Submitted:
05 July 2023
Posted:
10 July 2023
You are already at the latest version
Abstract
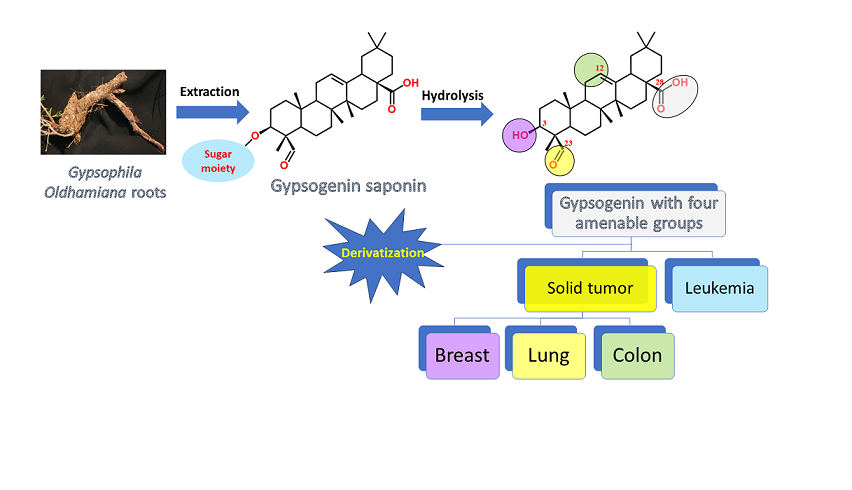
Keywords:
1. Introduction
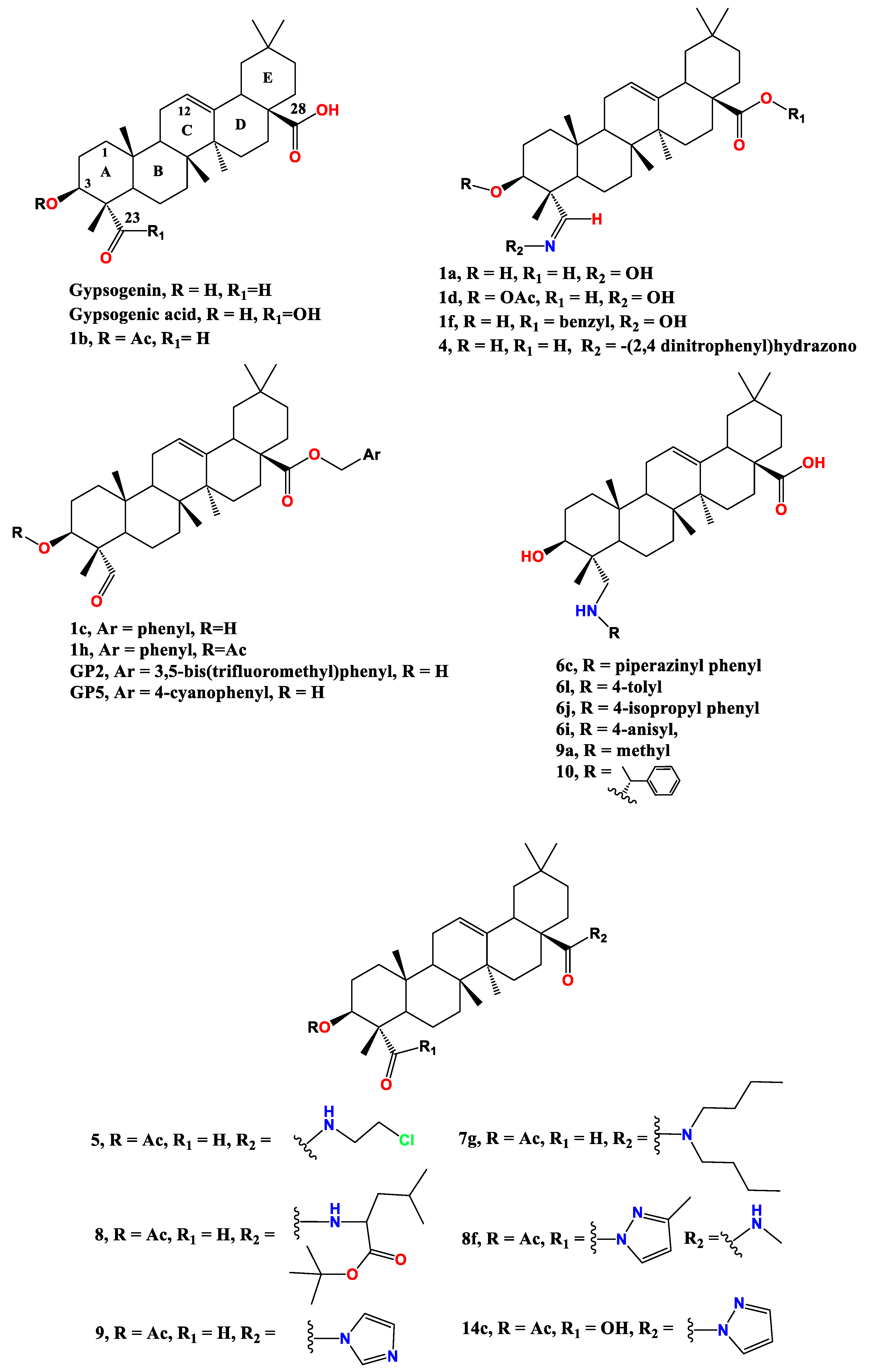
2. Gypsogenin extraction and chemical transformation:
3. Anti-cancer effect of gypsogenin, gypsogenic acid, and their semisynthetic derivatives
3.1. Anti-leukemic activity
3.2. Anti-breast cancer activity
3.3. Anti-lung cancer activity
3.4. Other anti-cancer activities
4. Conclusion and Future Directions
Acknowledgments
Conflicts of Interest
References
- J. Ferlay et al., “Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012,” Int. J. Cancer, vol. 136, no. 5, pp. E359-386, Mar. 2015. [CrossRef]
- F. Bray, J. Ferlay, I. Soerjomataram, R. L. Siegel, L. A. Torre, and A. Jemal, “Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries,” CA. Cancer J. Clin., vol. 68, no. 6, pp. 394–424, Nov. 2018. [CrossRef]
- C. Santucci et al., “Progress in cancer mortality, incidence, and survival: a global overview,” Eur. J. Cancer Prev. Off. J. Eur. Cancer Prev. Organ. ECP, vol. 29, no. 5, pp. 367–381, Sep. 2020. [CrossRef]
- J. Ferlay et al., “Cancer incidence and mortality patterns in Europe: Estimates for 40 countries and 25 major cancers in 2018,” Eur. J. Cancer Oxf. Engl. 1990, vol. 103, pp. 356–387, Nov. 2018. [CrossRef]
- S. Tan, D. Li, and X. Zhu, “Cancer immunotherapy: Pros, cons and beyond,” Biomed. Pharmacother. Biomedecine Pharmacother., vol. 124, p. 109821, Apr. 2020. [CrossRef]
- Z. Liu, Y. Ren, S. Weng, H. Xu, L. Li, and X. Han, “A New Trend in Cancer Treatment: The Combination of Epigenetics and Immunotherapy,” Front. Immunol., vol. 13, p. 809761, 2022. [CrossRef]
- B. Pereira, M. Billaud, and R. Almeida, “RNA-Binding Proteins in Cancer: Old Players and New Actors,” Trends Cancer, vol. 3, no. 7, pp. 506–528, Jul. 2017. [CrossRef]
- B. Bhinder, C. Gilvary, N. S. Madhukar, and O. Elemento, “Artificial Intelligence in Cancer Research and Precision Medicine,” Cancer Discov., vol. 11, no. 4, pp. 900–915, Apr. 2021. [CrossRef]
- M. O. Radwan et al., “New insight into the bioactivity of substituted benzimidazole derivatives: Repurposing from anti-HIV activity to cell migration inhibition targeting hnRNP M,” Bioorg. Med. Chem., pp. 117294–117294, Apr. 2023. [CrossRef]
- R. Rupaimoole and F. J. Slack, “MicroRNA therapeutics: towards a new era for the management of cancer and other diseases,” Nat. Rev. Drug Discov., vol. 16, no. 3, pp. 203–222, Mar. 2017. [CrossRef]
- A. Alqathama et al., “The in vitro cytotoxicity against human melanoma cells, tyrosinase inhibition and antioxidant activity of Grewia tenax leaves extracts,” Boletin Latinoam. Caribe Plantas Med. Aromat., vol. 22, no. 2, Art. no. 2, Nov. 2022. [CrossRef]
- Bader, A.; et al. “In Vitro Anticancer and Antibacterial Activities of the Essential Oil of Forsskal’s Basil Growing in Extreme Environmental Conditions,” Life, vol. 13, no. 3, Art. no. 3, Mar. 2023. [CrossRef]
