1. Introduction
1.1. General concept of solid solutions and motivations for the choice of NaClO3 - NaBrO3 system.
Solid solutions by substitution are often encountered among inorganic and metallic systems as soon as the change between the two species can be simply the replacement of an atom by another atom with a close atomic radius. Often, in such system, the lattice parameters of the solid solution for a given composition follow a Vegard’s rule i.e., there is a linear relation between the lattice parameters of the crystalline structure with the concentration of each of the components of the system [
1]. However, this empirical law was found not to be the general case, especially for metals [
2,
3].
Among inorganic solid solutions described in the literature, the NaClO
3-NaBrO
3 binary system (or NaClO
3 – NaBrO
3 – H
2O ternary system) has been widely studied. This may be partly due to the high crystallogenic character of the two components and their mixtures. Moreover, NaClO
3, NaBrO
3 and NaClO
3(x)BrO
3(1-x) intermediate compositions have low melting points [
5] and high (ClO
3-rich) to medium (BrO
3-rich) solubilities in water [
6,
7]. NaClO
3 has a melting point of 262°C and a solubility in water of 790g.l
−1. NaBrO
3 has a melting point of 347°C and a solubility in water of 275g.l
−1.
1.2. Crysal structure of NaClO3 and NaBrO3 pure compounds.
Sodium chlorate (NaClO
3, MW=106.44g.mol
−1, T
fus = 248°C) and sodium bromate (NaBrO
3, MW = 150.89g.mol
−1, T
fus = 381°C) have isomorphous crystal structures that were formerly investigated by Dickinson in 1921 [
8]. Both halates crystallize in the chiral space group
P2
13 (tertartohedral group in the cubic system), with crystalligraphic parameters
a = 6.57584(5) and
a = 6.70717(10) Angströms, respectively at RT [
9]. The space group
P2
13 is chiral thus, noncentrosymmetric in adequation with the property to exhibit second order nonlinear optical phenomena [
10,
11] (Savage, 1962; Simon, 1968).
1.3. Chirality and deracemization.
NaClO
3 and NaBrO
3 belong to achiral molecules that exhibit supramolecular chirality in the solid state [
12]. It was also found that optically dextro (D) rotatory NaClO
3 has the opposite configuration than the optically dextro (D) rotatory NaBrO
3, or, in other words (NaClO
3)
D has the same structural configuration as (NaBrO
3)
L [
13,
14,
15]. Thus, the (NaClO
3)
L/(NaClO
3)
D/(NaBrO
3)
L/(NaBrO
3)
D system is quaternary, when the solid phases are considered.
Deracemization (conversion of a racemic mixture to a pure enantiomer) in NaClO
3 supersaturated solution was first observed by Kipping and Pope [
16] (Kipping, 1898). Later on further experiments were conducted by Kondepudi et. al. on NaClO
3 [
17]. They observed that from a supersaturated solution under stirring, the resulting solid crystals were of the same chiral configuration. When the solution is stirred, fragments of a single configuration are dispersed in the media promoting their growth and inhibiting primary nucleation which normally generate an equal proportion of nuclei with opposite chiral configuration. Their work relies on the general conception of sensitivity of nonequilibrium systems [
18,
19,
20]. Buhse et. al. reported an interesting observation: deracemization can occur in NaClO
3 supersaturated solutions in which a NaBrO
3 crystal has been inoculated. NaBrO
3 acts as a template of crystallization for only one of the NaClO
3 chiral configuration (as NaClO
3 and NaBrO
3 are isomorphous) [
21].
In 2005, Viedma reported later that deracemization could also occur in suspension of NaClO
3 [
22]. He showed that continuous attrition on a suspension of sodium chlorate leads to the switch from a racemic population of lefthanded and right-handed crystals to a homochiral population of crystals. The possibility of a mechanism governed by reincorporation of clusters that are below the critical size of equilibrium is discussed. In 2011, Viedma and Cintas reported the use of dissolution-crystallization cycles (in boiling solutions) as an additional mechanism for the deracemization of NaClO
3 [
25]. The same phenomenon has been observed with different sources of energy crossing the suspension: Ultrasound, pressurization, and temperature cycling [
23]. Actually, for the latter, there is no need to form large temperature swings, continuous small oscillations in the temperature of the suspension lead to an irreversible evolution towards homochirality. Moreover, those different ways of deracemization could be coupled with agonist effects [
24]. Viedma et al. found that large aggregates formed by several crystals with only one chiral configuration could be obtained [
26]. With this method, aggregates can be simply separated manually under a polarized microscope as soon as their size was about 5-10mm. In 2020, Schindler et. al. found that the use of dithionate impurity, also known to be a habit modifier for NaClO
3 (see next paragraph) could, however, strongly delay this mechanism [
27].
Therefore, equilibrium in that system will impose simultaneously: the regular conditions for thermodynamic equilibrium (chemical equilibrium, thermal equilibrium, no segregation and a mechanical equilibrium) and homochirality.
1.4. Crystal growth of NaClO3 and NaBrO3 crystals in the presence of impurities.
Some decades ago, the general concept of crystal growth in the presence of impurities has attracted much attention. Explanation of anomalous optical phenomena, crystal habit modification and overall crystal symmetry breaking, including systems of solid solutions of both inorganic and organic materials were reported in the literature [
28,
29,
30,
31,
32,
33,
34,
35,
36]. Cases of habit modifications for NaClO
3 crystals have been reported since the 19
th Century (see discussions in ref. [
7,
41]).
In particular, the effect of Na
2S
2O
6 impurities was widely studied in the literature. Habit modification by dithionate impurity was first reported in 1930 to modify the habit of NaClO
3 crystals into a tetrahedral morphology [
37].
In the early 90′s Ristic et. al. studied the growth kinetics and morphology of NaClO
3 crystals from the solution in the presence of dithionate impurities [
38,
39]. They found that the cubic morphology easily switches towards a tetrahedral morphology as soon as a sufficient amount of dithionate impurities was added to the mixture (210ppm). This analysis was rationalized through the geometry of (ClO
3)
- ion being similar to that of one of the (SO
3)
- subgroups of the S
2O
62- ion on these specific tetrahedral faces, inhibiting the growth of these faces. Recently, Lan et. al. investigated deeply this phenomenon to validate a proposed model based on the nature of twinning (observed in NaClO
3 crystals) inducing by dithionate impurities [
40]. They found notably that at higher dithionate impurities level (1000ppm), twinned crystals can be formed with the particularity that the counter chiral configuration is produced at the twin interface occupied by dithionate impurities. This was due to the ionic structure of S
2O
62- which consists in two SO
3- moieties oppositely orientated. This work shows that if a crystal of a single chiral configuration can be produced by deracemization, the presence of dithionate impurities induces on the contrary that aggregates (twin crystals by inversion) are formed of crystalline domains of both chiral configurations.
Interestingly, BrO
3- ion has been reported to be a habit modifier of NaClO
3 (see discussions in refs. [
41,
42]) and to induce overall symmetry breaking for a wide range of NaClO
3(x)BrO
3(1-x) intermediate compositions.
1.5. NaClO3(x)BrO3(1-x) mixed crystals: kinetic ordering and deviation from ideal cubic structure.
In 1984, Raja et. al. [
43] analyzed a crystal with Na(ClO
3)
0.7(BrO
3)
0.3 composition and found that it crystallizes, also, in the cubic
P2
13 space group, confirming the existence of the solid solution. Nevertheless, many other works indicated different results. Since the 19
th century, polarized optical observations on NaClO
3(x)BrO
3(1-x) solid solution crystals revealed birefringence that is physically not allowed in isotropic cubic structures [
7,
41,
44]. The authors found that apparent single crystals exhibited sectors of different compositions (in both constituents of the solid solution) reducing the overall symmetry of these crystals to the monoclinic
P2
1 or triclinic
P1 space groups due to kinetic ordering of atoms during crystallization by water evaporation. Authors of the same research group also indicated a potential segregation of BrO
3- and ClO
3- microscale domains within an apparent ‘single’ crystal through the alternation of dextro- and levorotary domains [
42]. A complete study of the NaClO
3(x)BrO
3(1-x) system was also recently carried out by Su et. al. [
45]. Crystals of the solid solution between 14 to 81 wt. % NaBrO3 were found to be of worse optical quality i.e., opaque with cracks contrary to crystals obtained outside this range of composition. For these compositions a very weak diffraction peak at 13.3° 2θ was found and could be indexed as the (100) peak, forbidden for the
P2
13 space group (h00; h = 2n), indicating a slight deviation from an ideal cubic structure.
Nevertheless, it was reported that annealing close to the melting temperature leads to a homogenization of the crystals and hence to a return to the
P2
13 crystal structure [
42,
45,
46]. It was not reported whether the NaClO
3(x)BrO
3(1-x) solid solution crystals return to an equilibrium state or if a transition towards the cubic structure only possible at higher temperature could remain in a metastable state at room temperature.
1.6. Heterogeneous equilibria.
The first study of the NaClO
3 - NaBrO
3 system was performed in 1939 by Swenson and Ricci [
6], regardless the chirality. They published two isothermal studies of the ternary system NaClO
3 - NaBrO
3 – H
2O at 25°C and 50°C (
Figure 1, a & c). They showed that there might be a complete solid solution between the two components at 50°C (
Figure 1, d), but the possible existence of two solid solutions at 25°C was envisaged (
Figure 1, b). Indeed, careful examinations of (i) the solubility curve versus composition and (ii) the tie lines in this isotherm at 25°C reveal an odd behavior. In this work the authors suggested that the solid solution was apparently discontinuous. The solubility curve appeared to be divided in three parts corresponding to the following solid phases: (i) solid solution very close to pure NaBrO
3, (ii) a bromate solid solution containing few ppm to 5-10% of chlorate, and (iii) a chlorate solid solution containing up to 60-65% chlorate solid solution. Another authors’ suggestion was that this solid solution was continuous but with a marked tendency toward the formation of an isothermal invariant point.
1.7. Summary of the literature review and aim of that study
This literature review emphasizes the complex crystallization behavior by water evaporation for the NaClO3 - NaBrO3 system, especially when the NaClO3(x)BrO3(1-x) compositions are far from the respective pure phases. The problem is that crystallization is most often kinetically driven, both by the ability of ClO3-/BrO3- ions ordering as well as chirality ordering. Notably, both phenomena have been observed within an apparent well shaped single crystal.
The question whether the NaClO3(x)BrO3(1-x) mixed crystals belongs to a complete solution at room temperature (e.g., 25°C) is finally still open, the reason might be that equilibrium seems to be difficult to reach.
Before examining the system regarding the chirality and deracemization of mixed compositions, it appeared necessary to clarify the existence of heterogeneous equilibria between these two components.
2. Material and methods
2.1. Commercial material
Sodium chlorate was purchased from Alfa Aesar with purity of 99%. Sodium bromate was purchased from Merck with purity of 99%.
2.2. Crystallization by solvent evaporation
NaClO3 and NaBrO3 commercial powders were dissolved in water (40mg/mL) to make undersaturated solutions of different NaClO3/NaBrO3 ratios. The whole solutions were transferred to individual opened large crystallization beakers for evaporation at RT or in a temperature regulated ventilated oven. The crystals were collected at the end of evaporation in order to recover the same composition in NaClO3 and NaBrO3 that have been formerly introduced for the preparation of the samples. For some experiments at elevated temperatures, crystallizations were performed in opened vials under stirring.
2.3. Crystallization by spray-drying
The spray dryer used in this study is a Büchi B290 (conventional laboratory dryer designed with a co-current configuration). Compressed gas used is nitrogen and feeding solutions were aqueous solutions. The laboratory-scale spray dryer was equipped with a nozzle 0,7 mm in diameter (Tip = 0.7mm; Cap = 1.4mm).
