1. Introduction
The industrial revolution, rising per capita consumption, and population growth have all contributed to a sharp rise in global energy demand. [
1,
2,
3,
4] Around 80 % of the world's energy production comes from conventional or nonrenewable sources like coal and petroleum. Overuse of these fossil fuels harms both the environment and global economies. Burning coal and petroleum-based products releases harmful gases like CO
2, CH
4, and N
2O into the atmosphere, which has detrimental effects on both the environment and human health. Research into using eco-friendly and renewable energy sources has been intense due to the shortage and environmental issues caused by the use of fossil fuels. One of the key strategies for the global science and technology communities in response to the increase in energy demand is the development of clean, sustainable, and efficient energy storage and conversion technologies using renewable energy sources. Researchers and academics look for novel, functionalized advanced nanomaterials in a variety of dimensions that can support integrated energy storage and conversion processes that strike a balance between economic growth and environmental responsibility. [
5,
6,
7]
According to a power survey, there is insufficient energy storage, which results in the loss of about 30 % of the power that is generated. To solve these issues, it is crucial to create high-performance, substantial energy storage systems. [
8,
9] Alternative energy storage technologies include pumped hydroelectric storage, compressed air storage, flywheels, hydrogen storage, thermal energy storage, superconducting magnetic energy storage, and electrochemical energy storage. Industrial and automotive applications, including those for electric vehicles and the military, are paying more attention to electrochemical energy sources like batteries and electrochemical capacitors (ECs). [
10,
11] Batteries are the preferred option for applications requiring high energy density, despite their limited power output. In applications that call for high power but low energy density, conventional electrolytic capacitors are preferred. Research on new kinds of effective energy storage systems, such as electrochemical capacitors or supercapacitors, has been sparked by this (SCs). [
12] Future generations of energy storage devices will likely favor supercapacitors because of their superior specific capacitance, high power density, long lifespan, quick charge-discharge rate, excellent circulation features, low cost, and safety.
In a supercapacitor, the type of electrolyte and electrode surface area determines how much charge can be stored overall, whereas the size of the electrode and the total active mass are the limiting factors for the total charge that can be stored in a battery. Supercapacitors operate similarly to traditional capacitors. Supercapacitors have higher capacitance (F/g, F/cm
2) because their electrodes have a large surface area. Supercapacitors have a long-life cycle of about 5,00,000 cycles and can store 10-100 times more energy per unit volume than other rechargeable batteries. [
9,
13] In addition, SCs have a wider range of capacitance (12700 F) than conventional electrolytic capacitors, a lower equivalent series resistance (ESR 1/10th of electrolytic capacitor), and long cycle stability. [
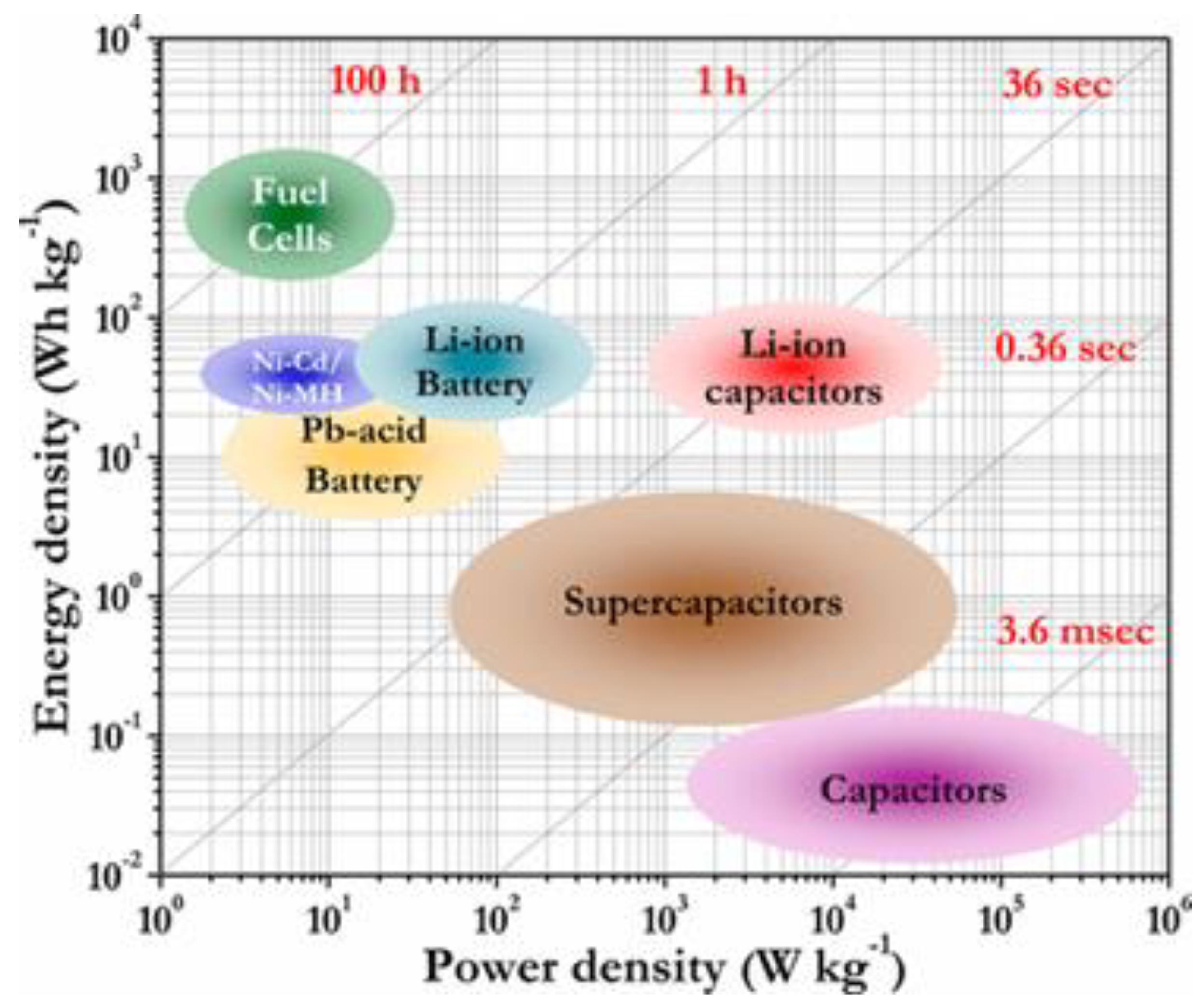
14] Consequently, as shown in the Ragone plot in
Figure 1, supercapacitors can act as a link between conventional capacitors and secondary ion batteries.
New electrolytes and electrode materials can increase the energy density of supercapacitors. Active electrode materials are suitable for supercapacitors, such as activated carbon, carbon nanotubes (CNTs), graphene, transition metal oxides, conducting polymers, etc. Different types of electrolytes, such as aqueous, non-aqueous, polymer-based, and ionic liquid-based electrolytes, can be used in supercapacitor devices. [
8] Active electrode materials play a crucial role in determining the performance of supercapacitors in terms of energy and power density, capacitance, and stability. Materials with high specific capacitance, such as activated carbon or transition metal oxides, can significantly enhance the energy density of supercapacitors, enabling them to store more energy per unit of mass or volume. Materials with high electrical conductivity are important for achieving high power density.
Capacitance and stability depend on the specific surface area and pore structure of the active electrode material. The redox properties of the active electrode materials determine the electrochemical performance of supercapacitors. Pseudocapacitive materials, such as metal oxides, hydrogens, or conducting polymers, undergo fast and reversible faradaic reactions at the electrode-electrolyte interface, enabling higher energy storage capacities than non-faradaic processes. The choice of active electrode materials affects the specific redox reactions, reversibility of the reactions, and charge transfer kinetics, thereby impacting the overall electrochemical performance of the supercapacitor. Active electrode materials significantly influence the cycling stability and lifespan of supercapacitors. Robust electrode materials with good mechanical stability and chemical resistance are necessary to ensure long-term performance and retain the supercapacitor's capacitance over a large number of cycles.
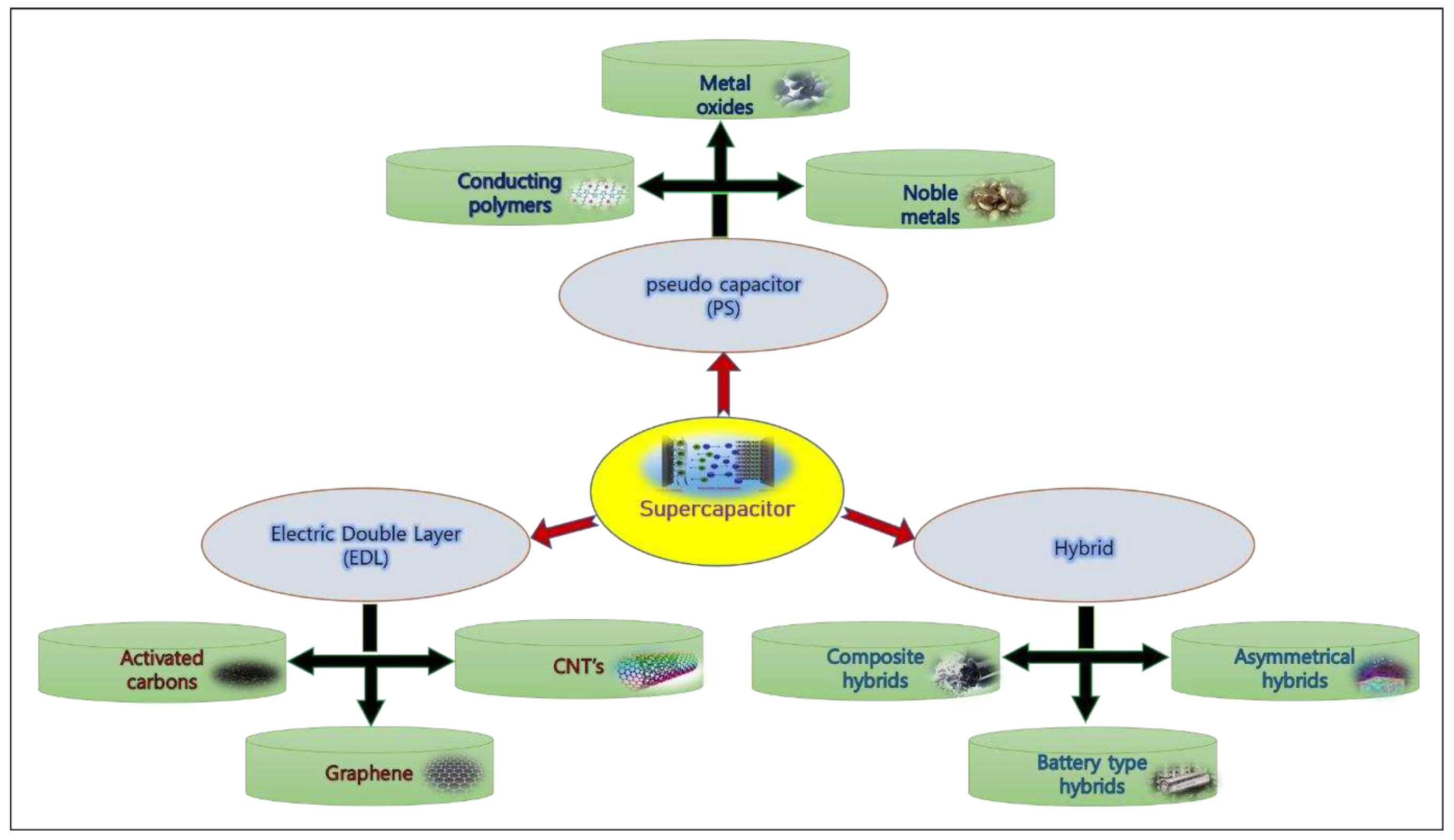
2. Classification of Supercapacitor Based on the energy storage mechanism
Two electrodes, the electrolyte and the separator, which are submerged in the electrolyte, make up supercapacitors. Supercapacitors may be classified into three groups based on their means of storing energy as shown in
Figure 2. electrochemical double-layer capacitors (EDLCs), pseudo-capacitors, and hybrid systems. The electrostatic interaction between ions on the substantial specific surface area of active electrode materials and electrolytes is the basis for the energy storage mechanism of EDLCs. The fast and reversible Faradaic redox reaction between the electrode materials and the electrolyte ions near the surface is the energy storage mechanism for pseudocapacitors. Pseudocapacitors typically have higher capacitance per gram than EDLCs, but their kinetics are slower because, in a pseudocapacitor, the energy storage process takes place both in the bulk of the electrode materials and on their surface, whereas in an EDLC, the charge/discharge process only takes place there. Hybrid Supercapacitors are a combination of EDL and Faradaic mechanisms provide a synergistic combination of EDL and pseudocapacitance, resulting in high energy density and improved cycling stability. [
16]
2.1. Electrochemical double-layer capacitors (EDLCs)
The electrostatic double layer that forms at the interface between the electrode and the electrolyte serves as the foundation for the energy storage mechanism in EDLCs. Ions from the electrolyte are drawn to the electrode surface when a voltage is applied, creating a double layer of charged species. The storage of energy results from this charge separation. The high-power density of EDLCs makes them capable of delivering and absorbing large amounts of power quickly. [
17,
18,
19,
20] Rapid charge and discharge rates are made possible by the absence of chemical reactions during charging and discharging, which makes EDLCs ideal for applications requiring quick energy delivery.
In comparison to other energy storage technologies like batteries, EDLCs typically have a lower energy density. The electrode's surface area and the capacitance of the double layer are the main determinants of energy storage. The goal of ongoing research is to create materials and designs that will increase the energy density of EDLCs.
An increase in the material's dielectric constant, surface area, and decrease in interplanar thickness can all increase the capacitance of a standard capacitor. [
21,
22] However, further modifying the material system and capacitor design can result in such an increase. For example, changing the particle size to the sub-nanometer scale can lead to the material's extraordinary electrochemical performance. Metal ion doping, i.e., Fe, Mn, Cr, and Co, can increase the electrical conductivity of the electrode material, which consequently increases the capacitance. For instance, if a capacitor has symmetric electrodes or its working principle is based on faradaic reactions, this supercapacitor can eventually have enhanced electrochemical performance. They are highly reversible due to their non-faradaic electrical mechanisms and have an extremely stable cycling ability of up to 10
6 cycles or more. The main disadvantage of EDLCs is the limited availability of electrode materials, which is now possible thanks to the development of ionic, conductive electrolytes. There are three main types of EDLCs in terms of their carbon content, which lead to different functions or roles in the device. carbon nanotubes (CNTs), graphene, and activated carbon are the most important features. [
23]These materials' porous structure offers a significant surface area for the formation of the double layer and increases capacitance. The excellent cycling stability of EDLCs typically enables them to withstand numerous charge-discharge cycles without significantly degrading. Energy storage in EDLCs doesn't involve any chemical reactions, which extends their life and reliability.
