1. Introduction
Brain tumours are abnormal and uncontrolled growth of cells in the human brain that affect usual brain functionality [
1]. Brain tumours are divided into primary and secondary. Primary brain tumours originate in the brain and can be subdivided into benign (non-cancerous) and malignant (cancerous). Secondary brain tumours are cancerous cells expanding to the brain from other parts of the human body [
2]. World Health Organization (WHO) classifies brain tumours into four grades. Grades 1 and 2 consist of less severe tumours such as meningiomas, while Grades 3 and 4 consist of more severe ones such as gliomas [
3]. Management and treatment of these brain neoplasms require an understanding of the location, size, and type of tumour. Various imaging modalities such as Magnetic Resonance Imaging (MRI), Positron Emission Tomography (PET), and Computed Tomography (CT) are used in the diagnosis of brain tumours. MRI is usually preferred as it is non-ionizing and non-invasive [
4]. However, manual segmentation and classification of these images are tedious processes, prone to human error, and can be subjective. To address these challenges, Clinical Decision Support Systems (CDSSs) are being used as a supportive tool for radiologists and clinicians to aid in the diagnosis and prognosis of brain tumours [
4].
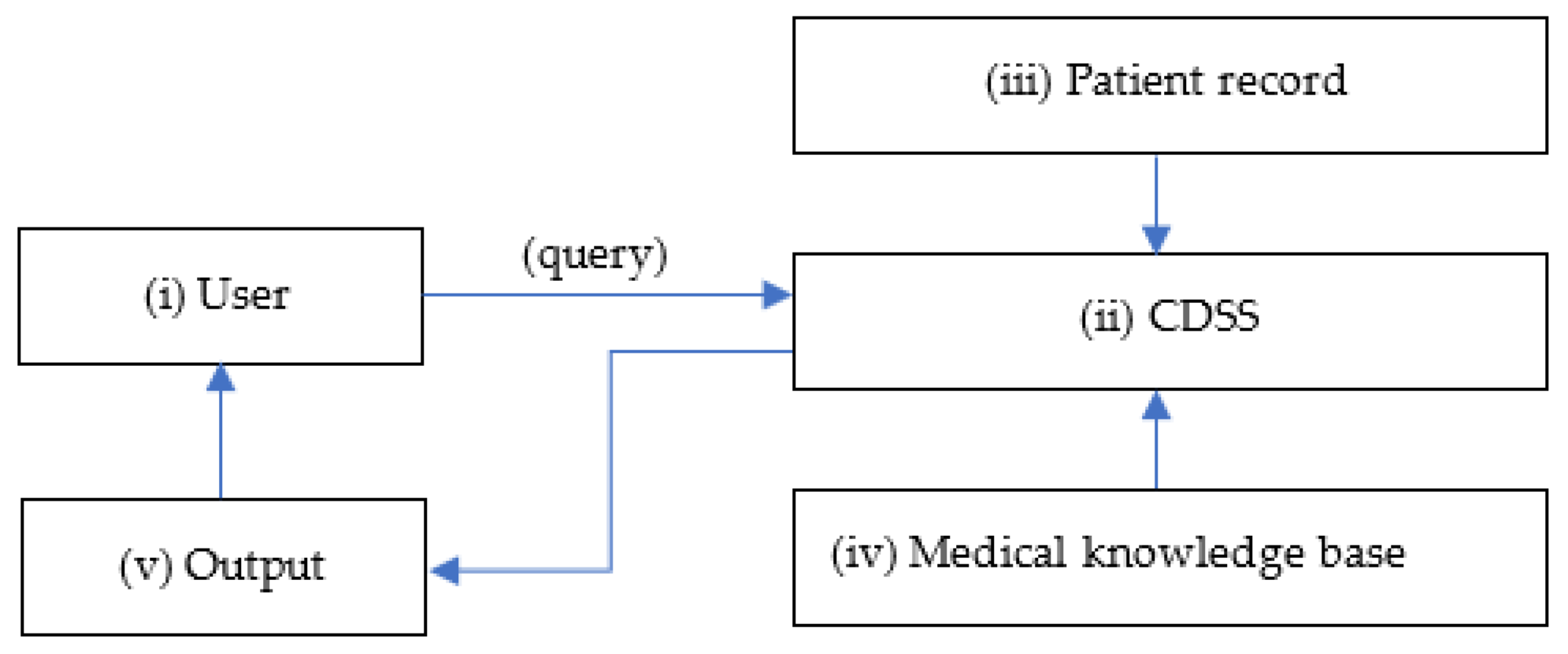
CDSSs are primarily designed for clinicians to use at-the-point of care shown in
Figure 1. A conventional CDSS is comprised of a software designed to match patient characteristics with a computerised medical knowledge base and present a patient-specific recommendation or evaluation to the clinician for making an informed decision [
5]. These computerised systems aid in early detection and characterisation of brain tumours by performing automatic tumour segmentation, differentiation, classification, and evaluation of brain imaging data [
6].