Several mixtures were dissolved in water, with the following parameters (
Table 1):
2.4. XRPD
XRPD patterns were obtained either with a Da Vinci D8 Bruker X-ray diffractometer with a Bragg-Brentano geometry (θ//θ)or a D8 diffractometer (Bruker, Germany) equipped with a modified goniometer of reverse-geometry (-θ/-θ) [
47](Coquerel, 2015). For both instruments, the incident X-ray beam consisted of the Cu Kα (λ = 1.5418 Å) with a tube voltage and amperage set at 40 kV and 40 mA respectively. The diffraction patterns were collected with a LynxEye
® linear detector (Bruker, Germany). Routine XRPD analyses were performed with a step of 0.04° (2θ), and a 12 s per step counting time from 3° to 30° (2θ). The system is monitored with DiffracPlus XRD commander software version 2.6.1. Data were processed with DIFFRAC.EVA software release 2018 (version 4.3.0.1).
2.5. TR-XRPD
Solid crystalline phases were analyzed by means of X-ray powder diffraction (XRPD) on D8 advance series II diffractometer (Bruker analytic X-ray Systems, Germany). The instrument is equipped with an X-ray tube containing a copper anticathode, (40 kV, 40 mA) and a LynxEye® linear detector for every XRD scan, the step size was fixed at 0.04° with a counting time of 0.5s/step over an angular range 10°- 40° (2-theta). Temperature was controlled and monitored using a TTK 450 heating stage (Anton Paar GmbH, Austria). Data were processed with Eva software version using DIFFRAC.EVA software release 2018 (version 4.3.0.1).
2.6. DSC
DSC experiments in the temperature range 0 °C to 400 °C were performed using a Netzsch DSC 204 F1 apparatus equipped with an intracooler. Each DSC run was performed with ca. 5-15 mg of a powdered sample in aluminum pans with pierced lids at 2 or 5 K/min heating rates. The atmosphere of the analyses was regulated by a helium flux (40 mL/min). Onset (respectively endset) temperatures are calculated from the intersection between the baseline and the slope of the first (respectively last) part of the endotherm.
2.7. SEM
Scanning electron microscopy (SEM) pictures were obtained with a JEOL JCM-5000 NeoScope instrument (secondary scattering electron) at an accelerated voltage of 10kV. Samples were stuck on a SEM stub with gloss carbon and coated with gold to reduce electric charges induced during analysis with a NeoCoater MP-19020NCTR.
2.8. SEM-EDX
Scanning Electron Microscopy (JEOL JSM 7200F) coupled with Energy Dispersive X-Ray spectrometer (EDX XFLASH 6160 from Bruker) was used to collect images and for quantitative chemical analysis. EDS spectra were collected under a voltage of 10 kV with a working distance of 10 mm. Although no conductive coatings were deposited, the experimental conditions were satisfactory to record BSE (Back Scattered Electrons) images and for X-ray mapping experiments.
2.9. Optical microscope
Polarized Optical Microscopy (POM) observation was conducted by using a Nikon SMZ-10A microscope (Nikon imaging Ltd.) coupled to CCD-camera connected to a computer. Data processing was run by using LEICA Microsystems Application Suite X software.
3. Results and discussion
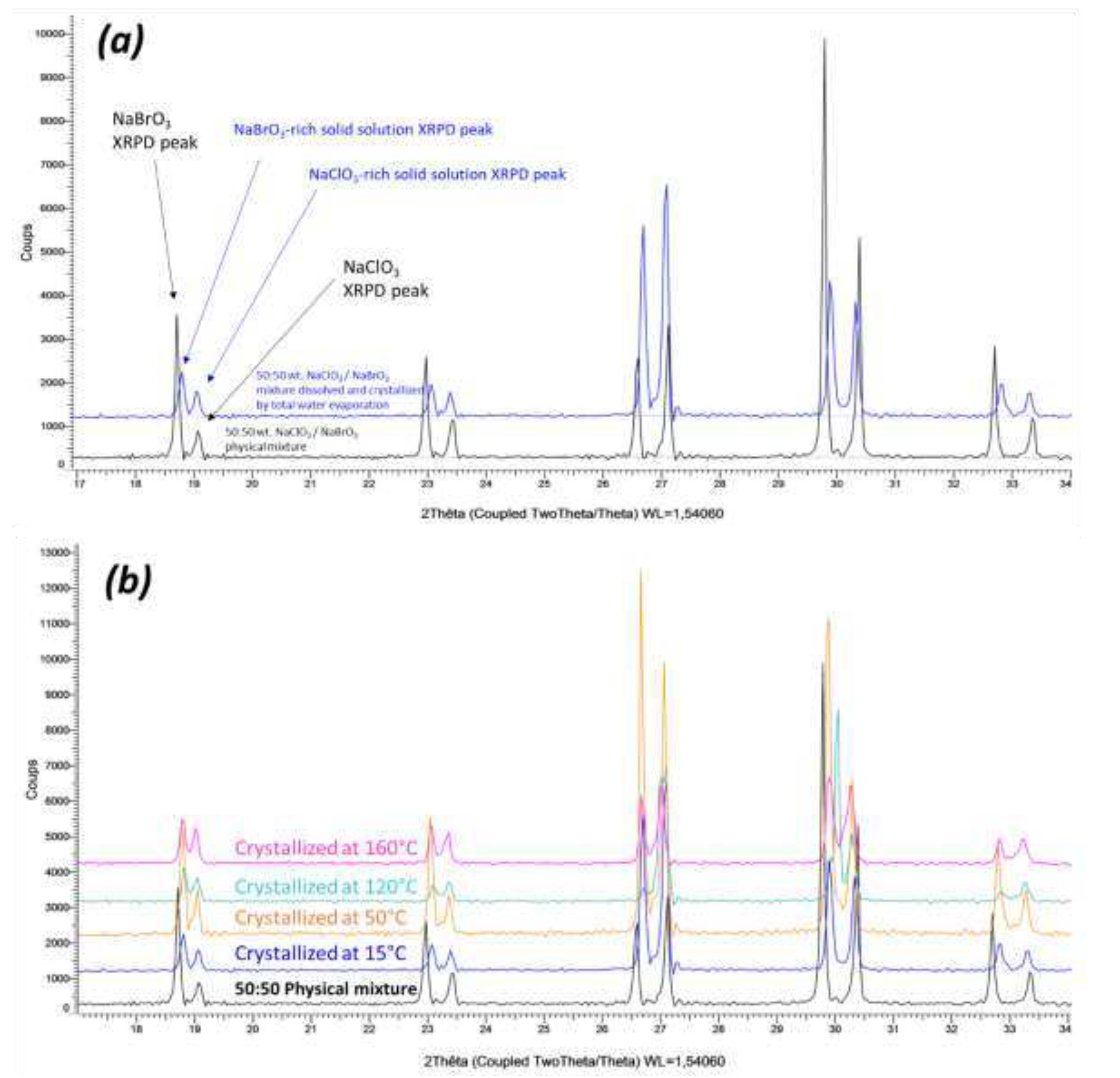
A. Confirmation of kinetic ordering through classical (slow) water-evaporation crystallization process evidence of a miscibility gap at 20°C.
In order to confirm the existence of a complete solid solution, or by contrast, the presence of two limiting solid solutions with a miscibility gap, several 50wt.% NaClO
3/NaBrO
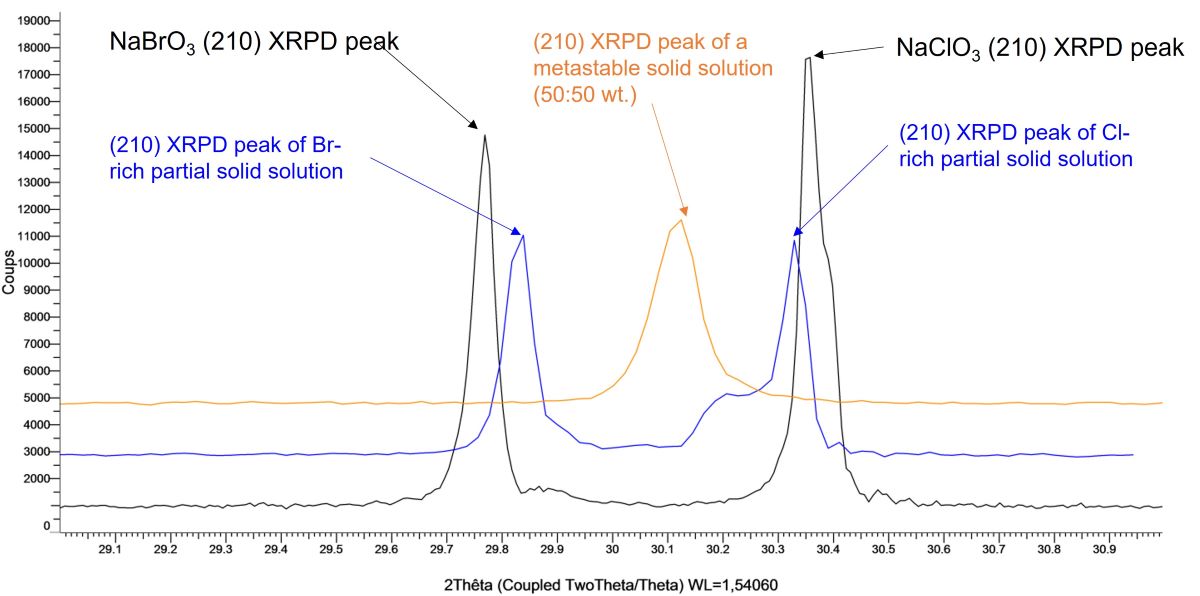
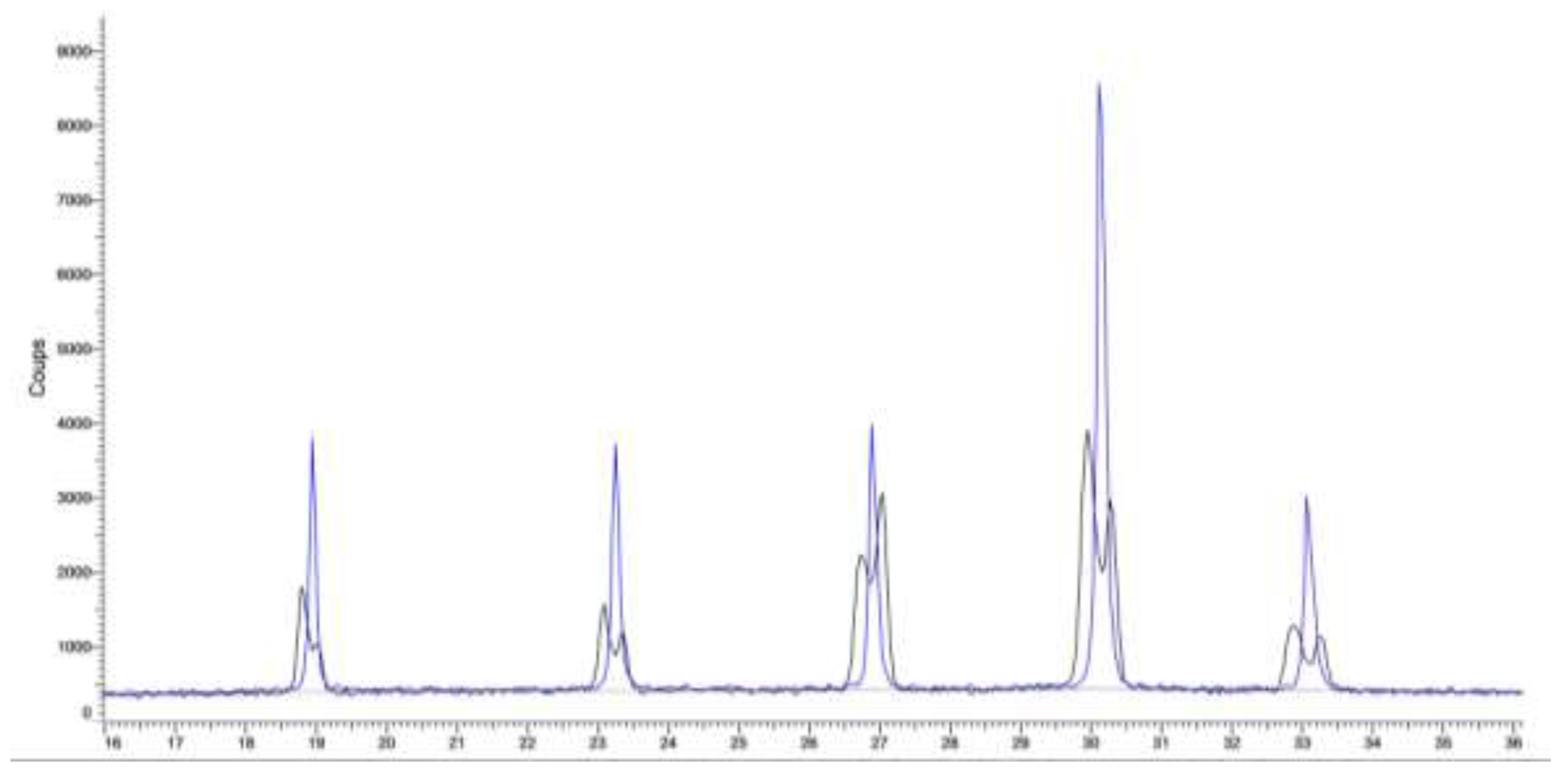
3 mixtures in aqueous solutions were dissolved and left under stagnant conditions to fully evaporate at: 20°C, 50°C, 80°C, 100°C, 120°C and 150°C. In all cases two closely related XRPRD patterns were obtained (
Figure 2). Despite the isomorphous character of NaClO
3 and NaBrO
3 single phases, the XRPD patterns of the 50wt.% powder sample obtained after evaporation at room temperature clearly indicated a miscibility gap in the solid state (
Figure 2, a). A careful examination of the peaks of the powder samples crystallized at RT revealed that the two solid phases obtained are not constituted by a physical mixture of pure NaClO
3 and pure NaBrO
3, but rather constituted by a physical mixture of two solid solutions with limiting domain of existence. Thus, a large miscibility gap exists from ca. 20wt.% NaBrO
3 to ca. 70wt.% NaBrO
3. This miscibility gap was confirmed to exist (at least) up to 160°C from the evaporation experiments conducted at elevated temperatures (
Figure 2, b).