2.2. Pseudocapacitors
Pseudocapacitance increases as a result of the electrolyte and electrode surface's quick oxidation and reduction. [
24] In redox reactions, reactants experience an alteration in the oxidation state. An object is said to be oxidized when an electron has been removed from it, whereas an object has been reduced when an electron has been added. Pseudo-capacitors made through this faradaic process have an increased energy density over EDLCs. The size of the electrode's surface areas and the kind of electrolyte used determine how much pseudo-capacitance there is. Electrons generated by the redox reaction are transferred across the electrolyte-electrode interface during the Faradaic process which leads to the accumulation of electrons at the electrode. [
25,
26] Theoretical metal oxide pseudo-capacitance can be calculated using the following formula:
where n represents the average number of electron transfers during the redox process, The Faraday constant is given by F, and the molar mass of the operating voltage window, and V is the metal oxide.
As shown in the following formula, a supercapacitor's energy density (E)
The formula for calculating a supercapacitor's maximum power density is
where R denotes the device's total equivalent series resistance.
High pseudo-capacitance is provided by transition metal oxides like titanium sulfide (TiS
2), manganese oxide (MnO
2), nickel oxide (NiO), and ruthenium oxide (RuO
2). Due to their high specific power and electrochemical stability, supercapacitors based on transition metal oxides perform extremely well electrochemically. Because of their high energy storage capacity, high pseudocapacitance value, low conducting resistance, low mass, and low price, conducting polymers have also become promising electrode materials for supercapacitors. The materials for electrodes can be any metal oxides, noble metals [
27], and conducting polymer, including polypyrrole, polyacetylene, poly (phenylenevinylene), polythiophene, polyaniline, etc. However, the life cycle and power density of pseudocapacitors are also shorter. These outcomes are the result of redox reactions occurring in the capacitors.
2.3. Hybrid
To create a hybrid supercapacitor, a single supercapacitor can simultaneously produce EDLC and pseudo-capacitance. With the ability to store charges in both non-Faradaic and Faradaic ways, hybrid supercapacitors can produce higher energy and power densities with good cycling stability. For instance, in a single supercapacitor, the non-Faradaic charge-discharge process occurs on one electrode while the redox reaction takes place on a different electrode. Composite hybrid [
28,
29,
30], battery-type hybrid [
31], and Asymmetric hybrid [
32,
33,
34] fall under this category.
2.4. Performance parameters of supercapacitor electrode materials
Specific capacitance (normalized by electrode mass, volume, or area), energy density, power density, rate capability (retained capacitance at a high current loading), and cycling stability are the major performance indicators of supercapacitors. [
35] It is preferable to raise the specific capacitance (C), and the operating voltage window (V), as well as lower the equivalent series resistance in a supercapacitor to increase its energy density and power density (R). The maximum operating voltage window (Vm) for EDLC supercapacitors is primarily determined by the electrolyte used, which is constrained by the electrolyte's stability. For supercapacitors based on aqueous electrolytes, Vm is typically 1 V. The creation of non-aqueous electrolytes with high Vm is one of the current research trends in supercapacitors. For instance, an electrolyte based on an ionic liquid can be operated at a Vm as high as 3.5 V. [
36]
✓Essential for rate capability and power density is high electronic conductivity.
✓increased specific surface area, which controls the specific capacitance
✓low costs for production and raw materials.
✓ideal electroactive locations that allow for pseudocapacitance.
✓High thermal and chemical stability have an impact on cyclic stability.
✓Rate capability and specific capacitance are impacted by controlled porosity.
These are all the characteristics that an ideal electrode should have. [
37]
In this review, we have discussed the most recent developments in Electrode Materials in supercapacitors. The paper describes the three materials used to improve supercapacitor efficiency: carbon-based materials for storage, metal oxide materials for high-performance applications, and advanced materials for high energy and power density applications. MXene and nanocomposite materials are suitable for increasing device power and energy density. that make them perfect for supercapacitor electrodes, as well as methods for producing the materials with appropriate performance.
3. Carbon-based materials for the high-performance supercapacitor
The research community is making great efforts to develop novel and high-performing electrode and electrolyte materials for solar cells (SCs). The widely accepted and popularized energy storage electrode material for SCs is carbon, which has low manufacturing cost, reduced size and weight, an abundance of availability, a large surface area, a controllable morphology, the intercalating capability of electrolytes within its high porosity, and high electrical conductivity. [
38,
39] Additionally, carbon materials can be post-treated to alter their structures and chemical and mechanical properties easily, which adds up to an advantage in improving their SC functioning and performance. With these added features and merits, virtually all SC manufacturers today make carbon or activated carbon (AC) their number one priority active electrode material component. [
40,
41] AC is a popular electrode material with a large surface area, good electrical characteristics, and a reasonable price. It can be produced from various carbonaceous materials, either physically or chemically. Physical activation involves heating carbon precursors to high temperatures, while chemical activation uses substances like sodium hydroxide, potassium hydroxide, zinc chloride, and phosphoric acid. AC has well-developed surface areas of up to 3000 m
2 g
-1 and varies in physiochemical properties depending on the activation techniques and carbon precursors used. [
42,
43] Specific capacitance and specific surface area (SSA) have been found to differ in AC, with a high SSA of 3000m
2 g
-1 resulting in a low capacitance. This implies that not all pores are productive when charge is accumulating. Performance of electrochemical reactions in carbon materials is also influenced by additional factors, such as pore size distribution. Large pore volumes, poor conductivity, and low material density are caused by excessive activation, and these factors lower the energy density and increase power loss. [
44,
45]
Because of its distinctive pore structure, mechanical and thermal stability, and superior electrical properties, carbon nanotubes (CNTs) have completely changed the science and engineering of carbon materials. Through the catalytic decomposition of hydrocarbons, CNTs are created, enabling the creation of nanostructures in different conformations and managing their crystalline structure. [
42] They are highly regarded for their electrical conductivity, support for active materials, and high-power electrode materials. Compared to AC, CNTs have a small surface area and a low energy density. Using potassium hydroxide for chemical activation can enhance specific capacitance.
The newly developed graphene has the highest specific surface area (SSA) of all carbon materials used as electrode materials in electrochemical double-layer capacitors, at about 2630 m
2 g
-1. [
46,
47,
48] Graphene can reach a capacitance of up to 550 F g
-1 if the entire SSA is utilized. The fact that both of the graphene sheet's major surfaces are exposed to the outside and are easily accessible to the electrolyte is another advantage of using graphene as an electrode material. The production of various types of graphene can be accomplished through a variety of processes, including chemical vapor deposition (CVD), micromechanical exfoliation, the arch discharge method, the unzipping of CNTs, epitaxial growth, electrochemical and chemical processes, and intercalation in graphite. [
49,
50,
51]
3.1. Bio derived carbon
Activated carbon, which is primarily made from conventional fossil fuels like coal, petroleum, and their derivatives, is distinct from biomass carbon. [
52,
53,
