.; Morri, C.; Orfanidis, S.; Pergent-Martini, C.; Semroud, R.; Serrano, O.; Thibaut, T.; Tomasello, A.; Verlaque, M. Climate change and Mediterranean seagrass meadows: a synopsis for environmental managers. Mediterr. Mar. Sci. 2014, 15(2), 462–473. [Google Scholar] [CrossRef]
- Bernier, P.; Guidi, J.-B.; Biittcher, M.E. 1997. Coastal progradation and very early diagenesis of ultramafic sands as a result of rubble discharge from asbestos excavations (northern Corsica, western Mediterranean). Mar. Geol. 1997, 144, 163–175. [Google Scholar] [CrossRef]
- Peirano, A.; Damasso, V.; Montefalcone, M.; Morri, C.; Bianchi, C.N. Effects of climate, invasive species and anthropogenic impacts on the growth of the seagrass Posidonia oceanica (L.) Delile in Liguria (NW Mediterranean Sea). Mar. Pollut. Bull. 2005, 50, 817–822. [Google Scholar] [CrossRef]
- de Mendoza, F.P.; Fontolan, G.; Mancini, E.; Scanu, E.; Scanu, S.; Bonamano, S.; Marcelli, M. Sediment dynamics and resuspension processes in a shallow-water Posidonia oceanica meadow. Mar. Geol. 2018, 404, 174–186. [Google Scholar] [CrossRef]
- Bonamano, S.; Piazzolla, D.; Scanu, S.; Mancini, E.; Madonia, A.; Piermattei, V.; Marcell, M. Modelling approach for the evaluation of burial and erosion processes on Posidonia oceanica meadows. Estuar. Coast. Shelf Sci. 2021, 254, 107321. [Google Scholar] [CrossRef]
- Mateo-Ramírez, Á.; Marina, P.; Martín-Arjona, A.; Bañares-España, E.; García Raso, J.E.; Rueda, J.L.; Urra, J. Posidonia oceanica (L.) Delile at its westernmost biogeographical limit (northwestern Alboran Sea): Meadow features and plant phenology. Oceans 2023, 4, 27–48. [Google Scholar] [CrossRef]
- Mutlu, E.; Olguner, C.; Özvarol, Y.; Gökoğlu, M. Spatiotemporalbiometrics of Cymodocea nodosa in a western Turkish Mediterranean coast. Biologia, 2022, 77(3), 649–670. [CrossRef]
- Mutlu, E.; Duman, G.S.; Karaca, D.; Özvarol, Y. , Şahin, A. Biometrical Variation of Posidonia oceanica with different bottom types along the entire Turkish Mediterranean coast. Ocean Sci. J. 2023, 58, 9. [Google Scholar] [CrossRef]
- Lawton, J.H. What do species do in ecosystems? Oikos 1994, 71, 367–374. [Google Scholar] [CrossRef]
- Ingrosso, G.; Abbiati, M.; Badalamenti, F.; Bavestrello, G.; Belmonte, G.; Cannas, R.; Benedetti-Cecchi, L.; Bertolino, M.; Bevilacqua, S.; Bianchi, C.N.; et al. Mediterranean Bioconstructions Along the Italian Coast. Adv. Mar. Biol. 2018, 79, 61–136. [Google Scholar]
- Edgar, G.J.; Shaw, C. The production and trophic ecology of shallow-water fish assemblages in southern Australia. 3. General relationships between sediments, seagrasses, invertebrates and fishes. J. Exp. Mar. Biol. Ecol. 1995, 194, 107–131. [Google Scholar] [CrossRef]
- Buia, M.C.; Gambi, M.C.; Zupo, V. Structure and functioning of Mediterranean seagrass ecosystems: An overview. Biol. Mar. Mediterr. 2000, 7, 167–190. [Google Scholar]
- Mazzella, L.; Scipione, M.B.; Buia, M.C. Spatio-temporal distribution of algal and animal communities in a Posidonia oceanica (L.) Delile meadow. Mar. Ecol. 1989, 10, 107–129. [Google Scholar] [CrossRef]
- Francour, P. Fish assemblages of Posidonia oceanica beds at Port-Cros (France, NW Mediterranean): Assessment of composition and long-term fluctuations by visual census. Mar. Ecol. 1997, 18, 157–173. [Google Scholar] [CrossRef]
- Dauby, P.; Bale, A.J.; Bloomer, N.; Canon, C.; Ling, R.D.; Norro, A.; Robertson, J.E.; Simon, A.; Théate, J.M.; Watson, A.J.; et al. Particle fluxes over a Mediterranean seagrass bed: A one-year sediment trap experiment. Mar. Ecol. Prog. Ser. 1995, 126, 233–246. [Google Scholar] [CrossRef]
- Mateo, M.A.; Sanchez-Lizaso, J.L.; Romero, J. Posidonia oceanica “banquettes”: A preliminary assessment for an ecosystem carbon and nutrient budget. Mar. Ecol. Prog. Ser. 2003, 151, 43–45. [Google Scholar] [CrossRef]
- Guala, I.; Simeone, S.; Buia, M.C.; Flagella, S.; Baroli, M.; De Falco, G. Posidonia oceanica ‘banquette’ removal: Environmental impact and management implications. Biol. Mar. Mediterr. 2006, 13, 149–153. [Google Scholar]
- Fonseca, M.S.; Koehl, M.A.R.; Kopp, B.S. Biomechanical factors contributing to self-organization in seagrass landscapes. J. Exp. Mar. Biol. Ecol. 2007, 340, 227–246. [Google Scholar] [CrossRef]
- Den Hartog, C. Structure, function, and classification in seagrass communities. In: McRoy CP, Helfferich C (eds) Seagrass ecosystems: a scientific perspective. Marcel Dekker, New York, 1977, pp 89–121.
