Submitted:
21 June 2023
Posted:
21 June 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
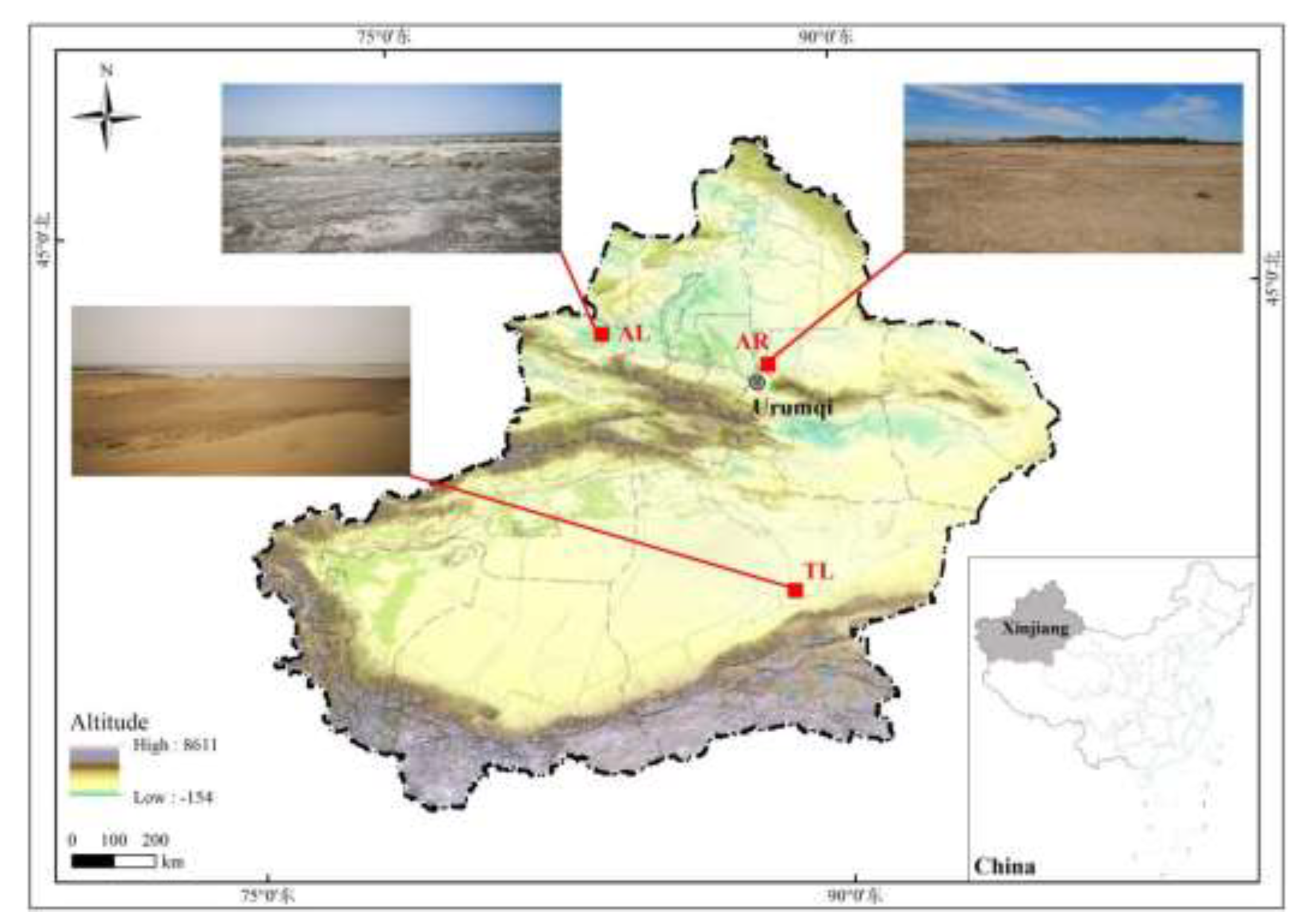
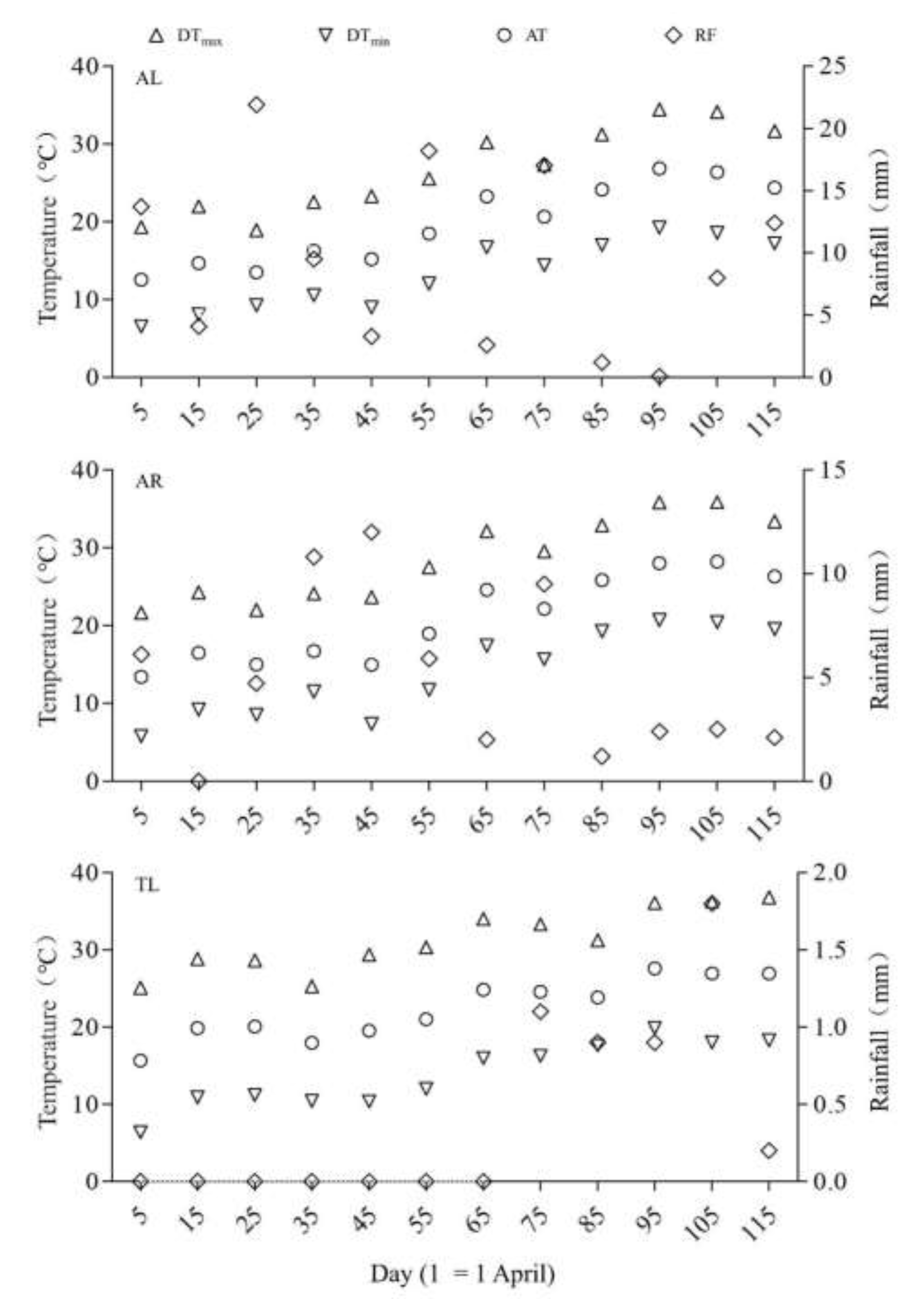
2.1. Study area
2.2. Data Source
2.3. Data collection and traits measurement
2.4. Statistical analysis
3. Results
3.1. Female reproductive traits among populations
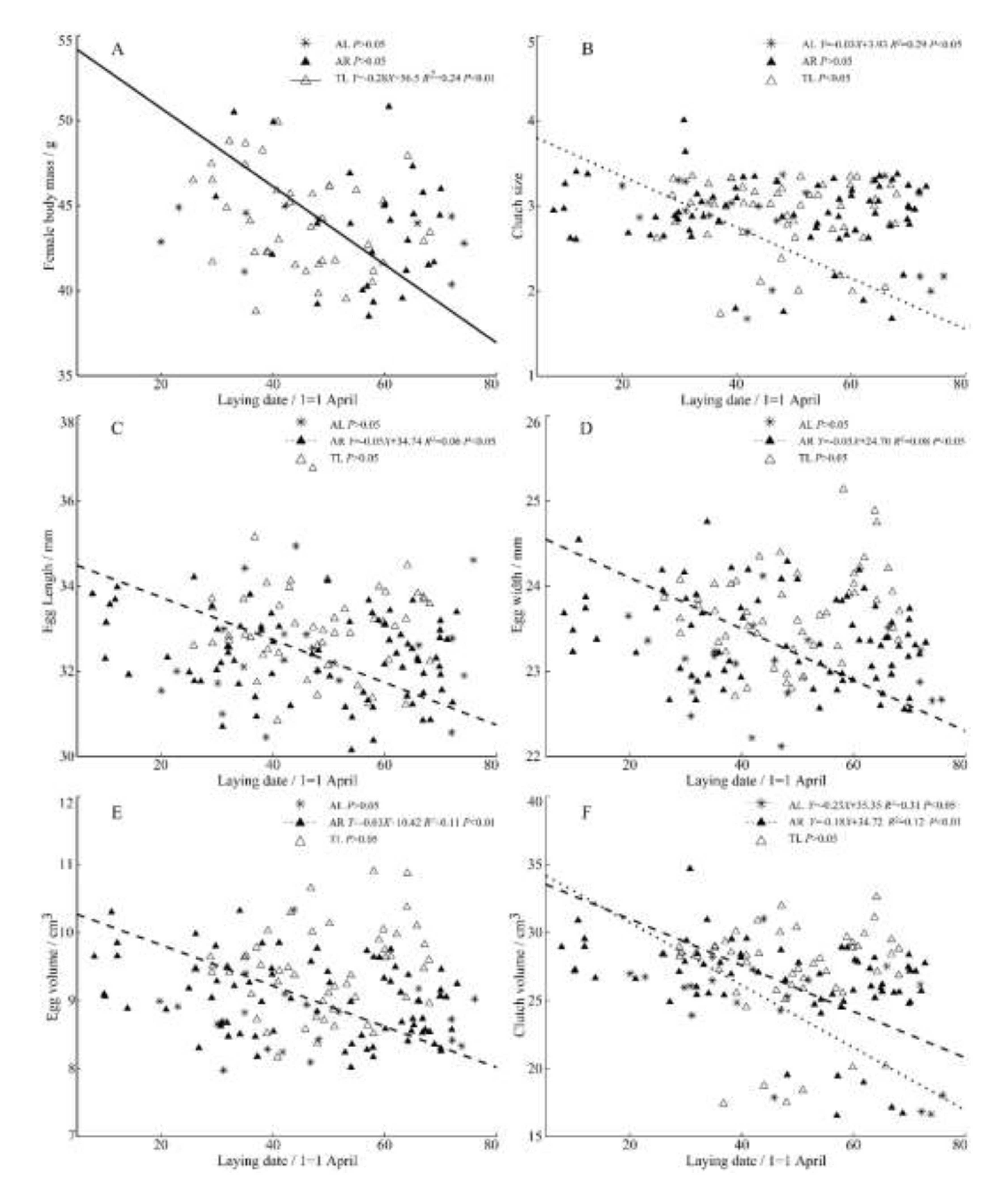
3.2. Laying date and reproductive traits
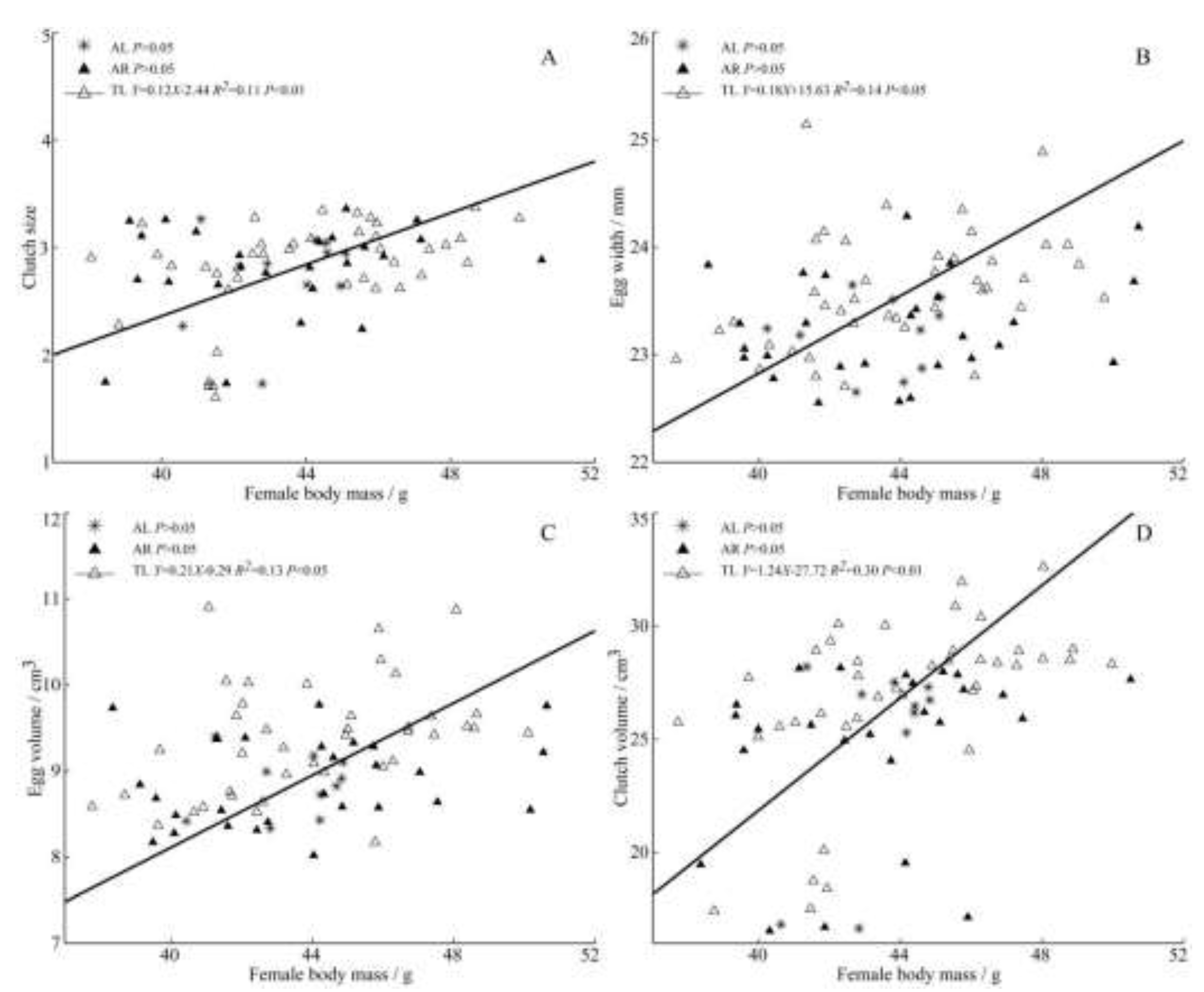
3.3. Reproductive output and female traits
3.4. Model selection
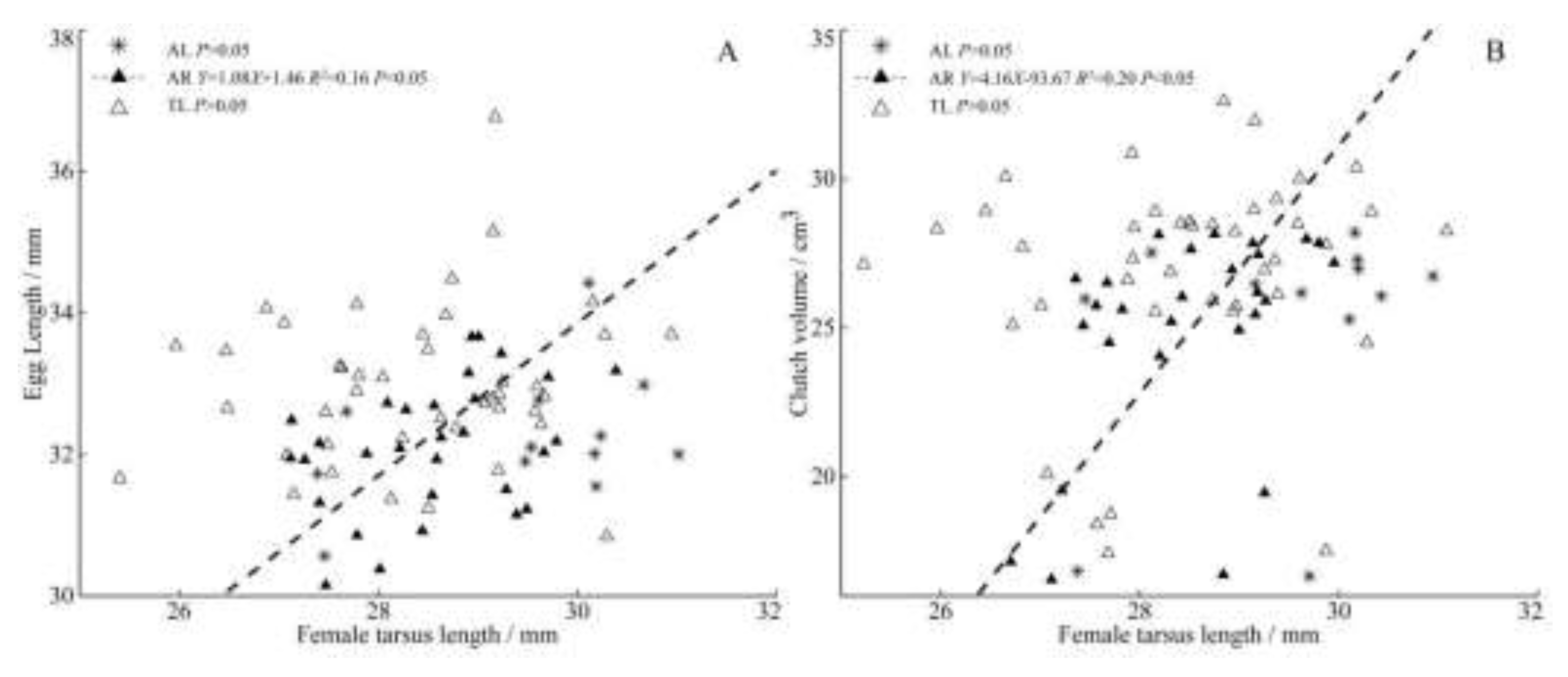
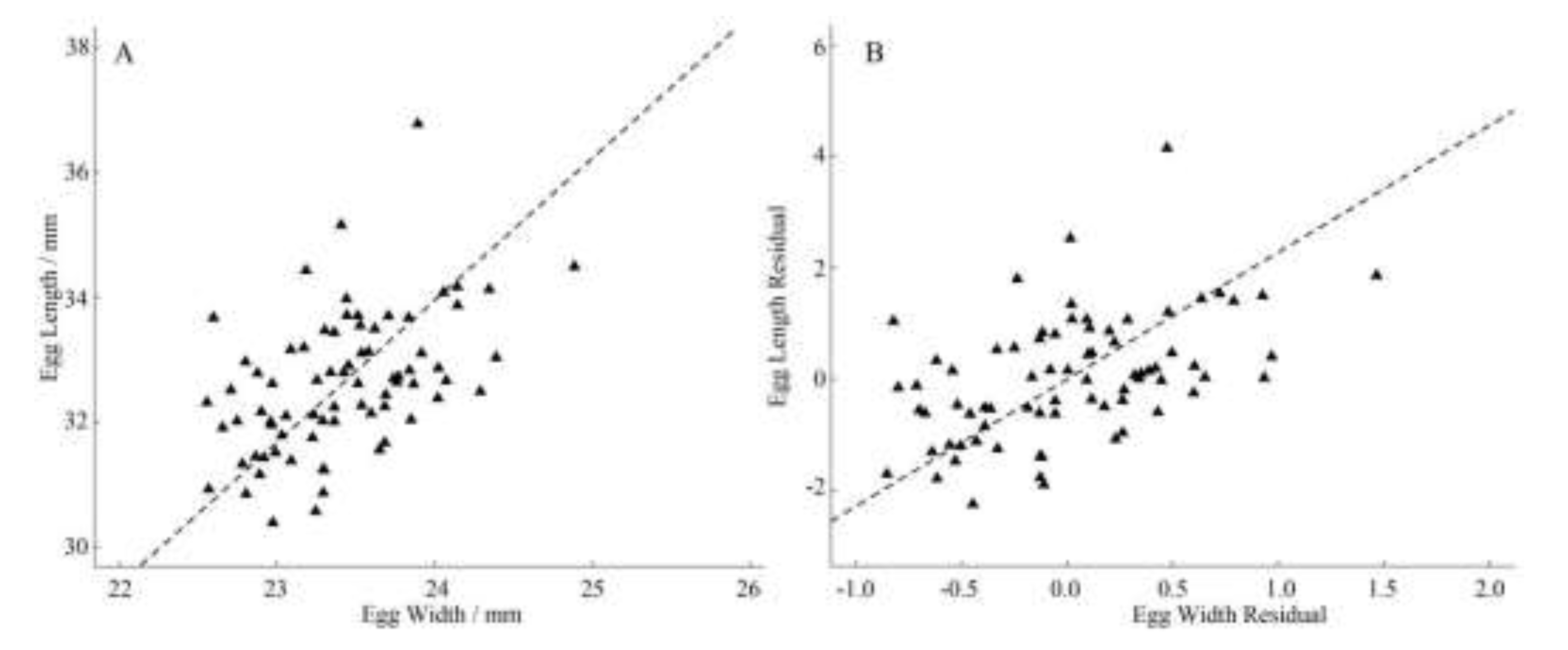
3.5. Allometry
4. Discussion
4.1. Differences of reproduction traits among populations
4.2. Relationship between reproductive traits and laying date
4.3. Egg size and reproductive output are affected by both maternal and environment conditions
4.4. The allometry of egg shape
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Roff, D.A. The evolution of life histories: Theory and analysis; Chapman & Hall: New York, USA, 1992. [Google Scholar]
