1. Introduction
Adventitious rooting (AR) is an organogenic process that involves cell fate reprogramming to initiate a root meristem. In woody species, clonal propagation of elite trees is often hampered by a decline in rooting ability as the tree ages [
1,
2]. Although maturation is a major factor limiting the cloning of recalcitrant adult trees, it may be possible to regenerate adventitious roots from mature trees by using ontogenetically young tissues from the base of the tree [
3,
4,
5].
For the development of adventitious roots and successful vegetative propagation, exogenous auxins are frequently used to initiate cell division and root primordia, including woody species [
6,
7,
8]. Indole-3-butyric acid (IBA), which is often used to induce adventitious roots in forest tree species, changes the expression of many genes at the whole plant level, although the gene response to auxin is highly dependent on the type of tissue [
9] and it is specific to the cell receiving the auxin signal [
10]. Canonical auxin signalling involves the perception of the hormone by the receptor complex formed by TIR1 and FB2, what leads to the degradation of Aux/IAA inhibitor proteins and releases Auxin Response Factors (ARFs), that will drive auxin-related gene expression [
11,
12]. However, increasing evidence suggest the existence of extranuclear perception mechanisms regulating auxin responses [
13]. In the case of AR, accumulation of asymmetrically distributed auxin in specific cells is mandatory to tag them as root founder cells, reprogramming their genetic program and entering a root developmental pathway [
14]. This asymmetric distribution is the result of the activity of the Polar Auxin Transport (PAT) machinery, which include several families of transport proteins [
15]. Therefore, modulation of PAT through the application of exogenous auxin or by means of specific PAT inhibitors enables the induction of AR or helps in the understanding of molecular mechanisms related to the induction of the process, respectively. N-1-naphthyl-phthalamic acid (NPA) is a PAT inhibitor widely used in research to characterize auxin-related responses and in horticulture and agriculture as an anti-auxin compound [
16].
Different model systems, including leaf petioles from ivy plants [
17,
18], cuttings from hypocotyls and epicotyls of
Pinus taeda,
P. radiata and Arabidopsis seedlings [
2,
19,
20,
21], juvenile and mature cuttings from eucalyptus [
22], black walnut [
23] or cuttings from pea plants of different ages [
24] have been used to study phase change and the maturation- or age-dependent decline of rooting competence.
On the basis of juvenile-mature gradient displayed in plants, an experimental system was developed with microshoots derived from basal sprouts and crown branches of the same chestnut tree [
1]. Both juvenile and mature shoot lines exhibited different
in vitro morphogenetic capacity and were used to study phase change and the maturation-related decline of AR [
5,
25,
26,
27,
28]. This system allowed for the identification of the
Scarecrow-like 1 gene (
CsSCL1), encoding a transcription factor from the GRAS family, which was upregulated by auxin within the first 24 h of treatment [
20]. GRAS proteins are involved in cell division, auxin signalling, root radial patterning and root meristem specification, among other processes [
29,
30,
31]. The localized expression of the gene in rooting competent cells of juvenile shoots indicates that
CsSCL1 plays an important role in the initiation of adventitious roots [
27]. Auxin-induced expression of
SCL1 was also detected in root progenitor cells only in juvenile cuttings during the AR of black walnut [
23]. Although the microshoot system is suitable for identifying rooting markers, the complexity of the shoots, in which several processes occur simultaneously in response to auxin treatment, can sometimes complicate the analysis of the results or mask the pursued responses. Auxin distribution at the base of wounded shoots, where exogenous auxin is applied to induce roots, may be altered by PAT from the shoot apex. For example, IAA levels during rooting were not positively correlated with AR ability in juvenile and mature shoots of chestnut [
25] and oak [
32].
Here we describe for the first time in chestnut a simple and useful leaf-based system to study adventitious rooting, taking into consideration auxin signaling and transport, as well as the loss of rooting competence on maturation. Auxin-induced expression of CsSCL1 was only detected in rooting competent leaves. NPA inhibition of PAT revealed a direct correlation between NPA-induced reduction of AR capacity and a down-regulation of the auxin-induced expression of CsSCL1, also associated to rooting competent cells.
3. Discussion
In the present study, we developed a simple method using leaves excised from shoots to study the histological and molecular mechanisms involved in regenerating adventitious roots. We demonstrated that the rooting rates in the leaves and the mother shoots from which they were excised were similar and correlated with the ontogenetic origin, consistent with the data previously reported in microshoots [
1,
25]. The decline in the morphogenetic potential of woody species with increasing physiological age and ontogenetic state during the ongoing process of phase change has been observed in various studies [
3,
33,
34,
35]. The strong influence of ontogenetic stage on rooting ability has also been demonstrated in pea cuttings [
24], and in chestnut it has been linked to changes in gene expression [
28,
36,
37]. The leaf system described here increased the potential number of samples by at least three times and also involved the use of less complex tissues, since roots arose from a small area at the base of the leaf petiole. In microshoots, roots regenerated from the basal part of the shoot and also along the stem. Thus, leaf system also prevents the potential side-effects caused by PAT in the shoot whereby endogenous auxin moves basipetally from the shoot apex and excludes other auxin-modulated developmental processes that may take place simultaneously in the shoot after IBA treatment. Overall, these advantages make the system highly suitable for studying the molecular mechanisms underlying adventitious root formation. Leaf explants have also been used to study AR in
Medicago truncatula [
38]. Different leaf protocols in Arabidopsis have been developed for studying AR, in which roots can be regenerated without auxin treatment [
39]. However, in our system, exogenous auxin is required to regenerate adventitious roots, as in most difficult-to-root woody species. In a leaf Arabidopsis system, it was shown that young leaves present a higher regeneration ability than mature ones [
40], which is in agreement with our findings in the chestnut leaf system.
Differences in regeneration ability between plants with contrasting ontogenetic state are believed to rely on distinct epigenetic status and hormone signalling, with the intertwined combination of both governing plant developmental plasticity and other processes [
41]. In a recent report, significant differences in hormone- and epigenetic-related signalling were found between auxin-treated chestnut microshoots with different ontogenetic state [
37]. Moreover, an age-related signalling pathway involving miRNAs and the
Squamosa Promoter Binding Protein-like (SPLs) genes governs the transition from the juvenile to the mature stage. High expression of
miR156 during juvenile period targets
SPLs genes for degradation, with eventual lower levels of the miRNAs allowing the expression of
SPLs and permitting the transition to the mature stage (reviewed in [
42]). Whether this age-related pathway or other mechanisms, such as histone modifications or hormone sensitivity, are linked to the differences found in the present work remains to be elucidated.
In a previous study with chestnut microshoots, we demonstrated that
CsSCL1 is induced by auxin in juvenile and mature microshoots, and is specifically expressed in cells involved in root initiation only in rooting competent shoots [
27]. In the present study, we demonstrated that
CsSCL1 expression is only induced in rooting-competent leaves, probably due to a reduced auxin signalling in mature detached leaves. We also described the specific distribution of mRNA transcripts associated with the activation of cells involved in initiating root meristems. By reducing the complexity of explants induced to root and excluding other auxin-regulated processes that occur in microshoots, auxin-induced
CsSCL1 expression was only detected in the responsive material, whereas the gene was also upregulated by auxin in mature microshoots (compare Figure 5 with Figure 3B and 3C in [
27]). Moreover,
CsSCL1 expression was induced when cell reorganization occurred and, occasionally, when the first asymmetric cell divisions were observed. The direct relationship between rooting ability and gene expression simplifies the molecular analysis and data interpretation. Expression of
SCL1 homologs has also been related to the AR process in pine, black walnut and eucalyptus, suggesting its role as a marker of rooting ability in forest species [
23,
43,
44]. The localized expression of
CsSCL1 in the QC and stem cells also indicates the involvement of the gene in maintaining the radial patterning of the root. This is also supported by the lack of an incipient columella in the round-shaped primordia developed after NPA treatment. The regulatory role of the GRAS family genes
SCR and
SHR in root radial patterning and maintenance of QC identity has been well documented [
45,
46,
47].
