1. Introduction
Arab countries are commonly defined as the 22 Arabic speaking member states of the League of Arab States across the Middle East and North Africa, with a total population of 444,517,783 in 2021 [
1].
The increasing cancer burden is a major health concern in Arab countries. Between 2018 and 2020, the estimated number of new cancer cases in Arab countries increased from 429,325 to 463,675, accounting for 2.4% of the global cancer incidence [
2]. In 2020, breast, colorectal, and liver cancers ranked highest in incidence among Arab women whereas lung, liver, and prostate cancers had the highest incidence in Arab men [
2,
3,
4].
Cancer is also a major cause of mortality in Arab countries, causing an estimated 281,656 total deaths in 2020, corresponding to 2.8% of global cancer deaths [
3]. Notably, the cancer mortality to incidence ratio (MIR) in Arab countries is higher than the global rates for both men (0.68 vs. 0.55) and women (0.54 vs. 0.48) [
2,
3].
In 2020, Yemen had the highest MIR for both men (0.79) and women (0.69) among Arab countries, whereas the lowest MIR was reported in the United Arab Emirates for both men (0.33) and women (0.47) [
2,
3].
The ethno-linguistic, sociocultural, and historical links between Arab countries may indicate genetic and lifestyle similarities among Arab populations [
5,
6]. According to the World Health Organization (WHO) 2020 report on regional cancer profiles, tobacco smoking, infections, obesity, and exposure to ultraviolet light were the common contributors to cancer cases or deaths in the Eastern Mediterranean Region (EMR) which includes 19 Arab countries (except Algeria, Comoros, and Mauritania) [
7]. However, Arab states demonstrate considerable differences in demography, socioeconomic and political status, population health and public health policies, healthcare services, and clinical research ecosystem [
4,
8,
9,
10], that can explain the country-level variations in cancer incidence and mortality among Arab men and women [
4,
11,
12]. For instance, there is a clear disparity in cancer burden between high- and low-income Arab countries. Colorectal, prostate, and lung cancers were the top three male cancers in high-income Arab countries in 2018. In contrast, stomach, esophageal, and lung cancer had the highest incidence in low-income Arab countries [
13]. Breast and uterus cancers had the highest incidence in high- and low-income countries, respectively, whereas thyroid and esophagus cancers ranked third in incidence in high- versus low-income Arab countries [
13].
Despite the growing cancer burden and the differential drivers and characteristics of various cancers within the region, oncology research output in Arab countries is low [
11,
14,
15,
16]. Overall, Arab institutions published 26,656 cancer-related studies from 2005 to 2019, contributing to 1.5% of global cancer publications during this period. Of note, >50% of cancer-related literature in the Arab region came from two countries, Egypt and Saudi Arabia, and most published articles related to breast (
n = 2241), colorectal (
n = 1169), or liver (
n = 1017) cancers [
16]. Additionally, there are numerous limitations in cancer screening, cancer registries, patient access, and oncology care systems in Arab countries. For example, high-quality national cancer registries were available in only 28% of EMR countries, and no country had high-quality mortality registration. In addition, the functionality of cancer control and oncology care systems in the EMR are subject to some limitations in infrastructure, workforce, and management capacity [
7].
Region-specific and country-level differences in genetic predisposition, environmental exposures, socioeconomic status, cancer treatment availability and access, and pharmacogenomics demand locally relevant clinical trials in Arab populations [
7,
17]. However, it is well noted that there is limited patient-level data and cancer trial participation in this region [
18]. This is a critical gap as the findings of clinical trials in Western populations are not necessarily applicable to the populations in Arab countries, posing challenges to cancer prevention, screening, diagnosis, treatment, and survivorship in the Arab states [
19]. Therefore, it is important to systematically assess the available evidence on oncology trials in Arab countries to identify the existing gaps and define key priorities in this area.
The present study aims to explore the therapeutic cancer trial landscape in the Arab region over the past 20 years. In this work, we relied on literature sources to identify published therapeutic cancer clinical trials involving centers from Arab countries and analyzed relevant characteristics and trends related to the trials and publication of their results to recognize persisting unmet needs that should drive future action.
