1. Introduction
Carcinoma or cancer of the rectum is a malignant tumour that accounts for 30-35% of all cancers of the large intestine, which in turn account for 10% of all cancers. Every year in Italy 12,000 people are diagnosed with rectal cancer and it is estimated that 40 out of every 100,000 inhabitants are affected, while 7,000 people die from the disease every year deaths related to the disease. The 80% of patients with CRC has a disease amenable to curative surgery (R0). Unfortunately, about 40% of these patients will develop a relapse, mainly within the first three years. Local recurrence (L.R.) is the reproduction of the tumour lesion at the site of previous surgery.
Local recurrence after curative surgery of rectum cancer remains a clinical challenge today that requires a multidisciplinary approach and careful selection of patients suitable for surgery.
The pelvis is the most frequent site of recurrence of local disease. Thanks to the introduction of total mesorectal excision (TME) and the integration of both adjuvant and, above all, neoadjuvant therapies, the incidence of pelvic recurrences in recent population studies reveals a decrease from 20-30% to 6-10%.
In 90% of cases where they occur, they appear during the first 5 years after primary surgical treatment, almost 70% in the first 2 years and 85% in the first 3 years [
1].
Surgical intervention R0, which is possible in more than 50% of these cases, is the only potentially curative treatment, with an estimated average 5-year survival rate of 30%. These operations are, sometimes, demolishing, expensive and burdened by a well-defined morbidity rate though the results are encouraging, even though only a relatively small number of patients can benefit from them.
The aim of this study is to frame the diagnostic-therapeutic problem of pelvic recurrence of rectum cancer, highlighting the current surgical standards and the possible role of the minimally invasive approach. It is necessary to identify an approach to the treatment of recurrences that may be shared. Although the method of presentation cannot be identical in the different patients, it is necessary to identify whether the approach using the laparoscopic technique, the robotic technique, the open-sky technique and/or chemotherapy or radiotherapy is better and when and how these resources can be harmonised. This retrospective examination of our surgical case study wishes to enter the debate in order to propose a possible line of action to adopt according to the site of the recurrence.
PELVIC RECURRENCE
1.1. Risk factors
Several factors have been related to an increased risk of local recurrence, both biological -those related to the neoplasm- and technical.
Incomplete removal of the meso rectum remains one of the most important risk factors. Quirke et al [
23] reported a very high recurrence rate (85%) in patients presenting with a circumferential margin infiltrated by the tumour. Conversely, TME is associated with a low recurrence incidence rate, as demonstrated by Heald's work (3.5% local recurrence) [
2]. A histopathological distance of less than 2 mm between the tumour and the circumferential resection margin is associated with a higher risk of recurrence (a 37% vs. a 6% risk) [
3]. The circumferential resection margin corresponds, in the case of TME, to the mesorectal band. A distance between the tumour and the menstrual band greater than/ equal to 6 mm, measured in the MRI images, correlates to a circumferential margin not involved in the histopathological examination, with a confidence interval of 97% [
4]. This shows that MRI is the most accurate pre-operative diagnostic method for predicting infiltration of the circumferential resection margin.
The stage of the neoplasm is another factor which affects the onset of recurrence, as well as survival. Melton, in a review of 51 published articles, analysing the follow-up of 10,465 patients, reported a frequency of recurrence in Dukes stages A, B and C by 8.5%, 16.3% and 28.6% respectively. [
5]. Lan et al. wrote of early recurrence rates (< 3 years) in patients with stage I and II T4 and stage III N2 rectum cancer [
6,
7].
Distal rectum cancers are most frequently associated with local recurrence. Already in 1963, Morson had observed that the local recurrence rate for cancers of the lower third of the rectum was 14.5%, higher than those of the middle third (8.3%) and upper third (5.2%) [
7].
A certain correlation between the incidence of local recurrence and the histotype of the primary tumour has been demonstrated. Several authors, including Wolfman and Umpleby [
8], observed a higher incidence of local recurrence for mucous adenocarcinomas.
There are conflicting data in the literature concerning the influence of the type of intervention on the risk of recurrence. Laparoscopic and robotic access is feasible and safe approaches and, compared to traditional surgery, show no differences in terms of disease-free survival [
9].
Anastomotic integrity seems to be another factor influencing the risk of local recurrence. Akyol et al. [
10] observed that the local recurrence rate is higher in patients with anastomosis dehiscence than in patients without this postoperative complication (46.9% versus 18.5%).