- F. Abo-Elghiet, M. H. Ibrahim, M. A. El Hassab, A. Bader, Q. M. A. Abdallah, and A. Temraz, “LC/MS analysis of Viscum cruciatum Sieber ex Boiss. extract with anti-proliferative activity against MCF-7 cell line via G0/G1 cell cycle arrest: An in-silico and in-vitro study,” J. Ethnopharmacol., vol. 295, p. 115439, Sep. 2022. [CrossRef]
- A. Bader et al., “Design and Synthesis of 4-O-Podophyllotoxin Sulfamate Derivatives as Potential Cytotoxic Agents,” Evid. Based Complement. Alternat. Med., vol. 2021, p. e6672807, Jan. 2021. [CrossRef]
- G. A. Gutiérrez-Rebolledo, A. G. Siordia-Reyes, M. Meckes-Fischer, and A. Jiménez-Arellanes, “Hepatoprotective properties of oleanolic and ursolic acids in antitubercular drug-induced liver damage,” Asian Pac. J. Trop. Med., vol. 9, no. 7, pp. 644–651, Jul. 2016. [CrossRef]
- G. B. Xu, Y. H. Xiao, Q. Y. Zhang, M. Zhou, and S. G. Liao, “Hepatoprotective natural triterpenoids,” Eur. J. Med. Chem., vol. 145, pp. 691–716, Feb. 2018. [CrossRef]
- T. B. Ayeleso, M. G. Matumba, and E. Mukwevho, “Oleanolic Acid and Its Derivatives: Biological Activities and Therapeutic Potential in Chronic Diseases,” Mol. Basel Switz., vol. 22, no. 11, p. 1915, Nov. 2017. [CrossRef]
- A. M. Aly, L. Al-Alousi, and H. A. Salem, “Licorice: A Possible Anti-inflammatory and Anti-ulcer Drug.,” AAPS PharmSciTech, vol. 6, no. 1, pp. E74-82, 2005. [CrossRef]
- S. J. Tsai and M. C. Yin, “Antioxidative and anti-inflammatory protection of oleanolic acid and ursolic acid in PC12 cells,” J. Food Sci., 2008. [CrossRef]
- M. O. Radwan, M. A. H. Ismail, S. El-Mekkawy, N. S. M. Ismail, and A. G. Hanna, “Synthesis and biological activity of new 18β-glycyrrhetinic acid derivatives,” Arab. J. Chem., vol. 9, no. 3, pp. 390–399, 16. May 2016. [CrossRef]
- L. I. Somova, F. O. Shode, P. Ramnanan, and A. Nadar, “Antihypertensive, antiatherosclerotic and antioxidant activity of triterpenoids isolated from Olea europaea, subspecies africana leaves,” J. Ethnopharmacol., vol. 84, no. 2–3, pp. 299–305, Feb. 2003. [CrossRef]
- L. O. Somova, A. Nadar, P. Rammanan, and F. O. Shode, “Cardiovascular, antihyperlipidemic and antioxidant effects of oleanolic and ursolic acids in experimental hypertension,” Phytomedicine, vol. 10, no. 2–3, pp. 115–121, Jan. 2003. [CrossRef]
- S. Zhang et al., “Antihypertensive activity of oleanolic acid is mediated via downregulation of secretory phospholipase A2 and fatty acid synthase in spontaneously hypertensive rats,” Int. J. Mol. Med., vol. 46, no. 6, pp. 2019–2034, Sep. 2020. [CrossRef]
- R. Pompei, S. Laconi, and A. Ingianni, “Antiviral Properties of Glycyrrhizic Acid and its Semisynthetic Derivatives,” Mini-Rev. Med. Chem., 2012. [CrossRef]
- Z.-G. Sun, T.-T. Zhao, N. Lu, Y.-A. Yang, and H.-L. Zhu, “Research Progress of Glycyrrhizic Acid on Antiviral Activity,” Mini-Rev. Med. Chem., vol. 19, no. 10, pp. 826–832, 19. May 2019. [CrossRef]
- M. J. Tohmé, M. C. Giménez, A. Peralta, M. I. Colombo, and L. R. Delgui, “Ursolic acid: A novel antiviral compound inhibiting rotavirus infection in vitro,” Int. J. Antimicrob. Agents, vol. 54, no. 5, pp. 601–609, Nov. 2019. [CrossRef]