- Catucci, E.; Scardi, M. Modeling Posidonia oceanica shoot density and rhizome primary production. Sci. Rep. UK. 2020, 10, 16978. [Google Scholar] [CrossRef] [PubMed]
- Marba, N.; Duarte, C.M.; Holmer, M.; Martinez, R.; Basterretxea, G.; Orfila, A.; Jordi, A.; Tintore, J. Effectiveness of protection of seagrass (Posidonia oceanica) populations in Cabrera national park (Spain). Environ. Conserv. 2002, 29(04), 509–518. [Google Scholar] [CrossRef]
- Orth, R.; Carruthers, T.; Dennison, W.; Duarte, C.; Fourqurean, J.; Heck, K.L.; Hughes, A.R.; Kendrick, G.A.; Kenworthy, W.J.; Olyarnik, S.; Short, F.T.; Waycott, M.; Williams, S.L. A global crisis for seagrass ecosystems. Bioscience 2006, 56, 987–996. [Google Scholar] [CrossRef]
- Gobert, S.; Sartoretto, S.; Rico-Raimondino, V.; Andral, B.; Chery, A.; Lejeune, P.; Boissery, P. Assessment of the ecological status of Mediterranean French coastal waters as required by the water framework directive using the Posidonia oceanica rapid easy index: PREI. Mar. Pollut. Bull. 2009, 58(11), 1727–1733. [Google Scholar] [CrossRef]
- Pergent, G.; Pergent-Martini, C.; Boudouresque, C.F. Utilisation de l’herbier a Posidonia oceanica comme indicateur biologique de la qualite du milieu littoral en Mediterranee: Etat des connaissances. Mesogee, 1995, 54, 3–27. [Google Scholar]
- Mutlu, E.; Karaca, D.; Duman, G.S.; Şahin, A.; Özvarol, Y.; Olguner, C.; 2023b. Seasonality and phenology of an epiphytic calcareous red alga, Hydrolithon boreale, on the leaves of Posidonia oceanica (L) Delile in the Turkish water. Environ. Sci. Pollut. Res. 2023, 30, 17193–17213. [Google Scholar] [CrossRef]
- Gobert, S.; Lefebvre, L.; Boissery, P.; Richir, J. A non-destructive method to assess the status of Posidonia oceanica meadows. Ecol. Indicat. 2020, 119, 106838. [Google Scholar] [CrossRef]
- McGonigle, C.; Grabowski, J.H.; Brown, C.J.; Weber, T.C.; Quinn, R. Detection of deep water benthic macroalgae using image-based classification techniques on multibeam backscatter at Cashes Ledge, Gulf of Maine, USA. Estuar. Coast. Shelf Sci. 2011, 91, 87–101. [Google Scholar] [CrossRef]
- Jaubert, J.M.; Chisholm, J.R.M.; Minghelli-Roman, A.; Marchioretti, M.; Morrow, J.H.; Ripley, H.T. Re-evaluation of the extent of Caulerpa taxifolia development in the northern Mediterranean using airborne spectrographic sensing. Mar. Ecol. Prog. Ser. 2003, 263, 75–82. [Google Scholar] [CrossRef]
- Robinson, K.A.; Ramsay, K.; Lindenbaum, C.; Frost, N.; Moore, J.; Wright, A.P.; Petrey, D. Predicting the distribution of seabed biotopes in the southern Irish Sea. Continent. Shelf Res. 2011, 31, 120–131. [Google Scholar] [CrossRef]
- Mielck, F.; Bartsch, I.; Hass, H.C.; Wölfl, A.-C.; Bürk, D.; Betzler, C. Predicting spatial kelp abundance in shallow coastal waters using the acoustic ground discrimination system RoxAnn. Estuar. Coast. Shelf Sci. 2014, 143, 1–11. [Google Scholar] [CrossRef]
- Noiraksar, T.; Sawayama, S.; Phauk, S.; Komatsu, T. Mapping Sargassum beds off the coast of Chon Buri Province, Thailand, using ALOS AVNIR-2 satellite imagery. Bot. Mar. 2014, 57(5), 367–377. [Google Scholar] [CrossRef]
- Randall, J.; Hermand, J.-P.; Ernould, M.-E.; Ross, J.; Johnson, C. Measurement of acoustic material properties of macroalgae (Ecklonia radiata). J. Exp. Mar. Biol. Ecol. 2014, 461, 430–440. [Google Scholar] [CrossRef]
- Randall, J.; Johnson, C.R.; Ross, J.; Hermand, J.-P. Acoustic investigation of the primary production of an Australian temperate macroalgal (Ecklonia radiata) system. J. Exp. Mar. Biol. Ecol. 2020, 524, 151309. [Google Scholar] [CrossRef]
- Ware, S.; Anna-Leena, D. Challenges of habitat mapping to inform marine protected area (MPA) designation and monitoring: An operational perspective. Mar. Pol. 2020, 111, 103717. [Google Scholar] [CrossRef]
- Vis, C.; Hudon, C.; Carignan, R. An evaluation of approaches used to determine the distribution and biomass of emergent and submerged aquatic macrophytes over large spatial scales. Aquat. Bot. 2003, 77, 187–201. [Google Scholar] [CrossRef]
- Hossain, M.S.; Mazlan, H. Potential of Earth Observation (EO) technologies for seagrass ecosystem service assessments. Inter. J. Appl. Earth Observ. Geoinform. 2019, 77, 15–29. [Google Scholar] [CrossRef]
- McCarthy, E.; Sabol, B. Acoustic characterization of submerged aquatic vegetation: military and environmental monitoring applications. In: Oceans 2000 MTS/IEEE Conference and Exhibition. Providence, USA, 2000, pp. 1957–1961.
- Fakiris, E.; Zoura, D.; Ramfos, A.; Spinos, E.; Georgiou, N.; Ferentinos, G.; Papatheodorou, G. Object-based classification of sub-bottom profiling data for benthic habitat mapping. Comparison with sidescan and RoxAnn in a Greek shallow-water habitat. Estuar. Coast. Shelf Sci. 2018, 208, 219–234. [Google Scholar] [CrossRef]
- Dimas, X.; Fakiris, E.; Christodoulou, D.; Georgiou, N.; Geraga, M.; Papathanasiou, V.; Orfanidis, S.; Kotomatas, S.; Papatheodorou, G. Marine priority habitat mapping in a Mediterranean conservation area (Gyaros, South Aegean) through multi-platform marine remote sensing techniques. Front. Mar. Sci. 2022, 9, 953462. [Google Scholar] [CrossRef]
- van Rein, H.; Brown, C.J.; Quinn, R.; Breen, J.; Schoeman, D. An evaluation of acoustic seabed classification techniques for marine biotope monitoring over broad-scales (>1 km2) and meso-scales (10 m2-1 km2). Estuar. Coast. Shelf Sci. 2011, 93, 336–349. [Google Scholar] [CrossRef]
- Urick, R.J. Principles of Underwater Sound. Third edition. Peninsula, California, 2013.
- Simmonds, J.; Maclennan, D. Fisheries acoustics: theory and practice. 2nd edition, Blackwell publishing, 2005, 456.