1.2. Classification
There is currently no agreement in international literature when it comes to a uniform classification of pelvic recurrence. The main classifications currently used are those of the Mayo Clinic and Memorial Sloan Kettering in the United States, of Yamada et al. in Japan, and of the Royal Marsden in Europe. The classification provided by the Mayo Clinic [
11] is based on the site of fixation (F1 to F3) and on the symptomatology (asymptomatic, symptomatic without pain and symptomatic with pain); that of Yamada [
12] on the degree and zone of fixation: localised, sacral or lateral and also defines the degree of survival. The Memorial Sloan Kettering group [
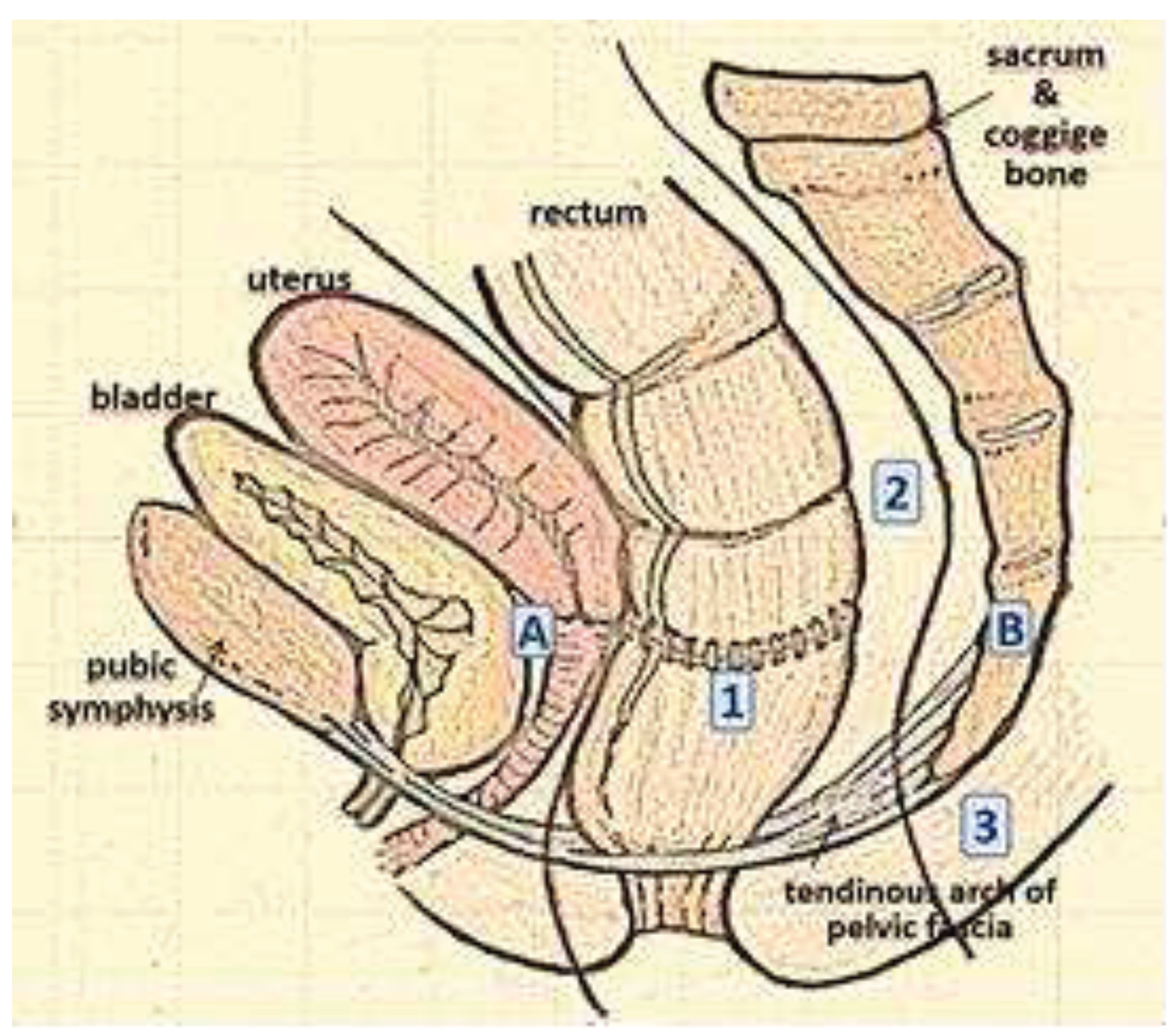
11] classified recurrence according to the zone of invasion (axial, anterior, and lateral or posterior) (
Figure 1).
Recently, the Royal Marsden Hospital provided a classification [
13] based on the degree of invasion of one or more of the seven pelvic compartments described in the pre-operative MRI, which correspond to the fascial boundaries and dissection plans, establishing a relationship between the site of recurrence and the possibility of recurrence of disease after resection. The seven compartments are: the central (C), posterior (P), inferior (I), anterior superior (AA) and inferior (AB), lateral (L) and peritoneal reflection (PR) zones. The classification of recurrence according to its location is of practical value, as it determines the type of surgical approach and eradication of the surrounding structures to be carried out (
Table 1).
In general, all classifications agree that central or axial recurrences are more easily resectable than posterior, lateral or anterior-reaching recurrences, which often require greater multi-organ resection.
1.3. Clinical presentation
The literature on this issue shows that 50% of patients complain of symptoms at diagnosis, while those of the remaining 50% are discovered during follow-up [
14].
Symptomatology is partly related to the type of intervention previously performed. After abdominal-perineal amputation, recurrence can be detected as a persistent mass or sinus in the perineum and/or vagina [
15]. After rectal-colic resection, the infiltration of the intestinal lumen causes changes in the alvo and rectorrhagia. However, some symptoms are constantly present, in particular sacral and/or perineal pain, the presence of which, at the time of diagnosis, has been linked to a worse-scenario prognosis.
1.4. Pre-operative diagnosis and summing up
The gold standard in the diagnosis of recurrence is biopsy of the tissue under suspicion. However, it is not always easy to obtain, except in the case of an intraluminal anastomotic recurrence which is generally accessible by endoscopy [
15]. When it is impossible to perform a biopsy, a clinical diagnosis conducted by a multidisciplinary team, should guide the therapeutic approach. The clinical evaluation should always include a digital exploration, which helps to discriminate between intra- or extra-luminous recurrence and its degree of fixity.
In selected cases, cystoscopy and colposcopy may be useful when seeking to assess invasion of adjacent organs. Endoscopic ultrasound is burdened with false positives due to its difficulty to discern between normal and neoplastic lymph nodes. Plasma levels of CEA can be useful and, if elevated, are associated with worse-scenario prognoses.