- Y. Yang et al., “Antifibrosis effects of triterpene acids of Eriobotrya japonica (Thunb.) Lindl. leaf in a rat model of bleomycin-induced pulmonary fibrosis,” J. Pharm. Pharmacol., vol. 64, no. 12, pp. 1751–1760, 2012. [CrossRef]
- M. K. Lee, K. Y. Lee, H. Y. Jeon, S. H. Sung, and Y. C. Kim, “Antifibrotic activity of triterpenoids from the aerial parts of Euscaphis japonica on hepatic stellate cells,” J. Enzyme Inhib. Med. Chem., vol. 24, no. 6, pp. 1276–1279, Dec. 2009. [CrossRef]
- H. Xiang et al., “A New Oleanolic Acid Derivative against CCl₄-Induced Hepatic Fibrosis in Rats,” Int. J. Mol. Sci., vol. 18, no. 3, p. 553, Mar. 2017. [CrossRef]
- C. Farina, M. Pinza, and G. Pifferi, “Synthesis and anti-ulcer activity of new derivatives of glycyrrhetic, oleanolic and ursolic acids,” Il Farm., vol. 53, no. 1, pp. 22–32, Jan. 1998. [CrossRef]
- L. B. Somensi et al., “Gastroprotective properties of Lupeol-derived ester: Pre-clinical evidences of Lupeol-stearate as a potent antiulcer agent,” Chem. Biol. Interact., vol. 321, pp. 108964–108964, Apr. 2020. [CrossRef]
- M. Chudzik, I. Korzonek-Szlacheta, and W. Król, “Triterpenes as Potentially Cytotoxic Compounds,” Molecules, vol. 20, no. 1, pp. 1610–1625, Jan. 2015. [CrossRef]
- Z.-Y. Tang, Y. Li, Y.-T. Tang, X.-D. Ma, and Z.-Y. Tang, “Anticancer activity of oleanolic acid and its derivatives: Recent advances in evidence, target profiling and mechanisms of action,” Biomed. Pharmacother., vol. 145, p. 112397, Jan. 2022. [CrossRef]
- J. A. R. Salvador et al., “Oleanane-, ursane-, and quinone methide friedelane-type triterpenoid derivatives: Recent advances in cancer treatment,” Eur. J. Med. Chem., vol. 142, pp. 95–130, Dec. 2017. [CrossRef]
- M. N. Laszczyk, “Pentacyclic triterpenes of the lupane, oleanane and ursane group as tools in cancer therapy,” Planta Med., vol. 75, no. 15, pp. 1549–1560, Dec. 2009. [CrossRef]
- M. H. Ghante and P. G. Jamkhande, “Role of Pentacyclic Triterpenoids in Chemoprevention and Anticancer Treatment: An Overview on Targets and Underling Mechanisms,” J. Pharmacopuncture, vol. 22, no. 2, pp. 55–67, Jun. 2019. [CrossRef]
- U. Shaheen, E. A. Ragab, A. N. Abdalla, and A. Bader, “Triterpenoidal saponins from the fruits of Gleditsia caspica with proapoptotic properties,” Phytochemistry, vol. 145, pp. 168–178, Jan. 2018. [CrossRef]
- J. Liese, B. A. Abhari, and S. Fulda, “Smac mimetic and oleanolic acid synergize to induce cell death in human hepatocellular carcinoma cells,” Cancer Lett., vol. 365, no. 1, pp. 47–56, Aug. 2015. [CrossRef]
- X. Wang et al., “Inhibitory effect of oleanolic acid on hepatocellular carcinoma via ERK-p53-mediated cell cycle arrest and mitochondrial-dependent apoptosis,” Carcinogenesis, vol. 34, no. 6, pp. 1323–1330, Jun. 2013. [CrossRef]
- D.-W. Mu, H.-Q. Guo, G.-B. Zhou, J.-Y. Li, and B. Su, “Oleanolic acid suppresses the proliferation of human bladder cancer by Akt/mTOR/S6K and ERK1/2 signaling,” Int. J. Clin. Exp. Pathol., vol. 8, no. 11, pp. 13864–13870, 2015.