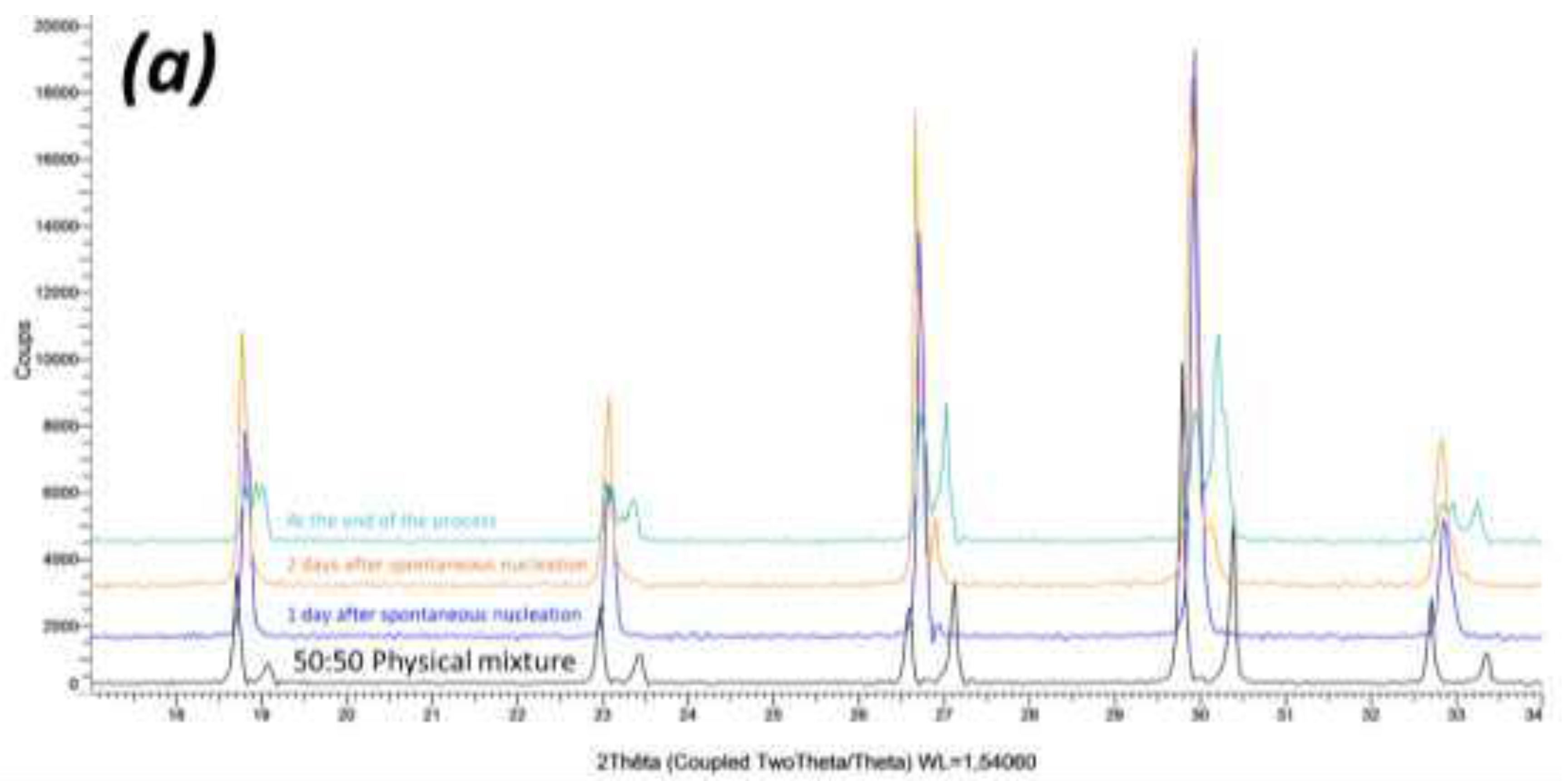
Crystallization pathway, from undersaturated solution towards total evaporation, was investigated on a 50wt.% NaClO
3/NaBrO
3 mixture dissolved in water (
Figure 3). During evaporation, several single particles were withdrawn at different times to investigate the solid phase. Results confirmed the solid-solid demixion (S.S.I + S.S.II + di-saturated saturated solution) during the last crystallization episodes.
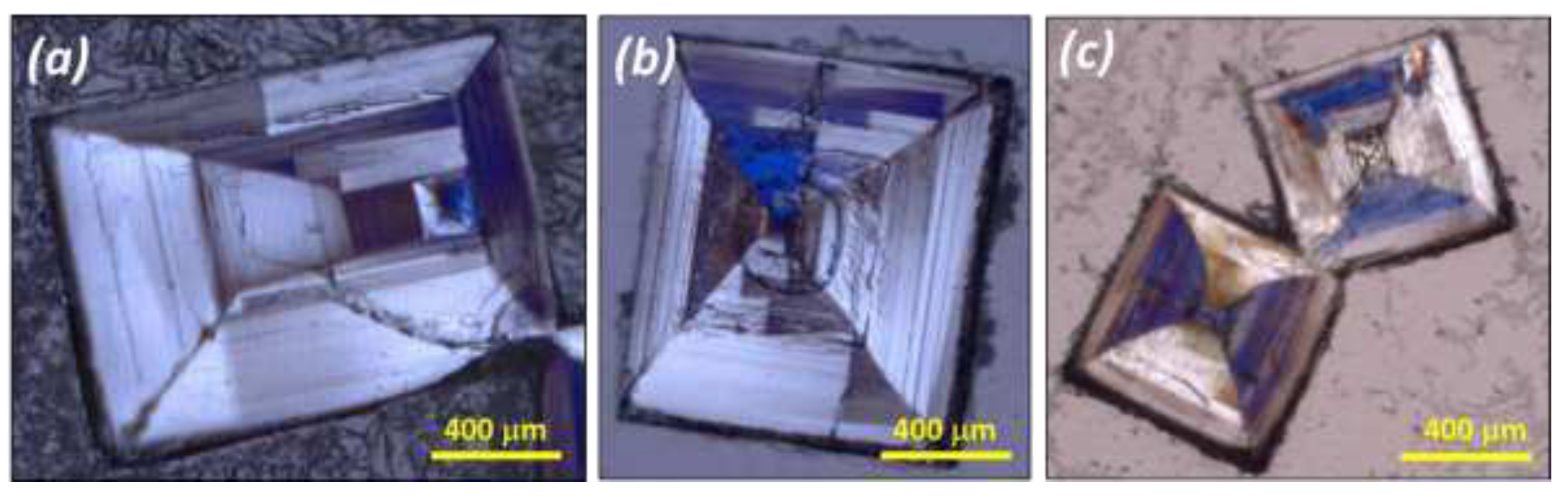
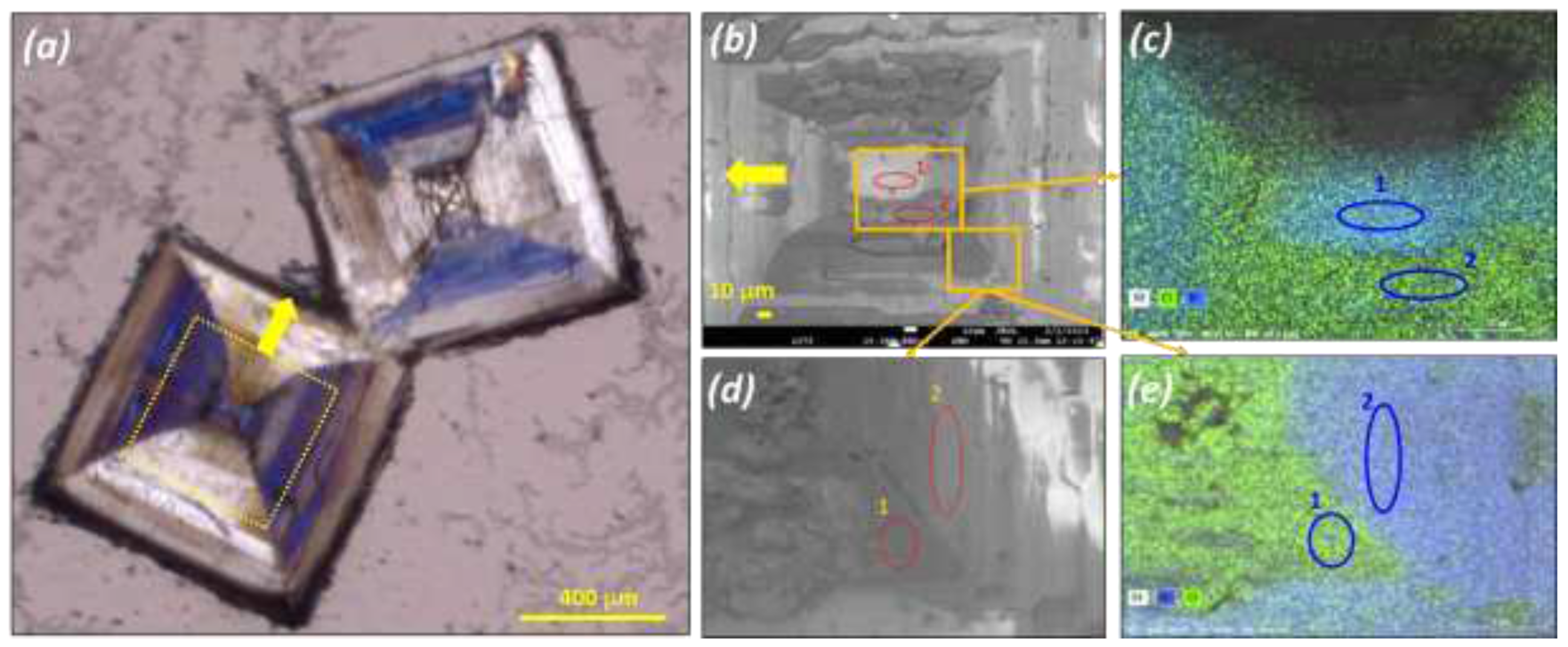
Large crystals were obtained by water evaporation at 20°C and submitted to a polarized microscopic inspection (Polarized Optical Microscopy: POM hereafter) (
Figure 4) and SEM-EDX analysis (
Figure 5).