- Depew, D.C.; Stevens, A.W.; Smith, R.E.H.; Hecky, Rt.E. Detection and characterization of benthic filamentous algal stands (Cladophora sp.) on rocky substrata using a high-frequency echosounder. Limnol. Oceanogr. Methods, 2009, 7, 693–705. [Google Scholar] [CrossRef]
- Monpert, C.; Legris, M.; Noel, C.; Zerr, B.; Caillec, J.M.L. Studying and modeling of submerged aquatic vegetation environments seen by a single beam echosounder. Proceedings of Meetings on Acoustics: Acoustical Society of America, 2012, 17, 070044. [Google Scholar] [CrossRef]
- Llorens-Escrich, S.; Tamarit, E.; Hernandis, S.; Sánchez-Carnero, N.; Rodilla, M.; Pérez-Arjona, I.; Moszynski, M.; Puig-Pons, V.; Tena-Medialdea, J.; Espinosa, V. Vertical configuration of a side scan sonar for the monitoring of Posidonia oceanica meadows. J. Mar. Sci. Engineer. 2021, 9, 1332. [Google Scholar] [CrossRef]
- Shao, H.; Minami, K.; Shirakawa, H.; Kawauchi, Y.; Matsukura, R.; Tomiyasu, M.; Miyashita, K. Target strength of a common kelp species, Saccharina japonica, measured using a quantitative echosounder in an indoor seawater tank. Fish. Res. 2019, 214, 110–116. [Google Scholar] [CrossRef]
- Minami, K.; Kita, C.; Shirakawa, H.; Kawauchi, Y.; Shao, H.; Tomiyasu, M.; Iwahara, Y.; Takahara, H.; Kitagawa, T.; Miyashita, K. Acoustic characteristics of a potentially important macroalgae, Sargassum horneri, for coastal fisheries. Fish. Res. 2021, 240, 105955. [Google Scholar] [CrossRef]
- Mutlu, E.; Balaban, C. New algorithms for the acoustic biomass estimation of Posidonia oceanica: a study in the Antalya gulf (Turkey). Fresen. Environ. Bull. 2018, 27(4), 2555–2561. [Google Scholar]
- Olguner, C.; Mutlu, E. Acoustic estimates of leaf height and biomass of Posidonia oceanica meadow in Gulf of Antalya, the eastern Mediterranean. COMU. J. Mar. Sci. Fish. 2020, 3(2), 79–94.
- Mutlu, E.; Olguner, C. Acoustic scattering properties of seagrass: In/ex situ measurements of Posidonia oceanica. Medit. Mar. Sci. 2023, 24(2), 272–291. [Google Scholar] [CrossRef]
- Mutlu, E.; Olguner, C. Acoustic scattering properties of a seagrass, Cymodocea nodosa: In-situ measurements. Bot. Mar. 2023 (under revision).
- Mutlu, E.; Olguner, C. Density-depended acoustical identification of two common seaweeds (Posidonia oceanica and Cymodocea nodosa) in the Mediterranean Sea. Thalassas. 2023. [Google Scholar] [CrossRef]
- Bakiera, D.; Stepnowski, A. Method of the sea bottom classification with a division of the first echo signal. Proceedings of the XIIIth Symposium on Hydroacoustics. 1996, 55–60. [Google Scholar]
- Bezdek, J.C. Pattern Recognition with Fuzzy Function Algorithms. Plenum Press. New York. 1981, 43–93. [Google Scholar]
- Burczynski, J. Bottom classification. BioSonics Inc. 4027 Leary Way NW, Seattle WA, 98107, USA. 1999, 14p.
- Stepnowski, A.; Moszynski, M.; Komendarczyk, R.; Burczynski, J. Visual real-time Bottom Typing System (VBTS) and neural networks experiment for seabed classification. Proceedings of the 3rd European Conference on Underwater Acoustics, Heraklion, Crete. 1996, 685-690.
- Chivers, R.C. New acoustic processing for underway surveying. The Hydrographic Journal. 1990, 56, 9–17. [Google Scholar]
- Orlowski, A. Application of multiple echoes energy measurements for evaluation of sea bottom type. Oceanologia. 1984, 19, 61–78. [Google Scholar]
- Pouliquen, E.; Lurton, X. Sea-bed identification using echo-sounder signals. Proceedings of the European Conference on Underwater Acoustics. Elsevier Applied Science, London and New York, 1992, 535-539.
- Tegowski, J. Acoustical classification of the bottom sediments in the southern Baltic Sea. Quaternary International. 2005, 130, 153–161. [Google Scholar] [CrossRef]
- Kruss, A.; Blondel, Ph.; Tegowski, J. Acoustic properties of macrophytes: comparison of single-beam and multibeam imaging with modeling results. Proceedings of the 11th European Conference on Underwater Acoustics. 2012, 168–175. [Google Scholar]
- Maceina, M.J.; Shireman, J.V. The use of a recording fathometer for determination of distribution and biomass of hydrilla. J. Aquat. Plant Manag. 1980, 18, 34–39. [Google Scholar]
- Winfield, I.; Onoufriou, C.; O’Connel, M.; Godlewska, M.; Ward, R.; Brown, A.; Yallop, M. Assessment in two shallow lakes of a hydroacoustic system for surveying aquatic macrophytes. Hydrobiologia. 2007, 584, 111–119. [Google Scholar] [CrossRef]
- Mutlu, E.; Balaban, C.; Gokoglu, M.; Ozvarol, Y.; Olguner, M.T. Acoustical density-dependent calibration of the dominant sea meadows and seagrasses and monitoring of their distribution. TUBITAK, Ankara, TUBITAK Project Final Report 110Y232, 2014, p 368.
- Mutlu, E.; Özvarol, Y.; Şahin, A.; Duman, G.S.; Karaca, D. Acoustical determination of biomass quantities and monitoring of distribution of Posidonia oceanica meadows on the Turkish entire coasts in the Eastern Mediterranean. TUBITAK, Ankara, TUBITAK Project Final Report, 117Y133, 2020, p 190.
- Marine Region. 20 March 2023. Available online: https://marineregions.org/accessed in March.
- EchoView. 20 December 2022. Available online: https://support.echoview.com/WebHelp/RH8_Popups/Phenomena/ Acoustic_beam_dead _zone_referenced.htm#:~:text=The%20deadzone %20is%20an%20area,areas%20with%20steep%20bottom%20topographyaccessed in December.