2. Materials and Methods
For this work, we considered Arab countries as defined by the World Bank, i.e., Algeria, Bahrain, Comoros, Djibouti, Egypt, Iraq, Jordan, Kuwait, Lebanon, Libya, Mauritania, Morocco, Oman, Palestinian Territories, Qatar, Saudi Arabia, Somalia, Sudan, Syria, Tunisia, UAE, and Yemen [
1].
2.1. Literature Search
We first searched for journal publications using PubMed on December 31, 2021, using the following search terms: (cancer*[tiab] OR tumour*[tiab] OR tumor*[tiab] OR neoplas*[tiab] OR malignan*[tiab] OR carcinom*[tiab]) AND ("middle East" OR "middle eastern" OR "Gulf states" OR "gulf state" OR Bahrain* OR Iraq* OR Kuwait* OR Oman* OR Qatar* OR "Saudi Arabia" OR "saudi arabian" OR "United Arab Emirates" OR UAE OR Emirati OR Algeria* OR Egypt* OR Jordan* OR Lebanon OR Lebanese OR Morocc* OR Tunisia* OR "north africa" OR "north african" OR "Comoros" OR Djibouti* OR Libya* OR Mauritania* OR Somalia* OR Palestine OR Palestinian* OR Sudan OR Sudanese OR Syria* OR Yemen* OR “Middle East"[Mesh] OR "Bahrain"[Mesh] OR "Iraq"[Mesh] OR "Kuwait"[Mesh] OR "Oman"[Mesh] OR "Qatar"[Mesh] OR "Saudi Arabia"[Mesh] OR "United Arab Emirates"[Mesh] OR "Algeria"[Mesh] OR "Egypt"[Mesh] OR "Jordan"[Mesh] OR "Lebanon"[Mesh] OR "Morocco"[Mesh] OR "Tunisia"[Mesh] OR "Africa, Northern"[Mesh] OR "Comoros"[Mesh] OR "Djibouti"[Mesh] OR "Libya"[Mesh] OR "Mauritania"[Mesh] OR "Somalia"[Mesh] OR "Sudan"[Mesh] OR "Syria"[Mesh] OR "Yemen"[Mesh]). The search was restricted to ‘Clinical Trial’ and to the years 2000 to 2021 to reflect a contemporary analysis. This retrieved 1428 journal publications that were reviewed by three authors (title + abstract and full-text publication when necessary) to exclude: (1) publications that did not report data from therapeutic cancer clinical trials; (2) secondary publications of clinical trials; and (3) publications of clinical trials that did not include a center from an Arab country for recruitment (even if the publication included an author from an Arab country). The latter was identified through the methods section, other in-text mentions or sources, ethical approvals, informative authorship and contributions, protocol supplement, or trial registry. For each journal publication of a clinical trial, we retrieved the following variables: year of publication, journal impact factor during the year of publication where available (retrieved through Journal Citation ReportsTM, Clarivate), number of citations (retrieved on March 29, 2022 through PubMed and Europe PubMed Central), Arab country authorship (no authors affiliated with Arab countries, authors(s) affiliated with a single Arab country, or author(s) affiliated with multiple Arab countries), non-Arab country authorship (no authors affiliated with non-Arab countries, or authors affiliated with Europe, North America, Central or South America, Asia/Australia, Africa, or multiple regions), first author(s) (affiliated with Arab country or not), and corresponding author(s) (affiliated with Arab country or not). We also retrieved the following information about the clinical trial: sample size, Arab country site(s) (single site from a single country, multiple sites from a single country, or multiple sites from multiple countries), non-Arab country site(s) (none, Europe, North America, Central or South America, Asia/Australia, Africa, or multiple regions), cancer type (breast, gynecologic, colorectal, upper gastrointestinal/liver/pancreas, lung, head and neck/thyroid, prostate, kidney/bladder, leukemia/lymphoma/myeloma, brain, soft tissue/bone, skin/melanoma, multiple, other/non-specific), intervention type (pharmacologic therapy, radiation therapy, surgical therapy, behavioral/psychological therapy, herbal/alternative therapy, other therapy, or multiple therapy), randomized controlled trial design (yes or no), and external funding (none/undisclosed, pharmaceutical, non-pharmaceutical, or both).
2.2. Statistical Analysis
Descriptive statistics were presented as median (range) or percentages. Bivariate comparisons were made using the Chi-squared test for categorical variables and Mann-Whitney U Test for continuous variables. All p-values were two-sided with the level of significance set at <0.05.