POM pictures reveal that what appear as single particles are actually a patchwork of domains of different sizes and geometries. POM reveals also that the chirality of those domains can differ even within the same crystallographic sector (
Figure 4).
EDX analysis on two spots belonging to two different domains reveal an almost inverted composition between Cl and Br (8.6 Cl & 30.5 Br atom % for spot 1) and (24.8 Cl and 6.2 Br atom % for spot 2). It can be surmised that those particles are in fact composed of four different domains: supramolecular left and right domains enriched in Bromate and supramolecular left and right domain enriched in chlorate. Due to the poor solubility of the bromate compared to the chlorate it is logical that the center of the particle is enriched in bromate.
From the above data we can conclude that, when the thermodynamic equilibrium is attained, sodium chlorate and sodium bromate do not form a complete solid solution from room temperature up to 160°C, but rather two partial solid solutions. It is likely that the miscibility gap in the solid-state extents to much higher temperatures
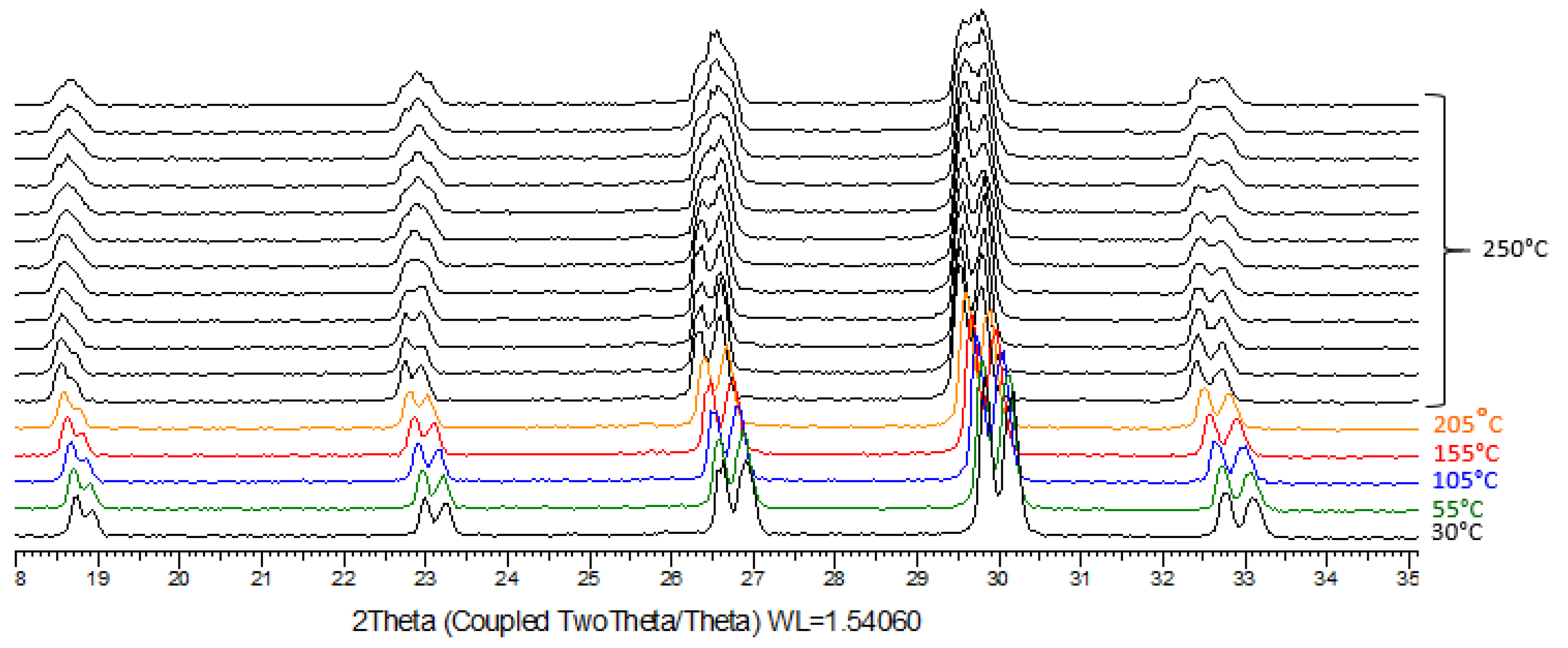
In order to see if the miscibility gap in the solid state intersects the solid liquid equilibria, temperature, resolved X-ray powder diffraction experiments (TR-XRPD) were run for the 50% (mass) composition (
Figure 6). Several heating ramps up to 250°C were used on the same sample. These thermal treatments with annealing for 11 h at high temperature, were interspersed with a flattening of the powder to avoid the retreat effect of sintering.
Figure 6 shows the third analysis on heating. A careful inspection of the evolution of the diffraction peaks versus temperature shows that the two patterns, related to the presence of a NaClO
3-rich and a NaBrO
3-rich solid solution, tend to collapse into a single one. Even if the annealing temperature was close to that of the solidus (see experimental points (filled circles) on
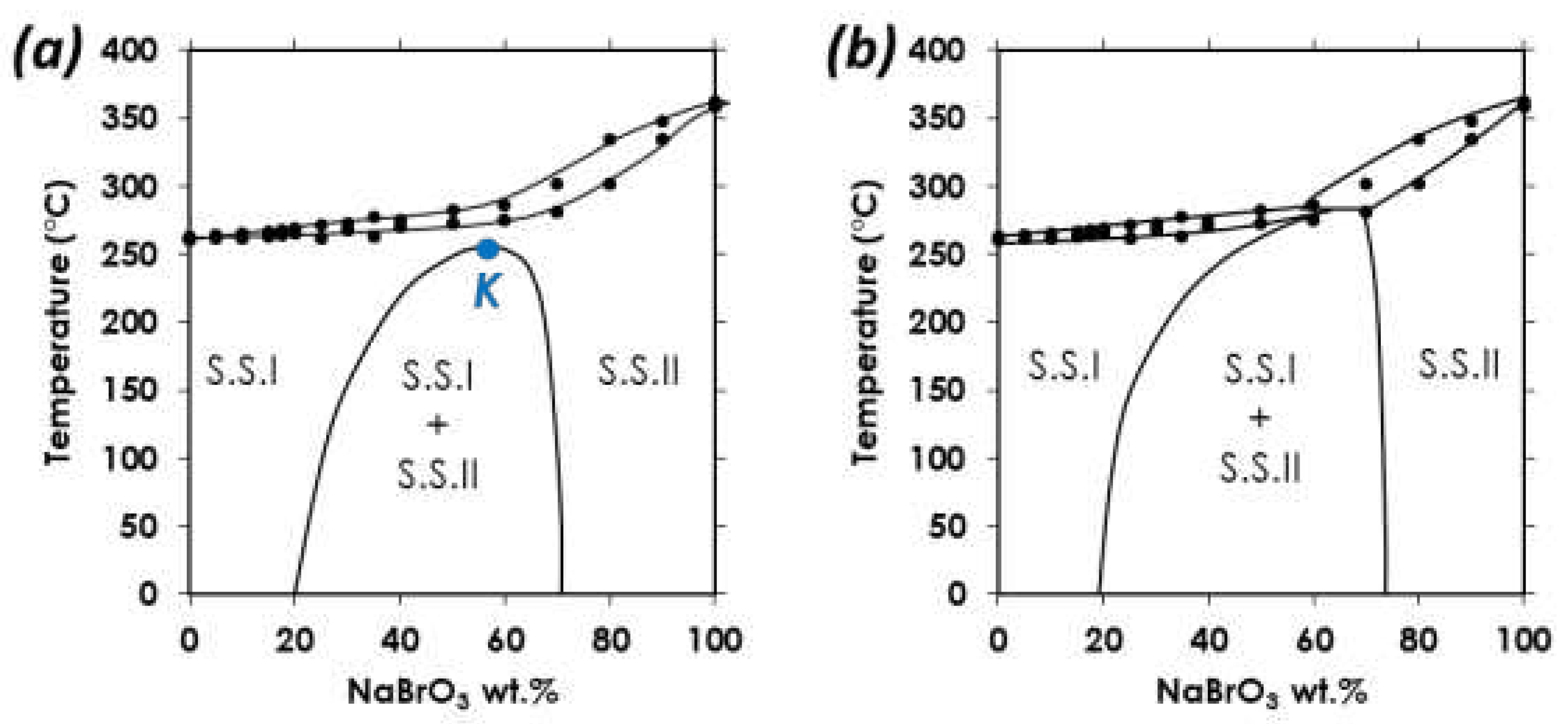
Figure 7), a genuine complete merge of the two patterns is not observed. Longer annealings have shown the beginning of decomposition and therefore could not be prolonged, furthermore. The opposite supramolecular chirality of the particle may not help the sintering. Therefore, this work leads to two hypotheses shown
Figure 7 (a) and
Figure 7 (b).
B. Crystallization of NaClO3/NaBrO3 mixtures by spray-drying. Evidence of a metastable complete solid solution.
Literature shows that homogeneity could be an issue in the preparation of samples with different compositions, therefore mixtures NaClO3(x)BrO3(1-x) 0 ≤ x ≤ 1 have been prepared by spray-drying according to Protocol 1.
It can be surmised that in every droplet a Kondepudi-like crystallization occurs. Accordingly, on drying, every droplet should give homochiral particles unless there is a bias in the nucleation process due to the presence of an active chiral impurity. The global result should be statistical nucleation of right-handed and left-handed particles, in other words: a racemic mixture. A recent article details the link between size and homochirality [
48].
