1. Introduction
Food security, energy supply, and climate change are among the biggest global challenges for the near future. For this reason, bioeconomy represents one of the major pillars for sustainable global development. The emerging bioeconomy basically involves the use of advanced biobased technologies to develop new sustainable processes and products from renewable materials [
1,
2,
3]. The conversion of lignocellulosic materials to fuel ethanol and other sustainable biofuels for the transport sector has a key role in facilitating the energy transition toward a net zero carbon emission to the atmosphere. Besides, by integrating the production of valuable biobased materials with biofuels and bioenergy, the overall profitability and productivity of biomass conversion processes can be potentially improved [
4].
Uruguay has large amount of eucalypt sawdust as a lignocellulosic agro-industrial residue with potential to produce cellulosic ethanol and other products within a forest biorefinery approach.
Eucalyptus spp. are fast-growing species that can achieve high biomass production and productivities.
E. grandis,
E. dunnii and
E. globulus are the most widely used species in Uruguayan pulp mills. Large-scale plantations of great economic importance are available for these species and their hybrids, and large quantities of wood residues are generated at the pulp mills during their processing [
5].
The conversion of lignocellulosic biomass to ethanol requires a pretreatment step, the enzymatic hydrolysis of plant polysaccharides (mostly glucans), and the conversion of fermentable sugars to fuel ethanol. The purpose of pretreatment is to increase the accessibility of enzymes to structural polysaccharides such as cellulose, preserving hemicellulose and lignin components for their subsequent recovery as co-products while minimizing yield losses to side reaction such as dehydration.
A wide variety of pretreatments have been used so far to breakdown the complex structure of the plant cell wall [
6,
7,
8,
9,
10,
11]. Steam explosion is known as an efficient, environmentally friendly, generally chemical-free industrially scalable pretreatment with which the biomass structure is altered by high pressure steaming followed by an explosive decompression [
11,
12,
13]. During pretreatment, furan compounds (furfural, HMF, and their derivatives), aliphatic organic acids (acetic, formic, levulinic acids), phenolic acids (p-hydroxybenzoic, syringic, ferulic, and p-coumaric acids), and xylooligomers can be formed by dehydration, hydrolysis and oxidation, and these may act synergistically as potential inhibitors for enzymatic hydrolysis and fermentation [
14,
15,
16]. To avoid such inhibitory effects, water-soluble sugars and degradation products can be separated by centrifugation and water washing of pretreatment solids. However, these operations increase production time and both electricity and water consumption in the process.
Working at high total solids (TS >15 wt%) increases the concentration of fermentable sugars and improves ethanol production, thereby reducing the energy costs of ethanol recovery by distillation [
11,
17,
18]. By contrast, working at low solids facilitates the mass transfer of enzymes and products, and reduces inhibition in both hydrolysis and fermentation stages. High TS technologies such as Very High Gravity (VHG) have been widely used in industrial scale to improve ethanol productivity from grains but not from lignocellulosic materials [
19].
The use of high TS causes various difficulties in handling and mixing of pretreated materials due to its high initial viscosity, which requires a powerful impeller system [
20,
21]. High enzyme dosages and the use of auxiliary enzymes such as xylanases is often necessary to improve enzymatic hydrolysis by decreasing the material viscosity and improving flow properties. Another alternative to increase enzyme accessibility and its catalytic activity, consequently increasing enzymatic hydrolysis yields without increasing enzyme dosage, is the use of additives such as non-catalytic proteins (BSA) or non-ionic surfactants (polyvinylpyrrolidone, polyethylene glycol, Tween) [
11,
22,
23,
24].
The high concentration of inhibitors in pretreated lignocellulosic materials, when working at high TS, is one of the limiting factors for achieving high yields of enzymatic hydrolysis and ethanol production. To this end, water washing represents an important process step since adding a detoxification stage can imply a substantial increase in the process costs [
25,
26,
27].
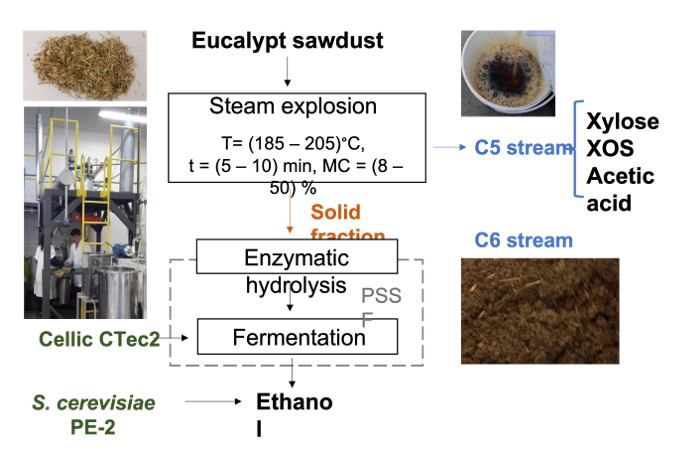
In this work, eucalypt sawdust was steam-exploded for cellulosic ethanol production within the biorefinery concept, aiming at maximizing process yields while minimizing the release of inhibitory compounds. The effect of water washing on enzymatic hydrolysis and fermentation using relatively high concentration of pretreated solids was investigated. A pre-saccharification and simultaneous fermentation strategy (PSSF) was used to reduce the medium viscosity during pre-hydrolysis, allowing better mixing in the subsequent simultaneous saccharification and fermentation (SSF) stage. The effect of other process variables was also evaluated on the final glucose concentration after enzymatic hydrolysis, such as sawdust initial moisture content, reaction temperature, and the biomass residence time into the steam reactor. All other process streams were characterized to evaluate the recovery yield of valuable co-products.
2. Materials and Methods
2.1. Raw Material
Eucalyptus grandis sawdust was obtained from a local pulp mill (UPM, Uruguay). The biomass was dried at 40°C until 8% moisture content and stored at room temperature. Material with particle size below 0.5 mm was discarded. The sieved sawdust chemical composition included 43.4 ± 1.2% glucans (mostly cellulose), 13.9 ± 0.4% hemicelluloses (mostly xylan) containing 0.3 ± 0.1% arabinosyl and 3.6 ± 0.1% acetyl groups, 26.1 ± 1.2% total lignin, 4.6 ± 0.1% total extractives and 0.8 ± 0.1% ash.
2.2. Sawdust Pretreatment
Steam explosion was carried out in a high-pressure batch steam reactor with a nominal volume of 10 L, provided with automatic sensors to control pressure, temperature, and reaction time [
28,
29]. Nearly 735 g of dry sawdust was used per each condition tested. A 2
3 factorial design was performed with three replicates at the central point using the following process variables: temperature (T) (185 and 205°C), reaction time (t) (5 and 10 min) and sawdust moisture content (MC) (8 and 50%). A 50% moisture content corresponds to the same humidity of fresh wood and 8% is the value achieved by ambient humidity equilibria in Uruguay. Two additional points were carried out in duplicate (cube face centers for moisture content, which were identified as assays EV10A, EV10B, EV11A, EV11B) to validate the model.
Table 1 shows the conditions tested in the 2
3 factorial design.
Moisture contents of 29 and 50% were achieved by soaking air-dried sawdust in distilled water and keeping the moisturized material in a closed plastic bag overnight at room temperature. Pretreatment was carried out after pre-heating the reactor vessel to the desired temperature and eliminating any steam condensate. Samples were loaded into the reactor vessel, the reactor was closed, and the temperature was raised up to the setpoint by injection of saturated steam. Once the stipulated residence time at the desired temperature was reached, the reactor was rapidly depressurized to atmospheric pressure. The exploded material was collected in a cyclone equipped with a stainless-steel sample collector. After this first decompression, a second explosion was performed to recover any residual material that may have been retained inside the reactor. The solid fraction was separated by centrifugation at 1000 rpm, followed by filtration through a nylon tissue in a Büchner funnel to remove most of the pretreatment liquor. The recovered solids were washed by immersion in distilled water (10 wt% TS) for 30 min at ambient temperature and filtered using a Büchner funnel once again; water washing was repeated four times until pH 5 in the wash waters. Raw material, pretreatment liquor and recovered solids were characterized following National Renewable Energy Laboratory (NREL) protocols [
30,
31,
32]. Xylo-oligosaccharides (XOS) were determined as the difference between xylose content before and after dilute sulfuric acid hydrolysis.