3. Results
A total of 320 therapeutic cancer clinical trial publications that contained evidence of participation of center(s) from Arab countries were included in this analysis. All included publications were published between the years 2000 and 2021, with an evident increase in the number of publications over time: 2000–2006 (n = 44, 13.8%), 2007–2013 (n = 105, 32.8%), and 2014–2021 (n = 171, 53.4%).
3.1. Publication-related Analyses
A summary of authorship for journal publications of a therapeutic clinical trial that included participation of a center from one of the included 22 Arab countries is provided in
Table 1. Most (91.3%) publications included author(s) from a single Arab country, with a slight increase in authorship from multiple Arab countries in recent years. Joint authorship with non-Arab countries was consistently low over the period analyzed (21.3%) and primarily included authors from Europe and North America. Notably, a higher proportion of publications included multiregional rather than single-country authorship in recent years. There was no significant difference in the frequency of joint non-Arab authorship between single or multiple Arab country authorship publications (20.9% vs. 25%,
p = 0.61).
A total of 270 (84.4%) trials were published in journals with a Journal Citation Reports impact factor (36/44 [81.8%] in 2000–2006, 80/105 [76.2%] in 2007–2013, and 154/171 [90.1%] in 2014–2021). The overall median impact factor was 2.57 (range, 0.08–35.9) and increased from 2000 to 2021: 1.87 (range, 0.34–11.12) in 2000–2006, 2.04 (range, 0.08–16.95) in 2007–2013, and 2.90 (range, 0.77–35.92) in 2014–2021. The journal impact factor was significantly higher in publications with joint non-Arab authorship (median, 3.3 [range, 0.49–35.9]) than those without (median, 2.49 [range, 0.08–10.1]) (p < 0.001). The overall median number of citations per article was 4 (range, 0–223) and was lower for more recent years: 5.5 (range, 1–139) in 2000–2006, 4 (range, 0–71) in 2007–2013, and 4 (range, 0–223) in 2014–2021. The number of citations was also significantly higher in publications with joint non-Arab authorship (median, 7 [range, 0–223]) than those without (median, 4 [range, 0–139]; p < 0.001).
3.2. Clinical Trial-related Analyses
Characteristics of the clinical trials featured in the journal publications are summarized in
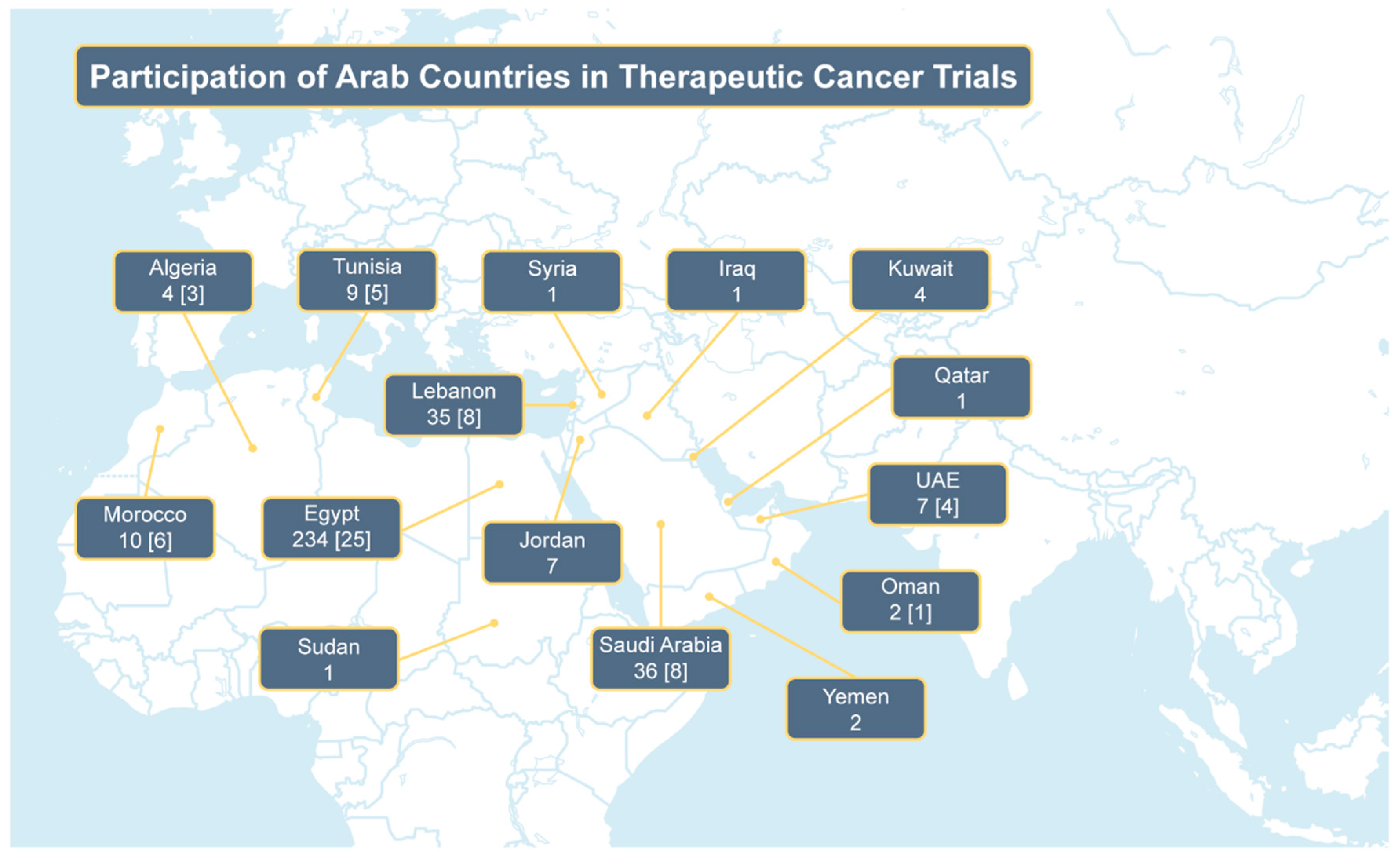
Table 2. The number of trials conducted by individual Arab countries is illustrated in