- Mello, L.G.S.; Rose, G.A. The acoustic dead zone: theoretical vs. empirical estimates, and its effect on density measurements of semi-demersal fish. ICES J. Mar. Sci. 2009, 66, 1364–1369. [Google Scholar] [CrossRef]
- De Robertis, A. ; Higginbottom,, I. A post-processing technique to estimate the signal-to-noise ratio and remove echosounder background noise. ICES J. Mar. Sci, 2007; 64(6), 1282–1291. [Google Scholar]
- Lee, W.S.; Lin, C.Y. Mapping of tropical marine benthic habitat: Hydroacoustic classification of coral reefs environment using single-beam (RoxAnn™) system. Continen. Shelf Res. 2018, 170, 1–10. [Google Scholar] [CrossRef]
- Lurton, X. An Introduction to Underwater Acoustics. Springer, 2002.
- Quintino, V.; Freitas, R.; Mamede, R.; Ricardo, F.; Rodrigues, A.M.; Mota, J.; Pe’rez-Ruzafa, A.; Marcos, C. Remote sensing of underwater vegetation using single-beam acoustics. ICES J. Mar. Sci. 2010, 67, 594–605. [Google Scholar] [CrossRef]
- Canals, M.; Ballesteros, E. Production of carbonate particles by phytobenthic communities on the Mallorca-Menorca shelf, northwestern Mediterranean Sea. Deep-Sea Res. Part II. 1997, 44, 611–629. [Google Scholar] [CrossRef]
- Terrados, J.; Medina-Pons, F.J. Inter-annual variation of shoot density and biomass nitrogen and phosphorus content of the leaves and epiphyte load of the seagrass Posidonia oceanica (L.) Delile off Mallorca Western Mediterranean. Sci. Mar. 2011, 75, 61–70. [Google Scholar] [CrossRef]
- Sghaier, Y.R.; Zakhama-Sraieb, R.Y.M.; Charfi-Cheikhrouha, F. Patterns of shallow seagrass (Posidonia oceanica) growth and flowering along the Tunisian coast. Aquat. Bot. 2013, 104, 185–192. [Google Scholar] [CrossRef]
- Mavko, G.; Mukerji, T.; Dvorkin, J. The rock physics handbook. Cambridge University Press. 1998. [Google Scholar]
- Merriam, C.O. Depositional history of lower Permian (Wolfcampian - Leonardian) carbonate buildups, Midland Basin, Upton County, Texas. M.S. thesis, Texas A&M University. 1999.
- Aleman, P.B. Acoustic impedance inversion of lower Permian carbonate buildups in the Permian Basin, Texas. Master Thesis, . The Office of Graduate Studies of Texas A&M University, 2004, 99.
- Mateo, M.A.; Romero, J.; Perez, M.; Littler, M.M.; Littler, D.S. Dynamics of millenary organic deposits resulting from the growth of the Mediterranean seagrass Posidonia oceanica. Estuar. Coast. Shelf Sci. 1997, 44, 103–110. [Google Scholar] [CrossRef]
- Enriquez, S.; Schubert, N. Direct contribution of the seagrass Thalassia testudinum to lime mud production. Nature Communications, 2004; I, 5, 3835. [Google Scholar]
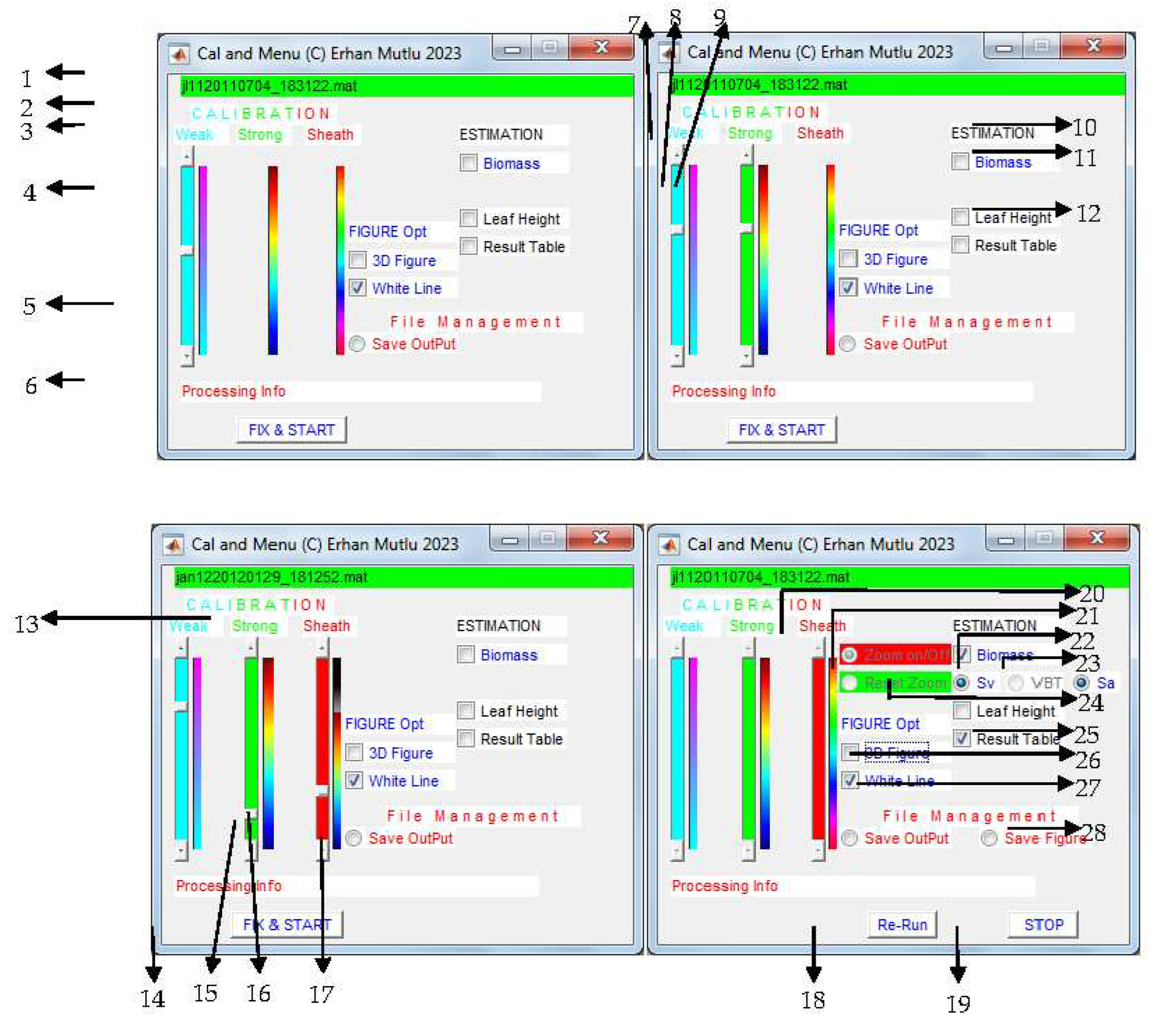
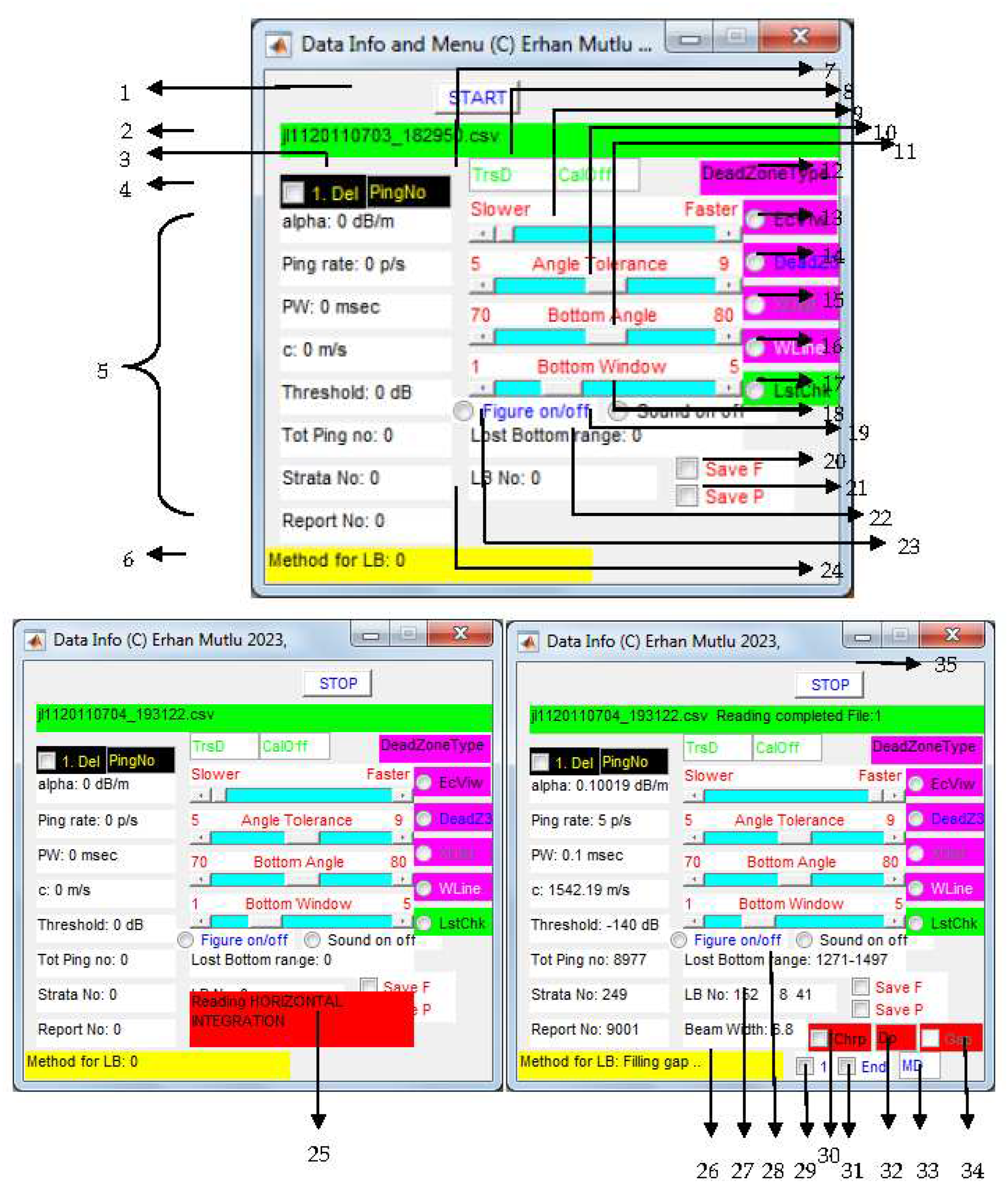
| No | Function | Case | Recommendation | Troubleshooting/negatives |
|---|---|---|---|---|
| 1 | Starting processing | First configure setting | Need to press START button after every 100 files processed | |
| 2 | Input data file name for process | |||
| 3 | Deletion of pings from the beginning | No bottom echo Bottom misestimated at the first ping |
Enter last ping no to delete | Possible to estimate wrong bottom |
| 4 | To delete the pings | Check the box | ||
| 5 | Data info of the acoustical file | Fixed during the data collection | See Appendix 4 | |
| 6 | Info for processing methods to estimate correct bottom | Auto-decision for cases (chirping, running average, filling gap) Process in cycle |
Allow auto-decision as long as possible Auto-switching to block solution in time |
Takes time; 1. for block lost bottoms 2. Single ping lost bottom in cycle |
| 7 | Transducer depth | Deployment Below surface | Required for real depth | Enter 0 if transducer at surface |
| 8 | Calibration offset | Deviation in echosounder calibration from reference ball | Acoustic data correction If less, + difference If more, - difference |
If not, uncorrected data Possible to misestimate biomass |
