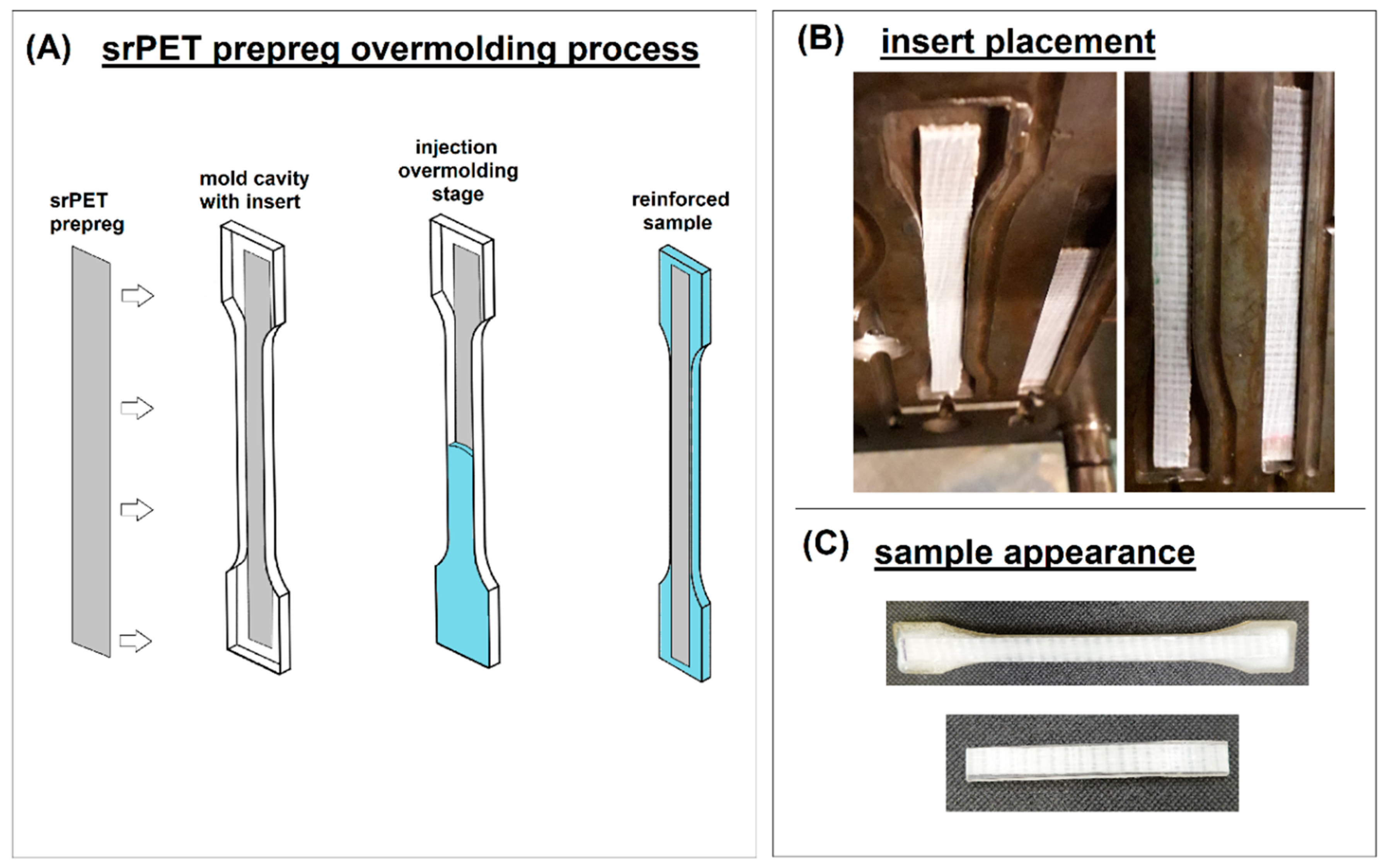
3.1. Mechanical performance – static tensile measurements and notched Charpy impact tests
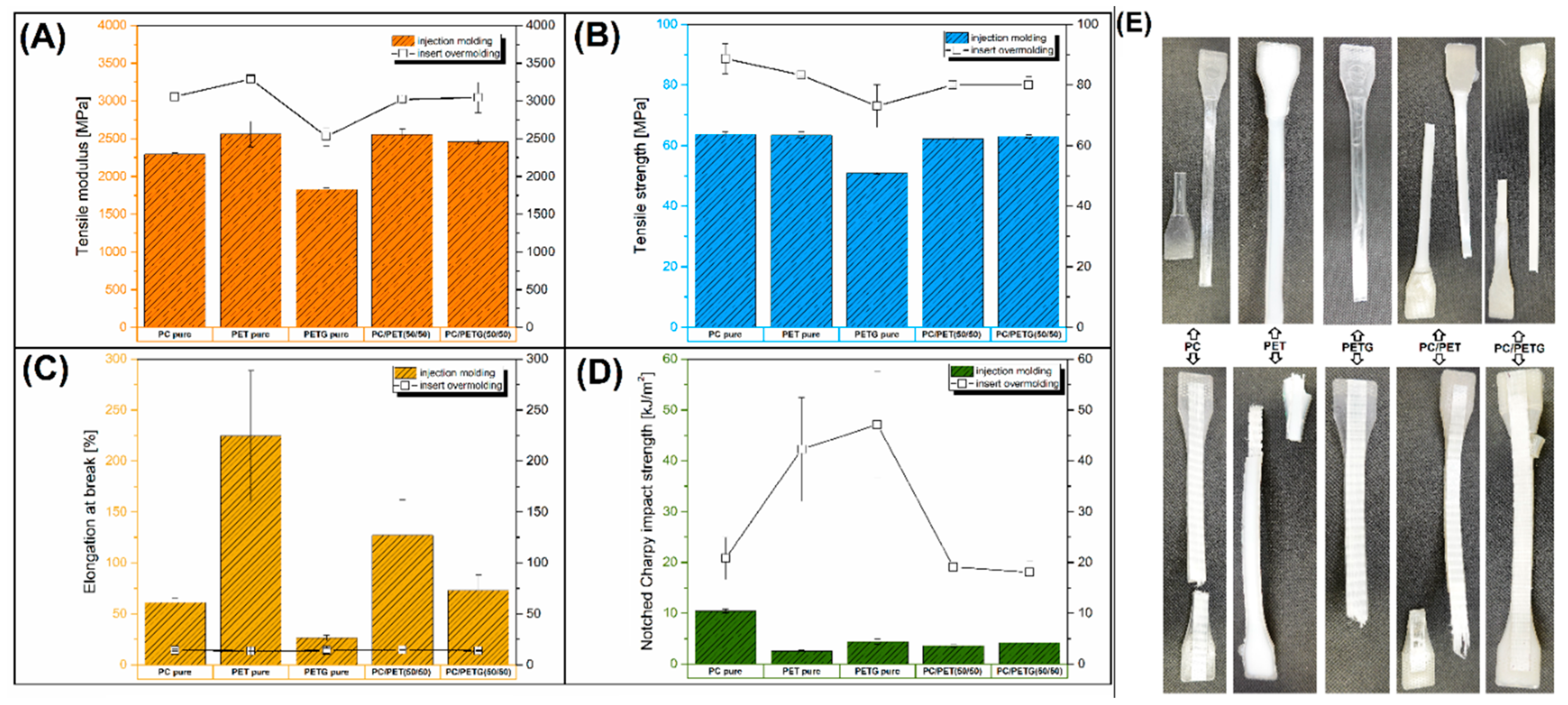
The results of the mechanical tests are shown in the form of plots in
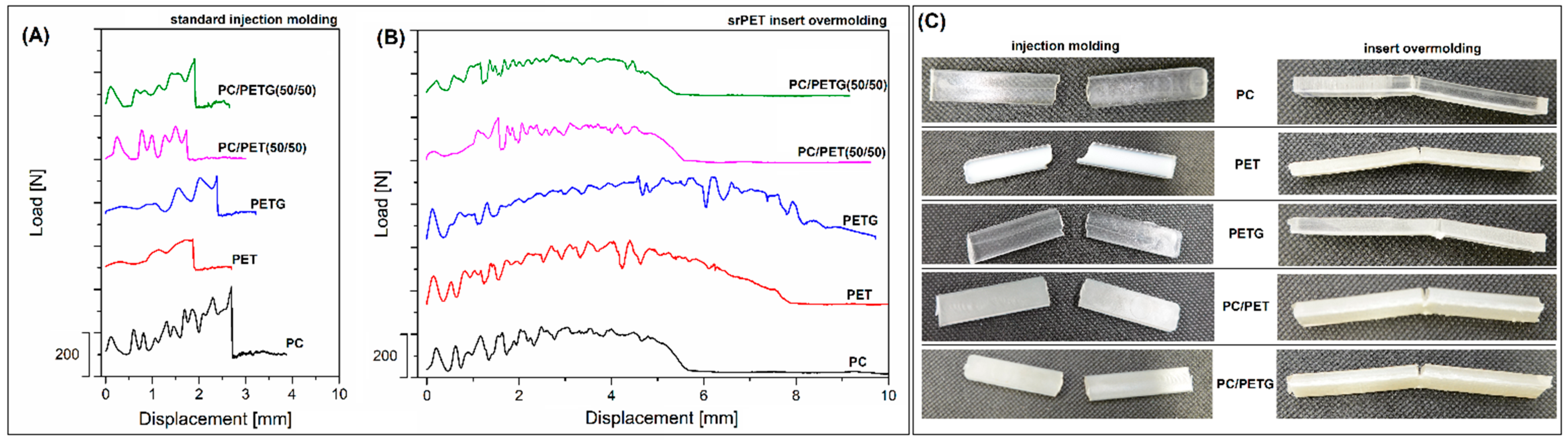
Figure 2, where the mean values of tensile modulus/strength together with elongation at break and impact strength are compared. Additionally, the results of the impact resistance measurements in the form of load/displacement plots and sample appearance are presented in
Figure 3.
