1. Introduction
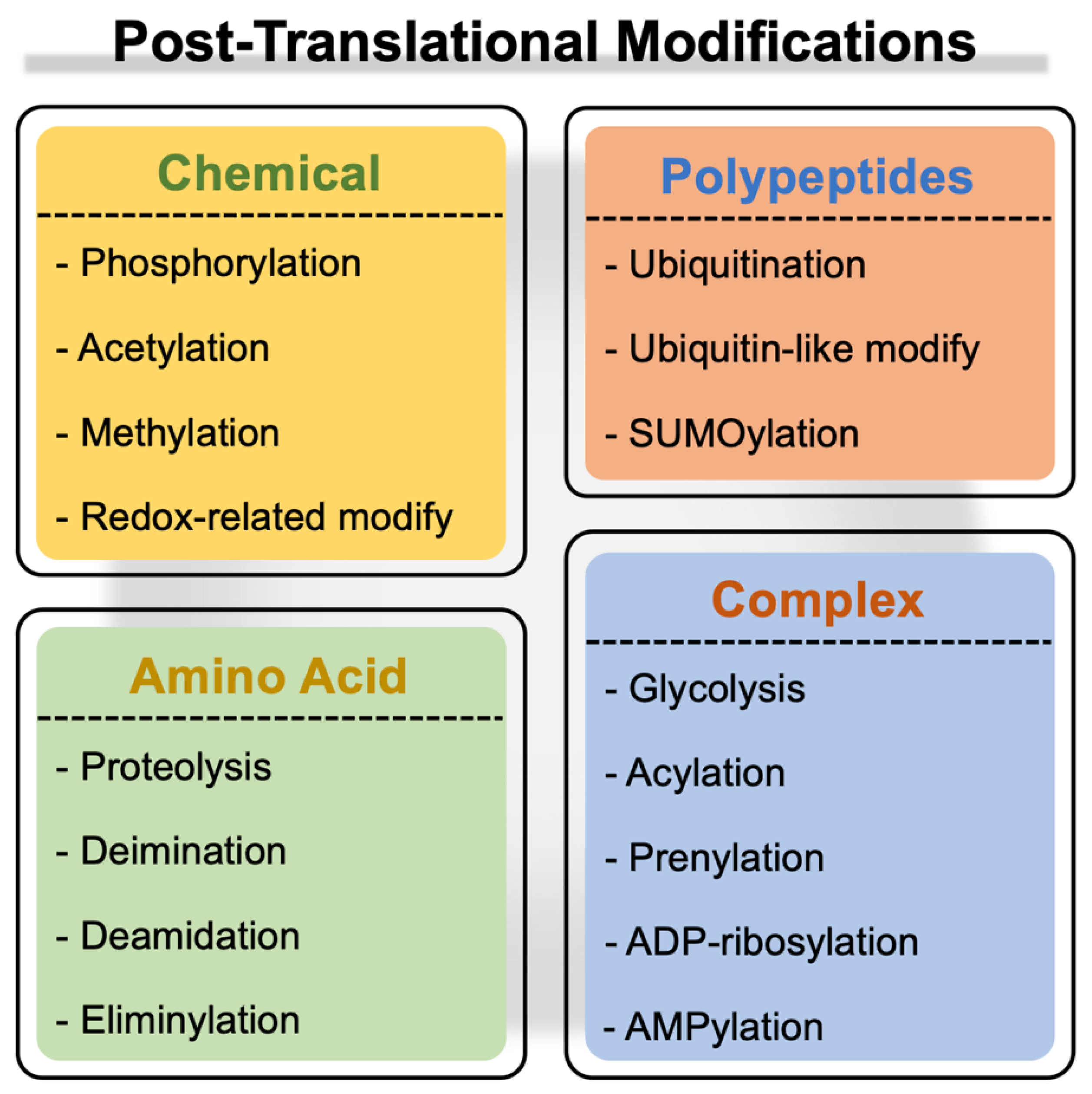
Post-translational modification (PTM) is the biochemical event that modulates the attributes of a protein during or following ribosomal synthesis. Generally, these modifications entail reversible chemical modifications (phosphorylation, acetylation, methylation, and redox-based modifications, including
S-nitrosylation,
S-sulfenation,
S-sulfination, and thiolation), enzymatic modifications (ubiquitination, ubiquitin-like modification, and SUMOylation), and complex molecules (glycosylation, ADP-ribosylation, AMPylation, and lipids attachment such as acylation and prenylation) and irreversible alterations such as deamidation, eliminylation, deimination, and proteolytic cleavage (
Figure 1) [
1]. The most frequently modified amino acids bear side chains containing hydroxy, amino, or thiol functional groups of serine, threonine, tyrosine, aspartate, asparagine, lysine, arginine, and cysteine [
1,
2]. A PTM can alter the chemical and biological attributes of the modified amino acid residues and/or neighboring polypeptide regions, leading to changes in the protein conformation, net charge, binding properties, and, ultimately, its function. Consequently, PTMs play a crucial role in a myriad of biological processes encompassing protein folding and stability, enzymatic activity, protein-protein interactions, cell signaling, and gene regulation [
2].