54] Biomass has a more logical three-dimensional hierarchical porous structure (with macro-, meso-, and micropore distributions) and easily modifiable surface functional groups. This will provide more active sites for supercapacitor applications, such as larger ion storage interfaces and pseudocapacitance generation through redox reactions. It has been suggested so far that biomass can be converted into clean renewable energy systems because of a variety of applications, including electrocatalysis, secondary batteries, energy conversion, and storage. [
55,
56,
57] Initial research efforts were concentrated on carbon produced synthetically from biomass. The structural variations of carbon derived from biomass and their application in energy storage have also been proposed. For instance, Pan focuses on the design of biomass-based carbon structures while Toupance emphasizes the use of biomass-based carbon in supercapacitors and hybrid solar cell applications. When presenting carbon derived from biomass or making references to particular applications, these reviews emphasize synthetic techniques. It is therefore still challenging and essential to address the ever-increasing research on biomass to present a thorough review in synthetic strategies, various dimensions, and applications in electrocatalysis, energy conversion, and storage of carbon derived from biomass.
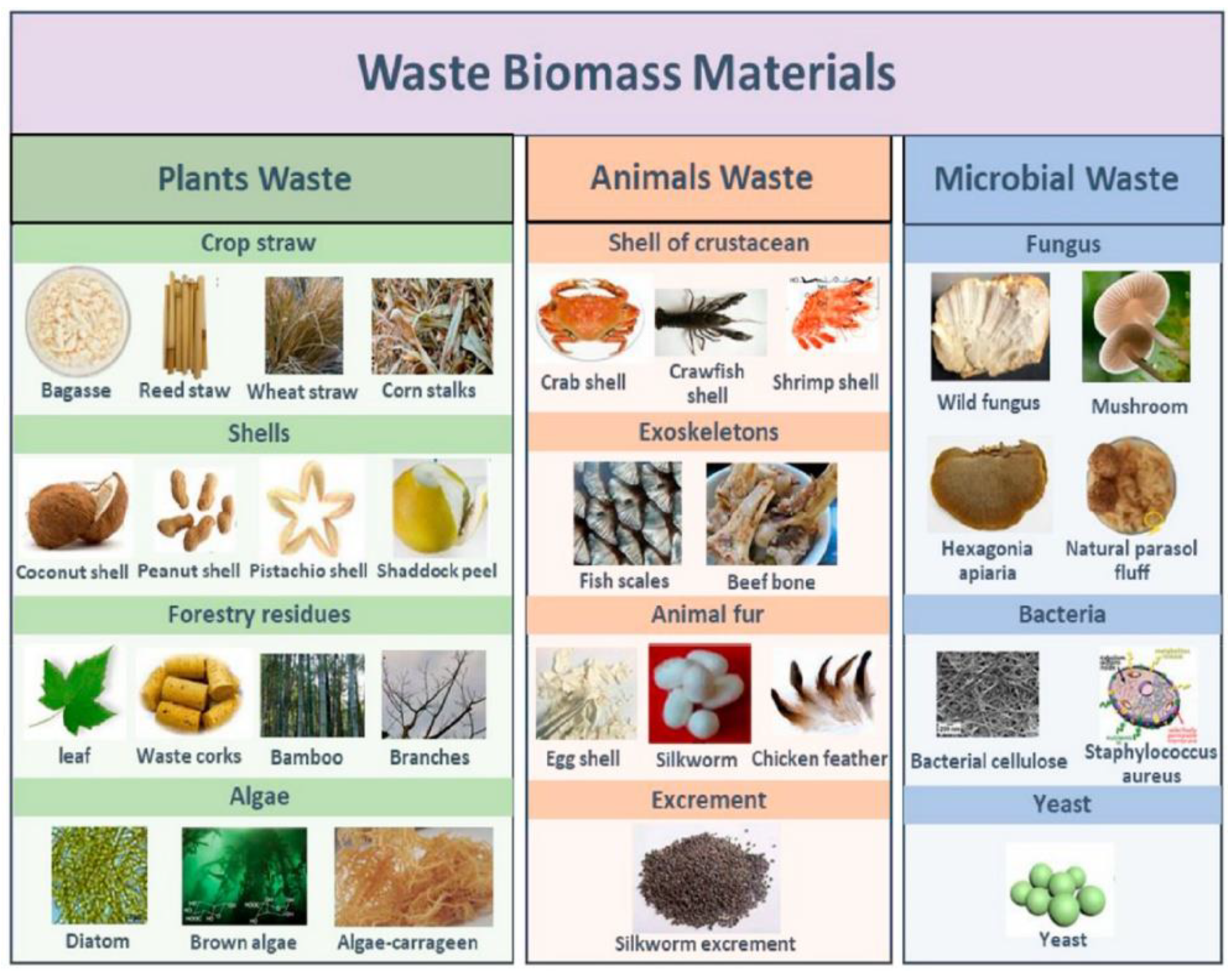
In addition, biomass is a sustainable and renewable source of carbon, making it a better choice for the environment than more conventional carbon sources like coal and petroleum. Additionally, a variety of natural sources, such as agricultural waste, forestry residues, and municipal solid waste, can be easily sourced for biomass (
Figure 3). The use of biomass porous carbon is positioned to play an increasingly significant role in meeting these needs as the demand for energy storage and conversion technologies grows. Biomass porous carbon is a promising material for the creation of high-performance supercapacitors and other energy storage devices because of its special properties and advantages in the economy and environment. Because it also results in the production of biomass oil as a byproduct, the process of preparing biomass carbon has a significant contribution to make to the economy. It possesses high thermochemical stability in addition to a large specific surface area and a hierarchical porous framework that is three-dimensional. It possesses a variety of surface functional groups, including oxygen, nitrogen, sulfur, and phosphorus-containing groups, and it is simple to modify these groups by the requirements of individual application scenarios. The vast majority of biomass materials are composed of alkali and alkaline earth metals, which not only speed up the activation reaction process but also offer an abundant supply of ion-binding sites for supercapacitors. Because of these characteristics, biomass porous carbon is undeniably an excellent candidate for energy storage and conversion processes. [
50,
51,
58]
Debika Gogoi used porous carbon made from coconut fiber as the anode and a nanocomposite made of CoFe
2O
4 nanoparticles (CF) immobilized within the pores of porous carbon (PC) as the cathode, created a high-performance all-solid-state flexible asymmetric supercapacitor device. The created device had a high energy density of 50.34 Wh kg
-1 at a power density of 1450 W kg
-1 and a good cycle life (retention of 91 % specific capacitance (CS) after 5000 cycles). [
60]
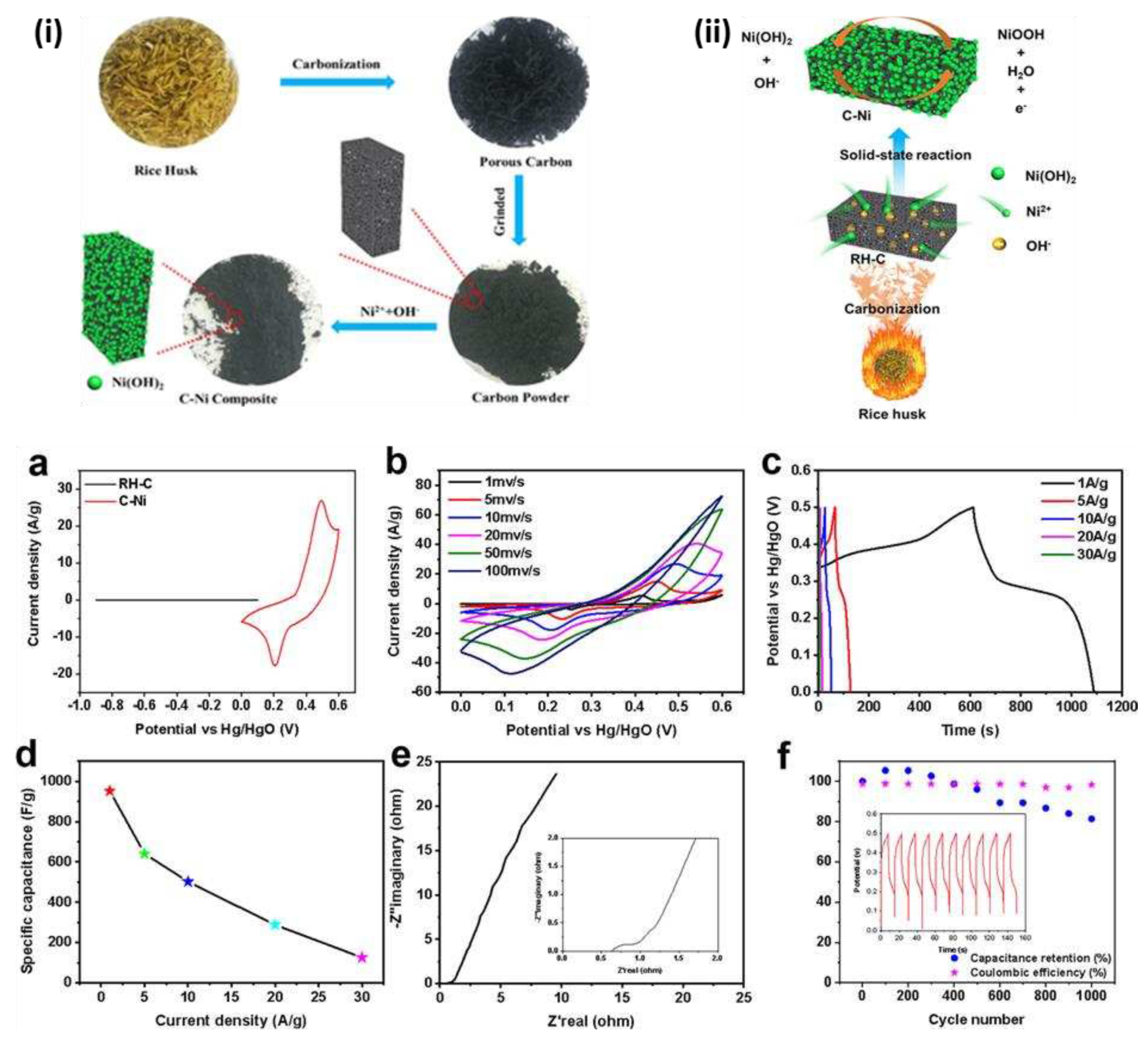
The successfully fabricated biomass-based carbon and β-Ni(OH)
2 hybrid electrode materials (
Figure 5 (i,ii)), and the gravimetric capacitance of C−Ni electrode materials reached ∼952 F g
-1 at 1.0 A g
-1 based on a three-electrode system configuration. The sample can still reach 126 F g
-1 even at a high current density of 30 A g
-1 (
Figure 5 (a-f)). [
61]
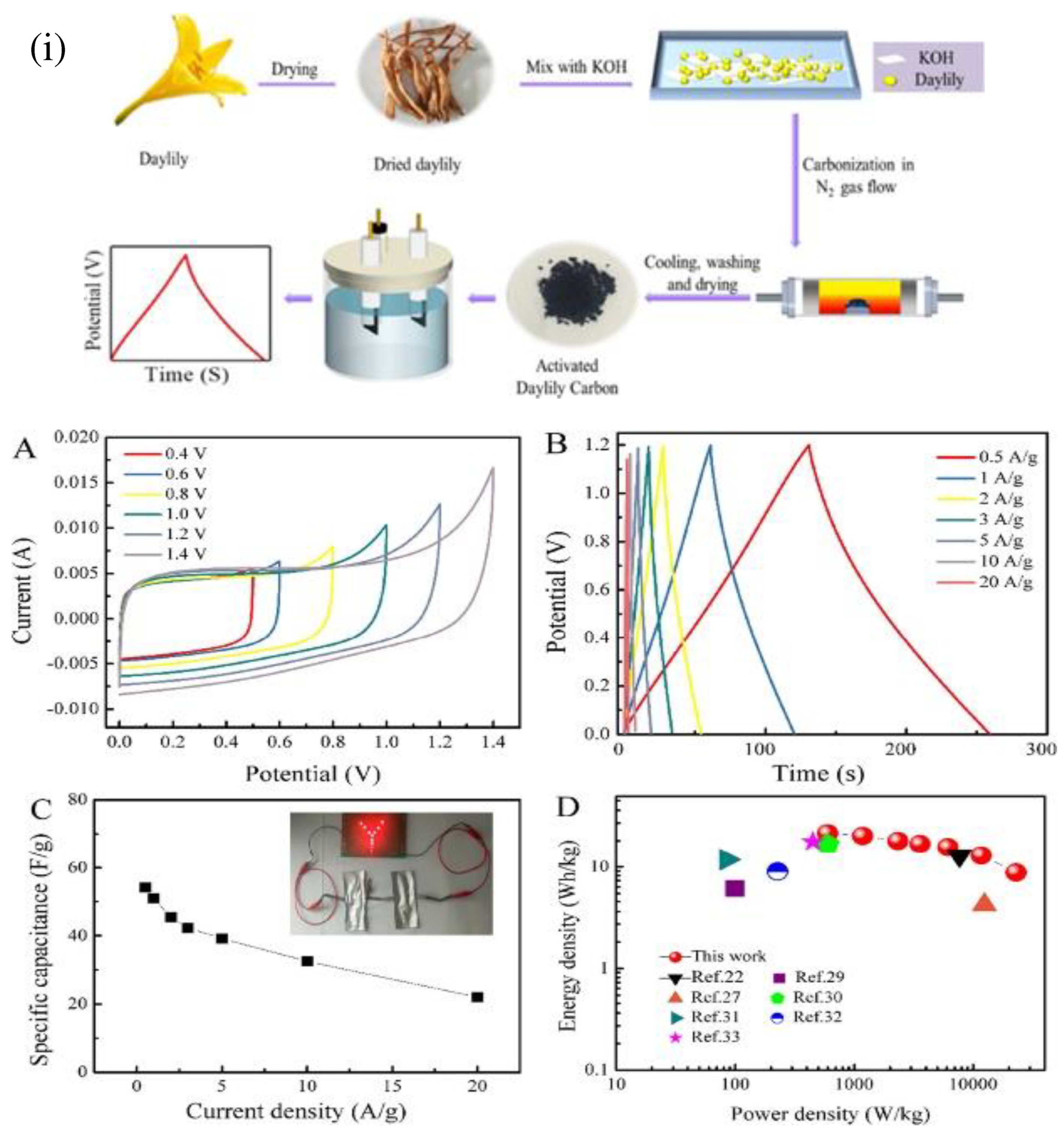
The selection of appropriate precursors is one of the most important steps involved in the production of high-performance activated carbon. Tianxiang Jin [
62] have chosen daylily due to the high levels of nitrogen and phosphorus that it contains, it is suitable for use as a precursor in the production of heteroatom-doped activated carbon (
Figure 6 (i)). A straightforward carbonization technique was used to successfully produce a porous carbon derived from daylilies. The resulting carbon had a large specific surface area and a high percentage of heteroatoms. The as-prepared carbon materials exhibited a remarkable specific capacitance of 299.1 F g
-1 at a current density of 0.5 A g
-1 and an excellent cycling stability of 99.6 % after 4000 cycles at a current density of 1 A g
-1 (
Figure 6 (a-c)). In addition to this, the assembled symmetric supercapacitor demonstrated a high-power density of 598.2 W/kg in 6 M KOH electrolyte while also exhibiting a high energy density of 21.6 W h kg
-1 (
Figure 6 (D)).
3.2. Synthetic derived Carbon:
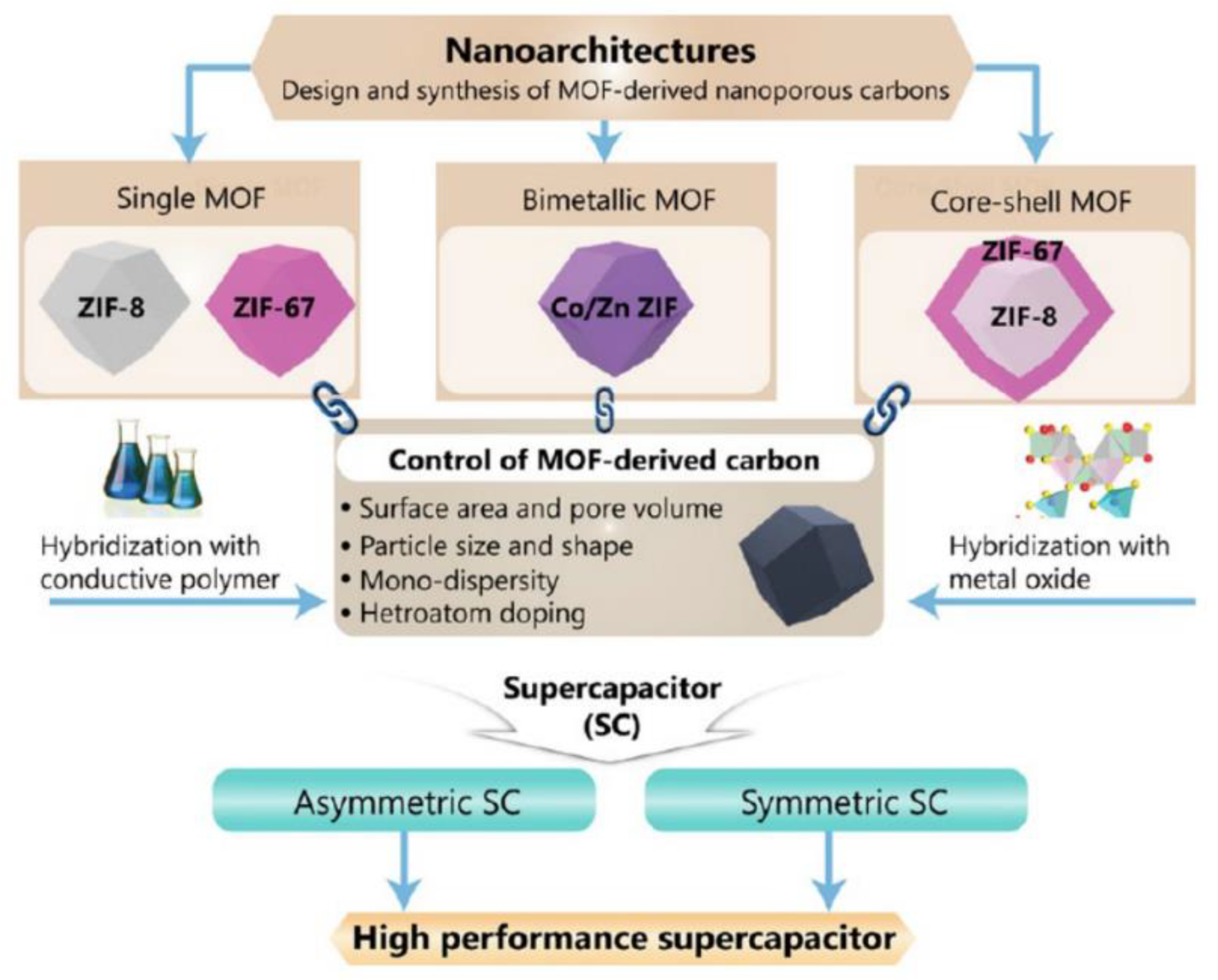
Porous materials are essential in various industries, including biomedicine, energy, adsorption, separation, and catalysis. Traditional porous materials, such as zeolite, mesoporous silica, carbon, metal oxide, and polymer, are amorphous, porous solids with irregular pores and ill-defined structures. Zeolites, a class of crystalline porous solids with superior stability, periodic structure, and intrinsic acidity, are widely used in industrial adsorption and catalysis. However, controlling acidic site distribution is challenging due to the various types of pore structures. Metal-organic frameworks (MOFs), a class of organic-inorganic hybrid and crystalline porous materials, have greatly expanded the field of porous materials. [
63,
64,
65] MOFs are fascinating materials due to their high porosity and atomic-level structures. However, their narrow pores limit their applications in diffusion control and large species processes. The development of hierarchically porous MOFs (HP-MOFs), composites, and derivatives has sparked interest in expanding traditional MOF-based carbon materials for energy storage applications (
Figure 7).
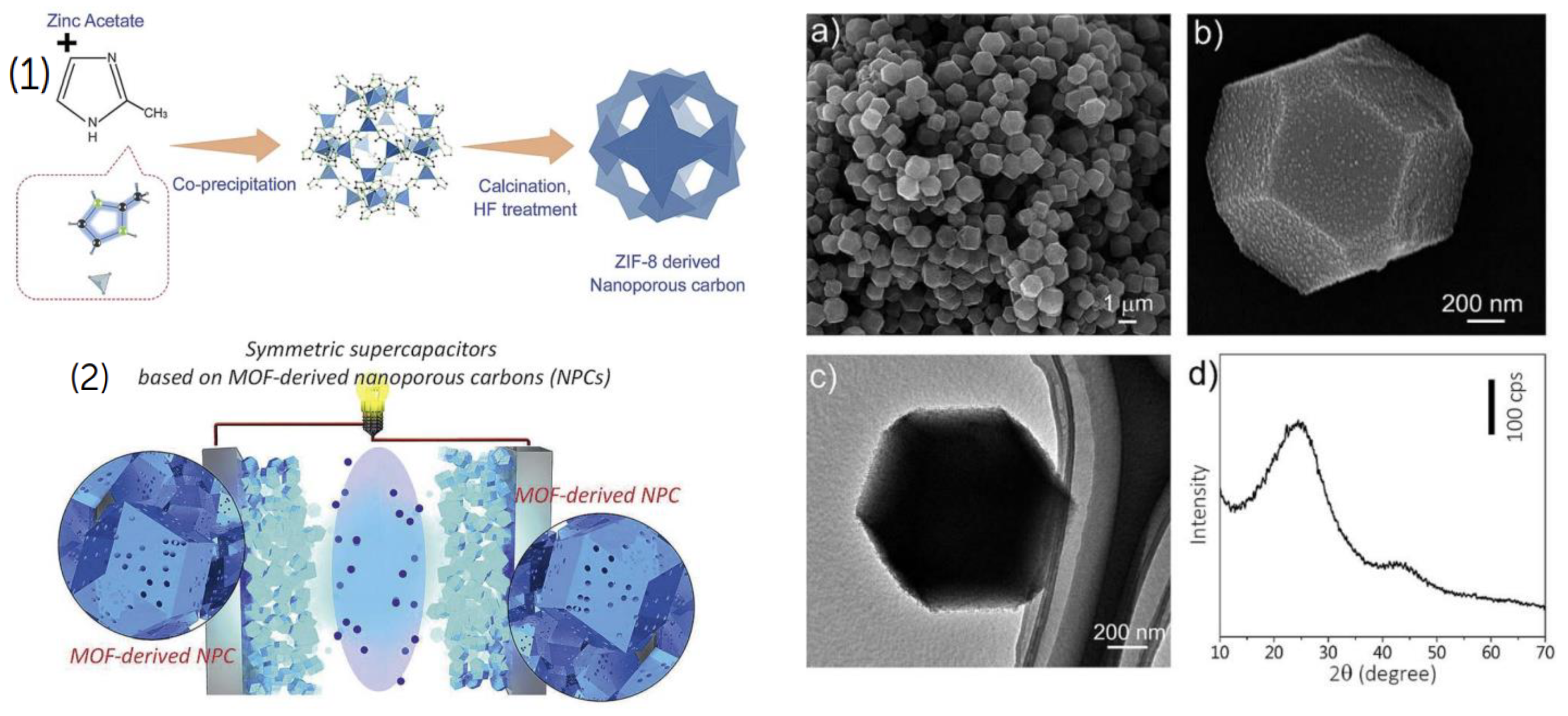
Rahul R. Salunkhe [
66] made MOF derived porous carbon (
Figure 8 (1,2)) and the uniformity of the large-sized NPC particles is good, and their average size is 1 mm. SEM images with high magnification display polyhedral shapes and symmetric geometry (
Figure 8 (a-d)). The obtained carbons are ZIF-8 morphologically authentic. The polyhedral shapes of small NPC particles make it simple to adjust particle size by altering reaction conditions. With a maximum capacitance of 251 F g
-1 at 5 mV s
-1, a specific energy of 10.86 W h kg
-1, and a specific power of 225 W kg
-1, the ZIF-8-derived NPC demonstrated impressive performance. Other NPC materials with improved pore architectures for next-generation energy storage applications could be created using this inexpensive technique. [
67]
Jing Yu developed nitrogen-doped porous carbons (NPCs) by treating the melamine-urea-formaldehyde resin with KOH. The addition of KOH increased the specific surface area and pore structure, resulting in high-performance supercapacitors with good electrochemical performance. The best sample had a large specific surface area of 2248 m
2 g
-1 and a high N content, resulting in a specific capacitance of 341 F g
-1 under 1 A g
-1 and a retention of 92 % after 5000 cycles. A symmetrical solid-state supercapacitor using NPCs as the electrode material had a maximum energy output of 9.60 W h kg
-1 at 1 A g
-1 (
Figure 9 (a-e)). NPCs offer potential benefits for high-performance supercapacitors and other energy storage technologies.[
68]
As we mentioned already supercapacitors are being investigated using materials like highly porous carbon black (CB), carbon nanotubes, carbon nanoflowers, carbon Nano horns, graphene, and their composites. However, low specific capacitance results from pure electrostatic charge accumulation, such as graphene's 200 F g
-1 in aqueous electrolytes. Short cycle life remains despite composites increasing specific capacitance through the redox process. Heteroatoms can increase specific capacitance invisibly by introducing heteroatoms to porous carbon materials like nitrogen and oxygen. This preserves the inherent benefits of carbon materials, such as high conductivity and a large specific surface. Heteroatom-doped carbon materials can be prepared using chemical vapor deposition, [
69] post-production processing, [
70] and direct carbonization, [
71] with direct carbonization being the preferred method due to its cost-effectiveness.