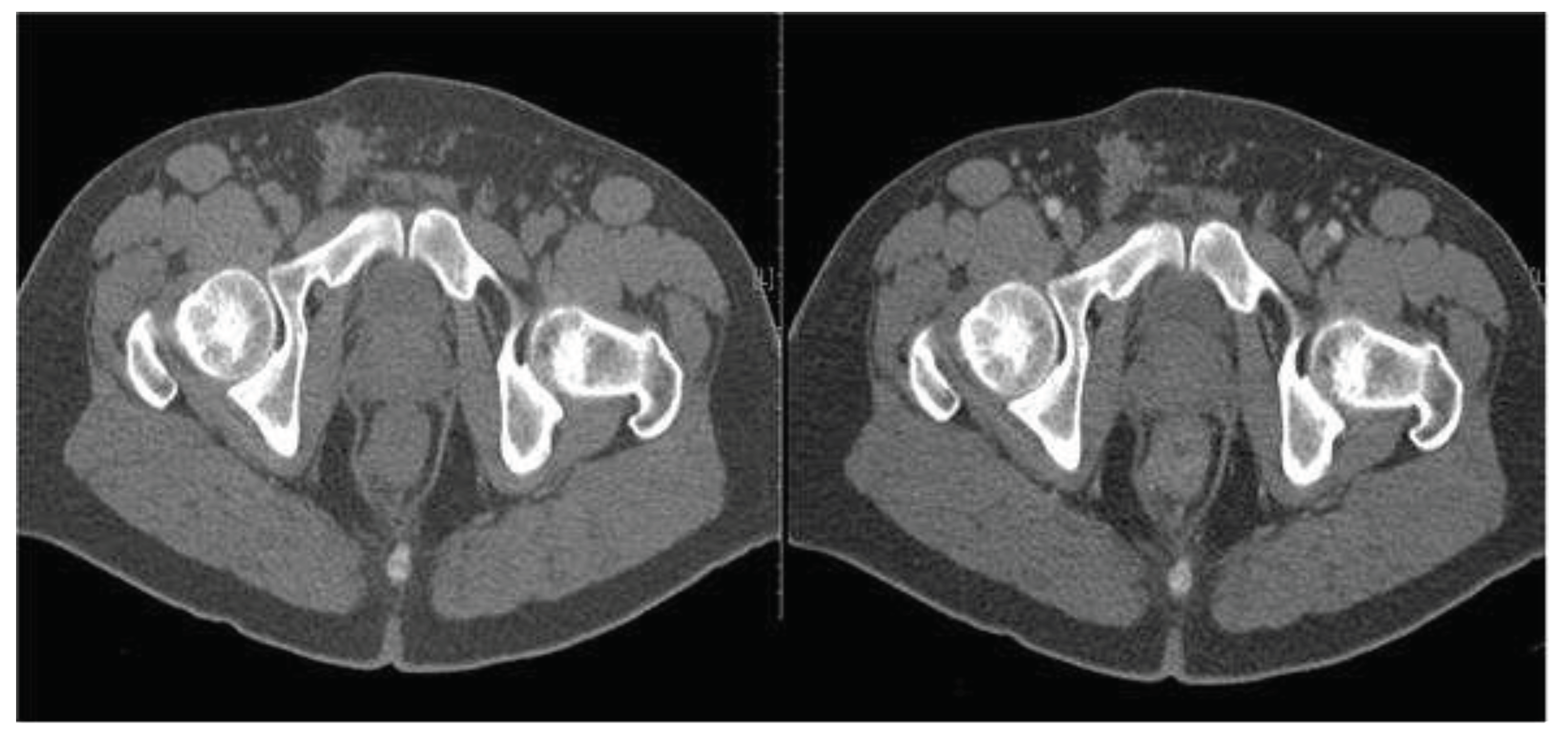
CT and MRI are two valid diagnostic and staging methods for pelvic recurrence. MRI of the pelvis with contrast medium, compared to CT, allows for a better differentiation of normal anatomical structures, such as the uterus or seminal vesicles, as well as differential diagnosis between fibrosis and recurrence (
Figure 3).
Figure 3.
TC examination before (A) and after the introduction of contrast medium by i.v. (B) (SE 45-year-old male). Recurrence of a neoformation in the distal rectum (originally a pT2bNx) shows the presence of a concentric thickening of the wall, more evident in the posterior and left lateral maximum thickness of 19 mm. This localised thickening at about 2.5 cm from the anal margin extends cranially for a stretch of about 5 cm and takes the contrast tenuously.
Figure 3.
TC examination before (A) and after the introduction of contrast medium by i.v. (B) (SE 45-year-old male). Recurrence of a neoformation in the distal rectum (originally a pT2bNx) shows the presence of a concentric thickening of the wall, more evident in the posterior and left lateral maximum thickness of 19 mm. This localised thickening at about 2.5 cm from the anal margin extends cranially for a stretch of about 5 cm and takes the contrast tenuously.
PET-TC-compared to CT and MRI, which are morphological tests - permits a functional evaluation. It allows one to detect occult lesions and differentiate between scar tissue and suspected neoplastic recurrence.
1.5. Surgical treatment
The treatment of recurrence of rectal carcinoma is multimodal. The location and extent of the recurrence, together with an evaluation of previous treatment, should be considered when seeking guidance for the choice of the appropriate treatment strategy to adopt.
In many of the studies in literature, about 40-50% of patients with local recurrence are considered candidates for surgical exploration, though only 30-40% undergo R0 resection [
14].
Whatever the type of resection, it should always be performed by removing the recurrence and the affected adjacent organs or structures en-bloc. Careful patient selection is essential in order to exclude formal contraindications to surgery (
Table 2).
The patient and his or her family should be adequately informed about the extent, sometimes largely demolishing, of the intervention and the related complications.
Surgical approaches
During the pre-TME era, recurrence often took place in the residual mesorectum or in anastomotic sites. On the contrary, today, relapses can be found in any pelvic compartment, often requiring extensive surgery.