2.3. Enzymatic Hydrolysis
The effect of pretreatment on enzymatic hydrolysis was evaluated by tests performed at 4 wt% TS using low enzyme loadings. These conditions were used to avoid compensating for an inefficient pretreatment by applying high enzyme loadings or masking an efficient pretreatment by diffusional restrictions attributable to high substrate concentrations. The pretreated solids were hydrolyzed in duplicate for 96 h at 48°C and 150 rpm using 100 mmol/L acetate buffer (pH 4.8), 0.1% (w/v) sodium azide and 5 FPU/g TS of Cellic CTec2 from Novozymes (Bagsværd, Denmark). Hydrolysis was carried out in 250 mL Erlenmeyer flasks containing 100 mL of suspended solids. Aliquots (1 mL) were withdrawn at different reaction times, heated for 5 min in a boiling water bath for enzyme denaturation, and centrifuged at 13000 rpm for 10 min. The supernatants were analyzed by HPLC according to the previously mentioned NREL protocol [
30].
2.4. Saccharification and Fermentation
After selecting the best pretreatment condition, enzymatic hydrolysis and fermentation were carried out using washed and unwashed exploded solids (solid obtained after filtration of the whole slurry) at two solids loading: 13 and 27 wt% TS. Glucose concentration for unwashed and washed exploded solids at 13 wt% TS were fitted by fractal kinetic modeling using the Equation 1:
where G(t) is the final glucose concentration (g/L), G
0 is the initial glucan concentration (expressed as glucose in g/L), t is the reaction time (h), k is the time dependent rate coefficient, and h is the fractal exponent.
Fermentations were carried out in duplicate using a PSSF strategy in 250 mL Erlenmeyer flasks containing 100 mL media. Pre-saccharification was performed at 48°C, 150 rpm for 24 h in citrate buffer 0.05 mol/L pH 4.8 containing 25 FPU/g glucan of CellicCTec2 and 20 mg/L chloramphenicol, while SSF was realized at 35°C, 100 rpm for 48 h, using
S. cerevisiae PE-2 at initial yeast concentration of 1x10
8 cells/mL [
7].
2.5. Statistical Analysis
The errors for each variable were estimated as standard deviations of replicated assays. Analysis of variance (ANOVA), regression equations and optimization by the desirability function was done with a 95% confidence level (α = 0.05) using the Statistica 8.0 software.
3. Results and Discussion
3.1. Pretreatment of Eucalypt Sawdust
Table 1 shows the results in terms of pH and solids recovery after pretreatment at different experimental conditions (t, T, MC), along with the compositional analysis of pretreatment liquors and water-washed solids, always expressed per 100 g sawdust (dry basis). Solids recovery ranged from 75 to 86%. As expected, these values decreased with an increase in the pretreatment severity factor (S
0). This decrease occurs due to a greater materials solubilization, mainly from the hemicellulosic fraction and easily degradable lignin. Additionally, mass losses may also be partially attributed to secondary by-products that were not quantified (including volatiles) and to residual materials that could not be recovered from the reactor vessel.
Hemicellulose sugars were partially recovered in the pretreatment liquor mostly in the oligomeric form. The recovery of water-soluble xylosaccharides (XS, xylose plus xylo-oligosaccharides expressed as xylose) were in the range of 24 to 55% (
Table 1). Martin-Sampedro et al. [
15] achieved a maximum XS recovery of 19% using eucalypt under similar pretreatment severities, while Park et al. [
34] reached 33% using eucalypt and NaOH-catalyzed steam explosion. On the other hand, Rochón et al. [
9] achieved a XS recovery of 82% (mainly as xylose), working at 180°C for 10 min in a semi-continuous steam explosion reactor. In our work, XS recovery in the liquid fraction was influenced significantly by pretreatment time and the T-t interaction at a 95% confidence level (
p < 0.05). By increasing the time and decreasing the T-t interaction, an increase in xylose recovery was observed in the liquid stream (
Figure S1 in the Supplementary Material). The highest average recovery values were reached for EV2A and EV4A, in which pretreatment was carried out at 185°C for 10 min with a sawdust moisture content of 8 and 50%, respectively.
Acetyl groups were recovered in high yields (between 71 and 97%) in the liquid stream. The highest value (97%) was obtained for the mildest pretreatment condition (185°C, 5 min), mainly linked to xylo-oligosaccharides (XOS), with a concentration of 20 g/L in the pretreatment liquor. Also, part of the acetyl groups was released as acetic acid from the xylan backbone by direct acid hydrolysis [
30]. In general, if isolated from pretreatment liquors in high yields, XOS are compounds with high added value mainly due to their application as prebiotics in the food and pharmaceutical industries [
35,
36,
37].
Other compounds such as HMF, furfural, formic acid and levulinic acids were obtained in small quantities in the pretreatment liquor, at concentrations of 0.1 g/L, 0.1 to 0.5 g/L and 0.1 to 2.8, 0.1 to 1.5 g/L, respectively. These values are higher than those obtained by steam explosion of olive pruning [
38],
E. globulus chips [
39,
40] and sugarcane bagasse [
41].
None of the variables studied (T, t, and MC) was statistically significant for the recovery of acetyl groups, furfural or HMF in the pretreatment liquor at a 95% confidence level (p < 0.05). Therefore, a model could not be established for these response functions in the present study. Besides, the concentration of furfural and HMF in liquid streams was either constant or undetectable for all conditions tested in the experimental design. Levulinic acid was the main by-product obtained from acid catalyzed hexose dehydration, ranging between 0.1 and 0.4 g per 100 g of sawdust except for pretreatment at the lowest severity (185°C for 5 min), where its production was not detected. The production of this acid increased positively with temperature, which was to be expected because more extreme pretreatment conditions (or higher pretreatment severities) favor side reactions involved in sugar dehydration.