The present study also provides evidence that the effect of NPA on the rooting response is highly dependent on the timing (when and for how long) of treatment. The onset of AR was always delayed by NPA, regardless of the type of treatment. A similar response has been observed in other species such as pine, oak and grapevine [
32,
48,
49]. In addition, auxin-induced activation of target-competent cells was not greatly impaired by the temporal (5 day) post-application of NPA, as once NPA was removed from the medium the cells were able to resume division and proceed to the rooting pathway. However, root emergence was prevented by the subsequent and continuous treatment with NPA (25 days) after the IBA treatment. This demonstrates that NPA not only inhibited the root primordia initiation but also root emergence and development, indicating a role for auxin transport in those processes. Moreover, the differentiation of rooting-competent cells to root initial cells was strongly inhibited by NPA when applied at the same time as IBA. It appears that NPA impaired the creation of an optimal auxin gradient in the target cells required for initiation of root meristem. In Arabidopsis, a maximum auxin gradient in the root stem cell niche is crucial for proper root development and patterning [
50,
51]. Finally, we demonstrated for the first time the NPA-mediated effect on gene expression during the adventitious rooting of chestnut leaves. The inhibitory effect of NPA on root initiation was correlated with the reduced levels of
CsSCL1 expression observed in leaves 24 h after simultaneous treatment with IBA and NPA, and the spatial expression pattern also varied in NPA treated leaves. These data confirm our previous findings regarding the involvement of
CsSCL1 in AR [
27], as well as the sensitivity to NPA that alters PAT and probably disrupts or impedes the creation of the auxin gradient required for the initiation of a root meristem. In Arabidopsis, gene expression related to de-novo root regeneration from leaves was blocked or impaired as a consequence of NPA treatment [
52].
The function of auxin signalling and transport in adventitious root formation have been reported in different species [
2,
53,
54,
55]. Inhibitors of plant auxin transport, such as NPA, were shown to delay and inhibit adventitious root formation [
32,
48,
49,
56,
57,
58]. A direct correlation between inhibition of AR by NPA and suppression of the 24-h peak of IAA during induction of adventitious roots was also demonstrated in petunia [
59]. In the model system described in the present work, NPA activity delayed and/or inhibited AR, depending on the mode of application. Therefore, blocking of PAT impedes the establishment of a new developmental program, although not irreversibly.
The ability of NPA to inhibit PAT has been known for a long time, and several possible explanations have been proposed [
60]. However, only recently the molecular basis of this process has begun to be unraveled. Several protein families are known to have the capacity to transport auxins, including PIN, PILs and ABCBs [
15], and they could therefore be potential targets of the NPA activity. Nonetheless, PAT-related auxin efflux from cells occurs by means of the activity of PIN proteins, a family of polarly localized transmembrane transporters. NPA was shown to specifically inhibit PIN1 mediated auxin transport [
61], while other report demonstrated that, once inside the cell, NPA can bind to different members of the PIN family through direct interaction with their inner domains, blocking their activity [
62]. Indeed, the activity of specific PIN proteins in the initial stages of AR has been shown in different species, like apple or olive tree [
63,
64]. In mature chestnut microshoots, increased activity of
CsPIN1 has been linked to an improved rooting response (Castro-Camba
et al., 2023, in preparation). Therefore, although other related action mechanisms cannot be ruled out, NPA might be blocking the activity of specific members of the PIN family in chestnut, impeding the establishment of auxin gradients. These gradients and the generated maxima and minima are essential in plants to deploy plastic developmental responses [
16].
In conclusion, the proposed in vitro leaf system in chestnut reproduces the physiological rooting response of the mother microshoots, which in turn is correlated with the ontogenetic stage of the tree material from which the microshoots were initiated. This system provides several advantages over the experimental microshoot system for studying the adventitious root organogenesis at the molecular level. Using the proposed new system, we demonstrated the inhibitory effect of NPA on root induction and root development. In addition, the negative effect of NPA on AR was directly correlated with the reduction in the auxin-induced expression of CsSCL1 observed in rooting competent cells. The system will also be useful for studying the effects of the chronological age of leaves on rooting ability, and could also eventually be extended to other woody species.
4. Materials and Methods
4.1. Plant Material
Stock shoot cultures of chestnut initiated from basal sprouts (BS) and crown branches (CR) of the same tree (P2 clone) were used in the study. These will hereafter be referred to as BS and CR. The techniques for
in vitro establishment and proliferation of shoots have already been described [
1]. Both lines have been maintained
in vitro in our laboratory for more than 20 years, and they retain different morphological characteristics as well as different rooting abilities associated with the maturation stage.
4.2. Adventitious root induction
Rooting experiments were performed on 1) shoots of 2.5 to 3.5 cm in length, harvested from 4-week-old proliferating cultures growing in multiplication medium, 2) the three youngest and fully expanded leaves with petioles detached from the upper thirds of these microshoots, and 3) leaf segments, to compare their rooting response versus whole leaves. Whole leaves were placed on the medium abaxial side down, with the cut ends of the petioles inoculated in the medium (see Supplementary Figure 1). Leaf segments were obtained from leaves that were cut transversely through the mid-vein into three segments: the basal part including the petiole, the middle segment and the upper part containing the leaf apex. The polarity of leaf segments was maintained and they were placed upright on the medium, with the cut surface in contact with the medium.
Root induction on shoots was performed by dipping the basal end of each shoot in 4.9 mM of IBA for 1 min. The shoots were subsequently transferred to Gresshoff and Doy [
65] medium supplemented with 1/3-strength macronutrients (1/3 GD) [
1]. Leaves and leaf segments were placed on 1/3 GD medium supplemented with 0 (control) or 25 µM IBA for 5 days in darkness before being transferred to IBA-free medium under a 16 h photoperiod.