| 9 | Manual intervention to speed up the process | Time consuming more than usual Takes time more than 10 mins |
Step-to-step slide forward until ‘Lost bottom range’ changes (see 28) | Speed up immediately faster resulted in misestimated bottom in some case |
| 10 | ± Bottom angle | Steepness of bottom | Use default setting Too steep, adjust with bottom angle together |
Suboptimum setting detects wrong bottom |
| 11 | Bottom angle | Cliff bottom No bottom echo, too deep |
Use default setting Increase angle at highly rough sea Increase or decrease angle to estimate the deepest depth |
Strong scatterers estimated as bottom |
| 12 | Label for dead zone estimation by 3 methods | Method of the present study embedded (see Figure 1). | Obligation | Angle estimation based on ping-to-ping, not distance |
| 13 | Echo View’s dead zone | Optional, EchoView [77] |
Click for future process | Works well if GPS reports every ping |
| 14 | Alternative dead zone | Optional, Mello and Rose [78] |
Click for future process | Works well, regardless of GPS reporting every ping |
| 15 | Calculation of dead zone based on GPS coordinate distance | Disabled due to its troubleshooting | The GPS reports geographical coordinates once every one second, not every ping | |
| 16 | White line | Optional, white area between bottom depth and dead zone | optional | |
| 17 | Last check to estimate real bottom last time | Misestimated bottom still available after the process | Optional if necessary | If bottom echo is weak, change the estimated bottom completely |
| 18 | ± next bottom tracking range | Steepness of cliff bottom | Narrowing and widening the window | Jump to the strong scatterers |
| 19 | Sounding on/off the angles | Slowing the process | ||
| 20 | Saving the output data | Use for the next algorithm | optional | If step up to the next analysis, must be on |
| 21 | Saving the enhanced echogram by the corrected bottom | Optional for see the results later | Check button when user does not monitor the process | |
| 22 | Current ping range of lost bottom detected | |||
| 23 | Showing root algorithm to detect availability of lost bottom | To see goodness of the estimation of correct bottom | optional | Slowing the process |
| 24 | Current number of lost bottom detected, Loop for auto-decision of lost-bottom correction |
|||
| 25 | Showing progress status in reading the data | |||
| 26 | See 6 | |||
| 27 | See 24 | |||
| 28 | See 22 | |||
| 29 | Recovery of the real bottom (see 31, 33) from the beginning of the data to 500 pings ahead | Bottom detected too shallow or too deep | Useful for the cases | Appear after data reading complete Misrecognize the water column depth as bottom if strong scattering layer exist above the depth |
| 30 | “Chirping” method for manual intervention to recover real bottom in lost bottom range | Time consuming more than usual Takes time more than 10 mins Lost bottom range in cycle and same ping values (see 9, 22, 28) |
Useful for the cases looking at function 27, 28 in cycle | Selection of the water column depth close to the bottom if strong scattering layer exist above the depth, resulted in misestimated bottom in some case (see 32) |
| 31 | Recovery of the real bottom (see 29, 33) from the last data to 500 pings backward | Bottom detected too shallow or too deep | Useful for the cases | Appear after data reading complete Selection the water column depth close to the bottom if strong scattering layer exist above the depth |
| 32 | Water column depth for cases in 30 | Bottom detected too shallow or too deep | Useful for the cases | Appear after a certain number of iteration of the loop in function no 22, 27, but early view when sliding the speed (no 9) toward the faster, Selection of the water column depth close to the bottom if strong scattering layer exist above the depth (see 30) |