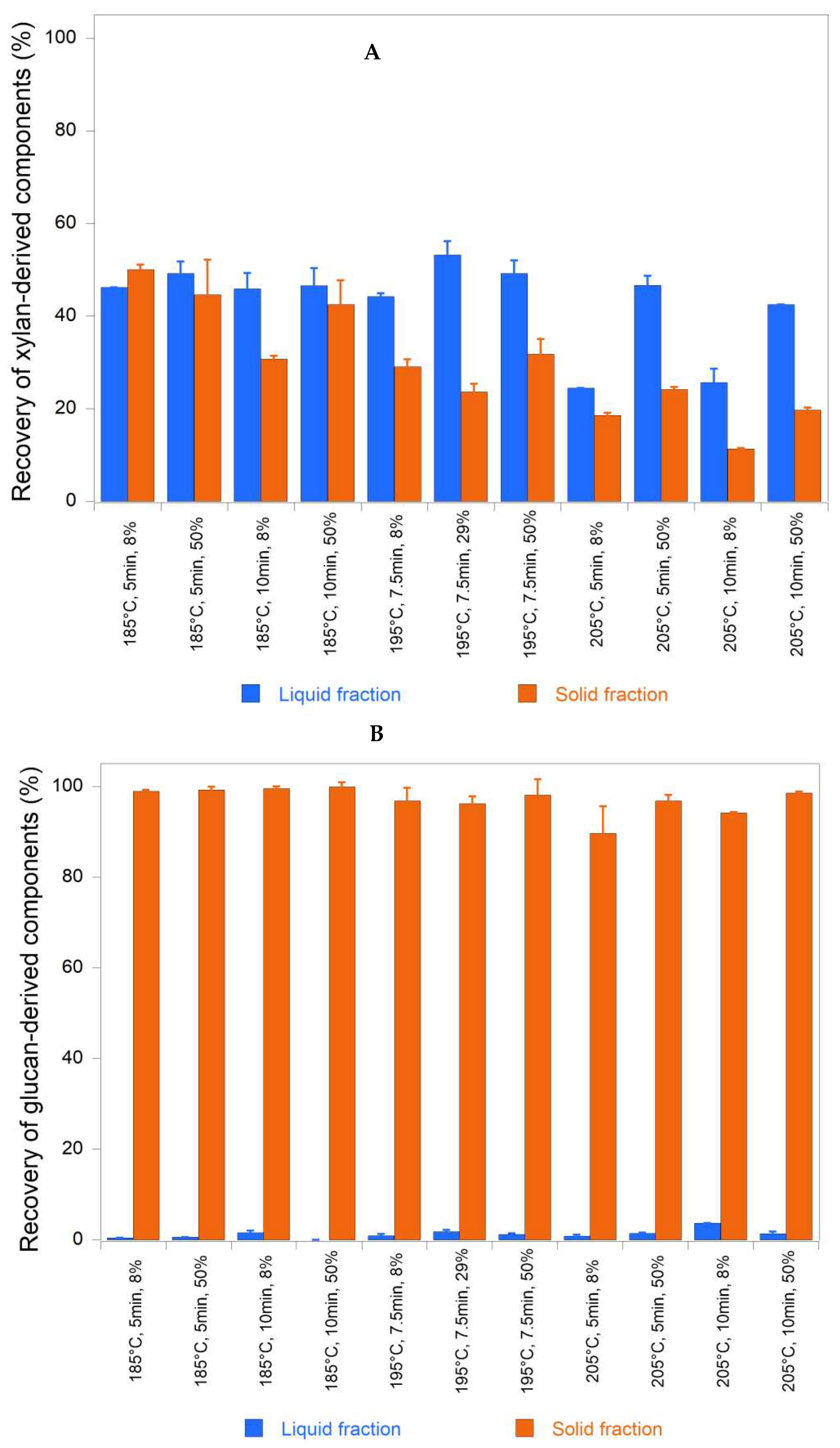
Glucans were the main component of the pretreatment solids, with percentages above 50% in all cases. Such glucan contents were aligned with those reported by other authors [
15,
42], which ranged from 50.3 to 54.8%. Glucan recovery in the pretreatment solids was between 90 and 100%, with an average of 97%, while xylan recovery was between 11 and 50%, with an average of 29% (
Figure 1). Park et al. [
34] found that glucan recovery remained relatively constant at ~84% when
E. grandis wood chips were steam-exploded at S
0 values ranging from 3.24 to 4.31, while Romaní et al.[
42] observed that glucan losses increased significantly for S
0 > 4.80.
Xylan content in pretreated solids was statistically affected only by temperature, while xylose release in the pretreatment liquor depended on the extent of xylan hydrolysis. Hence, based on the statistical data, temperature governed xylan hydrolysis, whereas time determined the amount of xylose remaining in solution. Xylan content in the pretreated solids were between 8.4 and 2.1% for S0 values from 3.20 to 4.09, while glucan represented 51.6 to 54.4% and total lignin varied from 36.8 to 42.3%, respectively.
Xylan removal from pretreated solids increased with pretreatment severity, but this was not accompanied by an increase in xylan components (both monomeric and oligomeric) in the pretreatment liquor, indicating mass losses normally due to dehydration. However, the quantification of furfural in this liquid stream did not explain the low xylan recovery values, since at most only 1% of potential furfural was recovered with respect to the original sawdust xylan composition. However, it is known that furans are partially lost as pretreatment volatiles or undergo condensation reactions forming acid-insoluble humins and/or pseudolignin [
43,
44,
45].
Temperature, time, and sawdust moisture content had no significant impact (p < 0.05) on both lignin and glucan contents of pretreated solids. The lignin content in pretreated solids varied from 33.0 to 43.7%, which corresponds to lignin recoveries higher than 100% (surplus between 2 and 25% depending on the pretreatment condition). This apparent lignin overestimation is due to the accumulation of humins and/or pseudolignin that are formed at high pretreatment severities and subsequently quantified as acid-insoluble lignin [
45,
46,
47], a feature that NREL protocols are not able to distinguish. Li et al. [
48] suggested that, at increased pretreatment severities, pseudolignin may account for as much as 50% of the acid-insoluble lignin found in steam-exploded aspen wood.
Although changes in sawdust moisture content (8 to 50%) had no statistically relevant impact on the analyzed response functions, excessive humidity may decrease pretreatment severity by slowing the heating rate inside the reaction chamber and eventually consume more steam to reach desired reaction temperature [
49]. This may have been the reason why air-dried sawdust (EV7A) resulted in ~4% more XS in the pretreatment liquor compared to using sawdust with a moisture content of 50% (EV9A).
3.2. Enzymatic Hydrolysis of Pretreated Eucalypt
Table 2 shows final glucose concentration, enzymatic hydrolysis efficiency and glucan conversion of unwashed exploded solids after incubation with a low enzyme loading of Cellic CTec2 for 72 h. Some glucan conversion efficiencies were higher than the corresponding hydrolysis efficiencies because the glucose present in the unwashed solids (therefore, in the retained pretreatment liquor) was considered in the former but not in the latter calculations. Enzymatic hydrolysis using only 5 FPU/g glucans released 3 to 13 g/L glucose in the substrate hydrolysate, while glucan conversion varied from 17 to 67% with maximum values achieved for pretreatments carried out at 205°C and 10 min (EV7A and EV9A). An improvement in hydrolysis efficiency could be observed by increasing both pretreatment temperature and retention time of the eucalypt sawdust inside the reactor vessel.
All pretreatment variables (temperature, time, and sawdust moisture content) had a positive influence in both enzymatic hydrolysis efficiency and glucan conversion (
Table 3; see also
Figure S2 in Supplementary Material). Temperature had the strongest effect on both response functions, followed by pretreatment residence time and sawdust moisture content. Also, when sawdust moisture content was high, glucan conversion was more sensitive to changes in pretreatment temperature (see
Supplementary Material).
Hemicellulose removal by acid hydrolysis improved both enzymatic hydrolysis efficiency and glucan conversion (glucose production from 100 g sawdust) (see
Figure S3 in Supplementary Material). The EV1A assay, which resulted in the lowest hydrolysis efficiency (17%), had the highest xylan concentration in the exploded solids (8.4%). However, other factors may have also been highly influential, such as changes in the distributions and in the physical and chemical properties of lignin.
As expected, hydrolysis efficiency increased when the enzyme dosage was increased from 9 to 25 FPU/g glucan. Improvements in enzymatic hydrolysis by adding more enzymes to the pretreated solids may not be economically viable, but ensures more enzyme in the medium to bind to cellulose chains. Working with an enzyme loading of 25 FPU/g glucan and 13 wt% TS raised hydrolysis efficiency with respect to the results achieved using 9 FPU/g glucan and 4 wt% TS. For example, after 24 h enzymatic hydrolysis of EV7A and EV9A unwashed pretreated substrates, hydrolysis efficiency was increased from 25 to 62% and from 44 to 57%, respectively. Also, changes in S
0, which combines both temperature and time in a single reaction ordinate, had a linear correlation with the mass recovery of pretreated solids and the parameters related to their enzymatic digestibility (final glucose concentration, hydrolysis efficiency, and glucan conversion) (see
Figure S4 in Supplementary Material).
A mathematical model was designed to reveal the effect of pretreatment variables on the following response functions: solids recovery, final glucose concentration, enzymatic hydrolysis efficiency, and glucan conversion (see
Table S1 in Supplementary Material). For hydrolysis efficiency and glucan conversion, both T.t and MC.t binary interactions were significant in the proposed model (
Table 3 and
Figure S5 in Supplementary Material). In the case of glucan conversion, a linear model was obtained with an excellent goodness-of-fit (R
2 of 0.999 and non-significant lack of fit). Likewise, a linear correlation was observed between experimental and theoretical values for glucan conversion (see
Figure S6 in Supplementary Material).
For glucan conversion efficiency (EGC), Equation 2 was developed with coded values considering only the statistically significant regression coefficients:
The highest glucan recovery from E. grandis sawdust was obtained at the highest pretreatment severity (205°C, 10 min), regardless of the sawdust original moisture content (66 and 67% in experiments EV7A and EV9A, respectively). Hence, the enzymatic hydrolysis performance of EV7A and EV9A unwashed substrates was investigated at 4, 13, and 27 wt% TS. Hydrolysis efficiency and glucan conversion remained almost unchanged (62.3 and 61.8%, respectively) for EV7A pretreated solids when experiments were carried out at 4 to 13 wt% TS, probably because any inhibitory effect, if existing, was resolved by increasing the enzyme dosage from 9 to 25 FPU/g. However, this behavior was different for assays carried out with EV9A or with EV7A at 27 wt% TS.