The access route is always a median navel-pubic laparotomy, preserving the lower epigastric vessels in case a rectus abdominis muscle flap is needed. After lysis adhesion, the abdominal cavity is explored to exclude secondarism undetected during preoperative imaging. There is often a marked pelvic fibrosis and, therefore, the first step involves identifying and isolating the ureters and iliac vessels at the level of the aortic bifurcation: these manoeuvres permit one to continue the pelvic dissection safely along the presacral fascia. Several intraoperative scenarios are possible [
17].
- (A)
Non-fixed recurrence located at the anastomotic site or in the central compartment of the pelvis or in the perineal wound. In these cases, radical resection can be achieved by "limited" surgery: extensive local excision or rescue AAP or, if feasible, rectocolic resection.
- (B)
Anterior pelvic recurrence. If the genitourinary tract is involved, an en-bloc resection is recommended. A limited involvement of structures, such as the bladder dome or the posterior wall of the vagina, can be managed by partial excision with negative margins. Conversely, relapses with invasion of the bladder trigon or prostate in males and the uterine cervix in females usually require total pelvic exenteration. The mortality rate of this operation is still high (2-14%), as is the morbidity rate (33-75%) [
19].
- (C)
Lateral pelvic recurrence. These relapses have a worse prognosis because it is extremely difficult to obtain tumour-free resection margins (< 19%) [
15]; sometimes these are impossible due to the involvement of larger vessels, the sciatic nerve, or pelvic bones.
- (D)
Dorsal recurrence. When the recurrence is localised in the dorsal compartment, an abdominal sacral resection can be performed. This operation is facilitated by a position of the patient in the ventral decubitus or "jack-knife" position Although numerous studies in literature demonstrate the feasibility and safety of sacral resections below the S2/S3 junction, the risk/benefit ratio of such interventions is still under discussion, since they expose the patient to a high risk of neurological lesions (high sacrectomies) and uncontrollable venous bleeding (
Table 3).
Moreover, it is not always easy to distinguish intraoperative bone infiltration from fibrous adhesions, as made evident by a recent series of 29 cases of recurrence reported by the Memorial-Sloan Kettering, where only 38% had real bone infiltration, while 68% regarded adhesions.
- (E)
Extended recurrences. The indication for pelvic exenteration combined with secretory surgery is a dependent institution. To date, the highest number of cases of exenteration with sacrectomy by institution is that of Solomon and Milne of the Royal Prince Alfred Hospital in Sydney, with 100 patients operated, with an R0 resection rate of 72%, with an average survival-rate of 45 months and 5 years for 38%, complications in 74%, in particular neurological complications (39%) in high sacrectomies [
19,
20].
1.6. The role of the minimally invasive approach
Laparoscopic surgery has become an accepted method of treatment for primary colorectal cancer. The growing experience of dedicated surgeons and the progressive improvement of technology have made it possible to extend the indications for laparoscopic surgery to patients with locally advanced rectal cancer.
There are very few follow-up studies in literature regarding the safety and feasibility of laparoscopic surgery for the treatment of locoregional pelvic recurrence (
Table 4) [
21,
22].
Only three studies proposed the laparoscopic approach for the treatment of recurrent colorectal cancer for a total of 29 patients.
The study by Park et a. [
23] compared 15 patients treated with laparoscopic access to 26 patients with open access. of the cases regarded anastomotic recurrences, ovarian and lateral pelvic lymph-node recurrences. The rate of resection in laparoscopy was 100% vs 84.6% of the open approach, with a lower rate of morbidity (13.3% vs 57.7%). The largest study is that by Nagasaki et a126 where 13 patients treated using the laparoscopic approach and 17 patients open intervention were compared. The laparoscopic approach was associated with a longer duration of surgery time (381' vs 241') but with an equivalent rate of RO resection (100% vs 94%) and postoperative morbidity (30.8% vs 23.5%), with only one case of conversion. These were central relapses or ones at the level of the lateral pelvic lymph nodes.
For the last five years or so, robotic surgery has been used more frequently, so that, as it has a greater power of resolution as an image and operational precision, it will replace both laparoscopic and open surgery. There are conditions to hope for greater survival.
The most recent series reported by Akiyoshi et a1. [
26] described 9 patients who underwent laparoscopic dissection of the lateral pelvic lymph nodes at the site of isolated recurrence after surgery for rectum cancer. R0 resection was possible in all patients, with acceptable morbidity rate (33%) [
27].
The authors of these studies comment that the minimally invasive approach has the advantage of magnified visibility, which facilitates and makes a more accurate dissection in a deep anatomical compartment such as the pelvis, with altered anatomical planes, possible. However, the operating times are longer and the experience required is greater. In addition, the laparoscopic approach seems to reduce blood loss, allow for early restoration of the intestinal function, and, oncologically speaking, can have equivalent rates of R0 resections [
23,
25].
Ultimately, this approach should not be used for extended multi-visceral resections but limited to salvaging treatment of isolated central recurrences or of those closer to the lateral pelvic lymph nodes.
1.7. Oncological results
In the absence of surgical treatment, an overall 5-year survival is 5% lower, with an approximate median survival of 7 months. An R0 resection is the main prognostic factor influencing survival. In a recent systematic review by Tanis et a1. [
28] which analysed 46 studies regarding the surgical treatment of pelvic recurrence from rectum cancer, the overall 5-year survival ranged from 11 to 39%, with an operating mortality of 2.2%. Harris et al report a case study of 533 patients from 5 high-volume oncology centres treated with pelvic exenteration, currently the most extensive and recent international experience. In their case history, 5-year survival after R0 resection is 44% and after RI resection 25% [
30].
1.8. The role of radiotherapy
If not previously performed, neoadjuvant radiotherapy may increase the number of patients eligible for radical surgery.
The combination of high-dose RT (50-60Gy) combined with chemotherapy has been shown to convert resectability by up to 80% of patients initially not eligible for surgical exploration [
3].