Available literature on CDSSs, used specifically for brain tumours, is limited to the best of our knowledge. This systematic review is, to the best of our knowledge, the first of its kind to evaluate different types of CDSSs used both for brain tumour diagnosis and prognosis, through medical imaging data. The research question is to identify what CDSSs are being used in the diagnosis and prognosis of brain tumours, to summarise the techniques used, and to evaluate their accuracy and outcomes.
2. Method
The methodology has been divided into (i) search strategy—databases used; (ii) study selection—keywords, and inclusion and exclusion criteria; (iii) data extraction—pre-defined data extraction proforma; (iv) study quality assessment—to assess the quality of included studies; (v) data synthesis—reasons for conducting a narrative and semi-quantitate review.
2.1. Search strategy
The literature search was conducted systematically in two academic databases viz., PubMed and Engineering Village. Both databases provided all the relevant studies needed in this area of research. For identifying medical literature in PubMed, Medical Subject Headings (MeSH) terms were used— ("decision support systems, clinical"[MeSH Terms] AND "Brain Neoplasms"[MeSH Terms]). In Engineering Village, both controlled vocabulary and general terms were used— (( (((((cancer)) OR ((((({Tumors} WN CV) OR ({Oncology} WN CV))))))) AND ((((({Decision support systems} WN CV))))))) AND brain). Studies published only in the English language were considered in this review. The search was not bound by any time frame.
2.2. Study selection
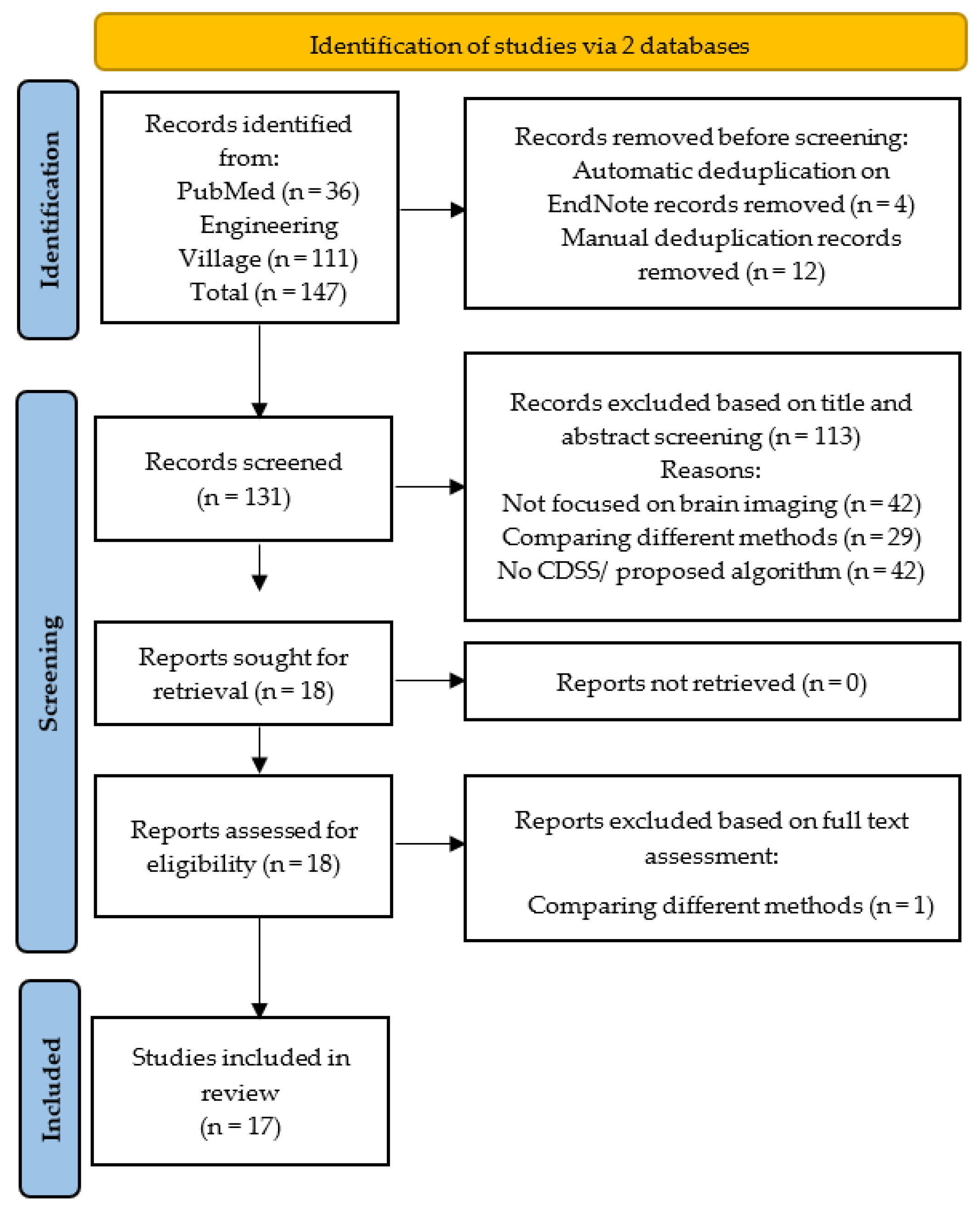
The systematic review was conducted according to the 2020 PRISMA protocol [
7]. The PRISMA flowchart has been shown in
Figure 2. The literature search was conducted without any timeframe to identify all studied published until May 2023.