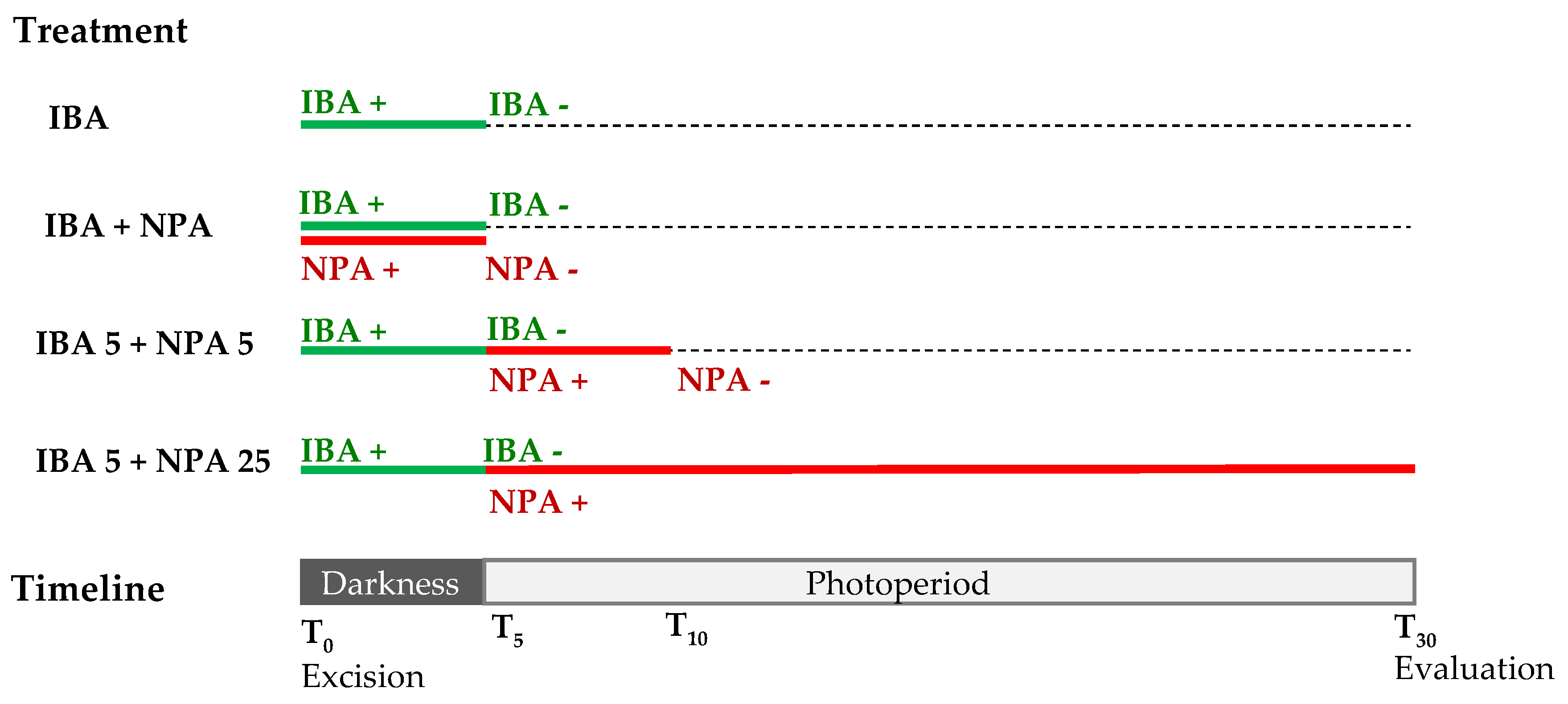
The effect of NPA on rooting was studied in both microshoots and leaves of the BS line. Microshoots treated with IBA were placed in 1/3 GD medium supplemented with 50 µM NPA for 0 (IBA) or 5 days (IBA+NPA5). The effect of NPA on root development was tested in auxin-treated leaves by applying 4 treatments designated as IBA, IBA+NPA, IBA5+NPA5 and IBA5+NPA25 (
Figure 10). Leaves were placed on 1/3 GD medium supplemented with 25 µM IBA for 5 days in darkness, and they were subsequently transferred to IBA-free medium under normal photoperiod (IBA). Following the same procedure, leaves were treated with 50 µM NPA simultaneously to IBA treatment and transferred to auxin- and NPA-free medium (IBA+NPA) or treated with NPA after IBA treatment for 5 (IBA5+NPA5) or 25 days (IBA5+NPA25).
Rooting rate (%), number of roots per rooted explant, % of non-basal roots, the longest root length, the time elapsed before the appearance of the first root, and the mean rooting time were recorded 30 days after the beginning of the experiment. Rooting time was evaluated as the mean day of root emergence of rooted explants. Leaf samples were also collected 6, 12, 24 and 72 h after each treatment for RNA extraction and/or in situ hybridization.
4.3. RNA extraction and quantification
Plant material was harvested, frozen in liquid nitrogen and stored at -70 ºC until use for RNA extraction. Axillary buds, internodes and leaves excised from BS microshoots were harvested at the end of the proliferation cycle. Adventitious roots were harvested 30 days after rooting induction and divided into three segments: apical (0-1 cm), middle (1-3 cm); and basal (3-4 cm) portions (see Supplementary Figure 2). IBA-treated leaves, control leaves and NPA-treated leaves were harvested 6, 12 and 24 h after treatments. Total RNA was extracted using the FavorPrep Plant Total RNA purification Mini Kit (for woody Plant) according to the manufacturer´s instructions.
4.4. Quantitative reverse transcriptase-polymerase chain reaction (qPCR)
For each sample, first strand cDNA synthesis was performed with 1 µg of total RNA in a final volume of 20 µl using the High Capacity cDNA Reverse Transcription kit (Applied Biosystems) according to the manufacturer´s instructions. The
CsSCL1 primers used in this study were previously designed for the analysis of chestnut microshoots [
27]. Three reference genes, Actin (ACT), Tubulin (TUB) and Polyubiquitin (UBI), were used as internal controls to normalize all data. The specificity of the primers and amplifications was confirmed by PCR and sequencing of the amplicons. The concentration of primers was adapted to each experiment and primer efficiency was tested using a standard curve for each gene. The genes, accession numbers, primer sequences and the amplicon length are outlined in the supplementary data (see Supplementary Table 1).
The qPCR analyses were carried out in an optical 48-well reaction plates with a StepOne Real-Time PCR System (Applied Biosystems) and with SYBR Green Master Mix (Applied Biosystems) to monitor PCR amplification. Reaction mixtures contained 1 X Power SYBR® Green, and 8.3 ng of cDNA as template, in a total volume of 15 µl. The concentration of
CsSCL1 primers was adjusted to 725 nM and 600 nM for the analyses carried out with samples of the different organs and from root induction experiments, respectively. Reaction mixtures were incubated at 95 ºC for 10 min, followed by 40 cycles of 95 ºC for 15 s and 60 ºC for 1 min. Three independent biological samples were used, and expression levels in each sample were based on 3 technical replicates. For quantification of
CsSCL1 in different organs, the results were expressed relative to the expression in internodes. In rooting experiments, data were expressed relative to the sample with lowest expression level in each biological replicate. Relative C
sSCL1 expression was expressed as a fold-change, as determined by the comparative Ct method [
66]. All calculations and normalizations were performed using DataAssist™ v3.0 software (Applied Biosystems).
4.5. In situ hybridization and histological analysis
To analyze the CsSCL1 expression pattern during the root induction phase and development of root primordia, BS leaves treated with IBA were harvested 12, 24, 72 h and 10 days after the treatments. Roots were also harvested 30 days after root induction. Leaves treated and not treated with IBA were also harvested 24 h after treatments. In addition, BS leaves simultaneously treated with IBA and NPA were harvested 24 h and 10 days after the start of the experiment. In leaves treated with IBA for 5 days and then transferred to the NPA containing medium, the material was harvested 10 days after starting the treatment. As most of the roots developed from this area, the basal part of each leaf containing the petiole was embedded and frozen in Jung tissue freezing medium (Leica Microsystems) on dry ice.
Cryosections (10 μm) were cut from the samples and placed on 3-aminopropyl-triethoxysilane glass slides, dried and fixed. A 650 bp fragment corresponding to the 5′ region of the
CsSCL1 gene (outside of the GRAS domain) was cloned into the PCR®II vector (Invitrogen) and amplified to generate
CsSCL1 specific probes. The PCR fragment, flanked by SP6 and T7 promoters, was used as a template for synthesising both sense and antisense digoxigenin (DIG)-labelled probes, with respectively T7 and SP6 polymerase, according to the manufacturer’s instructions (Roche Biochemicals). The probes were partially hydrolyzed by alkali treatment, to a mean length of 200 nucleotides. In situ hybridization was performed as described by Sánchez
et al. [
18] and the hybridization signal was detected using a DIG Nucleic Acid Detection kit (Roche Biochemicals) according to the manufacturer’s instructions.
For histological purposes, serial cryosections (10 µm) used in in situ experiments were stained in 0.05% toluidine blue O. Photographs were taken under bright-field illumination in a Nikon microscope equipped with an Olympus digital camera.