Figure 1. There was a cumulative increase in the median sample size of trials and a substantial increase in the proportion of randomized controlled trials over time (36.4% in 2000–2006, 46.7% in 2007–2013, and 75.4% in 2014–2021). Breast (25%), upper gastrointestinal/liver/pancreas (12.2%), and colorectal (8.1%) cancers were consistently the leading cancer types in the included clinical trial publications (
Table 2). Pharmacologic (50%), surgical (14.7%), and radiation (5.3%) therapy were the leading interventions in included clinical trial publications across the observation period, with multiple therapies used in 18.1% of publications. Most trials included only a single site from a single Arab country (82.8%) and did not receive external funding from pharmaceutical companies or other sources (82.8%). Overall, joint participation of non-Arab sites was consistently low over the years (12.5%); however, the proportion of trials with multiregional site participation increased over the years. The proportion of randomized controlled trials was comparable between trials with joint non-Arab country sites and those without (62.5% vs. 60.4%;
p = 0.80). However, external funding was significantly more common in trials with joint non-Arab country sites than in those without (72.5% vs. 9.3%;
p < 0.001). The trial sample size was also significantly higher in trials with collaborating sites in non-Arab countries (median, 50 [range, 20–657]) than in those without (median, 96 [range, 9–2577];
p = 0.003).
4. Discussion
This study provides several insights on the participation of Arab countries in therapeutic cancer clinical trials over the last 20 years. Overall, there was a steady increase in the number of trials conducted between the years 2000 and 2021. Although the clinical trials included a variety of interventions, they primarily reported data from trials in a few common cancers such as breast and gastrointestinal cancers. Most of the included publications were of small clinical trials conducted by single sites within a few individual countries. Although a considerable proportion of the published clinical trials were randomized clinical trials, the availability of external funding was low. For trials conducted in collaboration with non-Arab countries, the sample size was larger and external funding was more common than for trials conducted in Arab countries only. These observations indicate an expanding pool of investigators in Arab countries but also suggest some degree of reliance on Western countries for inclusion in large, externally funded studies. Moreover, the chance of collaboration between Arab and non-Arab countries remains low, despite marginal improvement in recent years.