- S. Amara, M. Zheng, and V. Tiriveedhi, “Oleanolic Acid Inhibits High Salt-Induced Exaggeration of Warburg-like Metabolism in Breast Cancer Cells,” Cell Biochem. Biophys., vol. 74, no. 3, pp. 427–434, Sep. 2016. [CrossRef]
- B. Chakravarti et al., “In vitro anti-breast cancer activity of ethanolic extract of Wrightia tomentosa: role of pro-apoptotic effects of oleanolic acid and urosolic acid,” J. Ethnopharmacol., vol. 142, no. 1, pp. 72–79, Jun. 2012. [CrossRef]
- X. Zhao, M. Liu, and D. Li, “Oleanolic acid suppresses the proliferation of lung carcinoma cells by miR-122/Cyclin G1/MEF2D axis,” Mol. Cell. Biochem., vol. 400, no. 1–2, pp. 1–7, Feb. 2015. [CrossRef]
- R. A. Furtado et al., “Ursolic acid and oleanolic acid suppress preneoplastic lesions induced by 1,2-dimethylhydrazine in rat colon,” Toxicol. Pathol., vol. 36, no. 4, pp. 576–580, Jun. 2008. [CrossRef]
- N. B. Janakiram, C. Indranie, S. V. Malisetty, P. Jagan, V. E. Steele, and C. V. Rao, “Chemoprevention of colon carcinogenesis by oleanolic acid and its analog in male F344 rats and modulation of COX-2 and apoptosis in human colon HT-29 cancer cells,” Pharm. Res., vol. 25, no. 9, pp. 2151–2157, Sep. 2008. [CrossRef]
- L. Žiberna et al., “Oleanolic Acid Alters Multiple Cell Signaling Pathways: Implication in Cancer Prevention and Therapy,” Int. J. Mol. Sci., vol. 18, no. 3, Art. no. 3, Mar. 2017. [CrossRef]
- V. R. Yadav, S. Prasad, B. Sung, R. Kannappan, and B. B. Aggarwal, “Targeting Inflammatory Pathways by Triterpenoids for Prevention and Treatment of Cancer,” Toxins, vol. 2, no. 10, pp. 2428–2466, Oct. 2010. [CrossRef]
- R. Borella et al., “Synthesis and Anticancer Activity of CDDO and CDDO-Me, Two Derivatives of Natural Triterpenoids,” Molecules, vol. 24, no. 22, p. 4097, Nov. 2019. [CrossRef]
- Y.-C. Hsu et al., “18β-glycyrrhetinic Acid Modulated Autophagy is Cytotoxic to Breast Cancer Cells,” Int. J. Med. Sci., vol. 20, no. 4, pp. 444–454, 2023. [CrossRef]
- J. Chen et al., “18β-Glycyrrhetinic-acid-mediated unfolded protein response induces autophagy and apoptosis in hepatocellular carcinoma,” Sci. Rep., vol. 8, no. 1, p. 9365, Jun. 2018. [CrossRef]
- Y. Sun, C. Dai, M. Yin, J. Lu, H. Hu, and D. Chen, “Hepatocellular carcinoma-targeted effect of configurations and groups of glycyrrhetinic acid by evaluation of its derivative-modified liposomes,” Int. J. Nanomedicine, vol. 13, pp. 1621–1632, 2018. [CrossRef]
- C. S. Lee, Y. J. Kim, M. S. Lee, E. S. Han, and S. J. Lee, “18beta-Glycyrrhetinic acid induces apoptotic cell death in SiHa cells and exhibits a synergistic effect against antibiotic anti-cancer drug toxicity,” Life Sci., vol. 83, no. 13–14, pp. 481–489, Sep. 2008. [CrossRef]
- H. Yamaguchi et al., “Novel effects of glycyrrhetinic acid on the central nervous system tumorigenic progenitor cells: induction of actin disruption and tumor cell-selective toxicity,” Eur. J. Med. Chem., vol. 45, no. 7, pp. 2943–2948, Jul. 2010. [CrossRef]
- A. Roohbakhsh, M. Iranshahy, and M. Iranshahi, “Glycyrrhetinic Acid and Its Derivatives: Anti-Cancer and Cancer Chemopreventive Properties, Mechanisms of Action and Structure- Cytotoxic Activity Relationship.,” Curr. Med. Chem., vol. 23, no. 5, pp. 498–517, 2016.
- S. Zafar et al., “Ursolic acid: a natural modulator of signaling networks in different cancers,” Cancer Cell Int., vol. 22, no. 1, p. 399, Dec. 2022. [CrossRef]
- T. J. Raphael and G. Kuttan, “Effect of naturally occurring triterpenoids ursolic acid and glycyrrhizic acid on the cell-mediated immune responses of metastatic tumor-bearing animals,” Immunopharmacol. Immunotoxicol., vol. 30, no. 2, pp. 243–255, 2008. [CrossRef]
- D.-K. Kim et al., “Apoptotic activity of ursolic acid may correlate with the inhibition of initiation of DNA replication,” Int. J. Cancer, vol. 87, no. 5, pp. 629–636, 2000. [CrossRef]
- X.-S. Liu and J. Jiang, “Induction of Apoptosis and Regulation of the MAPK Pathway by Ursolic Acid in Human Leukemia K562 Cells,” Planta Med., vol. 73, no. 11, pp. 1192–1194, Sep. 2007. [CrossRef]
- E. Pisha et al., “Discovery of betulinic acid as a selective inhibitor of human melanoma that functions by induction of apoptosis,” Nat. Med., vol. 1, no. 10, pp. 1046–1051, Oct. 1995. [CrossRef]
- A. Hordyjewska, A. Ostapiuk, A. Horecka, and J. Kurzepa, “Betulin and betulinic acid: triterpenoids derivatives with a powerful biological potential,” Phytochem. Rev., vol. 18, no. 3, pp. 929–951, Jun. 2019. [CrossRef]
- S. Fulda, “Betulinic acid: a natural product with anticancer activity,” Mol. Nutr. Food Res., vol. 53, no. 1, pp. 140–146, Jan. 2009. [CrossRef]
- E. Selzer et al., “Effects of betulinic acid alone and in combination with irradiation in human melanoma cells,” J. Invest. Dermatol., vol. 114, no. 5, pp. 935–940, 00. May 2020. [CrossRef]
- S. Fulda and K.-M. Debatin, “Sensitization for Anticancer Drug-Induced Apoptosis by Betulinic Acid,” Neoplasia N. Y. N, vol. 7, no. 2, pp. 162–170, Feb. 2005.