4.6. Statistical analysis
All rooting experiments were repeated three times with 18 explants per treatment. Percentage data were subjected to arcsine transformation before statistical analysis. Rooting parameters and the qPCR data were expressed as means ± SE (standard error) from three replicates. Multiple-group comparisons were evaluated by ANOVA followed by Duncan´s test. Differences were considered to be statistically significant at P ≤ 0.05.
Figure 1.
Root development in chestnut explants derived from basal sprouts (P2BS) 30 days after beginning of treatments. (a) Microshoots were dipped in 4.9 mM IBA solution for 1 min and then placed on IBA-free medium. (b) Leaves excised from microshoots were placed for 5 days on medium containing 25 µM IBA and then transferred to IBA-free medium. (c) The three different leaf segments, apical (al), middle (ml) and basal (bl), were treated as described for leaves.
Figure 1.
Root development in chestnut explants derived from basal sprouts (P2BS) 30 days after beginning of treatments. (a) Microshoots were dipped in 4.9 mM IBA solution for 1 min and then placed on IBA-free medium. (b) Leaves excised from microshoots were placed for 5 days on medium containing 25 µM IBA and then transferred to IBA-free medium. (c) The three different leaf segments, apical (al), middle (ml) and basal (bl), were treated as described for leaves.
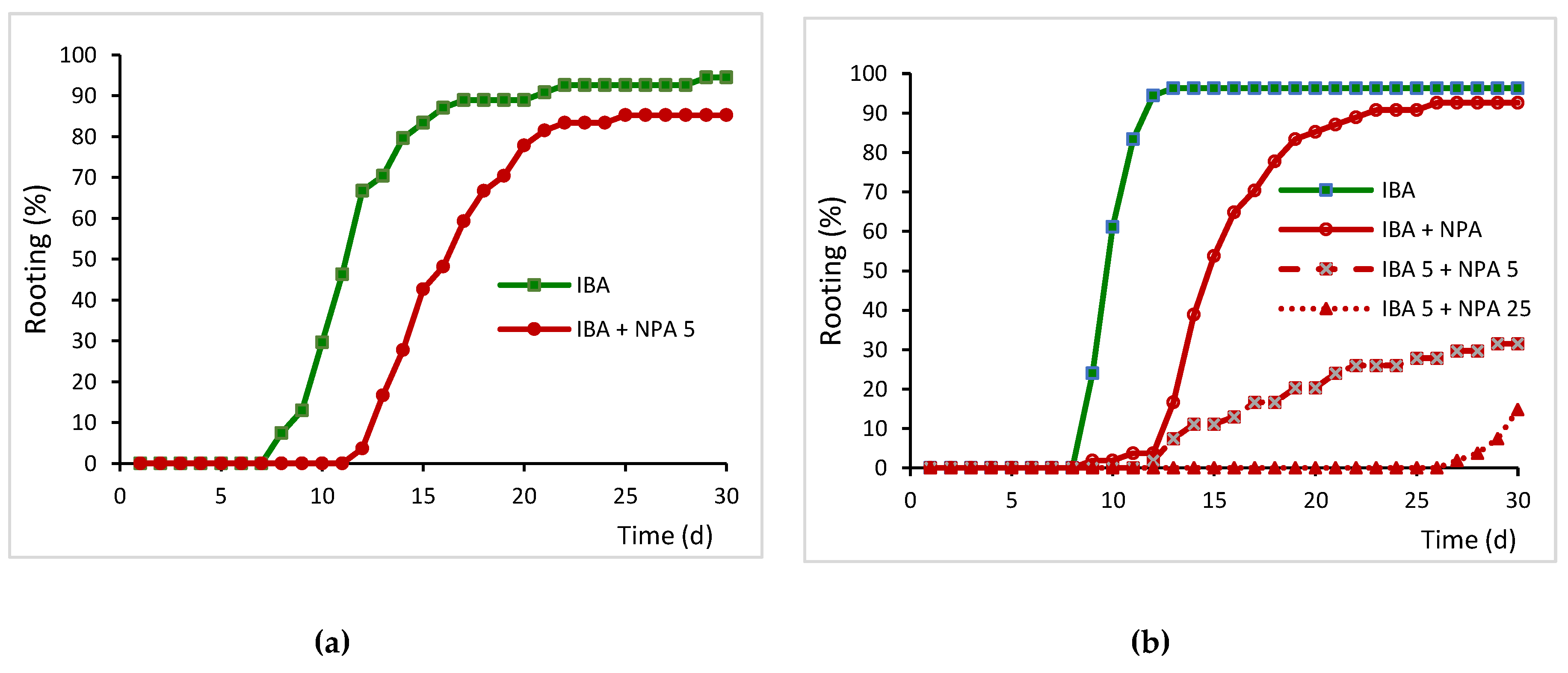
Figure 2.
Effect of NPA on the rooting response of juvenile chestnut microshoots and leaves. (a) Microshoots were dipped in 4.9 mM IBA for 1 min and then placed on 1/3 GD medium without NPA (IBA) or with 50 µM NPA for 5 days (IBA+NPA 5). (b) Leaves were treated with 25 µM IBA and transferred to IBA-free medium after 5 days (IBA). In addition, 50 µM NPA was added simultaneously to IBA treatment (IBA+NPA), during 5 days following IBA treatment (IBA 5+NPA 5), or during the 25 days following IBA treatment (IBA 5+NPA 25).
Figure 2.
Effect of NPA on the rooting response of juvenile chestnut microshoots and leaves. (a) Microshoots were dipped in 4.9 mM IBA for 1 min and then placed on 1/3 GD medium without NPA (IBA) or with 50 µM NPA for 5 days (IBA+NPA 5). (b) Leaves were treated with 25 µM IBA and transferred to IBA-free medium after 5 days (IBA). In addition, 50 µM NPA was added simultaneously to IBA treatment (IBA+NPA), during 5 days following IBA treatment (IBA 5+NPA 5), or during the 25 days following IBA treatment (IBA 5+NPA 25).