Eukaryotes, including plants and animals, undergo more complex PTMs, contrary to bacteria which can only manage limited PTMs such as phosphorylation, acetylation, methylation, and thiolation [
3]. Irregular PTMs leading to protein dysfunction have been implicated in a wide range of diseases, including cancers and neurodegenerative conditions such as Alzheimer’s [
4] and Parkinson’s [
5]. Despite the strong correlation between abnormal PTMs and diseases, PTMs remain a challenging topic to study due to their inherent complexity and diversity, various environmental conditions, and lack of the practical tool to explore these complex natural phenomena.
Hence, there is a growing demand for a versatile research platform to explore these intricate PTMs. The cell-free protein synthesis (CFPS) system has presented noteworthy progress in examining crucial PTMs. In this review, we revisit PTM investigations conducted using the CFPS system, aiming to unveil potential roadmaps for future studies and applications of PTMs in the realm of synthetic biology.
2. Cell-free protein synthesis system and protein biomanufacturing
2.1. Cell-free protein synthesis
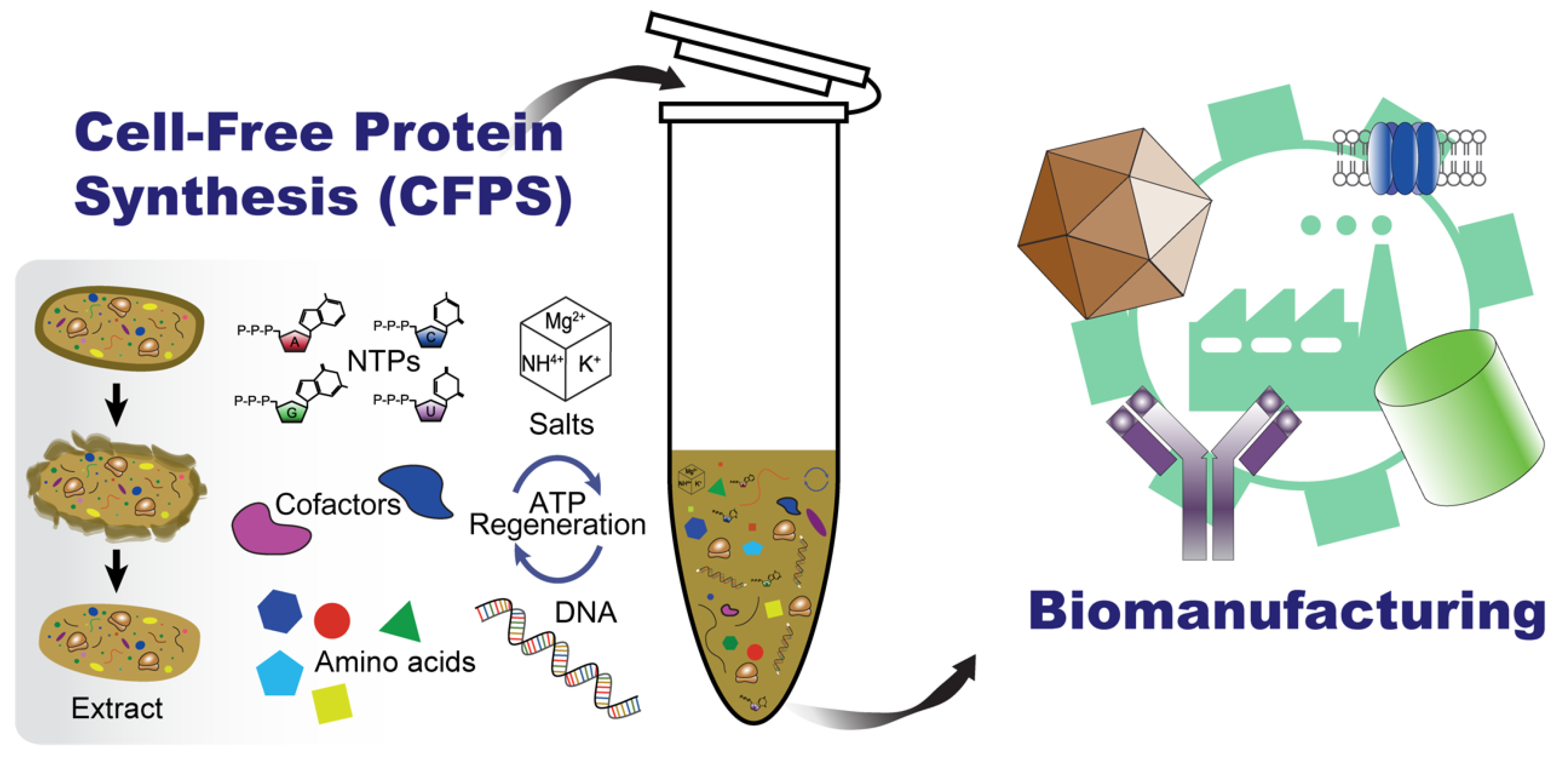
More than fifty years ago, Nirenberg and Matthaei introduced the CFPS system for the first time [
6], establishing a transformative platform that continues to advance our understanding of both fundamental and applied biology [
7]. Recent advances in synthetic biology further highlight CFPS as a rapid, high-yielding, and cost-effective production of various proteineous and non-proteineous bio-based products, which makes the system a compelling alternative to conventional cell-based biomanufacturing (
Figure 2) [
8,
9]. The open environment of the CFPS system serves as a major driving force that has enabled the direct addition of supplemental materials and control of cellular interactions. The versatility of CFPS positions this system as an optimal platform for producing complex and hard-to-synthesize proteins originating from higher organisms and functional proteins with precise PTMs [
9]. In addition, this system has shown promise in the development of synthetic genetic circuits [
10], unnatural amino acid incorporation [
11], and therapeutic protein production and prototyping [
8,
12].
2.2. Human proteins in CFPS biomanufacturing
The biopharmaceutical industry is predominantly focused on the development of protein therapeutics, such as monoclonal antibodies, peptides, and recombinant proteins, which represents the most proliferative category of emerging products [
13,
14]. Recently, a variety of notable human recombinant proteins have been produced in different types of CFPS systems. Sullivan et al. successfully produced both recombinant human erythropoietin (rhEPO) and human granulocyte-macrophage colony-stimulating factor (rhGM-CSF) in bacteria and yeast-based cell-free systems [