- Y. Lim et al., “Celastrol in cancer therapy: Recent developments, challenges and prospects,” Cancer Lett., vol. 521, pp. 252–267, Nov. 2021. [CrossRef]
- H. Yang, D. Chen, Q. C. Cui, X. Yuan, and Q. P. Dou, “Celastrol, a triterpene extracted from the Chinese ‘Thunder of God Vine,’ is a potent proteasome inhibitor and suppresses human prostate cancer growth in nude mice,” Cancer Res., vol. 66, no. 9, pp. 4758–4765, 06. May 2006. [CrossRef]
- M. Nagase, J. Oto, S. Sugiyama, K. Yube, Y. Takaishi, and N. Sakato, “Apoptosis induction in HL-60 cells and inhibition of topoisomerase II by triterpene celastrol,” Biosci. Biotechnol. Biochem., vol. 67, no. 9, pp. 1883–1887, Sep. 2003. [CrossRef]
- R. Kannaiyan et al., “Celastrol inhibits tumor cell proliferation and promotes apoptosis through the activation of c-Jun N-terminal kinase and suppression of PI3 K/Akt signaling pathways,” Apoptosis Int. J. Program. Cell Death, vol. 16, no. 10, pp. 1028–1041, Oct. 2011. [CrossRef]
- H. Zhang et al., “Synthesis of gypsogenin derivatives with capabilities to arrest cell cycle and induce apoptosis in human cancer cells,” R. Soc. Open Sci., vol. 5, no. 1, pp. 171510–171510, Jan. 2018. [CrossRef]
- C. Gampe and V. A. Verma, “Curse or Cure? A Perspective on the Developability of Aldehydes as Active Pharmaceutical Ingredients,” J. Med. Chem., vol. 63, no. 23, pp. 14357–14381, Dec. 2020. [CrossRef]