2. Materials and Methods
We examined, retrospectively, all the patients diagnosed with cancer of the rectum between 2008 and 2018 treated at the "Pietro Valdoni" Department of Surgery and monitored their follow-up for 5 years. The sample consists of 368 patients with rectal neoplasm, 136 females and 232 males, with an average age of 65.8 (the range was 37-86 years). In 103 of these cases the neoplasm was located in the upper rectum (28%), in 119 cases in the middle rectum (32.3%), in 102 cases in the lower rectum (27.7%), in 31 cases (8.4%) at the level of the right/sigma junction while, in 13 cases, it was not possible to identify the site with certainty (3.5%).
Curative surgery was possible in 288 of these patients (78.26%). Neoadjuvant chemotherapy was administered to 75 patients (20.4%). Of the 288 patients treated for rectal cancer, 31 (11%) presented with local recurrence. For the sake of completeness, 11 patients operated on in another hospital for rectal cancer were also evaluated, thus making the total 42 patients, including 26 males and 16 females, with an average age of 65.8 and an age range of 37 to 86.
For the purposes of our study 35 out of the 42 patients who were free from distant metastases at the time of their first surgical operation were examined (
Table 5).
As to the characteristics of the primary tumour, in 9 cases it was T4 (25.7%), in 18 cases T3 (51.4%), in 7 T2 (20%) and in one case Tl (2.8%). Surgery was conducted in all cases using the open technique: 21 patients underwent anterior resection (60%), 9 patients with perineal abdominal amputation according to Miles’s technique (25.7%), 4 patients using Hartmann's surgical approach (11.4%) and 1 patient with TEM (2.8%). A R0 resection was possible in 31 of the cases (88.5%), Rl-R2 in 4 (11.5%). In of the 6 patients (17.1%) neoadjuvant chemoradiation therapy was performed while 20 patients (57.1%) underwent adjuvant chemoradiation therapy.
With regard to the site of the recurrence, 13 patients developed an anastomotic recurrence (37.1%), 12 central-pelvic (31.4%), 9 presacral (25.7%), and 2 perineal (5.7%). The most frequently infiltrated organs were the ureters, bladder, sacral and pelvic bones, while no involvement of the sciatic nerve or iliac vessels was documented.
The interval between diagnosis of recurrence and previous surgery (disease-free survival) of the primary tumour was 13.4 months, with a range from 3 to 51 months. As for the onset symptoms, abdominal pain was the main symptom in 27 cases (77.1%), in 16 cases a palpable mass (45.7%) and in 12 patients (34.3%) the first sign was an increase in tumour markers (CEA).
3. Results
In 7 patients (20%), surgery was not possible because of diffused lung and liver metastases to (4 cases), or because of invasion of the sacral or pelvic bones. In 5 cases (14.3%) palliative surgical therapy (ileum/colostomy) was carried out, while in the remaining 23 cases (65.7%) curative surgical therapy was performed (
Table 6).
Of these 23 patients, 7 underwent a new recto-colic resection (all on a previous RAR), 10 underwent excision of the recurrence (5 on a previous RAR, 4 on an abdominoperineal amputation, 1 on a Hartmann intervention) and 6 with abdominoperineal amputation according to Miles (4 on a previous RAR, 1 on a Hartmann intervention, 1 on a TEM. All procedures were conducted using an open technique.
In 18 cases (64.2%) the resection was radical (RO), with a follow-up survival of 2 years, of 29.2 months on average, with a range of between 9 and 85 months after diagnosis of relapse.
In the other cases (Rl-R2) a progression of recurrence was observed in the following months (from 2 to 13, with an average of 5).
With regard to integrated therapy, 23 (78.5%) patients underwent adjuvant chemotherapy and radiotherapy.
Mortality was 7.1% and morbidity 42.8%, due mainly to anastomotic dehiscence (6 cases).
4. Discussion
Pelvic recurrence of rectum cancer has recently seen a considerable decline following the introduction of total mesorectal excision (TME) for rectal cancer and the integration of surgery with neoadjuvant chemotherapy-radiotherapy treatments.
Most of these recurrences occur within 2 years of surgery and in absence of treatment patients have an unfavourable prognosis, with significant impairment of their quality of life, due mainly to disabling pain.
Exclusively non-surgical therapies, such as radiotherapy or chemoradiation therapy, can have some palliative significance, often with little benefit and significant side effects.
Surgical treatment offers the best chance of survival if carried out for curative purposes and an RO resection is now possible for up to 50% of patients, the careful selection of whom is a prerequisite [
31].