15]. This achievement carries great significance because FDA approval of these proteins underscores the potential of CFPS to expedite cost-effective protein pharmaceutical production. In 2017, progress was marked by the successful production of recombinant streptokinase - a therapeutic protein crucial in dissolving blood clots - via a CFPS system derived from the Chinese hamster ovary (CHO) [
16]. In addition, the recombinant skin therapeutic protein, monomeric Filaggrin, was synthesized by Kim et al. in the
Escherichia coli-based CFPS system, representing another successfully biomanufactured human protein using CFPS [
17]. Despite multiple achievements in the production of human proteins via bacterial CFPS systems, these systems frequently suffer from complications related to incorrect protein folding and insufficient PTM incorporation. To counter these challenges, researchers have moved to more sophisticated CFPS systems that utilize eukaryotic organisms such as yeast, insect, CHO, and human (HeLa) cells, thereby facilitating correct protein folding and PTMs [
18].
3. Disulfide bond formation
Disulfide bonds are a type of PTM that consists of covalent linkages formed by the oxidation of two cysteine residues on the polypeptide chain [
19]. The presence of disulfide bonds is crucial for proper protein folding and serves as a vital factor that influences the structure and functionality of proteins. Any issues with disulfide bond formation can lead to misfolded proteins, which can have substantial implications, including disease development. Disulfide bonds are present in a wide range of proteins derived from various life forms. Some common types of disulfide bond-containing proteins include hormones, transport proteins, structural proteins, enzymes, and various classes of antibodies. The complexity of disulfide bonds can be described in terms of their patterns, which refer to the specific arrangement of disulfide bonds within a protein. These patterns can vary between different proteins, families, or organisms, with some containing only a few disulfide bonds while others contain many. Historically, the formation of these bonds in vitro has been a challenge due to the reducing conditions inside cells. However, recent advances in CFPS systems have provided technological advantages for producing proteins with disulfide bonds due to the system’s controlled and simplified environment. For example, in a CFPS system, the redox conditions can be precisely tuned to favor the formation of disulfide bonds. Moreover, components such as chaperones and disulfide isomerases, which assist in folding proteins and forming disulfide bonds, can be added in defined quantities. This level of control over the protein synthesis environment allows researchers to experiment with different conditions and components to optimize the production of proteins with specific disulfide bond patterns. Additionally, high-throughput approaches using CFPS systems can be useful for generating large libraries of proteins with varying disulfide bond patterns.