The results of the tensile modulus/strength measurements confirmed clearly that the reinforcing efficiency for all of the tested samples was a visible improvement. For most of the samples, the average tensile modulus increase was from 20 to 30%. For example, the initial modulus of pure PC and PET samples increased from initial 2295/2560 MPa to 3060/3290 MPa for srPET reinforced samples. Interestingly, the lowest tensile modulus for overmolded samples was recorded after using pure PETG as the main molded component; however, that fact is mainly caused by the lowest stiffness of that resin. Since the modulus value for pure PETG was around 1820 MPa, the overmolded sample reached 2530 MPa, which still can be considered a large reinforcement factor. The trends of changes for tensile strength are very similar to tensile modulus values. Again, the tensile strength for pure PC and PET-based samples was improved from 64/63 MPa to 89/83 MPa, while the results for PETG-based specimens were visibly lower. The results of strength/modulus results analysis for polymer blends revealed slightly similar results to pure PC and PET samples. The stiffness for injection molded and overmolded materials was almost similar for PC/PET and PC/PETG-based materials; the same conclusion applies to tensile strength values.
When comparing the results of the elongation at break values, it is clear that large differences appear for the injection molded materials, where the correlation between pure polymers is 61%, 225%, and 26%, respectively, for PC, PET, and PETG material. The results for the PC/PET(50/50) blends are the result of the properties of the component polymers reaching 127 %, while for PC/PETG(50/50) the elongation of 73% was higher than the PC and PETG resin. The observed effect is quite an unexpected result because, as a rule, for polymer mixtures, most of the mechanical properties are the result of the properties of individual components are reduced in relation to them. The factor explaining this phenomenon is the partial miscibility of this polymer system, in which case the presence of two types of polymer chains may cause a plasticizing effect, where the reduction of the entanglement density of the amorphous structure increases the chain mobility of the macromolecules. It is worth noting that compared to commonly used plasticizing compounds, the visible effect is quite negligible; therefore, the effectiveness of this phenomenon is rather not practical. Interestingly, it is difficult to find descriptions of similar effects in the literature; this phenomenon is partly described for oriented PETG/PC foils [
45].
It turns out, however, that the initial differences in the properties of polymers obtained using the traditional molding technique do not have a visible effect on the properties of the samples reinforced in the overmolding method. For all samples with srPET reinforcement, the elongation at break values was close to 14%. Considering that the maximum strain for the pure srPET prepreg of around 19% is slightly higher than for overmolded specimens, this behavior may suggest the occurrence of a partial delamination process before the final fracture of the sample. More complex results are detected during the analysis of the impact resistance values, where properties of overmolded samples seem to be the most beneficial. In the case of injection molded materials, the highest impact strength was recorded for pure PC samples (10 kJ/m2), while the for the rest of the materials, the strength values did not reach 5 kJ/m2. After the introduction of the srPET insert, the impact resistance for the PC-based sample was increased to 21 kJ/m2, while for PET and PETG-based samples, even 42 kJ/m2 and 47 kJ/m2, respectively. The results for the blends were again reduced to around 19 kJ/m2. Some additional results reflecting the impact resistance of specimens are presented in the form of load/displacement plots (see
Figure 3). The biggest area under the curve of the PC sample clearly revealed the highest energy necessary for sample breakage. For other samples, the distance before full fracture and maximum force of the impact was lower. Since the impact strength for overmolded samples is strongly improved, the plot appearance also differs from solid unreinforced materials. The main difference refers to a visibly longer distance before the final fracture of the specimen. The longest displacement was recorded for PET and PETG-based samples, while for PC-based material and both types of blends, the distance was visibly smaller. Interestingly, the maximum force difference was not significant. When considering the unreinforced samples, the maximum load reached around 300 N for pure PC, while the force did not reach 200 N for the rest of the materials. For insert reinforced PC, the maximum load reached 200 N, while for PC-based blends, the force values were lower (≈ 175 N). Visibly higher values of around 300 N were recorded for PET and PETG-based specimens. However, the load/force value difference cannot be considered significant since the fluctuations had a percentage dimension, while the change in fracture distance (time) was several times greater for the reinforced samples. The appearance of samples after the impact test is shown in
Figure 3C. The photos reveal brittle fracture for all of the injection molded materials; even for PC specimens, the fracture surface does not show plastic deformation as is usually the case for samples based on this polymer. The obvious reason for the deterioration of PC properties is the influence of secondary processing (recycling). The sample appearance for overmolded insert materials revealed a lack of srPET insert fracture, while the molded part of the specimen was cracked for most of the specimen.