These findings are also reflected in the associated journal publications, with ‘better’ publication outcomes, i.e., higher journal impact factor and article citations, noted when Arab countries are involved in trials in collaboration with non-Arab countries compared with conducting them alone.
The total number of publications (
N = 320) for cancer-related therapeutic trials hosted by or conducted in collaboration with Arab countries between 2000 and 2021 indicates a low research output for a region constituting 5.6% of the world population and contributing to 5% of global growth domestic product (GDP) in purchasing power standards [
20,
21]. The observed oncology trial publication output from Arab countries is low in the context of total clinical trials published in PubMed from 2000 to 2021 (
N = 110,281), all oncology trials (both observational and interventional) listed in the WHO International Clinical Trials Registry Platform for the period 1999–2021 (
N = 104,491), or all interventional cancer trials registered at ClinicalTrials.gov by December 2022 (
N = 75,629) [
22]. This is in line with the trends for the number of clinical trials by WHO regions for the period of 1999–2021, where the EMR (
N = 5,423) was behind the Western Pacific (
N = 16,860), Europe (
N = 14,879), America (
N = 12,969), and most recently, South-East Asia (
N = 9,374) regions [
22]. In addition, there is some inconsistency between the observed proportion of cancers targeted in the therapeutic trials conducted in Arab countries and those studies in oncology trials led by high- or middle-income countries. While breast and gastrointestinal cancers were the cancers most commonly included in the published articles in this analysis, hematologic, breast, gastrointestinal, lung, and urologic cancers were the leading targets in global phase III therapeutic trials published between 2014 and 2017 [
23].
The focus of Arab clinical trials on breast and gastrointestinal, including colorectal and liver cancer aligns with the high incidence and mortality of these cancers in the Arab population [
2,
3]. However, the number of clinical trials on lung, thyroid, gynecologic, and urologic cancers is disproportionately low despite their substantial burden in Arab men and women [
2,
3,
13,
24].
Furthermore, Arab countries had a lower share of gross domestic expenditure on research and development per GDP compared with the global research expenditure (0.64 vs. 1.93) in 2020, lagging behind Central and Eastern Europe (1.10), East Asia and Pacific (2.29), and North America and Western Europe (2.89) [
25]. Between 2005 and 2019, each Arab country (excluding Egypt) published <50 cancer-related articles per billion US
$ of their GDP, with the lowest contribution from low-income countries [
16]. Notably, the participation of middle-income Arab countries (Algeria, Egypt, Lebanon, Morocco, and Tunisia) in phase III oncology trials and their corresponding bibliometric research output were proportionally lower than non-Arab middle-income countries such as China, India, and Turkey [
23]. The lower expenditure on research translates into the lower cancer research output and clinical trial participation in Arab countries relative to the global trend.
There are multiple barriers to clinical cancer research in Arab countries, including: limited training and engagement of healthcare professionals, poor institutional collaborative research, a lack of clinical trial units and specifically designed system operating procedures for research, inconsistent regulatory processes for novel therapies in some countries, challenges surrounding patient enrollment in cancer trials, underrepresentation in, or restricted access to, global cancer-centric research and knowledge dissemination, and insufficient support or funding from the governments and pharmaceutical industry [
18,
23,
24,
26,
27,
28].
Overcoming the existing barriers in clinical cancer research and oncology care requires concerted multi-tiered actions across the Arab region. The lack of high-quality data from patients with cancer in Arab countries warrants optimization of national cancer registries and improvements in data access [
29,
30]. The development of designated oncology research centers in Arab countries can improve recruitment and retention of skilled researchers and clinicians who would otherwise seek new opportunities elsewhere [
28,
31]. Furthermore, the incorporation of research skills in the national medical education system, postgraduate training, and clinical practice can increase healthcare professional engagement in research and evidence generation [
18,
28,
32]. On an institutional level, initiatives to encourage multidisciplinary collaboration between the public and private sectors can strengthen local and international research networking, expanding clinical trial capacity and access in the region [
18,
30]. Additionally, public education and awareness plans can change misperceptions and trust issues about the clinical trials among patients and the wider community, potentially improving clinical trial acceptance and accrual [
18,
31]. Finally, clinical trial conduct in Arab countries can be further enhanced by the standardization of research review and approval processes [
33]. These advancements would address the global oncology community and pharma industry’s concerns over clinical cancer research capabilities in Arab countries, enabling efforts to include Arab institutions in pharma-sponsored clinical trials, and facilitating funding and support for locally led cancer trials in the region.