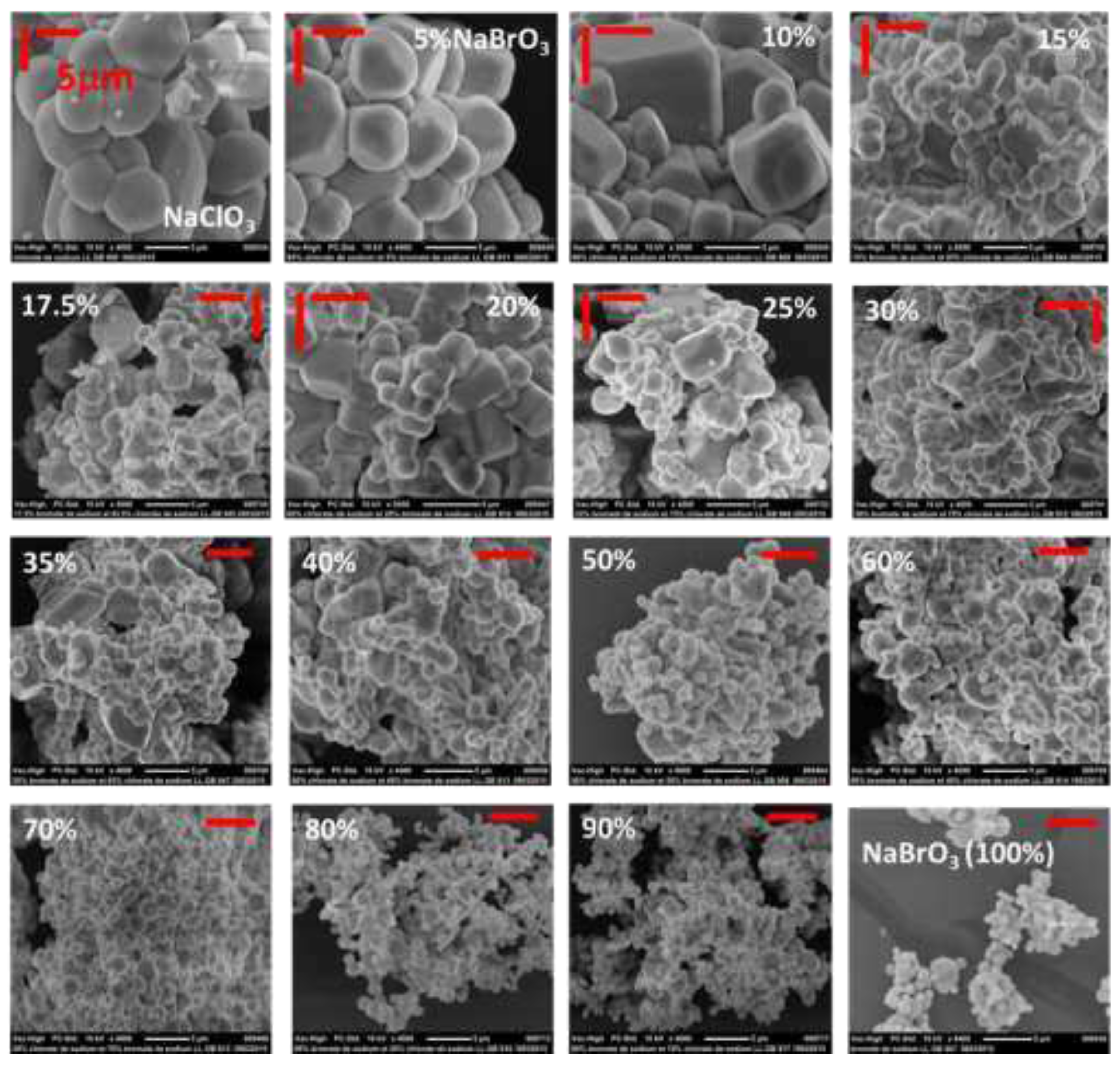
The obtained particles exhibit morphologies that are more isomorphous than crystallization from stagnant solution for which the habitus are flattened with characteristic defects (
Figure 4).
XRPD patterns on fresh spray-dried samples recorded at room temperature are displayed on
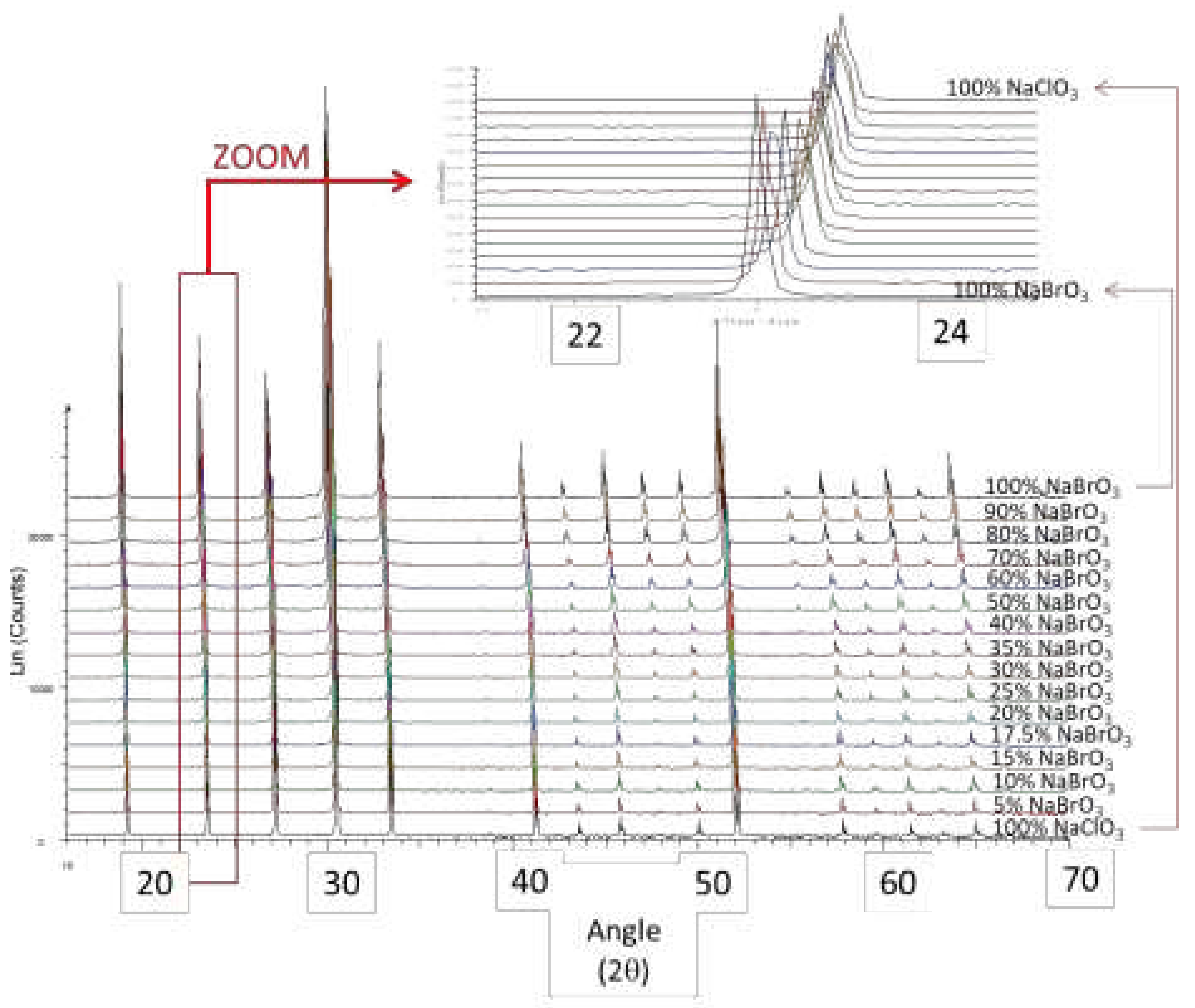
Figure 9. Contrary to powder samples obtained by slow water evaporation, the inlet shows the shift of the X-Ray diffraction peak versus composition in the full range 0 ≤ x ≤ 1. Furthermore,
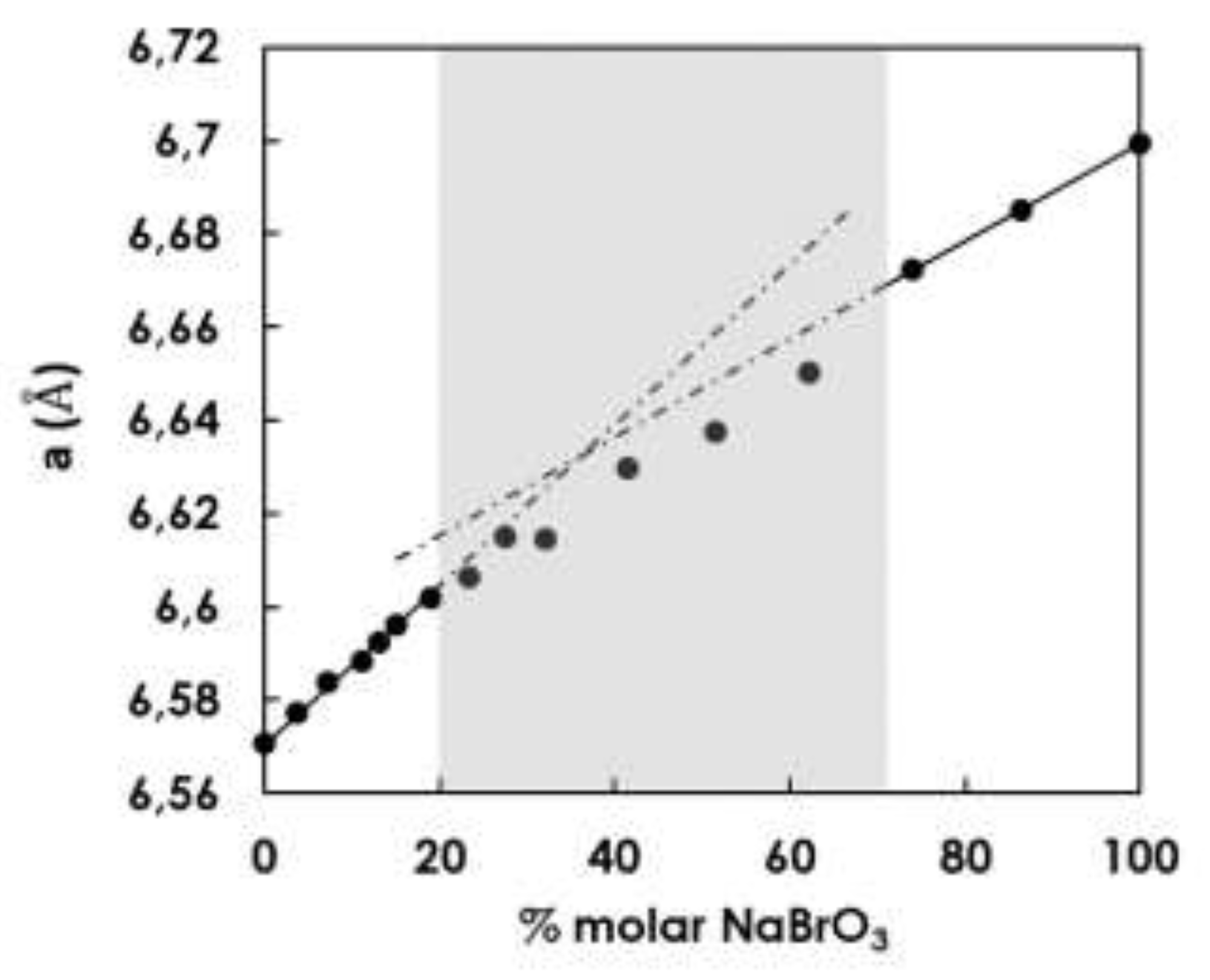
The evolution of the cubic unit-cell parameter at RT as function of the mol fraction of NaBrO
3 is represented on
Figure 10. Although the maximum difference between NaClO
3 and NaBrO
3 lattice parameter is only 1.92%, a discontinuity of the Vegard law is observable, that could reflect the metastable character of the solid solution. In the composition region ranging from ca. 20wt.% to 70wt.% (that is, by the way, closely similar to the limiting compositions of NaClO
3-rich and NaBrO
3-rich solid solution observed in the case of crystallization by slow solvent-evaporation), the density of the solid phases appears to be slightly higher than the two normal trends.