Enzyme inhibition caused a descensus in both efficiency parameters, perhaps due to the presence of organic acids and/or phenolic compounds since, as mentioned above, the solids were not previously washed with water. At 27 wt% TS, both hydrolysis efficiency and glucan conversion decreased more than half for both substrates (EV7A and EV9A) compared to experiments at 4 wt% TS. On the other hand, hydrolysis at 13 wt% TS ended at 48 h, reaching 85% glucose in 24 h. Other authors achieved more than 75% total glucose release within the first 24 h of hydrolysis, with yields continuing to increase with reaction time [
41,
46,
47].
Fockink et al. [
41] obtained similar glucan conversions at 24 h of hydrolysis (close to 60%) for steam-exploded sugarcane bagasse (S
0 of 3.67) using 15 wt% TS and 23.1 FPU/g glucan. However, their best result derived from hydrolysis at 20 wt% TS with 38.6 FPU/g glucan, in which a 99% glucan conversion was reached in 72 h [
41]. Regarding hydrolysis efficiency and glucan conversion of unwashed solids, the best results were obtained at 4 wt% TS with both EV7A and EV9A (65.5% and 67.1%, respectively).
The effect of water washing the pretreated solids was also analyzed. To this end, enzymatic hydrolysis was performed at high total solids (13 and 27 wt%) using EV7A and EV9A after a washing step. Water washing improved hydrolysis efficiency and glucan recovery due to the removal of enzyme inhibitors (
Table 4). For EV7A assay, glucose concentration, enzymatic hydrolysis efficiency and glucan conversion at 24 h increased by 15 and 10%, respectively, with respect to drained material without water washing. However, at 12 h of hydrolysis, there were no significant differences between unwashed and water-washed pretreated materials. In the case of the EV7A pretreated solids without water washing, the glucose concentration had an increase of 11% between 12 and 24 h of hydrolysis, while a 30% increase was observed for water-washed solids in the same hydrolysis timeframe.
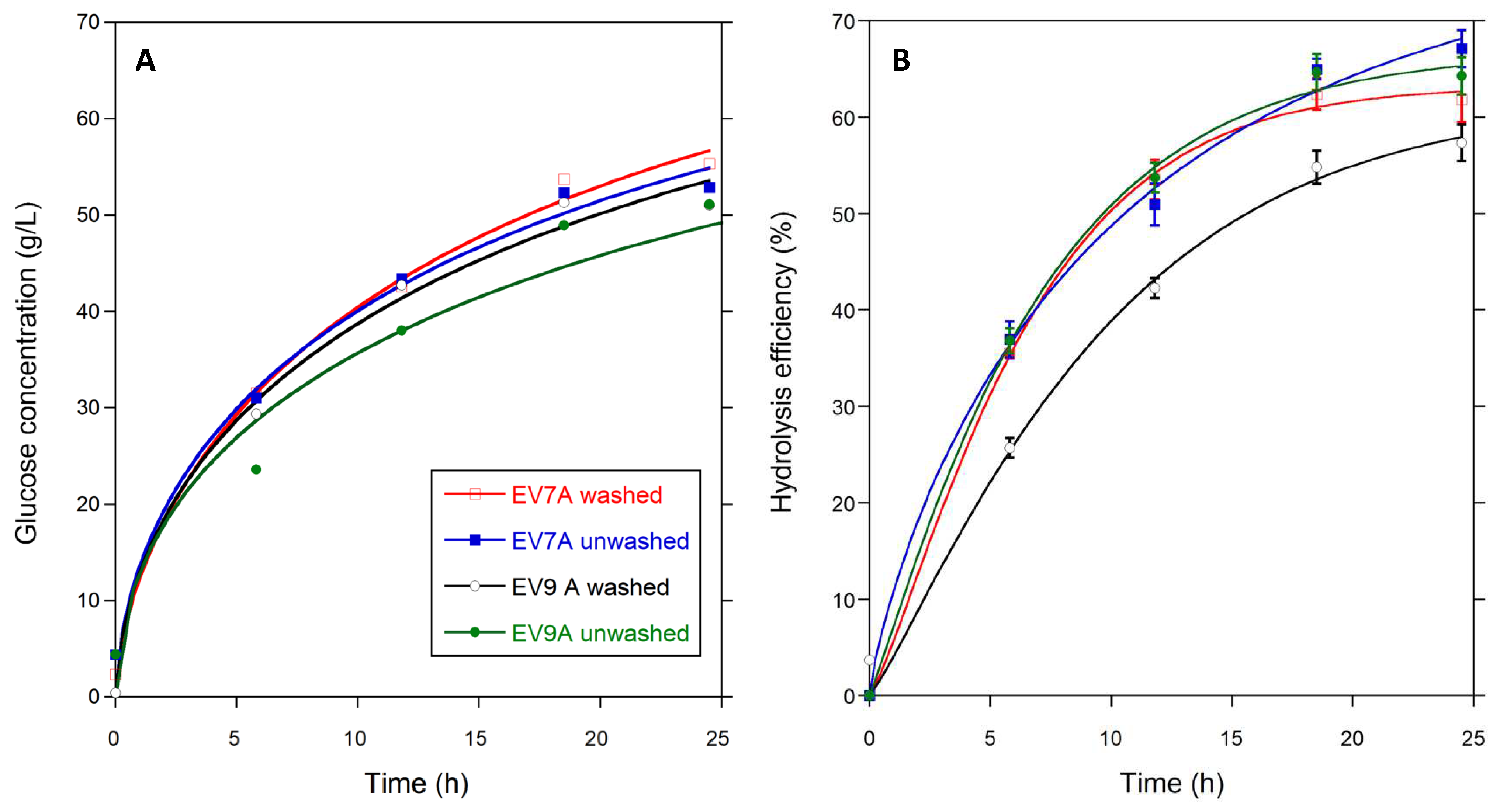
Table 5 contains the fractal kinetic parameters for the 24 h enzymatic hydrolysis of both unwashed and water-washed pretreated solids that were derived from experiments EV7A and EV9A (see Equation 1 for details), while
Figure 2 shows the corresponding enzymatic hydrolysis profiles. Similar hydrolysis profiles and comparable k values were obtained for unwashed and water-washed EV9A pretreated solids. However, EV9A unwashed solids had the highest h parameter, which corresponds to the worst enzymatic hydrolysis performance.
Unwashed solids presented higher h parameters due to the presence of hydrolysis and/or fermentation inhibitors, leading to poor ethanol production parameters (
Table 5). For the EV7A solids, there was only a 10% difference in glucan conversion for washed and unwashed solids. Hence, for this pretreatment condition, the advantages of water washing pretreated solids may be compensated by savings in capital costs, processing time, water requirements, and energy consumption.
3.3. Saccharification and Fermentation
In PSSF assays, hydrolysis of washed or unwashed pretreated solids was initially carried out for 24 h, when
S. cerevisiae PE-2 was added as inoculum to initiate ethanol production. Afterward, hydrolysis and fermentation were performed for another 52 h, giving a total processing time of 72 h for both steps. High TS (13 and 27 wt%) were used to achieve high ethanol concentrations. Enzymatic hydrolysis was performed for 24 h with an enzyme loading of 25 FPU/g glucan. After SSF for 12 to 18 h, ethanol concentration remained constant. The best results were obtained with 13 wt% TS, whereby 154 to 227 L of ethanol were produced per ton of sawdust (dry basis), depending on the condition and whether the pretreated material was water-washed or not. Increasing the substrate TS from 13 to 27 wt% did not produce an increase in the final ethanol concentration for both EV7A and EV9A pretreated solids, and ethanol productivities were also very similar (
Table 4). However, there was a decrease in ethanol yield in L per ton of sawdust. For 27 wt% TS, hydrolysis was partially inhibited resulting in less available glucose to ferment. Also, hydrolysis rate was decreased by decreasing the reaction temperature from 48 to 35°C during PSSF fermentation, while inhibitory effects may have been possibly enhanced. Hydrolysis efficiency dropped by a factor of 2 when the solids concentration was raised from 13 and 27 wt%, with the same effect being observed in the associated ethanol production. This suggests that the PE-2 yeast strain did not decrease productivity due to presence of possible inhibitors, but just because there was less glucose available in the medium for fermentation.