- Stearns, S.C. The Evolution of Life Histories; Oxford University Press: Oxford, UK, 1992. [Google Scholar]
- Newton, I. Lifetime Reproduction in Birds; Academic Press: London, UK, 1989. [Google Scholar]
- Xu, F.; Yang, W.K.; Li, Y.M. Enlarged egg size increases offspring fitness of a frog species on the Zhoushan archipelago of China. Sci. Rep. 2019, 9, 11653. [Google Scholar] [CrossRef]
- Armstrong, T.; Robertson, R.J. Parental investment based on clutch value: Nest desertion in response to partial clutch loss in dabbling ducks. Anim. Behav. 1988, 36, 941–943. [Google Scholar] [CrossRef]
- Verhoeven, M.A.; Loonstra, A.H.; McBride, A.D.; Tinbergen, J.M.; Kentie, R.; Hooijmeijer, J.C.; Both, C.; Senner, N.R.; Piersma, T. Variation in egg size of Black-tailed Godwits. Ardea 2019, 107, 291–302. [Google Scholar] [CrossRef]
- Ghalambor, C.K.; Martin, T.E. Fecundity-survival trade-offs and parental risk-taking in birds. Science 2001, 292, 494–497. [Google Scholar] [CrossRef]
- Martin, T.E. Age-related mortality explains life history strategies of tropical and temperate songbirds. Science 2015, 349, 966–970. [Google Scholar] [CrossRef] [PubMed]