3.1. Biomanufacturing monoclonal IgG antibodies
In 2019, Markami et al. utilized the PURE (protein synthesis using recombinant elements) CFPS system for producing disulfide-containing monoclonal IgG antibodies by optimizing the redox conditions and using specific disulfide catalysts and chaperones [
20]. The PURE system is a reconstituted CFPS system based on the protein synthesis machinery of
E. coli. Unlike the cell lysate-based system, the PURE system contains only purified factors involved in transcription, translation, and energy regeneration [
21]. This study revealed that the redox environment could be optimized by adjusting the ratio of GSH/GSSG supplemented to the system. Additionally, it was found that the use of the
E coli-derived disulfide bond catalyst, known as disulfide bond isomerase (DsbC), and the molecular chaperone, DnaK, was sufficient for functional IgG synthesis. Under optimal conditions, peak production of the anti-HER2 antibody trastuzumab reached 124 µg/mL, demonstrating the PURE systems’ potential for rapid and cost-effective production of therapeutic proteins. Moreover, the study’s findings highlight the flexibility of the CFPS approach. The ability to precisely control the reaction conditions in the CFPS system enabled the researchers to optimize the environment for efficient disulfide bond formation, a critical factor for the correct folding and function of many proteins.
3.2. Hydrophobins production
Hydrophobins, a class of small, surface-activated proteins produced by fungi, have unique biochemical properties [
22]. They have a strong affinity for hydrophilic and hydrophobic interfaces, which allows the hydrophobins to self-assemble into a coating that can change the behavior of a surface. This makes them potentially useful for a range of biotechnological applications, such as implant coatings and drug delivery systems. However, the production of hydrophobins has been challenging due to their complex structure, which includes multiple disulfide bonds. In 2020, Siddiquee et al. demonstrated six different types of natively folded hydrophobins in a CFPS system by adjusting the conditions of the CFPS system [
22]. This study showcased the functional expression of proteins with multiple disulfide bonds.
3.3. Recombinant tumor necrosis factor-alpha
Tumor Necrosis Factor-alpha (TNF-α) plays a crucial role in inflammatory responses, immune cell signaling, and apoptosis of cancer cells [
23]. In 2022, a team utilized CFPS to produce human TNF-α, which carries a single disulfide bond between cysteine residues 69 and 101 [
23]. The team optimized the yield of soluble TNF-α protein using response surface methodology and then tested the cytotoxicity of the recombinant TNF-α against three different human cancer cell lines, Caco-2, HepG-2, and MCF-7, as well as normal human cells. By optimizing the production process and confirming the protein’s therapeutic effects, this research highlighted the use of the CFPS system for the functional production of the therapeutic protein.
3.4. Prototyping disulfide bond-containing proteins
Dopp and Nigel reported a simple, functional, and cost-effective cell extract derived from a commercially available
E. coli strain SHuffle T7 Express
lysY that can express both T7 RNA polymerase and DsbC for in vitro prototyping of proteins with disulfide bonds [
24]. This strain provides the benefit of requiring only the optimization of IPTG induction and harvest times for the cell growth during cell extract preparation. In order to experimentally determine these optimized parameters, they used the Gaussia luciferase (GLuc), which carries five disulfide bonds and emits a strong luminescent signal, making it an ideal reporter for extract optimization. To demonstrate the versatility and rapid prototyping capability of the SHuffle extract, Dopp and Nigel screened the activity of four Luciferase candidates against ten luciferin analogues. Each of these analogues contained multiple disulfide bonds and acted on the substrate coelenterazine for bioluminescent activity assays. Beyond this, they also showcased the broad applicability of the developed cell extract by producing the enzymes, Hevamine, endochitinase A, and periplasmic AppA, each containing three disulfide bonds. The activity of Hevamine and ChitA was significantly improved in the developed system, showing 3.4x and 2.4x fold increase in activity, respectively.
4. Glycosylation
Glycosylation is one of the most common PTMs observed in eukaryotic organisms that plays an integral role in protein folding, movement, and signaling processes [
25]. This PTM entails the conjugation of a complex carbohydrate group, known as a “glycan,” to an amino acid residue within a protein [
26]. Asparagine-linked (N-linked) glycosylation and O-linked glycosylation are both highly prevalent types of glycosylation. N-linked glycosylation involves the attachment of a glycan to an asparagine residue, while O-glycosylation involves attachment to a serine, threonine, or hydroxylysine residue. In addition, both types of glycosylation can be distinguished from one another by their slightly different sugar peptide bonds. Although both N-linked and O-linked glycosylation are ubiquitously observed, N-glycosylation is comparatively more predominant. Proteins subjected to this PTM play crucial roles in regulating protein folding and sorting prior to their transport to the Golgi apparatus, thereby impacting immune responses, stem cell fate, and pharmacokinetics [
27]. Studying N-glycosylation presents substantial challenges due to the heterogeneity of resulting proteins, high cost, and reliance on mammalian cells, which comes with considerable expense and lower yield limitations. However, this PTM has been successfully challenged in the CFPS system.