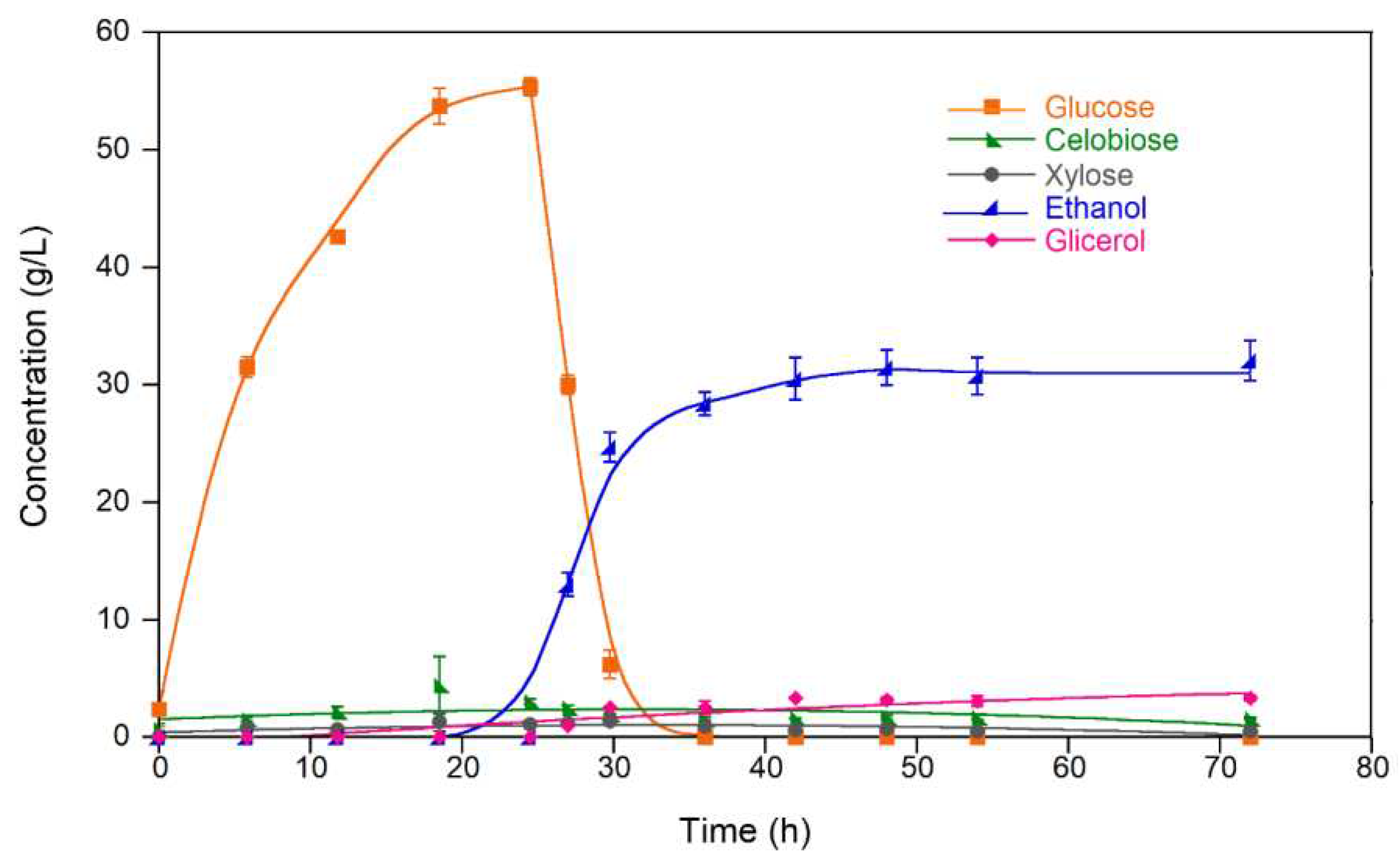
Figure 3 shows the concentration profiles of sugars, ethanol, and glycerol during PSSF of water-washed EV7A pretreated solids. Glycerol and cellobiose concentrations were always below 4 g/L, allowing the enzymes and yeast to perform well. Fermentation occurred rapidly, reaching the maximum ethanol concentration 12 h after inoculation. At this point, all available glucose was already consumed and both microbial growth and ethanol concentration remained constant until the end. Therefore, there was no need to extend hydrolysis and fermentation beyond 12 h. With this, the total processing time for optimal PSSF performance was reduced to only 36 h.
Ethanol yields and productivity increased with a decrease in initial sawdust moisture content. Unwashed steam-exploded solids from air-dried sawdust (EV7A) hydrolyzed faster in the first 12 h, reaching a final glucose concentration 16% higher than unwashed solids derived from sawdust with a 50% moisture content (EV9A). However, water-washed solids displayed a different behavior. This indicates that pretreatment was more severe for sawdust with a low moisture content, leading to a more extensive hydrolysis of water-soluble XOS that are highly detrimental to enzymatic hydrolysis. However, this rise in So was not strong enough to release fermentation inhibitors up to a critical level. For experiments carried out with high moisture content sawdust, inhibitory effects could be easily removed by water washing of the pretreated solids.
Romaní et al. [
42] used SSF to produce high glucan conversions (91%) and ethanol concentrations (51 g/L) from
E. globulus wood chips that were pretreated by steam explosion at 210°C for 10 min (S
o = 4.39) (
Table 6). Fermentation efficiencies between 50 and 76% were obtained using PSSF, which corresponds to an ethanol production of 154 to 227 L per ton of dry sawdust, respectively.
Neves et al. [
47] worked with steam-exploded sugarcane bagasse in three fermentation modalities and obtained the highest ethanol production yield (86%) by separate hydrolysis and fermentation (SHF), followed by 67% for SSF and 54% for PSSF. However, as in the present study, the best volumetric productivity was for PSSF (0.58 g/Lh). López-Linares et al. [
50] and Martín-Davison et al. [
51] obtained ethanol conversion efficiencies of 70 and 78% by SSF after pretreatment of rapeseed straw and poplar hybrid at 215 and 220°C, respectively (
Table 6). Chiarello et al. [
6,
52]achieved 83 and 88% ethanol conversion efficiencies from steam-exploded
E. urograndis wood chips that were pretreated at 210°C for 5 and 10 min, respectively, using the same equipment applied in this work. Despite using different saccharification and fermentation strategies, all these values are close to what was achieved from water-washed
E. grandis sawdust (76%). Also, McIntosh et al. [
26] used dilute sulfuric acid hydrolysis followed by steam explosion of
E. grandis wood chips to achieve similar ethanol yields in L per ton of dry matter. A higher ethanol yield of 304 L/ton was obtained by Castro et al. [
53] from phosphoric acid impregnated, steam-treated
E. benthamii wood chips but, in their studies, fermentation was carried out with a microorganism that was capable of fermenting both glucose and xylose to ethanol.
Glycerol is formed from an intermediate of glycolysis in response to high osmotic pressure due to high sugar concentration in the fermentation broth. This is why its production is increased at the beginning of fermentation and levels-off at longer reaction times in SSF experiments (
Figure 3).