Based on the purpose of this systematic review, there were 4 exclusion criteria (i) studies that did not use CDSS; (ii) studies that focused on comparing different methods/ techniques; (iii) studies that did not investigate brain tumours; (iv) studies that focused on the treatment of brain tumours. Additionally, papers that had insufficient information on results or limited/ poor methodology were also excluded.
2.3. Data extraction
Two authors independently performed data extraction based on a pre-defined data extraction proforma. Any conflicts or discrepancies between the authors were resolved by a third reviewer. Variables used for extraction of data were the year of study publication, study design, geographical location of the research conducted, sample size, modality used, CDSS features, techniques/ methods used, and CDSS output.
2.4. Study quality assessment
All studies included in this review have been assessed for the quality of their research. Keshav’s 5 Cs viz., category, context, clarity, correctness, and contribution [
8] were used to justify including papers in this review. Additionally, studies that had justifiable reasons for their sample size, patient selection criteria, and methodology used, were only considered.
2.5. Data synthesis
The identified studies were diverse in terms of their sample size, type of CDSS, and techniques used. Hence, a meta-analysis was not performed. Rather, both a narrative and semiquantitative summary of CDSSs used in brain tumour diagnosis and prognosis have been provided. Individual CDSSs have been broadly categorised, as and when necessary.
3. Results
All studies included in this review answer the research question; what are the available CDSSs being used in the diagnosis and prognosis of all types of brain tumours, what their features and techniques are, and finally what their accuracy and outcomes are. These are covered in the following sections.
3.1. Search results
The literature search conducted via two databases produced 146 studies out of which PubMed identified 36, while Engineering Village identified 111 studies. Automatic de-duplication in EndNote removed 4 studies while a manual scan removed an additional 14 duplicates, leaving 131 studies to be evaluated for the title and abstract screening
Figure 2. Based on the title and abstract 113 articles were removed. Out of the remaining 18 studies for full-text assessment, only 1 did not fulfil all the inclusion criteria and thus was removed. Finally, 17 articles were shortlisted for this systematic review.
3.2. Study characteristics
All 17 studies identified were full-text articles (100%); there were no abstracts from conference presentations. The types of study design within the review have been documented in
Table 1.
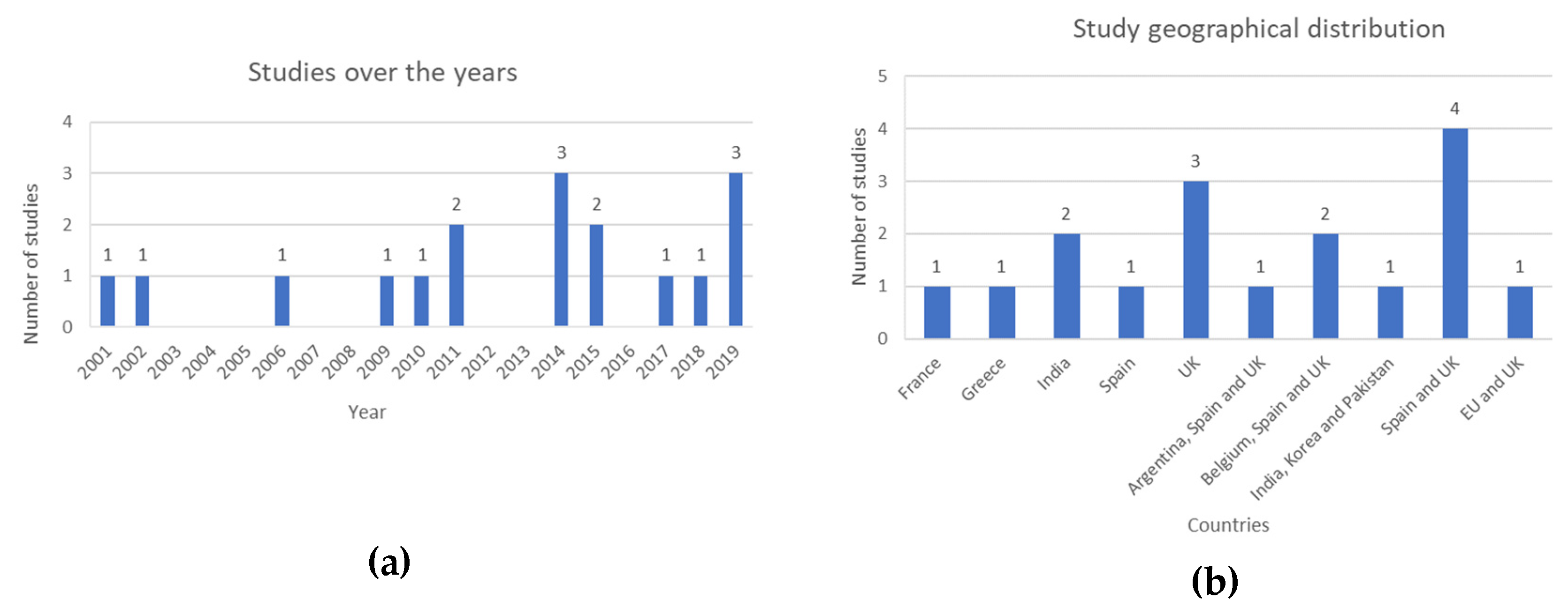
The search was not filtered by any time frame to include all available studies in this area until 30 May 2023. The distribution of studies published over time, and their geographical locations have been described in
Figure 3 (a) and (b) respectively.