The solid mixtures obtained by spray-drying (
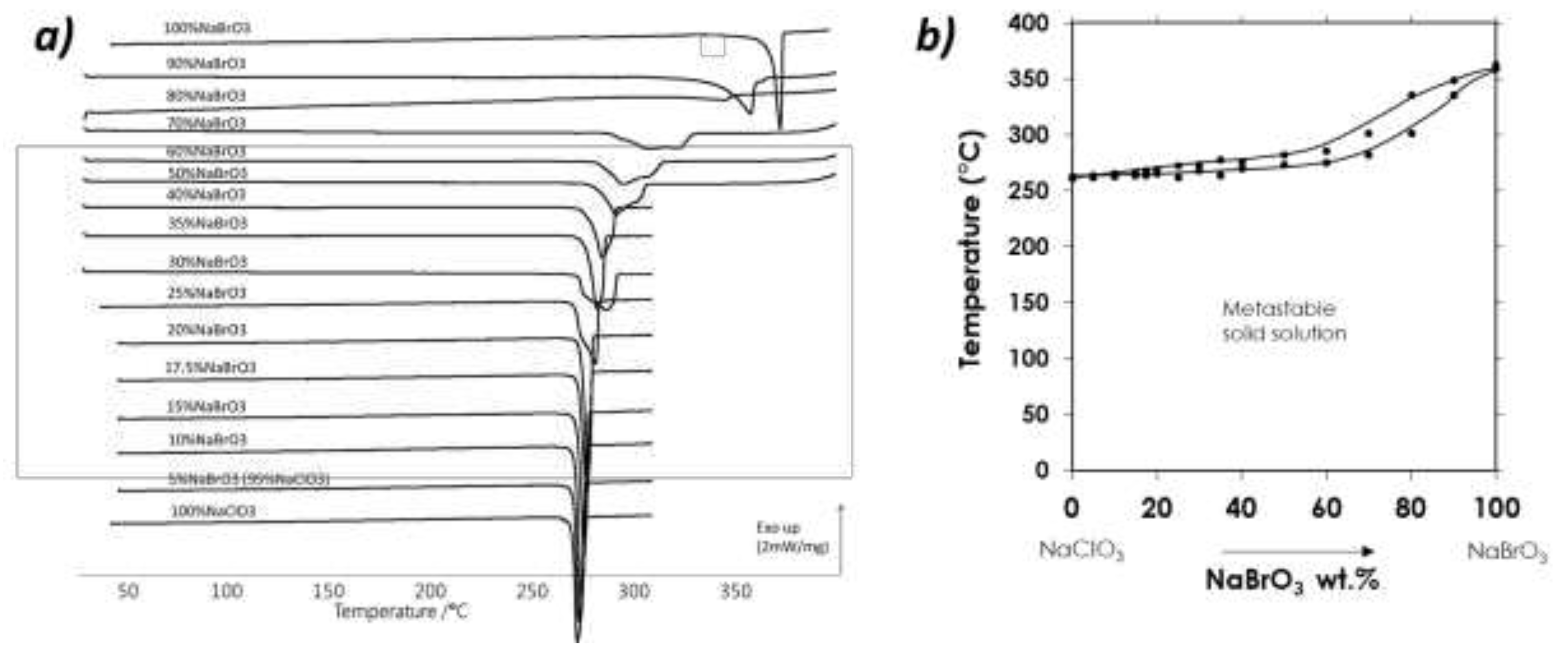
Protocol 1) were analyzed by DSC at 5K/min heating rate.
Figure 11 (a) shows the profiles versus composition on heating.
Figure 11 (b) collects the onset and endset of melting peak (solid filled circles). The resulting binary system includes a solidus and a liquidus which are rather flat up to 60wt.% NaBrO
3. This seems consistent with the tie-lines of the ternary system at 25°C determined by Swenson and Ricci [
6]. The binary system is consistent with a continuous solid solution by means of substitution.
In order to increase the productivity of spray-drying, a mixture of 50% (mass) was used with protocol 2 (concentration 8 times higher than with protocol 1 and the temperature of the feeding liquid was 60°C). With these new parameters, part of the solid was stuck on the upper part of the drying chamber. The rest was stuck on the outlet of the drying chamber or in the collector bowl.
XRPD analyses of the solid in the drying chamber and the collector bowl are displayed in
Figure 12 (The solid stuck at the outlet of the drying chamber appeared identical to that in the collector bowl). The two XRPD patterns shows a clear difference: (i) the powder stuck in the upper part of the drying chamber is duplicated testifying the presence of two similar solid solutions (ii) the powder sampled from the collector bowl is a single phase with diffraction peaks having intermediate d-spacings in comparison with the two partial solid solutions. For the former the full and fast dehydration is aborted resulting in two solid solutions, for the latter a fast and completed dehydration leads to a unique solid solution with the same composition as the feeding solution. For this solid, this is the same result as when using protocol 1.
If the powder obtained after spray-drying in the collector bowl is put for one day under 84% relative humidity at RT, the single XRPD signature spits into two resembling XRPD patterns just like what is obtained by complete slow evaporation.
These results demonstrate that, whatever the composition, the two components could be forced to mix completely if the kinetics of crystallization is fast enough and if the drying is completed in a single step such as during spray-drying using protocol 1. Of course, under these conditions homochirality cannot be controlled; the final product is a random distribution of chiral particles -probably close to a racemic mixture. Thus, the binary section presented in
Figure 11 (b) is merely a metastable racemic section of the quaternary system:
NaClO3\\ - NaClO3//- NaBrO3\\ - NaBrO3//, The symbols: \\ and//refer to opposite supramolecular chirality. The crystallographic features of the two components: same space group and a small difference only of the cubic parameter (less than 2% at RT) render possible the existence of two mirror image metastable complete solid solutions.
4. Conclusion & Perspectives
Spray-drying is a technique which favorizes access to metastable states because the entire crystallization -including nucleation, growth and complete drying- takes place in the order of one second. By using this energetic and swift process, the sodium chlorate and sodium bromate appear fully miscible from RT to fusion but the two-mirror-image complete solid solutions are actually, in a metastable state. Strangely enough, the metastable part of the solid solution corresponds to a slightly denser crystal lattice than the mean of the two stable limiting solid solutions. When the relative humidity exceeds a threshold ca. 75-84% the two (lefthanded and righthanded) complete solid solutions spit into mirror- image partial solid solutions.
For big particles such as those obtained by slow evaporation without stirring, this work evidences the presence of domains rich in bromate and others rich in chlorate which have epitaxial relationships. At 20°C, as a rough approximation, the composition range of NaClO3-rich solid solution is circa: 0%- 20% wt%, the composition range of NaBrO3-rich solid solution is circa 70%-100% wt%.
The construction of the genuine stable phase diagram does not appear that straightforward. Indeed, in addition to the formations of the partial solid solutions it is necessary to reach homochirality. This could be ensured by a continuous grinding (but the pollution by material snatched off from the beads could be an issue) or temperature cycling assisted or not by ultrasounds. The kinetics of the attainment of the ‘true’ equilibrium depends on the doses of those treatments (I.e. intensity versus time).
Future work will focus on deracemization ssNaClO3 rich and ss ssNaBrO3 rich up to their limits in compositions. It will also be interesting to test the deracemization inside the miscibility gap of these two partial solid solutions. Actually, is there an influence of the two ssNaClO3 rich and ssNaBrO3 rich in order to reach identical supramolecular chirality so: homo-homochirality or by contrast: hetero-homochirality?
Author Contributions
Conceptualization, Gerard Coquerel; Data curation, Sylvain Marinel; Investigation, SIMON Florent, Nicolas Couvrat, Christelle Bilot and Gerard Coquerel; Methodology, Gerard Coquerel; Resources, Sylvie Malo; Writing – original draft, SIMON Florent and Gerard Coquerel; Writing – review & editing, Gerard Coquerel.
Conflicts of Interest
The authors declare no conflict of interest and agreed on the last version of the manuscript.
References
- Vegard, L. The constitution of the solid solution and the space filling of the atoms. Zeitschrift für Phys. 1921, 5, 17–26. [Google Scholar] [CrossRef]
- King, H.W. Qualitative size-factors for metallic solid solutions. J. Mater. Sci. 1966, 1, 79–90. [Google Scholar] [CrossRef]
- Denton, A.R.; Ashcroft, N.W. Vegard’s law. Phys. Rev. A. 1991, 43, 3161–3164. [Google Scholar] [CrossRef]
- Chandrasekaran, K.S.; Monhanlal, S.K. The x-ray anomalous dispersion and optical rotation in the crystalline solid solution NaClO3:NaBrO3. Pramana 1976, 7, 152–159. [Google Scholar] [CrossRef]
- Subhadra, K.G.; Hussain, K.A. Melting Temperatures of NaClO3-NaBrO3 Mixed Crystals. Cryst. Res. Technol. 1987, 22, 6–827. [Google Scholar] [CrossRef]
- Swenson, T.; Ricci, J.E. The Ternary Systems KBrO3-KClO3-H2O at 25 °C and NaBrO3-NaClO3-H2O at 25 and 50 °C. J. Am. Chem. Soc. 1939, 61, 1974–1977. [Google Scholar] [CrossRef]
- Crundwell, G.Y.; Gopalan, P.; Bakulin, A.; Peterson, M.L.; Kahr, B. Effect of Habit Modification on Optical and X-ray Structures of Sodium Halate Mixed Crystals: The Etiology of Anomalous Double Refraction. Acta Cryst. 1997, B53, 189–202. [Google Scholar] [CrossRef]
- Dickinson, R.G.; Goodhue, E.A. The Crystal Structure of Sodium Chlorate and Sodium Bromate. J. Am. Chem. Soc. 1921, 43, 2045–2055. [Google Scholar] [CrossRef]
- Abrahams, S.C.; Bernstein, J.L. Remeasurement of Optically Active NaClO3 and NaBrO3. Acta Cryst. 1977, B33, 3601–3604. [Google Scholar] [CrossRef]
- Savage, A.; Miller, R.C. Measurements of Second Harmonic Generation of Ruby Laser Line in Piezoelectric Crystals. Appl. Opt. 1962, 1, 661–664. [Google Scholar] [CrossRef]
- Simon, H.J.; Bloembergen, N. Second-Harmonic Light Generation in Crystals with Natural Optical Activity. Phys. Rev. 1968, 171, 1104–1114. [Google Scholar] [CrossRef]
- Matsuura, T.; Koshima, H. Review: Introduction to chiral crystallization of achiral compounds. Spontaneous generation of chirality. J. Photochem. Photobiol. 2005, 6, 7–24. [Google Scholar] [CrossRef]
- Ramachandran, G.N.; Chandrasekaran, K.S. The Absolute Configuration of Sodium Chlorate. Acta Crystallogr. 1957, 10, 671–675. [Google Scholar] [CrossRef]
- Beurkskens-Kerssen, G.; Kroon, J.; Endeman, H.J.; Van Laar, J.; Bijvoet, J.M. Absolute Configuration and Rotatory Power of the Crystals of NaBrO3 and NaClO3. In Crystallography and Crystal Perfection; Ramachandran, G.N., Ed.; London Academic Press, 1963; pp. 225–236. [Google Scholar]
- Chandrasekhar, S.; Madhava, M.S. Optical roratory dispersion of crystals of sodium chlorate and sodium bromate. Acta Cryst. 1967, 23, 911. [Google Scholar] [CrossRef]
- Kipping, F.S.; Pope, W.J. Enantiomorphism. J. Chem. Soc. Trans. 1898, 73, 606–617. [Google Scholar] [CrossRef]
- Kondepudi, D.K.; Kaufman, R.J.; Singh, N. Chiral symmetry breaking in sodium chlorate crystallization. Science 1990, 250. [Google Scholar] [CrossRef] [PubMed]
- Kondepudi, D.K.; Prigogine, I. Sensitivity of nonequilibrium systems. Phys. A Stat. Mech. Appl. 1981, 107, 1–24. [Google Scholar] [CrossRef]