4.1. One-pot glycoprotein synthesis
In 2018, a collaboration between the DeLisa and Jewett groups synthesized a glycopeptide using a bacterial CFPS system [
27]. The
E. coli cell extract, which is enriched with a glycosyltransferase enzyme and a sugar substrate, facilitated the synthesis of a glycopeptide with a single sugar moiety attached. This “one-pot glycoprotein synthesis system” was a major stride in glycoengineering. The team used the gram-negative bacterium
Campylobacter jejuni as a model system which carries a bacterial protein glycosylation locus (PGL), directing cells to execute N-glycosylation akin to eukaryotic cells.
E. coli strain CLM24, previously optimized for N-glycosylation by inactivating O-glycosylation, was used to prepare the crude cell extract. Subsequently, the team tested whether glycans other than
C. jejuni would be comparable with the system. They found that native and engineered
Campylobacter lari glycans, native
Wolinella succinogenes glycans, and many engineered
E. coli and
Klebsiella pneumoniae glycans, all with diverse structures, supported in vitro glycosylation successfully. Finally, the team developed a genuinely “one-pot” system by creating a cell extract from the CLM24 cells overexpressing the oligosaccharide donors and enzymes utilized previously. This enabled complete glycosylation of the DQNAT motif at single-chain Fv antibody (scFv13-R4
DQNAT) and superfolder Green fluorescent Protein (sfGFP
217-DQNAT) without adding supplements to the CFPS system. The “one-pot” system gave researchers great flexibility over the components being utilized to create the most efficient system possible. Additionally, the system was ten times more cost-effective than the commercial glycoprotein synthesis system [
27].
4.2. Recombinant immunotoxin synthesis
In 2022, Krebs and colleagues worked to produce a recombinant immunotoxin (RIT) based on the Pseudomonas exotoxin A, PE24 [
28]. This strong RIT can potentially induce cell death, therefore the CFPS system provides a suitable production environment. However, producing this immunotoxin is challenging because the anti-CD7 antibody located in the RIT requires N-glycosylation. The team utilized CHO cell and
E. coli-based CFPS systems to address this. Following liquid scintillation, SDS-PAGE, autoradiography, ELISA, and MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assay, the team confirmed the successful production of RIT. It was later observed that both CHO and
E. coli cell-free synthesized RITs were highly effective in killing cells with CD7 and to a lesser extent, cells without CD7 expression. This work holds significant promise for the treatment of diseases associated with CD7 expression, including T-cell acute lymphoblastic leukemia, acute myeloid leukemia, and others.
4.3. GlycoCAP
Recently, the Jewett group successfully synthesized proteins with noncanonical glycan attachments, utilizing the
E. coli-based CFPS system [
29]. Lectins are proteins that bind to specific glycans, recognized for their potential use in treating allergies and autoimmune disorders. However, it is difficult to create glycan-based drugs because scientists are unable to rapidly create a variety of structures necessary to formulate the drugs [
29]. Currently, researchers use mammalian cell culture to create glycans, leading to heterogenous populations, although homogenous ones would be preferred. To address this problem, the team developed the GlycoCAP system, which facilitated the installation of noncanonical glycans onto various proteins. This showcased the application of four different glycans onto the dust mite allergen - α2,3 C5-azido-sialyllactose, α2,3 C9-azido-sialyllactose, α2,6 C5-azido-sialyllactose, and α2,6 C9-azido-sialyllactose. This breakthrough opens the door for the development of novel allergy treatments and enhances our understanding of certain neurodegenerative diseases.
5. Other PTMs available in the CFPS system
5.1. Phosphorylation
Phosphorylation is a type of reversible PTM often used to activate or deactivate proteins [
1,
2]. The addition of a phosphate group introduces a negative charge, rendering the protein more hydrophilic, and in turn, changing the shape of the protein. This modification influences various cellular processes, such as cell growth, apoptosis, and signal transduction. Current methods include using chemical analogs or modifying target sites to mimic phosphorylation, but these are all limited by low yields [
30]. Notably, in 2015 Oza et al. demonstrated the site-specific incorporation of phosphoserine into human mitogen-activated ERK activating kinase 1 (MEK1) utilizing an
E. coli-based CFPS system [
30]. They successfully produced mono- and double-phosphorylated MEK1 in milligram quantities and tested the activity in a recreated in vitro signaling cascade. This work suggests that the CFPS system can be a high-yielding production platform for the direct phosphorylation on proteins. In addition, this marked a significant advancement towards a better understanding of the human phosphoproteome and the exploration of disease-related phosphorylated proteins and potential therapeutic small molecule inhibitors.