- A. Weiss et al., “FGF401, A First-In-Class Highly Selective and Potent FGFR4 Inhibitor for the Treatment of FGF19-Driven Hepatocellular Cancer,” Mol. Cancer Ther., vol. 18, no. 12, pp. 2194–2206, Dec. 2019. [CrossRef]
- “Covalent docking of large libraries for the discovery of chemical probes | Nature Chemical Biology.” https://www.nature.com/articles/nchembio.1666 (accessed Jun. 29, 2023).
- L. Heller, S. Schwarz, B. A. Weber, and R. Csuk, “Gypsogenin derivatives: an unexpected class of inhibitors of cholinesterases,” Arch. Pharm. (Weinheim), vol. 347, no. 10, pp. 707–716, Oct. 2014. [CrossRef]
- N. A. J. C. Furtado et al., “Pentacyclic Triterpene Bioavailability: An Overview of In Vitro and In Vivo Studies,” Molecules, vol. 22, no. 3, Art. no. 3, Mar. 2017. [CrossRef]
- S. Emirdağ-Öztürk, T. Karayıldırım, A. Çapcı-Karagöz, Ö. Alankuş-Çalışkan, A. Özmen, and E. Poyrazoğlu-Çoban, “Synthesis, antimicrobial and cytotoxic activities, and structure-activity relationships of gypsogenin derivatives against human cancer cells,” Eur. J. Med. Chem., vol. 82, pp. 565–573, Jul. 2014. [CrossRef]
- K.-P. Sun, T.-T. Zhao, L. Liu, X.-D. Mu, and J.-Y. Sun, “Anticancer Structure-activity Relationships and Potential Target Exploration of the Natural Product Gypsogenin,” ChemistrySelect, vol. 8, no. 10, p. e202300072, 2023. [CrossRef]