| 33 | Water column depth for recovery of the real bottom (see 29, 31) | Bottom detected too shallow or too deep | Useful for the cases | Appear after data reading complete Selection of the water column depth close to the bottom if strong scattering layer exist above the depth |
| 34 | “Filling Gap” method for manual intervention to recover real bottom in lost bottom range | Disabled | Not used currently | |
| 35 | Stopping the analysis | User stops the analysis | Do not stop if misestimating bottom depth in some data files, process again later when all files completed |
Stop after the current file processing is finished |
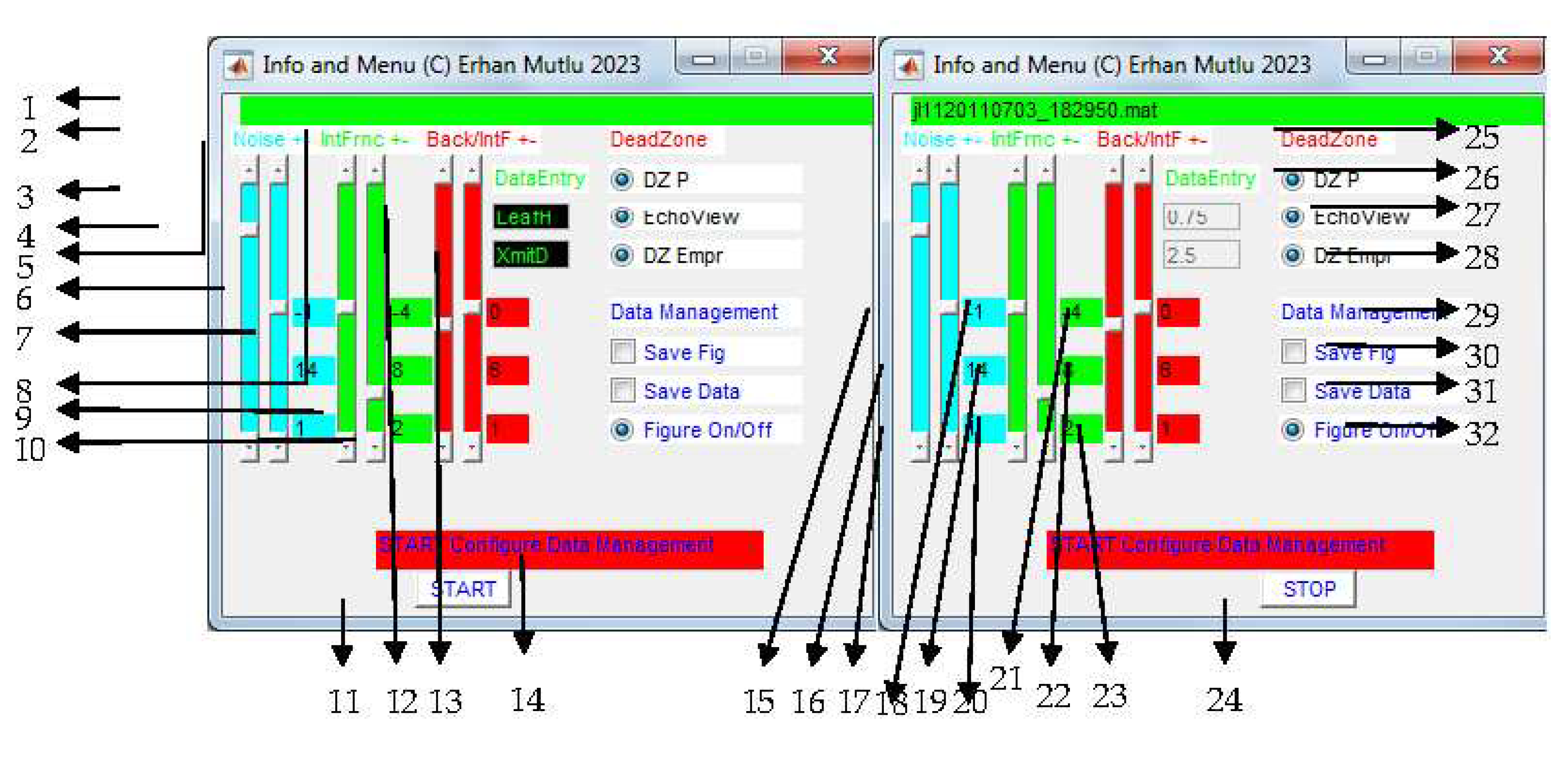
| No | Function | Case | Recommendation | Troubleshooting/negatives |
|---|---|---|---|---|
| 1 | Data file name to read | Label name | ||
| 2 | Background noise ± | label | ||
| 3 | Background noise threshold | Could adjust threshold during analysis if noise not organized well for removal | Slide up and down accordingly | Depending on background noise data measured in listening mode and signal-to-noise ratio estimated (S/N) Possible spatiotemporal changes in the noise measurements |
| 4 | ± background noise threshold for removal | threshold insufficient to detect background noise | Slide up and down to increase and decrease range of the threshold fixed (see 3) | The faster adjustment the less targets (seagrass) |
| 5 | Interference & reverberation noise ± | Label name | ||
| 6 | Interference & reverberation noise threshold | Could adjust threshold during analysis if such noise not detected well | Slide up and down accordingly | Depending on Interference & reverberation noise data measured in listening mode Spatiotemporal changes in the noise measurements |
| 7 | ± Interference & reverberation noise threshold for removal of spurious targets | threshold insufficient to detect Interference & reverberation noise | Slide up and down to increase and decrease range of the threshold fixed (see 6) | The faster adjustment the less targets (seagrass) If no noise data file for Interference & reverberation noise, background noise data file needed |
| 8 | background noise /interference & reverberation noise ratio ± | Label name | ||
| 9 | background noise /interference & reverberation ratio | Alternative method for removal such noises Could adjust threshold during analysis if noise not detected well |
Slide up and down accordingly looking at echogram | Depending on both background and Interference & reverberation noise data measured in listening mode Spatiotemporal changes in the noise measurements |
| 10 | ± background noise /interference & reverberation noise threshold for removal | threshold insufficient to detect Interference & reverberation noise | Slide up and down to increase and decrease range of the threshold fixed (see 9) | The faster adjustment the less targets (seagrass) If no noise data file for Interference & reverberation noise, background noise data file needed |
| 11 | Start analysis | Start button | After all setting done in data entry (see 12, 13) | Cannot change the data entry after functioning (see 12, 13) |
| 12 | Canopy height of seagrass | Change the default setting if necessary | First look at the canopy height at maxima in the echogram for a survey | Need a rough value, but not more than 1 m After starting analysis the entry disabled |
| 13 | Transducer depth at draft of R/V | Deployment depth below surface | Measurement for real bottom depth | Enter 0 if transducer at surface After starting analysis the entry disabled |
| 14 | info to start or stop | Label name | ||
| 15 | Info for lower limit of background S/N ratio | No detection still if correct S/N threshold set up | Use 3 and 4 in the case | Upper limit setting not independent |
| 16 | Info for background S/N ratio | No detection still if correct S/N threshold set up | Use 3 and 4 in the case | Lower and upper limit setting not independent |