Table 1 lists capacitive data for carbonaceous precursors, including synthetic and natural sources like polyindole, polyaniline and polyacrylonitrile (
Figure 10 a-f).
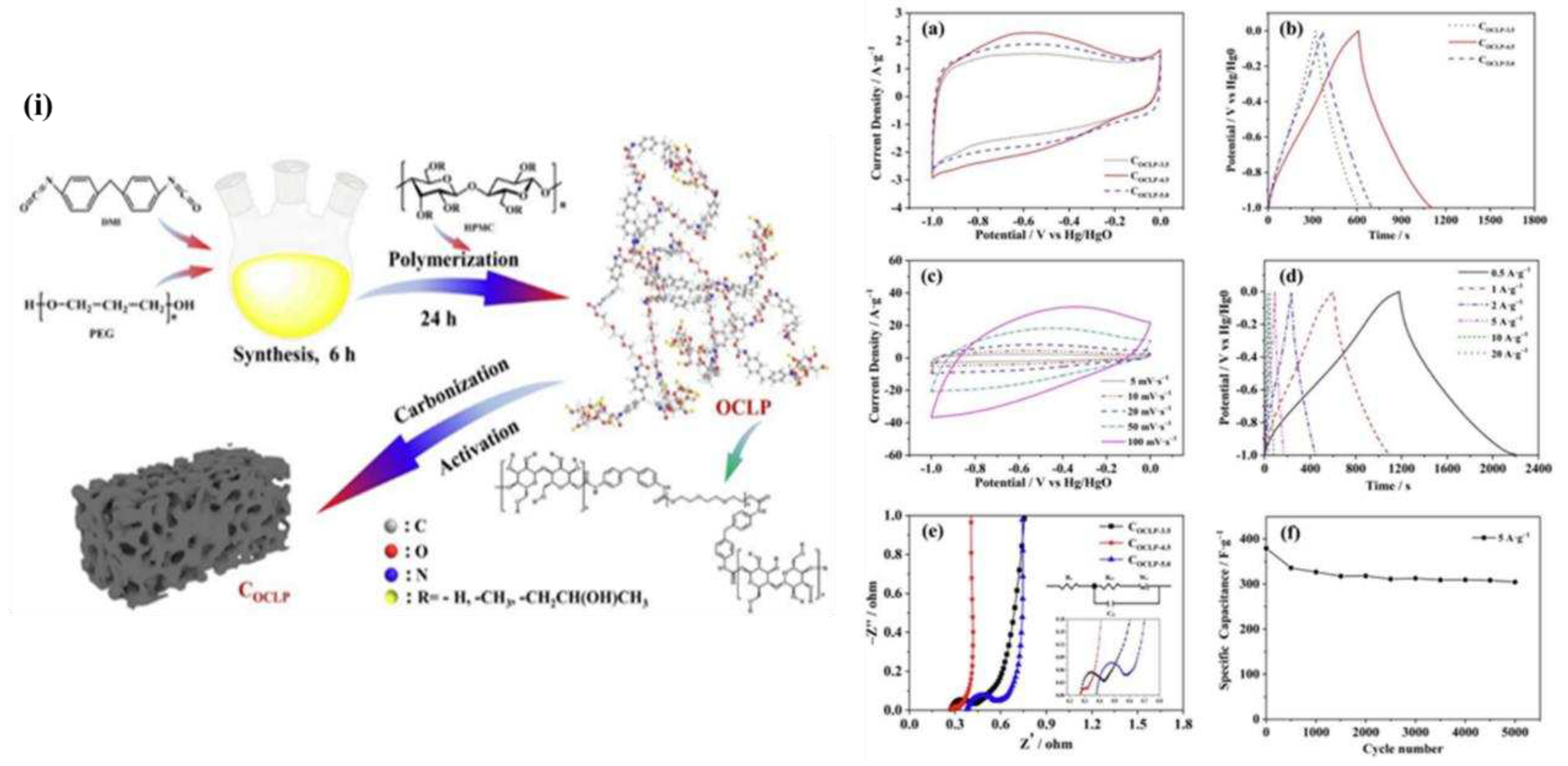
In order to create N/O-doped porous COCLPs (optimized porous carbon material), Jianhao Lao developed a network-structured organic crosslinked polymer COCLP-4.5, which exhibited exceptional electrochemical performance. The specific capacitance reached 522 Fg-1 at 0.5 Ag-1 and 309 Fg-1 at 20 Ag-1 in a three-electrode system. Additionally, the symmetric capacitor attained a power density of 200.0 W kg-1 and an energy density of 18.04 Wh kg-1. The COCLPs benefited from the hierarchical porous carbon formed by organic crosslinked polymers' net structure as well as from the pseudocapacitance that heteroatoms added.
4. Recent Metal oxide materials on supercapacitor
The metal oxides-based supercapacitors are much important to the super capacitor applications. The advantages of the metal oxides are as follows, (i) good conductivity, (ii) more stability, (iii) mechanical strength, (iii) high energy and power density. The combination of multi metal oxide materials may enhances the synergetic effect of the material, these would be modified by the optimization of various metal oxide materials. The multi metal oxide composition has more advantage to enhance the synergetic effect of the materials, however it needs to optimize the proper composition by the experimental procedure. Here some of the multi metal oxide advanced electrode materials for the supercapacitor applications.
4.1. Bi-metal oxide
The manganese and molybdenum based hierarchical MnMoO
4.H
2O@MnO
2 core-shell Nano arrays were used for the high-performance asymmetric supercapacitor (
Figure 11 (a-n)). The bi metal oxide material of MnMoO
4.H
2O@MnO
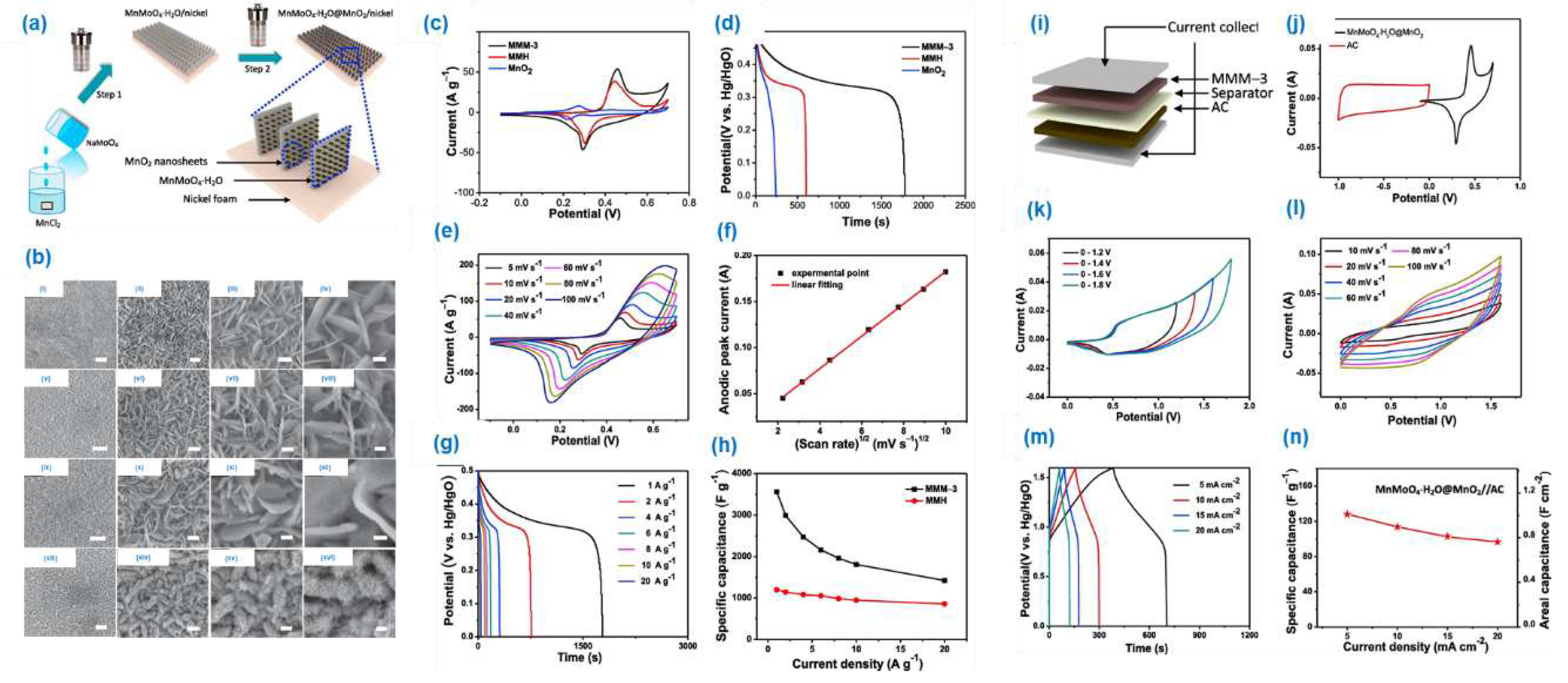
2 was prepared by using two step hydrothermal method. To enhance the specific capacitance the bimetal oxide material was directly coated on the nickel foam substrate. The FE-SEM and the TEM analysis were demonstrates the core-shell nanosheet array structure of the material. The material was confirmed by proper characterization techniques such as P-XRD, XPS and elemental mapping analysis. The electrochemical performance of the material studied through 2,3-electrode system. The 3-electrode system impels the maximum specific capacitance of the material was 3560.2 F/g at 1 A/g. The obtained result is considerable high in the recently reported material in supercapacitor area. The 2-electrode system possess the energy and power density of the material are 45.6 Wh k/g and 507.3 W k/g. The synergetic effect of the bi-metal oxide could be the reason for the high efficiency of the material.
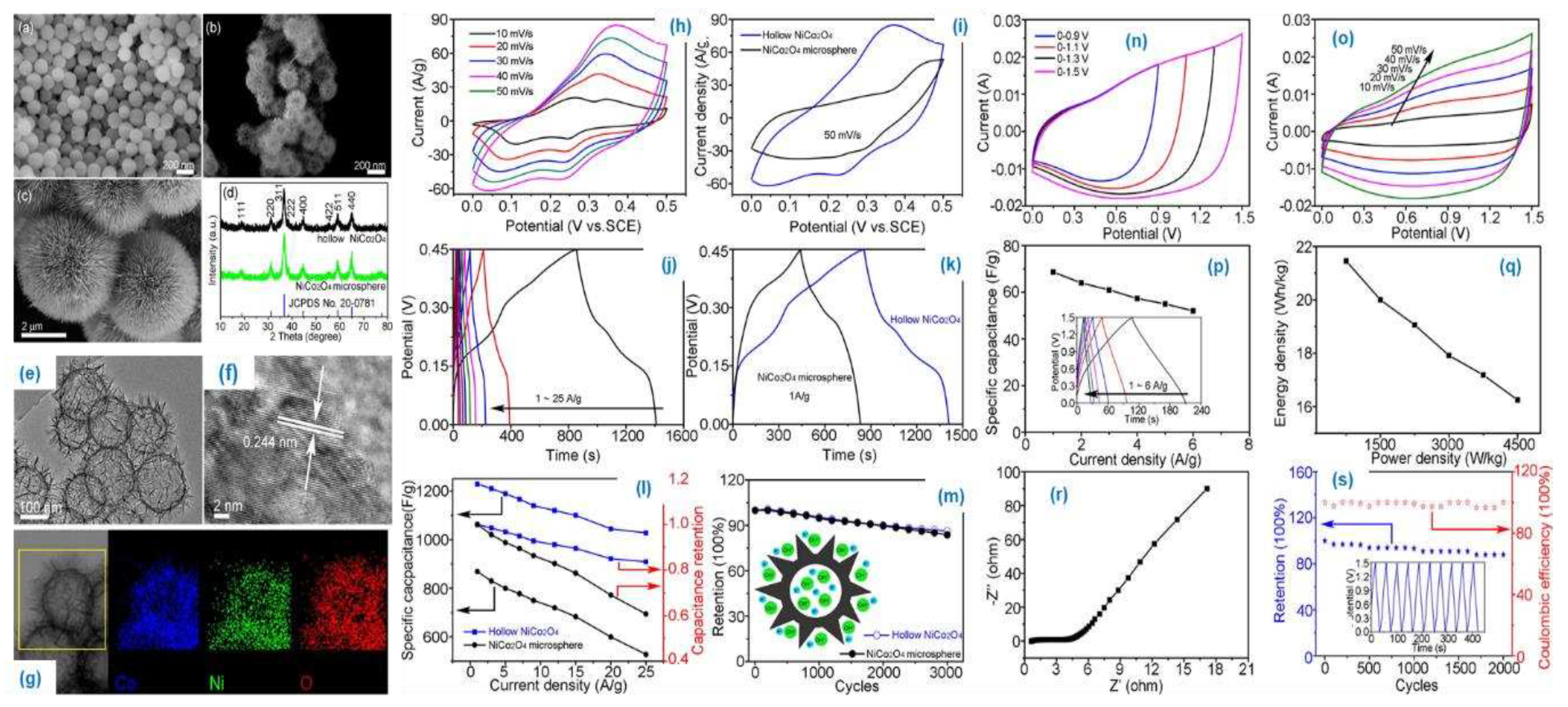
The hollow structured NiCoO
4 nano sphere type material was prepared by modified Stober and hydrothermal method. The material confirmed by P-XRD, XPS analysis and the surface area was obtained by BET method. The FE-SEM and HR-TEM analysis suggest as the material shows the hallow nanosphere structure with the diameter of ~200-220 nm. The obtained surface area of the NiCoO
4 is 166.1 m
2/g and the pore size distribution are 3.8 - 4.3 nm. The electrochemical performance of the material studied systematically (
Figure 12 (a-s)). The specific capacitance of the material is 1229 F/g at 1 A/g with the rate performance of ~83.6 %. The material shows specific capacitance of 68.7 F/g, the attained energy and power density are 21.5 W h/kg, 4500 W/kg. The results suggest as the array formation on the nanosphere could enhances the specific capacitance, due to the increase in surface area of the material.