- Kondepudi, D.K.; Nelson, G.W. Chiral symmetry breaking in nonequilibrium systems. Phys. Rev. Lett. 1983, 50, 1023–1026. [Google Scholar] [CrossRef]
- Kondepudi, D.K.; Nelson, G.W. Weak neutral currents and the origin of biomolecular chirality. Nature 1985, 314, 438–441. [Google Scholar] [CrossRef]
- Buhse, T.; Durand, D.; Kondepudi, D.; Laudadio, J.; Spilker, S. Chiral symmetry breaking in crystallization: the role of convection. Phys. Rev. Lett. 2000, 84, 4405–4408. [Google Scholar] [CrossRef] [PubMed]
- Viedma, C. Chiral symmetry breaking during crystallization: complete chiral purity induced by nonlinear autocatalysis and recycling. Phys. Rev. Lett. 2005, 94, 065504. [Google Scholar] [CrossRef] [PubMed]
- Suwannasang, K.; Flood, A.; Rougeot, C.; Coquerel, G. Using Programmed Heating-Cooling with Racemization in Solution for Complete Symmetry Breaking of a Conglomerate Forming System. Cryst. Growth Des. 2013, 13, 3498–3504. [Google Scholar] [CrossRef]
- Rougeot, C.; Guillen, F.; Plaquevent, J.-C.; Coquerel, G. Ultrasound-Enhanced Deracemization: Towards the Existence of Agonist Effects in the Interpretation of Spontaneous Symmetry Breaking. Cryst. Growth Des. 2015, 15, 2151–2155. [Google Scholar] [CrossRef]
- Viedma, C.; Cintas, P. Homochiraliry beyond grinding: deracemizing chrial crystals by temperature gradient under boiling. Chem. Commun. 2011, 47, 12786–12788. [Google Scholar] [CrossRef]
- Viedma, C.; McBride, J.M.; Kahr, B.; Cintas, P. Enantiomeric-Specific Oriented Attachment: Formation of Macroscopic Homochiral Crystal Aggregates from a Racemic System. Angew. Chem. Int. Ed. 2013, 52, 10545–10548. [Google Scholar] [CrossRef] [PubMed]
- Schindler, M.; Brandel, C.; Kim, W.-S.; Coquerel, G. Temperature Cycling Induced Deracemization of NaClO3 under the Influence of Na2S2O6. Cryst. Growth Des. 2020, 20, 1–414. [Google Scholar] [CrossRef]
- Akizuki, M.; Hampar, M.S.; Zusman, J. An explanation of anomalous optical properties of topaz. Mineral. Mag. 1979, 43, 237–241. [Google Scholar] [CrossRef]
- Akizuki, M. Crystal symmetry and order-disorder structure of brewsterite. Am. Mineral. 1987, 72, 645–648. [Google Scholar]
- Akikuzi, M.; Konno, H. Internal texture and abnormal optical property of apophyllite. N. Jahrb. Mineral. Abh. 1985, 151, 99–115. [Google Scholar]
- McBride, J.M.; Bertmann, S.B. Using Crystal Birefringence to Study Molecular Recognition. Angew. Chem. 1989, 28, 330–333. [Google Scholar] [CrossRef]
- Weisinger-Lewin, Y.; Frolow, F.; McMullan, R.K.; Koetzle, T.F.; Lahav, M.; Leiserowitz, L. Reduction of crystal symmetry of a solid solution: a neutron diffraction study at 15K and the host/guest system asparagine/aspartic acid. J. Am. Chem. Soc. 1989, 111, 1035–1040. [Google Scholar] [CrossRef]
- Weissbuch, I.; Lahav, M.; Leiserowitz, L.; Meredith, G.R.; Vanherzeele, H. Centrosymmetric crystals as host matrices for second-order optical nonlinear effect. Chem. Mater. 1989, 1, 114–118. [Google Scholar] [CrossRef]
- Shimon, L.J.W.; Vaida, M.; Frolow, F.; Lahav, M.; Leiserowitz, L.; Weissinger-Lewin, Y.; McMullan, R.K. Symmetry lowering in crystalline solid solutions: a study of cinnamamide-thienylacrylamide by X-ray and neutron diffraction and solid state photochemistry. Faraday Discuss. 1993, 95, 307. [Google Scholar] [CrossRef]
- Kahr, B.; McBride, J.M. Optically Anomalous Crystals. Angew. Chem. Int. Ed. Engl. 1992, 31, 1–26. [Google Scholar] [CrossRef]
- Kahr, B.; Gurney, R.W. Dyeing crystals. Chem. Rev. 2001, 101, 893–951. [Google Scholar] [CrossRef]
- Buckley, H.E. 2. The Influence of RO4 and Related Ions on the Crystalline Form of Sodium Chlorate. Zeitschrift für Krist. 1930, 15–31. [Google Scholar]
- Ristic, R.; Sherwood, J.N.; Wojciechowskf, K. Morphology and growth kinetics of large sodium chlorate crystals grown in the presence and absence of dithionate impurity. J. Phys. Chem. 1993, 97, 10774–10782. [Google Scholar] [CrossRef]
- Ristic, R.; Shekunov, B.Y.; Sherwood, J.N. Growth of the tetrahedral faces of sodium chlorate crystals in the presence of dithionate impurity. J. Cryst. Growth. 1994, 139, 336–343. [Google Scholar] [CrossRef]
- Lan, Z.-P.; Lai, X.; Roberts, K.; Klapper, H. X-ray Topographic and Polarized Optical Microscopy Studies of Inversion Twinning in Sodium Chlorate Crystals Grown in the Presence of Sodium Dithionate Impurities. Cryst. Growth Des. 2014, 14, 6084–6092. [Google Scholar] [CrossRef]
- Gopalan, P.; Peterson, M.L.; Crundwell, G.; Kahr, B. Reevaluating Structures for Mixed Crystals of Simple Isomorphous Salts: NaClxBr1-xO3. J. Am. Chem. Soc. 1993, 115, 3366–3367. [Google Scholar] [CrossRef]
- Kaminsky, W.; Claborn, K.; Kahr, B. Polarimetric imaging of crystals. Chem. Soc. Rev. 2004, 33, 514–525. [Google Scholar] [CrossRef]
- Sanjeevi Raja, C.; Mohanlal, S.K.; Chandrasekaran, K.S. An X-ray diffraction study of the size effect on a crystalline solid solution of sodium halates. Zeitschrift für Krist. 1984, 166, 121–127. [Google Scholar]
- Shtukenberg, A.G.; Rozhdestvenskaya, I.V.; Popov, D.Y.; Punin, Y.O. Kinetic ordering of atoms in sodium chlorate-bromate solid solutions. J. Solid State Chem. 2004, 177, 4732–4742. [Google Scholar] [CrossRef]
- Su, J.; Song, Y.; Zhang, D.; Chang, X. Characterization of unidirectionally grown NaCl1−xBrxO3 crystals. Powder Diffr. 2009, 24, 234–238. [Google Scholar] [CrossRef]
- Hulliger, J.; Wüst, T.; Brahimi, K.; Burgener, M.; Aboufadl, H. A stochastic principle behind polar properties of condensed molecular matter. New J. Chem. 2013, 37, 2229–2235. [Google Scholar] [CrossRef]
- Coquerel, G.; Sanselme, M.; Lafontaine, A. Method and Measuring Scattering of X-Rays, Its Applications and Implementation Device. US Patent 9,955,898, 2016. [Google Scholar]
- Bak, S.Y.; Coquerel, G.; Kim, W.-S.; Park, B.J. Solution Volume Effects on Spontaneous Chiral Symmetry Breaking of Sodium Chlorate Crystals. J. Phys. Chem. Lett. 2023, 14, 785–790. [Google Scholar] [CrossRef]
Figure 1.
(a)(c) Ternary isothermal sections at 25°C and 50°C proposed by Swenson and Ricci (adapted from ref. (Swenson, 1939)) Note: No XRD data were collected during this work, the recovered solid compositions were determined by titration, so the interpretations of the data can be manifold. (b) Swenson and Ricci’s proposal for biphasic domains in the ternary isotherm at 25°C. At this temperature and to be in accordance with Gibbs phase rule, those three biphasic domains need to be interlaced by triphasic domains (triangular white domains). The three regions are represented with different colors. The green domain encompasses, by far, the largest part of the two-phase region from 0% bromate (100% chlorate) to ca. 60-65 wt.% NaBrO3 in the solid phase and from A to B for the corresponding saturated solution. The rather thin region colored in grey, corresponds to a possible ordered domain ranging from 90-95% to almost 100% bromate in the solid phase and from B to C for the corresponding saturated solution. The last region colored in blue behaves like a pure component; the tie lines are likely to converge to nearly pure sodium bromate and the corresponding saturated solution ranging from C to D. (d) Swenson and Ricci’s proposal for equilibria at 50°C showing a continuous solid solution.
Figure 1.
(a)(c) Ternary isothermal sections at 25°C and 50°C proposed by Swenson and Ricci (adapted from ref. (Swenson, 1939)) Note: No XRD data were collected during this work, the recovered solid compositions were determined by titration, so the interpretations of the data can be manifold. (b) Swenson and Ricci’s proposal for biphasic domains in the ternary isotherm at 25°C. At this temperature and to be in accordance with Gibbs phase rule, those three biphasic domains need to be interlaced by triphasic domains (triangular white domains). The three regions are represented with different colors. The green domain encompasses, by far, the largest part of the two-phase region from 0% bromate (100% chlorate) to ca. 60-65 wt.% NaBrO3 in the solid phase and from A to B for the corresponding saturated solution. The rather thin region colored in grey, corresponds to a possible ordered domain ranging from 90-95% to almost 100% bromate in the solid phase and from B to C for the corresponding saturated solution. The last region colored in blue behaves like a pure component; the tie lines are likely to converge to nearly pure sodium bromate and the corresponding saturated solution ranging from C to D. (d) Swenson and Ricci’s proposal for equilibria at 50°C showing a continuous solid solution.
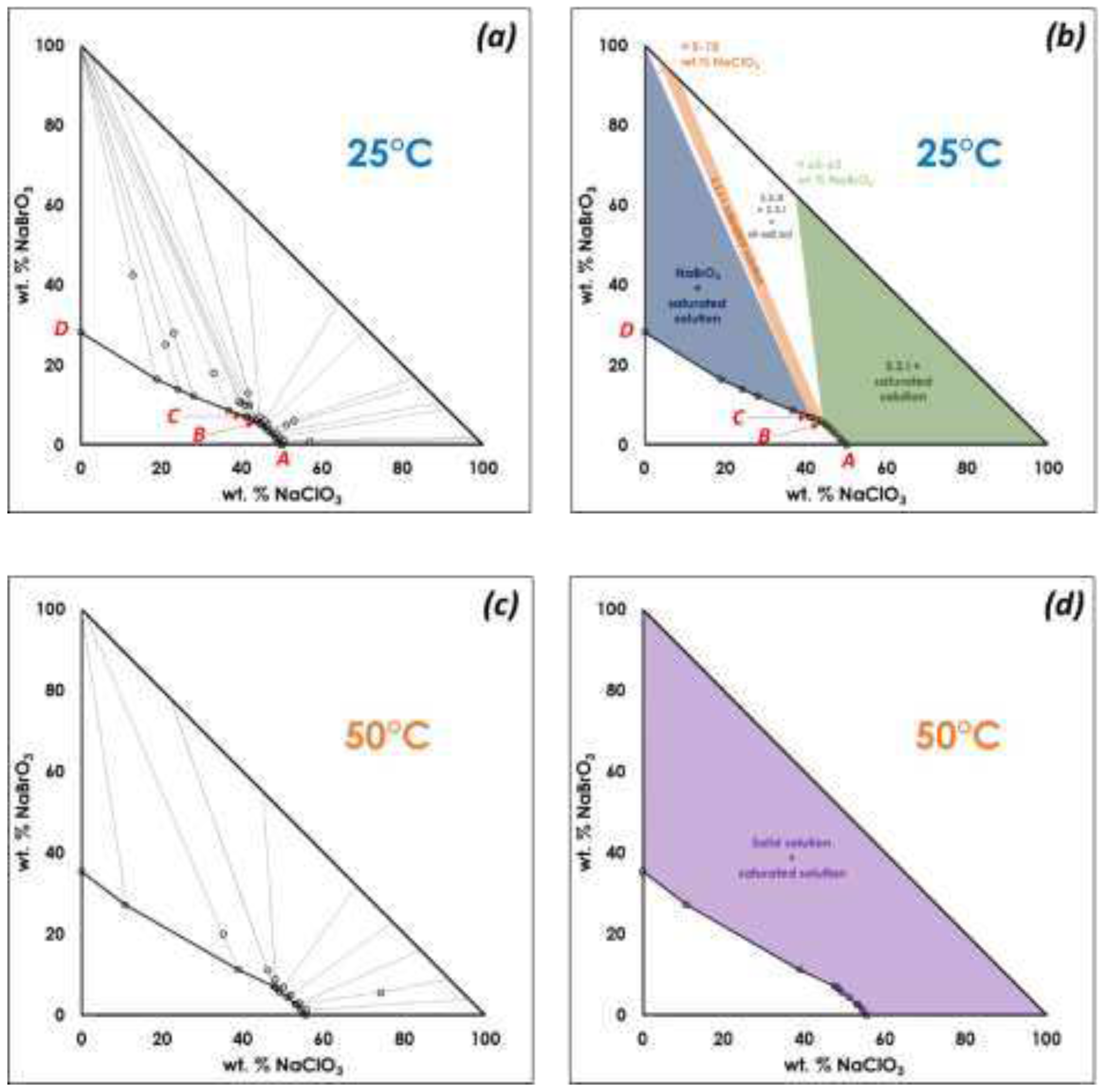
Figure 2.