3.2. Thermomechanical properties – DMTA analysis/HDT tests
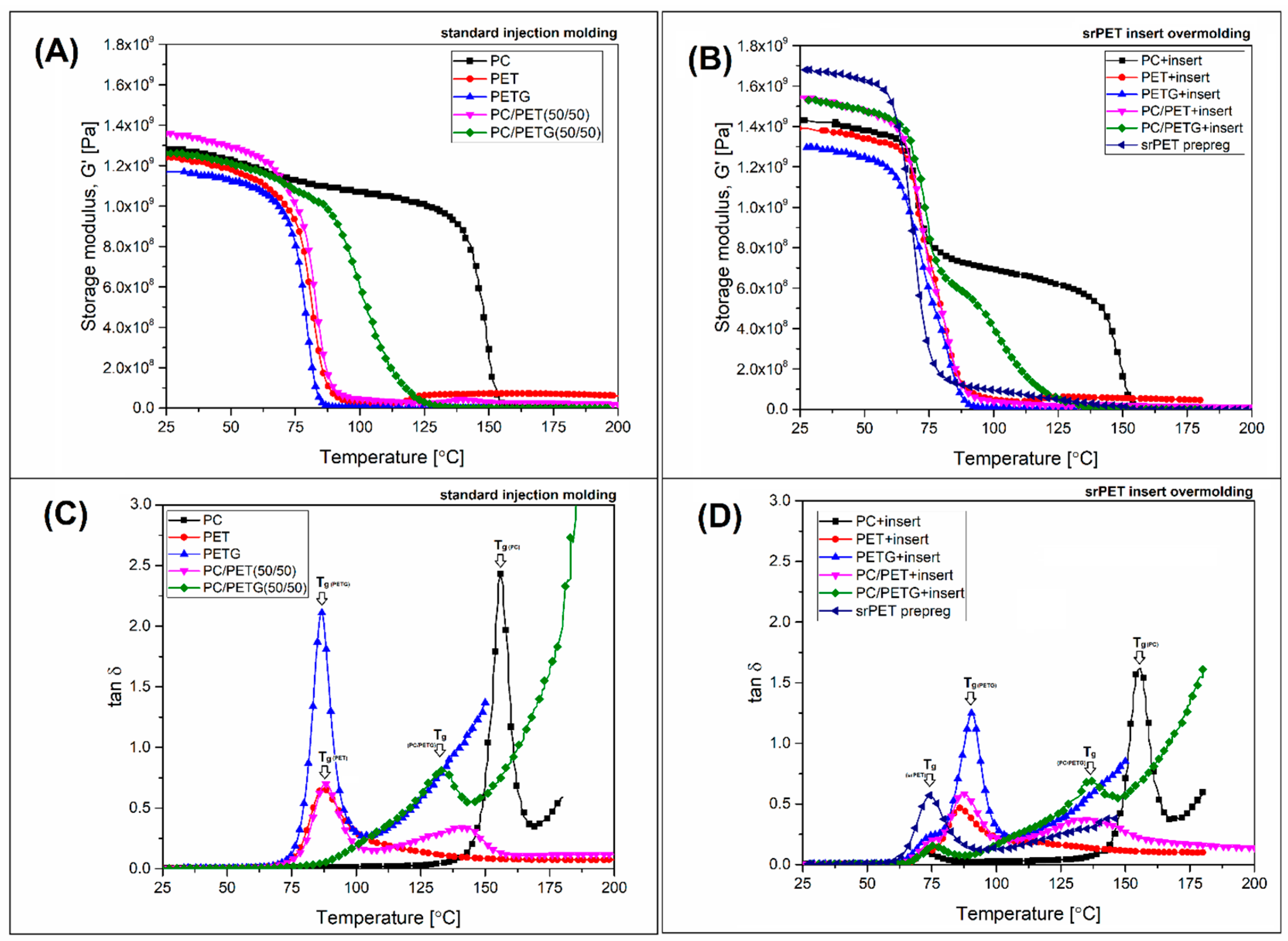
One of the main reasons for using PC in the presented study was its high thermomechanical properties, including HDT/Vicat test results. The analysis of the prepared materials was conducted using DMTA measurements and heat deflection tests (HDT). The storage modulus and tan δ plots are collected in
Figure 4, while the recorded HDT temperature results are revealed in
Figure 5.
The results of the storage modulus analysis for pure polymers (see
Figure 4A) revealed a large difference between polycarbonate (PC) resin and both types of polyesters (PET, PETG). The plateau region where the stiffness of samples can be considered as stable for PC reached around 130 °C, while for both polyesters, its around 60°C; exceeding this limit defines the range of the glass transition, where due to the increase in mobility of polymer chains the storage modulus drops significantly. Interesting differences can be noticed for PC-based blends where the plot appearance was different depending on the polyester type. For PC/PET material, the initial value of the storage modulus was slightly higher than for other samples; however, considering the small magnitude of this difference, this fact can be neglected. More importantly, the stiffness drop starts in the same temperature range as that of pure PET sample, and despite the use of 50% PC in the structure, the decrease in stiffness is almost identical to that of pure PET or PETG. The modulus value at 100 °C does not exceed 10% of the value obtained at room temperature. Different behavior applies to PC/PETG samples, where the appearance of the curve suggests clear changes in the phase characteristics. For this material, the initial drop of stiffness at around 60 °C cannot be confirmed; the storage modulus plateau reached almost 90 °C; however, even above this limit, the stiffness drop is not so evident as for PC/PET blend since the rubbery state plateau is reached at around 130 °C. Changes of this type clearly indicate differences in the miscibility of PC/PET and PC/PETG systems, which is confirmed by the tan δ curve analysis results.