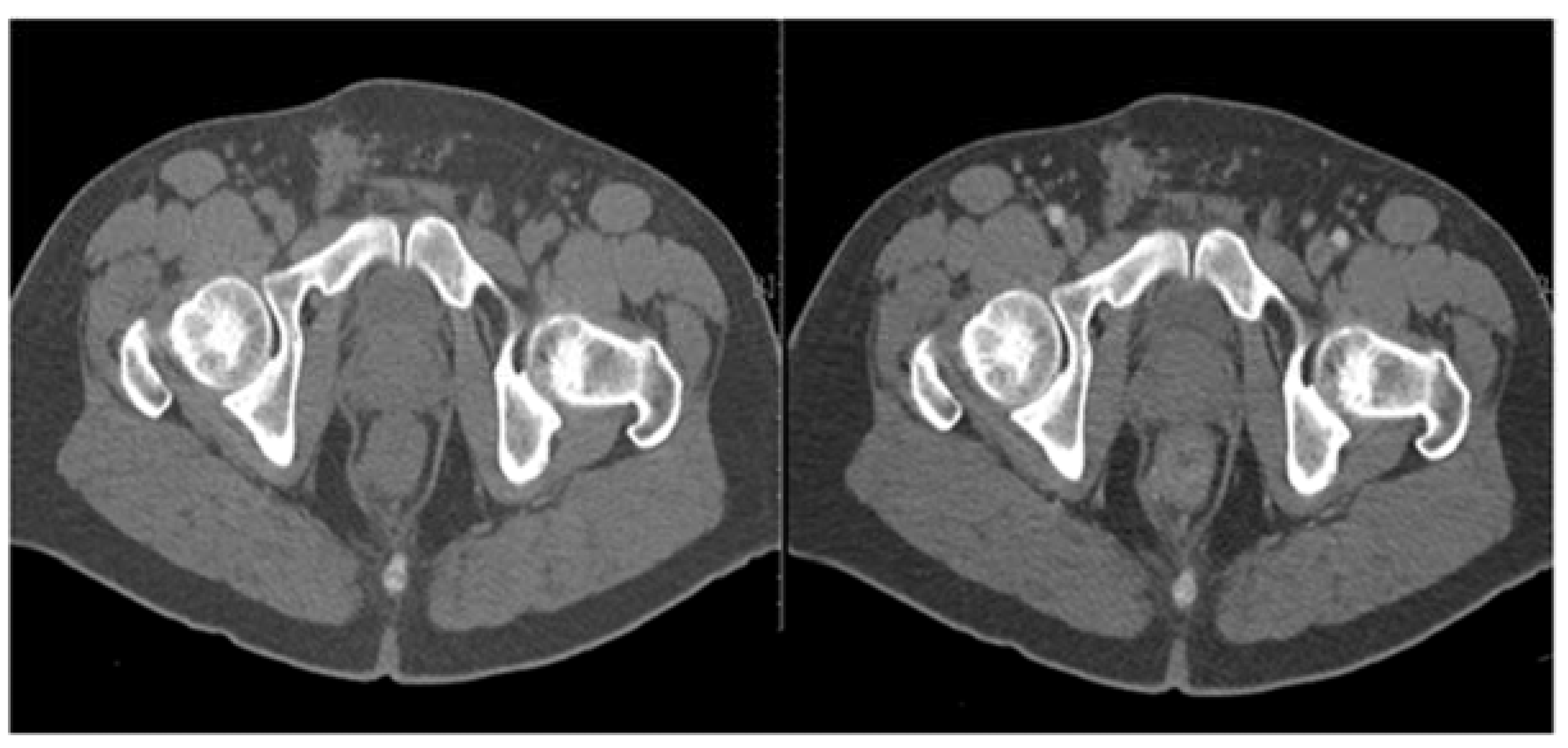
Imaging diagnostics, pelvic MRI, in particular, allows for the precise definition of the site of recurrence and the anatomical relationships with the surrounding structures, when planning the most appropriate type of surgery to perform (
Figure 2).
Figure 2.
MRI Neo Formation Ca Colon-Sigma (A) MRI Axial T2weight FR FSE and (B) Sagittal T1 FSE LAVA-FLEX HR (AG, female 77years old).
Figure 2.
MRI Neo Formation Ca Colon-Sigma (A) MRI Axial T2weight FR FSE and (B) Sagittal T1 FSE LAVA-FLEX HR (AG, female 77years old).
Outcomes of left hemicolectomy with packaging of ileo-stomy in left iliac fossa. At today's follow-up control, a concentric transmural parietal thickening of the rectal stump is evident (maximum parietal thickness about 13mm; about 47mm from the anal margin) extending longitudinally for about 44mm, prevalent on the posterior side; the thickening is characterised by a marked restriction of the signal in diffusion and post-contrast vascularisation. There are evident signs of extra-parietal encroachment at the level of the right postero-lateral sector. Inhomogeneity and thickening of the mesorectum and the mesorectal fascia.
Limited surgery is possible in the case of isolated anastomotic and central peri-anastomotic relapses, which have the most favourable prognosis overall, with a 5-year survival of up to 65%.
If the recurrence is ventral, dorsal, or lateral and involves the pelvic viscera or neighbouring bone structures, such as the sacrum, the exeresis should be extended and en bloc. In the latter case, these are highly demolitive operations, burdened by numerous complications and therefore to be carried out in dedicated high-volume centres and by an experienced multi-disciplinary team that includes not only the oncological surgeon but also the neurosurgeon, orthopaedist, plastic surgeon, and other dedicated figures. Even these destructive interventions must aim at an RO resection which can guarantee an average 5-year survival of up to 44% [
32].
In our case study, the rate of RO resection was 65.7%, in line with the numerous experiences reported in literature (
Table 6); the surgical interventions performed were largely "limited" to the favourable site of recurrence, with acceptable rates of mortality and morbidity.
The role of minimally invasive surgery is becoming increasingly popular today but, if on the one hand it has the undeniable advantage of enlarging the anatomical structures and allowing a more accurate dissection, on the other it does not seem applicable to perform extensive multivisceral resections which, only with the open approach, are they achievable with the utmost radicality. Finally, the concept of multidisciplinary therapy needs to be applied, not only to the management of cancer of the primary rectum but also to its recurrence. The integration of radio-chemotherapeutic surgery increases the rate of resectability and leads to encouraging results in terms of percentages of recurrence.
5. Conclusions
Local recurrence of rectum cancer is still the main complication of this disease today. If untreated, it results in an average survival of about 8 months and poor quality of life. Advanced stage and non-radical surgery (R>O) of the primary tumour seem to be the main risk factors. At present, we need to focus our attention on two fundamental aspects: early diagnosis and the most appropriate treatment of recurrence. Early diagnosis can be accomplished thanks to a valid follow-up to be carried out especially during the first 2-3 years after surgery. If a recurrence is diagnosed, the patients need to be carefully rehabilitated and followed by a multidisciplinary oncological team that, by mutual agreement, chooses the most appropriate treatment.