- Szekely, T.; Karsai, I. , Williams, T.D. Determination of clutch size in the Kentish Plover Charadrius alexandrinus. Ibis 1994, 136, 341–348. [Google Scholar] [CrossRef]
- Tian, L.; Liu, Y.; Zhou, Z.; Zhou, H.; Lu, S.; Zhang, Z. Reproductive success of a tropical barn swallow Hirundo rustica population is lower than that in temperate regions. Animals 2023, 13, 62. [Google Scholar] [CrossRef] [PubMed]
- Szekely, T.; Cuthill, I.C. Trade-off between mating opportunities and parental care: Brood desertion by female Kentish Plovers. P ROY SOC B-BIOL SCI 2000, 267, 2087–2092. [Google Scholar] [CrossRef]
- Zheng, G.M. Ornithology, 2nd ed.; Beijing Normal University Press: Beijing, China, 2012; pp. 260–276. [Google Scholar]
- Monaghan, P. Resource allocation and life history strategies in birds. Acta Zool. Sin. 2004, 50, 942–947. [Google Scholar]
- Karagicheva, J.; Rakhimberdiev, E.; Saveliev, A.; Piersma, T. Annual chronotypes functionally link life histories and life cycles in birds. Funct. Ecol. 2018, 32, 2369–2379. [Google Scholar] [CrossRef]