3.4. Mass Balance
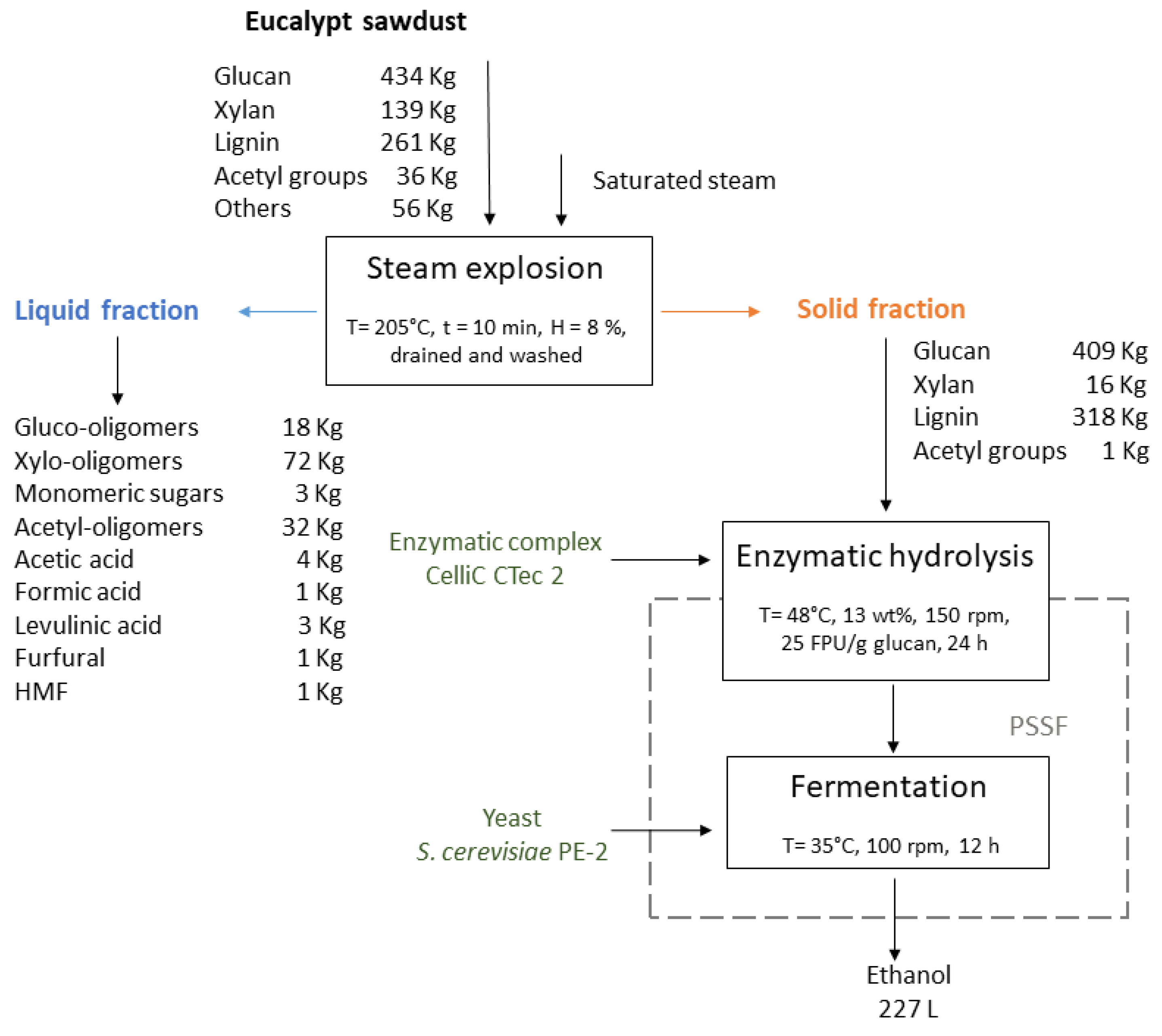
Steam explosion of air-dried
E. grandis sawdust (8% moisture content, 205°C, 10 min) followed by PSSF fermentation produced 227 L ethanol, 73 kg XS (xylose plus XOS), 36 kg acetic acid, 7 kg glycerol, 1 kg 5-HMF plus furfural, and 3 kg levulinic acid per ton of dry biomass (
Figure 4). The XS recovery was similar to those obtained by Cebreiros et al. [
54] and Romaní et al. [
55] using eucalypt and steam explosion, however, XOS in our work were obtained in higher yields. These XOS have a great potential to be used as prebiotics in food and feed applications [
35,
37]. High ethanol productions per dry ton of eucalypt sawdust are obtained. Similar values were reported by Chiarello et al. [
6] working with
E. urograndis wood chips and steam explosion.
Steam explosion allowed for a selective fractionation of
E. grandis sawdust with respect to plant cell wall polysaccharides. Lignin remains mostly with the enzymatic sludge, where it may be recovered from for its subsequent valorization. Some reports indicate that this type of lignin is more activated than other sources of technical lignins [
56].
5. Conclusion
Steam explosion of eucalypt sawdust was an effective pretreatment technique for biomass fractionation. Sawdust moisture content had little impact on pretreatment yields and the residence time into the steam reactor was the main variable to improve the recovery of xylosaccharides. Pretreatment increased enzyme accessibility to plant cell wall polysaccharides (mostly cellulose), resulting in reasonably high glucose yields (61%) at 24 h of hydrolysis using low enzyme loadings. Temperature had the greatest impact on enzymatic hydrolysis performance and water washing of the exploded solid increased the overall glucan conversion efficiency. The PSSF strategy at high total solids using water-washed steam-exploded solids improved ethanol yields. The best pretreatment condition allows to potentially obtain 227 L ethanol and 73 kg xylosaccharides from one ton of dry sawdust.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on
Preprints.org.
Author Contributions
Conceptualization, M.G., J.G., M.N.C., C.L. and L.P.R.; Formal analysis, M.G., J.G., M.N.C., C.L., M.D.F. and L.P.R.; Funding acquisition, M.G., M.N.C. and L.P.R.; Investigation, M.G., J.G., L.M.C., M.N.C., M.V. and M.D.F.; Methodology, M.G., J.G., L.M.C., M.N.C., M.V., M.D.F. and L.P.R.; Project administration, M.N.C; Resources, M.N.C.; Supervision, M.N.C. and C.L.; Validation, M.N.C., C.L., M.D.F. and L.P.R.; Writing – original draft, M.G, J.G., M.N.C., C.L., M.D.F. and L.P.R.; Writing – review & editing, M.G, M.N.C., C.L., M.D.F. and L.P.R.
Funding
This research was funded by the Agencia Nacional de Investigación e Innovación (ANII-FSE-2014-102701, Uruguay), by the Comisión Sectorial de Investigación Científica (CSIC, MIA2016-3-260, Uruguay) and by the Conselho Nacional de Desenvolvimento Científico e Tecnológico (grants 309506/2017-4 and 315930/2021-7, Brazil).