4.2. Tri-metal oxide
The tri-metal oxides-based materials are much interest in battery type super capacitor applications. These materials show fast charge diffusion and reversible faradaic reactions; hence it provides high specific capacitance, energy and power density. Many available metal oxides such as Co3O4, MnO4, Fe2O3, MoO3, V2O5 and ZnO could be applicable for the preparation of tri-metal oxides-based materials. The advantages of theses metal oxide materials include cost effective and environmentally friendly as well.
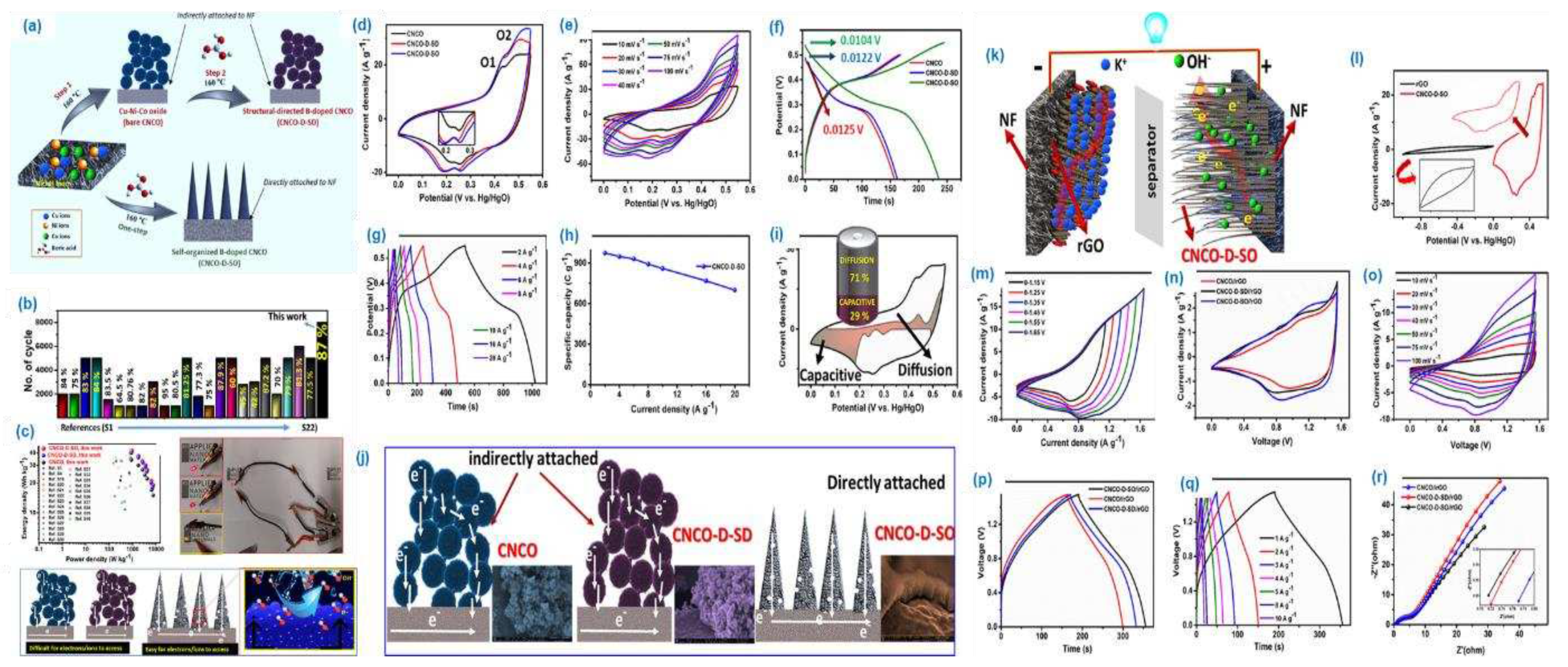
The Cu-Ni-Co oxides was used to prepare tri metal oxide materials and followed by boron doping was adopted for the high-performance super capacitor application. The FE-SEM analysis shows needle like morphology was obtained for trimetal oxide materials, while the agglomerated needle like morphology obtained for boron doped metal oxide materials (
Figure 13 (a-r)). The obtained specific capacitance of the doped trimetaloxide is 974 C /g at 2 A/g, and the materials shows 700 C /g at 20 A/g this suggest as the material shows high rate capability. The energy and power density of the material was calculated by using coin cell method. The rGO was used as a negative electrode, while the prepared material was used as a positive electrode. The obtained energy and power density of the trimetal oxide material is 42 Wh /kg and 926 W /kg. The materials stability and the electrochemical performance of the tri metal oxide material are increased after the boron doping. The results suggest as the doping of hetero atom onto the metal oxide material could enhance the electrochemical performance of the material.
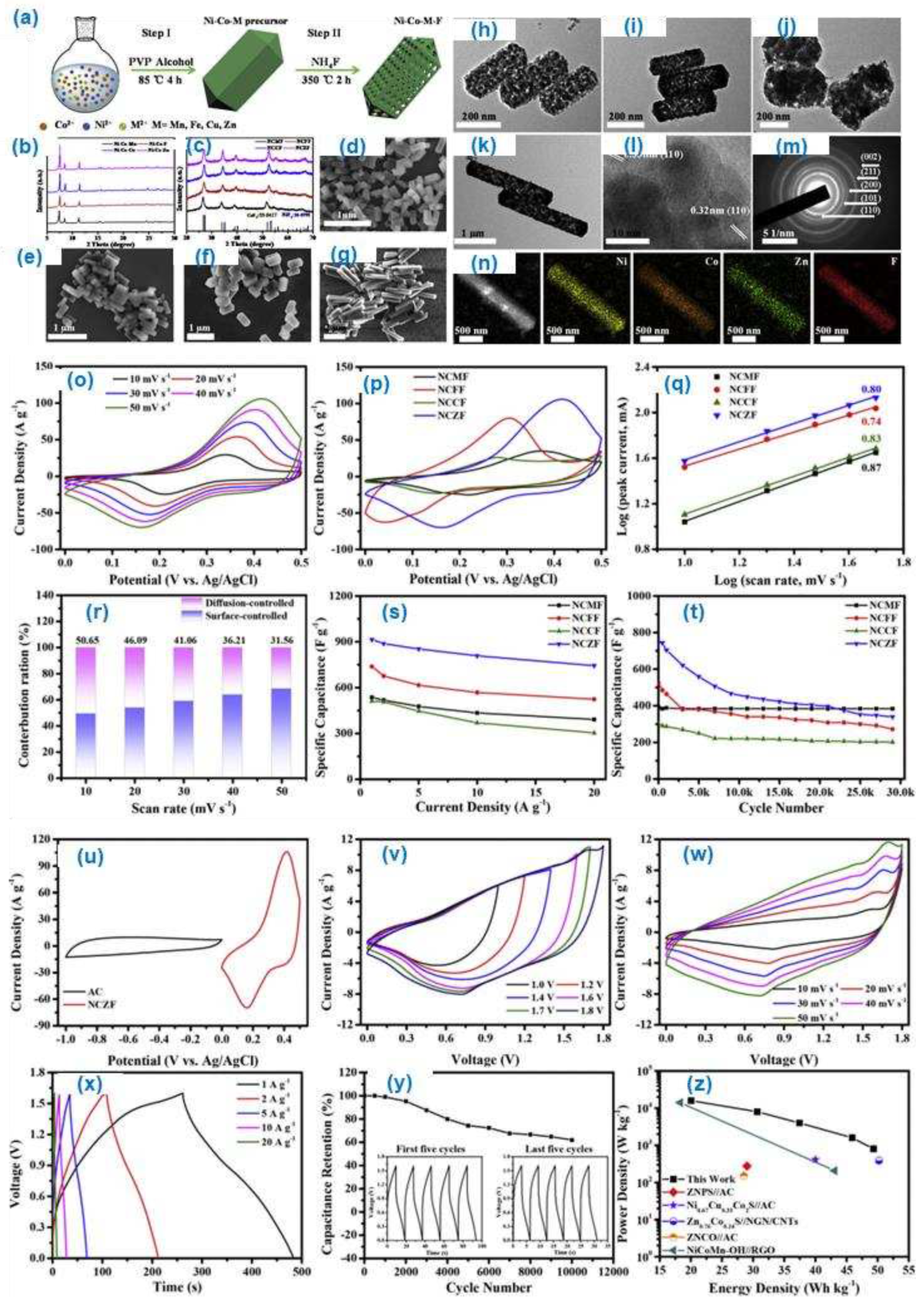
The Ni-Co-M (M=Mn, Fe, Zn) based porous trimetallic fluoride materials prepared as an electrode material for the high-performance asymmetric supercapacitors (
Figure 14 (a-z)). The obtained material shows high rate capability and recycle stability. Among the various composition the Ni-Co-Zn fluoride shows highest specific capacitance of 914.4 F/g at 1A/g with 81.3 % capacitance retention at 20 A/g. The fluoride doped trimetal oxide material shows the high energy density of 49.3 Wh/kg at 800 W/kg. The reason for the high energy density is due to the superior synergetic effect between the metal oxide materials. The obtained fluoride doped transition metal oxide materials suggest as the doping of halogen group element to the transition metal oxide materials could enhances the specific capacitance of the electrode materials.
5. Advanced materials for the high power and energy density application
5.1. Carbon metal oxide composites
Due to their substantial surface area, low price, and simplicity of processing, activated carbons (AC) are frequently used as electrode materials. However, their rate and energy storage capabilities are subpar. On the other hand, pseudo-capacitance can achieve higher capacitance values but has poor electrical conductivity, which leads to low power density and unstable cycling. Both activated carbons and pseudocapacitive materials need to be developed in order to improve supercapacitors. Through the cooperative action of EDLC and pseudocapacitance mechanisms, recent composite materials that combine carbon-based materials with metal oxides, hydrocarbons, or conducting polymers have demonstrated improved capacitive performance and cycle life. These composites improve each component's inherent qualities, such as mechanical stability, electrical and ionic conductivity, and electrochemical reactivity. Hybrid designs can also successfully reduce the surface energy of active nanomaterials. In recent publications, electrodes made of activated carbon or metal oxide-hydroxide were used in a variety of applications.
Because metal oxides, such as RuO
x, CoO
x, SnO
x, FeO
x, MnO
x, NiO
x, etc., possess pseudocapacitance that results from a quick redox reaction between metal oxides and electrolytes in addition to the regular double-layer capacitance, they have a higher capacitance than carbon materials. [
76] For instance, it was determined that Co
3O
4 and NiO have theoretical capacitances of 3560 and 2584 F g
-1, respectively, which are significantly higher than those of carbon electrodes. [
26,
77] However, due to their poor electrical conductivity, severe agglomeration, and short cycle life, metal oxides are frequently ineffective when used directly in high-performance supercapacitors. As an alternative, both academia and industry have highlighted composite materials made of porous carbon and metal oxides that are used as electrodes for supercapacitors. Superior electrochemical performances are the result of the synergistic interactions between hierarchical porous carbons and pseudocapacitive metal oxides, which can both increase electrical conductivity and provide channels for charge transport. For instance, Zheng et al. created a 3D binder-free electrode using hierarchically porous carbons loaded with Co
3O
4 nanoarrays and morpho butterfly wing scales to achieve an exceptional specific capacitance of 978.9 F g
-1 at 0.5 A g
-1. [
78]
Zhiwei Tian [
79] reported a manganese oxide/carbon composite with high specific capacitance and conductivity (
Figure 15 (a-i)). Utilizing homemade porous carbon and KMnO
4 as raw materials, manganese oxides/carbon composites have been created. Due to the Mn-O-C interaction, these composites have high specific metal oxide capacitance and high carbon conductivity. At current densities of 1, 2, 3, 5, and 10 A g
-1, the optimized composite electrode exhibits extremely high specific capacitance values of 550, 520, 481, 402, and 318 Fg
-1. The completed asymmetric supercapacitor (ASC) device exhibits excellent rate performance at 1600, 2297, 3715, and 6890 W/kg and an energy density of 50.2 Wh kg
-1 at 800 Wkg
-1. After 10,000 cycles, the ASC device still maintained 90.5% capacitance, demonstrating excellent cycling stability. Energy storage uses for these ASC devices could include powering 35 lights and an electronic stopwatch. The material is a promising candidate for manganese/carbon composite supercapacitors, which are anticipated to be produced in large quantities commercially due to their straightforward preparation methods and ultra-high performance.