This study does not come without limitations. We relied on English-language journal publications in PubMed to identify clinical trials, which would exclude trials that were not published in a PubMed-indexed journal (results never published, results published in non-indexed journals, results published as congress abstracts only, and ongoing trials without results) and those published in non-English language. However, retrieving publications through an additional Google scholar search was not feasible or practical considering the broad nature of the topic investigated. Moreover, relying on information from congress abstracts or clinicaltrials.gov listings would not have allowed for the retrieval of all variables of interest. Lastly, there is a chance that some trials were not retrieved through our search as no reference to Arab countries was made in their keyword indices.
5. Conclusion
Despite the rapidly increasing burden of cancer among Arab populations, cancer research productivity and clinical trial capacity are still low in Arab countries. The number of cancer trials published in high-impact journals and the proportion of randomized controlled trials led by, or enrolling patients from, Arab countries is increasing. However, the lack of external funding and limited international collaboration are important limiting factors for clinical trial conduct and publication outcomes in Arab countries. These shortcomings can be addressed by developing a research-enabling oncology care system and enhancing cancer data utilization, with subsequent beneficial impact on cancer care and patient outcomes.
Author Contributions
Conceptualization, H.O.A.-S., I.A.-G. and K.M.M.; methodology, H.O.A.-S. and K.M.M.; software, K.M.M.; validation, K.M.M.; formal analysis, K.M.M., K.S. and N.E.T.; investigation, K.M.M., K.S. and N.E.T.; resources, K.M.M.; data curation, K.M.M.; writing—original draft preparation, H.O.A.-S., I.A.-G., K.S., N.E.T. and K.M.M.; writing—review and editing, H.O.A.-S., I.A.-G., K.S., N.E.T. and K.M.M.; visualization, K.M.M.; supervision, K.M.M.; project administration, K.S. and N.E.T.; funding acquisition, N/A. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Pfizer Inc.
Data Availability Statement
Data presented in this study are based on analysis of publicly available data from PubMed and are available on request from the corresponding author.
Acknowledgments
Medical writing and editorial support was provided by Roham Sadeghimakki, MD, PhD, Megan Christian, and Rosie Henderson, all of Onyx, (a Prime Global agency, London, UK) and funded by Pfizer according to Good Publication Practice guidelines (Link).
Conflicts of Interest
K.S. is an employee of and owns stock in Pfizer. N.E.T. is an employee of Pfizer. H.O.A.-S., I.A.-G. and K.M.M. declare no conflict of interest. The Sponsor was involved in the study design, collection, analysis, and interpretation of data of information provided in the manuscript. However, ultimate responsibility for opinions, conclusions, and data interpretation lies with the authors.
References
- The World Bank. World Development Indicators: Arab World. Available online: https://data.worldbank.org/country/1A (accessed on 20 December 2022).
- Mahdi, H.; Mula-Hussain, L.; Ramzi, Z.S.; Tolba, M.; Abdel-Rahman, O.; Abu-Gheida, I.; Khorshid, O.; Al Sukhun, S.; Siddiqi, N.P.; Al Mandhari, Z. Cancer Burden Among Arab-World Females in 2020: Working Toward Improving Outcomes. JCO Glob Oncol 2022, 8, e2100415. [Google Scholar] [CrossRef] [PubMed]
- Mula-Hussain, L.; Mahdi, H.; Ramzi, Z.S.; Tolba, M.; Zaghloul, M.S.; Benbrahim, Z.; Abusanad, A.; Al-Shamsi, H.; Bounedjar, A.; Jazieh, A.-R. Cancer Burden Among Arab World Males in 2020: The Need for a Better Approach to Improve Outcome. JCO Glob Oncol 2022, 8, e2100407. [Google Scholar] [CrossRef]
- Al-Shamsi, H.O.; Iqbal, F.; Abu-Gheida, I.H. Introduction. In Cancer in the Arab World, Al-Shamsi, H.O., Abu-Gheida, I.H., Iqbal, F., Al-Awadhi, A., Eds.; Springer Nature: Singapore, 2022; pp. 1–14. [Google Scholar] [CrossRef]
- Mandil, A.; Chaaya, M.; Saab, D. Health status, epidemiological profile and prospects: Eastern Mediterranean region. Int J Epidemiol 2013, 42, 616–626. [Google Scholar] [CrossRef]
- Sharara, E.; Akik, C.; Ghattas, H.; Makhlouf Obermeyer, C. Physical inactivity, gender and culture in Arab countries: a systematic assessment of the literature. BMC Public Health 2018, 18, 639. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization Regional Office for Eastern Mediterranean (WHO-EMRO). Cancer Regional Profile 2020. Available online: https://cdn.who.int/media/docs/default-source/ncds/ncd-surveillance/cancer-profiles-2020/emro-cancer-profile-2020.pdf?sfvrsn=533e7d92_3 (accessed on 20 December 2022).