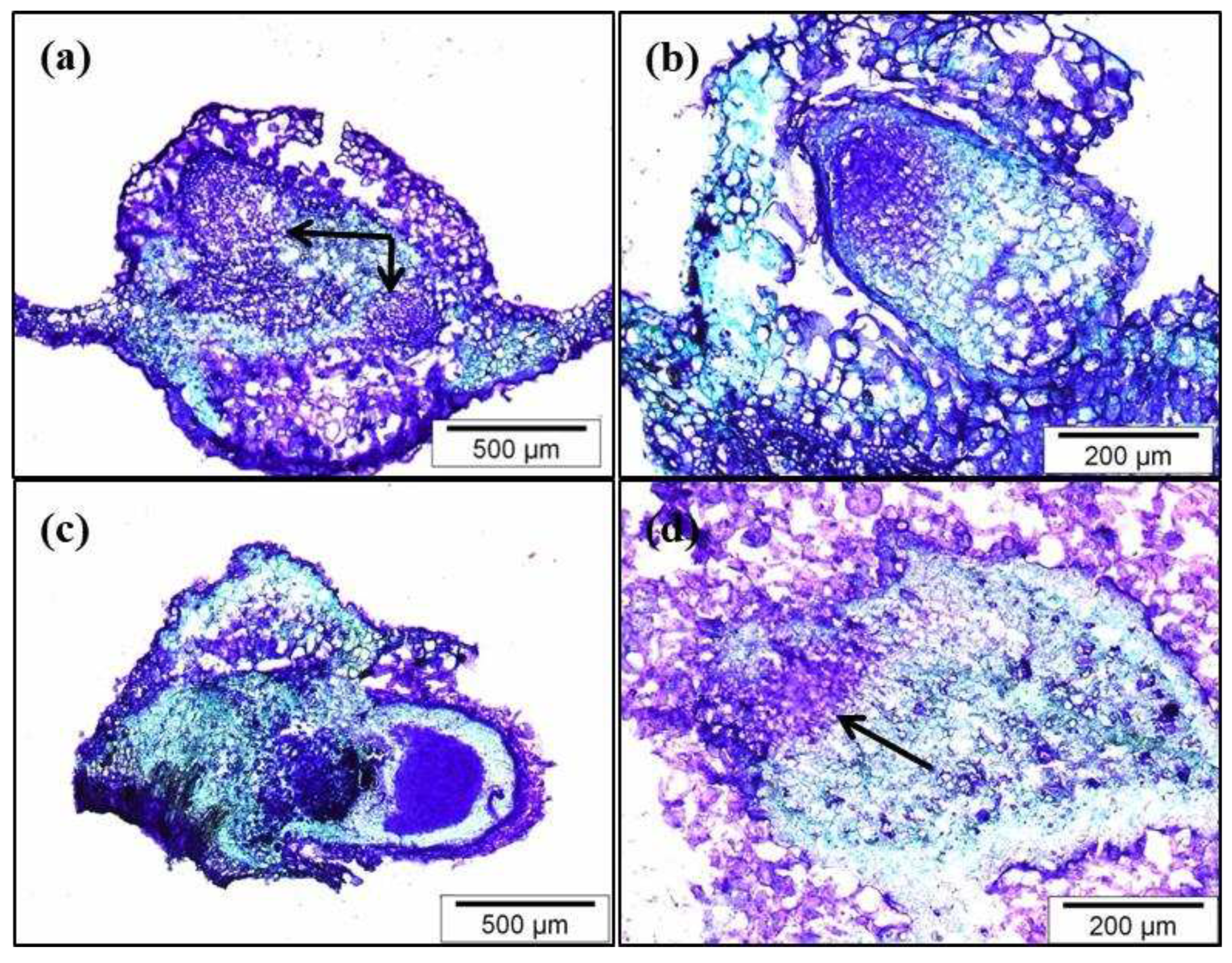
Figure 3.
Effect of NPA on the development of adventitious roots in leaves 10 days after the start of treatments. (a-b) Leaves were treated with 25 µM IBA for 5 days and then transferred to IBA-free medium. (c) Leaves were simultaneously treated with 25 µM IBA and 50 µM NPA for 5 days. (d) Leaves were treated with 25 µM IBA for 5 days and then transferred to IBA-free medium supplemented with 50 µM NPA for 5 days. Sections of 10 µm were stained with toluidine blue. Root primordia in (a) and the incipient meristem in (d) are indicated by arrows.
Figure 3.
Effect of NPA on the development of adventitious roots in leaves 10 days after the start of treatments. (a-b) Leaves were treated with 25 µM IBA for 5 days and then transferred to IBA-free medium. (c) Leaves were simultaneously treated with 25 µM IBA and 50 µM NPA for 5 days. (d) Leaves were treated with 25 µM IBA for 5 days and then transferred to IBA-free medium supplemented with 50 µM NPA for 5 days. Sections of 10 µm were stained with toluidine blue. Root primordia in (a) and the incipient meristem in (d) are indicated by arrows.
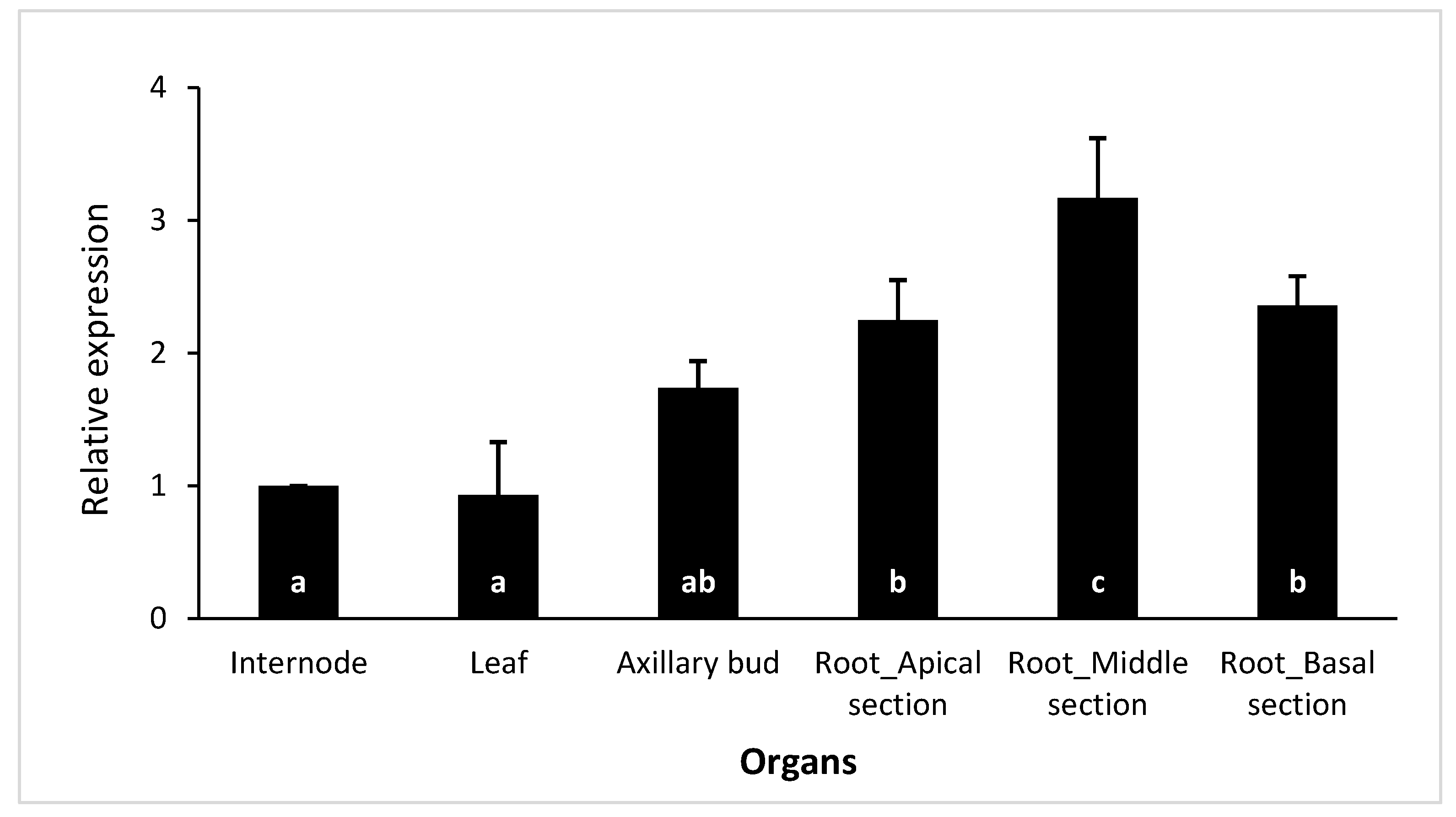
Figure 4.
Expression analysis of CsSCL1 by qPCR in different organs: internode, leaf, axillary bud, and the apical, middle and basal sections of the adventitious root. Results are expressed as a meanvalues ±SE of three biological replicates, and the relative expression was normalized to shoot internode levels. The same letter at the bottom of the bars indicates no significant difference at P≤ 0.05.
Figure 4.
Expression analysis of CsSCL1 by qPCR in different organs: internode, leaf, axillary bud, and the apical, middle and basal sections of the adventitious root. Results are expressed as a meanvalues ±SE of three biological replicates, and the relative expression was normalized to shoot internode levels. The same letter at the bottom of the bars indicates no significant difference at P≤ 0.05.