5.2. MXenes based materials for the high-performance supercapacitor application
MXenes are a group of two-dimensional advanced functional nanomaterials that have been investigated for use in the synthesis of fuel and clean energy, among other applications. They have distinct layered structures that enhance electrolyte ion transport in addition to offering active transition metal redox sites on the surface. MXenes are particularly promising as high-performance electrodes for electrochemical capacitors because of these qualities like conductivity, aspect ratio etc., [
80] With aqueous electrolytes like (NH
4)
2SO
4 and MgSO
4, electric double-layer capacitors (EDLC) have a significant impact on the charge/discharge tests of MXene electrodes. Because hydrated ions fill the interlayer spacing, the EDLC dominates these tests. [
81,
82] A higher capacitance is seen when H
2SO
4 is used, suggesting that protons are the predominant charge carriers. H
2SO
4's impact on the storage mechanism is still debatable, necessitating additional theoretical research and combined characterization. Redox reactions, intercalation, and underpotential deposition are blamed for pseudocapacitance. In an H2SO4 electrolyte, MXene cathodes that are connected to conductive polymer-based anodes produce pseudocapacitance. Intercalation stores alkali metal cations in MXene-based supercapacitors that use organic electrolytes. Although in situ characterization tools can shed light on the structural development and chemical environments of MXene-based electrodes, there are still many unanswered questions regarding ion dynamics in supercapacitors. [
83,
84]
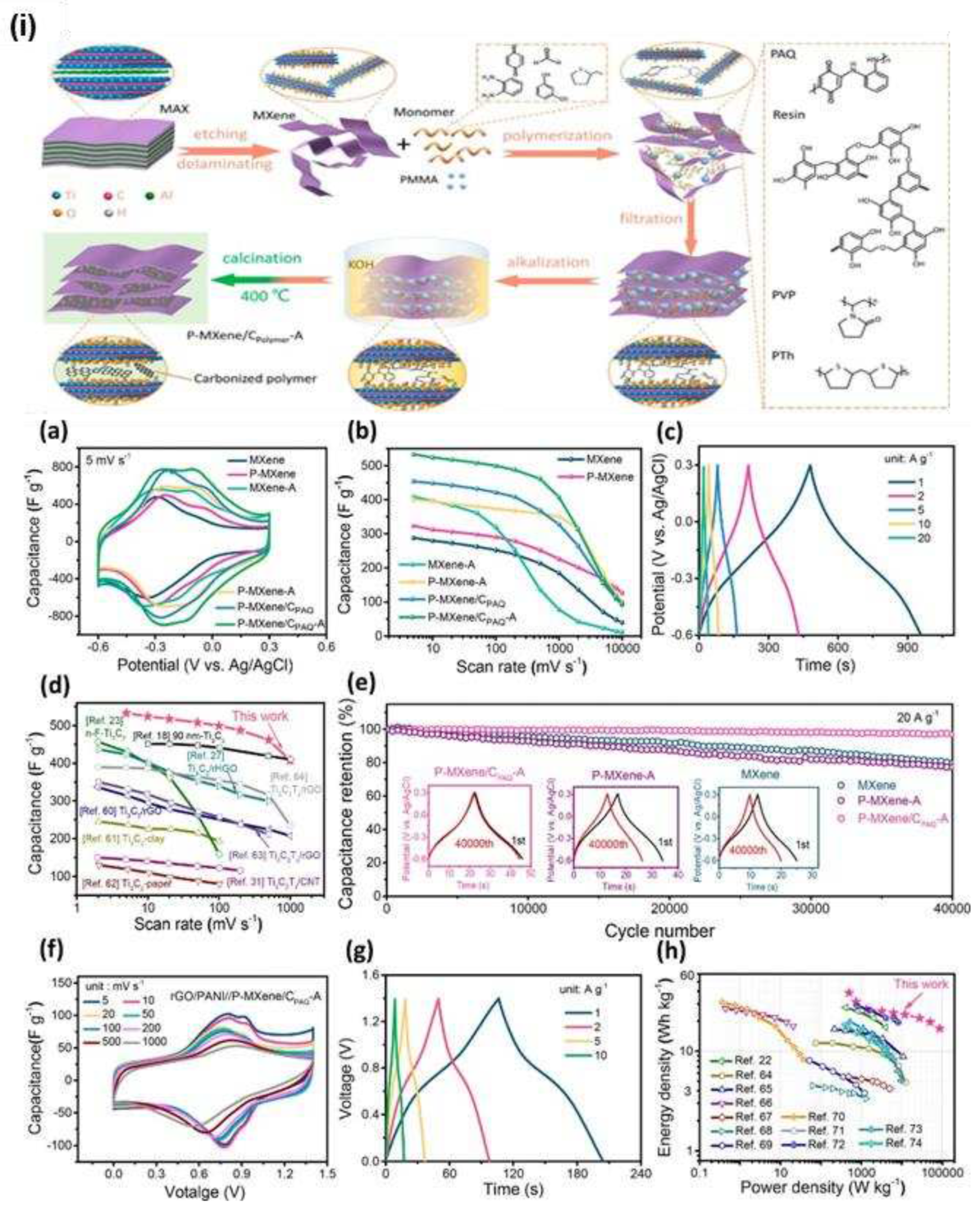
MXene-based electrocatalysts require innovative methods for synthesizing monolayer MXenes with large transverse dimensions and thoughtful structural modifications in order to achieve excellent activity and selectivity. Rui Ma [
85] developed Ti
3C
2T
x MXene-based supercapacitor electrodes with improved capacitance, rate capability, cyclic stability, and mechanical flexibility. He combined engineering the electrode structure, surface chemistry, and fabrication process through an optimized integration approach. The P-MXene/CPAQ-A electrode, made of quinone-amine polymer, achieved a capacitance of 532.9 F g
-1 at 5 mV s
-1 and better rate performance and cyclic stability (97.1 % retention after 40,000 cycles at 20 A g
-1) (
Figure 16 (i), (a-h)). This electrode outperformed pristine MXene and P
MXene electrodes in terms of retention and cyclic stability. The optimized techniques produce more active sites, quicker ion accessibility, better chemical stability, and good mechanical flexibility. The P-MXene/C
polymer electrodes show capacitance enhancements due to enlarged interlayer spaces, nanostructured carbonized polymers, PMMA sacrificing templates, and intercalated K
+ ions. MXene surface modifications include F removal, a decrease in OH, an increase in O, K
+ intercalation, and some carbonized polymers possessing electrochemically active groups. Improved rate capability is due to enlarged interlayer spaces and porous or hierarchical nanostructured polymers, which circumvent restacking and accelerate ion accessibility. However, undesirable nanostructures may affect performance at higher scan rates. Improved cyclic stability is likely due to enhanced chemical stability and repressed oxidation.
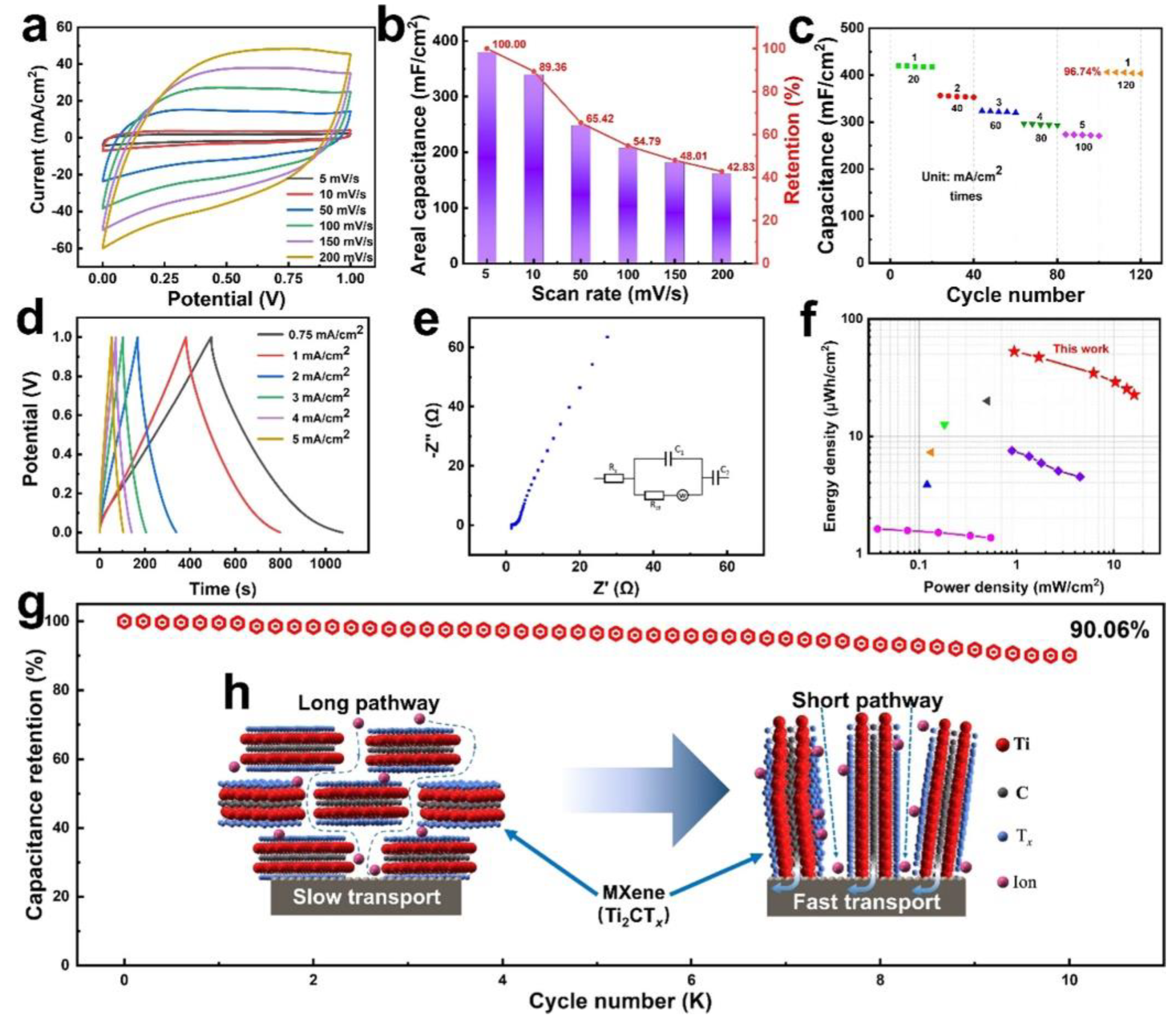
Bao Shi [
86] successfully synthesized self-supported and vertically aligned Ti
2CT
x MXene nanosheets on the surface of carbon fibers (CF), which required the use of the molten salt method in conjunction with the selective etching method (
Figure 17 (a-h)). This method reduces the risk by using CFs as a flexible substrate and carbon resource without the need to collect powder samples from HF mixtures. Additionally, the Ti
2CT
x MXene's vertical alignment solves the stacking issue with conventional MXene film electrodes made using the filtration method, which shortens and streamlines the ion transport path and improves the performance of zinc-ion n hybrid supercapacitors (HSC). With 90% capacity retention after 10,000 cycles, a super low self-discharge rate of 0.53 mV h
-1, and a high areal capacitance of 380 mF Cm
-2 at a scan rate of 5 mV s
-1, the Zn-ion HSCs made from Ti
2CT
x MXene@CF/Zn@Cu@CF exhibit excellent stability.
Table 1.
The electrochemical performance results of some recently reported martials.
Table 1.
The electrochemical performance results of some recently reported martials.
| Materials |
Specific capacitance (F g−1) |
Energy density
(Wh kg−1) |
Power density |
Ref. |
| Biomass derived Carbon |
258.8 at 1 Ag−1 |
7.11 |
125.46 |
[87] |
| Biomass derived Carbon |
382 at 1 Ag−1 |
23.13 |
300 |
[88] |
| polyindole |
293 at 0.2 A g−1
|
15 |
- |
[89] |
| polyaniline |
∼290 at 1 A g−1
|
10.3 |
- |
[90] |
| polyacrylonitrile |
252 at 0.1 A g−1
|
8.8 |
- |
[91] |
| Metal Oxide (Cu-doped Co3O4 NP) |
- |
64.1 |
800 |
[92] |
| Metal Oxide (Mn3O4 triangular structures) |
751.3 at 1 A g− 1
|
91.7 |
899.5 |
[93] |
Carbon/metal oxide composite
(ZnO/ NiO@MWCNT) |
1988.8 |
43.59 |
4000 |
[94] |
Carbon/metal oxide
(NiO@Co3O4-Activated C) |
800.9 at 1.0 A. g− 1
|
136.6 |
- |
[95] |
| MXene (Ti3C2Tx/V2O5) |
-319.1 at 5 A g− 1
|
18.43 |
603.2 |
[96] |
| MXene (Ti3AlC2/polyaniline/Co Ni LDH) |
1200 at 1 A g− 1
|
399.95 |
39.33 |
[97] |
6. Conclusion
In a comparable form, supercapacitors (SCs) are examined in detail in this paper, including their structure, operating principles, specifications, materials, and applications. The classification of supercapacitors based on mechanisms was briefly discussed as well. It examines their benefits, contemporary technologies, and suppliers. The SC model, a real-time application, and performance evaluations are also shared in the paper. The article discusses the most recent advances in material technologies, overcoming application challenges, and enhancing the marketability and efficacy of SCs. The composites of carbon-metal oxide and carbon-MXene are expected to be promising composites in the future because of their excellent performance, environmental friendliness, simple manufacturing procedures, and commercial viability.
Acknowledgments
S.C. Kim greatly acknowledges the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (2020R1I1A3052258) for financial support.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Rashidi, S.; Esfahani, J.A.; Hormozi, F. Classifications of Porous Materials for Energy Applications. Encycl. Smart Mater. 2021, 774–785. [Google Scholar] [CrossRef]
- AL Shaqsi, A.Z.; Sopian, K.; Al-Hinai, A. Review of Energy Storage Services, Applications, Limitations, and Benefits. Energy Reports 2020, 6, 288–306. [Google Scholar] [CrossRef]
- Grim, R.G.; Huang, Z.; Guarnieri, M.T.; Ferrell, J.R.; Tao, L.; Schaidle, J.A. Transforming the Carbon Economy: Challenges and Opportunities in the Convergence of Low-Cost Electricity and Reductive CO2 Utilization. Energy Environ. Sci. 2020, 13, 472–494. [Google Scholar] [CrossRef]
- Mohanty, B.; Jena, B.K.; Basu, S. Single Atom on the 2D Matrix: An Emerging Electrocatalyst for Energy Applications. ACS Omega 2020, 5, 1287–1295. [Google Scholar] [CrossRef] [PubMed]
- Inagaki, M.; Konno, H.; Tanaike, O. Carbon Materials for Electrochemical Capacitors. J. Power Sources 2010, 195, 7880–7903. [Google Scholar] [CrossRef]
- Raj, B.; Padhy, A.K.; Basu, S.; Mohapatra, M. Review—Futuristic Direction for R&D Challenges to Develop 2D Advanced Materials Based Supercapacitors. J. Electrochem. Soc. 2020, 167, 136501. [Google Scholar] [CrossRef]
- Miao, L.; Song, Z.; Zhu, D.; Li, L.; Gan, L.; Liu, M. Recent Advances in Carbon-Based Supercapacitors. Mater. Adv. 2020, 1, 945–966. [Google Scholar] [CrossRef]
- Béguin, F.; Presser, V.; Balducci, A.; Frackowiak, E. Carbons and Electrolytes for Advanced Supercapacitors. Adv. Mater. 2014, 26, 2219–2251. [Google Scholar] [CrossRef]
- Theerthagiri, J.; Senthil, R.A.; Nithyadharseni, P.; Lee, S.J.; Durai, G.; Kuppusami, P.; Madhavan, J.; Choi, M.Y. Recent Progress and Emerging Challenges of Transition Metal Sulfides Based Composite Electrodes for Electrochemical Supercapacitive Energy Storage. Ceram. Int. 2020, 46, 14317–14345. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, H.; Li, C.; Wang, K.; Sun, X.; Ma, Y. Recent Advances in Porous Graphene Materials for Supercapacitor Applications. RSC Adv. 2014, 4, 45862–45884. [Google Scholar] [CrossRef]
- Huang, S.; Zhu, X.; Sarkar, S.; Zhao, Y. Challenges and Opportunities for Supercapacitors. APL Mater. 2019, 7. [Google Scholar] [CrossRef]
- Najib, S.; Erdem, E. Current Progress Achieved in Novel Materials for Supercapacitor Electrodes: Mini Review. Nanoscale Adv. 2019, 1, 2817–2827. [Google Scholar] [CrossRef] [PubMed]
- Revankar, S.T. Chemical Energy Storage; Elsevier Inc., 2019; ISBN 9780128139752.