For both types of pure polyesters, the glass transition temperature (T
g) was at the same level of 85 °C; the differences in the peak height reflect the content of the crystalline phase in PET resin. The T
g peak for the PC sample reached 160 °C, while the dominating amorphous character of this resin is also confirmed by the relatively higher maximum point of the transition peak. The interesting details are revealed for blended samples, where for the PC/PET sample, two peaks can be distinguished, while for PC/PETG sample, only one peak. This fact clearly confirms the difference in phase miscibility for this type of blend. In the case of PC/PET material, the first peak at around 85 °C is strongly corresponding with the T
g of the PET phase, an interesting phenomenon is the surface area under the tan δ curve in the glass transition region, where the area for PET and PC/PET blend coincides. Such behavior would suggest a significant increase in the content of the amorphous phase of the PET phase in the mixture, but it is one of the assumptions regarding the observed phenomenon. During the further temperature increase, another tan δ peak appears at around 140 °C. The location of the peak near the T
g range for PC clearly confirmed the miscibility of the PC-PET system; however, it is also evident that this phenomenon is also limited. The lack of changes in the glass transition of the PET phase shows that a significant volume of this polymer is not miscible with the PC phase; such a phenomenon is typical for semi-crystalline polymers where the crystalline phase domains are surrounded by the rigid amorphous fraction (RAF) [
46,
47]. In this case, only a part of the PET chains in the form of a mobile amorphous fraction can be part of a homogeneous PC/PET mixture.
Contrary to the PC/PET blend, where the miscibility phenomenon seems to be present only for part of the mixture, the tan δ plots confirmed that for PC/PETG materials, the blending procedure led to the formation of a homogenous polymer mixture. The full miscibility is confirmed by the presence of a single tan δ peak at around 130 °C. Compared to pure PET resin, PETG copolymer is characterized by a fully amorphous structure, which means that the chain mobility on not limited by the presence of rigid amorphous fraction (RAF). Both PETG and PC chains can interact during the extrusion blending process, which leads to the formation of a homogenous melt. During the cooling of the material, there is no separation of the crystalline phase, as in the case of the PC-PET system.
The comparison of the storage modulus and tan δ plots for injection molded samples (
Figure 4A, C) reflects the reference viscoelastic properties of pure polymers and prepared blends. The rest of the plots present the DMA analysis for insert reinforced specimens, where the properties of the samples are the resultant of the injection molded part and the srPET insert. For comparison purposes, the DMTA plots for pure srPET prepreg are also attached. The results of the storage modulus analysis clearly confirmed the reinforcing effect of the overmolding procedure since the initial values of the modulus recorded close to the room temperature are visibly higher than for unreinforced samples. In addition to the increase in stiffness, there are also clear differences in the modulus values for individual samples. The highest stiffness was recorded for pure srPET prepreg; however, the results for PC/PET and PC/PETG samples are only slightly lower. Another decrease in modulus was detected for PC and PET material, while the lowest stiffness refers to PETG sample. It may be somewhat surprising that the stiffness for the srPET prepreg under these conditions is only slightly higher than the value for the molded samples. However, in the case of DMTA measurements in the torsion mode, the anisotropy of the tested sample is of great importance, which is unfavorable for the tested prepregs, because it does not allow testing the stiffness samples along the reinforcement fibers. Taking into account that the analysis is to indicate the trends of changes in the modulus value, the exact determination of the modulus value in all directions would be a redundant procedure. Due to the nature of the analyzed thermomechanical properties, a more important fact is the change in the softening process initiation temperature of the tested materials, which is slightly lower after the introduction of the prepreg. The onset of the storage modulus reduction was recorded at around 65 °C for most of the samples, even for PC overmolded specimens. It is lower than 75°C for standard injection molding procedure.
It is clear that this type of behavior was caused by the presence of insert reinforcement, especially the LPET copolymer used as the matrix for oriented PET fibers. When shifted to tan δ plots, it is clear that the first peak at around 75°C reflects the Tg for LPET matrix in srPET insert. The most visible signal for LPET is visible for the sample made of the srPET prepreg alone; however, similar peaks of slightly lower intensity are recorded for each of the samples. The second of the visible Tg peaks area is recorded around 85 °C and corresponds to samples where for overmolding procedure, we use PET, PETG, and PC/PET blend. The remaining tan δ peaks are also related to the previously analyzed tan δ curves, where for PC+insert samples, a clear Tg peak occurs at 160°C, while for the PC/PETG mixture, the Tg value is again around 130°C. It is worth noting that a common feature of the tan δ diagrams for overmolded samples is a slightly smaller area under the curves in the area of glass transitions; this phenomenon is a consequence of replacing part of the molded material with a layer of srPET prepreg.
Summarizing the results of the DMTA analysis for overmolded samples, it is worth noting that for most materials, the introduction of the prepreg did not improve the thermomechanical properties since only for samples prepared using pure PC and PC/PETG blend the storage modulus values above the main glass transition region was relatively high. For these two materials, the stiffness drop takes place in two stages. The first loss in thermomechanical properties should be associated with the softening range of the srPET prepreg, while the second final decrease is associated with the loss of properties by the overmoulded material. The expected heat resistance for the rest of the prepared samples should be lower than for injection molded samples since the onset of the softening process occurs at lower temperatures. Additionally, the storage modulus loss takes place in one step since the main Tg area of the LPET matrix is very close to overmolded PET, PETG, and PC/PET material.