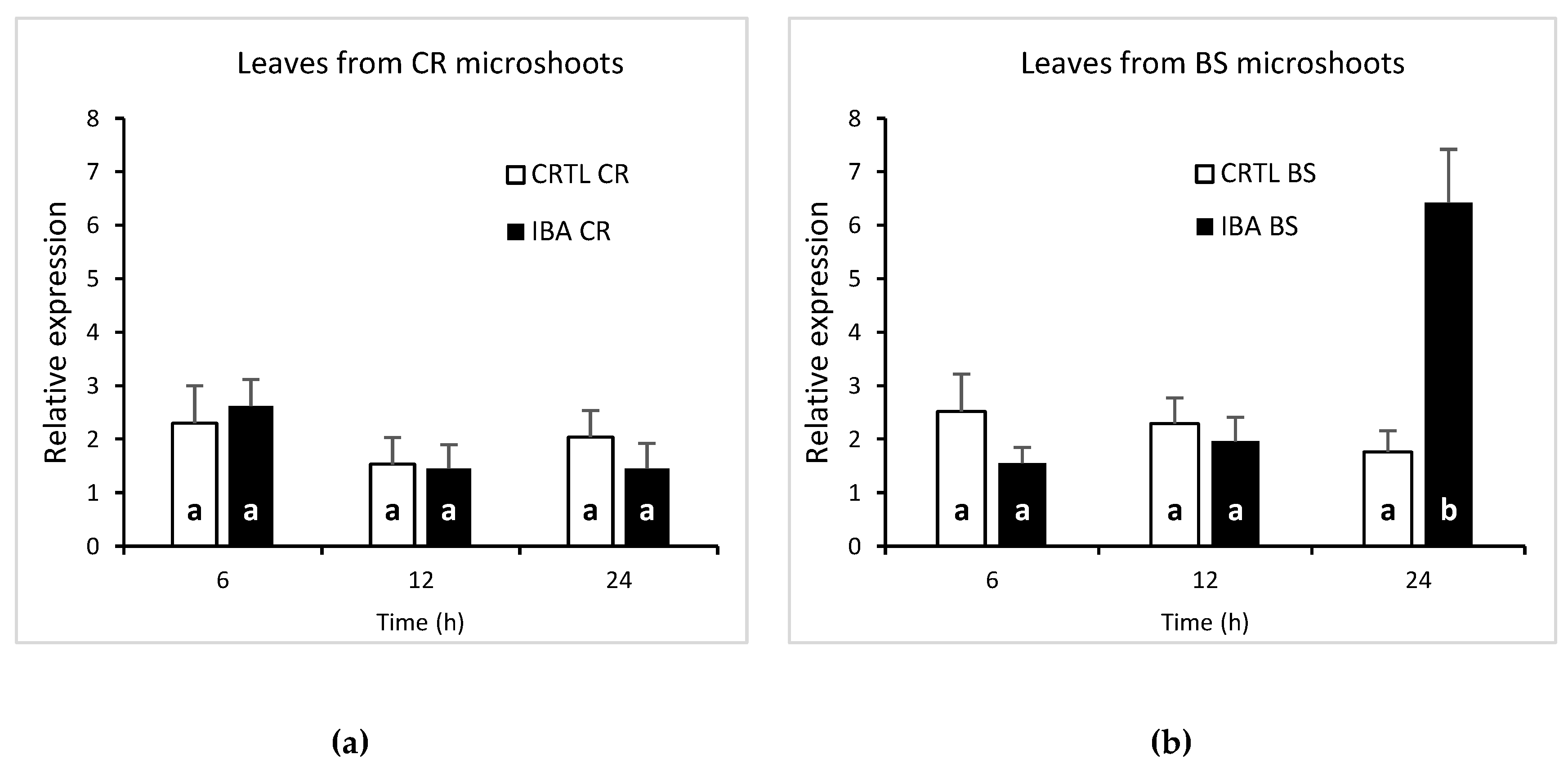
Figure 5.
Expression of CsSCL1 during adventitious root induction of leaves excised from microshoots derived from crown branches (a) and from basal sprouts (b). Leaves were treated with 25 µM IBA (IBA) or were not treated (CTRL) and harvested at 6, 12 and 24 h. The increase in gene expression at 24 h after IBA treatment in BS leaves was significant at P≤ 0.05. The interaction time x treatment was significant at P≤ 0.001.
Figure 5.
Expression of CsSCL1 during adventitious root induction of leaves excised from microshoots derived from crown branches (a) and from basal sprouts (b). Leaves were treated with 25 µM IBA (IBA) or were not treated (CTRL) and harvested at 6, 12 and 24 h. The increase in gene expression at 24 h after IBA treatment in BS leaves was significant at P≤ 0.05. The interaction time x treatment was significant at P≤ 0.001.
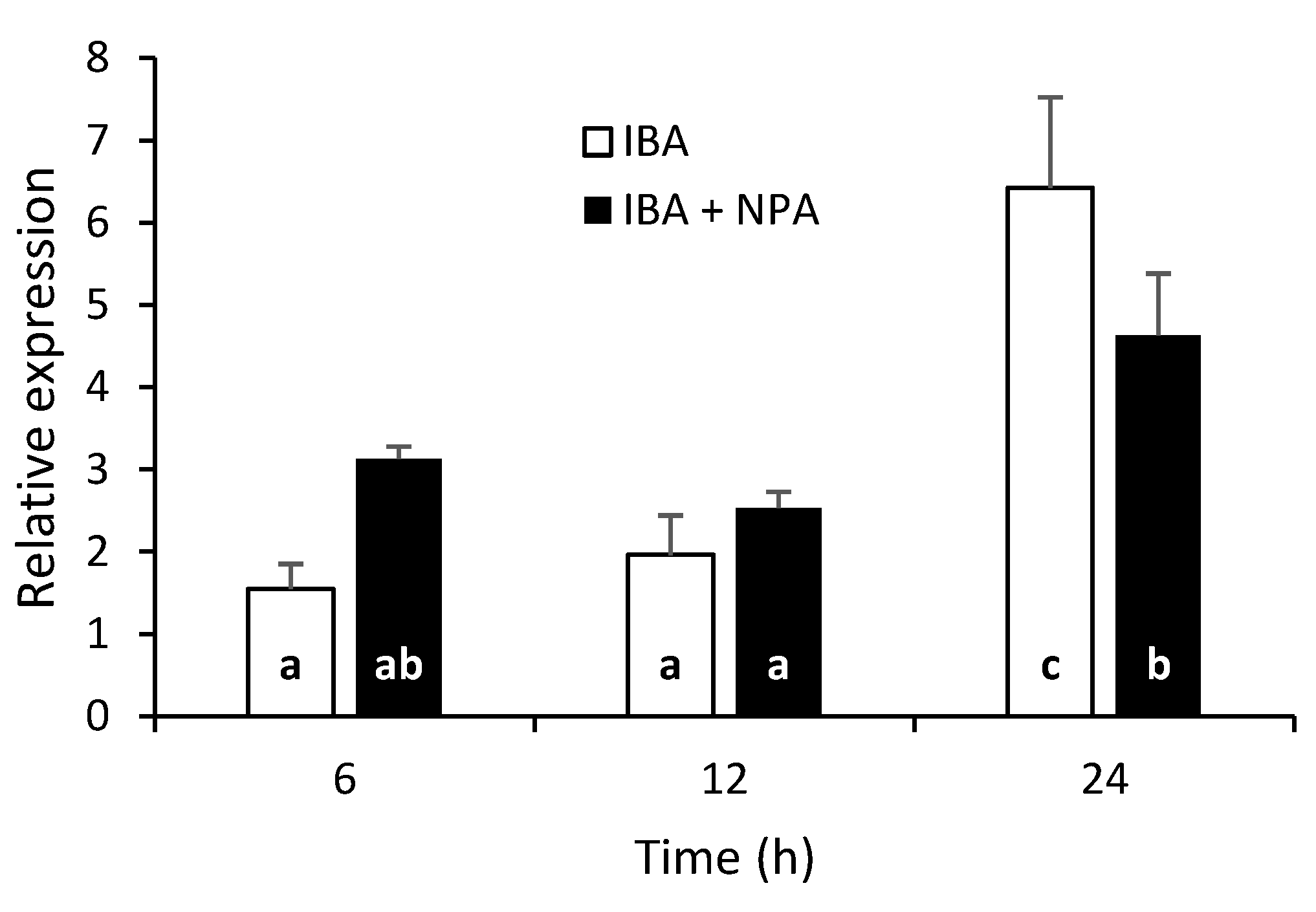
Figure 6.