- G. Wu, H. Chu, J. Wang, Y. Mu, and J. Sun, “Synthesis of gypsogenin and gypsogenic acid derivatives with antitumor activity by damaging cell membranes,” New J. Chem., vol. 43, no. 47, pp. 18898–18914, Dec. 2019. [CrossRef]
- H. I. Ciftci et al., “EGFR-Targeted Pentacyclic Triterpene Analogues for Glioma Therapy,” Int. J. Mol. Sci., vol. 22, no. 20, pp. 10945–10945, Oct. 2021. [CrossRef]
- N. G. Ulusoy et al., “Design, semi-synthesis and examination of new gypsogenin derivatives against leukemia via Abl tyrosine kinase inhibition and apoptosis induction,” Int. J. Biol. Macromol., vol. 222, pp. 1487–1499, Dec. 2022. [CrossRef]
- H. I. Ciftci et al., “Design, Synthesis and Biological Evaluation of Pentacyclic Triterpene Derivatives: Optimization of Anti-ABL Kinase Activity,” Molecules, vol. 24, no. 19, pp. 3535–3535, Sep. 2019. [CrossRef]
- I. Lee et al., “Cytotoxicity of triterpenes isolated from Aceriphyllum rossii,” Chem. Pharm. Bull. (Tokyo), vol. 55, no. 9, pp. 1376–1378, Sep. 2007. [CrossRef]
- I. Krasteva, M. Yotova, D. Yosifov, N. Benbassat, K. Jenett-Siems, and S. Konstantinov, “Cytotoxicity of gypsogenic acid isolated from Gypsophila trichotoma,” Pharmacogn. Mag., vol. 10, no. Suppl 2, pp. S430–S433, 2014. [CrossRef]
- S. Emirdağ-Öztürk, İ. Babahan, and A. Özmen, “Synthesis, characterization and in vitro anti-neoplastic activity of gypsogenin derivatives,” Bioorganic Chem., vol. 53, pp. 15–23, Apr. 2014. [CrossRef]