7 studies (41%) were based on publicly available datasets, 3 studies (18%) were conducted based on international datasets, 2 studies (12%) used regional data, and 5 studies (29%) were conducted at a single center.
3.3. CDSSs used in the diagnosis and prognosis of brain tumours
The different types of CDSSs identified through this review, alongside the sample size, modality, sub specific type of brain tumour, techniques, accuracy, and outcome have been listed in
Table 2.
3.3.1. Diagnostic support systems
MRI-based brain tumour classifier systems were proposed by [
10,
11]. Both studies utilised the publicly available Brain Tumor Segmentation (BraTS) dataset. Features were extracted by Gray Level Co-occurrence Matrix (GLCM) method and Run Length of Centralized Patterns (RLCP) respectively. Accuracy of classification of tumours performed by Boosting Support Vector Machine (BSVM) algorithm was 97.69% [
10] as compared to 96.47% using Naïve Bayes (NB) [
11]. When comparing the two classifiers, BSVM can be considered to have superior capabilities as it performs well with even with larger, high dimension datasets and the algorithm’s complexity does not increase with reducing training time. Another study by [
12], based on a hospital dataset of 30 patients, with an accuracy of 99.00% was able to determine size, shape, and location of tumour, utilised Speeded Up Robust Features (SURF) enhanced Bag of Words (BoW) feature extraction method combined with SVM classifier. The 3D visualisation capability of this CDSS outperformed available state-of-the-art tools such as ITK-SNAP and 3D-Doctor according to a subjective comparative analysis. Based on a subjective evaluation undertaken by two separate expert raters, the proposed diagnostic support system can be implemented at local and remote levels. Finally, a study by [
13] proposed a computerised decision support framework with a sample size of 48 patients for automatic tumour discrimination between Glioblastoma Multiforme (GBM) and solitary Metastasis (MET) using MRI. The novel segmentation method (D-SEG) along with a neural networks-based classifier achieved an accuracy of 97.92%. However, using a semi-automatic segmentation method and a relatively smaller dataset can be seen as limitations of the proposed CDSS.
Studies [
14] and [
15] used data from
Magnetic Resonance Spectroscopy (MRS) for automatic classification of
1H MR spectra from brain tumour samples. The multi-stage classifier based on decision trees, LDA and k-NN, reduced bias and classification errors, and had superior prediction capabilities [
14] as compared to using only LDA in [
15]. Both studies successfully categorised tumours into benign vs. malignant, and low-grade vs. high-grade with higher than 90.00% classification accuracy.
Paper [
16] conducted a prospective parallel-randomized pilot study to evaluate
Curiam BT—a CDSS for the diagnosis of brain tumours based on
1H MRS. Curiam BT included four predictive models: M1 with Short Echo Time (STE) classifier to discriminate between aggressive, meningioma, and low-grade glial tumours with an accuracy of 88%; M2 with both STE and Long Echo Time (LTE) to discriminate between aggressive, meningioma, and low-grade glial tumours with an accuracy of 92%; M3 with STE to discriminate between high-grade tumours and low-grade tumours with an accuracy of 83%; M4 with STE to discriminate between meningiomas and non-meningioma with an accuracy of 91%. All models were based on Fisher LDA and Peak Integration. The pilot study conducted with a sample size of 55 confirmed Curiam BT improved diagnostic accuracy and can be used as an effective tool to train and assist novice radiologists to diagnose brain tumours. To optimize the CDSS for routine practice, conducting a clinical trial with a larger sample size, and integrating the CDSS within the Picture Archiving and Communication System (PACS) of the hospital are a few recommendations provided by [
16].
Study [
17] developed a Fast Spectroscopic Multiple Analysis (
FASMA) system based on various combinations of multiparametric MRI data for brain tumour classification. This CDSS was designed with a SVM classifier and integrated data from 3T
1H-MRS, DWI, DTI and PWI, for characterisation of brain tumours. Highest accuracy in classification of tumours was obtained when all the above-mentioned MR parameters are considered. It was also seen that k-NN and LDA had inferior classification accuracies as compared to SVM classifier. SVM produced an accuracy score of >90.00% in intra-tumoral area and >80.00% in peri-tumoral area. FASMA provides additional information regarding tumour characteristics and can be used as an assistive tool for tumour diagnosis and grading.