| 17 | Info for upper limit of background S/N ratio | No detection still if correct S/N threshold set up | Use 3 and 4 in the case | lower limit setting not independent |
| 18 | Info for lower limit of Interference & reverberation S/N ratio | No detection still if correct S/N threshold set up | Use 6 and 7 in the case | Upper limit setting not independent |
| 19 | Info for Interference & reverberation S/N ratio | No detection still if correct S/N threshold set up | Use 6 and 7 in the case | Lower and upper limit setting not independent |
| 20 | Info for upper limit of Interference & reverberation S/N ratio | No detection still if correct S/N threshold set up | Use 6 and 7 in the case | lower limit setting not independent |
| 21 | Info for background noise /interference & reverberation S/N ratio | No detection still if correct S/N threshold set up | Use 9 and 10 in the case | Upper limit setting not independent |
| 22 | Info for background noise /interference & reverberation S/N ratio | No detection still if correct S/N threshold set up | Use 9 and 10 in the case | Lower and upper limit setting not independent |
| 23 | Info for upper limit of background noise /interference & reverberation S/N ratio | No detection still if correct S/N threshold set up | Use 9 and 10 in the case | lower limit setting not independent |
| 24 | Stopping the analysis | User wants to stop the analysis | Do not stop if misestimated noise removal in some data files, process that later again when all files completed |
Stop after the current file processing, not immediately |
| 25 | Dead Zone and methods | Label name | ||
| 26 | Dead zone estimated by the present study | Optional, Present study, |
Click on to involve that into average Dead Zone calculation, or not | Angle estimation based on ping-to-ping, not distance, Need to include the most precise methods of dead zone for average, At least one of three methods required for the next analysis |
| 27 | Echo View’s dead zone | Optional, EchoView [77] |
If available in the input data, option enable | Works well if GPS reports every ping, At least one of them required for the next analysis |
| 28 | Alternative dead zone | Optional, Mello and Rose [78] |
If available in the input data, option enable | Works well, regardless of GPS reporting every ping, At least one of them required for the next analysis |
| 29 | Data management to save output data, or figure, or to show S/N solution figures | Label name | ||
| 30 | Saving the enhanced echogram by the corrected bottom | Optional to see the results later | Check button when user does not monitor the process | |
| 31 | Saving the processed data | Use for the next algorithm | optional | If stepping to the next analysis, must be on |
| 32 | Only S/N solution figures | To show the figures | optional | Slow down the analysis |
| No | Function | Case | Recommendation | Troubleshooting/negatives |
|---|---|---|---|---|
| 1 | Current input file name on process | Updating by file to file | ||
| 2 | Title for removal of spurious weak and strong scatterers, and sheaths | label | ||
| 3 | Label of weak scattering removal | label | ||
| 4 | Calibration threshold setting to remove weak scatterers | High background noise, some weak zooplankton layers | Adjust neither less nor more looking at the enhanced echogram | A good calibration data for showing clear seagrass |
| 5 | Color scale to guide user to slide up or down for weak scatterers removal | colorbar | Colors cannot fit to the acoustical data echogram in some cases, looking at the echogram | |
| 6 | current info while processing the data | Strong scattering removal takes time | ||
| 7 | Label of strong scattering removal | label | ||
| 8 | Calibration threshold setting to remove strong scatterers | Reverberation, interference, Fishes and schools, compact zooplankton layers | Adjust neither less nor more looking at the enhanced echogram | A good calibration data needed for showing clear seagrass, appears after function 4 completed |