5.2. In vitro prenylation
In 2022, Kai et al. developed a prenylated protein synthesis system using the
E. coli-based CFPS [
31], leveraging the eukaryotic prenylation machinery, CFpPS, a similar concept to the cell-free one-pot glycoprotein synthesis platform previously developed in the DeLisa and Jewett groups [
27]. Protein prenylation is an irreversible PTM that transfers a farnesyl (15 carbon) or geranylgeranyl (20 carbon) group to a cysteine residue located in a C-terminal consensus sequence (CaaX box) of the target protein. This reaction is catalyzed by farnesyltransferase (Ftase) and geranylgeranyl transferase type 1 (GGTase-1), respectively. The CFpPS allowed for the co-translational expression of several model proteins, including Kras, Hras, RhoA, RhoC, Rac1, and Cdc4. The system successfully demonstrated soluble protein expression and membrane binding ability. This advancement provides compelling evidence for the practical use of the bacterial CFPS system for studying complex eukaryotic PTMs.
5.3. ALiCE
The Almost Living Cell-free Expression (ALiCE) system derived from the tobacco plant was developed to overcome some of the limitations inherent to bacterial CFPS systems [
32]. The AliCE system employs microsomal vesicles native to the cells, which allows the system to perform PTMs more efficiently. The team successfully utilized the AliCE system to produce various eukaryotic proteins that are typically difficult to express, such as a Hepatitis B core antigen model Virus-Like Particle, glucose oxidase (enzyme containing multiple disulfide bonds), SARS-CoV2 spike protein, and anti-tumor necrosis factor alpha monoclonal antibody adalimumab, which requires multiple glycosylation sites and disulfide bonds. Moreover, this team was the first to express the human cannabinoid receptor type II (CB2) in the ALiCE system. Lastly, the team was able to produce these proteins by minimizing batch-to-batch variability with scalable yields.
6. Concluding remarks and future challenges
This review summarizes the advances in cell-free systems for PTMs, which have given researchers unprecedented control over the modification processes and the ability to overcome the challenges faced with traditional in vivo systems. Using the CFPS system, proteins with unique modifications that are otherwise difficult or impossible to produce in vivo can now be synthesized efficiently. This extends not only to research purposes, such as understanding protein function and interactions, but also to practical applications, such as industrial pharmaceutical production. The ability to produce therapeutic proteins using the CFPS system at reduced cost and increased yield could revolutionize drug development, making novel therapeutics more accessible and affordable for end users. However, it is crucial to continue optimizing and developing the system, focusing on improving yield and reproducibility, and ensuring the functional activity of the target proteins. In conclusion, the CFPS system holds great potential for the study and production of post-translationally modified proteins. As the scientists continue to improve and expand the PTM repertoire available in the CPFS system, we can expect the system’s significant contribution to the biomanufacturing industry as well as synthetic biology research.
Author Contributions
Y.-C.K. conceived the contents. K.B.P. and C.E.L. wrote the original manuscript K.B.P., C.E.L., and Y.-C.K. revised and edited the manuscript. K.B.P. and C.E.L. contributed equally. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the Louisiana Board of Regent (RCS, Grant No. LEQSF(2020-23)RD-A-01) and USDA National Institute of Food and Agriculture (HATCH, Accession No. 1021535, Project No. LAB94414).
Ethics Statement
Not applicable.
Informed Consent Statement
Not applicable.