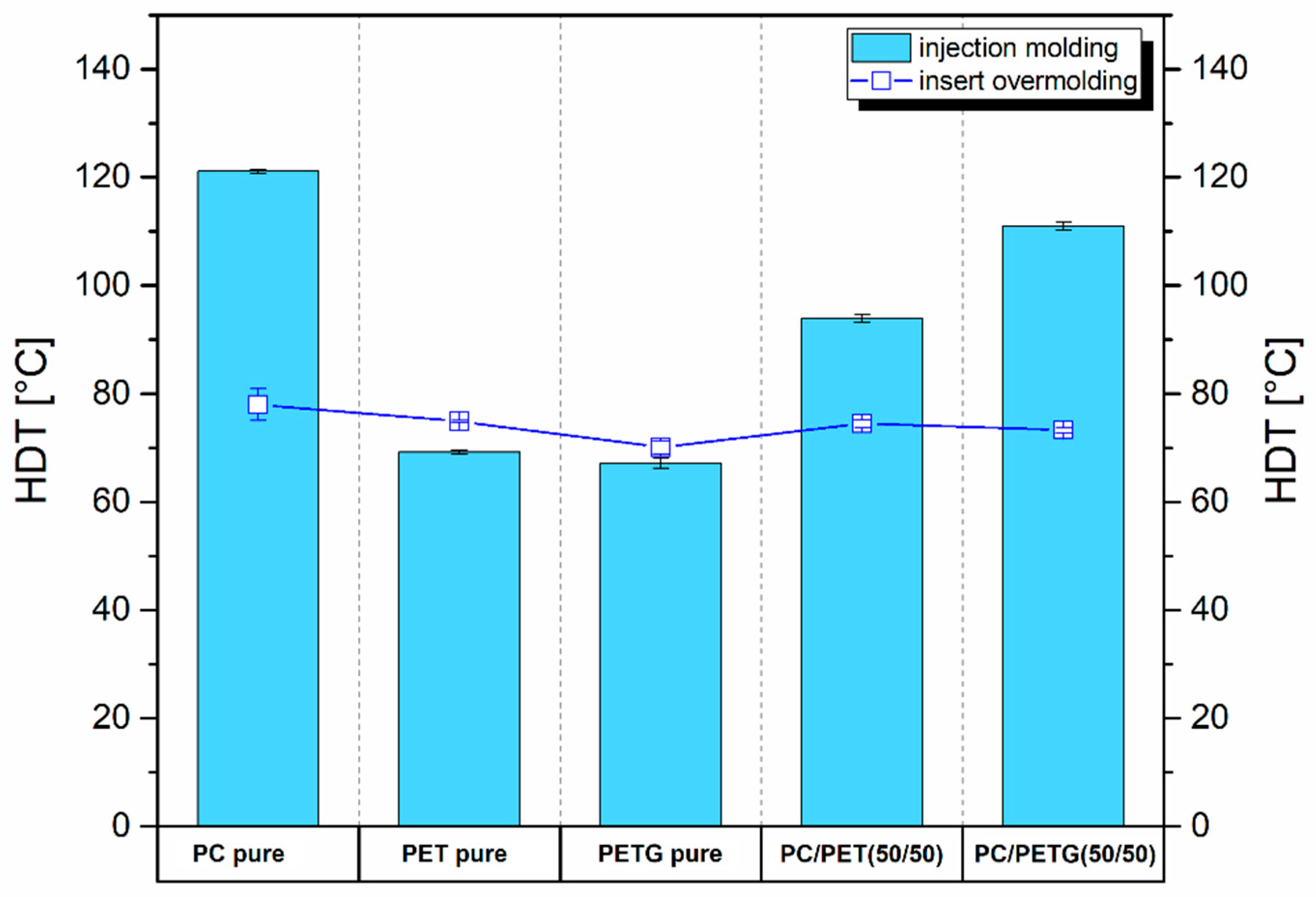
The DMTA analysis is complemented by the HDT studies presented in
Figure 5. The results of the standard injection molded samples are compared with counterparts obtained by overmolding process. The results for standard specimens confirmed the highest HDT for pure PC material (121 °C), while for both polyesters, the heat resistance was visibly lower, reaching 69 °C and 67 °C, respectively, for PET and PETG material. It is clear that the HDT values are strongly correlated with the T
g area of pure polymers. For prepared blends, the HDT coefficient values are the result of the components' properties, where the HDT results for PC/PET were around 94 °C, and for PC/PETG, 111°C. In particular, the result for the PC/PETG sample reflects the positive effect of miscibility for this polymer system, which allows for better properties than the other samples. It is worth noting that the measurements were carried out with a load of 1.8 MPa, as for technical plastics such as polyamide or epoxy resins, so the obtained results can be considered very good from the perspective of applications. Unfortunately, comparing the reference results with the samples subjected to the overmolding process, it is clearly visible that the prepreg insertion has a negative impact on the obtained thermomechanical properties, which was also partly suggested by the DMTA analysis. The highest HDT was recorded again for overmolded PC sample; however, the recorded deflection point reached only 78 °C; for the rest of the samples, the results oscillated between in the range of 70° - 75°C, which for most of the materials is lower than the reference value. It is clear that the obtained reduction in heat resistance was caused by the presence of srPET prepreg since the HDT value for this material was around 72.5 °C.