- Gautham Prasad, G.; Shetty, N.; Thakur, S.; Rakshitha; Bommegowda, K.B. Supercapacitor Technology and Its Applications: A Review. IOP Conf. Ser. Mater. Sci. Eng. 2019, 561. [Google Scholar] [CrossRef]
- Winter, M.; Brodd, R.J. What Are Batteries, Fuel Cells, and Supercapacitors? Chem. Rev. 2004, 104, 4245–4269. [Google Scholar] [CrossRef]
- Ho, M.Y.; Khiew, P.S.; Isa, D.; Tan, T.K.; Chiu, W.S.; Chia, C.H. A Review of Metal Oxide Composite Electrode Materials for Electrochemical Capacitors. Nano 2014, 9, 1–25. [Google Scholar] [CrossRef]
- Ellenbogen, J.C. Supercapacitors : A Brief Overview. 2006.
- Jayalakshmi, M.; Balasubramanian, K. Simple Capacitors to Supercapacitors - An Overview. Int. J. Electrochem. Sci. 2008, 3, 1196–1217. [Google Scholar] [CrossRef]
- Kiamahalleh, M.V.; Zein, S.H.S.; Najafpour, G.; Sata, S.A.; Buniran, S. Multiwalled Carbon Nanotubes Based Nanocomposites for Supercapacitors: A Review of Electrode Materials. Nano 2012, 7, 1–27. [Google Scholar] [CrossRef]
- Choi, H.; Yoon, H. Nanostructured Electrode Materials for Electrochemical Capacitor Applications. Nanomaterials 2015, 5, 906–936. [Google Scholar] [CrossRef]
- Salunkhe, R.R.; Kaneti, Y.V.; Kim, J.; Kim, J.H.; Yamauchi, Y. Nanoarchitectures for Metal-Organic Framework-Derived Nanoporous Carbons toward Supercapacitor Applications. Acc. Chem. Res. 2016, 49, 2796–2806. [Google Scholar] [CrossRef]
- Tang, J.; Salunkhe, R.R.; Liu, J.; Torad, N.L.; Imura, M.; Furukawa, S.; Yamauchi, Y. Thermal Conversion of Core-Shell Metal-Organic Frameworks: A New Method for Selectively Functionalized Nanoporous Hybrid Carbon. J. Am. Chem. Soc. 2015, 137, 1572–1580. [Google Scholar] [CrossRef]
- Gu, W.; Yushin, G. Review of Nanostructured Carbon Materials for Electrochemical Capacitor Applications: Advantages and Limitations of Activated Carbon, Carbide-Derived Carbon, Zeolite-Templated Carbon, Carbon Aerogels, Carbon Nanotubes, Onion-like Carbon, and Graphene. Wiley Interdiscip. Rev. Energy Environ. 2014, 3, 424–473. [Google Scholar] [CrossRef]
- Mohapatra, S.; Acharya, A.; Roy, G.S. The Role of Nanomaterial for the Design of Supercapacitors. Lat. Am. J. Phys. Educ. 2012, 6, 380–384. [Google Scholar]
- Beidaghi, M.; Wang, C. Micro-Supercapacitors Based on Interdigital Electrodes of Reduced Graphene Oxide and Carbon Nanotube Composites with Ultrahigh Power Handling Performance. Adv. Funct. Mater. 2012, 22, 4501–4510. [Google Scholar] [CrossRef]
- Chen, T.; Dai, L. Carbon Nanomaterials for High-Performance Supercapacitors. Mater. Today 2013, 16, 272–280. [Google Scholar] [CrossRef]
- Yan, Y.; Wang, T.; Li, X.; Pang, H.; Xue, H. Noble Metal-Based Materials in High-Performance Supercapacitors. Inorg. Chem. Front. 2017, 4, 33–51. [Google Scholar] [CrossRef]
- Bao, L.; Zang, J.; Li, X. Flexible Zn2SnO4/MnO2 Core/Shell Nanocable-Carbon Microfiber Hybrid Composites for High-Performance Supercapacitor Electrodes. Nano Lett. 2011, 11, 1215–1220. [Google Scholar] [CrossRef]
- Ghosh, D.; Giri, S.; Moniruzzaman, M.; Basu, T.; Mandal, M.; Das, C.K. α MnMoO4/Graphene Hybrid Composite: High Energy Density Supercapacitor Electrode Material. Dalt. Trans. 2014, 43, 11067–11076. [Google Scholar] [CrossRef] [PubMed]
- Neiber, R.R.; Kumar, J.; Soomro, R.A.; Karkus, S.; Abo-Dief, H.M.; Alanazi, A.K.; Altalhia, A.A.; El-Bahy, Z.M. NiZnCoO4/CoWO4 Hybrid Composite with Improved Electrochemical Performance for Supercapacitor Application. J. Energy Storage 2022, 52. [Google Scholar] [CrossRef]
- Iro, Z.S.; Subramani, C.; Dash, S.S. A Brief Review on Electrode Materials for Supercapacitor. Int. J. Electrochem. Sci. 2016, 11, 10628–10643. [Google Scholar] [CrossRef]
- Wu, N.; Bai, X.; Pan, D.; Dong, B.; Wei, R.; Naik, N.; Patil, R.R.; Guo, Z. Recent Advances of Asymmetric Supercapacitors. Adv. Mater. Interfaces 2021, 8, 1–17. [Google Scholar] [CrossRef]
- Zheng, S.; Wu, Z.S.; Wang, S.; Xiao, H.; Zhou, F.; Sun, C.; Bao, X.; Cheng, H.M. Graphene-Based Materials for High-Voltage and High-Energy Asymmetric Supercapacitors. Energy Storage Mater. 2017, 6, 70–97. [Google Scholar] [CrossRef]
- Shao, Y.; El-Kady, M.F.; Sun, J.; Li, Y.; Zhang, Q.; Zhu, M.; Wang, H.; Dunn, B.; Kaner, R.B. Design and Mechanisms of Asymmetric Supercapacitors. Chem. Rev. 2018, 118, 9233–9280. [Google Scholar] [CrossRef] [PubMed]
- Stoller, M.D.; Ruoff, R.S. Best Practice Methods for Determining an Electrode Material’s Performance for Ultracapacitors. Energy Environ. Sci. 2010, 3, 1294–1301. [Google Scholar] [CrossRef]
- Balducci, A.; Dugas, R.; Taberna, P.; Simon, P.; Plee, D.; Mastragostino, M.; Passerini, S.; Balducci, A.; Dugas, R.; Taberna, P.; et al. High Temperature Carbon – Carbon Supercapacitor Using Ionic Liquid as Electrolyte To Cite This Version : HAL Id : Hal-03592593. 2022.
- Fields, E.; Fields, S.E. Encyclopedia of Applied Electrochemistry; 2014; ISBN 9781441969965.
- Mahankali, K.; Thangavel, N.K.; Ding, Y.; Putatunda, S.K.; Arava, L.M.R. Interfacial Behavior of Water-in-Salt Electrolytes at Porous Electrodes and Its Effect on Supercapacitor Performance. Electrochim. Acta 2019, 326, 134989. [Google Scholar] [CrossRef]
- Liu, X.; Xu, W.; Zheng, D.; Li, Z.; Zeng, Y.; Lu, X. Carbon Cloth as an Advanced Electrode Material for Supercapacitors: Progress and Challenges. J. Mater. Chem. A 2020, 8, 17938–17950. [Google Scholar] [CrossRef]
- Tutlienė, S.; Petrulevičienė, M.; Pilipavičius, J.; Žarkov, A.; Selskis, A.; Stanionytė, S.; Juodkazytė, J.; Vilčiauskas, L. Electrochemical Performance of Sol-Gel Synthesized NaTi 2 (PO 4 ) 3 - Carbon Composites as Aqueous Na-Ion Battery Anodes. J. Electrochem. Soc. 2021, 168, 060545. [Google Scholar] [CrossRef]
- Lan, G.; Yang, J.; Ye, R.P.; Boyjoo, Y.; Liang, J.; Liu, X.; Li, Y.; Liu, J.; Qian, K. Sustainable Carbon Materials toward Emerging Applications. Small Methods 2021, 5, 1–18. [Google Scholar] [CrossRef]
- Pandolfo, A.G.; Hollenkamp, A.F. Carbon Properties and Their Role in Supercapacitors. J. Power Sources 2006, 157, 11–27. [Google Scholar] [CrossRef]
- Syarif, N.; Ivandini Tribidasari; Wibowo, W. Direct Synthesis Carbon/Metal Oxide Composites for Electrochemical Capacitors Electrode. Int. Trans. J. Eng. Manag. Appl. Sci. Technol. 2012, 3, 21–34. [Google Scholar]
- Lota, K.; Sierczynska, A.; Acznik, I.; Lota, G. Effect of Aqueous Electrolytes on Electrochemical Capacitor Capacitance. Chemik 2013, 67, 1138–1145. [Google Scholar]
- Lufrano, F.; Staiti, P. Mesoporous Carbon Materials as Electrodes for Electrochemical Supercapacitors. Int. J. Electrochem. Sci. 2010, 5, 903–916. [Google Scholar] [CrossRef]
- Tamilarasan, P.; Mishra, A.K.; Ramaprabhu, S. Graphene/Ionic Liquid Binary Electrode Material for High Performance Supercapacitor. 2011 Int. Conf. Nanosci. Technol. Soc. Implic. NSTSI11 2011, 1–5. [Google Scholar] [CrossRef]
- John R. Miller, R.A. Outlaw, B.C.H. Graphene Electric Double Layer Capacitor with Ultra-High-Power Performance. Electrochimica Acta (2011) 56(28) 10443-10449.
- Kim, T.Y.; Lee, H.W.; Stoller, M.; Dreyer, D.R.; Bielawski, C.W.; Ruoff, R.S.; Suh, K.S. High-Performance Supercapacitors Based on Poly(Ionic Liquid)-Modified Graphene Electrodes. ACS Nano 2011, 5, 436–442. [Google Scholar] [CrossRef] [PubMed]
- Apriwandi, A.; Taer, E.; Farma, R.; Setiadi, R.N.; Amiruddin, E. A Facile Approach of Micro-Mesopores Structure Binder-Free Coin/Monolith Solid Design Activated Carbon for Electrode Supercapacitor. J. Energy Storage 2021, 40, 102823. [Google Scholar] [CrossRef]
- Gou, G.; Huang, F.; Jiang, M.; Li, J.; Zhou, Z. Hierarchical Porous Carbon Electrode Materials for Supercapacitor Developed from Wheat Straw Cellulosic Foam. Renew. Energy 2020, 149, 208–216. [Google Scholar] [CrossRef]
- Du, J.; Zhang, Y.; Lv, H.; Hou, S.; Chen, A. Yeasts-Derived Nitrogen-Doped Porous Carbon Microcapsule Prepared by Silica-Confined Activation for Supercapacitor. J. Colloid Interface Sci. 2021, 601, 467–473. [Google Scholar] [CrossRef]
- Zhao, X.Y.; Huang, S.S.; Cao, J.P.; Xi, S.C.; Wei, X.Y.; Kamamoto, J.; Takarada, T. KOH Activation of a HyperCoal to Develop Activated Carbons for Electric Double-Layer Capacitors. J. Anal. Appl. Pyrolysis 2014, 105, 116–121. [Google Scholar] [CrossRef]
- Zhao, X.Y.; Huang, S.S.; Cao, J.P.; Wei, X.Y.; Magarisawa, K.; Takarada, T. HyperCoal-Derived Porous Carbons with Alkaline Hydroxides and Carbonate Activation for Electric Double-Layer Capacitors. Fuel Process. Technol. 2014, 125, 251–257. [Google Scholar] [CrossRef]
- Zhang, W.L.; Xu, J.H.; Hou, D.X.; Yin, J.; Liu, D.B.; He, Y.P.; Lin, H.B. Hierarchical Porous Carbon Prepared from Biomass through a Facile Method for Supercapacitor Applications. J. Colloid Interface Sci. 2018, 530, 338–344. [Google Scholar] [CrossRef]
- Zhao, G.; Yu, D.; Zhang, H.; Sun, F.; Li, J.; Zhu, L.; Sun, L.; Yu, M.; Besenbacher, F.; Sun, Y. Sulphur-Doped Carbon Nanosheets Derived from Biomass as High-Performance Anode Materials for Sodium-Ion Batteries. Nano Energy 2020, 67, 104219. [Google Scholar] [CrossRef]
- Luo, H.; Chen, M.; Cao, J.; Zhang, M.; Tan, S.; Wang, L.; Zhong, J.; Deng, H.; Zhu, J.; Lu, B. Cocoon Silk-Derived, Hierarchically Porous Carbon as Anode for Highly Robust Potassium-Ion Hybrid Capacitors. Nano-Micro Lett. 2020, 12, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.; Balasubramanian, R.; Srinivasan, M.P. Hydrothermal Conversion of Biomass Waste to Activated Carbon with High Porosity: A Review. Chem. Eng. J. 2016, 283, 789–805. [Google Scholar] [CrossRef]
- Gao, F.; Shao, G.; Qu, J.; Lv, S.; Li, Y.; Wu, M. Tailoring of Porous and Nitrogen-Rich Carbons Derived from Hydrochar for High-Performance Supercapacitor Electrodes. Electrochim. Acta 2015, 155, 201–208. [Google Scholar] [CrossRef]
- Yuancheng Huang, Zheng Tang, Siyu Zhou, Hong Wang, Yougen Tang, D.S. and H.W. Renewable Waste Biomass-Derived Carbon Materials for Energy Storage. Yuancheng Huang et Al 2022 J. Phys. D: Appl. Phys. 55 313002.