Data Availability Statement
Acknowledgments
The authors thank UPM Fray Bentos for kindly supplying the eucalypt sawdust and to Novozymes (Bagsværd, Denmark) for supplying the enzymes for hydrolysis. Also, the authors wish to thank students and staff of the Department of Chemistry, Federal University of Paraná.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Carus, M.; Dammer, L. The Circular Bioeconomy - Concepts, Opportunities, and Limitations. Ind. Biotechnol. 2018, 14, 83–91. [Google Scholar] [CrossRef]
- Motola, V.; De Bari, I.; Pierro, N.; Giocoli, A. IEA Bioenergy Task42. Bioeconomy and Biorefining Strategies in the EU Member States and Beyond; 2018. [Google Scholar]
- De Bari, I.; Liuzzi, F.; Ambrico, A.; Trupo, M. Arundo Donax Refining to Second Generation Bioethanol and Furfural. Processes 2020, 8, 1–15. [Google Scholar] [CrossRef]
- Kim, J.S.; Lee, Y.Y.; Kim, T.H. A Review on Alkaline Pretreatment Technology for Bioconversion of Lignocellulosic Biomass. Bioresour. Technol. 2016, 199, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Boscana, M.; Boragno, L. Actualidad Del Sector Forestal. Dirección General Forestal - MGAP. Anu. 2018 Análisis Sect. y cadenas Product. 2019, 229–239. [Google Scholar]
- Chiarello, L.M.; Ramos, C.E.A.; Neves, P.V.; Ramos, L.P. Production of Cellulosic Ethanol from Steam-Exploded Eucalyptus Urograndis and Sugarcane Bagasse at High Total Solids and Low Enzyme Loadings. Sustain. Chem. Process. 2016, 4, 15. [Google Scholar] [CrossRef]
- Guigou, M.; Cabrera, M.N.; Vique, M.; Bariani, M.; Guarino, J.; Ferrari, M.D.; Lareo, C. Combined Pretreatments of Eucalyptus Sawdust for Ethanol Production within a Biorefinery Approach. Biomass Convers. Biorefinery 2018. [Google Scholar] [CrossRef]
- Romaní, A.; Ruiz, H.A.; Teixeira, J.A.; Domingues, L. Valorization of Eucalyptus Wood by Glycerol-Organosolv Pretreatment within the Biorefinery Concept: An Integrated and Intensified Approach. Renew. Energy 2016, 95, 1–9. [Google Scholar] [CrossRef]
- Rochón, E.; Cabrera, M.N.; Scutari, V.; Cagno, M.; Guibaud, A.; Martínez, S.; Böthig, S.; Guchin, N.; Ferrari, M.D.; Lareo, C. Co-Production of Bioethanol and Xylosaccharides from Steam-Exploded Eucalyptus Sawdust Using High Solid Loads in Enzymatic Hydrolysis: Effect of Alkaline Impregnation. Ind. Crops Prod. 2022, 175. [Google Scholar] [CrossRef]
- Park, J.; Jones, B.; Koo, B.; Chen, X.; Tucker, M.; Yu, J.; Pschorn, T.; Venditti, R.; Park, S. Use of Mechanical Refining to Improve the Production of Low-Cost Sugars from Lignocellulosic Biomass. Bioresour. Technol. 2016, 199, 59–67. [Google Scholar] [CrossRef]
- Broda, M.; Yelle, D.J.; Serwańska, K. Bioethanol Production from Lignocellulosic Biomass—Challenges and Solutions. Molecules 2022, 27. [Google Scholar] [CrossRef]
- Yu, Y.; Wu, J.; Lau, H.; Resaei, H.; Takada, M.; Bi, X.; Sokhansanj, S. Steam Explosion of Lignocellulosic Biomass for Multiple Advanced Bioenergy Processes: A Review. Renew. Sustain. Energy Rev. 2022, 154, 111871. [Google Scholar] [CrossRef]
- Zhang, B.; Li, H.; Chen, L.; Fu, T.; Tang, B.; Hao, Y.; Li, J.; Li, Z.; Zhang, B.; Chen, Q.; et al. Recent Advances in the Bioconversion of Waste Straw Biomass with Steam Explosion Technique: A Comprehensive Review. Processes 2022, 10, 1–14. [Google Scholar] [CrossRef]
- Cebreiros, F.; Guigou, M.D.; Cabrera, M.N. Integrated Forest Biorefineries: Recovery of Acetic Acid as a by-Product from Eucalyptus Wood Hemicellulosic Hydrolysates by Solvent Extraction. Ind. Crops Prod. 2017, 109, 101–108. [Google Scholar] [CrossRef]
- Martin-Sampedro, R.; Revilla, E.; Villar, J.C.; Eugenio, M.E. Enhancement of Enzymatic Saccharification of Eucalyptus Globulus: Steam Explosion versus Steam Treatment. Bioresour. Technol. 2014, 167, 186–191. [Google Scholar] [CrossRef]
- Kundu, C.; Lee, J.W. Bioethanol Production from Detoxified Hydrolysate and the Characterization of Oxalic Acid Pretreated Eucalyptus (Eucalyptus Globulus) Biomass. Ind. Crops Prod. 2016, 83, 322–328. [Google Scholar] [CrossRef]
- Cunha, J.T.; Romaní, A.; Costa, C.E.; Sá-Correia, I.; Domingues, L. Molecular and Physiological Basis of Saccharomyces Cerevisiae Tolerance to Adverse Lignocellulose-Based Process Conditions. Appl. Microbiol. Biotechnol. 2019, 103, 159–175. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, D.; Michael, B.; Hirth, T.; Rupp, S.; Zibek, S. High Solids Enzymatic Hydrolysis of Pretreated Lignocellulosic Materials with a Powerful Stirrer Concept. Appl. Biochem. Biotechnol. 2014, 172, 1699–1713. [Google Scholar] [CrossRef] [PubMed]
- Zabed, H.; Sahu, J.N.; Suely, A.; Boyce, A.N.; Faruq, G. Bioethanol Production from Renewable Sources: Current Perspectives and Technological Progress. Renew. Sustain. Energy Rev. 2017, 71, 475–501. [Google Scholar] [CrossRef]
- Du, J.; Cao, Y.; Liu, G.; Zhao, J.; Li, X.; Qu, Y. Identifying and Overcoming the Effect of Mass Transfer Limitation on Decreased Yield in Enzymatic Hydrolysis of Lignocellulose at High Solid Concentrations. Bioresour. Technol. 2017, 229, 88–95. [Google Scholar] [CrossRef]
- Jawad Kadhum, H.; Murthy, G.S. Novel System Design for High Solid Lignocellulosic Biomass Conversion. Bioresour. Technol. 2022, 350, 126897. [Google Scholar] [CrossRef]
- Cai, C.; Qiu, X.; Zeng, M.; Lin, M.; Lin, X.; Lou, H.; Zhan, X.; Pang, Y.; Huang, J.; Xie, L. Using Polyvinylpyrrolidone to Enhance the Enzymatic Hydrolysis of Lignocelluloses by Reducing the Cellulase Non-Productive Adsorption on Lignin. Bioresour. Technol. 2017, 227, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Yao, F.; Tian, D.; Shen, F.; Hu, J.; Zeng, Y.; Yang, G.; Zhang, Y.; Deng, S.; Zhang, J. Recycling Solvent System in Phosphoric Acid plus Hydrogen Peroxide Pretreatment towards a More Sustainable Lignocellulose Biorefinery for Bioethanol. Bioresour. Technol. 2019, 275, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Vasic, K.; Knez, Z.; Leitgeb, M. Bioethanol Production by Enzymatic Hydrolysis from Different. Molecules 2021, 26, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Qin, L.; Li, X.; Liu, L.; Zhu, J.Q.; Guan, Q.M.; Zhang, M.T.; Li, W.C.; Li, B.Z.; Yuan, Y.J. Dual Effect of Soluble Materials in Pretreated Lignocellulose on Simultaneous Saccharification and Co-Fermentation Process for the Bioethanol Production. Bioresour. Technol. 2017, 224, 342–348. [Google Scholar] [CrossRef] [PubMed]
- McIntosh, S.; Zhang, Z.; Palmer, J.; Wong, H.-H.; Doherty, O.S.; Vancov, T. Pilot-Scale Cellulosic Ethanol Production Using Eucalyptus Biomass Pre-Treated by Dilute Acid and Steam Explosion. Biofuels, Bioprod. Biorefining 2016, 10, 346–358. [Google Scholar] [CrossRef]
- Zimbardi, F.; Viola, E.; Arcieri, G.; Valerio, V.; Carnevale, M. A Novel Method to Detoxify Steam-Exploded Biomass and Produce a Substrate for Biorefinery. Processes 2022, 10. [Google Scholar] [CrossRef]
- Ramos, L.P.; da Silva, L.; Ballem, A.C.; Pitarelo, A.P.; Chiarello, L.M.; Silveira, M.H.L. Enzymatic Hydrolysis of Steam-Exploded Sugarcane Bagasse Using High Total Solids and Low Enzyme Loadings. Bioresour. Technol. 2015, 175, 195–202. [Google Scholar] [CrossRef]