| 9 | Color scale to guide user to slide up or down for strong scatterers removal | colorbar | Colors cannot fit to the acoustical data echogram in some cases, looking at the echogram | |
| 10 | Title to show results in figure and table of the estimation | label | ||
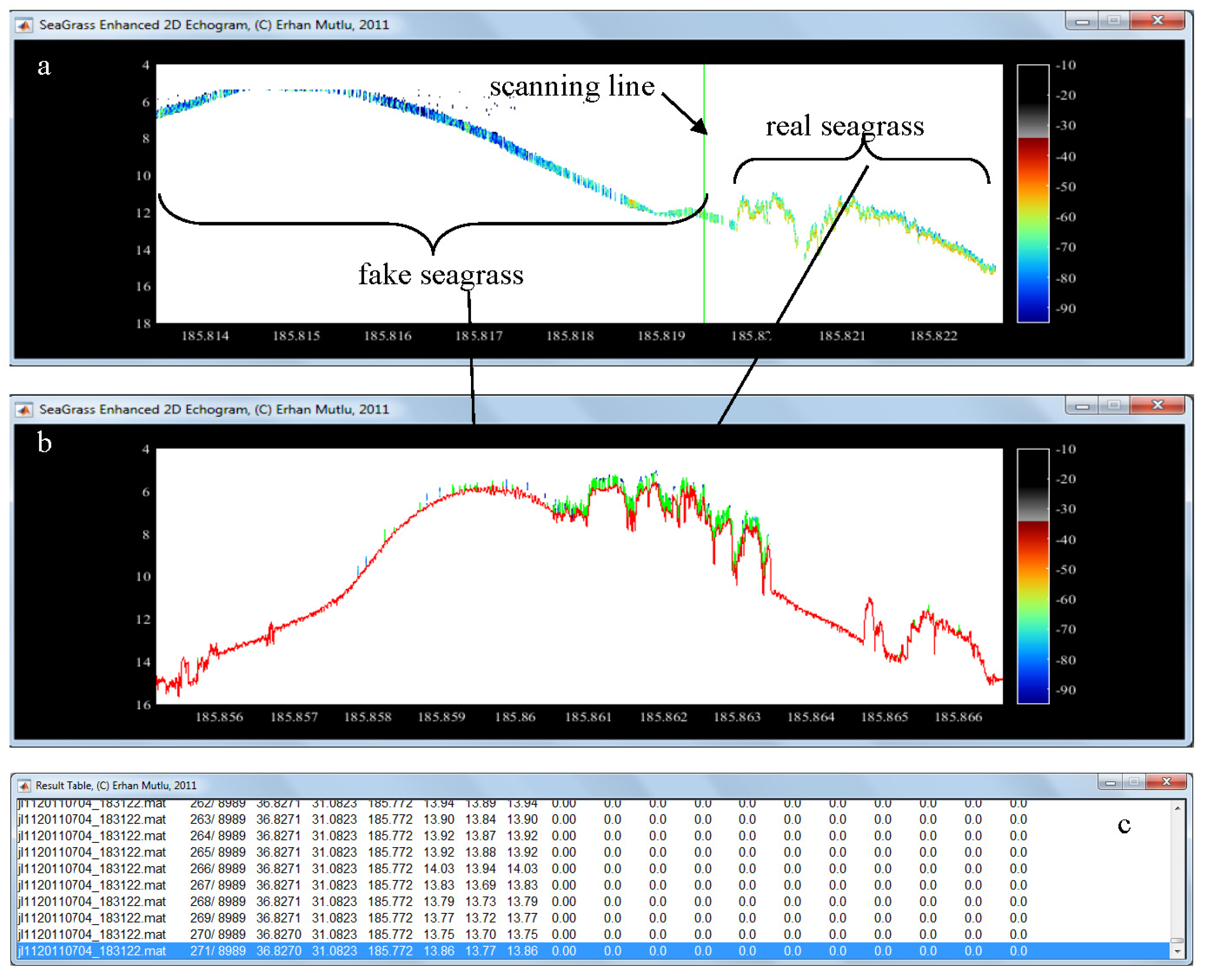
| 11 | Showing figure for estimated biomass on map with trackline | Optional, | Use “tools” after the analysis completed | Slowing the analyses, trackline width constant |
| 12 | Showing figure for estimated leaf (canopy) length on map with trackline | Optional, | Use “tools” after the analysis completed | Slowing the analyses, trackline width constant |
| 13 | Label of sheaths fixation and removal | label | ||
| 14 | Fixing the settings and then start the analysis | All settings completed | Functions in 20 and 21 will be disabled after the start, Press START button after every 100 files processed |
|
| 15 | Calibration threshold setting to fix and remove the sheath | Calibration to fix sheath threshold | Adjust neither less nor more looking at the enhanced echogram until sheath disappear, Fix threshold ligule if sheath too short in time, use functions 20 and 21, if not well fixing settings, restart from function 4 |
A good calibration data needed for showing clear seagrass with sheaths, too short sheath could not be detected in time of year, appears after function 8 completed |
| 16 | Color scale to guide user to slide up or down for fixing and removing sheath | colorbar | Colors cannot fit to the acoustical data echogram in some cases, looking at the echogram | |
| 17 | Saving outputs into file in *.xls format | For mapping the biomass and canopy height later | Use the” tools” or other mapping software | Three different biomass estimation, some could be different from each other |
| 18 | Re-start the analysis from the beginning | Not well works for the estimates | Use the option when case needed | All settings needed from the beginning |
| 19 | Stopping the analysis | User stops the analysis | Use in case of function 18 | Stop after the current file completed, not immediately |
| 20 | Zooming on the main echogram | Fixing sheaths better for large file | Use the options | No way for zoom back to previous appearance, Use function 19, Disabled after starting analysis |
| 21 | Zoom back to original size of the echogram | To select better sheaths on the echogram | Use the option when case needed | Disabled after starting analysis |
| 22 | Method VBT to estimate biomass | Disabled | Need of the VBT software | |
| 23 | Method to map estimate biomass based on Sa | Only estimates using regression between biomass and Sa | use if necessary | Slowing down the processing, appear only after checking function 11, Estimates (Sa and Sv) always available in the output file |
| 24 | Method to map estimate biomass based on Sv | Only estimates using regression between biomass and Sv | Use if necessary | Slowing down the processing, appear only after checking function 11, Estimates (Sa and Sv) always available in the output file |
| 25 | Show the complex estimates with many variables in a scrolling Table | optional | optional | Slowing down the processing, Click off/on not to show the results |
| 26 | Show 3D curtain enhanced echogram | optional | optional | Slowing down the processing, |
| 27 | White line on 2D echogram | Optional, white area between bottom depth and dead zone | optional | Not in 3D echogram |
| 28 | Save figures for mapping biomass or leaf height | When function 11 or 12 checked, respectively or both | Optional, use the “tools” or other mapping software later | Saving after finishing all files processing, see function 11 and 12, appear only after checking function 11 or 12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