Paper [
18] designed a Modular Medical Image Region of Interest Analysis Tool and Repository (
MIROR) for childhood cancer diagnosis. The study was conducted on a cohort of 48 children. The CDSS used advanced MRI data to differentiate between benign and malignant tumours. 10-fold cross-validation was performed to compare SVM and k-NN classifiers. When utilizing all extracted features, SVM based classification model achieved an accuracy of 89% while k-NN based model achieved an accuracy of 93%. The repository also aims to increase children’s brain tumour dataset and add medical information from previous cases to assist clinicians in decision making.
The
HealthAgents project, funded by the European Union, included studies [
19], [
20], and [
21]. The HealthAgents network is a globally distributed repository of information and knowledge regarding brain tumour diagnosis and prognosis [
20]. An interactive user interface of HealthAgents to facilitate classification of children’s brain tumours was designed by [
19]. The study was conducted on a cohort of 33 children with cerebellar tumours. MR spectral data was used to provide diagnostic information on brain tumours. For a three-class classifier, principal component analysis followed by LDA achieved a classification accuracy of 91.00%. A leave-one-out analysis for two-class classifier achieved a classification accuracy of 94.00%. Through these techniques, clinicians are provided with flexibility to use MRS data for childhood brain tumour diagnosis. The first release of the HealthAgents DSS was presented in study [
20]. It was based on a sample size of 182 with feature extraction performed by LDA, SVM and LSVM. STE and LTE models combined achieved >90.00% classification accuracy and had significant improvement over using models based on STE or LTE separately. The study concluded that in vivo MRS data when combined with ex vivo/ in vitro High-Resolution Magic Angle Spinning Nuclear Magnetic Resonance (HR-MAS) and gene expression, has the potential to improve brain tumour classification and produce novel prognostic biomarkers [
20]. A study conducted by [
21] developed an independent automatic classification framework as a part of the pattern recognition technique development of the HealthAgents project. This study also suggested including HR-MAS or gene expression data such as DNA microarrays could improve the diagnostic capability of the proposed framework.
International Network for Pattern Recognition of Tumours Using Magnetic Resonance (
INTERPRET) DSS was evaluated by [
22]. A multi-centre European collaboration from 2000 to 2002 called the INTERPRET project developed a DSS to assist neuroradiologist who had no prior experience of using MRS data to diagnose and grade brain tumours. It was seen that STE classifier performed better than LTE with a classification accuracy of 89.00%. [
23] evaluated the second version of INTERPRET DSS. This study confirmed the added value of using
1H MRS data for brain tumour characterisation. Version 2.0 is integrated with an additional long-TE classifier as opposed to only short-TE in version 1.0. To use Version 2.0 expert knowledge was not required in spectroscopy or any specific protocol. [
24] evaluated the third version of INTERPRET DSS. It had a larger embedded database, and improved diagnostic differentiation capabilities. Three LDA-based classifiers— short, long, and concatenated short+long TE differentiated between common types of tumours. The combined LTE and STE classifier achieved the highest accuracy with 89.20%. The CDSS also successfully differentiated between tumour and pseudo-tumoral disease. The combined LTE and STE achieved a classification accuracy of 92.10%. A study by [
25] evaluated the INTERPRET prototype DSS to classify brain tumours of 334 patients based on in vivo
1H single-voxel spectral data of different types of brain tumours. The study concluded that using MRS data for brain tumour diagnosis over MRI data alone showed significant improvement in diagnosis. The combined use of LTE and STE also improved accuracy of classification. The LDA-based classifier integrated within this prototype DSS successfully differentiated between three tumour groups— meningiomas, LGGs and HGGs.
3.3.2. Prognostic support systems
Study [
9] was the only work identified through this systematic review that designed a CDSS which predicts tumour diameters under Temozolomide (TMZ) chemotherapy and provides a prognosis on when to stop treatment. The study was conducted with a sample size of 42 patients with Diffuse Low-Grade Gliomas (DLGG) and was based on two mathematical models—linear and exponential. The input variables were tumour diameters and the time of acquisition of the MRI scan since the starting of the treatment. The linear model with an average accuracy of 89.00% prevailed. However, the limited number of available DLGG cases did not allow model validation on a separate dataset. Hence, increasing the size of the dataset and additionally including molecular factors that affect tumour growth are recommended.
4. Discussion
It should be emphasized that a CDSS does not completely substitute a clinician’s diagnostic decision, rather assists clinicians in dealing with a large amount of complex medical imaging data in a shorter time span. A well-designed CDSS not only improves diagnostic capabilities, but also should be easily implemented within routine clinical practice, optimizing care delivery and decision making [
26].
The various types of CDSSs identified through this review for brain tumour diagnosis and prognosis were Curiam BT [
16], FASMA [
17], MIROR [
18], HealthAgents [
19,
20,
21] and INTERPRET [
22,
23,
24,
25]. There have been some significant achievements during the development of such systems worth mentioning. While designing and developing INTERPRET, a vast repository of brain tumours was created containing 304 histopathological STE low-grade gliomas, meningiomas, and high-grade malignant tumours. Another achievement of this project was to define a data acquisition protocol to standardize data collection from different centers. MIROR helps in providing the latest techniques and findings in the diagnosis of brain tumours improving the skillset of the clinicians.