- Stoffel, R.B.; Neves, P.V.; Felissia, F.E.; Ramos, L.P.; Gassa, L.M.; Area, M.C. Hemicellulose Extraction from Slash Pine Sawdust by Steam Explosion with Sulfuric Acid. Biomass and Bioenergy 2017, 107, 93–101. [Google Scholar] [CrossRef]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D. Determination of Sugars, Byproducts, and Degradation Products in Liquid Fraction Process Samples. Natl. Renew. Energy Lab. 2008, 1–14. [Google Scholar]
- Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, A.; Sluiter, J.; Templeton, D. Preparation of Samples for Compositional Analysis Laboratory Analytical Procedure (LAP) I. Natl. Renew. Energy Lab. 2008, 1–9. [Google Scholar]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D.; Nrel, D.C. Determination of Structural Carbohydrates and Lignin in Biomass Determination of Structural Carbohydrates and Lignin in Biomass. 2012, 2011. [Google Scholar]
- Overend, R.; Chornet, E. Fractionation of Lignocellulosics by Steam-Aqueous Pretreatment. Phil Trans R Soc L. 1987, 321, 523–536. [Google Scholar] [CrossRef]
- Park, J.Y.; Kang, M.; Kim, J.S.; Lee, J.P.; Choi, W.I.; Lee, J.S. Enhancement of Enzymatic Digestibility of Eucalyptus Grandis Pretreated by NaOH Catalyzed Steam Explosion. Bioresour. Technol. 2012, 123, 707–712. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, R.; Winters, A.; Bryant, D.N.; Bosch, M.; Clifton-Brown, J.; Leak, D.; Gallagher, J. Pilot-Scale Production of Xylo-Oligosaccharides and Fermentable Sugars from Miscanthus Using Steam Explosion Pretreatment. Bioresour. Technol. 2020, 296, 122285. [Google Scholar] [CrossRef] [PubMed]
- Gullón, P.; González-Muñoz, M.J.; Parajó, J.C. Manufacture and Prebiotic Potential of Oligosaccharides Derived from Industrial Solid Wastes. Bioresour. Technol. 2011, 102, 6112–6119. [Google Scholar] [CrossRef]
- Míguez, B.; Gullón, P.; Cotos-Yáñez, T.; Massot-Cladera, M.; Pérez-Cano, F.J.; Vila, C.; Alonso, J.L. Manufacture and Prebiotic Potential of Xylooligosaccharides Derived From Eucalyptus Nitens Wood. Front. Chem. Eng. 2021, 3. [Google Scholar] [CrossRef]
- Negro, M.J.; Alvarez, C.; Ballesteros, I.; Romero, I.; Ballesteros, M.; Castro, E.; Manzanares, P.; Moya, M.; Oliva, J.M. Ethanol Production from Glucose and Xylose Obtained from Steam Exploded Water-Extracted Olive Tree Pruning Using Phosphoric Acid as Catalyst. Bioresour. Technol. 2014, 153, 101–107. [Google Scholar] [CrossRef]
- Martín-Sampedro, R.; Eugenio, M.E.; García, J.C.; Lopez, F.; Villar, J.C.C.; Diaz, M.J.J.; Martı, R. Steam Explosion and Enzymatic Pre-Treatments as an Approach to Improve the Enzymatic Hydrolysis of Eucalyptus Globulus. Biomass and Bioenergy 2012, 42, 97–106. [Google Scholar] [CrossRef]
- Martin-Sampedro, R.; Eugenio, M.E.; Moreno, J.A.; Revilla, E.; Villar, J.C. Integration of a Kraft Pulping Mill into a Forest Biorefinery: Pre-Extraction of Hemicellulose by Steam Explosion versus Steam Treatment. Bioresour. Technol. 2014, 153, 236–244. [Google Scholar] [CrossRef]
- Fockink, D.H.; Urio, M.B.; Sánchez, J.H.; Ramos, L.P. Enzymatic Hydrolysis of Steam-Treated Sugarcane Bagasse: Effect of Enzyme Loading and Substrate Total Solids on Its Fractal Kinetic Modeling and Rheological Properties. Energy and Fuels 2017, 31, 6211–6220. [Google Scholar] [CrossRef]
- Romaní, A.; Garrote, G.; Ballesteros, I.; Ballesteros, M. Second Generation Bioethanol from Steam Exploded Eucalyptus Globulus Wood. Fuel 2013, 111, 66–74. [Google Scholar] [CrossRef]
- de Carvalho, D.M. Study on the Structure and Properties of Xylan Extracted from Eucalyptus, Sugarcane Bagasse and Sugarcane Straw, KTH Royal Institute of Technology Fibre and Polymer Science, 2015.
- Hu, F.; Jung, S.; Ragauskas, A. Pseudo-Lignin Formation and Its Impact on Enzymatic Hydrolysis. Bioresour. Technol. 2012, 117, 7–12. [Google Scholar] [CrossRef]
- Sannigrahi, P.; Kim, D.H.; Jung, S.; Ragauskas, A. Pseudo-Lignin and Pretreatment Chemistry. Energy Environ. Sci. 2011, 4, 1306–1310. [Google Scholar] [CrossRef]
- Cara, C.; Ruiz, E.; Ballesteros, M.; Manzanares, P.; Negro, M.J.; Castro, E. Production of Fuel Ethanol from Steam-Explosion Pretreated Olive Tree Pruning. Fuel 2008, 87, 692–700. [Google Scholar] [CrossRef]
- Neves, P.V.; Pitarelo, A.P.; Ramos, L.P. Production of Cellulosic Ethanol from Sugarcane Bagasse by Steam Explosion: Effect of Extractives Content, Acid Catalysis and Different Fermentation Technologies. Bioresour. Technol. 2016, 208, 184–194. [Google Scholar] [CrossRef]
- Li, J.; Henriksson, G.; Gellerstedt, G. Lignin Depolymerization/Repolymerization and Its Critical Role for Delignification of Aspen Wood by Steam Explosion. Bioresour. Technol. 2007, 98, 3061–3068. [Google Scholar] [CrossRef] [PubMed]
- Ramos, L.P.; Breuil, C.; Kushner, D.J.; Saddler, J.N. Steam Pretreatment Conditions for Effective Enzymatic Hydrolysis and Recovery Yields of Eucalyptus Viminalis Wood Chips. Holzforschung 1992, 46, 149–154. [Google Scholar] [CrossRef]
- López-Linares, J.C.; Ballesteros, I.; Tourán, J.; Cara, C.; Castro, E.; Ballesteros, M.; Romero, I. Optimization of Uncatalyzed Steam Explosion Pretreatment of Rapeseed Straw for Biofuel Production. Bioresour. Technol. 2015, 190, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Martín-Davison, J.S.; Ballesteros, M.; Manzanares, P.; Sepúlveda, X.P.B.; Vergara-Fernández, A. Effects of Temperature on Steam Explosion Pretreatment of Poplar Hybrids with Different Lignin Contents in Bioethanol Production. Int. J. Green Energy 2015, 12, 832–842. [Google Scholar] [CrossRef]
- Chiarello, L.M.; Carlos, C.E.; Dos Santos, L.F.F.; Silveira, M.H.L.; Zaccaron, S.; Schiehser, S.; Santo, M.E.; Guimarães, F.E.G.; De Azevêdo, E.R.; Polikarpov, I.; et al. Characterization of Pretreated Fractions and Cellulosic Ethanol Production from Steam-Exploded Eucalyptus Urograndis. Energy and Fuels 2020, 34, 535–545. [Google Scholar] [CrossRef]
- Castro, E.; Nieves, I.U.; Mullinnix, M.T.; Sagues, W.J.; Hoffman, R.W.; Fernández-Sandoval, M.T.; Tian, Z.; Rockwood, D.L.; Tamang, B.; Ingram, L.O. Optimization of Dilute-Phosphoric-Acid Steam Pretreatment of Eucalyptus Benthamii for Biofuel Production. Appl. Energy 2014, 125, 76–83. [Google Scholar] [CrossRef]
- Cebreiros, F.; Ferrari, M.D.; Lareo, C. Cellulose Hydrolysis and IBE Fermentation of Eucalyptus Sawdust for Enhanced Biobutanol Production by Clostridium Beijerinckii DSM 6423. Ind. Crop. Prod. 2019, 134, 50–61. [Google Scholar] [CrossRef]
- Romaní, A.; Larramendi, A.; Yáñez, R.; Cancela, Á.; Sánchez, Á.; Teixeira, J.A.; Domingues, L. Valorization of Eucalyptus Nitens Bark by Organosolv Pretreatment for the Production of Advanced Biofuels. Ind. Crops Prod. 2019, 132, 327–335. [Google Scholar] [CrossRef]
- Chio, C.; Sain, M.; Qin, W. Lignin Utilization: A Review of Lignin Depolymerization from Various Aspects. Renew. Sustain. Energy Rev. 2019, 107, 232–249. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).