- Kronfol, N.M. Delivery of health services in Arab countries: a review. East Mediterr Health J 2012, 18, 1229–1238. [Google Scholar] [CrossRef]
- Khoja, T.; Rawaf, S.; Qidwai, W.; Rawaf, D.; Nanji, K.; Hamad, A. Health Care in Gulf Cooperation Council Countries: A Review of Challenges and Opportunities. Cureus 2017, 9, e1586. [Google Scholar] [CrossRef]
- Blair, I.; Grivna, M.; Sharif, A.A. The "Arab World" is Not a Useful Concept When Addressing Challenges to Public Health, Public Health Education, and Research in the Middle East. Front Public Health 2014, 2, 30. [Google Scholar] [CrossRef]
- Hamadeh, R.R.; Borgan, S.M.; Sibai, A.M. Cancer Research in the Arab World: A review of publications from seven countries between 2000–2013. Sultan Qaboos Univ Med J 2017, 17, e147–e154. [Google Scholar] [CrossRef]
- AlHarthi, F.S.; Qari, A.; Edress, A.; Abedalthagafi, M. Familial/inherited cancer syndrome: a focus on the highly consanguineous Arab population. NPJ Genom Med 2020, 5, 3. [Google Scholar] [CrossRef]
- Siddiqui, A.A.; Amin, J.; Alshammary, F.; Afroze, E.; Shaikh, S.; Rathore, H.A.; Khan, R. Burden of Cancer in the Arab World. In Handbook of Healthcare in the Arab World; Springer: 2021; pp. 495–519. [CrossRef]
- Znaor, A.; Fouad, H.; Majnoni d'Intignano, F.; Hammerich, A.; Slama, S.; Pourghazian, N.; Eser, S.; Piñeros Petersen, M.; Bray, F. Use of cancer data for cancer control in the Eastern Mediterranean Region: Results of a survey among population-based cancer registries. Int J Cancer 2021, 148, 593–600. [Google Scholar] [CrossRef] [PubMed]
- Abdul-Sater, Z.; Shamseddine, A.; Taher, A.; Fouad, F.; Abu-Sitta, G.; Fadhil, I.; Saab, R.; Sullivan, R.; Adib, S.M.; Saleh, S.; et al. Cancer Registration in the Middle East, North Africa, and Turkey: Scope and Challenges. JCO Glob Oncol 2021, 1101–1109. [Google Scholar] [CrossRef] [PubMed]
- Machaalani, M.; El Masri, J.; El Ayoubi, L.M.; Matar, B. Cancer research activity in the Arab world: a 15-year bibliometric analysis. J Egypt Public Health Assoc 2022, 97, 26. [Google Scholar] [CrossRef]
- Drake, T.M.; Knight, S.R.; Harrison, E.M.; Søreide, K. Global Inequities in Precision Medicine and Molecular Cancer Research. Front Oncol 2018, 8, 346. [Google Scholar] [CrossRef] [PubMed]
- Al-Shamsi, H.O. Barriers and Facilitators to Conducting Oncology Clinical Trials in the UAE. Clin Pract 2022, 12, 885–896. [Google Scholar] [CrossRef] [PubMed]
- Hofmarcher, T.; Ahmad, A.; Lindgren, P.; Wilking, N. Cancer Care in the Middle East and Africa. Available online: https://ihe.se/en/publicering/cancer-care-in-the-middle-east-and-africa-2/ (accessed on 20 December 2022).