Effect of NPA on CsSCL1 expression during adventitious root induction in leaves excised from P2BS microshoots. Leaves were treated with 25 µM IBA (IBA) or simultaneously with IBA and 50 µM of NPA (IBA+NPA) and harvested 6, 12 and 24 h after treatment. The same letter at the bottom of the bars indicates no significant difference at P≤ 0.05. The interaction time x treatment was significant at P≤ 0.05.
Figure 6.
Effect of NPA on CsSCL1 expression during adventitious root induction in leaves excised from P2BS microshoots. Leaves were treated with 25 µM IBA (IBA) or simultaneously with IBA and 50 µM of NPA (IBA+NPA) and harvested 6, 12 and 24 h after treatment. The same letter at the bottom of the bars indicates no significant difference at P≤ 0.05. The interaction time x treatment was significant at P≤ 0.05.
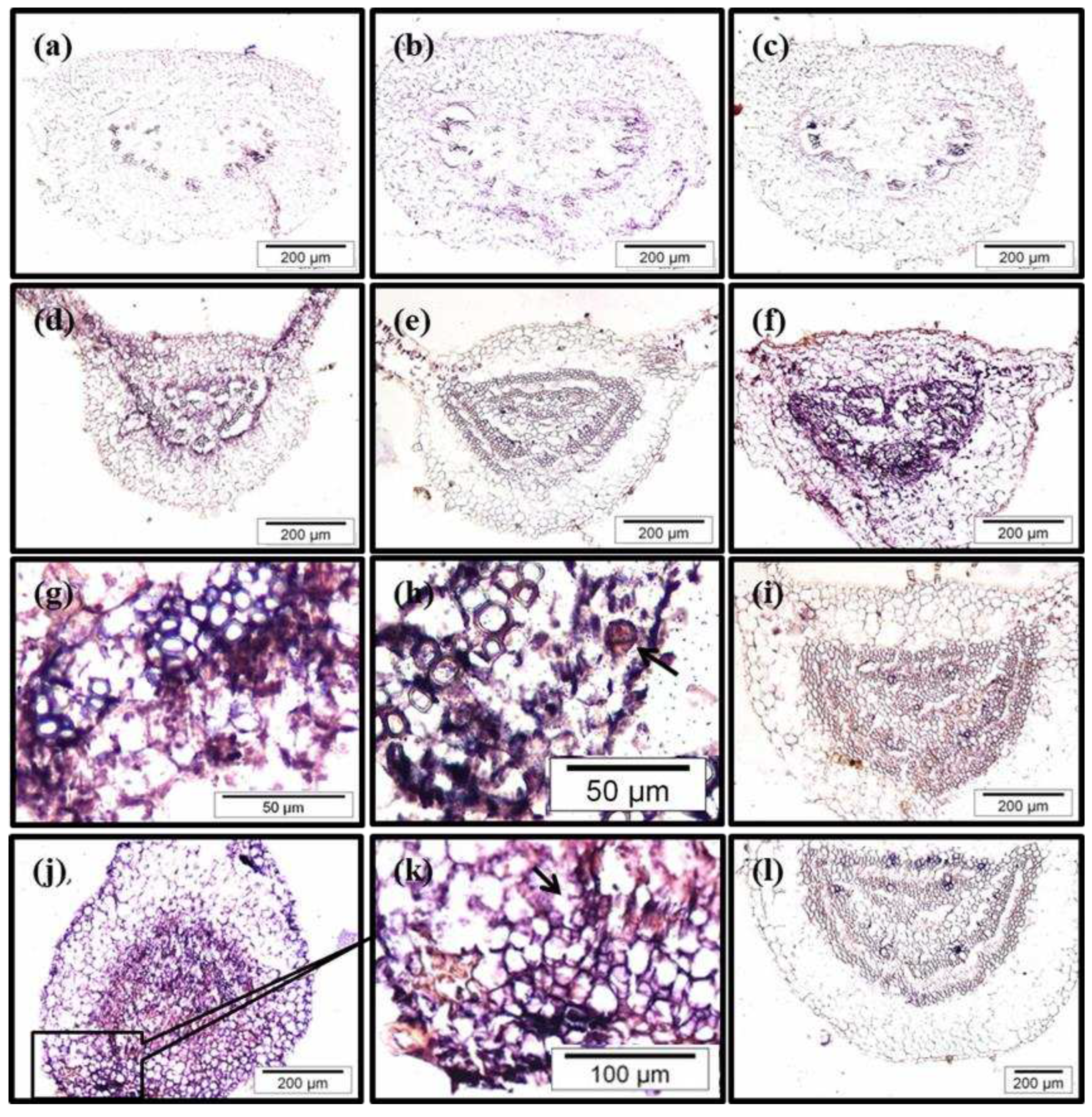
Figure 7.
In situ localization of CsSCL1 mRNA in sections of leaves excised from crown (a, b, c) and basal (d, e, f, g, h, i, j, k, l) microshoots. Leaves were treated with 0 µM IBA (b, e), 25 µM IBA (a, c, d, f, g, h, j, k) or with 25 µM IBA and 50 µM NPA simultaneously (i, l). Leaves were harvested 12 h (d), 24 h (a, b, c, e, f, g, h, i, l) or 72 h (j, k) after the start of treatments. Sections were hybridized with the antisense probe (b-k) or the sense probe (a, l). CsSCL1 expression is localized to cells surrounding the vascular tissues in IBA-treated juvenile leaves (d, f, g). Putative asymmetric cell division (h) and organized cell divisions (k) are indicated by arrows.
Figure 7.
In situ localization of CsSCL1 mRNA in sections of leaves excised from crown (a, b, c) and basal (d, e, f, g, h, i, j, k, l) microshoots. Leaves were treated with 0 µM IBA (b, e), 25 µM IBA (a, c, d, f, g, h, j, k) or with 25 µM IBA and 50 µM NPA simultaneously (i, l). Leaves were harvested 12 h (d), 24 h (a, b, c, e, f, g, h, i, l) or 72 h (j, k) after the start of treatments. Sections were hybridized with the antisense probe (b-k) or the sense probe (a, l). CsSCL1 expression is localized to cells surrounding the vascular tissues in IBA-treated juvenile leaves (d, f, g). Putative asymmetric cell division (h) and organized cell divisions (k) are indicated by arrows.