A major area of concern is not just designing the CDSS software but also implementation and acceptance of use by clinicians in routine clinical practice. Acceptance of use will only be possible if the clinicians view CDSS both as a tool and a process. Clinicians are also more likely to use the CDSS if their own decision-making matches with the system’s [
26]. The systems often lack transparency regarding how the output was achieved can be another reason why there is lack of user acceptance [
27].
Considering CDSSs as prognostic support systems, more research is needed due to a limited number of articles in this review to show the overall capability of such systems. More focus has been given on designing diagnostic support systems as opposed to prognostic support systems, which leaves the latter to be explored further.
The strengths of this systematic review are the use of PRISMA protocol in reporting of studies, the use of Keshav’s 5Cs method in assessing quality of included studies, and the level of details extracted from the included studies. Not bound by a timeframe to include all relevant studies and having the search strategy highly specific on CDSS used in brain tumour diagnosis and prognosis based on medical imaging data are other strengths of this review. However, a limitation should be mentioned. This review only considered studies published in English language; there could be others CDSSs being developed that are published in different languages.
There is scope for future work that can be recommended, based on this systematic review. Presence of a global standard protocol may increase CDSSs translation into routine clinical practice. Additionally, including clinicians’ feedback, user needs and expectations while designing the CDSS may improve its acceptance at the point of care. Finally, if there is a stage where the CDSS reveals their decision-making process, it will allow clinicians to increase their engagement with the systems and accelerate CDSS adoption. This type of a multi-task CDSS can be fully embedded within a clinicians’ regular workflow. This will give rise to a more trainable system that is capable of accepting feedback, revise recommendations and provide alternative clinical decisions for improve healthcare delivery.
5. Conclusions
Management and treatment of brain tumours require an early and accurate diagnosis, while prognostic understanding can also be beneficial in the choice of care planning for the patient. Advances in neuro-oncology imaging techniques have improved both detection and treatment planning of these tumours. Leveraging advanced imaging technologies, vastly available medical knowledge, and patient-specific information, a CDSS provides evidence-based recommendations to assist clinicians at the point of care. It reduces medical errors, enhances diagnostic capabilities, and has the potential to improve healthcare delivery. Presence of a global standard or guideline specific to CDSSs on brain tumour diagnosis and prognosis is recommended. Increased effort must be taken not only in developing such CDSSs but also implementing them into routine clinical practice to increase clinicians’ engagement and CDSS adoption. To be able to do so, highlighting a few of areas of improvement are necessary. Although a CDSS improves diagnostic capabilities, and healthcare delivery, there is a lack of specific evidence or studies to support these claims. The absence of empirical data slows down both user acceptance and evaluation of actual impact of CDSS on brain tumour management. Instead of emphasizing on the advantages of implementing CDSS, highlighting its potential drawbacks or limitations could improve decision-making. Embedding CDSS into routine clinical practice may increase complexity, requiring additional funding and investment in latest technology, infrastructure, and training of healthcare professionals. With each patient condition being unique, a well-tailored patient-specific recommendation is needed which is a drawback of current CDSS as it lacks customization. Ethical and legal considerations such as patient privacy and safety, consent, and liability should be given importance alongside the technical aspects. Overall, to design and implement a CDSS, apart from highlighting potential benefits, providing empirical evidence, addressing drawbacks and challenges, and considering ethical and legal implications will increase CDSS usability and acceptance.
Author Contributions
Conceptualization, T.M. (Teesta Mukherjee), O.P. (Omid Pournik) and T.N.A. (Theodoros N. Arvanitis); methodology, T.M. and O.P.; validation, T.M., O.P. and T.N.A.; data curation, T.M. and O.P.; writing—original draft preparation, T.M., S.N.L.C.K. (Sarah N. Lim Choi Keung); writing—review and editing, O.P., S.N.L.C.K.; visualization, S.N.L.C.K. and T.N.A.; supervision, T.N.A.; project administration, T.N.A.; funding acquisition, T.N.A. All authors have read and agreed to the published version of the manuscript.
Funding
Teesta Mukherjee receives funding through a PhD Scholarship at the Department of Electronic, Electrical and Systems Engineering, School of Engineering, College of Engineering and Physical Sciences, University of Birmingham, Edgbaston, Birmingham B15 2TT, United Kingdom.
Acknowledgments
The authors would like to thank the Department of Electronic, Electrical and Systems Engineering, School of Engineering, College of Engineering and Physical Sciences, University of Birmingham, Edgbaston, Birmingham B15 2TT, United Kingdom for Teesta Mukherjee’s PhD.
Conflicts of Interest
This is to declare that all authors have no conflict of interest, and all funding details have been provided in this manuscript.