- Gogoi, D.; Makkar, P.; Das, M.R.; Ghosh, N.N. CoFe2O4 Nanoparticle Decorated Hierarchical Biomass Derived Porous Carbon Based Nanocomposites for High-Performance All-Solid-State Flexible Asymmetric Supercapacitor Devices. ACS Appl. Electron. Mater. 2022, 4, 795–806. [Google Scholar] [CrossRef]
- Cai, J.; Zhang, D.; Ding, W.P.; Zhu, Z.Z.; Wang, G.Z.; He, J.R.; Wang, H.B.; Fei, P.; Si, T.L. Promising Rice-Husk-Derived Carbon/Ni(OH)2 Composite Materials as a High-Performing Supercapacitor Electrode. ACS Omega 2020, 5, 29896–29902. [Google Scholar] [CrossRef]
- Jin, T.; Su, J.; Luo, Q.; Zhu, W.; Lai, H.; Huang, D.; Wang, C. Preparation of N,P Co-Doped Porous Carbon Derived from Daylily for Supercapacitor Applications. ACS Omega 2022, 7, 37564–37571. [Google Scholar] [CrossRef]
- Kreno, L.E.; Leong, K.; Farha, O.K.; Allendorf, M.; Van Duyne, R.P.; Hupp, J.T. Metal-Organic Framework Materials as Chemical Sensors. Chem. Rev. 2012, 112, 1105–1125. [Google Scholar] [CrossRef]
- Islamoglu, T.; Goswami, S.; Li, Z.; Howarth, A.J.; Farha, O.K.; Hupp, J.T. Postsynthetic Tuning of Metal-Organic Frameworks for Targeted Applications. Acc. Chem. Res. 2017, 50, 805–813. [Google Scholar] [CrossRef]
- Xiao, J.D.; Jiang, H.L. Metal-Organic Frameworks for Photocatalysis and Photothermal Catalysis. Acc. Chem. Res. 2019, 52, 356–366. [Google Scholar] [CrossRef]
- Salunkhe, R.R.; Kamachi, Y.; Torad, N.L.; Hwang, S.M.; Sun, Z.; Dou, S.X.; Kim, J.H.; Yamauchi, Y. Fabrication of Symmetric Supercapacitors Based on MOF-Derived Nanoporous Carbons. J. Mater. Chem. A 2014, 2, 19848–19854. [Google Scholar] [CrossRef]
- Torad, N.L.; Hu, M.; Kamachi, Y.; Takai, K.; Imura, M.; Naito, M.; Yamauchi, Y. Facile Synthesis of Nanoporous Carbons with Controlled Particle Sizes by Direct Carbonization of Monodispersed ZIF-8 Crystals. Chem. Commun. 2013, 49, 2521–2523. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Fu, N.; Zhao, J.; Liu, R.; Li, F.; Du, Y.; Yang, Z. High Specific Capacitance Electrode Material for Supercapacitors Based on Resin-Derived Nitrogen-Doped Porous Carbons. ACS Omega 2019, 4, 15904–15911. [Google Scholar] [CrossRef] [PubMed]
- Iyyamperumal, E.; Wang, S.; Dai, L. Vertically Aligned BCN Nanotubes with High Capacitance. ACS Nano 2012, 6, 5259–5265. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Lee, J.S.; Zhu, Q.; Liu, J.; Wang, Y.; Dai, S. Ammonia-Treated Ordered Mesoporous Carbons as Catalytic Materials for Oxygen Reduction Reaction. Chem. Mater. 2010, 22, 2178–2180. [Google Scholar] [CrossRef]
- Qie, L.; Chen, W.M.; Wang, Z.H.; Shao, Q.G.; Li, X.; Yuan, L.X.; Hu, X.L.; Zhang, W.X.; Huang, Y.H. Nitrogen-Doped Porous Carbon Nanofiber Webs as Anodes for Lithium Ion Batteries with a Superhigh Capacity and Rate Capability. Adv. Mater. 2012, 24, 2047–2050. [Google Scholar] [CrossRef]
- Xu, J.; Sun, Y.; Lu, M.; Wang, L.; Zhang, J.; Qian, J.; Liu, X. Fabrication of Hierarchical MnMoO4·H2O@MnO2 Core-Shell Nanosheet Arrays on Nickel Foam as an Advanced Electrode for Asymmetric Supercapacitors. Chem. Eng. J. 2018, 334, 1466–1476. [Google Scholar] [CrossRef]
- Xu, K.; Yang, J.; Hu, J. Synthesis of Hollow NiCo2O4 Nanospheres with Large Specific Surface Area for Asymmetric Supercapacitors. J. Colloid Interface Sci. 2018, 511, 456–462. [Google Scholar] [CrossRef]
- Hussain, I.; Mak, T.; Zhang, K. Boron-Doped Trimetallic Cu-Ni-Co Oxide Nanoneedles for Supercapacitor Application. ACS Appl. Nano Mater. 2021, 4, 129–141. [Google Scholar] [CrossRef]
- Zhou, X.; Dai, H.; Huang, X.; Ren, Y.; Wang, Q.; Wang, W.; Huang, W.; Dong, X. Porous Trimetallic Fluoride Ni–Co–M (M = Mn, Fe, Cu, Zn) Nanoprisms as Electrodes for Asymmetric Supercapacitors. Mater. Today Energy 2020, 17, 1–8. [Google Scholar] [CrossRef]
- Cottineau, T.; Toupin, M.; Delahaye, T.; Brousse, T.; Bélanger, D. Nanostructured Transition Metal Oxides for Aqueous Hybrid Electrochemical Supercapacitors. Appl. Phys. A Mater. Sci. Process. 2006, 82, 599–606. [Google Scholar] [CrossRef]
- Guo, X.L.; Li, G.; Kuang, M.; Yu, L.; Zhang, Y.X. Tailoring Kirkendall Effect of the KCu7S4 Microwires towards CuO@MnO2 Core-Shell Nanostructures for Supercapacitors. Electrochim. Acta 2015, 174, 87–92. [Google Scholar] [CrossRef]
- Zheng, Y.; Li, Z.; Xu, J.; Wang, T.; Liu, X.; Duan, X.; Ma, Y.; Zhou, Y.; Pei, C. Multi-Channeled Hierarchical Porous Carbon Incorporated Co3O4 Nanopillar Arrays as 3D Binder-Free Electrode for High Performance Supercapacitors. Nano Energy 2016, 20, 94–107. [Google Scholar] [CrossRef]
- Zhiwei Tian a, 1;, Huiling Li a, 1; B, W.Y.; B, S.J.; Chunmei Zhang c Shaohua Jiang a, B. Fabrication of Manganese Oxides/Carbon Composites for High Energy Density Asymmetric Supercapacitor.
- Chen, Y.; Yang, H.; Han, Z.; Bo, Z.; Yan, J.; Cen, K.; Ostrikov, K.K. MXene-Based Electrodes for Supercapacitor Energy Storage. Energy and Fuels 2022, 36, 2390–2406. [Google Scholar] [CrossRef]
- Sugahara, A.; Ando, Y.; Kajiyama, S.; Yazawa, K.; Gotoh, K.; Otani, M.; Okubo, M.; Yamada, A. Negative Dielectric Constant of Water Confined in Nanosheets. Nat. Commun. 2019, 10, 1–7. [Google Scholar] [CrossRef]
- Hu, M.; Hu, T.; Li, Z.; Yang, Y.; Cheng, R.; Yang, J.; Cui, C.; Wang, X. Surface Functional Groups and Interlayer Water Determine the Electrochemical Capacitance of Ti3C2 T x MXene. ACS Nano 2018, 12, 3578–3586. [Google Scholar] [CrossRef] [PubMed]
- Boota, M.; Chen, C.; Van Aken, K.L.; Jiang, J.; Gogotsi, Y. Organic-Inorganic All-Pseudocapacitive Asymmetric Energy Storage Devices. Nano Energy 2019, 65, 104022. [Google Scholar] [CrossRef]
- Luo, J.; Zhang, W.; Yuan, H.; Jin, C.; Zhang, L.; Huang, H.; Liang, C.; Xia, Y.; Zhang, J.; Gan, Y.; et al. Pillared Structure Design of MXene with Ultralarge Interlayer Spacing for High-Performance Lithium-Ion Capacitors. ACS Nano 2017, 11, 2459–2469. [Google Scholar] [CrossRef] [PubMed]
- Ma, R.; Zhang, X.; Zhuo, J.; Cao, L.; Song, Y.; Yin, Y.; Wang, X.; Yang, G.; Yi, F. Self-Supporting, Binder-Free, and Flexible Ti3C2TxMXene-Based Supercapacitor Electrode with Improved Electrochemical Performance. ACS Nano 2022, 16, 9713–9727. [Google Scholar] [CrossRef]
- Shi, B.; Chen, L.; Jen, T.C.; Liu, X.; Li, L.; Chen, A.; Shen, G. Vertical Arrangement of Ti2CTx MXene Nanosheets on Carbon Fibers for High-Performance and Flexible Zn-Ion Supercapacitors. ACS Appl. Nano Mater. 2023, 6, 315–322. [Google Scholar] [CrossRef]
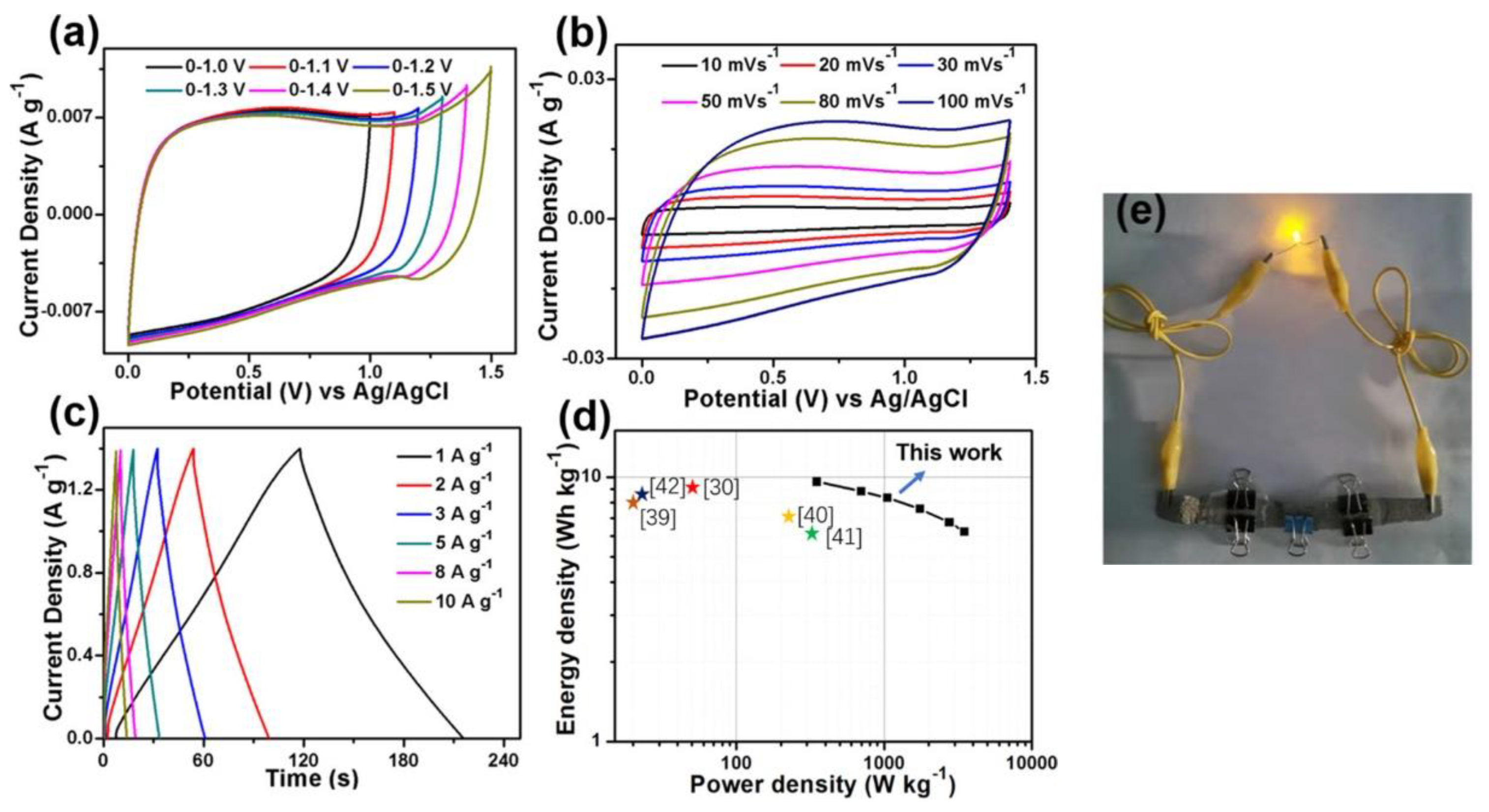
- Farma, R.; Indriani, A.; Apriyani, I. Hierarchical-Nanofiber Structure of Biomass-Derived Carbon Framework with Direct CO2 Activation for Symmetrical Supercapacitor Electrodes. J. Mater. Sci. Mater. Electron. 2023, 34, 1–15. [Google Scholar] [CrossRef]
- Palanisamy, R.; Karuppiah, D.; Rengapillai, S.; Abdollahifar, M.; Ramasamy, G.; Wang, F.M.; Liu, W.R.; Ponnuchamy, K.; Shim, J.; Marimuthu, S. A Reign of Bio-Mass Derived Carbon with the Synergy of Energy Storage and Biomedical Applications. J. Energy Storage 2022, 51, 104422. [Google Scholar] [CrossRef]
- Wu, J.; Zhou, W.; Jiang, F.; Chang, Y.; Zhou, Q.; Li, D.; Ye, G.; Li, C.; Nie, G.; Xu, J.; et al. Three-Dimensional Porous Carbon Derived from Polyindole Hollow Nanospheres for High-Performance Supercapacitor Electrode. ACS Appl. Energy Mater. 2018, 1, 4572–4579. [Google Scholar] [CrossRef]
- Zhu, T.; Zhou, J.; Li, Z.; Li, S.; Si, W.; Zhuo, S. Hierarchical Porous and N-Doped Carbon Nanotubes Derived from Polyaniline for Electrode Materials in Supercapacitors. J. Mater. Chem. A 2014, 2, 12545–12551. [Google Scholar] [CrossRef]
- Shu, Y.; Maruyama, J.; Iwasaki, S.; Maruyama, S.; Shen, Y.; Uyama, H. Fabrication of N-Doped and Shape-Controlled Porous Monolithic Carbons from Polyacrylonitrile for Supercapacitors. RSC Adv. 2017, 7, 43172–43180. [Google Scholar] [CrossRef]
- Liu, S.; Kang, L.; Hu, J.; Jung, E.; Zhang, J.; Jun, S.C.; Yamauchi, Y. Unlocking the Potential of Oxygen-Deficient Copper-Doped Co3O4Nanocrystals Confined in Carbon as an Advanced Electrode for Flexible Solid-State Supercapacitors. ACS Energy Lett. 2021, 6, 3011–3019. [Google Scholar] [CrossRef]
- Yesuraj, J.; Vajravijayan, S.; Yang, R.; Nandhagopal, N.; Gunasekaran, K.; Selvam, N.C.S.; Yoo, P.J.; Kim, K. Self-Assembly of Hausmannite Mn3O4Triangular Structures on Cocosin Protein Scaffolds for High Energy Density Symmetric Supercapacitor Application. Langmuir 2022, 38, 2928–2941. [Google Scholar] [CrossRef] [PubMed]
- Hariharan, G.; Arunpandiyan, S.; Shanmugapriya, V.; Bharathi, S.; Babu, M.; Selvakumar, B.; Arivarasan, A. Role of Various Redox Additive Electrolytes on the Electrochemical Performances of Mixed Metal Oxide Loaded Multiwalled Carbon Nanotube Based Supercapacitors. J. Energy Storage 2023, 57, 106178. [Google Scholar] [CrossRef]
- Gouda, M.S.; Shehab, M.; Helmy, S.; Soliman, M.; Salama, R.S. Nickel and Cobalt Oxides Supported on Activated Carbon Derived from Willow Catkin for Efficient Supercapacitor Electrode. J. Energy Storage 2023, 61, 106806. [Google Scholar] [CrossRef]
- Luo, W.; Sun, Y.; Lin, Z.; Li, X.; Han, Y.; Ding, J.; Li, T.; Hou, C.; Ma, Y. Flexible Ti3C2Tx MXene/V2O5 Composite Films for High-Performance All-Solid Supercapacitors. J. Energy Storage 2023, 62, 106807. [Google Scholar] [CrossRef]
- Lin, Z.; Li, X.; Li, S.; Li, B.; Ding, J.; Han, Y.; Li, T.; Hou, C.; Ma, Y. Highly Flexible, Foldable Carbon Cloth/MXene/Polyaniline/CoNi Layered Double Hydroxide Electrode for High-Performance All Solid-State Supercapacitors. J. Energy Storage 2023, 64, 107116. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).