- Weiser, E.L.; Brown, S.C.; Lanctot, R.B.; Gates, H.R.; Abraham, K.F.; Bentzen, R.L.; Bêty, J.; Boldenow, M.L.; Brook, R.W.; Donnelly, T.F.; English, W.B.; Flemming, S.A.; Franks, S.E.; Gilchrist, H.G.; Giroux, M.; Johnson, A.; Kennedy, L.V.; Koloski, L.; Kwon, E.; Lamarre, J.; Lank, D.B.; Lecomte, N.; Liebezeit, J.R.; McKinnon, L.; Nol, E.; Perz, J.; Rausch, J.; Robards, M.; Saalfeld, S.T.; Senner, N.R.; Smith, P.A.; Soloviev, M.; Solovyeva, D.; Ward, D.H.; Woodard, P.F.; Sandercock, B.K. Life-history tradeoffs revealed by seasonal declines in reproductive traits of Arctic-breeding shorebirds. J. Avian Biol. 2018, 49, e01531. [Google Scholar] [CrossRef]
- Lu, X.; Ke, D.; Zeng, X.; Yu, T. Reproductive ecology of two sympatric Tibetan snowfinch species at the edge of their altitudinal range: Response to more stressful environments. J Arid Environ 2009, 73, 1103–1108. [Google Scholar] [CrossRef]
- Luo, R.S.; Su, T.P.; Niu, N.; Liang, W. Effect of temperature on timing of reproduction and nesting success in a tropical population of Barn Swallows in south China. Chin. J. Ecol. 2016, 8, 2159–2163. [Google Scholar] [CrossRef]