(a) XRPD pattern of powder sample (crystallized by slow evaporation under stirring from 50wt.% NaClO3/NaBrO3 mixtures in aqueous solution) in comparison with pure NaClO3 and NaBrO3 commercial samples (physical mùixture). Based on the peak position, a rough estimation of the limiting composition of NaClO3-rich and NaBrO3-rich solid solution indicated 20wt.% and 70wt.% in NaBrO3, respectively. Thus two partial solid solutions exist: one NaClO3-rich solid solution (hereafter labelled S.S.I) ranging from 0wt.% to 20wt.% NaBrO3, and one NaBrO3-rich solid solution (hereafter labelled as S.S.II) ranging from 70wt.% to 100wt.% NaBrO3. (b) XRPD patterns of powder samples crystallized by slow evaporation from 50wt.% NaClO3/NaBrO3 mixtures in aqueous solution at different temperature: RT, 15°C, 50°C, 120°C, and 160°C) confirmed that the miscibility gap is still present at elevated temperature.
Figure 2.
(a) XRPD pattern of powder sample (crystallized by slow evaporation under stirring from 50wt.% NaClO3/NaBrO3 mixtures in aqueous solution) in comparison with pure NaClO3 and NaBrO3 commercial samples (physical mùixture). Based on the peak position, a rough estimation of the limiting composition of NaClO3-rich and NaBrO3-rich solid solution indicated 20wt.% and 70wt.% in NaBrO3, respectively. Thus two partial solid solutions exist: one NaClO3-rich solid solution (hereafter labelled S.S.I) ranging from 0wt.% to 20wt.% NaBrO3, and one NaBrO3-rich solid solution (hereafter labelled as S.S.II) ranging from 70wt.% to 100wt.% NaBrO3. (b) XRPD patterns of powder samples crystallized by slow evaporation from 50wt.% NaClO3/NaBrO3 mixtures in aqueous solution at different temperature: RT, 15°C, 50°C, 120°C, and 160°C) confirmed that the miscibility gap is still present at elevated temperature.
Figure 3.
XRPD patterns of single particles withdrawn from an open crystallizer at different times during water evaporation: (black XRPD pattern): 50:50 NaClO3/NaBrO3 physical mixture (reference); (blue XRPD pattern) particle withdrawn after 1 day after spontaneous nucleation; (orange XRPD pattern) after two days after spontaneous nucleation; (green XRPD pattern) at the end of the process (total evaporation). Only one particle was withdrawn at a time so the overall composition of the system remained unchanged.
Figure 3.
XRPD patterns of single particles withdrawn from an open crystallizer at different times during water evaporation: (black XRPD pattern): 50:50 NaClO3/NaBrO3 physical mixture (reference); (blue XRPD pattern) particle withdrawn after 1 day after spontaneous nucleation; (orange XRPD pattern) after two days after spontaneous nucleation; (green XRPD pattern) at the end of the process (total evaporation). Only one particle was withdrawn at a time so the overall composition of the system remained unchanged.
Figure 4.
Polarized-light optical microscopy (POM) of selected NaClO3(x)BrO3(1-x) mixed crystals grown by complete evaporation at 20°C from 50wt.% NaClO3/NaBrO3 mixtures in aqueous solution. (a), (b) and (c) show apparent single crystals revealing domains of different handedness. Hopper defects (concave crystals) were often found, because crystal edges and corners grow faster than surfaces for large crystals grown at high supersaturation without stirring.
Figure 4.
Polarized-light optical microscopy (POM) of selected NaClO3(x)BrO3(1-x) mixed crystals grown by complete evaporation at 20°C from 50wt.% NaClO3/NaBrO3 mixtures in aqueous solution. (a), (b) and (c) show apparent single crystals revealing domains of different handedness. Hopper defects (concave crystals) were often found, because crystal edges and corners grow faster than surfaces for large crystals grown at high supersaturation without stirring.
Figure 5.
Mapping by EDX of a large particle obtained by slow aqueous evaporation at room temperature from a 50wt.% NaClO3/NaBrO3. Mixture. (a) Polarized Optical Microscopy POM pictures showing the particle. (b) and (d) SEM pictures of selected area of the selected particle. (c) and (e) corresponding composition mapping by EDX showing different concentration of Cl and Br atoms depending on the selected zone on the particle: (c) zone 1: molar Br ratio = 30.5/(30.5+8.6) = 78%; zone 2: molar Br ratio = 20%; (e) zone 1: molar Br ratio = 21%; zone 2: molar Br ratio = 81%.
Figure 5.
Mapping by EDX of a large particle obtained by slow aqueous evaporation at room temperature from a 50wt.% NaClO3/NaBrO3. Mixture. (a) Polarized Optical Microscopy POM pictures showing the particle. (b) and (d) SEM pictures of selected area of the selected particle. (c) and (e) corresponding composition mapping by EDX showing different concentration of Cl and Br atoms depending on the selected zone on the particle: (c) zone 1: molar Br ratio = 30.5/(30.5+8.6) = 78%; zone 2: molar Br ratio = 20%; (e) zone 1: molar Br ratio = 21%; zone 2: molar Br ratio = 81%.
Figure 6.
TR-XRPD of a 50% Mass Composition from 85°C to 250°C, annealing of the 11 last patterns (11h) at 250°C.
Figure 6.
TR-XRPD of a 50% Mass Composition from 85°C to 250°C, annealing of the 11 last patterns (11h) at 250°C.
Figure 7.
(a) Putative homochiral binary phase diagram between NaClO3 and NaBrO3 showing a stable miscibility gap up to a critical point (K), above which a long period of diffusion time could succeed in mixing the two components in the full range of composition (i.e., from 0 to 100% NaBrO3). Note that homochirality is supposed to appear to give left-handed or right-handed crystals only. (b) Putative homochiral binary phase diagram between NaClO3 and NaBrO3 showing a stable miscibility gap that does not close up at elevated temperature but intersects the liquid-solid equilibria, implying a three-phase invariant (peritectic transition). Note: for both (a) and (b), filled circles corresponds to experimental points determined by DSC (present work).
Figure 7.
(a) Putative homochiral binary phase diagram between NaClO3 and NaBrO3 showing a stable miscibility gap up to a critical point (K), above which a long period of diffusion time could succeed in mixing the two components in the full range of composition (i.e., from 0 to 100% NaBrO3). Note that homochirality is supposed to appear to give left-handed or right-handed crystals only. (b) Putative homochiral binary phase diagram between NaClO3 and NaBrO3 showing a stable miscibility gap that does not close up at elevated temperature but intersects the liquid-solid equilibria, implying a three-phase invariant (peritectic transition). Note: for both (a) and (b), filled circles corresponds to experimental points determined by DSC (present work).
Figure 8.
SEM pictures--with the same magnification- of spray-dried samples obtained by using Protocol 1 from different initial compositions (ranging from 0% NaBrO3 (100% NaClO3) to 100% NaBrO3 (0% NaClO3)). As NaBrO3 is less soluble than NaClO3, particles were found to be smaller.
Figure 8.
SEM pictures--with the same magnification- of spray-dried samples obtained by using Protocol 1 from different initial compositions (ranging from 0% NaBrO3 (100% NaClO3) to 100% NaBrO3 (0% NaClO3)). As NaBrO3 is less soluble than NaClO3, particles were found to be smaller.
Figure 9.
XRPD pattern versus composition of NaClO3(x)BrO3(1-x) (mass fraction) prepared by spray-drying -protocol 1- The inlet is a zoom on the second peak at ca. 23 ° (2θ scale Cu Kα.).
Figure 9.
XRPD pattern versus composition of NaClO3(x)BrO3(1-x) (mass fraction) prepared by spray-drying -protocol 1- The inlet is a zoom on the second peak at ca. 23 ° (2θ scale Cu Kα.).
Figure 10.
Crystallographic parameter a of NaClO3(x)BrO3(1-x) (molar fraction) prepared by spray-drying -using protocol 1 (precise values are reported in Table SI-1). A discontinuity of the Vegard law seems to be present for compositions ranging from ca. 20% to ca. 70% NaBrO3, for which the cubic cell parameter seems to be slightly lower than the mean of parameters limiting the partial solid solutions.
Figure 10.
Crystallographic parameter a of NaClO3(x)BrO3(1-x) (molar fraction) prepared by spray-drying -using protocol 1 (precise values are reported in Table SI-1). A discontinuity of the Vegard law seems to be present for compositions ranging from ca. 20% to ca. 70% NaBrO3, for which the cubic cell parameter seems to be slightly lower than the mean of parameters limiting the partial solid solutions.
Figure 11.
(a) DSC analyses (heating rate 5K.min−1) of powder samples prepared by spray-drying (Protocol 1). (b) Racemic binary section of the quaternary system: (NaClO3)L/(NaClO3)D/(NaBrO3)L/(NaBrO3)D. for which a metastable solid solution could be obtained.
Figure 11.
(a) DSC analyses (heating rate 5K.min−1) of powder samples prepared by spray-drying (Protocol 1). (b) Racemic binary section of the quaternary system: (NaClO3)L/(NaClO3)D/(NaBrO3)L/(NaBrO3)D. for which a metastable solid solution could be obtained.
Figure 12.
50% mass after Spray drying protocol 2: (black pattern) solid collected in the upper part of the drying chamber; (blue pattern): solid from the collector bowl.
Figure 12.
50% mass after Spray drying protocol 2: (black pattern) solid collected in the upper part of the drying chamber; (blue pattern): solid from the collector bowl.
Table 1.
Spray-drying parameters for two sets of experiments.
Table 1.
Spray-drying parameters for two sets of experiments.
| Parameters |
Protocol 1 |
Protocol 2 |
| Concentration of the NaClO3 (1-x)-NaBrO3(x) aqueous solutions |
2g/150mL water |
6g/50mL water |
| Inlet temperature of the drying nitrogen |
220°C |
220°C |
| Feed flow rate (peristaltic pump) |
360mL/h |
300mL/h |
| Temperature of the feed solution |
Room temperature |
Ca. 60°C |
| Atomizing airflow rate |
473L/h |
500L/h |
| Aspiration |
40m3/h |
40m3/h |
| Location of the powder at the end of the process |
Mainly in the bowl collector |
In the drying chamber, cyclone and bowl collector |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).