Abbreviations
The following abbreviations have been used in this review.
| BSVM |
Boosting Support Vector Machine |
| CDSS |
Clinical Decision Support System |
| CT |
Computed Tomography |
| DLGG |
Diffuse Low-Grade Glioma |
| DSS |
Decision Support System |
| FASMA |
Fast Spectroscopic Multiple Analysis |
| GLCM |
Gray-Level Co-Occurrence Matrix |
| HGG |
High-Grade Glioma |
| HR-MAS |
High-Resolution Magic Angle Spinning Nuclear Magnetic Resonance |
| INTERPRET |
International Network for Pattern Recognition of Tumours Using MR |
| k-NN |
K-Nearest Neighbors Algorithm |
| LDA |
Linear Discriminant Analysis |
| LGG |
Low-Grade Glioma |
| LTE |
Long Echo Time |
| MeSH |
Medical Subject Headings |
| MIROR |
Modular Medical Image Region of Interest Analysis Tool and Repository |
| MRI |
Magnetic Resonance Imaging |
| MR |
Magnetic Resonance |
| MRS |
Magnetic Resonance Spectroscopy |
| NB |
Naïve Bayes |
| NNW |
Neural Network |
| PACS |
Picture Archiving and Communication System |
| PET |
Positron Emission Tomography |
| PRISMA |
Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| QDA |
Quadratic Discriminant Analysis |
| STE |
Short Echo Time |
| SVM |
Support Vector Machine |
| TMZ |
Temozolomide |
| WHO |
World Health Organization |
References
- Khan, M.A.; Khan, A.; Alhaisoni, M.; Alqahtani, A.; Alsubai, S.; Alharbi, M.; Malik, N.A.; Damaševičius, R. Multimodal brain tumor detection and classification using deep saliency map and improved dragonfly optimization algorithm. International Journal of Imaging Systems and Technology 2022, 33, 572–587. [Google Scholar] [CrossRef]
- Özkaraca, O.; Bağrıaçık, O.İ.; Gürüler, H.; Khan, F.; Hussain, J.; Khan, J.; Laila, U.E. Multiple brain tumor classification with dense CNN architecture using brain MRI images. Life 2023, 13, 349. [Google Scholar] [CrossRef] [PubMed]
- Louis, D.N.; Perry, A.; Wesseling, P.; Brat, D.J.; Cree, I.A.; Figarella-Branger, D.; Hawkins, C.; Ng, H.K.; Pfister, S.M.; Reifenberger, G.; et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A summary. Neuro Oncol 2021, 23, 1231–1251. [Google Scholar] [CrossRef]
- Kang, J.; Ullah, Z.; Gwak, J. MRI-based brain tumor classification using ensemble of deep features and Machine Learning Classifiers. Sensors 2021, 21, 2222. [Google Scholar] [CrossRef] [PubMed]
- Sutton, R.T.; Pincock, D.; Baumgart, D.C.; Sadowski, D.C.; Fedorak, R.N.; Kroeker, K.I. An overview of clinical decision support systems: Benefits, risks, and strategies for Success. Npj Digital Medicine 2020, 3, 17. [Google Scholar] [CrossRef] [PubMed]
- Tsolaki, E; Kousi, E; Svolos, P; Kapsalaki, E; Theodorou, K; Kappas, C; Tsougos, I. Clinical decision support systems for brain tumor characterization using advanced magnetic resonance imaging techniques. World Journal of Radiology 2014, 6, 72–81. [Google Scholar] [CrossRef]
- Page M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews BMJ 2021, 372. [CrossRef]
- Keshav, S. How to read a paper. ACM SIGCOMM Computer Communication Review 2007, 37, 83–84. [Google Scholar] [CrossRef]
- Abdallah, M.B.; Blonski, M.; Wantz-Mezieres, S.; Gaudeau, Y.; Taillandier, L.; Moureaux, J.M.; Darlix, A.; deChampfleur, N.M.; Duffau, H. Data-driven predictive models of diffuse low-grade gliomas under chemotherapy. IEEE Journal of Biomedical and Health Informatics 2019, 23, 38–46. [Google Scholar] [CrossRef]
- Arasi, P.R.; Suganthi, M. A clinical support system for brain tumor classification using soft computing techniques. Journal of Medical Systems 2019, 43, 1–11. [Google Scholar] [CrossRef]
- Gupta, N.; Bhatele, P.; Khanna, P. Identification of gliomas from brain MRI through adaptive segmentation and run length of centralized patterns. Journal of Computational Science 2017, 25, 213–220. [Google Scholar] [CrossRef]
- Mehmood, I.; Sajjad, M.; Muhammad, K.; Shah, S.I.; Sangaiah, A.K.; Shoaib, M.; Baik, S.W. An efficient computerized decision support system for the analysis and 3D visualization of brain tumor. Multimedia Tools and Applications 2019, 78, 12723–12748. [Google Scholar] [CrossRef]
- Yang, G.; Jones, T.L.; Barrick, T.R.; Howe, F.A. Discrimination between glioblastoma multiforme and solitary metastasis using morphological features derived from the p:q tensor decomposition of diffusion tensor imaging. NMR in Biomedicine 2014, 27, 1103–1111. [Google Scholar] [CrossRef]