The measurements clearly confirm that despite the use of materials with increased thermomechanical properties at the stage of overmolding, the low softening point for the used srPET reinforcing insert is the main reason for the deterioration of heat resistance.
3.3. Phase transition measurements – DSC analysis
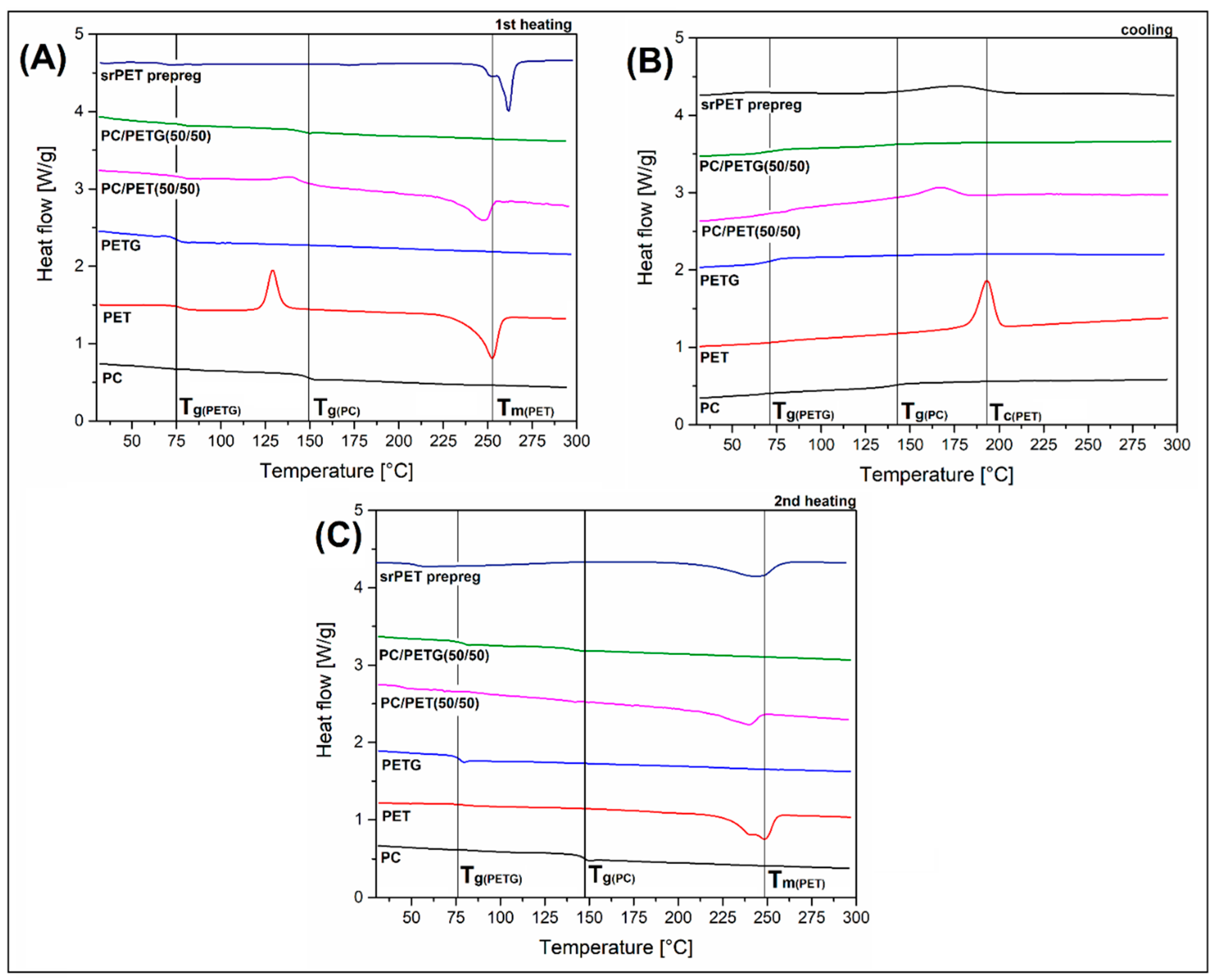
The DSC measurements are presented in the form of 1
st heating, cooling, and 2
nd heating thermograms (see
Figure 6). Tests were conducted for samples prepared by the injection molding process, while for comparison, the plots are combined with the results for srPET prepreg. The main reason for conducting these measurements was the evaluation of the glass transition regions for amorphous polymers and the melting temperature for the PET phase. The figures with more detailed plots of the DSC measurements are presented in the Supplementary Information section, separately for 1
st heating (
Figure S1), cooling (
Figure S2) and 2
nd heating (
Figure S3).