- H. I. Ciftci et al., “The First Pentacyclic Triterpenoid Gypsogenin Derivative Exhibiting Anti-ABL1 Kinase and Anti-chronic Myelogenous Leukemia Activities,” Biol. Pharm. Bull., vol. 41, no. 4, pp. 570–574, 2018. [CrossRef]
- G. Tian et al., “Experimental studies of the therapeutic effect of Gypsophila oldhamiana gypsogenin on Lewis lung cancer in mice,” Chin. J. Clin. Oncol., vol. 5, no. 3, pp. 206–210, Jun. 2008. [CrossRef]
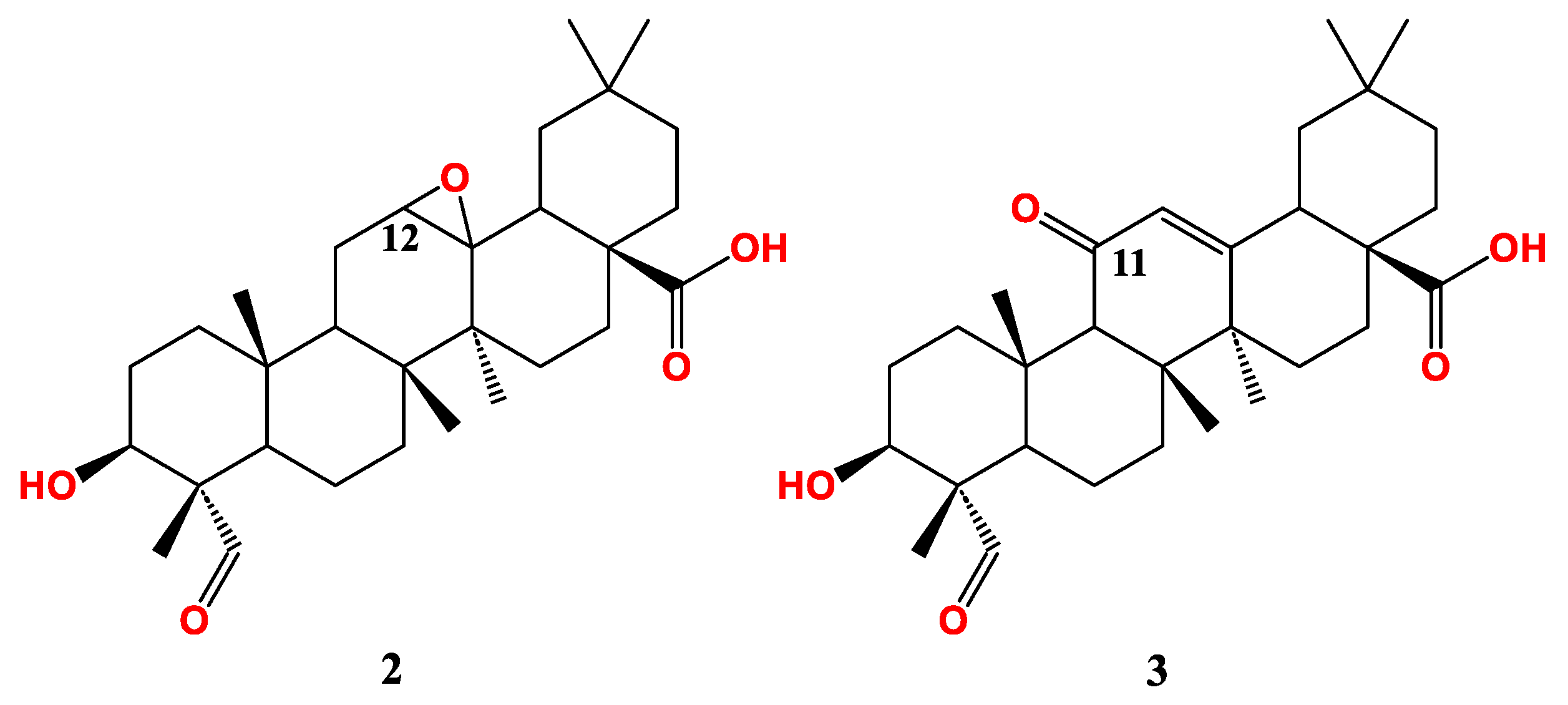
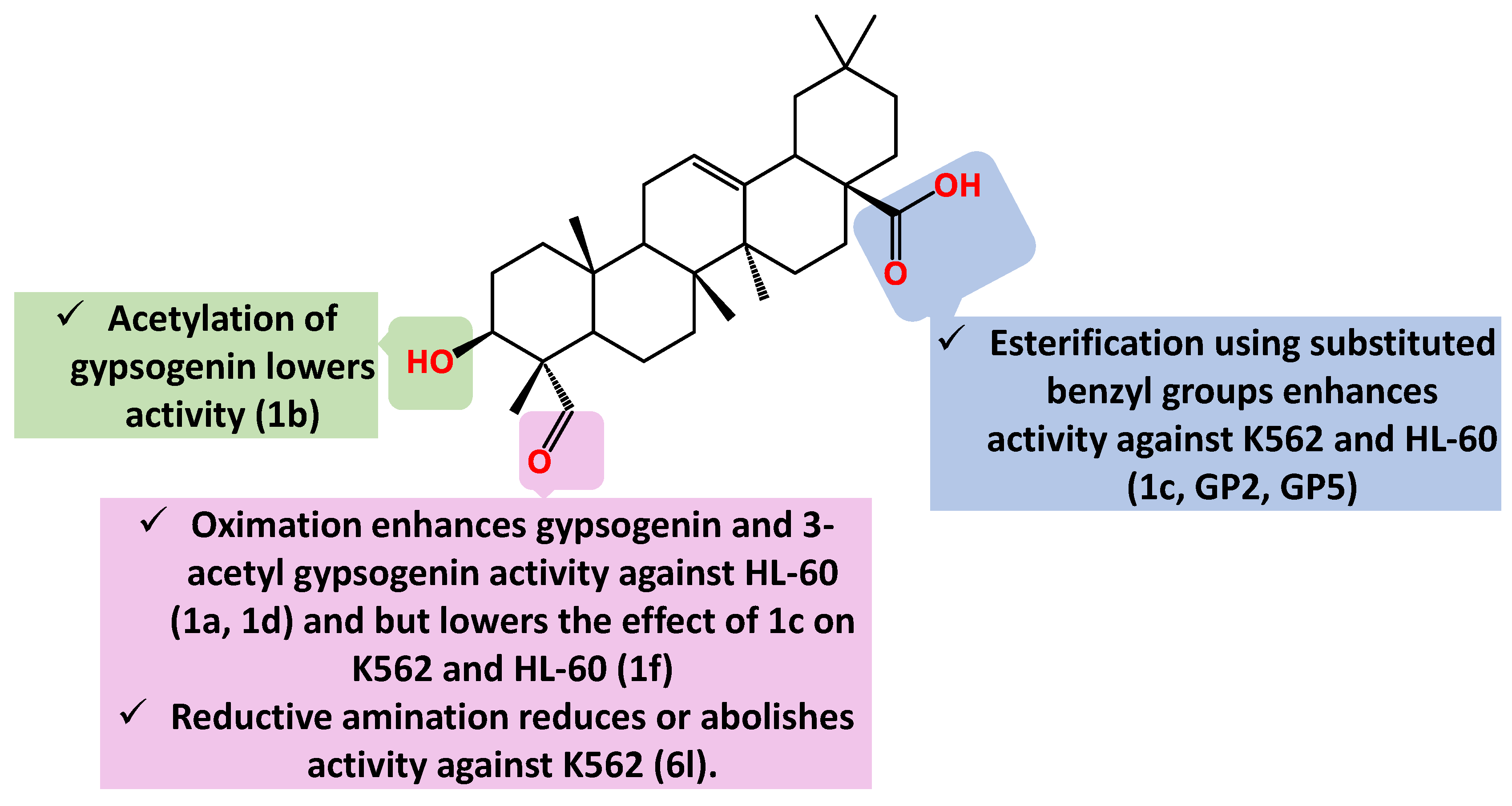
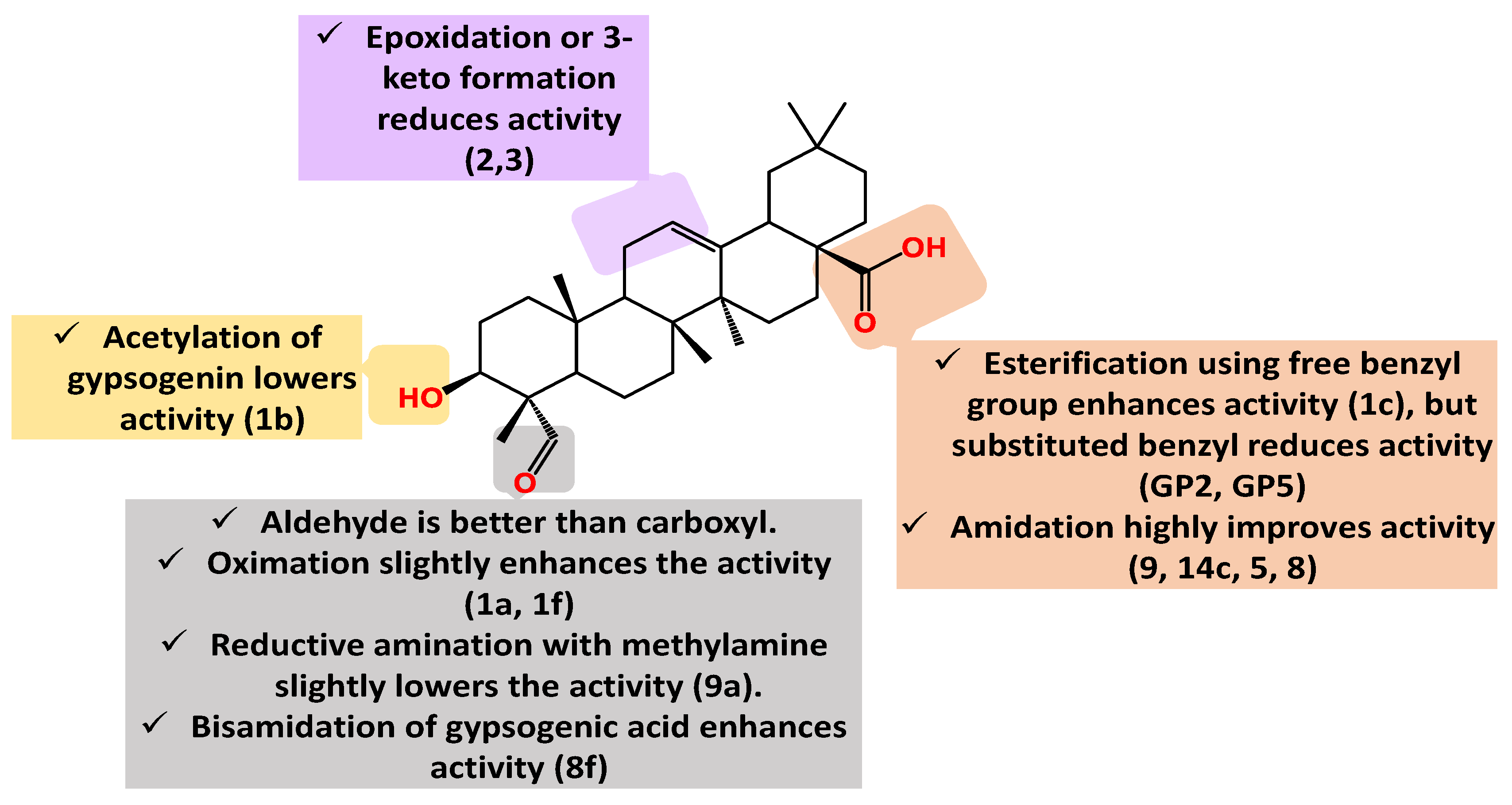
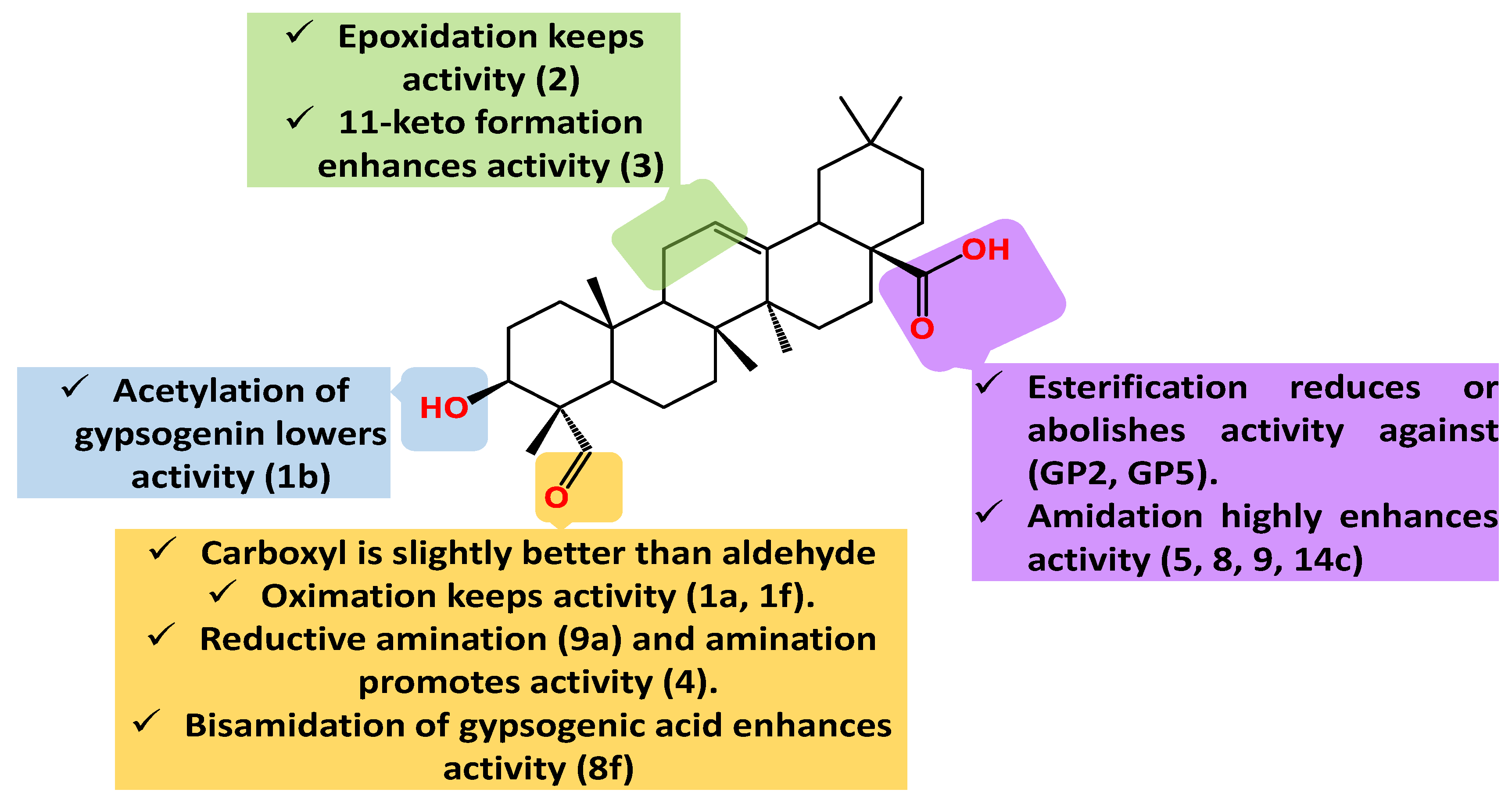
| Compound Cell line name and IC50 µM | |||
|---|---|---|---|
| HT-29 [74] | Saos-2 [82] | HeLa [74] | |
| Gypsogenin | 10.4 | 7.8 | 22.4 |
| 1a | 10.8 | 7.9 | 8.7 |
| 1b | 11.1 | 8.2 | 35.0 |
| 1d | 6.7 | 8.9 | >100 |
| LOVO [75] | |||
| 2 | > 30 | ||
| 3 | 17.8 | ||
| 5 | 7.2 | ||
| 8 | 0.8 | ||
| 9a | 5.8 | ||
| LOVO [68] | HePG2 [68] | SKOV3 [68] | |
| 4 | 2.9 | 10.0 | 9.7 |
| 7g | 3.5 | 12.5 | 13.1 |
| HepG2 [76] | TE-1 [76] | MC3-8 [76] | |
| 8f | 3.6 | 5.4 | 4.8 |
| 9 | 4.0 | 4.7 | 2.9 |
| 14c | 2.2 | 4.2 | 2.6 |
| HeLa [79] | |||
| GP2 | 35.2 | ||
| GP5 | 5.6 | ||
| U251 | T98G | U87 | |
| 10 [77] | 5.8 | 8.1 | 17.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