- Remeš, V.; Matysioková, B.; Vrána, J. Adaptation and constraint shape the evolution of growth patterns in passerine birds across the globe. Front. Zool. 2020, 17, 29. [Google Scholar] [CrossRef] [PubMed]
- Hille, S.M.; Cooper, C.B. Elevational trends in life histories: Revising the pace-of-life framework. Biol. Rev. 2015, 90, 204–213. [Google Scholar] [CrossRef] [PubMed]
- Lu, X. Reproductive ecology of blackbirds (Turdus merula maximus) in a high-altitude location, Tibet. J Ornithol. 2015, 146, 72–78. [Google Scholar] [CrossRef]
- Lu, X. , Gong, G., Zeng, X. Reproductive ecology of Brown-cheeked Laughing Thrushes (Garrulus henrici) in Tibet. J Field Ornithol. 2008, 79, 152–158. [Google Scholar] [CrossRef]
- Boyle, A.W. , Sandercock, B.K., Martin, K. Patterns and drivers of intraspecific variation in avian life history along elevational gradients: A meta-analysis. Biological Reviews 2015, 91, 469–482. [Google Scholar] [CrossRef]
- Deeming, D.C. Importance and evolution of incubation in avian reproduction. — In: Avian incubation: Behaviour, environment, and evolution (Deeming, D.C., ed.). Oxford University Press: Oxford, 2002.