Acknowledgments
K.B.P. and C.E.L. thank the support of the Donald W. Clayton Engineering Excellence Award (Graduate Assistantship) from the College of Engineering at Louisiana State University.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Spoel, S.H. Orchestrating the proteome with post-translational modifications. J Exp Bot 2018, 69, 4499–4503. [Google Scholar] [CrossRef] [PubMed]
- Macek, B.; Forchhammer, K.; Hardouin, J.; Weber-Ban, E.; Grangeasse, C.; Mijakovic, I. Protein post-translational modifications in bacteria. Nat Rev Microbiol 2019, 17, 651–664. [Google Scholar] [CrossRef] [PubMed]
- Ramazi, S.; Zahiri, J. Post-translational modifications in proteins: resources, tools and prediction methods. Database 2021, 2021, baab012. [Google Scholar] [CrossRef] [PubMed]
- Ercan-Herbst, E.; Ehrig, J.; Schöndorf, D.C.; Behrendt, A.; Klaus, B.; Gomez Ramos, B.; Prat Oriol, N.; Weber, C.; Ehrnhoefer, D.E. A post-translational modification signature defines changes in soluble tau correlating with oligomerization in early stage Alzheimer’s disease brain. Acta Neuropathol Commun 2019, 7, 192. [Google Scholar] [CrossRef]
- Zhang, J.; Li, X.; Li, J.D. The roles of post-translational modifications on α-synuclein in the pathogenesis of Parkinson’s diseases. Front Neurosci 2019, 13, 381. [Google Scholar] [CrossRef]
- Nirenberg, M.W.; Matthaei, J.H. The dependence of cell-free protein synthesis in E. coli upon naturally occurring or synthetic polyribonucleotides. Proc Natl Acad Sci USA 1961, 47, 1588–1602. [Google Scholar] [CrossRef]
- Silverman, A.D.; Karim, A.S.; Jewett, M.C. Cell-free gene expression: an expanded repertoire of applications. Nat Rev Genet 2020, 21, 151–170. [Google Scholar] [CrossRef]
- Zawada, J.F.; Burgenson, D.; Yin, G.; Hallam, T.J.; Swartz, J.R.; Kiss, R.D. Cell-free technologies for biopharmaceutical research and production. Curr Opin Biotechnol 2022, 76, 102719. [Google Scholar] [CrossRef]
- Khambhati, K.; Bhattacharjee, G.; Gohil, N.; Braddick, D.; Kulkarni, V.; Singh, V. Exploring the potential of cell-free protein synthesis for extending the abilities of biological systems. Front Bioeng Biotechnol 2019, 7, 248. [Google Scholar] [CrossRef]
- Jeong, D.; Klocke, M.; Agarwal, S.; Kim, J.; Choi, S.; Franco, E.; Kim, J. Cell-free synthetic biology platform for engineering synthetic biological circuits and systems. Methods Protoc 2019, 2, 39. [Google Scholar] [CrossRef]
- Martin, R.W.; Des Soye, B.J.; Kwon, Y.C.; Kay, J.; Davis, R.G.; Thomas, P.M.; Majewska, N.I.; Chen, C.X.; Marcum, R.D.; Weiss, M.G.; et al. Cell-free protein synthesis from genomically recoded bacteria enables multisite incorporation of noncanonical amino acids. Nat Commun 2018, 9, 1203. [Google Scholar] [CrossRef]
- Wu, Y.; Cui, Z.; Huang, Y.-H.; de Veer, S.J.; Aralov, A.V.; Guo, Z.; Moradi, S.V.; Hinton, A.O.; Deuis, J.R.; Guo, S.; et al. Towards a generic prototyping approach for therapeutically-relevant peptides and proteins in a cell-free translation system. Nat Commun 2022, 13, 260. [Google Scholar] [CrossRef]
- Durocher, Y.; Butler, M. Expression systems for therapeutic glycoprotein production. Curr Opin Biotechnol 2009, 20, 700–707. [Google Scholar] [CrossRef]
- Ho, R.J.Y.; Chien, J. Trends in translational medicine and drug targeting and delivery: new insights on an old concept—targeted drug delivery with antibody–drug conjugates for cancers. J Pharm Sci 2014, 103, 71–77. [Google Scholar] [PubMed]
- Sullivan, C.J.; Pendleton, E.D.; Sasmor, H.H.; Hicks, W.L.; Farnum, J.B.; Muto, M.; Amendt, E.M.; Schoborg, J.A.; Martin, R.W.; Clark, L.G.; et al. A cell-free expression and purification process for rapid production of protein biologics. Biotechnol J 2016, 11, 238–248. [Google Scholar] [CrossRef] [PubMed]
- Tran, K.; Gurramkonda, C.; Cooper, M.A.; Pilli, M.; Taris, J.E.; Selock, N.; Han, T.-C.; Tolosa, M.; Zuber, A.; Peñalber-Johnstone, C.; et al. Cell-free production of a therapeutic protein: expression, purification, and characterization of recombinant streptokinase using a CHO lysate. Biotechnol Bioeng 2018, 115, 92–102. [Google Scholar] [CrossRef]