- Minguillón, J.; Tate, A.R.; Arús, C.; Griffiths, J.R. Classifier combination for in vivo magnetic resonance spectra of brain tumours. Multiple Classifier Systems: Third International Workshop, MCS 2002, 3, 282–292. [Google Scholar] [CrossRef]
- Underwood, J. Tate, A.R.; Luckin, R.; Majós, C.; Capdevila, A.; Howe, F.; Griffiths, J.; Anús, C. A prototype decision support system for MR spectroscopy-assisted diagnosis of brain tumours. MEDINFO, IOS Press, 2001; pp. 561–565. [CrossRef]
- Sáez, C.; Martí-Bonmatí, L.; Alberich-Bayarri, Á.; Robles, M.; García-Gómez, J.M. Randomized pilot study and qualitative evaluation of a clinical decision support system for brain tumour diagnosis based on sv 1H MRS: Evaluation as an additional information procedure for novice radiologists. Computers in Biology and Medicine 2014, 45, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Tsolaki, E. Svolos, P.; Kousi, E.; Kapsalaki, E.; Fezoulidis, I.; Fountas, K.; Theodorou, K.; Kappas, C.; Tsougos, I. Fast Spectroscopic Multiple Analysis (FASMA) for Brain Tumor Classification: A clinical decision support system utilizing multi-parametric 3T mr data. International Journal of Computer Assisted Radiology and Surgery 2015, 10, 1149–1166. [CrossRef]
- Zarinabad, N.; Meeus, E.M.; Manias, K.; Foster, K.; Peet, A. Automated Modular Magnetic Resonance Imaging Clinical Decision Support System (MIROR): An application in pediatric cancer diagnosis (preprint). JMIR Medical Informatics 2018, 6, e30. [Google Scholar] [CrossRef] [PubMed]
- Gibb, A.; Easton, J.; Davies, N.; Sun, Y.; MacPherson, L.; Natarajan, K.; Arvanitis, T.; Peet, A. The development of a graphical user interface, functional elements and classifiers for the non-invasive characterization of childhood brain tumours using magnetic resonance spectroscopy. The Knowledge Engineering Review 2011, 26, 353–363. [Google Scholar] [CrossRef]
- González-Vélez, H.; Mier, M.; Julià-Sapé, M.; Arvanitis, T.N.; García-Gómez, J.M.; Robles, M.; Lewis, P.H.; Dasmahapatra, S.; Dupplaw, D.; Peet, A.; et al. HealthAgents: Distributed multi-agent brain tumor diagnosis and prognosis. Applied Intelligence 2009, 30, 191–202. [Google Scholar] [CrossRef]
- Sáez, C.; García-Gómez, J.M.; Vicente, J.; Tortajada, S.; Luts, J.; Dupplaw, D.; Van Huffel, S.; Robles, M. A generic and Extensible Automatic Classification Framework applied to brain tumour diagnosis in HealthAgents. The Knowledge Engineering Review 2011, 26, 283–301. [Google Scholar] [CrossRef]
- Julià-Sapé, M.; Griffiths, J.R.; Tate, R.A.; Howe, F.A.; Acosta, D.; Postma, G.; Underwood, J.; Majós, C.; Arús, C. Classification of brain tumours from mr spectra: The interpret collaboration and its outcomes. NMR in Biomedicine 2015, 29, 371. [Google Scholar] [CrossRef]
- Julià-Sapé, M.; Majós, C.; Camins, À.; Samitier, A.; Baquero, M.; Serrallonga, M.; Doménech, S.; Grivé, E.; Howe, F.A.; Opstad, K.; et al. Multicentre evaluation of the interpret decision support system 2.0 for Brain tumour classification. NMR in Biomedicine 2014, 27, 1009–1018. [Google Scholar] [CrossRef]
- Pérez-Ruiz, A.; Julià-Sapé, M.; Mercadal, G.; Olier, I.; Majós, C.; Arús, C. The interpret decision-support system version 3.0 for evaluation of Magnetic Resonance Spectroscopy data from human brain tumours and other abnormal brain masses. BMC Bioinformatics 2010, 11. [Google Scholar] [CrossRef]
- Tate, A.R.; Underwood, J.; Acosta, D.M.; Julià-Sapé, M.; Majós, C.; Moreno-Torres, À.; Howe, F.A.; van der Graaf, M.; Lefournier, V.; Murphy, M.M.; et al. Development of a decision support system for diagnosis and grading of brain tumours using in vivo magnetic resonance single voxel spectra. NMR in Biomedicine 2006, 19, 411–434. [Google Scholar] [CrossRef] [PubMed]
- Khairat, S.; Marc, D.; Crosby, W.; Al Sanousi, A. Reasons for physicians not adopting clinical decision support systems: Critical Analysis (preprint). JMIR Med Inform 2018, 6, e24. [Google Scholar] [CrossRef] [PubMed]
- Belard, A.; Buchman, T.; Forsberg, J.; Potter, B.K.; Dente, C.J.; Kirk, A.; Elster, E. Precision diagnosis: A view of the clinical decision support systems (CDSS) landscape through the lens of critical care. Journal of Clinical Monitoring and Computing 2017, 31, 261–271. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).