- Colwell, M.A. B.; Millett, J.J.; Meyer, J.N.; Hall, S.J.; Hurley, S.E.; Mcallister, A.; Transou, R.R. LeValley. Snowy plover reproductive success in beach and river habitats. J. Field Ornith. 2005; 76, 373–382. [Google Scholar] [CrossRef]
- Chalfoun, A.D., K. A. Schmidt. Adaptive breeding habitat selection: Is it for the birds? Auk 2012, 129, 589–599. [Google Scholar] [CrossRef]
- Dunn, P. Breeding dates and reproductive performance. Advances in Ecological Research, 2004; 35, 69–87. [Google Scholar] [CrossRef]
- Caro, T. Antipredator defenses in birds and mammals. Chicago: University of Chicago Press; 2005.
- Luo, R.S.; Su, T.P.; Niu, N.; Liang, W. Effect of temperature on timing of reproduction and nesting success in a tropical population of Barn Swallows in south China. Chin. J. Ecol. 2016, 8, 2159–2163. [Google Scholar] [CrossRef]
- Song, S.; Chen, J.N.; Jiang, B.; Liu, N.F. Variation in egg and clutch size of the Black Redstart (Phoenicurus ochruros) at the northeastern edge of the Qinghai-Tibetan Plateau. Avian Res. 2016, 7, 20. [Google Scholar] [CrossRef]
- Boyle, A.W.; Sandercock, B.K.; Martin, K. Patterns and drivers of intraspecific variation in avian life history along elevational gradients: A meta-analysis. Biol. Rev. 2016, 91, 469–482. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Xiao, H.; Han, H.; Wei, W.; Zhou, H.; Zhang, Z. Comparison of breeding strategies of two sympatric thrush species in an alpine environment. Front. Ecol. Evol. 2022, 10, 1049983. [Google Scholar] [CrossRef]
- Hille, S.M.; Cooper, C.B. Elevational trends in life histories: Revising the pace-of-life frame. Biol. Rev. 2015, 90, 204–213. [Google Scholar] [CrossRef]
- Jetz, W.; Sekercioglu, C.H.; Bohning-Gaese, K. The worldwide variation in avian clutch size across species and space. PLoS Biol. 2008, 12, 2650–2657. [Google Scholar] [CrossRef] [PubMed]
- Stoddard, M.C.; Yong, E.H.; Akkaynak, D.; Sheard, C.; Tobias, J.A.; Mahadevan, L. Avian egg shape: Form, function, and evolution. Science 2017, 356, 1249–1254. [Google Scholar] [CrossRef]
- Du, B.; Liu, C.J.; Yang, M.; Bao, S.J.; Guan, M.M.; Liu, N.F. Horned larks on the Tibetan Plateau adjust the breeding strategy according to the seasonal changes in the risk of nest predation and food availability. J. Avian Biol. 2014, 45, 466–474. [Google Scholar] [CrossRef]
- Hasegawa, M.; Arai, E. Convergent evolution of the tradeoff between egg size and tail fork depth in swallows and swifts. J. Avian Biol. 2018, 49, e01684. [Google Scholar] [CrossRef]
- Charnov, E.L.; Downhower, J.F.; Brown, L.P. Optimal offspring sizes in small litters. Evol. Ecol. 1995, 9, 57–63. [Google Scholar] [CrossRef]
- Hargitai, R.; Török, J.; Tóth, L.; Hegyi, G.; Rosivall, B.; Szigeti, B.; Szöllősi, E. Effects of environmental conditions and parental quality on inter- and intraclutch egg-size variation in the Collared Flycatcher (Ficedula albicollis). Auk 2005, 122, 509–522. [Google Scholar] [CrossRef]
- Christians, J.K. Avian egg size: Variation within species and inflexibility within individuals. Biol. Rev. 2002, 77, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Ruffino, L.; Salo, P.; Koivisto, E.; Banks, P.B.; Korpimaki, E. Reproductive responses of birds to experimental food supplementation: A meta-analysis. Front. Zool. 2014, 11, 80. [Google Scholar] [CrossRef]
- Hallmann, K.; Griebler, E.M. An exploration of differences in the scaling of life history traits with body mass within reptiles and between amniotes. Ecol. Evol. 2018, 8, 5480–5494. [Google Scholar] [CrossRef] [PubMed]
- Yanagi, S.; Tuda, M. Female size constrains egg size via the influence of reproductive organ size and resource storage in the seed beetle Callosobruchus chinensis. J. Insect Physiol. 2012, 58, 1432–1437. [Google Scholar] [CrossRef] [PubMed]
- Krist, M. Egg size and offspring quality: A meta-analysis in birds. Biol. Rev. 2011, 86, 692–716. [Google Scholar] [CrossRef]
- Bouakkaz, A.; Belhassini, T.; Bensouilah, M.A. Bensouilah & M. Houhamdi. Breeding behaviour of the Kentish Plover (Charadrius alexandrinus) in a salt marsh frothe Eastern High Plateaux, northeast Algeria. Journal of King Saud University-Science, 2017; 29, 291–301. [Google Scholar] [CrossRef]
- Liang, T.; Zhou, L.; He, W.F.; Shi, L. Variations in the reproductive strategies of three populations of Phrynocephalus helioscopus in China. Peer J 2018, 6, e5705. [Google Scholar] [CrossRef]
- Székely, T. Why study plovers? The significance of non-model organisms in avian ecology, behaviour and evolution. J. Ornithol. 2019, 160, 923–933. [Google Scholar] [CrossRef]
- Colwell, M.A.; Haig, S.M. The population ecology and conservation of Charadrius Plovers. Taylor & Francis Group: Florida, USA, 2019; pp. 91–127.