- The World Bank. World Development Indicators: Population, total. Available online: https://data.worldbank.org/indicator/SP.POP.TOTL (accessed on 20 December 2022).
- The World Bank. World Development Indicators: GDP, PPP (current international $). Available online: https://data.worldbank.org/indicator/NY.GDP.MKTP.PP.CD (accessed on 20 December 2022).
- World Health Organization. Number of clinical trial registrations by location, disease, phase of development, age and sex of trial participants (1999-2021). Available online: https://www.who.int/observatories/global-observatory-on-health-research-and-development/monitoring/number-of-trial-registrations-by-year-location-disease-and-phase-of-development (accessed on 18 January 2023).
- Rubagumya, F.; Hopman, W.M.; Gyawali, B.; Mukherji, D.; Hammad, N.; Pramesh, C.S.; Zubaryev, M.; Eniu, A.; Tsunoda, A.T.; Kutluk, T.; et al. Participation of Lower and Upper Middle–Income Countries in Oncology Clinical Trials Led by High-Income Countries. JAMA Netw Open 2022, 5, e2227252. [Google Scholar] [CrossRef]
- Hamadeh, R.R.; Jahrami, H.; Nazzal, K. Cancer Research in the Arab World. In Cancer in the Arab World, Al-Shamsi, H.O., Abu-Gheida, I.H., Iqbal, F., Al-Awadhi, A., Eds.; Springer: Singapore, 2022; pp. 395–408. [Google Scholar] [CrossRef]
- UNESCO Institute for Statistics. Science,technology and innovation: Gross domestic expenditure on R&D (GERD), GERD as a percentage of GDP, GERD per capita and GERD per researcher. Available online: http://data.uis.unesco.org/?queryid=74# (accessed on 8 December 2022).
- Benhima, N.; El Fadli, M.; Essâdi, I.; Belbaraka, R. What does it take to conduct clinical trials in African countries? Insights from Morocco. Ecancermedicalscience 2022, 16, 1411. [Google Scholar] [CrossRef]
- Alemayehu, C.; Mitchell, G.; Nikles, J. Barriers for conducting clinical trials in developing countries- a systematic review. Int J Equity Health 2018, 17, 37. [Google Scholar] [CrossRef]
- Abdul-Sater, Z.; Menassa, M.; El Achi, N.; Abdul-Khalek, R.A.; Abu-Sittah, G.; Mukherji, D. Strengthening capacity for cancer research in conflict settings: key informant insights from the Middle East. Ecancermedicalscience 2020, 14, 1153. [Google Scholar] [CrossRef]
- Abdul-Sater, Z.; Mukherji, D.; Adib, S.M.; Shamseddine, A.; Abu-Sitta, G.; Fadhil, I.; Sullivan, R.; Omari, A.A.; Saleh, S.; Taher, A. Cancer registration in the Middle East, North Africa, and Turkey (MENAT) region: A tale of conflict, challenges, and opportunities. Front Oncol 2022, 12, 1050168. [Google Scholar] [CrossRef] [PubMed]
- Znaor, A.; Eser, S.; Anton-Culver, H.; Fadhil, I.; Ryzhov, A.; Silverman, B.G.; Bendahou, K.; Demetriou, A.; Nimri, O.; Yakut, C. Cancer surveillance in northern Africa, and central and western Asia: challenges and strategies in support of developing cancer registries. Lancet Oncol 2018, 19, e85–e92. [Google Scholar] [CrossRef]
- Al-Shamsi, H.O.; Abyad, A.M.; Rafii, S. A Proposal for a National Cancer Control Plan for the UAE: 2022–2026. Clin Pract 2022, 12, 118–132. [Google Scholar] [CrossRef] [PubMed]
- Khaled, H.M.; Soliman, A.S. Medical Oncology Education in Egypt Over the Past 50 Years: the Experience of the National Cancer Institute of Cairo University. J Cancer Educ 2021, 36, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Department of Health - Abu Dhabi. DOH guidelines for conducting clinical trials with investigational products and medical devices. Available online: https://doh.gov.ae/-/media/40B18D052C3E40B2A079ABD7571E2260.ashx (accessed on 20 December 2022).
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).