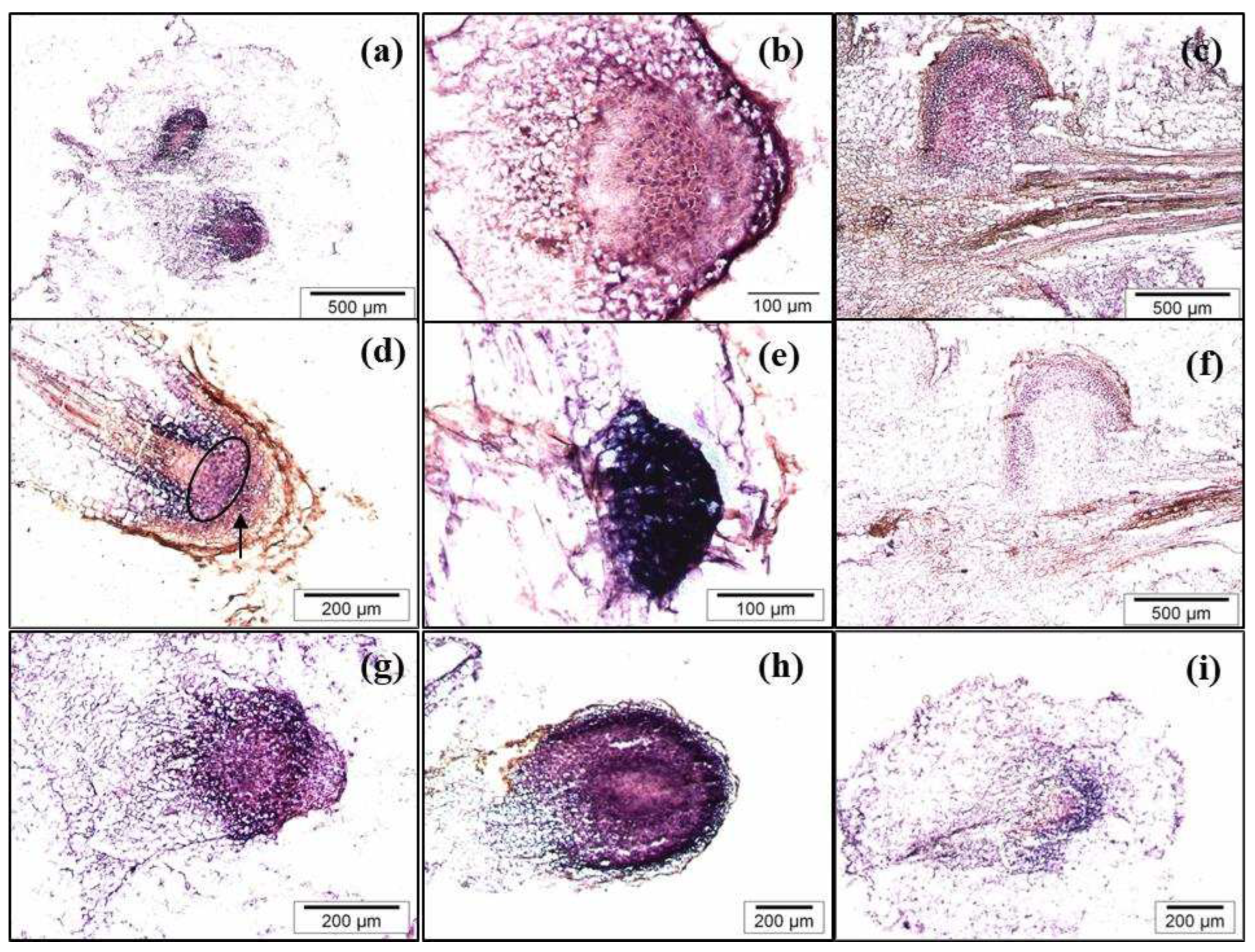
Figure 8.
In situ localization of CsSCL1 mRNA in cross (a, b, f, g, h, i) and longitudinal sections (c, d, e, f) of roots developed from BS leaves. Leaves were treated with IBA (a, b, c, d, e, f, g), treated simultaneously with IBA and NPA (h) or treated with IBA and then transferred to NPA containing medium (i). Sections were hybridized with the antisense (a, b, c, d, e, g, h, i) or the sense probe (f). The arrow in 8d indicates the columella progenitor cells.
Figure 8.
In situ localization of CsSCL1 mRNA in cross (a, b, f, g, h, i) and longitudinal sections (c, d, e, f) of roots developed from BS leaves. Leaves were treated with IBA (a, b, c, d, e, f, g), treated simultaneously with IBA and NPA (h) or treated with IBA and then transferred to NPA containing medium (i). Sections were hybridized with the antisense (a, b, c, d, e, g, h, i) or the sense probe (f). The arrow in 8d indicates the columella progenitor cells.
Figure 9.
Adventitious rooting in leaves excised from in vitro proliferating shoots of cork oak (a), and birch (b, c). (a, b) Leaves were treated with 25 µM IBA for 5 days and then transferred to IBA-free medium. (c) Adventitious root formation in birch leaf not treated with IBA.
Figure 9.
Adventitious rooting in leaves excised from in vitro proliferating shoots of cork oak (a), and birch (b, c). (a, b) Leaves were treated with 25 µM IBA for 5 days and then transferred to IBA-free medium. (c) Adventitious root formation in birch leaf not treated with IBA.
Figure 10.
Indole-3-butyric acid (IBA) and N-1-naphthyl-phthalamic acid (NPA) treatments applied to chestnut leaves. Leaves excised from chestnut microshoots were treated with 25 µM IBA for 5 days in darkness and transferred to IBA-free medium under normal photoperiod. NPA (50 µM) was applied simultaneously to IBA treatment for 5 days or after IBA treatment for 5 or 25 days. Evaluation was performed at day 30. IBA +, application of IBA; IBA -, transference to IBA-free medium. NPA +, application of NPA; NPA -, transference to NPA-free medium.
Figure 10.
Indole-3-butyric acid (IBA) and N-1-naphthyl-phthalamic acid (NPA) treatments applied to chestnut leaves. Leaves excised from chestnut microshoots were treated with 25 µM IBA for 5 days in darkness and transferred to IBA-free medium under normal photoperiod. NPA (50 µM) was applied simultaneously to IBA treatment for 5 days or after IBA treatment for 5 or 25 days. Evaluation was performed at day 30. IBA +, application of IBA; IBA -, transference to IBA-free medium. NPA +, application of NPA; NPA -, transference to NPA-free medium.
Table 1.
Rooting response of P2 BS chestnut microshoots derived from basal sprouts, leaves excised from BS microshoots and leaf segments. Microshoots were dipped in 4.9 mM IBA solution for 1 min and then placed on IBA-free medium. Leaves and leaf segments were placed for 5 d on medium containing 25 µM IBA and then transferred to IBA-free medium. Each value represents the mean (±SE) from three experiments with 18 replicates of each type of explant. NBR:Non-basal roots; LRL: longest root length; MRT: Mean rooting time.
Table 1.
Rooting response of P2 BS chestnut microshoots derived from basal sprouts, leaves excised from BS microshoots and leaf segments. Microshoots were dipped in 4.9 mM IBA solution for 1 min and then placed on IBA-free medium. Leaves and leaf segments were placed for 5 d on medium containing 25 µM IBA and then transferred to IBA-free medium. Each value represents the mean (±SE) from three experiments with 18 replicates of each type of explant. NBR:Non-basal roots; LRL: longest root length; MRT: Mean rooting time.
| Explant |
Rooting(%) |
Nº Roots |
NBR (%) |
LRL(cm) |
MRT (days) |
| Shoot |
94.8±3.2 (a) |
8.4±0.9 (a) |
51.7±19.8 (a) |
2.6±1.8 (b) |
11.3±0,7 (b) |
| Leaf |
96.3±1.9 (a) |
5.8±0.4 (b) |
8.7±0.8 (b) |
3.7±3.1 (a) |
9.3±0.3 (a) |
| Leaf segment |
69.1±5.0 (a) |
2.9±0.2 (c) |
2.9±1.5 (b) |
2.5±0.8 (b) |
11.1±0.2 (b) |