- Elgar, M.A.; Heaphy, L.J. Covariation between clutch size, egg weight and egg shape: Comparative evidence for chelonians. J. Zool. 1989, 219, 137–152. [Google Scholar] [CrossRef]
- Kratochvíl, L.; Frynta, D. Egg shape and size allometry in geckos (Squamata: Gekkota), lizards with contrasting eggshell structure: Why lay spherical eggs? [J]. Journal of Zoological Systematics and Evolutionary Research 2010, 44, 217–222. [Google Scholar] [CrossRef]
- Fraga, R.M.; Amat, J.A. Breeding biology of a Kentish plover (Charadrius alexandrinus) population in an inland saline lake. Ardeola 1996, 43, 69–85. [Google Scholar]
- Fojt, E.; Triplet, P.; Robert, J.C.; Stillman, R.A. Comparison of the breeding habitats of Little Ringed Plover Charadrius dubius and Kentish plover Charadrius alexandrinus on a shingle bed. Bird Study 2000, 47, 8–12. [Google Scholar] [CrossRef]
- del Hoyo, J.; Wiersma, P.; Kirwan, G.M. Kentish plover (Charadrius alexandrinus), version 1.1. In Birds of the World; Keeney, B.K. Cornell Lab of Ornithology: Ithaca, NY, USA, 2021. [Google Scholar] [CrossRef]
- Alrashidi, M.; Kosztolanyi, A.; Shobrak, M.; Kupper, C.; Szekely, T. Parental cooperation in an extreme hot environment: Natural behaviour and experimental evidence. Anim. Behav. 2011, 82, 235–243. [Google Scholar] [CrossRef]
- Zheng, G.M. A Checklist on the Classification and Distribution of the Birds of China, 3rd ed.; Science Press: Beijing, China, 2017; p. 68. [Google Scholar]
- Wang, D.; Zheng, S.C.; Wang, P.; Matsiko, J.; Sun, H.Z.; Hao, Y.F.; Li, Y.M.; Zhang, Z.W.; Que, P.J.; Meng, D.R.; Zhang, Q.H.; Jiang, G.B. Effects of migration and reproduction on the variation in persistent organic pollutant levels in Kentish plovers from Cangzhou Wetland, China. Sci. Total Environ. 2019, 670, 122–128. [Google Scholar] [CrossRef]
- Zeraoula, A.; Bensouilah, T.; Brahmia, H.; Bouslama, Z.; Houhamdi, M. Breeding biology of the European Blackbird Turdus merula in orange orchards. J King Saud Univ Sci 2015, S1018364715000981. [Google Scholar] [CrossRef]
- Potapov, R.L. Adaptation of birds to life in high mountains in Eurasia. Acta Zool Sin 2004, 50, 970–977. [Google Scholar]
- Ghalambor, C.K.; Martin, T.E. Fecundity-survival trade-offs and parental risk-taking in birds. Science 2001, 292, 494–497. [Google Scholar] [CrossRef] [PubMed]
- Martin, T.E. Age-related mortality explains life history strategies of tropical and temperate songbirds. Science 2015, 349, 966–970. [Google Scholar] [CrossRef]
- Martin, K. and Wiebe, K.L. Coping mechanisms of alpine and arctic breeding birds: Extreme weather and limitations to reproductive resilience. Integr Comp Biol 2004, 44, 177–185. [Google Scholar] [CrossRef]
- Badyaev, A.V.; Ghalambor, C. K. 2001. Evolution of life histories along elevational gradients: Trade-off between parental care and fecundity. Ecology 2001, 8282, 2948–2960. [Google Scholar] [CrossRef]
- Johnson, L.S.; Ostlind, E.; Brubaker, J.L.; Balenger, S.L.; Johnson, B.G.P.; Golden, H. Changes in egg size and clutch size with elevation in a Wyoming population of Mountain Bluebirds. Condor 2006, 108, 591. [Google Scholar] [CrossRef]
- Bears, H.; Martin, K.; White, G.C. Breeding in high-elevation habitat results in shift to slower life-history strategy within a single species. J. Anim. Ecol. 2009, 78, 365–375. [Google Scholar] [CrossRef]
- Amat, J.A.; Fraga, R.M.; Arroyo, G.M. Intraclutch egg-size variation and offspring survival in the Kentish plover Charadrius alexandrinus. Ibis 2001, 143, 17–23. [Google Scholar] [CrossRef]
- Rocha, A.D.; Fonseca, D.; Masero, J.A. Coastal saltpans are a good alternative breeding habitat for Kentish plover Charadrius alexandrinus when umbrella species are present. J. Avian Biol. 2016, 47, 824–833. [Google Scholar] [CrossRef]
- Bouakkaz, A.; Belhassini, K.; Bensouilah, T.; Bensouilah, M.A.; Houhamdi, M. Breeding behaviour of the Kentish plover (Charadrius alexandrinus) in a salt marsh from the Eastern High Plateaux, northeast Algeria. J. King Saud Univ. Sci. 2017, 29, 291–301. [Google Scholar] [CrossRef]
- Que, P.; Chang, Y.J.; Eberhart-phillips, L.J.; Liu, Y.; Székely, T. Low nest survival of a breeding shorebird in Bohai Bay, China. J. Ornithol. 2015, 156, 297–307. [Google Scholar] [CrossRef]
- Song, Z.T.; Lin, X.; Que, P.J.; Halimubieke, N.; Huang, Q.J.; Zhang, Z.W.; Székely, T.; Liu, Y. The allocation between egg size and clutch size depends on local nest survival rate in a mean of bet-hedging in a shorebird. Avian Res. 2020, 11, 40. [Google Scholar] [CrossRef]
- Ma, M. A Checklist on the Distribution of the Birds in Xinjiang. Science Press: Beijing, China, 2011; pp. 49–50.