Rescue surgery is technically feasible and justified in a selected group of patients. An aggressive multimodal approach guarantees disease-free survival rates of up to 30% of selected cases. Surgical resection has a higher cost/benefit ratio than non-surgical treatment. The refinement of the diagnostic and staging methods, the operating technique, the chemoradiation therapy and the availability of multidisciplinary teams will be able to improve the results of surgical intervention, further.
Author Contributions
P.I., S.I. and C.D.I. contributed to manuscript writing and editing and data collection; A.P., D.C. and S.S contributed to data analysis; L.I. and M.C. contributed to conceptualization and supervision; all authors have read and approved the final manuscript.
Funding
This research received no external funding.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Moore, H. G., Shoup, M., Riedel, E., Minsky, B. D., Alektiar, K. M., Ercolani, M., Paty, P. B., Wong, W. D., & Guillem, J. G. (2004). Colorectal cancer pelvic recurrences: determinants of resectability. Diseases of the colon and rectum, 47(10), 1599–1606. [CrossRef]
- Heald, R. J., Husband, E. M., & Ryall, R. D. (1982). The mesorectum in rectal cancer surgery--the clue to pelvic recurrence? The British journal of surgery, 69(10), 613–616. [CrossRef]
- Rödel, C., Grabenbauer, G. G., Matzel, K. E., Schick, C., Fietkau, R., Papadopoulos, T., Martus, P., Hohenberger, W., & Sauer, R. (2000). Extensive surgery after high-dose preoperative chemoradiotherapy for locally advanced recurrent rectal cancer. Diseases of the colon and rectum, 43(3), 312–319. [CrossRef]
- Beets-Tan, R. G., Beets, G. L., Vliegen, R. F., Kessels, A. G., Van Boven, H., De Bruine, A., von Meyenfeldt, M. F., Baeten, C. G., & van Engelshoven, J. M. (2001). Accuracy of magnetic resonance imaging in prediction of tumour-free resection margin in rectal cancer surgery. Lancet (London, England), 357(9255), 497–504. [CrossRef]
- Melton, G. B., Paty, P. B., Boland, P. J., Healey, J. H., Savatta, S. G., Casas-Ganem, J. E., Guillem, J. G., Weiser, M. R., Cohen, A. M., Minsky, B. D., Wong, W. D., & Temple, L. K. (2006). Sacral resection for recurrent rectal cancer: analysis of morbidity and treatment results. Diseases of the colon and rectum, 49(8), 1099–1107. [CrossRef]
- Lan, Y. T., Chang, S. C., Yang, S. H., Lin, C. C., Wang, H. S., Jiang, J. K., Chen, W. S., Lin, T. C., Chiou, S. H., & Lin, J. K. (2014). Comparison of clinicopathological characteristics and prognosis between early and late recurrence after curative surgery for colorectal cancer. American journal of surgery, 207(6), 922–930. 207, 6, 922–930. [CrossRef]
- Nagasaki, T., Akiyoshi, T., Ueno, M., Fukunaga, Y., Nagayama, S., Fujimoto, Y., Konishi, T., & Yamaguchi, T. (2014). Laparoscopic salvage surgery for locally recurrent rectal cancer. Journal of gastrointestinal surgery: official journal of the Society for Surgery of the Alimentary Tract, 18(7), 1319–132. [CrossRef]
- Umpleby, H. C., Ranson, D. L., & Williamson, R. C. (1985). Peculiarities of mucinous colorectal carcinoma. The British journal of surgery, 72(9), 715–718. [CrossRef]
- García-Granero, E., Navarro, F., Cerdán Santacruz, C., Frasson, M., García-Granero, A., Marinello, F., Flor-Lorente, B., & Espí, A. (2017). Individual surgeon is an independent risk factor for leak after double-stapled colorectal anastomosis: An institutional analysis of 800 patients. Surgery, 162(5), 1006–1016. [CrossRef]
- Akyol, A. M., McGregor, J. R., Galloway, D. J., Murray, G. D., & George, W. D. (1991). Anastomotic leaks in colorectal cancer surgery: a risk factor for recurrence? International journal of colorectal disease, 6(4), 179–183. [CrossRef]
- PelvEx Collaborative (2019). Surgical and Survival Outcomes Following Pelvic Exenteration for Locally Advanced Primary Rectal Cancer: Results From an International Collaboration. Annals of surgery, 269(2), 315–321. [CrossRef]
- Yamada, K., Ishizawa, T., Niwa, K., Chuman, Y., Akiba, S., & Aikou, T. (2001). Patterns of pelvic invasion are prognostic in the treatment of locally recurrent rectal cancer. The British journal of surgery, 88(7), 988–993. [CrossRef]
- Georgiou, P. A., Tekkis, P. P., Constantinides, V. A., Patel, U., Goldin, R. D., Darzi, A. W., John Nicholls, R., & Brown, G. (2013). Diagnostic accuracy and value of magnetic resonance imaging (MRI) in planning exenterative pelvic surgery for advanced colorectal cancer. European journal of cancer (Oxford, England: 1990), 49(1), 72–81. [CrossRef]
- Bakx, R., Visser, O., Josso, J., Meijer, S., Slors, J. F., & van Lanschot, J. J. (2008). Management of recurrent rectal cancer: a population-based study in greater Amsterdam. World journal of gastroenterology, 14(39), 6018–6023. [CrossRef]
- Ferenschild, F. T., Vermaas, M., Verhoef, C., Dwarkasing, R. S., Eggermont, A. M., & de Wilt, J. H. (2009). Abdominosacral resection for locally advanced and recurrent rectal cancer. The British journal of surgery, 96(11), 1341–1347. [CrossRef]