- Kim, J.; Copeland, C.E.; Seki, K.; Vögeli, B.; Kwon, Y.C. Tuning the cell-free protein synthesis system for biomanufacturing of monomeric human filaggrin. Front Bioeng Biotechnol 2020, 8, 590341. [Google Scholar] [CrossRef] [PubMed]
- Zemella, A.; Thoring, L.; Hoffmeister, C.; Kubick, S. Cell-free protein synthesis: pros and cons of prokaryotic and eukaryotic systems. Chembiochem 2015, 16, 2420–2431. [Google Scholar] [CrossRef] [PubMed]
- Weiss, K.; Racho, J.; Riemer, J. Compartmentalized disulfide bond formation pathways. In Redox Chemistry and Biology of Thiols, 1st ed.; Alvarez, B., Comini, M.A., Salinas, G., Trujillo, M., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 321–340. [Google Scholar]
- Murakami, S.; Matsumoto, R.; Kanamori, T. Constructive approach for synthesis of a functional IgG using a reconstituted cell-free protein synthesis system. Sci Rep 2019, 9, 671. [Google Scholar] [CrossRef]
- Shimizu, Y.; Inoue, A.; Tomari, Y.; Suzuki, T.; Yokogawa, T.; Nishikawa, K.; Ueda, T. Cell-free translation reconstituted with purified components. Nat Biotechnol 2001, 19, 751–755. [Google Scholar] [CrossRef]
- Siddiquee, R.; Choi, S.S.-c.; Lam, S.S.; Wang, P.; Qi, R.; Otting, G.; Sunde, M.; Kwan, A.H.-y. Cell-free expression of natively folded hydrophobins. Protein Expr Purif 2020, 170, 105591. [Google Scholar] [CrossRef] [PubMed]
- El-Baky, N.A.; EL-Fakharany, E.M.; Sabry, S.A.; El-Helow, E.R.; Redwan, E.M.; Sabry, A. A de novo optimized cell-free system for the expression of soluble and active human tumor necrosis factor-alpha. Biology 2022, 11, 157. [Google Scholar] [CrossRef] [PubMed]
- Dopp, J.L.; Reuel, N.F. Simple, functional, inexpensive cell extract for in vitro prototyping of proteins with disulfide bonds. Biochem Eng J 2020, 164, 107790. [Google Scholar] [CrossRef]
- Shrimal, S.; Cherepanova, N.A.; Gilmore, R. Cotranslational and posttranslocational N-glycosylation of proteins in the endoplasmic reticulum. Semin Cell Dev Biol 2015, 41, 71–78. [Google Scholar] [CrossRef]
- Reily, C.; Stewart, T.J.; Renfrow, M.B.; Novak, J. Glycosylation in health and disease. Nat Rev Nephrol 2019, 15, 346–366. [Google Scholar] [CrossRef]
- Jaroentomeechai, T.; Stark, J.C.; Natarajan, A.; Glasscock, C.J.; Yates, L.E.; Hsu, K.J.; Mrksich, M.; Jewett, M.C.; DeLisa, M.P. Single-pot glycoprotein biosynthesis using a cell-free transcription-translation system enriched with glycosylation machinery. Nat Commun 2018, 9, 2686. [Google Scholar] [CrossRef] [PubMed]
- Krebs, S.K.; Stech, M.; Jorde, F.; Rakotoarinoro, N.; Ramm, F.; Marinoff, S.; Bahrke, S.; Danielczyk, A.; Wüstenhagen, D.A.; Kubick, S. Synthesis of an anti-CD7 recombinant immunotoxin based on PE24 in CHO and E. coli cell-free systems. Int J Mol Sci 2022, 23, 13697. [Google Scholar] [CrossRef]
- Thames, A.H.; Moons, S.J.; Wong, D.A.; Boltje, T.J.; Bochner, B.S.; Jewett, M.C. GlycoCAP: a cell-free, bacterial glycosylation platform for building clickable azido-sialoglycoproteins. ACS Synth Biol 2023, 12, 1264–1274. [Google Scholar] [CrossRef]
- Oza, J.P.; Aerni, H.R.; Pirman, N.L.; Barber, K.W.; ter Haar, C.M.; Rogulina, S.; Amrofell, M.B.; Isaacs, F.J.; Rinehart, J.; Jewett, M.C. Robust production of recombinant phosphoproteins using cell-free protein synthesis. Nat Commun 2015, 6, 8168. [Google Scholar] [CrossRef]
- Kai, L.; Sonal; Heermann, T. ; Schwille, P. Reconstitution of a reversible membrane switch via prenylation by one-pot cell-free expression. ACS Synth Biol 2023, 12, 108–119. [Google Scholar] [CrossRef]
- Gupta, M.D.; Flaskamp, Y.; Roentgen, R.; Juergens, H.; Gimenez, J.A.; Albrecht, F.; Hemmerich, J.; Arfi, Z.A.; Neuser, J.; Spiegel, H.; et al. ALiCE: a versatile, high yielding and scalable eukaryotic cell-free protein synthesis (CFPS) system. bioRxiv 2022. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).