- Vincze, O.; Kosztolányi, A.; Barta, Z.; Küpper, C.; Alrashidi, M.; Amat, J.A. Parental cooperation in a changing climate: Fluctuating environments predict shifts in care division. Global Ecol. Biogeogr. 2017, 26, 347–358. [Google Scholar] [CrossRef]
- Abuduwaili, J. , Zhang, Z.Y., Jiang, F.Q. Assessment of the distribution, sources and potential ecological risk of heavy metals in the dry surface sediment of Aibi Lake in Northwest China. PLoS ONE 2015, 10, e0120001. [Google Scholar] [CrossRef]
- Ma, M.; Bayahen, K.; Li, F.; Hu, B.W.; Wu, J.Q.; Douglas, M.; Gao, X.; Amanjiang, R.; Chen, Y.; Mei, Y.; Ding, P. List of birds and count of Autumn migration in Ebinur Wetland Nature Reserve. Sichuan, J. Zool. 2010, 6, 912–918. [Google Scholar]
- Ye, Z.; Chen, S.; Zhang, Q.; Liu, Y.; Zhou, H. Ecological water demand of Taitema Lake in the lower reaches of the Tarim River and the Cherchen River. Remote Sens. 2022, 14, 832. [Google Scholar] [CrossRef]
- Zuo, T.; Chen, Y.; Ding, J. Research on vegetation coverage dynamics and prediction in the Taitema Lake region. Water 2022, 14, 725. [Google Scholar] [CrossRef]
- Szekely, T.; Kosztolanyi, A.; Kupper, C. Practical guide for investigating breeding ecology of Kentish plover Charadrius alexandrinus. University of Bath: Bath, UK, 2008.
- Hoyt, D.F. Practical methods of estimating volume and fresh weight of bird eggs. Auk 1979, 96, 73–77. [Google Scholar] [CrossRef]
- Norte, A.C.; Ramos, J.A. Nest-site selection and breeding biology of Kentish plover Charadrius alexandrinus on sandy beaches of the Portuguese west coast. Ardeola 2004, 51, 255–268. [Google Scholar]
- Weggler, M. Constraints on, and determinants of, the annual number of breeding attempts in the multi-brooded Black Redstart Phoenicurus ochruros. Ibis 2006, 148, 273–284. [Google Scholar] [CrossRef]
- Bears, H.; Martin, K.; White, G.C. Breeding in high-elevation habitat results in shift to slower life-history strategy within a single species. J. Anim. Ecol. 2009, 78, 365–375. [Google Scholar] [CrossRef]
- Martin, T.E. , 2008. Egg size variation among tropical and temper-ate songbirds: An embryonic temperature hypothesis. Proc. Natl Acad. Sci. 2008, 105, 9268–9271. [Google Scholar] [CrossRef]
- Yampolsky, L.Y.; Scheiner, S.M. Why larger offspring at lower temperatures? A demographic approach. Am. Nat. 1996, 147, 86–100. [Google Scholar] [CrossRef]
- Boulton, R.L.; Cassey, P. How avian incubation behaviour influences egg surface temperatures: Relationships with egg position, development and clutch size. J. Avian Biol. 2012, 43, 289–296. [Google Scholar] [CrossRef]
- Cooney, C.R.; Sheard, C.; Clark, A.D.; Healy, S.D.; Liker, A.; Street, S.E.; Troisi, C.A.; Thomas, G.H.; Székely, T.; Hemmings, N.; Wright, A.E. Ecology and allometry predict the evolution of avian developmental durations. Nat. Commun. 2020, 11, 2383. [Google Scholar] [CrossRef]
- Barta, Z.; Szekely, T. The optimal shape of avian eggs. Funct. Ecol. 1997, 11, 656–662. [Google Scholar] [CrossRef]
- Duursma, D.E.; Gallagher, R.V.; Price, J.J.; Griffith, S.C. Variation in avian egg shape and nest structure is explained by climatic conditions. Sci. Rep. 2018, 8, 4141. [Google Scholar] [CrossRef] [PubMed]
| Traits | AL(n=20) | AR(n=77) | TL(n=61) | F-level and | P-value |
|---|---|---|---|---|---|
| Female body mass *#, g | 43.42±1.52 | 43.79±3.37 | 43.95±2.93 | F2, 75=0.127, | P=0.881 |
| Female tarsometatarsus length *#, mm | 29.47±1.12 a | 28.51±0.84 b | 28.38±1.23 b | F2, 81=4.602, | P=0.012 |
| Egg length †, mm | 32.38±1.30 B | 32.36±1.08 B | 33.01±1.24 A | F2, 437=15.810, | P<0.001 |
| Egg width #, mm | 23.10±0.55 Bb | 23.38±0.55 Ba | 23.64±0.54 A | F2, 436=23.070, | P<0.001 |
| Egg shape # | 0.71±0.03 | 0.72±0.03 | 0.72±0.03 | F2, 436=3.961 | P=0.020 |
| Egg volume #, cm3 | 8.80±0.60 Bb | 9.02±0.58 Ba | 9.40±0.64 A | F2, 436=28.790 | P<0.001 |
| Clutch size † | 2.75±0.43 | 2.96±0.31 | 2.87±0.34 | χ2=5.929 | P=0.052 |
| Clutch volume †, cm3 | 24.57±4.02 Bb | 26.39±3.19 a | 27.07±3.50 A | χ2=12.249 | P=0.002 |
| Incubation period &, day | / | 26±1.33 a | 25.09±0.51 b | U=54.50 | P=0.037 |
| Parameter | Optimization model | |
|---|---|---|
| Egg volume | Clutch volume | |
| Clutch size | ||
| Female body mass | + | + |
| Female tarsometatarsus length | + | |
| Laying date | ||
| Egg length | + | |
| Egg width | ||
| Average temperature | + | + |
| Daily temperature difference | ||
| Daily maximum temperature | ||
| Rainfall | ||
| Population | ||
| AICc | -197.50 | -197.00 |
| Weight | 0.948 | 0.611 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