In the case of pure amorphous materials, which refer to PC and PETG resin, the T
g appears as the inflection point of the DSC signal curve. There is a clear difference between PC, where inflection was recorded at 148°C, and while for PETG sample at 75°C. In the case of DSC results, the indication of the glass transition temperature may have a slightly different value than for mechanical measurements such as DMTA or TMA; however, in the case of changes in the T
g temperature range, they are visible for each of the thermal analysis techniques. Interestingly, for the amorphous PC/PETG blend, the position of T
g inflection points related to the PC and PETG phase were not shifted; however, the intensity of the baseline signal change has clearly decreased, which results from the decrease in the volume of the analyzed phase in relation to the pure material. For PET-based samples, the presence of a partly crystalline structure leads to the appearance of two visible peaks. The first exothermic peak with the maximum point at around 130 °C refers to the cold crystallization (T
cc) phenomenon, while the final melting of the PET crystalline phase was recorded at 255°C. It is clear that for blended samples, the peak position was slightly shifted. The temperature of the melting point was lower, while the cold crystallization peak appeared at a higher temperature. That kind of behavior is related to the disturbance in the PET crystalline phase structure. The presence of the PC chains limits the freedom of PET polymer chains movement, which consequently causes the formation of a crystalline structure with disturbed morphology and a shorter length of lamellae [
48,
49]. The analysis of the DSC signal for the srPET prepreg reveals some interesting information about this material. Since the matrix phase for this polymer consists of a low melting type of polyester resin (LPET), the T
g region was close to other polyester-based samples; however, compared to PET or PETG specimens, the inflection of the plot was slightly reduced to around 65°C. Since the reinforcing prepreg was made from highly oriented PET fibers (HTPET), the cold crystallization phenomenon did not occur. Another noteworthy fact was related to the position of the melting point area, where the peak position was visibly higher than for standard PET resin. The position of the peak maximum was recorded at around 262 °C. That behavior is typical during the melting of oriented materials since the macromolecular structure of the fiber is characterized by a high level of crystallinity and resulting in improved thermal stability.
The cooling thermograms presented in
Figure 6B did not reveal any untypical features. For amorphous PC and PETG samples, the glass transition area was at a similar level compared to 1
st heating stage. More complex results are revealed for PET-based samples, where the exothermic peaks are recorded at different temperatures. Unlike the injection molded samples, the PET phase used for the preparation of srPET prepreg was made from a different type of resin, which is why the properties cannot be compared to other samples. Interestingly, for pure PET and PC/PET samples, the crystallization peak position also differs; however, it is relatively clear to combine this behavior with the formation of a partly miscible polymer melt structure where the formation of crystal lamellae is hindered [
30,
33,
36].
The 2
nd heating stage of the DSC measurements, presented in
Figure 6C, revealed that most of the properties observed during the initial heating are confirmed, especially regarding the glass transition region. The basic differences are related to the remelting of the sample structure and the subsequent cooling at a relatively slow rate (10/°C) compared to the molding procedure. A typical phenomenon for measurements of the 2nd heating stage is the disappearance of the cold crystallization peaks, resulting from the already mentioned slow cooling rate and crystallization of the PET phase. The melting point for the pure PET sample was slightly shifted to 247°C; however, there is an additional peak that appears at around 240°C. That phenomenon is quite typical for thermoplastic polyesters, especially PET or PLA since the crystalline phase has the tendency to formation of less organized structures. The additional peak reflects the presence of smaller and less ordered crystalline forms. Interestingly, for PC/PET blend, the temperature of the main melting peak coincides with the additional peak for the pure PET sample. This behavior again indicates the presence of limited mobility of polymer chains in PC/PET systems, which results in the dominance of smaller lamellar structures of the PET crystalline phase. The difference between the appearance of the 1
st and 2
nd heating plot also refers to the srPET material. The main visible change, besides the lack of a cold crystallization peak, is the shifting of the melting point. Similar to other samples, the peak position was lower than for the unheated specimen; however, this time, the difference reached around 20°C. Such a large difference in the melting point cannot be solely due to differences in the size of the forming crystal structures. Therefore, it is worth explaining that the high melting point for the structures tested during the 1st heating stage results in both from the higher stability of the crystalline structures of the fibers, but also from the stresses inside the composite structure, which are caused by the stretching of the fibers during the bonding process. It is worth explaining that the phenomenon of a double peak occurring during the melting of the crystalline structure of PET fibers for the srPET sample has a slightly different character than for the PET material shaped by injection molding. Since the fiber grades of PET resin have lower molecular weight, the formation of the crystalline structure is facilitated. For this reason, the crystalline structure is more homogeneous, and there are no variations in the size of the lamellae, leading to double peak formation.
Summarizing the results of the DSC analysis, it is worth emphasizing that the macromolecular structure for the prepared polymer blends is clearly different from that represented by pure materials. In the case of PC/PET and PC/PETG systems, the occurrence of at least partial miscibility is confirmed, which affects the interactions between polymer chains and the formation of the phase system after the production process.