- van Hooft, J. E., van Halsema, E. E., Vanbiervliet, G., Beets-Tan, R. G., DeWitt, J. M., Donnellan, F., Dumonceau, J. M., Glynne-Jones, R. G., Hassan, C., Jiménez-Perez, J., Meisner, S., Muthusamy, V. R., Parker, M. C., Regimbeau, J. M., Sabbagh, C., Sagar, J., Tanis, P. J., Vandervoort, J., Webster, G. J., Manes, G., … European Society of Gastrointestinal Endoscopy (2014). Self-expandable metal stents for obstructing colonic and extracolonic cancer: European Society of Gastrointestinal Endoscopy (ESGE) Clinical Guideline. Endoscopy, 46(11), 990–1053. [CrossRef]
- Suzuki, K., Dozois, R. R., Devine, R. M., Nelson, H., Weaver, A. L., Gunderson, L. L., & Ilstrup, D. M. (1996). Curative reoperations for locally recurrent rectal cancer. Diseases of the colon and rectum, 39(7), 730–736. [CrossRef]
- Hajibandeh, S., Hajibandeh, S., Morgan, R., & Maw, A. (2020). The incidence of right-sided colon cancer in patients aged over 40 years with acute appendicitis: A systematic review and meta-analysis. International journal of surgery (London, England), 79, 1–5. [CrossRef]
- Moriya, Y., Akasu, T., Fujita, S., & Yamamoto, S. (2004). Total pelvic exenteration with distal sacrectomy for fixed recurrent rectal cancer in the pelvis. Diseases of the colon and rectum, 47(12), 2047–2054. [CrossRef]
- Weber, K. L., Nelson, H., Gunderson, L. L., & Sim, F. H. (2000). Sacropelvic resection for recurrent anorectal cancer. A multidisciplinary approach. Clinical orthopaedics and related research, (372), 231–240. [CrossRef]
- Izzo, L., Impara, L., Pugliese, F., Mansour, M. A., De Felice, F., De Dominicis, C., De Santis, A., De Felice, C., Gabriele, R., Basso, L., Di Cello, P., Caputo, M., & Izzo, P. (2015). Preoperative staging of resectability of colon cancer using virtual colonoscopy: correlation with surgical results. Our experience. Annali italiani di chirurgia, 86, 432–436.
- Lee, D. J., Sagar, P. M., Sadadcharam, G., & Tan, K. Y. (2017). Advances in surgical management for locally recurrent rectal cancer: How far have we come? World journal of gastroenterology, 23(23), 4170–4180. [CrossRef]
- Park, S. Y., Choi, G. S., Jun, S. H., Park, J. S., & Kim, H. J. (2011). Laparoscopic salvage surgery for recurrent and metachronous colorectal cancer: 15 years' experience in a single center. Surgical endoscopy, 25(11), 3551–3558. [CrossRef]
- Lu, A. G., Wang, M. L., Hu, W. G., Li, J. W., Zang, L., Mao, Z. H., Dong, F., Feng, B., Ma, J. J., Zong, Y. P., & Zheng, M. H. (2006). Zhonghua wai ke za zhi [Chinese journal of surgery], 44(9), 597–599.Mcall JL, Cox MR, Wattchow DA (1995) Analysis of local recurrence rate after surgery for rectal cancer. Int J Colorect Dis 1995; 10: 126-132.
- Kim, S. H., Neve, R. S., & Joh, Y. G. (2008). Multimedia article. Relaparoscopy for salvage surgery in anastomotic recurrence of rectal cancer: feasible and safe. Diseases of the colon and rectum, 51(11), 1712–1713. [CrossRef]
- Akiyoshi, T., Nagata, J., Nagasaki, T., Konishi, T., Fujimoto, Y., Nagayama, S., Fukunaga, Y., & Ueno, M. (2015). Laparoscopic salvage lateral pelvic lymph node dissection for locally recurrent rectal cancer. Colorectal disease: the official journal of the Association of Coloproctology of Great Britain and Ireland, 17(10), O213–O216. [CrossRef]
- Milne, T., Solomon, M. J., Lee, P., Young, J. M., Stalley, P., Harrison, J. D., & Austin, K. K. (2014). Sacral resection with pelvic exenteration for advanced primary and recurrent pelvic cancer: a single-institution experience of 100 sacrectomies. Diseases of the colon and rectum, 57(10), 1153–1161. [CrossRef]
- Tanis, P. J., Doeksen, A., & van Lanschot, J. J. (2013). Intentionally curative treatment of locally recurrent rectal cancer: a systematic review. Canadian journal of surgery. Journal canadien de chirurgie, 56(2), 135–144. [CrossRef]
- Sagar, P. M., Gonsalves, S., Heath, R. M., Phillips, N., & Chalmers, A. G. (2009). Composite abdominosacral resection for recurrent rectal cancer. The British journal of surgery, 96(2), 191–196. [CrossRef]
- Harris, L. J., Phillips, B. R., Maxwell, P. J., Isenberg, G. A., & Goldstein, S. D. (2010). Outcomes of low anterior resection anastomotic leak after preoperative chemoradiation therapy for rectal cancer. The American surgeon, 76(7), 747–751.
- Barbaro, B., Leccisotti, L., Vecchio, F. M., Di Matteo, M., Serra, T., Salsano, M., Poscia, A., Coco, C., Persiani, R., Alfieri, S., Gambacorta, M. A., Valentini, V., Giordano, A., & Bonomo, L. (2017). The potential predictive value of MRI and PET-CT in mucinous and nonmucinous rectal cancer to identify patients at high risk of metastatic disease. The British journal of radiology, 90(1069), 20150836. [CrossRef]
- Platt, E., Dovell, G., & Smolarek, S. (2018). Systematic review of outcomes following pelvic exenteration for the treatment of primary and recurrent locally advanced rectal cancer. Techniques in coloproctology, 22(11), 835–845. 83. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).